Abstract
Background
Adverse drug reactions (ADRs) are estimated to be the fifth cause of hospital death. Up to 50% are potentially preventable and a significant number are recurrent (reADRs). Clinical decision support systems have been used to prevent reADRs using structured reporting concerning the patient's ADR experience, which in current clinical practice is poorly performed. Identifying ADRs directly from free text in electronic health records (EHRs) could circumvent this.
Aim
To develop strategies to identify ADRs from free‐text notes in electronic hospital health records.
Methods
In stage I, the EHRs of 10 patients were reviewed to establish strategies for identifying ADRs. In stage II, complete EHR histories of 45 patients were reviewed for ADRs and compared to the strategies programmed into a rule‐based model. ADRs were classified using MedDRA and included in the study if the Naranjo causality score was ≥1. Seriousness was assessed using the European Medicine Agency's important medical event list.
Results
In stage I, two main search strategies were identified: keywords indicating an ADR and specific prepositions followed by medication names. In stage II, the EHRs contained a median of 7.4 (range 0.01‐18) years of medical history covering over 35 000 notes. A total of 318 unique ADRs were identified of which 63 were potentially serious and 179 (sensitivity 57%) were identified by the rule. The method falsely identified 377 ADRs (positive predictive value 32%). However, it also identified an additional eight ADRs.
Conclusion
Two key strategies were developed to identify ADRs from hospital EHRs using free‐text notes. The results appear promising and warrant further study.
Keywords: adverse drug event, adverse drug reaction, clinical decision support, clinical decision support system, drug allergy, free‐text, natural language processing, text‐mining
What is already known about this subject
Recurrent adverse drug reactions (ADRs) are common and significantly influence morbidity, mortality and medical costs. Many recurrent ADRs are preventable and can be attributed to unintended represcription. Clinical decision support systems were implemented to prevent unintended represcription, but such systems only function when the ADR is registered as structured information, which in current clinical practice is poorly done.
What this study adds
Identifying ADRs directly from hospital free‐text electronic health record (EHR) notes using an automated tool is promising, although sensitivity and specificity need further improvement. Nearly a third of all registered ADRs could, however, not be found in physician notes. The seriousness influences the chance the rule‐based model finds the ADR.
1. INTRODUCTION
Adverse drug reactions (ADRs), including allergic responses, frequently occur and significantly influence morbidity, mortality and medical costs. 1 , 2 About 3.6‐6.5% of hospitalizations are related to an ADR. 3 , 4 , 5 , 6 , 7 Furthermore, 10‐15% of patients develop an ADR during hospitalization 4 , 8 resulting in death in 0.05‐0.25% of cases. 4 , 9 ADRs are the fifth most common cause of hospital deaths. 4 , 10 Moreover, 28‐56% of all ADRs are potentially preventable. 5 , 7 , 11 , 12 Different approaches have been used to define preventability, and most of these present difficulties for translation into interventions. A significant number of potentially preventable ADRs are recurrent ADRs (reADRs) (10‐30% of all ADRs, 13 , 14 13‐50% of medication‐related hospitalizations 14 , 15 , 16 , 17 ). reADRs have a different form of preventability compared to first occurrence ADRs, introducing knowledge on a patient's response to a drug in a certain dose in a certain context, making them easier to prevent. Preventable reADRs have multiple origins. The most important cause is unintended represcription, defined as the represcription of medication previously intentionally stopped due to an ADR (eg, the represcription of hydrochlorothiazide stopped due to hyponatraemia in an elderly woman). 15 , 18 To prevent unintended represcriptions and the risk of reADRs, clinical decision support systems (CDSSs) have been implemented to alert prescribers when a medication is represcribed after it was previously stopped due to an ADR. 19 Currently, however, CDSSs only function when the ADR is registered as structured information at the level of the individual patient within an ADR module linked to or part of the computerized physician order entry (CPOE) system of the electronic health record (EHR). In current clinical practice, this is poorly performed due to time constraints, inadequate IT systems, a lack of peer support and failing to acknowledge the importance of structurally registering ADRs. 20 Healthcare professionals frequently describe ADRs in clinical notes and discharge summaries using free‐text entries, 18 which is not effective in preventing unintended represcription. 16 , 21 , 22
Identifying ADRs directly from free‐text EHR notes could solve the issue of underreporting in a structured format by healthcare professionals. In recent years, progress has been made in identifying ADRs from free text. Honingman et al developed an algorithm to screen primary care records. 23 Iqbal et al developed and validated an algorithm to detect specific ADRs related to antidepressants and antipsychotics in psychiatric hospital EHRs. 24 Aramaki et al developed and tested an ADR identification algorithm using Japanese discharge summaries. 25 The sensitivity of the different algorithms was approximately 60% for general ADR identification 23 , 25 and up to 90% for specific ADRs. 24
Previous studies have focused on specific medication, 24 , 26 specific ADRs, 27 , 28 selected notes 25 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 and specific settings. 23 , 24 No studies have been identified that use a general approach to detect ADRs from all free text available in a hospital EHR system. Investigating specific notes may result in identifying only a fraction of the reported ADRs; focusing on specific ADRs automatically overlooks other ADRs. Moreover, previous studies have not assessed the causality and seriousness of the identified results. Therefore, the aim of this study was to develop strategies to identify ADRs from free‐text notes in hospital EHRs.
2. METHODS
2.1. Design and setting
The study was performed at the Catharina Hospital, Eindhoven, the Netherlands, a 696‐bed teaching hospital that used CS‐EZIS (version 5.2, Chipsoft B.V., Amsterdam, the Netherlands) for its EHR system. This EHR system was implemented in stages, launching in 2008 and adopting paperless recording from 2015 onwards. Medical records before 2008 are available (as scanned PDFs) as part of the multimedia module. Within the EHR system there are distinct modules. For example, a CPOE module, a module for structured ADR registration, a CDSS module and a module for free‐text EHR notes. Inside the free‐text EHR module, different types of EHR notes may be distinguished (eg, physician notes, nursing notes, pathology notes, radiology notes and operation notes). An EHR note is registered at a specific time and could contain multiple entries such as medical history, physical examinations, additional findings, summaries and therapeutic plans. Figure 1 provides a graphical representation of the EHR structure. To supply additional, medication‐related, clinical decision support the hospital uses Gaston Pharma (Gaston Medical, Eindhoven, the Netherlands), which is linked to the EHR database.
FIGURE 1.
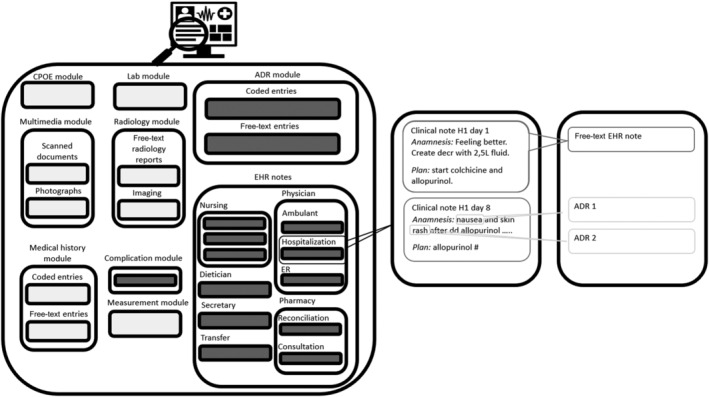
On the left is a graphical representation of the EHR including the different modules. The free‐text notes included from the different modules are marked grey. On the right is an example of a free‐text EHR note with two potential ADRs
2.2. Stage I: Identification of search strategies
To discover strategies for identifying ADRs, the EHRs of 10 random patients from internal medicine and geriatric departments were manually screened and supplemented with strategies devised by the researchers. ADRs retrieved from the manual review were categorized into key identification strategies. These were subsequently fine‐tuned by adding different words with the same meaning, commonly used abbreviations for these words, and typing and common spelling errors. Based on the false negatives, letter combinations or text strings were identified which were to be ignored, followed by variations, abbreviations, typing and spelling errors. The strategies were programmed into a rule‐based model using the available CDSS, Gaston Pharma. The model output included a text string containing the identified keywords (determined by the strategy), the entire free‐text EHR note and the EHR notes without the disregarded text strings. One output could contain one or more ADR.
2.3. Stage II: Inclusion of patients' EHRs
The performance of the rule‐based model was assessed using 45 additional EHRs, which were compared to a manual EHR review. The EHRs of 45 consecutive patients were included in the study when the patients were hospitalized for over 24 hours to the departments of geriatrics (15), internal medicine (15) or oncology (15). The inclusion order was based on reverse chronological discharges before 1 June 2018. A complete history of the free‐text EHR notes was included. Scanned or imported (PDF) documents were excluded.
2.4. Stage II: EHR reviews, definitions and classification
The manual EHR review was performed independently by two assessors (a clinical pharmacist and a physician in training) using a predefined protocol, included in Supporting Information. The EHRs were searched for free‐text notes containing potential ADRs. The ADRs were defined according to the World Health Organization (WHO): “a response to a drug which is noxious and unintended, and which occurs at doses normally used in man for the prophylaxis, diagnosis, or therapy of disease, or for the modifications of physiological function”. 37 Type A‐‐D potential ADRs were included: type A, augmented pharmacological effects; type B, bizarre, including allergic and nonimmune drug sensitivities; type C, chronic effects; and type D, delayed effects, including carcinogenesis and teratogenesis. 38 In cases where the two assessors did not reach a consensus, a third assessor (a member of the Dutch Pharmacovigilance Centre, LAREB) gave the final decision. Symptoms or diseases with multifactorial causes, including medication, were included as potential ADRs (eg, “hyponatraemia due to malnutrition and hydrochlorothiazide use”). Duplicate entries (ie, the same ADR occurring during the same hospitalization) were not included. reADRs were scored separately. An ADR was considered recurrent if the medication was represcribed or the ADR occurred during a separate hospitalization. Free‐text and CPOE were not searched to find out if special measures were taken to modify risk of recurrence when represcribed (eg, dose reduction). Anatomical Therapeutic Chemical (ATC) classification was used to code the medication associated with the ADR. An ADR having more than one medication as the possible cause was included as a single ADR with all separately coded medications. In contradiction to pharmacovigilance requirements, ADRs without specific drug names mentioned, but with a drug group mentioned (eg, hyponatraemia due to antidepressant use) were included in the study, as these can still present important additions to the care process and medical history. The rule‐based model used the Dutch G‐standard database, including all generic medicine names, trade names and group names registered in the Netherlands. 39 The ADRs were classified using the Medical Dictionary for Regulatory Activities (MedDRA version 23.1). MedDRA provides validated standardized hierarchical structure terminology, which is used by regulatory authorities, post‐marketing pharmacovigilance institutes and pharmaceutical manufacturers. The ADRs were classified using the lowest hierarchy, being the lower‐level term, while the preferred term, used in the summary of product characteristics (SmPC), was matched to obtained references to ADRs. For example, the lower‐level terms tingling of extremity, pins and needles and peripheral neuropathy all fall within the preferred term paraesthesia. ADRs were categorized as potentially serious using the corresponding European Medicines Agency's Important Medical Events list. 40 The causality of the ADRs was assessed by a clinical pharmacist trained in pharmacovigilance using the Naranjo algorithm. Only ADRs with a Naranjo score of ≥1 were included. 41
2.5. Stage II: Data collection
At the moment of hospitalization, characteristics such as gender, age, total medications (including over‐the‐counter medications) and treatment specialisms were collected from the patients' EHRs. Medical history and laboratory results were collected to calculate the Charlson Comorbidity Index. Moreover, the following information was collected to characterize the data: the number of hospitalizations (≥24 hours) and ambulant visits (including hospitalizations <24 hours), medical specialisms, record history, the number of EHR notes and the number of words (calculated using spaces) and characters used. The following data was collected for each EHR note containing ADRs: ADRs, medication involved, search strategy, surrounding paragraph or context (including the space between words, date, form and type of healthcare professional). Research Manager (Cloud9, Deventer) was used to record, edit and save the anonymized data. Venn diagram plotter version 1.5.5 was used to construct the Venn diagram.
2.6. Stage II: Data analysis
If an alert generated by the rule contained multiple ADRs, they were all considered to be identified. True positives (TPs) were ADRs identified by the manual and rule‐based EHR reviews. False positives (FPs) were identifications by the rule‐based EHR review but not the manual review. False negatives (FNs) were ADRs not identified by the rule‐based EHR review. Sensitivity was calculated as TPs/(TPs + FNs). The positive predictive value (PPV) was calculated as TPs/(TPs + FPs). FNs and FPs were further analysed to improve the applied search strategies and search for additional strategies to improve future versions of the tool. FPs were also analysed to provide recommendations for improving the list of disregarded text strings and context.
3. RESULTS
3.1. Stage I: Identification of search strategies
Based on the 10 EHR records, five key strategies for identifying EHR notes containing ADRs were identified (Table 1). Supporting Information Table S1 provides a full overview, including disregarded text strings and original Dutch words. The first strategy (S1) used keywords implying one or multiple ADRs, including conjugations of (a) drug‐induced, (b) allergy, (c) side effect, (d) intolerance, (e) reaction and (f) toxicity. The second strategy (S2) included a search of 13 different prepositions followed by drug groups, names, therapies or their abbreviations. An example could be “pins and needles after FOLFOX cycle.” Supporting information Table S1 provides a full list of the added abbreviations used in S1 and S2. The third strategy (S3) used free‐text entries titled allergy and anaphylaxis. Such free‐text entries were used when the ADR module was introduced in 2015. The fourth strategy (S4) searched the complication registration module for drug‐related complications. The final strategy (S5) searched for ADRs registered in the ADR module, including coded and free‐text entries.
TABLE 1.
Summary of ADR identification strategies used in the rule
| Number | Search strategies | Included trigger words e | |
|---|---|---|---|
| S1 | Keywords implying an ADR | Conjugations of | Drug‐induced |
| Allergy | |||
| Side‐effect | |||
| Intolerance | |||
| Toxicity | |||
| Reaction | |||
| S2a | Prepositions followed closely b by a drug group c , a generic drug name, a drug brand, trade name or abbreviation a drug c or drug therapy d | Conjugations of | By |
| With | |||
| After | |||
| Of | |||
| On | |||
| Since | |||
| S2b | Abbreviations using the included prepositions | Conjugations of | a.r. (as a result) |
| b.o. (based on) | |||
| a.c.o. (as a consequence of) | |||
| S3 | Content of forms labelled a | Conjugations of | Allergies: |
| Anaphylaxis: | |||
| S4 | Content of complication registration containing key field drug‐induced | … | … |
| S5 | Content of ADR module | … | … |
Notes: The maximum number of characters (ie, proximity between a preposition and a drug name) was set at 16.
Abbreviations: ADR, adverse drug reaction.
Forms labelled allergy and anaphylaxis using free‐text entries were employed prior to the introduction of the ADR module in 2015.
The maximal number of characters between the preposition and the drug name was 16.
The drug group names were based on the Anatomical Therapeutic Chemical (ATC) therapeutic subgroup, pharmacological subgroup, chemical subgroup or chemical substance (ie, second to fifth levels of ATC main groups classified by WHO).
Examples are PPI (proton‐pump inhibitor), HCTZ (hydrochlorothiazide), FOLFOX (combination therapy of fluorouracil and oxaliplatin). A full list of abbreviations is provided in Table S2.
English translations of the trigger words are presented here; Dutch trigger words are presented in Table S1.
3.2. Stage II: Patient and data characteristics
Table 2 presents the patient and data characteristics included in the 45 EHRs. The mean age of the patients was 68 years (range 21‐92) and 64.4% were female. During the most recent hospitalization, patients had a median Charlson Comorbidity Index score of 5 (range 0‐13) and used a median of eight (range 0‐20) different medications. Patients had a median of three hospitalizations (range 1‐39) and 60 ambulant visits (ie, hospital stay <24 hours) (range 2‐433), resulting in a median medical history of 7.4 years (range 0.01‐18). The median number of free‐text EHR notes per patient was 585 (range 41‐2820). These were formed of a median of 41 921 words (range 4070‐259 750) constructed by a median of 449 179 (22 027‐2 594 750) characters. This resulted in approximately 35 000 free‐text EHR notes for review.
TABLE 2.
Patient and data characteristics
| Variable | Range | |
|---|---|---|
| Mean age in years | 68 | 21‐92 |
| Female (%) | 29 (64.4) | n/a |
| Variable | Median | Range |
| Charlson comorbidity index at last hospitalization | 5 | 0‐13 |
| Unique medication used at last hospitalization (n) | 8 | 0‐20 |
| Hospitalizations a | 3 | 1‐39 |
| Ambulant visits b | 60 | 2‐433 |
| Medical record history (years) | 7.4 | 0.01‐18 |
| FT EHR notes per patient | 585 | 41‐2820 |
| Words c per patient | 41 921 | 4070‐259 750 |
| Characters per patient | 449 179 | 22 027‐2 594 750 |
Abbreviations: EHR, electronic health record; FT, free text.
Hospitalizations were >24 hours; hospitalizations <24 hours were included as ambulant visits.
Ambulant visits included telephone and video consultations.
The number of spaces was used to estimate the number of words.
3.3. Stage II: Inclusion of ADRs
Figure 2 provides a flowchart showing the inclusion of potential ADRs discovered during the manual EHR review. A total of 643 potential ADRs were identified. During matching, 39 potential reADRs were detected and the remaining potential ADRs (n = 269) were identified as duplicates. Excluding the duplicates and reADRs resulted in 326 unique potential ADRs. After excluding eight (n = 8) potential ADRs with a Naranjo score <1, 318 unique ADRs remained.
FIGURE 2.
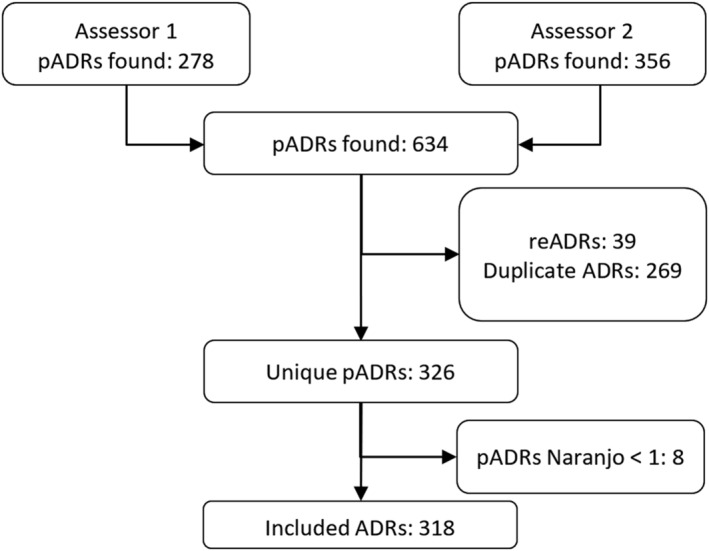
Inclusion and exclusion of potential ADRs. pADRs, potential adverse drug reactions; reADRs, recurrent adverse drug reacions
3.4. Stage II: Type of EHR notes
ADRs were found in different types of EHR notes. Most ADRs (68%, n = 216) were cited in physician notes, including ambulant, ER and admission notes. However, 17% (n = 55) of ADRs were only found in nursing notes. The remaining identified ADRs were only found in other types of EHR notes, such as dietician or pharmacist notes. ADRs included in the study were recorded by 206 individual healthcare professionals, dived over 12 medical specialisms.
3.5. Stage II: ADR characteristics
The median Naranjo score for the included ADRs was 4 (range 1‐6). Fifteen ADRs were judged probable (score 5‐8) and no ADRs were scored definite (score ≥9). Overall, patients had a median of four ADRs (range 0‐32). The median number of ADRs was six (range 1‐32) in the oncology EHRs and two (range 0‐26) in the internal medicine and geriatric EHRs. Supporting Information Table S3 provides an overview of the number of ADRs per system organ class. A fifth (19.8%, n = 63) of all ADRs were classified as potentially serious. Supporting Information Table S4 provides an overview of all potentially serious ADRs and related medication. Twenty of these were related to chemotherapy, six to myelosuppression, seven to polyneuropathy, three to hepatotoxicity and one to a pulmonary embolism. Serious ADRs not related to chemotherapy were renal failure (n = 6), myelosuppression (n = 5), hepatotoxicity (n = 4) and ileus (n = 2). Cardiac problems were frequently registered, including bradycardia (n = 3), QT prolongation (n = 2), ventricular tachycardia (VT) (n = 1) and cardiovascular collapse (n = 1). One anaphylactic reaction and one case of allergic angioedema were also identified.
Most ADRs (87%, n = 278) were stated in the SmPC of a causative medicine. Five percent (n = 14) could have been related to the ADRs cited in the SmPC, albeit not at the preferred term level, 3% (n = 10) of symptoms had no specific drug mentioned in the ADR entry, so no match was possible, and 5% (n = 16) of symptoms had no reference in the SmPC. A few examples of ADRs without reference in the SmPC were paraesthesia due to exemestane, polyneuropathy due to oxycodone and urine retention due to midazolam. Supporting Information Table S5 provides the full list of ADRs with no reference in the SmPC.
A total 39 of reADRs were identified, representing over 10% of the identified ADRs, distributed over 17 patients. Twelve of the 39 reADRs were potentially serious, seven of which were associated with chemotherapy. One reADR resulted in an acute hypersensitivity reaction due to represcription during hospitalization.
3.6. Stage II: Comparison of rule‐based and manual EHR reviews
The rule‐based model identified 556 potential ADRs, 179 unique identifications matched the ADRs obtained from the manual EHR review and 377 potential ADRs were identified as FPs. Of the 318 ADRs identified in the manual EHR review, 179 were also identified by the rule‐based review, resulting in a sensitivity of 57% and a PPV of 32%. However, the rule identified eight additional ADRs with a Naranjo score ≥1, of which one ADR was classified as serious. Figure 3 presents a Venn diagram of the EHR review methods and the overlap therein.
FIGURE 3.
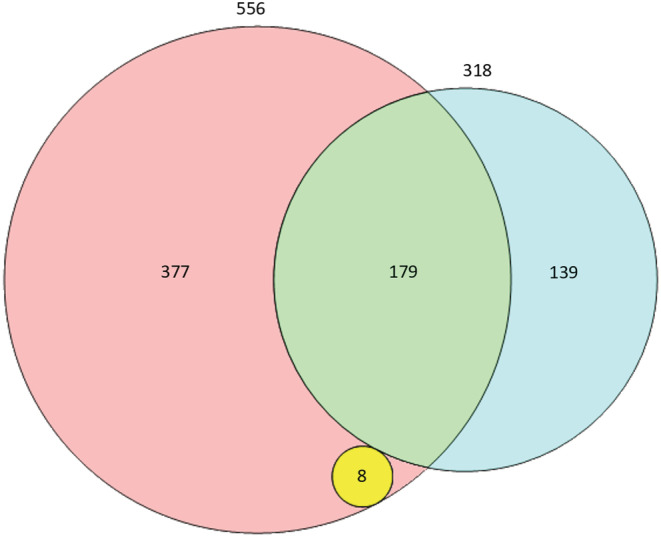
Venn diagram presenting unique adverse drug reactions (ADRs). The blue circle (n = 318), including the green portion, represents the total number of unique ADRs identified by the manual electronic health record (EHR) review. The red circle (n = 556) including the green and yellow portions represents the total number of unique ADRs identified by the rule‐based EHR review (true positives + false positives). The red section (n = 377) represents the false positives. The green section (n = 179) represents the number of true positives. The yellow circle represents ADRs found only by the rule‐based EHR review
3.7. Stage II: Analysis of rule‐based EHR strategies
Table 3 presents the TPs, FPs and PPVs for the different search strategies, including their stratifications. The total TPs per strategy is higher than the number of unique ADRs that were correctly identified using multiple search strategies. The rule‐based model correctly identified 179 unique ADRs. Of these 179 ADRs, 159 were identified using only one strategy, 19 were identified using two strategies (S2 with + S1 drug‐induced, n = 7; S2 with + S2 various, n = 7; S1 drug‐induced + S2 of, n = 2; S2 by + S2 of, n = 2; S1 drug‐induced + S1 allergy, n = 1) and four were identified using three strategies (S1 drug‐induced + S1 toxicity + S2 of, n = 2; S1 allergy + S1 reaction + S2 on, n = 1; S2 by + S2 with + S2b a.c.o. [as a consequence of], n = 1), which adds‐up to 206 true positive identifications.
TABLE 3.
True positives and false positives per search strategy identifying ADRs
| Number | Search strategies | Included trigger words | TPs | FPs | PPV | |||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | % | ||||
| S1 | Keywords implying an ADR | Conjugations of | Drug‐induced | 20 | 10 | 5 | 1 | 80 |
| Allergy | 17 | 8 | 80 | 21 | 18 | |||
| Side effect | 21 | 10 | 10 | 3 | 68 | |||
| Intolerance | 0 | 0 | 0 | 0 | n/a | |||
| Reaction | 1 | 0 | 2 | 1 | 33 | |||
| Toxicity | 13 | 6 | 1 | 0 | 93 | |||
| S1 Total/overall ADRs found by keywords | 72 | 35 | 98 | 26 | 42 | |||
| S2a | Prepositions followed closely b by a drug group c , a generic drug name, a drug brand, trade name or abbreviation a drug, or drug therapy d | Conjugations of | By | 16 | 8 | 19 | 5 | 41 |
| With | 55 | 27 | 44 | 12 | 56 | |||
| After | 12 | 6 | 67 | 18 | 15 | |||
| Of | 31 | 15 | 62 | 16 | 33 | |||
| On | 5 | 2 | 73 | 19 | 6 | |||
| Since | 2 | 1 | 12 | 3 | 14 | |||
| S2b | Abbreviations using the included prepositions | Conjugations of | As a result of/because of | 0 | 0 | 1 | 0 | 0 |
| Based on | 3 | 1 | 0 | 0 | 100 | |||
| As a consequence of | 4 | 2 | 1 | 0 | 80 | |||
| S2 Total/overall ADRs found by a combination of predisposition and drug name | 128 | 62 | 278 | 74 | 31 | |||
| S3 | Content of forms labelled a | Conjugations of | Allergies: | 0 | 0 | 0 | 0 | n/a |
| Conjugations of | Anaphylaxis: | 0 | 0 | 0 | 0 | n/a | ||
| S3 Total/overall ADRs found in labelled allergy and anaphylaxis forms | 0 | 0 | 0 | 0 | n/a | |||
| S4 | Content of complication registration containing key field drug‐induced | … | … | 0 | 0 | 0 | 0 | n/a |
| S5 | Content of ADR module | … | … | 6 | 3 | 0 | 0 | 100 |
| S5 Total/overall ADRs found in ADR module | 6 | 3 | 0 | 0 | 100 | |||
| Total ADRs identified | 206 | 100 | 377 | 100 | 35 | |||
Abbreviations: Adr, adverse drug reaction; FP, false positive; PPV, positive predicted value; TP, true positive.
Forms labelled allergy and anaphylaxis using free‐text entries were applied prior to the ADR module's introduction in 2015.
The maximum number of characters (ie, proximity between a preposition and drug name) was set at 16.
The drug group names were based on the Anatomical Therapeutic Chemical (ATC) therapeutic subgroup, pharmacological subgroup, chemical subgroup or chemical substance (ie, second to fifth levels of ATC main groups classified by WHO).
Examples are PPI (proton‐pump inhibitor), HCTZ (hydrochlorothiazide), FOLFOX (combination therapy of fluorouracil and oxaliplatin). A full list of abbreviations is provided in Table S2.
Overall the search strategy using prepositions followed by a drug name or group (S2) accounted for 62% (n = 125) of the identified ADRs, while using keywords (S1) accounted for 35% (n = 72), and only 3% (n = 6) were identified using the ADR module (S5). Within S1 the most effective keyword was toxicity (PPV of 93%), which identified 6% (n = 13) of the ADRs. Less effective, although with a higher yield, were the keywords drug‐induced (PPV 80%, n = 20) and side effect (PPV 68%, n = 21). Within S2, the preposition with the highest yield was with (46 TPs, PPV 32%). The term based on had a PPV of 100%, although it identified only two ADRs. The FPs related to S2 were responsible for 74% of the total ADRs, followed by words forming abbreviations of allergy (21%, n = 80). Naranjo causality score and SmPC reference did not markedly increase or decrease the sensitivity of the rule‐based review, nor did the system organ class or the type of medication. However, ADR potential seriousness increased the sensitivity to 67% (41/66) compared to 55% (138/252) for nonserious ADRs.
3.8. Stage II: False negatives analysis
Table 4 shows the analysis and categorization of the false negatives (n = 139). S2 accounted for most the of the false negatives, 41% (n = 57). Within S2, abbreviations of drug names (31.7%, n = 44) were the most common cause and missing abbreviations (4.3%, n = 6) were the second most common cause. Other search strategies using S1 and S3 accounted for six (4.3%) and three (2.2%) false negatives, respectively. Two additional strategies were uncovered from the analysis of the false negatives. The most promising additional strategy (aS6) was usage of MedDRA terms combined with drug names in close proximity to each other (16 characters), which identified an additional 29 ADRs. The second additional strategy was adding abbreviations of ‘cannot tolerate’, which added an additional two positively identified ADRs. For 30.2% (n = 38) of false‐negative ADRs, no simple rule‐based identification strategies were uncovered. ADRs not mentioned in physician notes were less likely to be identified and were responsible for 44% (54/122) of the FNs.
TABLE 4.
Analysis of false negatives
| Number | Search strategies (n = 139) | n | % | |
|---|---|---|---|---|
| Missing conjugations of | Drug‐induced | 3 | 2.2 | |
| Allergy | 0 | 0.0 | ||
| Side effect | 3 | 2.2 | ||
| Intolerance | 0 | 0.0 | ||
| Reaction | 0 | 0.0 | ||
| Toxicity | 0 | 0.0 | ||
| S1 | Potential improvement: Using keywords implying ADRs | 6 | 4.4 | |
| >16 characters between the preposition and drug name | 2 | 1.4 | ||
| Missing synonyms for drug names | 44 | 31.7 | ||
| Missing abbreviations | 6 | 4.3 | ||
| Missing prepositions | 2 | 1.4 | ||
| DD | 3 | 2.2 | ||
| S2 | Potential improvement: Using prepositions followed closely a by a drug group b , d , a generic drug name, a drug brand, trade name or abbreviation a drug, or drug therapy c , d | 57 | 41.0 | |
| S3 | Potential improvement: In ADRs found in labelled allergy and anaphylaxis forms | 0 | 0.0 | |
| Specific missing complication fields | 3 | 2.2 | ||
| S4 | Potential improvement: Content of complication registration containing key field drug‐induced | 3 | 2.2 | |
| S5 | Potential improvement: Found in ADR module | 0 | 0.0 | |
| MedDRA + drug name | 3 | 2.2 | ||
| Drug name + MedDRA | 24 | 17.3 | ||
| Missing synonym of MedDRA term | 2 | 1.4 | ||
| aS6 | Opportunity for additional strategy: MedDRA term mentioned in text combined with drug name d | 29 | 20.9 | |
| Cannot tolerate | 2 | 1.6 | ||
| aS7 | Opportunity for additional strategy: Abbreviations of cannot tolerate | 2 | 1.6 | |
| No obvious additional strategy e | 42 | 30.2 | ||
Abbreviations: ADR, adverse drug reactions; DD, differential diagnosis.
The maximum number of characters between the preposition and drug was 16.
Drug group names used were based on the ATC therapeutic subgroup, pharmacological subgroup, chemical subgroup or chemical substance (ie, second to fifth levels of ATC main groups classified by WHO).
Examples being PPI (proton‐pump inhibitor), HCTZ (hydrochlorothiazide), FOLFOX (combination therapy of fluorouracil and oxaliplatin). A full list of abbreviations is provided in Table S2.
MedDRA term and drug name are mentioned within 16 characters of each other.
No simple rule‐based strategy was thought of to identify these ADRs.
4. DISCUSSION
This study describes the first steps in the development of an automated tool for identifying ADRs using free text in hospital EHRs. To our knowledge, this is the first study describing strategies to identify ADRs from a hospital EHR using all types of free‐text EHR notes and including all types of ADRs. Furthermore, it is the first to consider the causality of the potential ADRs. During stage I, the manual review of 10 EHRs, two promising strategies were identified: keywords indicating an ADR and specific prepositions followed closely by medication names. In stage II, 45 complete EHR histories were manually reviewed and compared to strategies built into a rule‐based model. Despite the early development stage, the rule‐based model achieved a sensitivity of 57% and a PPV of 32%. Analysis of the FNs revealed that S1 as well as S2 could potentially be significantly further improved.
Studies of previous ADRs involving hospitalizations have demonstrated that each patient handover is accompanied by information loss, particularly during handovers from hospitals to primary care. 22 , 42 This study supports these findings within the same hospital and EHR setting. In 32% of cases, no reference was found in physician notes to ADRs recorded by nurses, pharmacy technicians, pharmacists or other healthcare professionals. These findings also support the hypothesis that focusing on specific EHR notes only partially identifies previous ADRs. Moreover, only 2% of the ADRs had a structured registration, enabling CDSS alerting. Recurrency of ADRs was common: 17 of the 45 patients studied experienced a reADR and one patient had three recurrences. One of these reADRs resulted in an acute hypersensitivity reaction due to unintended represcription during hospitalization. While not formally assessed, at least 10% of the ADRs appeared to have been preventable, with a warning during represcription.
Analysis of the FNs revealed possibilities for fine‐tuning discovered strategies, such as extending the library of synonyms and abbreviated medication names. However, the analysis also revealed that additional strategies are needed to achieve the desired sensitivity. An obvious strategy would be to include symptoms and side effects followed or preceded by medication names. While powerful, this strategy may be prone to falsely identifying disease symptoms as an ADR. Moreover, although an extensive, coded database of ADRs was readily available, many of the ADRs were either misspelled, abbreviated or described in such a way that they were not readily identified in the MedDRA database. As with the G‐standard medication database used in the developed tool, the MedDRA database will require extension to include frequently used synonyms and abbreviations. The FP analysis demonstrated that natural language processing techniques are required to understand the context of trigger words, for example recognizing when effects are considered positive (eg, hypernatremia resolved after starting hydrochlorothiazide). However, several simple modifications could potentially significantly reduce FPs. The first would be to extend the library of disregarded text strings; this could reduce the number of FPs resulting from the trigger word allergy in particular. Second, medicine names or abbreviations therefore must be screened to identify those having additional meanings.
One of the limitations of the study methodology was that the identification strategies were based on EHRs originating from a single hospital, using one EHR system, while language use may differ between hospitals and regions. Furthermore, only EHRs of patients recently hospitalized to a ward focusing on internal medicine were included. The language used by healthcare professionals may vary according to their specialisms. Scanned documents were discarded, thereby potentially missing ADRs, particularly since referral letters often contain ADR information. These limitations may have resulted in a failure to discover key identification strategies and an overestimation of the rule's performance. Nevertheless, the EHR history contained notes from ambulant visits and hospitalizations related to several medical specialisms (n = 12) and the ADRs were recorded by a large number of diverse healthcare professionals (n = 206). The Naranjo algorithm was used to assess causality of the ADRs. There is, however, much debate on the reliability of this and other algorithms to assess causality because of problems with reproducibility and validity. However, “no method is universally accepted for causality assessment of ADRs”. 43 The Naranjo algorithm was chosen as it is still the preferred method for causality assessment by pharmacovigilance authorities and healthcare professionals in the Netherlands. At least possible (≥0) ADRs were included. It could be argued that only a score of ≥5 or even ≥9 should be used for inclusion. However, the primary aim of the study was to discover strategies identifying EHR notes possibly containing ADRs, and for this purpose it is useful to include all possible ADRs. Excluding type E and F ADRs could be seen as a limitation. Current CDSSs, however, would not be able to generate alerts on E‐ and F‐type ADRs.
The first step to further develop the tool would be to translate the search strategies and logic to programming more suitable for natural language processing (eg, Python or R). This process would also create the possibility of adding fuzzy logic and using artificial intelligence techniques such as machine learning. The developed rule‐based model retrieved items of text referring to ADRs, but it did not extract and code the ADRs and associated medication, which would be required to avoid duplicating identified ADRs and is essential before feeding ADRs back to the EHR for use in a CDSS. Therefore, the second step would be to automatically extract and code the ADRs from the identified text strings. Also, for a tool to fully utilize all available free text in the EHR, optical character recognition software must be considered before processing the text. At the back end of the tool, a CDSS could be used to extract valuable information to contextualize the retrieved ADR. For example, if the tool returns hypernatremia due to diuretic, the CDSS can retrieve the specific medication and dose used from the CPOE. After such developments, the tool should be tested on a different hospital EHR to study the generalizability and usability of the tool.
Using ADRs registered in free text as input for CDSS to alert physicians would be a considerable advance to reduce the number unintended represcriptions. It is important to consider, however, that there is also an overall underreporting of ADRs by healthcare professionals, 44 therefore the implementation of tools to detect ADRs from free text will never solve the entire problem. Considerable attention should thus also be given to directly improving ADR registration by patients as well as healthcare professionals. Education and electronic reminders can help to improve the feeling of support from social environment and recognition of the importance of correct ADR registration. 45 , 46 Also, improving EHR systems in such a way as to make it easier and less time‐consuming to properly register an ADR can markedly improve registration. 47 Introducing patient self‐reporting within the EHR patient portal would possibly also increase the number of ADRs registered. 48
5. CONCLUSION
Two key strategies were developed to identify ADRs from free text in a hospital EHR. These strategies show promise, warranting further study and the development of a tool to alert healthcare professionals to previously experienced ADRs.
CONFLICT OF INTEREST
None declared.
CONTRIBUTORS
A.Wa., A.We,, M.K., R.G. and C.vdL. contributed to the conception and initial design of the study. T.E. and E.K. contributed to the revised study design. A.Wa. and A.We. contributed to the acquisition of the data. N.J. contributed to the performing causality assessment of the potential adverse drug events and validation of MedDRA coding. A.Wa., A.We. and B.vdB. contributed to the analysis of the data and drafting of the manuscript. N.J., E.K., T.E., A.B., M.K., R.G. and C.vdL. critically revised the interpretation and analysis of the data and manuscript. All authors agree to be fully accountable for ensuring the integrity and accuracy of the work. All authors have read and approved the final manuscript.
6.
Supporting information
SUPPORTING INFORMATION TABLE S1 Complete overview of Dutch keywords used in the rule‐based model identification of ADRs, including ignored text strings
SUPPORTING INFORMATION TABLE S2 Complete list of abbreviations of drugs, drug groups and drug therapies
SUPPORTING INFORMATION TABLE S3 ADRs per MedDRA system organ class
SUPPORTING INFORMATION TABLE S4 Number of serious ADRs per system organ class and related medication
SUPPORTING INFORMATION TABLE S5 Adverse drug reactions found without a reference in the SmPC, sorted by Naranjo score
ACKNOWLEDGEMENTS
We would like to thank Wilma Compagner and Paul de Clerq for their contribution in programming and helping to implement the rule‐based model.
Wasylewicz A, van de Burgt B, Weterings A, et al. Identifying adverse drug reactions from free‐text electronic hospital health record notes. Br J Clin Pharmacol. 2022;88(3):1235-1245. doi: 10.1111/bcp.15068
DATA AVAILABILITY STATEMENT
Freely available after contact with first author.
REFERENCES
- 1. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta‐analysis of prospective studies. JAMA. 1998;279:1200‐1205. [DOI] [PubMed] [Google Scholar]
- 2. Lundkvist J, Jonsson B. Pharmacoeconomics of adverse drug reactions. Fundam Clin Pharmacol. 2004;18:275‐280. [DOI] [PubMed] [Google Scholar]
- 3. Miller RR. Hospital admissions due to adverse drug reactions. A report from the Boston Collaborative Drug Surveillance Program. Arch Intern Med. 1974;134:219‐223. [PubMed] [Google Scholar]
- 4. Bouvy JC, De Bruin ML, Koopmanschap MA. Epidemiology of adverse drug reactions in Europe: a review of recent observational studies. Drug Saf. 2015;38:437‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274:29‐34. [PubMed] [Google Scholar]
- 6. Kohn LT, Corrigan JM, Donaldson MS (Eds). To Err is Human: Building a Safer Health System. Washington (DC); 2000. [PubMed] [Google Scholar]
- 7. Leendertse AJ, Egberts AC, Stoker LJ, van den Bemt PM, Harm Study Group . Frequency of and risk factors for preventable medication‐related hospital admissions in the Netherlands. Arch Intern Med. 2008;168:1890‐1896. [DOI] [PubMed] [Google Scholar]
- 8. Seidl LG, Thornton GF, Cluff LE. Epidemiological studies of adverse drug reactions. Am J Public Health Nations Health. 1965;55:1170‐1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Juntti‐Patinen L, Neuvonen PJ. Drug‐related deaths in a university central hospital. Eur J Clin Pharmacol. 2002;58:479‐482. [DOI] [PubMed] [Google Scholar]
- 10. European Commission . Strengthening pharmacovigilance to reduce adverse effects. In, 2008. https://ec.europa.eu/commission/presscorner/detail/en/MEMO_08_782
- 11. Bates DW, Leape LL, Petrycki S. Incidence and preventability of adverse drug events in hospitalized adults. J Gen Intern Med. 1993;8:289‐294. [DOI] [PubMed] [Google Scholar]
- 12. Jha AK, Kuperman GJ, Teich JM, et al. Identifying adverse drug events: development of a computer‐based monitor and comparison with chart review and stimulated voluntary report. J am Med Inform Assoc. 1998;5:305‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Welk B, Richard L, Winick‐Ng J, Shariff SZ, Clemens KK. The burden of repeat prescribing medications after a related adverse drug event. Healthc Q. 2018;21:36‐39. [DOI] [PubMed] [Google Scholar]
- 14. Zhang M, Holman CD, Preen DB, Brameld K. Repeat adverse drug reactions causing hospitalization in older Australians: a population‐based longitudinal study 1980‐2003. Br J Clin Pharmacol. 2007;63:163‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van der Linden CM, Jansen PA, van Marum RJ, Grouls RJ, Korsten EH, Egberts AC. Recurrence of adverse drug reactions following inappropriate re‐prescription: better documentation, availability of information and monitoring are needed. Drug Saf. 2010;33:535‐538. [DOI] [PubMed] [Google Scholar]
- 16. Lesar TS, Briceland L, Stein DS. Factors related to errors in medication prescribing. JAMA. 1997;277:312‐317. [PubMed] [Google Scholar]
- 17. Parameswaran Nair N, Chalmers L, Bereznicki BJ, Curtain CM, Bereznicki LR. Repeat adverse drug reaction‐related hospital admissions in elderly Australians: A retrospective study at the Royal Hobart Hospital. Drugs Aging. 2017;34:777‐783. [DOI] [PubMed] [Google Scholar]
- 18. van der Linden CM, Jansen PA, van Geerenstein EV, et al. Reasons for discontinuation of medication during hospitalization and documentation thereof: a descriptive study of 400 geriatric and internal medicine patients. Arch Intern Med. 2010;170:1085‐1087. [DOI] [PubMed] [Google Scholar]
- 19. van der Linden CM, Jansen PA, Grouls RJ, et al. Systems that prevent unwanted represcription of drugs withdrawn because of adverse drug events: a systematic review. Ther Adv Drug Saf. 2013;4:73‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Geeven IPAC, Jessurun NT, Wasylewicz ATM, Drent M, Spuls PI, Hoentjen F, van Puijenbroek EP, Vonkeman HE, Grootens KP, van Doorn MBA, van den Bemt BJF, Bekkers CL. Barriers and facilitators for structurally registering adverse drug reactions in electronic health records: a qualitative study with healthcare professionals. PRISMA symposium Online, 2021. https://www.knmp.nl/downloads/overzicht-parallelsessies-30-april-2021.pdf/at_download/file [DOI] [PubMed]
- 21. Gandhi TK, Burstin HR, Cook EF, et al. Drug complications in outpatients. J Gen Intern Med. 2000;15:149‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van der Linden CM, Kerskes MC, Bijl AM, Maas HA, Egberts AC, Jansen PA. Represcription after adverse drug reaction in the elderly: a descriptive study. Arch Intern Med. 2006;166:1666‐1667. [DOI] [PubMed] [Google Scholar]
- 23. Honigman B, Lee J, Rothschild J, et al. Using computerized data to identify adverse drug events in outpatients. J am Med Inform Assoc. 2001;8:254‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iqbal E, Mallah R, Rhodes D, et al. ADEPt, a semantically‐enriched pipeline for extracting adverse drug events from free‐text electronic health records. PLoS One. 2017;12:e0187121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aramaki E, Miura Y, Tonoike M, et al. Extraction of adverse drug effects from clinical records. Stud Health Technol Inform. 2010;160:739‐743. [PubMed] [Google Scholar]
- 26. Hazlehurst B, Naleway A, Mullooly J. Detecting possible vaccine adverse events in clinical notes of the electronic medical record. Vaccine. 2009;27:2077‐2083. [DOI] [PubMed] [Google Scholar]
- 27. Epstein RH, St Jacques P, Stockin M, Rothman B, Ehrenfeld JM, Denny JC. Automated identification of drug and food allergies entered using non‐standard terminology. J Am Med Inform Assoc. 2013;20:962‐968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goss FR, Plasek JM, Lau JJ, Seger DL, Chang FY, Zhou L. An evaluation of a natural language processing tool for identifying and encoding allergy information in emergency department clinical notes. AMIA Annu Symp Proc. 2014;2014:580‐588. [PMC free article] [PubMed] [Google Scholar]
- 29. Chapman WW, Bridewell W, Hanbury P, Cooper GF, Buchanan BG. A simple algorithm for identifying negated findings and diseases in discharge summaries. J Biomed Inform. 2001;34:301‐310. [DOI] [PubMed] [Google Scholar]
- 30. Doan S, Bastarache L, Klimkowski S, Denny JC, Xu H. Integrating existing natural language processing tools for medication extraction from discharge summaries. J Am Med Inform Assoc. 2010;17:528‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gold S, Elhadad N, Zhu X, Cimino JJ, Hripcsak G. Extracting structured medication event information from discharge summaries. AMIA Annu Symp Proc. 2008;237‐241. [PMC free article] [PubMed] [Google Scholar]
- 32. Jagannathan V, Mullett CJ, Arbogast JG, et al. Assessment of commercial NLP engines for medication information extraction from dictated clinical notes. Int J Med Inform. 2009;78:284‐291. [DOI] [PubMed] [Google Scholar]
- 33. Hahn U, Romacker M, Schulz S. MEDSYNDIKATE – a natural language system for the extraction of medical information from findings reports. Int J Med Inform. 2002;67:63‐74. [DOI] [PubMed] [Google Scholar]
- 34. Fiszman M, Chapman WW, Aronsky D, Evans RS, Haug PJ. Automatic detection of acute bacterial pneumonia from chest X‐ray reports. J c Med Inform Assoc. 2000;7:593‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Geniaux H, Assaf D, Miremont‐Salame G, et al. Performance of the standardised MedDRA(R) queries for case retrieval in the French spontaneous reporting database. Drug Saf. 2014;37:537‐542. [DOI] [PubMed] [Google Scholar]
- 36. Combi C, Zorzi M, Pozzani G, Moretti U, Arzenton E. From narrative descriptions to MedDRA: automagically encoding adverse drug reactions. J Biomed Inform. 2018;84:184‐199. [DOI] [PubMed] [Google Scholar]
- 37. WHO Meeting on International Drug Monitoring: the Role of National Centres . International Drug Monitoring – The Role of the National Centres. World Health Organization Technical Report Series No. 498. Geneve: World Health Organization; 1972. [PubMed] [Google Scholar]
- 38. Baldo BA, Pham NH. Adverse Reactions to Drugs and Drug Allergy: Scope of This Book. In: Drug Allergy: Clinical Aspects, Diagnosis, Mechanisms, Structure‐Activity Relationships. New York, NY: Springer New York; 2013:1‐13. [Google Scholar]
- 39. Royal Dutch Association for the Advancement of Pharmacy (KNMP) G‐standard Dutch National Drug Database. In, 2018.
- 40. EMA Important Medical Events list. MedDRA version 23.1. In: EMA/484057/2020 corr1, 2020.
- 41. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239‐245. [DOI] [PubMed] [Google Scholar]
- 42. van der Linden CM, Jansen PA, van Marum RJ, Grouls RJ, Egberts TC, Korsten EH. An electronic system to document reasons for medication discontinuation and to flag unwanted represcriptions in geriatric patients. Drugs Aging. 2012;29:957‐962. [DOI] [PubMed] [Google Scholar]
- 43. Agbabiaka TB, Savovic J, Ernst E. Methods for causality assessment of adverse drug reactions: a systematic review. Drug Saf. 2008;31:21‐37. [DOI] [PubMed] [Google Scholar]
- 44. Hazell L, Shakir SA. Under‐reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29:385‐396. [DOI] [PubMed] [Google Scholar]
- 45. Potlog Shchory M, Goldstein LH, Arcavi L, Shihmanter R, Berkovitch M, Levy A. Increasing adverse drug reaction reporting‐How can we do better? PLoS One. 2020;15:e0235591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tillman EM, Suppes SL, Feldman K, Goldman JL. Enhancing pediatric adverse drug reaction documentation in the electronic medical record. J Clin Pharmacol. 2021;61:181‐186. [DOI] [PubMed] [Google Scholar]
- 47. Ribeiro‐Vaz I, Silva AM, Costa Santos C, Cruz‐Correia R. How to promote adverse drug reaction reports using information systems – a systematic review and meta‐analysis. BMC Med Inform Decis Mak. 2016;16(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Weingart SN, Carbo A, Tess A, et al. Using a patient internet portal to prevent adverse drug events: a randomized, controlled trial. J Patient Saf. 2013;9:169‐175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION TABLE S1 Complete overview of Dutch keywords used in the rule‐based model identification of ADRs, including ignored text strings
SUPPORTING INFORMATION TABLE S2 Complete list of abbreviations of drugs, drug groups and drug therapies
SUPPORTING INFORMATION TABLE S3 ADRs per MedDRA system organ class
SUPPORTING INFORMATION TABLE S4 Number of serious ADRs per system organ class and related medication
SUPPORTING INFORMATION TABLE S5 Adverse drug reactions found without a reference in the SmPC, sorted by Naranjo score
Data Availability Statement
Freely available after contact with first author.


