Abstract
Objectives
South Africa has made remarkable progress in increasing the coverage of antiretroviral therapy (ART) among pregnant women; however, viral suppression among pregnant women receiving ART is reported to be low. Access to routine viral load testing is crucial to identify women with unsuppressed viral load early in pregnancy and to provide timely intervention to improve viral suppression. This study aimed to determine the coverage of maternal viral load monitoring nationally, focusing on viral load testing, documentation of viral load test results, and viral suppression (viral load < 50 copies/mL). At the time of this study, the first‐line regimen for women initiating ART during pregnancy was non‐nucleoside reverse transcriptase (NNRTI)‐based regimen.
Methods
Between 1 October and 15 November 2019, a cross‐sectional survey was conducted among 15‐ to 49‐year‐old pregnant women attending antenatal care in 1589 nationally representative public health facilities. Data on ART status, viral load testing and viral load test results were extracted from medical records. Logistic regression was used to examine factors associated with coverage of viral load testing.
Results
Of 8112 participants eligible for viral load testing, 81.7% received viral load testing, and 94.1% of the viral load test results were documented in the medical records. Of those who had viral load test results documented, 74.1% were virally suppressed. Women initiated on ART during pregnancy and who received ART for three months had lower coverage of viral load testing (73%) and viral suppression (56.8%) compared with women initiated on ART before pregnancy (82.8% and 76.1%, respectively). Initiating ART during pregnancy rather than before pregnancy was associated with a lower likelihood of receiving a viral load test during pregnancy (adjusted odds ratio = 1.6, 95% confidence interval: 1.4–1.8).
Conclusions
Viral load result documentation was high; viral load testing could be improved especially among women initiating ART during pregnancy. The low viral suppression among women who initiated ART during pregnancy despite receiving ART for three months highlights the importance of enhanced adherence counselling during pregnancy. Our finding supports the WHO recommendation that a Dolutegravir‐containing regimen be the preferred regimen for women who are newly initiating ART during pregnancy for more rapid viral suppression.
Keywords: pregnant women, South Africa, viral load monitoring, viral load result documentation, viral load testing, viral suppression
INTRODUCTION
Globally, the prevention of vertical HIV transmission programmes have made tremendous progress in reducing paediatric HIV infection. Despite the progress made, achieving the elimination of vertical HIV transmission target has been challenging for most Sub‐Saharan African countries due to the high burden of HIV among reproductive‐age women in these countries [1]. Reaching the elimination of vertical HIV transmission target requires sustained effort to increase early antiretroviral therapy (ART) initiation and viral suppression (viral load < 50 copies/mL) throughout the pregnancy and breastfeeding period [2]. The risk of vertical HIV transmission is directly correlated with the level of viraemia during pregnancy and breastfeeding [2, 3. Initiation of ART before pregnancy and maintaining viral suppression throughout pregnancy and breastfeeding can eliminate the risk of vertical HIV transmission, while late initiation of ART and elevated viral load level during pregnancy and breastfeeding are associated with high risk of vertical HIV transmission [2, 3. South Africa has made remarkable progress in increasing the coverage of ART among pregnant women; however, viral suppression among pregnant women receiving ART is reported to be low – up to 30% of women receiving ART are not virally suppressed at the time of delivery [4, 5
Access to routine viral load testing is crucial to identify women with unsuppressed viral load early in pregnancy and to provide them timely adherence intervention and, where necessary, antiretroviral regimen change to improve viral suppression [6]. The World Health Organization (WHO) recommends routine viral load monitoring be provided to pregnant women on ART at entry to antenatal care or at 3 months after ART initiation for pregnant women initiating ART during pregnancy, and at delivery for all pregnant women [6]. South Africa adopted this WHO guideline in 2004 [7]. Despite the early adoption of the WHO viral load monitoring guideline, the implementation of viral load testing has been suboptimal in South Africa. In 2019, an estimated 27% of adults on ART in South Africa had not received the recommended one viral load testing per annum [8]. Among pregnant women, analysis assessing the coverage of viral load testing has not been done at a national level; therefore, the coverage of viral load monitoring in this population is not known. Although there are a few small studies that have been conducted to assess maternal viral load monitoring during antenatal care, these studies have limited generalizability as they target a few clinics or focus on a particular segment of the population [9, 10
In South Africa, point‐of‐care viral load testing is not available at public facilities. Viral load testing relies on a series of critical steps that must be carried out to provide a viral load test result. These include drawing of blood specimens from patients and sending to central laboratories; performing viral load testing at central laboratories and returning results to clinics; recollecting blood specimens (for repeat testing) when specimens are rejected or have an invalid result; documenting viral load results in patients’ files, reviewing the results, and taking action based on this review. Actions may include the provision of enhanced adherence counselling and repeat viral load testing for those with high viral load count. These steps are called ‘the viral load cascade’. Breakdown in any one of these steps can cause failure to monitor viral load. Understanding at which step(s) of the viral load cascade breakdown occurs is essential to addressing the gaps that contribute to low viral load monitoring, which will assist in fast‐tracking progress towards viral suppression targets for elimination of vertical HIV transmission.
This study evaluated the coverage of maternal viral load monitoring in South Africa and identified gaps in the viral load cascade steps focusing on viral load testing, documentation of viral load results, and viral suppression among pregnant women attending antenatal care at nationally selected public facilities during the 2019 South African antenatal sentinel survey.
METHODS
Study setting
All (> 4000) primary healthcare facilities in South Africa provide antenatal care services. Prevention of vertical HIV transmission services, including viral load testing, are provided in all antenatal care facilities. At the time of this survey (October to November 2019), the South African viral load monitoring guidelines recommended that all women on ART should have viral load testing at their first antenatal care visit or upon confirmation of pregnancy [11]. For newly diagnosed women initiating ART during pregnancy, viral load testing was recommended at 3 months after ART initiation. For all HIV‐positive pregnant women, repeat viral load testing was recommended at delivery and 6 months after delivery. For women who were not virally suppressed, depending on their viral load level, viral load testing was to be repeated in 8–10 weeks (for viral load 50–999 copies/mL) or 4–6 weeks (for viral load ≥ 1000 copies/mL) after the initial unsuppressed viral load result. Although viral load testing services have been available in South Africa since 2004 [7], the coverage of viral load testing increased in recent years following WHO’s endorsement of viral load monitoring as the primary method for monitoring response to ART [6]. At the time of this survey, viral load testing for public health facilities in South Africa was provided at 17 laboratories using both the Roche and Abbott assays [12]. Results are returned via hard copy, short message system (SMS) printers or accessed through web portals [13]. At the time of this study, the first‐line regimen for women initiating ART during pregnancy was a non‐nucleoside reverse transcriptase (NNRTI)‐based ‐ most often efavirenz (EFV) ‐based ‐ regimen.
Study design and participants
The antenatal survey is a cross‐sectional survey conducted biennially in South Africa to primarily monitor HIV prevalence among pregnant women but also to evaluate the performance of the prevention of vertical HIV transmission programme. The survey aimed to enrol 36 015 pregnant women (regardless of HIV status) from 1589 public health facilities selected from each of the 52 districts in South Africa, biennially. For this sub‐analysis, only HIV‐positive pregnant women who either initiated ART before pregnancy or were taking ART for ≥ 3 months at enrolment were included in the analysis.
Sampling and data collection procedures for the antenatal survey
Health facilities that took part in the 2019 antenatal survey were selected using a stratified probability proportional to size (PPS) sampling method from each district. The 2019 survey was conducted between 1 October and 15 November 2019. During this period, consenting pregnant women aged 15–49 years, attending the antenatal clinic for the first time or for follow‐up visits during their current pregnancy were consecutively enrolled (regardless of HIV or ART status) until either the required sample size or the end of the study was reached. Health workers providing antenatal care services in the selected facilities collected demographic and clinical data (including maternal education, relationship with the father of the child, and gravidity) through interviews. Data were extracted from medical records, which included: age of the woman, gestational age at booking, gestational age today, HIV status (per rapid test performed at the clinic at the time of first antenatal care visit, during follow‐up visit, or test done before pregnancy if participants were already on ART at the time of pregnancy), the timing of ART initiation as a categorical response (i.e. initiated before pregnancy, at the first, second or third trimester), viral load test done during pregnancy, whether viral load test result was documented, and latest viral load test result as a categorical response (i.e. < 50, 50–1000, and > 1000 copies/mL). If more than one viral load test was done during the pregnancy, only information on the most recent viral load test was extracted. On the day of the survey, blood specimens were collected from each participant, regardless of prior knowledge of HIV status, and tested for HIV at regional laboratories using two serial imunoassays (IAs) following the standard guideline [14]. Detailed descriptions of the methodology of the survey have been published previously [15, 16
Sub‐study analysis
The coverage of the following three viral load cascade indicators was estimated: viral load testing; viral load test result documentation; and viral suppression (defined as viral load < 50 copies/mL).
Viral load testing
This indicator measured the proportion of HIV‐positive women eligible for viral load testing who received a viral load test in their current pregnancy (if there was more than one viral load test, the most recent viral load test was assessed). Participants were considered as ‘eligible for viral load testing’, according to the national guideline, if they initiated ART before pregnancy, or if they were newly initiated on ART during pregnancy but had received ART for at least 3 months. For the latter group, as the timing of ART initiation was reported by trimester (i.e. as first, second or third trimester), duration on ART was calculated by subtracting the mid‐week of the trimester that ART was initiated from the participants’ gestational age at survey enrolment. For participants initiated on ART in the first trimester, the gestational age/week of their first antenatal care visit was considered as the time of ART initiation, as almost all participants started antenatal care after the mid‐week of the first trimester; and where gestational age at the first visit is not reported, time of ART initiation was set at 10 weeks as most women do not attend antenatal care before 10 weeks.
Participants whose ART status was not reported, those who reported not initiating ART, and those whose rapid test or IA test was HIV‐negative were excluded from this analysis, regardless of their response to the viral load monitoring questions. This study also excluded participants whose reported timing of ART initiation was less than 3 months from their gestational age at enrolment in the survey.
Documentation of viral load test result
This indicator measured the percentage of participants who had undergone a viral load test with a result (from a test provided during pregnancy) that was documented on the medical record – if there was more than one viral load test, documentation of the most recent viral load test result was assessed (the denominator for this indicator was the number of HIV‐positive women with viral load test done).
Viral suppression
This indicator measured the percentage of participants with a documented viral load test result of < 50 copies/mL (using participants with documented viral load test result as a denominator). In the case where participants had more than one viral load test result during pregnancy, the most recent result was extracted from the medical record. The three viral load cascade indicators were analysed at the national level and in stratified groups. χ2 test was used to assess significant associations. A sensitivity analysis was conducted to validate the viral load data extracted from the medical record using laboratory data (these data are presented under Supplementary Box 1).
We fitted a multivariable survey logistic regression model to assess factors associated with not receiving a viral load test. All variables significant at a P‐value cut‐off point of 0.2 in a bivariable analysis were included in multivariable analysis. Variables significant at a P‐value cut‐off point of 0.05 and other variables that have ≥ 10% effect on other significant variables were kept in the final model. Adjusted odds ratios (AORs) and 95% confidence intervals (CIs) were reported. All analyses took into account the survey design (i.e. all analyses adjusted for the different sampling stages: stratification and clustering within primary sampling units, and for the finite number of primary sampling units). All analyses were also weighted for sample size realization and the Statistics South Africa 2019 midyear population size of women of reproductive age (15–49 years) to adjust for differential population size across provinces and for different sample size achievement at district level [17]
Ethical considerations
Participation in the survey was voluntary, requiring written informed consent. Ethical approval was obtained from the University of the Witwatersrand Human Research Ethics Committee (Medical) (ethics clearance number: M170556), and the nine provincial health research ethics committees. The study protocol was reviewed following the Centers for Disease Control and Prevention, United States (CDC‐US) human research protection procedures.
RESULTS
Of 41 598 participants approached, 11 518 were HIV‐positive per laboratory (IA) test conducted as part of the antenatal survey (Fig. 1). Of those who tested positive by IA, 95.9% (11 046) knew their HIV status before enrolment into the survey from a test done during a prior antenatal care visit or before pregnancy. Of those who knew their HIV‐positive status before enrolment in the survey, 65.2%(7207) had initiated ART before pregnancy and 8.2% (905) had initiated ART during pregnancy and received ART for at least 3 months – in total 73.4% (8112) of participants with known HIV status were eligible to receive a viral load test before the survey.
FIGURE 1.
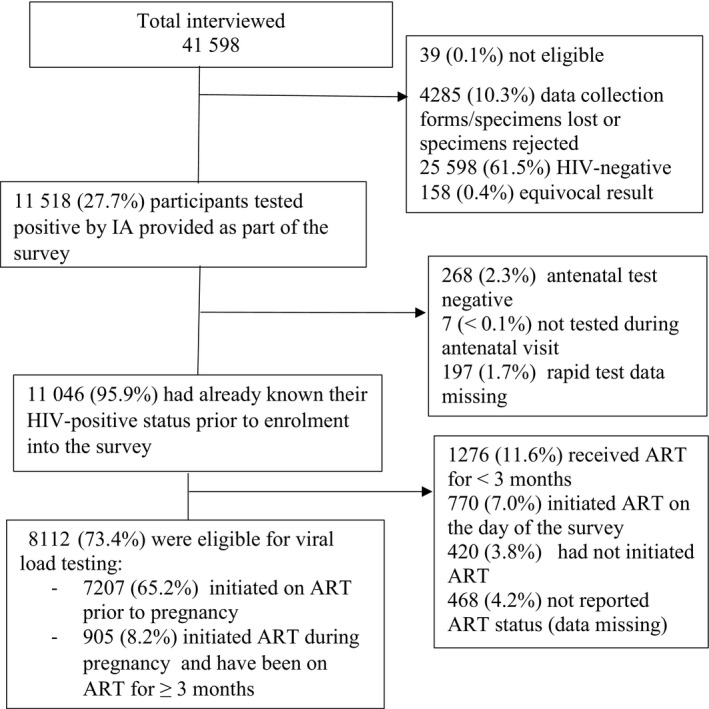
Flow chart of participants due for viral load testing in the National Antenatal Sentinel Survey, 2019. Percentages are unweighted. ART, antiretroviral therapy; IA, immunoassay. Unweighted percentages
Characteristics of participants eligible for viral load testing
Participants eligible for a viral load test had a median age of 26 years interquartile range (IQR): 22–31]. Participants who started ART before pregnancy were slightly older (median age 30 vs. 27 years) and were more likely to have at least one prior pregnancy history than participants who started ART during pregnancy (Table 1). The median gestational ages at first antenatal care booking and enrolment in the survey were 14 (IQR: 10–20) and 26 weeks (IQR: 18–34), respectively. Most (92.3%) pregnant women initiated antenatal care after 6 weeks of gestational age. At the time of the survey, participants who started ART during pregnancy included in this analysis had already received ART for a median duration of 16 weeks (IQR: 14–21) (these excluded participants who received ART for < 3 months) at a median gestational age of 34 weeks (IQR: 30–36). The majority of facilities (> 60%) included in the study were large clinics from the urban area.
TABLE 1.
Characteristics of eligible participants for viral load testing in the 2019 antenatal survey, South Africa
|
All (N = 8112) |
(A) Started ART before pregnancy (N = 7207) |
(B) Started ART during pregnancy (ART initiated ≥ 3 months before enrolment in the survey) (N = 905) |
(C) Survey‐based χ2 test (P value) comparing column A with B |
|
|---|---|---|---|---|
| Age | ||||
| 15–24 years | 1397 (18.1) | 1138 (16.6) | 259 (29.7) | < 0.01 |
| 25–34 years | 4405 (58.4) | 3910 (58.3) | 495 (59.7) | |
| 35–49 years | 1743 (23.5) | 1653 (25.2) | 90 (10.6) | |
| Marital status | ||||
| Married | 1192 (14.9) | 1095 (15.4) | 97 (11.0) | |
| Cohabiting | 2416 (31.9) | 2172 (32.2) | 244 (29.5) | < 0.01 |
| In a relationship, living apart | 4137 (50.1) | 3641 (49.6) | 496 (53.7) | |
| Single | 263 (3.2) | 208 (2.8) | 55 (5.8) | |
| Education | ||||
| None | 103 (1.2) | 91 (1.2) | 12 (1.3) | < 0.01 |
| Primary | 1007 (12.9) | 911 (13.1) | 96 (12.1) | |
| Secondary | 6199 (76.4) | 5530 (76.9) | 669 (72.9) | |
| Tertiary | 743 (9.4) | 620 (8.9) | 123 (13.8) | |
| Gestational age at enrolment in the survey (weeks) [median (IQR)] | 26 (18–34) | 25 (16–32) | 34 (30–36) | < 0.01* |
| Gestational age at first booking (weeks) [median (IQR)] | 14 (10–20) | 14 (10–20) | 13 (9.5–18) | < 0.01* |
| Duration of ART (weeks) [median (IQR)] | 16 (14–21) | |||
| Gravidity | ||||
| Primigravida (1) | 953 (11.6) | 717 (9.7) | 236 (25.6) | |
| Multigravida (2+) | 7039 (88.4) | 6381 (90.3) | 658 (74.4) | < 0.01 |
| Facility location | ||||
| Urban | 4679 (61.2) | 4113 (60.6) | 566 (65.3) | |
| Rural | 2711 (30.8) | 2439 (31.2) | 272 (27.7) | 0.01 |
| Peri‐urban | 722 (8.1) | 655 (8.2) | 67 (7.0) | |
| Type of facility | ||||
| CHC | 1469 (18.6) | 1307 (18.7) | 162 (17.8) | |
| Clinic | 6616 (81.2) | 5876 (81.1) | 740 (82.0) | |
| Hospital | 24 (0.2) | 21 (0.2) | 3 (0.2) | |
| Mobile | 3 (< 0.1) | 3 (< 0.1) | 0 | 0.66 |
| Size of facility | ||||
| Small | 554 (7.4) | 494 (7.5) | 60 (7.1) | |
| Medium | 1988 (25.0) | 1776 (25.1) | 212 (24.1) | |
| Large | 5570 (67.6) | 4937 (67.5) | 633 (68.8) | 0.67 |
| Province | ||||
| Eastern Cape | 1381 (11.6) | 1246 (11.8) | 135 (10.0) | |
| Free State | 673 (5.5) | 594 (5.4) | 79 (5.6) | |
| Gauteng | 969 (22.1) | 841 (21.7) | 128 (25.4) | |
| KwaZulu‐Natal | 2639 (31.2) | 2368 (31.6) | 271 (27.9) | |
| Limpopo | 393 (6.6) | 341 (6.5) | 52 (7.5) | |
| Mpumalanga | 786 (8.7) | 695 (8.6) | 91 (8.7) | |
| Northern Cape | 224 (1.3) | 196 (1.3) | 28 (1.4) | |
| North West | 576 (6.7) | 512 (6.7) | 64 (6.5) | |
| Western Cape | 471 (6.4) | 414 (6.4) | 57 (6.8) | 0.02 |
Data are presented as n (%) unless noted otherwise. Weighted percentages. Missing data excluded.
Abbreviations: CHC, community health centre; IQR, interquartile range.
Used equality of median test.
Viral load tests done and viral suppression
Of 8112 participants eligible for a viral load test, 81.7% (95% CI: 80.7–82.6%) received a viral load test, and 94.1% (95% CI: 93.4–94.8) of these test results had been returned and documented in the participants’ medical record (Table 2; Fig. 2). Of those whose viral load test result was documented on the medical record, 74.1% (95% CI: 73.0–75.2) were virally suppressed (i.e. their viral load was < 50 copies/mL), 16.4% had viral load between 50 and 1000 copies/mL, and 9.5% had a viral load > 1000 copies/mL. Among participants who initiated ART during pregnancy, both participants with viral loads > 1000 and ≤ 1000 copies/mL received ART for a median duration of 16 weeks.
TABLE 2.
Viral load cascade among HIV‐positive pregnant women in the 2019 antenatal survey, South Africa
|
All (N = 8112) |
Started ART before pregnancy (N = 7207) |
Started ART during pregnancy (N = 905) |
Survey‐based χ2 test b P‐value |
|
|---|---|---|---|---|
| I. Viral load test | ||||
| Viral load test done | 6542 (81.7) | 5887 (82.8) | 655 (73.0) | < 0.01 |
| Viral load test not done | 1095 (14.0) | 957 (13.8) | 138 (15.8) | |
| Reported as viral load test not due a | 319 (4.3) | 222 (3.4) | 97 (11.2) | |
| Not reported c | 156 | 141 | 15 | |
| II. Viral load test documentation on medical record | 0.5 | |||
| Documented | 5969 (94.1) | 5371 (94.1) | 598 (93.6) | |
| Not documented | 358 (5.9) | 321 (5.9) | 37 (6.4) | |
| Not reported c | 215 | 195 | 20 | |
| III. Viral load test results (copies/mL) | ||||
| < 50 | 4277 (74.1) | 3948 (76.1) | 329 (56.8) | |
| 50−1000 | 944 (16.4) | 784 (15.1) | 160 (28.0) | < 0.01 |
| > 1000 | 556 (9.5) | 465 (8.8) | 91 (15.2) | |
| Not reported c | 192 |
174 |
18 | |
Data are presented as n (%) unless noted otherwise. Weighted percentages.
These were reported as viral load test not due even though according to their timing of antiretroviral therapy (ART) initiation they were due for viral load testing.
The χ2 test compares participants who initiated ART during pregnancy with those who initiated ART before pregnancy.
Missing data excluded from percentage calculations. The denominator for viral load testing was the 8112 participants due for viral load (which comprises 7207 participants who initiated ART before pregnancy and 905 who initiated ART during pregnancy). The denominator for documentation of viral load result was the number who had viral load testing (N = 6542) and the denominator for viral suppression was the number who had viral load results documented (N = 5969).
FIGURE 2.
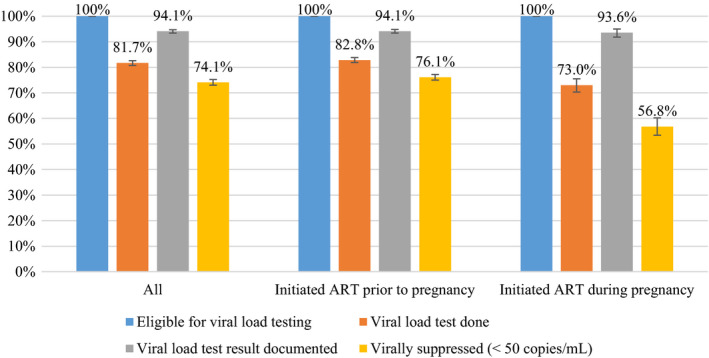
Viral load cascade among pregnant women in the National Antenatal Sentinel Survey, 2019, South Africa. ART, antiretroviral therapy. Weighted percentages The percentage for each bar is calculated using the previous bar as the denominator
Participants who initiated ART during pregnancy had lower coverage of viral load testing (73.0%, 95% CI: 70.3–75.5) compared with participants who initiated ART before pregnancy (82.8%, 95% CI: 81.9–83.8). Viral suppression was low among participants who initiated ART during pregnancy, with nearly half (43.2%, 95% CI: 39.8–46.6) of these participants not achieving viral suppression despite receiving ART for 3 months. Viral suppression was 76.1% (95% CI: 75.0–77.2) among women who initiated ART before pregnancy.
By province, the coverage of viral load testing ranged from 77.0% in Western Cape to 86.3% in Mpumalanga (Fig. S1). Viral load testing was higher in rural facilities (87.7%, 95% CI: 83.2–86.0) than in urban (80.1%, 95% CI: 78.7–81.4) facilities. Viral load testing did not vary by size or type of facility. Greater than 87% of viral load results were documented in all nine provinces. Viral suppression varied by province, ranging from 61.1% in Limpopo to 79.7% in KwaZulu‐Natal. Documentation of viral load result and viral suppression did not vary by location (i.e. rural‐urban categories), size or type of facility.
In a multivariable analysis, after adjusting for education and marital status, women who initiated ART during pregnancy were less likely to receive a viral load test than women who initiated ART before pregnancy (AOR = 1.6, 95% CI: 1.4–1.8) (Table 3). This study also found a modest association between attending urban facilities and not receiving a viral load test (AOR = 1.3, 95% CI: 1.1–1.5). Primigravida women (AOR = 1.3, 95% CI: 1.1–1.5) and younger women (15–24 years, AOR = 1.3, 95% CI: 1.1–1.5) were less likely to receive a viral load test than multigravida and older women (35–49 years), respectively.
TABLE 3.
Factors associated with not receiving a viral load test in the 2019 antenatal survey, South Africa (N = 8112)
| Univariate odds ratio (95% CI) | Adjusted odds ratio (95% CI) | |
|---|---|---|
| Gravidity | ||
| Primigravida (1) | 1.5 (1.3–1.7) | 1.3 (1.1–1.5) |
| Multigravida (2+) | Ref. | Ref. |
| Facility location | ||
| Urban | 1.4 (1.2–1.6) | 1.3 (1.1–1.5) |
| Peri‐urban | 1.2 (0.9–1.5) | 1.1 (0.8–1.4) |
| Rural | Ref. | Ref. |
| Timing of ART initiation | ||
| Prior to pregnancy | Ref. | Ref. |
| During pregnancy | 1.8 (1.6–2.0) | 1.6 (1.4–1.8) |
| Marital status | ||
| Married | Ref. | Ref. |
| Cohabiting | 1.1 (1.0–1.3) | 1.0 (0.8–1.2) |
| In a relationship, living apart | 1.0 (0.9–1.2) | 0.9 (0.8–1.1) |
| Single | 1.5 (1.1–2.0) | 1.3 (0.9–1.7) |
| Education | ||
| None | Ref. | Ref. |
| Primary | 1.5 (0.9–2.4) | 1.7 (0.9–2.0) |
| Secondary | 1.1 (0.7–1.7) | 1.1 (0.7–1.9) |
| Tertiary | 1.0 (0.6–1.7) | 1.0 (0.6–1.7) |
| Age | ||
| 15–24 years | 1.4 (1.2–1.7) | 1.3 (1.1–1.5) |
| 25–34 years | 1.0 (0.9–1.1) | 1.0 (0.9–1.1) |
| 35–49 years | Ref. | Ref. |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval. Missing data are excluded. N = 7183 observations (88.5% of data) were included in multivariable analysis. For each predictor, the group selected as ‘Ref.’ was selected so that the group/factor that increased the likelihood of not receiving viral load testing could be reported.
DISCUSSION
In this first‐ever national‐level analysis of the coverage of viral load monitoring among pregnant women in South Africa, this study found that most pregnant women (81.7%) received a viral load test, and the viral load test result was documented for nearly all participants who received viral load testing (94.1%). However, viral suppression was low, particularly among participants who initiated ART during pregnancy (56.8%). Women who initiated ART before pregnancy were more likely to receive viral load testing and had higher viral suppression than women who initiated ART during pregnancy. Among women eligible for a viral load test, there was a modest association between not receiving viral load testing and attending antenatal care at urban facilities, being younger (15–24 years) and primigravid.
Both viral load testing and documentation of viral load test result were much higher in this study compared with data previously reported in South Africa and elsewhere in Sub‐Saharan African countries. For pregnant women, previous studies reported between 30% and 72% coverage of viral load testing [9, 18. Among adults in the general population, viral load monitoring is reported to be 73% in South Africa [8]. While the relatively higher coverage of viral load testing in this study is encouraging, almost one in five pregnant women eligible for viral load testing in this study had not received a viral load test.
In the literature, lack of training, inadequate knowledge (among health workers), and poor adherence to the guideline have been identified as the main reasons for missed opportunities to provide a viral load test [21, 22. Also, for women initiating ART during pregnancy, as each woman starts ART at a different time, keeping track of the month that each woman is due for a viral load test in a consistent manner is difficult, which contributes to low viral load testing [21]. Implementing simple and innovative best practices, such as the use of stickers (placed on the patient medical record) that easily identify the month a woman is due for viral load testing, could help to track viral load testing ‘due dates’ more efficiently [21]. Educating pregnant women about the importance of viral load testing could also be of benefit, as patient‐level factors (such as missing appointments for antenatal care) could contribute to a missed opportunity for viral load testing [22].
It is notable that, despite receiving ART for 3 months, about 43% of participants who initiated ART during pregnancy were not virally suppressed and a substantial percentage (15.2%) of them had viral load > 1000 copies/mL. Suboptimal ART adherence and drug resistance could be potential reasons for the observed low viral suppression among women who initiated ART during pregnancy [23]. About 28% of participants who initiated ART during pregnancy and received ART for 3 months had viral load in the range 50–999 copies/mL. The lack of viral suppression among these women could be due to insufficient time to achieve viral suppression in 3 months with current first‐line antiretroviral (ARV) regimens. At the time of this survey, an EFV‐based regimen was used as a first‐line regimen for pregnant women in South Africa. Studies have shown that a dolutegravir (DTG)‐based regimen is associated with faster achievement of viral suppression (a median time to viral suppression of 28 days) compared with an EFV‐based regimen (median time to viral suppression of 84 days) [24, 25.
In light of the identified slow viral suppression rate with an EFV‐based regimen used at the time of this survey for women initiating treatment during pregnancy, our finding supports the recommendation (by WHO and local government) that a DTG‐containing regimen be the preferred regimen for women who are newly initiating ART during pregnancy [11]. Dolutegravir has a high genetic barrier to drug resistance and enables faster achievement of viral suppression [26]. Although there is a slightly higher risk of neural tube defect with initiation of DTG before 6 weeks of pregnancy (0.19%) compared with an EFV‐based regimen (0.17%)[27], in this study, most (92.3%) pregnant women initiated antenatal care after 6 weeks of gestational age, indicating that most infants born from women who initiate a DTG‐based regimen during pregnancy will not be exposed to an increased risk of neural tube defect.
This study did not assess the treatment regimen participants were on at the time of the viral load testing. However, the rollout of DTG‐based regimens did not start in South Africa until December 2019[28], and anecdotal reports indicate that the rollout may have been carried out slowly due to the shift in focus to manage the COVID‐19 pandemic.
The higher viral suppression among women who initiated ART before pregnancy in this study was expected and likely to be due to the longer duration of ART, as most (74%) participants who initiated ART before pregnancy reported that they knew about their HIV‐positive status during the pregnancy before the current one.
This study did not assess all viral load cascade steps that are crucial for the achievement of suppressed viral load. For instance, the study did not collect data on the timing of a viral load test, which would have given more information on the timeliness of the viral load test done. Other studies in South Africa report late provision of viral load testing as one of the barriers to timely and appropriate corrective action [29, 30. This study also did not assess turnaround time for results and the challenges encountered in specimen transportation. Loss of specimens and cold chain breakdown during transportation of specimens contribute to the delay in viral load testing in South Africa [13, 31. Assessing the feasibility of implementing more permanent solutions such as point‐of‐care viral load testing is essential as it would overcome the challenges associated with transportation of specimens. Point‐of‐care viral load testing is particularly critical at the time of delivery as viral load results need to be available immediately after delivery to decide on the appropriate ART prophylaxis for the infant [32]. The study did not look at the actions taken to improve viral suppression among participants with unsuppressed viral load. After viral load testing is done, prompt use of the viral load test result for patient management is the most crucial step (including enhanced adherence counselling and/or ARV regimen switch), as a lack of timely action reduces the benefit of viral load monitoring.
While those who initiated ART during pregnancy represented a relatively small percentage of HIV‐positive participants in this study, incorrect reporting of gestational age could affect the estimate for viral load testing coverage. Just over a quarter of known HIV‐positive participants were also excluded from this analysis due to missing data or short duration of ART. These participants had similar demographic characteristics as participants included in the analysis. Due to the cross‐sectional design of the study, we did not follow up participants to collect data on viral load at delivery and pregnancy outcomes.
In conclusion, while most participants received a viral load test, and the viral load test result was documented for nearly all participants, viral suppression was low, particularly among those who initiated ART during pregnancy and received ART for 3 months. This highlights the urgent need to strengthen adherence counselling and to monitor the effectiveness of treatments (ART) received within antenatal care services. We recommend fast‐tracking the rollout of DTG among newly diagnosed HIV‐positive women during pregnancy, as it is critical for all HIV‐positive women to achieve viral suppression as early as possible in the pregnancy to reduce the risk of vertical HIV transmission. The coverage of viral load testing could also be improved further by regular training of health workers who are managing antenatal care clients, implementation of innovative best practice that can help to easily identify women due for a viral load test, and incorporation of messages on the importance of viral load testing during health education of pregnant women.
CONFLICT OF INTEREST
The authors declare there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
SAW: conceptualization, data curation, formal analysis, funding acquisition, methodology, project administration, supervision, writing of the original draft, and review and editing of the manuscript. TK‐C: conceptualization, methodology and review and editing of the manuscript. CL: conceptualization, methodology and review and editing of the manuscript. SM: conceptualization, methodology and review and editing of the manuscript. MC: conceptualization, writing and review and editing of the manuscript. KA: conceptualization, writing and review and editing of the manuscript. AP: conceptualization, funding acquisition, methodology, project administration, resources, supervision, validation, and review and editing of the manuscript.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGEMENTS
We thank Dr G. Hunt and Ms E. Cutler from NICD for coordinating all laboratory aspects of the survey as well as facilitating the setup of the Optical Mark Recognition (OMR) software for electronic capturing of data and for overseeing data capturing. We thank all data clerks and their managers (Ms K. Richards and Ms C. Harrison) for their superb job in performing data quality checks on completed data collection forms and communicating data quality problems in a timely manner to the central team. We thank Dr H. Vreede and Dr T. Bell for extracting the laboratory data bi‐weekly from the NHLS laboratory information system. We thank Ms L. Jankelowitz and Ms M. Sibanyoni from the South African HIV Clinicians Society (SAHCS) for their role in organizing and facilitating the training of provincial survey coordinators, recruiting data clerks, and providing telephonic technical support to provincial survey coordinators during the implementation of the survey.
Our appreciation goes to the provincial survey coordinators: Mr Z. Merile (Eastern Cape), Ms M. Nophale, Ms M. Mothibi (Free State), Dr B. Ikalafeng (Gauteng), N. Mbana, Ms G. Khumalo (KwaZulu–Natal), Ms F. Ngobeni, Ms M. Monwa (Limpopo), Mr J. Sigudla, Dr T. Mona (Mpumalanga), Ms N. Kopang, Ms T. Nondanyana, Mr B. Mashute (Northern Cape), Ms M. Motswasele, Ms N. Mangonyane (North West), Ms V. Mudaly (Western Cape) and their teams who coordinated the survey in their respective provinces and districts. Sincerest gratitude is also extended to the National Health Laboratory Services (NHLS) and NICD testing laboratories and coordinators: Ms B. Singh, Ms Z. Brukwe (NICD, Gauteng), Ms H. Vilakazi, Mr P. Moyeni, Mr B Tembe (Eastern Cape), Ms M. Nkonyane, Mr P. Letanta, Ms H. Potgieter, Mr M. D. Morobadi (Free State), Ms I. Chetty, Mr M. Ellapen (Inkosi Albert Luthuli Hospital, University of KwaZulu–Natal), Ms R. Diokana, Mr J. Ngwenya (Dr George Mukari, Limpopo and North West), Mr I. Mofokeng (Rob Ferreira Nelspruit, Mpumalanga), and Dr H. Vreede, Mr Z. Isaacs and Ms A. Stewart (Groote Schuur Hospital, Western Cape and Northern Cape).
Note that the findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the funding agencies.
Woldesenbet SA, Kufa‐Chakezha T, Lombard C, et al. Coverage of maternal viral load monitoring during pregnancy in South Africa: Results from the 2019 national Antenatal HIV Sentinel Survey. HIV Med. 2021;22:805–815. 10.1111/hiv.13126
Funding information
This project was supported by the President's Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) (under the terms of cooperative agreement=5 NU2GGH001631). The World Health Organization (WHO), the National Department of Health (NDoH), and the National Institute for Communicable Diseases (NICD) funded the data collection for the survey.
REFERENCES
- 1. Goga A, Singh Y, Jackson D, et al. Is elimination of vertical transmission of HIV in high prevalence settings achievable? BMJ 2019; 364: l687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mandelbrot L, Tubiana R, Le Chenadec J, et al. No perinatal HIV‐1 transmission from women with effective antiretroviral therapy starting before conception. Clin Infect Dis. 2015; 61 (11): 1715‐1725. [DOI] [PubMed] [Google Scholar]
- 3. European CS. Mother‐to‐child transmission of HIV infection in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005; 40 (3): 458‐465. [DOI] [PubMed] [Google Scholar]
- 4. Moyo F, Haeri Mazanderani A, Murray T, et al. Characterizing viral load burden among HIV‐infected women around the time of delivery: findings from four tertiary obstetric units in Gauteng, South Africa. J Acquir Immune Defic Syndr. 2020; 83 (4): 390‐396. [DOI] [PubMed] [Google Scholar]
- 5. Woldesenbet SA, Kufa T, Barron P, et al. Viral suppression and factors associated with failure to achieve viral suppression among pregnant women in South Africa. AIDS. 2020; 34 (4): 589‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization . Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva Switzerland: World Health Organization, 2013. http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf. [PubMed] [Google Scholar]
- 7. Lecher S, Ellenberger D, Kim A, et al. Scale‐up of HIV Viral Load Monitoring — Seven Sub‐Saharan African Countries. Morbidity and Mortality Weekly Report. November 27, 2015 / 64(46);1287‐1290 US. [DOI] [PubMed]
- 8. Diseko L, Overmeyer R. South African DSD update. 12 November 2019. South Africa National Department of Health. [cited 15/ 4/2020]. Available from: http://www.differentiatedcare.org/Portals/0/adam/Content/LUn23I85V0aDet59YdligA/File/Overmeyer_Final.pdf.
- 9. Moyo F, Haeri Mazanderani A, Bhardwaj S, et al. Near‐real‐time tracking of gaps in prevention of mother‐to‐child transmission of HIV in three districts of KwaZulu‐Natal Province, South Africa. S Afr Med J. 2018; 108 (4): 319‐324. [DOI] [PubMed] [Google Scholar]
- 10. Euvrard J, Schulz T, Hilderbrand K, et al. How accurately do routinely reported HIV viral load suppression proportions reflect progress towards the 90–90‐90 target in the population on antiretroviral treatment in Khayelitsha, South Africa? S Afr Med J 2019; 109 (3): 174. [DOI] [PubMed] [Google Scholar]
- 11. Guideline for the Prevention of Mother to Child Transmission of Communicable Infections (HIV, Hepatitis, Listeriosis, Malaria, Syphilis and TB) 2019 [cited:12/03/20]. Available from: http://www.nicd.ac.za/wp‐content/uploads/2019/11/Guidelines‐for‐the‐Prevention‐of‐Transmission‐of‐Communicable‐Diseases‐from‐mother‐to‐child_28‐October.pdf.
- 12. Cassim N, Coetzee LM, Stevens WS, Glencross DK. Addressing antiretroviral therapy‐related diagnostic coverage gaps across South Africa using a programmatic approach. Afr J Lab Med. 2018; 7 (1): 681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lecher S, Williams J, Fonjungo P, et al. Progress with Scale‐Up of HIV Viral Load Monitoring — Seven Sub‐Saharan African Countries, January 2015–June 2016. Morbidity and Mortality Weekly Report. December 2, 2016 / Vol. 65 / No. 47 US. [DOI] [PubMed]
- 14. World Health Organization (2019). Consolidated guidelines on HIV testing services for a changing epidemic (cited 27/08/20). Available from: https://www.who.int/publications/i/item/consolidated‐guidelines‐on‐hiv‐testing‐services‐for‐a‐changing‐epidemic.
- 15. Woldesenbet SA, Kufa T, Lombard C, et al. The 2017 National Antenatal Sentinel HIV Survey, South Africa, National Department of Health [cited: 02/03/19]. Available from: https://www.nicd.ac.za/wp‐content/uploads/2019/07/Antenatal_survey‐report_24July19.pdf.
- 16. Woldesenbet S, Kufa T, Cheyip M, et al. Awareness of HIV‐positive status and linkage to treatment prior to pregnancy in the "test and treat" era: A national antenatal sentinel survey, 2017, South Africa. PLoS One 2020; 15 (3): e0229874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rao JNK, Thomas DR. Analysis of Categorical Response Data from Complex Surveys: An Appraisal and Update. Analysis of Survey Data 2003. p. 85‐108.
- 18. Cisse CAT, Inzale MA, Wade NF, Niang MM, Diallo D, Ndiaye SN. Screening and management of HIV infection in pregnant women in Dakar. Med Sante Trop. 2018; 28 (2): 186‐192. [DOI] [PubMed] [Google Scholar]
- 19. Swannet S, Decroo T, de Castro S , et al. Journey towards universal viral load monitoring in Maputo, Mozambique: many gaps, but encouraging signs. Int Health. 2017; 9 (4): 206‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sandbulte M, Brown M, Wexler C, et al. Maternal viral load monitoring: Coverage and clinical action at 4 Kenyan hospitals. PLoS One 2020; 15 (5): e0232358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peters R, Kekana M, Cole‐Hamilton S, Mongwe MW, Railton J. Towards the HIV 90–90‐90 target: A simple and low‐cost intervention to improve viral load completion. HIV Nursing Matters. 2016; 7: 35. [Google Scholar]
- 22. Making viral load routine successes and challenges in the implementation of routine HIV viral load monitouring. Part 1 programatic strategies [cited 23/06/20]. Available from: https://www.msf.org/sites/msf.org/files/making_viral_load_routine_part_1_programmatic_strategies.pdf
- 23. Redd AD, Mukonda E, Hu NC, et al. ART adherence, resistance, and long‐term HIV viral suppression in postpartum women. Open Forum Infect Dis. 2020; 7(10): ofaa346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir‐lamivudine for the treatment of HIV‐1 infection. N Engl J Med 2013; 369 (19): 1807‐1818. [DOI] [PubMed] [Google Scholar]
- 25. Waitt C, Orrell C, Walimbwa S, et al. Safety and pharmacokinetics of dolutegravir in pregnant mothers with HIV infection and their neonates: A randomised trial (DolPHIN‐1 study). PLoS Medicine 2019; 16 (9): e1002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Inzaule SC, Hamers RL, Doherty M, Shafer RW, Bertagnolio S, Rinke de Wit TF. Curbing the rise of HIV drug resistance in low‐income and middle‐income countries: the role of dolutegravir‐containing regimens. Lancet Infect Dis. 2019; 19 (7): e246‐e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zash R, Holmes L, Diseko M, et al. Update on neural tube defects with antiretroviral exposure in the Tsepamo study, Botswana. Presented at: International AIDS Conference. 6–10 July 2020. Virtual Conference. Oral late breaker abstract OAXLB0102. Available from: https://cattendee.abstractsonline.com/meeting/9289/presentation/3500.
- 28. New HIV drug combo rolled out in South Africa. News medical life science. Accessed 23 October 2020. Available from: https://www.news‐medical.net/news/20191202/New‐HIV‐drug‐combo‐rolled‐out‐in‐South‐Africa.aspx.
- 29. Fox MP, Brennan AT, Nattey C, et al. Delays in repeat HIV viral load testing for those with elevated viral loads: a national perspective from South Africa. J Int AIDS Soc. 2020; 23 (7): e25542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kubheka SE, Archary M, Naidu KK. HIV viral load testing coverage and timeliness after implementation of the wellness anniversary in a paediatric and adolescent HIV clinic in KwaZulu‐Natal, South Africa. South Afr J HIV Med. 2020; 21 (1): 1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stevens WS, Marshall TM. Challenges in implementing HIV viral load testing in South Africa. J Infect Dis 2010; 201(Supplement_1): S78‐S84. [DOI] [PubMed] [Google Scholar]
- 32. Lesosky M, Glass T, Mukonda E, Hsiao NY, Abrams EJ, Myer L. Optimal timing of viral load monitoring during pregnancy to predict viraemia at delivery in HIV‐infected women initiating ART in South Africa: a simulation study. J Int AIDS Soc. 2017; 20 (Suppl): 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


