Abstract
Sustained virologic response at posttreatment Week 12 (SVR12) is the widely accepted efficacy endpoint for direct‐acting antiviral agents. Those with hepatitis C virus (HCV) are presenting younger with milder liver disease, potentially reducing need for long‐term liver posttreatment monitoring. This analysis aimed to determine the positive predictive value (PPV) of SVR at posttreatment Week 4 (SVR4) for achieving SVR12 in patients with HCV, without cirrhosis or with compensated cirrhosis, receiving glecaprevir/pibrentasvir (G/P) in clinical trials. An integrated dataset from 20 Phase 2 and 3 clinical trials of G/P was evaluated in patients with 8‐, 12‐ or 16‐week treatment duration consistent with the current label (label‐consistent group), and in all patients regardless of treatment duration consistency with the current label (overall group). Sensitivity analyses handled missing data either by backward imputation or were excluded. SVR4 PPV, negative predictive value (NPV), sensitivity and specificity were calculated for achieving SVR12 in both groups, and by treatment duration in the label‐consistent group. SVR was defined as HCV ribonucleic acid <lower limit of quantification. The label‐consistent group and overall group included 2890 and 4390 patients, respectively. PPV of SVR4 for SVR12 was >99% in both groups regardless of treatment duration. Not achieving SVR4 had 100% NPV and sensitivity for all groups. SVR4 measure had 79.5% specificity for identifying patients who did not achieve SVR12. Across 20 Phase 2/3 clinical trials of G/P, SVR4 was highly predictive of SVR12. Long‐term follow‐up to confirm SVR may not be necessary for certain populations of patients with HCV.
Keywords: care cascade, concordance, direct‐acting antivirals, hepatitis C virus, sustained virologic response
Abbreviations
- APRI
aspartate aminotransferase to platelet ratio index
- CC
compensated cirrhosis
- DAA
direct‐acting antiviral
- EASL
European Association for the Study of the Liver
- G/P
glecaprevir/pibrentasvir
- GT
genotype
- HCV
hepatitis C virus
- LLOQ
lower limit of quantification
- LTFU
lost to follow‐up
- NC
non‐cirrhotic
- NPV
negative predictive value
- PPV
positive predictive value
- PRS
prior treatment with regimens containing interferon, pegylated interferon, ribavirin, and/or sofosbuvir
- RNA
ribonucleic acid
- SVR
sustained virologic response
- WHO
World Health Organization
Significance statement.
Patients with hepatitis C virus (HCV) are presenting younger and with milder liver disease, potentially reducing the need for long‐term liver posttreatment monitoring. This study of 20 Phase 2/3 clinical trials of glecaprevir/pibrentasvir (G/P) found that sustained virologic response at posttreatment Week 4 (SVR4) was highly predictive of SVR12 in patients with HCV, without cirrhosis or with compensated cirrhosis. The concordance between SVR4 and SVR12 for patients treated with G/P for treatment durations as short as 8 weeks provides reassurance that long‐term follow‐up of patients with HCV may not be necessary for patients without ongoing risk for advanced liver disease.
1. INTRODUCTION
Approximately 71 million people have chronic hepatitis C virus (HCV) infection globally. 1 In 2016, the World Health Organization (WHO) set an international target of reducing new chronic HCV infections by 90%, and mortality from HCV by 65%, by 2030. 2 To meet these targets, the HCV care cascade needs to be simple, targeted and patient‐centred. 3
WHO guidelines recommend that all adults with chronic HCV receive treatment with pangenotypic direct‐acting antivirals (DAAs). 4 Combinations of these drugs result in high levels of sustained virologic response (SVR) at posttreatment Week 12 (SVR12), with minimal safety concerns and short treatment durations. 5 , 6 , 7 , 8 , 9 , 10
Glecaprevir/pibrentasvir (G/P) is a fixed‐dose pangenotypic DAA combination approved for the treatment of HCV genotypes (GT) 1–6. 5 , 11 Data from clinical trials and real‐world studies have shown G/P to be highly effective and well tolerated in a broad range of patients without cirrhosis and those with compensated cirrhosis (CC), 12 , 13 , 14 , 15 , 16 over treatment durations as short as 8 weeks. 5 , 17 , 18
Ensuring patients initiate and maintain HCV treatment without becoming lost to follow‐up (LTFU) is a concern for treatment prescribers. 19 Although the numbers of patients becoming LTFU during clinical trials for pangenotypic regimens tend to be low, 20 real‐world data suggest LTFU rates for patients receiving DAA therapy can be much higher in clinical practice. 21 This can significantly impact on the HCV care cascade, 22 with studies suggesting that becoming LTFU can contribute to a lack of SVR. 21
The characteristics of patients with HCV is evolving, with patients becoming progressively younger and with less advanced liver disease, potentially reducing the need for long‐term posttreatment monitoring. 23 , 24 Consequently, patients and/or providers may be less motivated to adhere to robust posttreatment visit schedules. Furthermore, marginalized patients, who are often disengaged from health care, constitute a large and rapidly growing subgroup of newly diagnosed HCV infections. 25 This, coupled with an increase in HCV treatment providers practicing in nontraditional settings, 26 means that long‐term follow‐up may present challenges for some.
The length of posttreatment follow‐up to define cure, previously defined as SVR at posttreatment Week 24 (SVR24), was shortened upon demonstration of high concordance rates of SVR12 with DAAs. 27 , 28 However, due to the high SVR12 rates expected across all groups of patients treated with pangenotypic DAAs, the utility of SVR12 monitoring has been questioned with the latest clinical practice guideline updates from the European Association for the Study of the Liver (EASL) indicating that SVR12 may be omitted if the patient is adherent to therapy (except in those with high‐risk behaviours and risk of reinfection). 29 Nevertheless, there remains an evidence gap on how early response correlates with SVR12; understanding the frequency of late relapse (after posttreatment Week 4) would provide reassurance to treatment providers that long‐term follow‐up may be dispensable if proven to be low. This could particularly benefit marginalized patients in whom long‐term follow‐up can be problematic.
This analysis aimed to determine the positive predictive value (PPV) of SVR at posttreatment Week 4 (SVR4) for achieving SVR12 in patients with HCV without cirrhosis or with CC receiving G/P in clinical trials.
2. METHODS
2.1. Study design
Two analyses were performed on the same integrated dataset collected from 20 Phase 2 and 3 clinical trials of G/P (Table S1): (1) patients receiving G/P for treatment durations (8, 12 or 16 weeks) consistent with then current prescribing information in the region the patient received treatment 11 , 30 (label‐consistent group); and (2) all patients, regardless of whether their treatment duration was consistent with then current prescribing information in the region the patient received treatment 11 , 30 (overall group). Clinical trials included in this analysis represented a wide range of patient types, including paediatric patients as young as age 12 years; patients with compensated cirrhosis, HIV coinfection, chronic kidney disease and organ transplantation; and patients enrolled in East Asian countries.
2.2. Study population
Study population included patients with HCV ribonucleic acid (RNA) measures available both at posttreatment Week 4 and at posttreatment Week 12. For this analysis, patients with HCV reinfection, those who received doses of G/P other than 300/120 mg once daily, or any combination that also included ribavirin or sofosbuvir were excluded. Lastly, patients who were DAA‐experienced to NS3/4A protease inhibitors or NS5A inhibitors were also excluded.
2.3. Outcomes
The PPV, negative predictive value (NPV), sensitivity and specificity of SVR4 were calculated for achieving SVR12 in both the label‐consistent and the overall group (Figure 1), as well as by treatment duration (8, 12 or 16 weeks) in the label‐consistent group.
FIGURE 1.
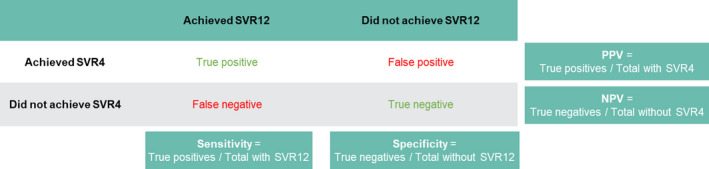
Definition of PPV, NPV, sensitivity and specificity for SVR4 versus SVR12. Abbreviations: NPV, negative predictive value; PPV, positive predictive value; SVR4, sustained virologic response at posttreatment Week 4; and SVR12, sustained virologic response at posttreatment Week 12
2.4. Statistical definitions and analysis
PPV was defined as the proportion of patients with SVR12 among those with SVR4, while NPV was defined as the proportion of patients without SVR12 among those without SVR4. Sensitivity was defined as the proportion of patients with SVR4 among those with SVR12, and specificity was defined as the proportion of patients without SVR4 among those without SVR12 (Figure 1). The SVR rate was calculated as the number of patients with HCV RNA less than the lower limit of quantification (LLOQ)/total number of patients in the intention‐to‐treat population. For SVR measures, the last value in the analysis time window was used. A sensitivity analysis handled missing data as treatment failure.
All statistical analyses were conducted using the SAS® software package (version 9.4; SAS Institute Inc., Cary, NC, USA).
3. RESULTS
3.1. Baseline demographics
A total 4390 patients were included in the overall group and 2890 in the label‐consistent group with 2582, 218 and 90 patients receiving G/P for 8, 12 and 16 weeks, respectively. Overall, the baseline demographics of both groups were closely matched, with the majority of patients being male (54.9% and 54.9%, respectively), treatment‐naive (78.9% and 78.4%, respectively) and with HCV GT1 (47.7% and 52.4%, respectively). The only discernible difference was in the G/P duration received by each group. A total of 58.8% of patients received 8‐week G/P in the overall group compared with 89.3% in the label‐consistent group (Table 1).
TABLE 1.
Demographic and clinical characteristics
| Characteristic | Overall group (n = 4390) | Label‐consistent group (n = 2890) |
|---|---|---|
| Male | 2412 (54.9) | 1587 (54.9) |
| Age (years), median (range) | 54.0 (12.0–88.0) | 54.0 (12.0–88.0) |
| HCV genotype | ||
| 1 | 2096 (47.7) | 1513(52.4) |
| 2 | 962 (21.9) | 651 (22.5) |
| 3 | 884 (20.1) | 485 (16.8) |
| 4/5/6 | 230 (5.2)/56 (1.3)/162 (3.7) | 103 (3.6)/24 (0.8)/114 (3.9) |
| Baseline HCV RNA (IU/ml) | ||
| <1,000,000 | 1628 (37.1) | 1007 (34.8) |
| ≥1,000,000 | 2762 (62.9) | 1883 (65.2) |
| Prior HCV treatment history | ||
| Treatment‐naïve | 3464 (78.9) | 2266 (78.4) |
| Treatment‐experienced | 926 (21.1) | 624 (21.6) |
| DAA compliance a | 4026 (91.7) | 2652 (91.8) |
| Cirrhosis | 907 (20.7) | 515 (17.8) |
| Injection drug use | ||
| Yes, unknown | 285 (6.5) | 189 (6.5) |
| Yes, ≤12 months | 62 (1.4) | 35 (1.2) |
| Yes, >12 months | 941 (21.4) | 564 (19.5) |
| No | 3102 (70.7) | 2102 (72.7) |
| G/P regimen | ||
| 8 weeks | 2582 (58.8) | 2582 (89.3) |
| 12 weeks | 1718 (39.1) | 218 (7.5) |
| 16 weeks | 90 (2.1) | 90 (3.1) |
All results are n (%) unless otherwise stated; percentages are calculated from non‐missing values.
Abbreviations: DAA, direct‐acting antiviral; G/P, glecaprevir/pibrentasvir; HCV, hepatitis C virus; RNA, ribonucleic acid.
Compliance was calculated as the percentage of tablets taken relative to the total number of tablets expected to be taken. Compliance was defined as a calculated percentage between 80% and 120%.
3.2. SVR4 and SVR12 rates
In the overall group, 99.2% of patients achieved SVR4 and 99.0% achieved SVR12, whereas in the label‐consistent group, the rates were 99.1% and 98.9%, respectively. Within the label‐consistent group, the proportion of patients achieving SVR was >93% for all treatment durations at both posttreatment Weeks 4 and 12.
3.3. The PPV, NPV, sensitivity and specificity of SVR4 for achieving SVR12
The PPV of SVR4 for achieving SVR12 was >99% in both the label‐consistent and overall groups and did not differ by treatment duration, indicating that >99% of patients who achieved SVR4 went on to achieve SVR12. No patients who failed to achieve SVR4 went on to achieve SVR12, as demonstrated by the NPV of 100% for both groups (Figure 2).
FIGURE 2.
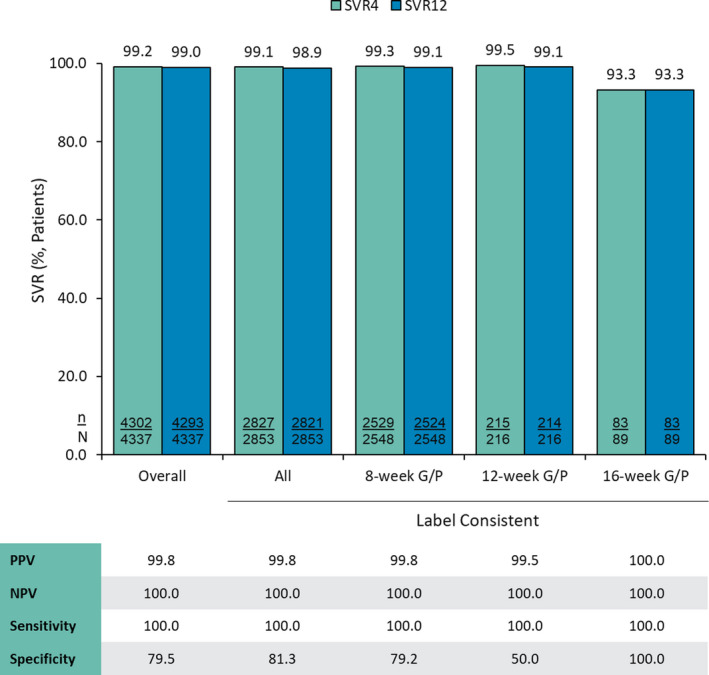
Percentage of patients achieving SVR4 and SVR12 by dataset and treatment duration and concordance of SVR at posttreatment Weeks 4 and 12. Abbreviations: G/P, glecaprevir/pibrentasvir; NPV, negative predictive value; PPV, positive predictive value; SVR, sustained virologic response; SVR4, SVR at posttreatment Week 4; and SVR12, SVR at posttreatment Week 12
Sensitivity was 100% for both groups, indicating that all patients who achieved SVR12 had also achieved SVR4. The SVR4 measure had 79.5% specificity for identifying patients who did not achieve SVR12, indicating that only 1 of 5 patients who relapse do so after the SVR4 timepoint. (Figure 2).
3.4. Sensitivity analysis
When missing data were treated as failures, similar results were seen across all groups. PPV and NPV were ≥99.1% and ≥98.4%, respectively. Sensitivity was >99.9%, and specificity ranged from 50.0% in the label‐consistent group treated with 12‐week G/P to 100% in the label‐consistent group treated with 16‐week G/P (Figure 3).
FIGURE 3.
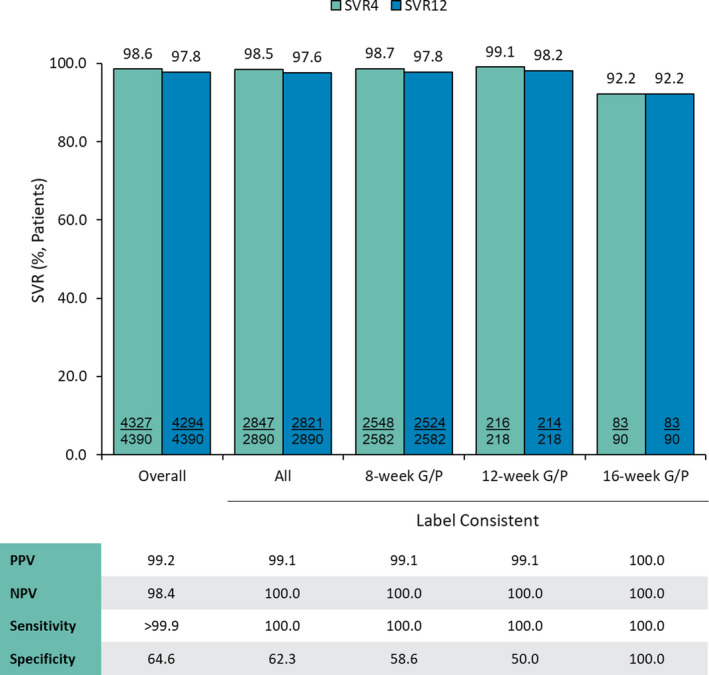
Percentage of patients achieving SVR4 and SVR12 by dataset and treatment duration and concordance of SVR at posttreatment Weeks 4 and 12 (sensitivity analysis—imputation of missing data as failures). Abbreviations: G/P, glecaprevir/pibrentasvir; NPV, negative predictive value; PPV, positive predictive value; SVR, sustained virologic response; SVR4, SVR at posttreatment Week 4; and SVR12, SVR at posttreatment Week 12
3.5. Characteristics of non‐SVRs after posttreatment week 4
Among 4337 patients in the overall analysis, 9 of those that achieved SVR4 did not achieve SVR12, including 1 patient with reinfection detected by phylogenetic analysis after the database lock and clinical study report finalization, and 1 patient who discontinued treatment after just 18 days of G/P. Taking this into consideration, the adjusted overall specificity of SVR4 for SVR12 was 83.3%.
Among the remaining 7 patients who relapsed (1 GT1a, 2 GT2, 2 GT3a, 1 GT3k and 1 GT5a), there were no distinctive commonalities predictive of late relapse. Treatment durations included 3 each receiving 8‐ and 12‐week G/P, and 1 receiving 16‐week G/P. Five had F0–1 fibrosis, 1 had F2 fibrosis, and 1 had F4 fibrosis. The mean platelet count was 194 × 109/L, and the mean baseline viral load was 6.89 log10 IU/mL. All 7 patients were <LLOQ on treatment at Week 4, and all were DAA compliant.
4. DISCUSSION
Data from both clinical trials and real‐world studies have demonstrated that G/P is a well‐tolerated and effective therapy in patients with chronic HCV and CC and in those without cirrhosis. 31 Treatment failure can be due to on‐treatment breakthrough but is more often related to posttreatment relapse. 32 This current analysis found that for patients receiving G/P treatment within a clinical trial setting, most relapses occur within the first 4 weeks of treatment completion and achieving SVR4 was highly predictive of SVR12, regardless of treatment duration. These findings are similar to those from previous studies of patients treated with sofosbuvir‐containing regimens where PPV of SVR4 for achieving SVR12 was >98% and NPV was 100%. 28
During the present analysis, all measures of concordance were similar between the overall group and the label‐consistent group, regardless of treatment duration received. This included SVR4 and SVR12 rates >99% and high PPV (99.8%) and NPV (100%) for the 8‐week treatment duration group, indicating the high effectiveness of this short treatment regimen. These results are to be expected because 8‐week G/P has demonstrated high rates of SVR12 in various subgroups of patients with or without CC, in both clinical trial and real‐world settings. 12 , 17 , 18 , 33
The high degree of concordance between SVR4 and SVR12 observed in the present study provide reassurance for both patients and providers that posttreatment SVR monitoring and long‐term follow‐up may be not necessary for some populations of patients with HCV treated with G/P. This is in line with the latest EASL guidelines which deem that SVR12 ‘can be omitted’ across all groups of patients without cirrhosis or with CC, if adherent to pangenotypic DAA therapy. 29 However, for those patients with unexplainable abnormal liver function, more advanced liver disease, ongoing high‐risk behaviours, or hepatocellular carcinoma, follow‐up for reinfection and/or disease progression remains important. 29 , 34 , 35
In the past, HCV treatment has necessitated a high degree of continuous care, to which many at‐risk populations found it difficult to follow. Removing the need for posttreatment SVR monitoring could potentially allow for the expansion of treatment into populations where extensive follow‐up is more difficult to conduct, such as persons who inject drugs, homeless patients and other marginalized groups. This approach is supported by a previous study, which showed that a lack of posttreatment follow‐up had no impact on SVR rates when compared with those with adequate follow‐up. 36 However, it is important to note that marginalized patient groups are those at highest risk of reinfection, 37 and for whom further interval testing may yield greatest benefit through early re‐treatment and reinforcement of harm reduction measures, along with identification and treatment of infection among injecting peers.
There are several initiatives to simplify the model of care for HCV that are in operation globally. These include the use of telemedicine and treatment delivered by nonmedical primary care providers (such as addiction specialists and social workers). 3 Reducing the need for long‐term follow‐up for some patient populations would further simplify HCV care which, when combined with the shorter treatment durations associated with DAA therapy, may lead to an increase in patient populations who were previously unwilling or unable to access treatment engaging with HCV treatment programmes. 2 The simplification of care has the potential to allow for better resource allocation, enabling for further expansions in the number of health care providers and facilitating improvements in access and linkage to HCV care. 26
A strength, and perhaps also a limitation for real‐world interpretation of these data, is that these datasets were collected from G/P clinical trials in which adherence to study visits contributed to minimal missing data. Under these conditions, where compliance was documented, SVR4 was shown to robustly predict SVR12. However, it is important to note that extrapolating datasets from clinical trials to populations where compliance may not be assured, such as people who inject drugs, must be done with caution. The sensitivity analysis including missing data as a nonresponder continued to demonstrate high PPV of SVR4 for SVR12.
The simplification of the HCV care cascade has the potential to improve outcomes for a wide range of patients. Our analysis demonstrates concordance between SVR4 and SVR12 for patients treated with G/P for treatment durations as short as 8 weeks, providing reassurance that long‐term follow‐up of patients with HCV is no longer necessary. Simplification of the HCV care cascade has the potential to improve outcomes for a wide range of patients by allowing for the reallocation of healthcare resources and reducing the risk of patients becoming LTFU, thereby contributing towards the elimination of HCV as a major public health threat.
CONFLICT OF INTEREST
Edward Gane is a member of advisory board participant: AbbVie, Aligos, Arbutus, Assembly, Clear B Therapeutics, Dicerna, Gilead Sciences, Janssen, Merck, Roche and Vir Bio, and is a lead investigator for an AbbVie funded study. Victor de Ledinghen is a member of advisory board participant and/or speaker: MyrPharma, AbbVie, Gilead, BMS, MSD, Tillotts, SuperSonic Imagine, Intercept Pharma, Orphalan, Echosens. Douglas E. Dylla is an employee of AbbVie and may hold stock/share options. Giuliano Rizzardini is a paid consultant: AbbVie, Angelini, Bristol‐Myers Squibb, Gilead, MSD, and ViiV, and is a contributor to research funding through ASST Fatebenefratelli‐Sacco University Hospital: AbbVie, Gilead, MSD, and ViiV Healthcare. Mitchell L. Shiffman is a speaker: AbbVie, Eisai, Daiichi Sankyo, Gilead, Intercept, and Shionogi; advisor: AbbVie, Eisai, Gilead, HepQuant, Intercept, Mallinckrodt, and Shionogi; involved in grant support: Aurora, Celgene, Conatus, CymaBay, Enanta, Exalenz, Genfit, Gilead, HepQuant, Intercept, Madrigal, Mallinckrodt, NGM Bio, and Viking Therapeutics; and involved in stock options: Exalenz. Stephen T. Barclay is a speaker and teaching fees, grant/research support, advisory boards: AbbVie and Gilead; advisory boards: Intercept. Jose Luis Calleja is a consultant and lecturer: AbbVie, Gilead Sciences, and Intercept. Zhenyi Xue is an employee of AbbVie Inc., and may hold stock/share options. Margaret Burroughs is an employee of AbbVie Inc., and may hold stock/share options. Julio A. Gutierrez is a speaker and scientific advisor: AbbVie, Alexion, Exelexis, Echosens, Gilead Sciences, Intercept, MediciNova, and Seal Rock. He is an investigator or has research grants from 3V Bio, Cymabay, Forma, Genfit, Metacrine, Merck, Paraxel, Pfizer, Regeneron, and Sorrento.
AUTHOR CONTRIBUTIONS
All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship. Glecaprevir was identified by AbbVie and Enanta.
ETHICS APPROVAL
The study protocol was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines approved by the institutional review board (Ethics Committee of Ärztekammer Westfalen‐Lippe; reference number 2014–395‐f‐S). All participants were required to provide written informed consent before being enrolled in the registry.
Supporting information
Table S1
ACKNOWLEDGMENTS
Medical writing support was provided by Sean Littlewood, MRes and Ugo Battaglia, PhD, of Fishawack Communications, Ltd; funded by AbbVie.
Gane E, de Ledinghen V, Dylla DE, et al. Positive predictive value of sustained virologic response 4 weeks posttreatment for achieving sustained virologic response 12 weeks posttreatment in patients receiving glecaprevir/pibrentasvir in Phase 2 and 3 clinical trials. J Viral Hepat. 2021;28:1635–1642. 10.1111/jvh.13600
Funding information
AbbVie sponsored the study, contributed to its design and participated in the collection, analysis and interpretation of the data and in the writing, reviewing and approval of the manuscript
DATA AVAILABILITY STATEMENT
An access to individual datasets is not available due to the informed consent. Upon request, aggregated data can be made available.
REFERENCES
- 1. World Health Organization (WHO) . Global Hepatitis Report 2017. Accessed 24th February 2021. http://apps.who.int/iris/bitstream/10665/255016/1/9789241565455‐eng.pdf
- 2. World Health Organization (WHO) . Global Health Sector Strategy on Viral Hepatitis. 2016–2021. 2016. Accessed 24th February 2021. https://apps.who.int/iris/bitstream/handle/10665/246177/WHO‐HIV‐2016.06‐eng.pdf;jsessionid=B789DC827B02AF7A18F792AB221CF0D5?sequence=1
- 3. Lazarus JV, Pericas JM, Picchio C, et al. We know DAAs work, so now what? Simplifying models of care to enhance the hepatitis C cascade. J Intern Med. 2019;286:503‐525. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization (WHO) . Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection. 2018. Accessed 24th February 2021. https://www.who.int/hepatitis/publications/hepatitis‐c‐guidelines‐2018/en/ [PubMed]
- 5. AbbVie Inc . Maviret prescribing information. May 2020.
- 6. Gilead Sciences Inc . VOSEVI prescribing information. November 2019.
- 7. Merck Sharpe & Dohme Corp . ZEPATIER prescribing information. December 2019.
- 8. Gilead Sciences Inc . EPCLUSA prescribing information. March 2020.
- 9. AbbVie Inc . VIEKIRA PAK prescribing information. December 2019.
- 10. Gilead Sciences Inc . HARVONI prescribing information. March 2020.
- 11. AbbVie Inc . Maviret Summary of Product Characteristics. June 2020.
- 12. Zeuzem S, Foster GR, Wang S, et al. Glecaprevir‐Pibrentasvir for 8 or 12 weeks in HCV genotype 1 or 3 infection. N Engl J Med. 2018;378:354‐369. [DOI] [PubMed] [Google Scholar]
- 13. Asselah T, Kowdley KV, Zadeikis N, et al. Efficacy of Glecaprevir/Pibrentasvir for 8 or 12 weeks in patients with hepatitis C virus genotype 2, 4, 5, or 6 infection without cirrhosis. Clin Gastroenterol Hepatol. 2018;16:417‐426. [DOI] [PubMed] [Google Scholar]
- 14. Aghemo A, Alberti A, Andreone P, et al. Effectiveness and safety of glecaprevir/pibrentasvir in chronic hepatitis C patients: Results of the Italian cohort of a post‐marketing observational study. Dig Liver Dis. 2020. doi: 10.1016/j.dld.2020.08.007 [DOI] [PubMed] [Google Scholar]
- 15. Berg T, Naumann U, Stoehr A, et al. Real‐world effectiveness and safety of glecaprevir/pibrentasvir for the treatment of chronic hepatitis C infection: data from the German Hepatitis C‐Registry. Aliment Pharmacol Ther. 2019;49:1052‐1059. [DOI] [PubMed] [Google Scholar]
- 16. Lampertico P, Carrion JA, Curry M, et al. Real‐world effectiveness and safety of glecaprevir/pibrentasvir for the treatment of patients with chronic HCV infection: a meta‐analysis. J Hepatol. 2020;72:1112‐1121. [DOI] [PubMed] [Google Scholar]
- 17. Lampertico P, Mauss S, Persico M, et al. Real‐world clinical practice use of 8‐week glecaprevir/pibrentasvir in treatment‐naive patients with compensated cirrhosis. Adv Ther. 2020;37:4033‐4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown RS Jr, Buti M, Rodrigues L, et al. Glecaprevir/pibrentasvir for 8weeks in treatment‐naive patients with chronic HCV genotypes 1–6 and compensated cirrhosis: the EXPEDITION‐8 trial. J Hepatol. 2020;72:441‐449. [DOI] [PubMed] [Google Scholar]
- 19. Aleman S, Soderholm J, Busch K, Kovamees J, Duberg AS. Frequent loss to follow‐up after diagnosis of hepatitis C virus infection: a barrier towards the elimination of hepatitis C virus. Liver Int. 2020;40:1832‐1840. [DOI] [PubMed] [Google Scholar]
- 20. Zuckerman E, Gutierrez JA, Dylla DE, et al. Eight weeks of treatment with glecaprevir/pibrentasvir is safe and efficacious in an integrated analysis of treatment‐naive patients with hepatitis C virus infection. Clin Gastroenterol Hepatol. 2020;18(2544–2553):e2546. [DOI] [PubMed] [Google Scholar]
- 21. Pham TT, Keast SL, Farmer KC, et al. Sustained Virologic response and costs associated with direct‐acting antivirals for chronic hepatitis C infection in Oklahoma Medicaid. J Manag Care Spec Pharm. 2018;24:664‐676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Darvishian M, Wong S, Binka M, et al. Loss to follow‐up: a significant barrier in the treatment cascade with direct‐acting therapies. J Viral Hepat. 2020;27:243‐260. [DOI] [PubMed] [Google Scholar]
- 23. European Centre for Disease Prevention and Control . Annual Epidemiological Report for 2018, Hepatitis C. Accessed 24th February 2021. https://www.ecdc.europa.eu/sites/default/files/documents/HEPC_AER_2018_Report.pdf
- 24. Ryerson AB, Schillie S, Barker LK, Kupronis BA, Wester C. Vital signs: newly reported acute and chronic hepatitis C cases ‐ United States, 2009–2018. MMWR Morb Mortal Wkly Rep. 2020;69:399‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. American Association for the Study of Liver Diseases (AASLD) and Infectious Diseases Society of America (IDSA) . Identification and Management of HCV in People Who Inject Drugs. 2019. Accessed 24th February 2021. https://www.hcvguidelines.org/unique‐populations/pwid
- 26. Pawlotsky JM, Ramers CB, Dillon JF, Feld JJ, Lazarus JV. Simplification of care for chronic hepatitis C virus infection. Semin Liver Dis. 2020;40:392‐402. doi: 10.1055/s-0040-1713657 [DOI] [PubMed] [Google Scholar]
- 27. Chen J, Florian J, Carter W, et al. Earlier sustained virologic response end points for regulatory approval and dose selection of hepatitis C therapies. Gastroenterology. 2013;144(1450–1455):e1452. [DOI] [PubMed] [Google Scholar]
- 28. Yoshida EM, Sulkowski MS, Gane EJ, et al. Concordance of sustained virological response 4, 12, and 24 weeks post‐treatment with sofosbuvir‐containing regimens for hepatitis C virus. Hepatology. 2015;61:41‐45. [DOI] [PubMed] [Google Scholar]
- 29. European Association for the Study of the Liver . EASL recommendations on treatment of hepatitis C: Final update of the series. J Hepatol. 2020;73:1170‐1218. [DOI] [PubMed] [Google Scholar]
- 30. AbbVie Inc . MAVYRET (US package insert). May 2020.
- 31. Liu X, Hu P. Efficacy and safety of Glecaprevir/Pibrentasvir in patients with chronic HCV infection. J Clin Transl Hepatol. 2021;9:125‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buti M, Esteban R. Management of direct antiviral agent failures. Clin Mol Hepatol. 2016;22:432‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grebely J, Dore GJ, Alami NN, et al. Safety and efficacy of glecaprevir/pibrentasvir in patients with chronic hepatitis C genotypes 1–6 receiving opioid substitution therapy. Int J Drug Policy. 2019;66:73‐79. [DOI] [PubMed] [Google Scholar]
- 34. Lybeck C, Brenndorfer ED, Sallberg M, Montgomery SM, Aleman S, Duberg AS. Long‐term follow‐up after cure from chronic hepatitis C virus infection shows occult hepatitis and a risk of hepatocellular carcinoma in noncirrhotic patients. Eur J Gastroenterol Hepatol. 2019;31:506‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lin C‐P, Liang P‐C, Huang C‐I, et al. Concordance of SVR12, SVR24 and SVR durability in Taiwanese chronic hepatitis C patients with direct‐acting antivirals. PLoS One. 2021;16:e0245479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kottilil S, Kamat M, Gupte A, Shah S. Does Lost to follow up for HCV Treatment Matter Anymore? Noverl Strategy for HCV Elimination. Poster presented at: American Association for the Study of Liver Disease; 2020. [Google Scholar]
- 37. Martinello M, Grebely J, Petoumenos K, et al. HCV reinfection incidence among individuals treated for recent infection. J Viral Hepat. 2017;24:359‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
An access to individual datasets is not available due to the informed consent. Upon request, aggregated data can be made available.


