Summary
Background
The rising incidence of early onset colorectal cancer (EOCRC) might reflect a novel tumour entity.
Aims
To evaluate clinicopathological characteristics of sporadic EOCRC (in patients < 50 years old) and investigate changes over time
Methods
All patients with sporadic EOCRC between 1989 and 2016 were included and divided by age: 20‐29 years (group I), 30‐39 years (group II) and 40‐49 years (group III).
Results
We included 6400 patients. The presence of signet‐ring cells and more poorly differentiated tumours were more common in the younger age groups: 5.4% and 3.7% for signet‐ring cells in group I and II vs 1.4% in group III (P < 0.01), and 28.5% and 20.3% for poorly differentiated in group I and II vs 16.6% in group III, (P < 0.01 group I; P = 0.07 group II). Positive lymph nodes were more frequently observed in the younger age groups: 16.2% in group I vs 9.3% in group II (P = 0.01) and 7.9% (P < 0.01) in group III. Over time, a greater proportion of CRCs were diagnosed in women in group I (34.5% < 2004 vs 54.9%>2005, P = 0.09), and a higher percentage of rectal cancer was found in age group III (34.3% < 2004 vs 40.7% > 2005, P < 0.01). Mean overall survival was 6.3 years and improved over time.
Conclusions
EOCRC is not only characterised by age of onset but also by the more frequent presence of signet‐ring cells, more poorly differentiated tumours, and higher risk of lymph node metastases. In the most recent years, a higher proportion of rectal cancer was found from the age of 30 years, and a higher proportion of CRCs were diagnosed in females below the age of 30 years.
Clinicopathological characteristics of early onset colorectal cancer.

1. INTRODUCTION
Colorectal cancer (CRC) incidence and mortality are decreasing in adults older than 50 years due to screening and improvements in CRC treatment in both the US and Europe. 1 , 2 Conversely, CRC incidence in young adults, early‐onset CRC (EOCRC), is rising in several parts of the world. 2 , 3 It is known that individuals with Lynch syndrome (LS) or familial adenomatous polyposis (FAP) are more likely to develop CRC at a relatively young age. However, this group accounts for only 2%‐3% of all CRC cases. 4 Most of EOCRCs are sporadic cases. The underlying factors contributing to the increasing incidence of sporadic CRC in young adults are still incompletely understood but seem to include obesity, lack of physical activity, alcohol intake and cigarette smoking. 5 , 6 , 7 Also, several drugs have been reported to be associated with CRC risk. The use of oral antibiotics is associated with an increased CRC risk, while the use of statin and aspirin might decrease this risk. 8 , 9 , 10 Association studies on sporadic EOCRC show that male gender, being black or Asian, having inflammatory bowel disease (IBD) or a family history of CRC might be associated with an increased EOCRC risk. 11 To fully elucidate causes and mechanisms of EOCRC, it is important to have more insight into both patient and tumour characteristics of these CRCs. Data on location, histology, and tumour stages of sporadic EOCRC compared to late‐onset CRC are scarce and conflicting. Some studies indicate a higher prevalence of right‐sided CRC in EOCRC while other studies showed a higher prevalence of a more distal location. 12 , 13 Signet‐ring cells were described to be more prominent in EOCRC, while conflicting studies were published on KRAS, NRAS and BRAF mutations among EOCRC patients. 14 , 15 These conflicting data might be a result of differences between and within EOCRC cohorts. For example, the very young patients (below the age of 30 years) might have a different type of CRC than the slightly older EOCRC patients (30‐50 years of age). The latter might resemble more the sporadic CRC in adults above the age of 50 years of age. Furthermore, it is questioned whether the rising incidence of sporadic EOCRC might reflect the rise of a novel tumour entity. Therefore, the aim of this study was to assess the clinicopathological characteristics of sporadic EOCRCs within different age categories (20‐29 years vs 30‐39 years vs 40‐49 years) and investigate changes over time.
2. METHODS
2.1. Study population
All CRC patients below the age of 50 years were identified from the Netherlands Cancer Registry (NKR) and the Dutch national pathology registry PALGA, the nationwide network and registry of histo‐ and cytopathology in the Netherlands between 1989 and 2016 with follow‐up of each case until 31 January 2018. EOCRCs were defined as sporadic cancers of the colon or rectum in individuals under the age of 50 years that were tested for LS and showed an MSS phenotype. Patients were divided into three age groups: group I (20‐29 years); group II (30‐39 years) and group III (40‐49 years). All patients with an adenocarcinoma located in the colon and/or rectum were included. Excluded from this study were patients with LS tumours, neuroendocrine tumours, neuroendocrine carcinomas and squamous cell carcinomas.
The study was conducted in accordance with the Declaration of Helsinki Principles and approved by the ethical committee of the Erasmus University Medical Center, Rotterdam (MEC‐2020‐0048).
2.2. Data source
Data on age‐related histopathological features were retrieved from the NKR and the Dutch national pathology registry PALGA. 16 , 17 NKR complies clinical data of all newly diagnosed patients with cancer in the Netherlands since 1989. The PALGA database covers all pathology laboratories in the Netherlands. Summaries of all histopathology and cytopathology reports are generated automatically at the laboratories and transferred to the central databank of PALGA.
2.3. Data collection
Tumours on which molecular analyses were performed and were negative for a hereditary disorder, were defined as sporadic CRC. Clinical characteristics included gender, age at diagnosis, tumour location and tumour stage. Tumour location was grouped by primary site, where cecum to sigmoid (ICD‐O‐3 codes C180, C182‐C187 and C199) was defined as colon and rectum (C209) was defined separately. Pathological characteristics included histopathology, degree of differentiation, presence of (lymph node) metastasis, lymphatic invasion and angioinvasion. For N stage the UICC 7th edition was used. 18 Lymph node metastasis were categorised in two groups: patients with no or <7 lymph nodes (≤N2a) or patients with >7 lymph nodes (N2b).
TNM stage was based on histopathologic examination (pTNM). In case pTNM stage was not available, TNM stage before treatment (cTNM) was used. Data on the presence of lymphatic invasion and angioinvasion was only available for the years 2015 and 2016. Furthermore, the prevalence of the following genes was examined: BRAF, NRAS and KRAS. Overall survival (OS) was defined as the time between the date of diagnosis to the date of death from any cause or the end of follow‐up.
2.4. Statistical analyses
The proportions between age categories were compared using chi‐squared or Fishers exact tests when appropriate. Group‐wise comparisons were performed when the overall P‐value of a group was P < 0.10.
To elucidate the clinical and histopathological characteristics of patients with sporadic EOCRC over time, the study period was divided into two time periods (period 1: 1989‐2004 and period 2: 2005‐2018) comparing the first 15 years of data to the second 15 years. Differences between the time periods were compared using the chi‐squared test.
Kaplan‐Meier curves and log‐rank tests were used to evaluate differences in survival. A two‐sided P‐value of less than 0.05 was considered statistically significant. Data analyses were performed using spss version 25.
3. RESULTS
3.1. Baseline characteristics
In total, 15 925 CRC patients under the age of 50 years were identified between 1989 and 2016 (52% male, mean age 43 years, SD 5.8) (Figure 1). No molecular diagnostics were performed on 7.905 (49.6%) patients. Differences in characteristics between patients with and without molecular diagnostics are depicted in Table S1. Patients tested for MSI were slightly older 43.5 years vs 42.7 years (P < 0.01), were more often females 49.5% vs 46.5% (P < 0.01), had more often more than seven positive lymph nodes (8.1% vs 5.9%, P < 0.01) and had a well‐differentiated tumour (80.1% vs 78.1%, P < 0.01).
FIGURE 1.
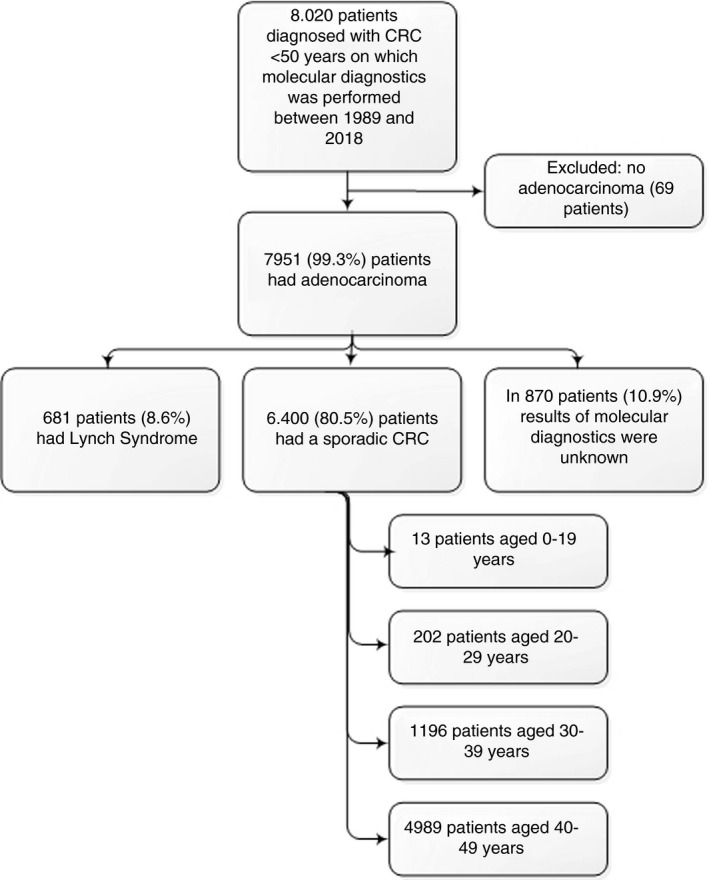
Flowchart
Of the other 8020 patients, 69 patients were excluded because the tumour was not an adenocarcinoma.
Of the remaining 7951 patients with an adenocarcinoma and MSI tested, 6400 (80.5%) was a sporadic EOCRC, 681 patients (8.6%) were diagnosed with LS, and of 870 patients (10.9%) the result of molecular diagnostics was unknown.
3.1.1. Sporadic EOCRC
When focusing on the 6400 sporadic EOCRC patients, 49.2% was male with a mean age of 43 years (SD 5.6). In total, 202 (3%) patients were diagnosed at the age of 20‐29 years old (group I); 1196 (19%) patients at the age of 30‐39 years old (group II) and 4.989 (78%) patients at the age of 40‐49 years old (group III). Due to the low number of patients in age group 0‐19 years of age (n = 13 [0.2%]), clinicopathological features were described and not included in the comparison analyses.
3.2. Clinical and pathological characteristics of patients with sporadic EOCRC
3.2.1. Characteristics per age group
In the youngest sporadic EOCRC age group (0‐19 years) patients had a mean age of 16 years (SD 2·2), 61.5% was female, and in 38.5% the tumour was located in the rectum. CRC was poorly differentiated in 46.2% and in 38.5% signet‐ring cell carcinoma was present.
Between age groups I, II and III no difference in gender (P = 0.43) and location (P = 0.10) was observed (Table 1). More often positive lymph nodes were diagnosed in group I, 16.2% vs 9.3% in group II (P = 0.01) and 7.9% (P < 0.01) in group III. Also, in group I more poorly differentiated tumours 28.5% were found, followed by 20.3% in group II and 16.6% in group III (P < 0.01). Both in groups I and II more signet‐ring cell carcinomas 5.4% and 3.7% vs 1.4% in group III (P < 0.01) were present (Figure 2). The only differences between age groups and TNM stage, were more prevalent TNM stage I tumours in age group III compared to age group II (13.0% vs 11.1%, P = 0.04) and more frequently diagnosed TNM stage III tumours in age group II compared to age group III (9.9% vs 6.8%, P < 0.01). No differences in the number of metastases were observed between the age groups. Also, no difference in the number of mucinous carcinoma and presence of angioinvasion was observed. Lymphatic invasion was more commonly found in groups I and II compared to group III, 33.3% and 28.0% vs 20.3% (P = 0.09) respectively. No difference was observed in the number of KRAS, NRAS and BRAF mutations.
TABLE 1.
Clinical and pathological features of sporadic EOCRC divided into three age groups
| Characteristic of EOCRC patients |
Group I 20‐29 years |
Group II 30‐39 years |
Group III 40‐49 years |
P‐value | Group I vs group II | Group I vs group III | Group II vs group III |
|---|---|---|---|---|---|---|---|
| Total number | 202 | 1196 | 4989 | ||||
| Gender | |||||||
| Male | 103 (51.0) | 569 (47.6) | 2470 (49.5) | 0.43 | |||
| Female | 99 (49.0) | 627 (52.4) | 2519 (50.5) | ||||
| Location | |||||||
| Colon | 133 (68.6) | 714 (61.0) | 2977 (61.0) | 0.10 | . | . | . |
| Rectum | 61 (31.4) | 456 (39.0) | 1905 (39.0) | ||||
| Mucinous histology | |||||||
| Absent | 188 (93.1) | 1126 (94.1) | 4741 (95.0) | 0.25 | |||
| Present | 14 (6.9) | 70 (5.9) | 248 (5.0) | ||||
| Signet‐ring cell histology | |||||||
| Absent | 191 (94.6) | 1152 (96.3) | 4919 (98.6) | <0.01 b | 0.23 | <0.01 | <0.01 |
| Present | 11 (5.4) | 44 (3.7) | 70 (1.4) | ||||
| Differentiation grade | |||||||
| Well/moderate | 108 (71.5) | 721 (79.7) | 3206(83.4) | <0.01 b | 0.02 | <0.01 | <0.01 |
| Poor | 43 (28.5) | 184 (20.3) | 636 (16.6) | ||||
| TNM stage | |||||||
| I | 30 (14.9) | 133 (11.1) | 668 (13.0) | 0.08 b | 0.13 | 0.55 | 0.04 |
| II | 12 (5.9) | 71 (5.9) | 238 (4.8) | 0.21 | |||
| III | 13 (6.4) | 118 (9.9) | 340 (6.8) | <0.01 b | 0.12 | 0.83 | <0.01 |
| IV | 26 (12.9) | 174(14.5) | 633 (12.7) | 0.23 | |||
| Number of metastasis | |||||||
| 0 | 146 (72.3) | 886 (74.1) | 3795 (76.1) | 0.19 | |||
| 1 | 35 (17.3) | 204 (17.1) | 745 (14.9) | 0.14 | |||
| 2 | 11 (5.4) | 71 (5.9) | 306 (6.1) | 0.90 | |||
| 3 | 9 (4.5) | 31 (2.6) | 130 (2.6) | 0.27 | |||
| Number of positive lymph nodes | |||||||
| <7 positive lymph nodes | 129 (83.8) | 816 (90.7) | 3599 (92.1) | <0.01 b | 0.01 | <0.01 | 0.16 |
| >7 positive lymph nodes | 25 (16.2) | 84 (9.3) | 307 (7.9) | ||||
| Lymphatic invasion a | |||||||
| No | 16 (66.7) | 67 (72.0) | 468 (79.7) | 0.09 b | 0.61 | 0.12 | 0.09 |
| Yes | 8 (33.3) | 26 (28.0) | 119 (20.3) | ||||
| Angioinvasion a | |||||||
| No | 14 (66.7) | 41 (69.5) | 331 (74.4) | 0.56 | |||
| Yes | 7 (33.3) | 18 (30.5) | 114 (25.6) | ||||
| KRAS mutation | |||||||
| Absent | 14 (58.3) | 72(63.2) | 261 (55.9) | 0.37 | |||
| Present | 10 (41.7) | 42 (36.8) | 206 (44.1) | ||||
| NRAS mutation | |||||||
| Absent | 13 (92.9) | 64(98.5) | 244 (94.6) | 0.38 | |||
| Present | 1(7.1) | 1 (1.5) | 14 (5.4) | ||||
| BRAF mutation | |||||||
| Absent | 18 (100) | 73 (93.6) | 299 (91.8) | 0.42 | |||
| Present | 0 (0) | 5 (6.4) | 26 (8.0) | ||||
Data of lymphatic invasion and angioinvasion was only available for years 2015 and 2016.
A two‐sided P‐value of less than 0.05 was considered statistically significant.
FIGURE 2.
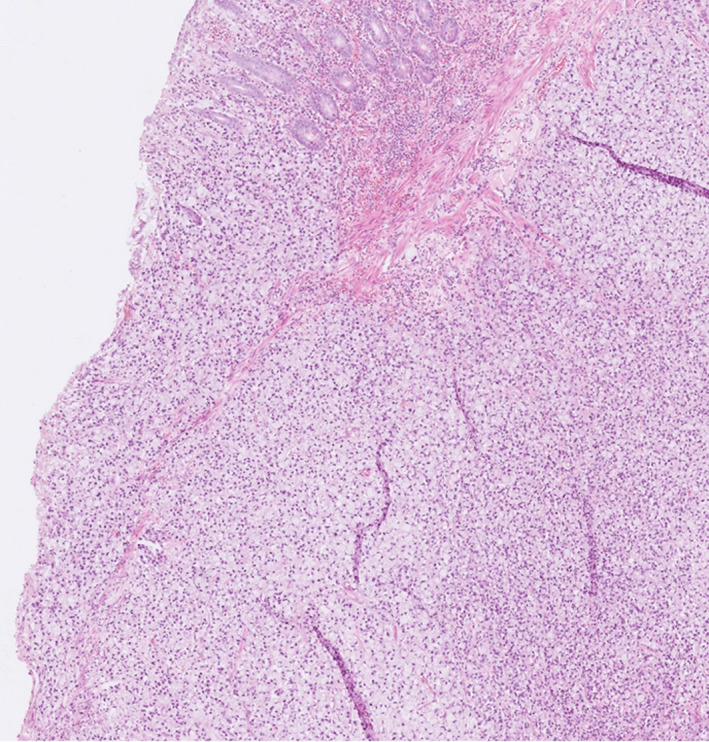
Microscopic image of a signet‐ring cell carcinoma in the colon
3.2.2. EOCRC characteristics over time
In age group I, 34.5% of the cancers were diagnosed in women in time period 1989‐2004 compared to 54.9% in time period 2005‐2018 (P = 0.01) (Figure 3 and Table S2). In age groups II and III no differences in gender were observed over time. For tumour location age group I showed the highest percent of cancers located in the colon in both men and women, and this did not change over time. In age group II the percent of rectal cancer was 33.8% in time period 1989‐2004 and 41.6% in period 2005‐2018 (P = 0.01) and in age group III the percent of rectal cancer was 34.3% in period 1989‐2004 and 40.7% in period 2005‐2018 (P < 0.01). The percent of poorly differentiated CRCs remained stable in age group I. In age groups II and III a decline over time was observed, 25.1% of the patients were diagnosed with a poorly differentiated CRC in age group II between 1989 and 2004 and declined to 17.4% between 2005 and 2018 (P = 0.05) and in age group III 20.3% had a poorly differentiated CRC between 1989 and 2004 and declined to 15.0% between 2005 and 2018 (P < 0.01). A higher proportion of patients had lymph nodes metastases after 2005 in all three age groups.
FIGURE 3.
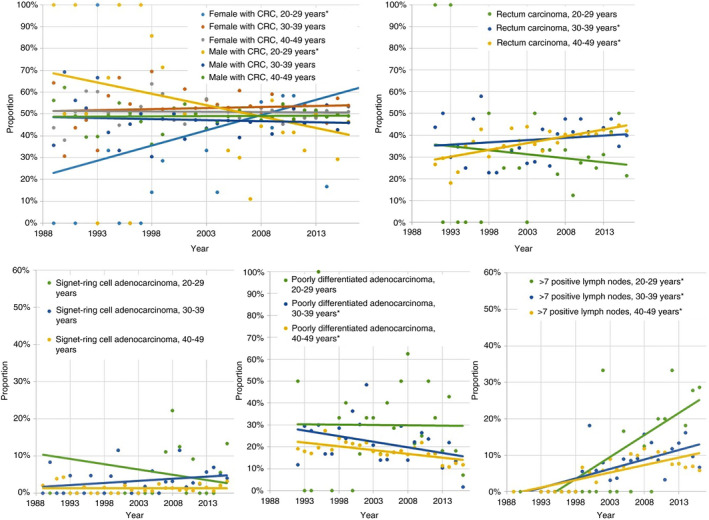
Proportion of female and male patients with colorectal cancer (CRC), rectum carcinomas, signet‐ring cell adenocarcinomas, poorly differentiated CRC and CRC with more than 7 positive lymph nodes over time divided into three age groups. *Significant difference
3.2.3. Overall survival outcome
Mean OS time was 6.3 years (SD 6.2). Overall 5‐year disease‐free survival rates were 60.9% in group I, 62.7% in group II, and 64.2% in group III. OS did not significantly differ between the three groups (P = 0.72) (Figure 4).
FIGURE 4.
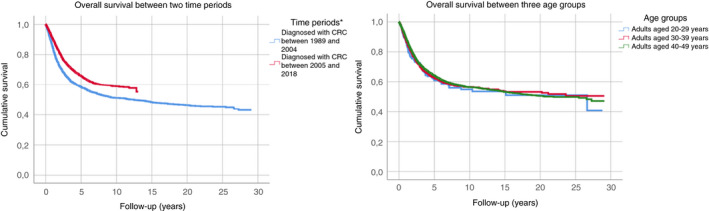
Overall disease‐free survival analyses in sporadic early‐onset colorectal cancer (EOCRC) patients per time period (1989‐2004 vs 2005‐2018) and per age group. *Significant difference
A better survival rate was found for patients diagnosed with CRC between 2005 and 2018, with an overall 5‐year disease‐free survival rate of 65.8% vs 58.4% for patients diagnosed between 1989 and 2004 (P < 0.01; Figure 4).
4. DISCUSSION
This study presents a nationwide analysis of clinical and histopathological characteristics of CRC in patients <50 years of age over the past 30 years. Poorly differentiated tumours, presence of signet‐ring cells, and higher number of lymph node metastasis were significantly more prevalent in 20‐39 years old compared to the 40‐49 years old. Over time, a higher proportion of EOCRCs were diagnosed in women below the age of 30 years, while a higher proportion of tumours were located in the rectum in the older group, 30‐49 years old. OS was 6.3 years and improved over time.
This is the first study to assess clinicopathological features between different age groups of true sporadic EOCRC patients, without obscuration of patients with LS‐CRC. Identification of EOCRC remains a major challenge and is expected to become more prevalent in the upcoming years. Insights about EOCRC both from a patient and tumour perspective may help to better recognise EORCC patients.
The results from our study confirm the observations of two other studies from the US. In one study 55 EOCRC patients below the age of 40 years were compared to sporadic CRC patients older than 40 years of age. 15 In the other US study, more than 36 000 patients were included. 19 Both studies showed a higher prevalence of signet‐ring cell carcinomas and a higher proportion of tumours located in the left side of the colon or in the rectum in the youngest age group. 15 , 19 In addition, we found that sporadic EOCRC patients <40 years of age had more often lymph nodes metastases. Another study using the SEER 9 Registries concluded that EOCRC were more often found at an advanced stage and were more often mucinous carcinomas. 20 However, in this study they were unable to exclude LS patients which may have biased the results.
A consistent finding is that the incidence of rectal cancer in EOCRC patients increased over time. In a previous study, it was shown that the incidence of rectal cancer in patients <40 years of age over two time periods (1992‐1996 and 2010‐2014) increased from 2.7 per 100 000 to 4.4 per 100 000 patients. 21 The incidence rates, however, of carcinoid carcinomas located in the rectum increased more steeply than adenocarcinomas. This may partly explain the rapid rise of rectal carcinomas, especially for those studies that did not assess cancers by histological subtypes. 22
We found that a higher proportion of CRCs were diagnosed in women aged 20‐29 years old in more recent years. A true increase in incidence could however not be calculated because of the missing population numbers of women per time period. It is known that men are at greater risk for late‐onset CRC, but recent studies revealed that men also have a higher risk for EOCRC. 10 , 23 These studies however did not stratify by age or ethnicity. An American study for example found that rural Non‐Hispanic black women had the highest incidence rate ratios, which was primarily driven by colon cancers. 24 Differences may possibly explained by differences in genetic make‐up and life style factors, such as obesity and red meat consumption, but does not fully explain the gender difference in EOCRC. 25 More research is required, stratifying groups by age, ethnicity and tumour site (colon vs rectal cancer) to elucidate explanations that may better clarify gender differences in EOCRC. Furthermore, a remarkable finding was the decline of poorly differentiated EOCRC over time, while more positive lymph nodes were found over time. The latter could be explained by the fact that the evaluation of lymph nodes became a quality measure for colon cancer care, since the number of lymph nodes examined is positively associated with the survival of patients. 26 Another explanation for the higher proportion of patients with positive lymph nodes could be the improved techniques to harvest lymph nodes, such as fat clearance. 27
Our study included data on KRAS, NRAS and BRAF genes. KRAS is a common gene in CRC patients and has the ability to promote tumour proliferation and suppress differentiation. As biomarker, KRAS predicts response to anti‐EGFR therapies. 28 , 29 NRAS is less prevalent in CRC patients and are able to suppress apoptosis. 28 BRAF genes are found in 7% of the tumours and is considered as a driver in the serrated pathway. 30 Previous literature showed conflicting results regarding the prevalence of KRAS, NRAS and BRAF genes in EOCRC patients. A review from Italy included 46 articles, of which ten studies reported on prevalence of KRAS genes in EOCRC. 14 Seven studies reported a lower prevalence of KRAS genes in EOCRC compared to older CRC patients, two studies showed a similar prevalence and one study had a higher prevalence. The prevalence of BRAF genes was reported to be similar among EOCRC compared to older patients. 14 NRAS mutation prevalence in EOCRC patients was only reported in one study with a small patient population, they reported three NRAS mutations in 69 patients. 31 Our results showed no difference in KRAS, NRAS and BRAF genes between the different EOCRC age groups.
There is controversy around the prognosis of patients with sporadic EOCRC, varying from worse to better outcome compared to late‐onset CRC patients. 20 , 32 , 33 , 34 , 35 The latter might be explained by the mixture with LS‐CRC patients in these studies. Although OS increased over time, our study observed no difference in OS between the age groups in EOCRC. The increased OS over time may be explained by improved diagnostic modalities and treatment options. 36 But also more early diagnosis of CRC in time may have contributed to the increased survival. Unfortunately, we were not able to analyse the CRC specific mortality due to the retrospective design of this study.
One could theorise that the low survival rate of EOCRC patients is the result of a patient‐ or doctor delay in diagnosing CRC, whereas for patients known with a hereditary disease awareness of CRC occurrence exists. Young patients seek medical attention at a later stage because they neglect their symptoms or delay seeking medical attention. Doctors may attribute the alarm symptoms of young patients with CRC to benign causes without further examination. However, some characteristics of sporadic EOCRC could not be subjected to patient or doctor delay, like gender, location of the tumour and type of histology. Therefore, it is reasonable that differences in tumour features suggestive of differences in tumourigenesis may play a role in clinical outcome. The question what is causing the histopathological changes is still unanswered.
Previous studies on EOCRC have pooled the data of all CRC patients under the age of 40 or 50 years. 37 , 38 This study provides a more in‐depth clinical and histopathological characterisation of young adults with sporadic CRC aged 20‐29 years, 30‐39 years and 40‐49 years. We found that poor prognosis features of EOCRC were more prevalent in 20‐ to 29‐year‐old adults, followed by 30‐ to 39‐year‐old and less prevalent in 40‐ to 49‐year‐old adults. This makes a period effect resulting from external factors that equally affect all age groups at a particular time period less likely. In literature, it is hypothesised that the increased trend of EOCRC follows the pattern of a cohort effect where the youngest generation is more susceptible for the development of a different, more aggressive type of CRC. While CRC detected in adults aged 40‐49 years are more comparable to the CRC found in the general population with comparable clinical and pathological features. The cause of the cohort effect is still unknown. Possible risk factors may be the increasing prevalence of obese individuals in the last decades or alterations in gut microbiota due to a more frequent use of antibiotics. 39 But also germline variants of multiple genes could be associated with increased EOCRC risk. One study revealed that EOCRC patients have unique molecular features, with less BRAF V600 mutations compared to patients with late‐onset CRC, and the presence of more subtypes of CMS1 and CMS2. 19 Another study showed a high prevalence (16%) of germline mutations in patients with EOCRC. 40 Both studies however included LS patients. A recent published study showed that EOCRC exhibits a different genetic risk compared to late‐onset CRC due to low‐penetrance common genetic polymorphisms, with a stronger association in patients without a CRC family history. 41 Though genetic factors probably play a role in the increased risk of EOCRC, most likely multiple (risk) factors are involved.
Strength of this study was the large nationwide database covering all patients diagnosed with CRC below the age of 50 years over the past 30 years in the Netherlands on which molecular analyses were performed. This study also has several limitations. First, the retrospective design of the study. This could have led to information and selection bias or misclassification of data. To ensure that LS patients were not included, we excluded all patients in who no molecular diagnostics was performed. Comparing the MSI tested group with the non‐tested group, significantly more women were molecularly tested for LS. This may have been caused by the fact that women had more often features of LS. Although we identified significant differences between the tested and non‐tested group, the clinical relevance of this selection bias is less clear than including all patients, including unidentified LS patients. Ideally, one would like to follow a cohort of young adults over a long period of time. Although prospective studies should be initiated, it takes time before conclusions can be drawn and recommendations are given. With the increase in EOCRC incidence in different parts of the world, it is important to gather information at this moment in order to understand this trend and attempt to reverse it. This large retrospective study will help to contribute to the understanding of EOCRC. Second, because of the retrospective design of this study, we had no access to data regarding risk factors (e.g. smoking status, obesity, use of antibiotics). Also, no information was available regarding family history and ethnicity. Third, no linear analyses overtime were possible due to the small sample size in the youngest age groups.
To conclude, this study revealed clinicopathological differences within the groups defined as EOCRC in the last 30 years. The proportion of rectal cancer increased from the age of 30 years in more recent years, while in patients below the age of 30 years a higher proportion of CRC was found in females and characterised by a more frequent presence of signet‐ring cells and poor histological features. Clinicians should be aware of these differences in clinicopathological characteristics to optimise (early) detection and eventually targeted CRC treatment.
AUTHORSHIP
Guarantor of the article: M.C.W. Spaander.
Author contributions : FV: study concept and design, acquisition of data, analysis and interpretation of data, statistical analysis, drafting of the manuscript, critical revision of the manuscript; SN: analysis and interpretation of the data, drafting of the manuscript, critical revision of the manuscript; IN: critical revision of the manuscript; EK: critical revision of the manuscript, study supervision; MS: study concept and design, critical revision of the manuscript, study supervision. All authors approved the final version of the manuscript.
Supporting information
Tables S1‐S2
ACKNOWLEDGEMENTS
The authors thank the registration team of the Netherlands Comprehensive Cancer Organization (IKNL) for the collection of data for the Netherlands Cancer Registry as well as IKNL staff for scientific advice. We also thank the Dutch pathology registry (PALGA) for the collection of data and their scientific advice.
Declaration of personal interests: None.
Declaration of funding interests: None.
Vuik FER, Nieuwenburg SAV, Nagtegaal ID, Kuipers EJ, Spaander MCW. Clinicopathological characteristics of early‐onset colorectal cancer. Aliment Pharmacol Ther. 2021;54:1463–1471. doi: 10.1111/apt.16638
The Handling Editor for this article was Dr Colin Howden, and it was accepted for publication after full peer‐review.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Vuik FE, Nieuwenburg SA, Bardou M, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut. 2019;68:1820–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States. J Natl Cancer Inst. 2017;109:1974–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aaltonen LA, Salovaara R, Kristo P, et al. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med. 1998;338:1481–1487. [DOI] [PubMed] [Google Scholar]
- 5. Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta‐analysis. JAMA. 2008;300:2765–2778. [DOI] [PubMed] [Google Scholar]
- 6. Wei EK, Giovannucci E, Wu K, et al. Comparison of risk factors for colon and rectal cancer. Int J Cancer. 2004;108:433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta‐analysis of prospective studies. Am J Clin Nutr. 2007;86:556–565. [DOI] [PubMed] [Google Scholar]
- 8. Zhang J, Haines C, Watson AJM, et al. Oral antibiotic use and risk of colorectal cancer in the United Kingdom, 1989–2012: a matched case‐control study. Gut. 2019;68:1971–1978. [DOI] [PubMed] [Google Scholar]
- 9. Cheung KS, Chen L, Chan EW, Seto WK, Wong ICK, Leung WK. Statins reduce the progression of non‐advanced adenomas to colorectal cancer: a postcolonoscopy study in 187 897 patients. Gut. 2019;68:1979–1985. [DOI] [PubMed] [Google Scholar]
- 10. Low EE, Demb J, Liu L, et al. Risk factors for early‐onset colorectal cancer. Gastroenterology. 2020;159:492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gausman V, Dornblaser D, Anand S, et al. Risk factors associated with early‐onset colorectal cancer. Clin Gastroenterol Hepatol. 2019;S1542–3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Silla IO, Rueda D, Rodriguez Y, Garcia JL, de la Cruz VF, Perea J. Early‐onset colorectal cancer: a separate subset of colorectal cancer. World J Gastroenterol. 2014;20:17288–17296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Savas N, Dagli U, Akbulut S, Yuksel O, Sahin B. Colorectal cancer localization in young patients: should we expand the screening program? Dig Dis Sci. 2007;52:798–802. [DOI] [PubMed] [Google Scholar]
- 14. Mauri G, Sartore‐Bianchi A, Russo AG, Marsoni S, Bardelli A, Siena S. Early‐onset colorectal cancer in young individuals. Mol Oncol. 2019;13:109–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chang DT, Pai RK, Rybicki LA, et al. Clinicopathologic and molecular features of sporadic early‐onset colorectal adenocarcinoma: an adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod Pathol. 2012;25:1128–1139. [DOI] [PubMed] [Google Scholar]
- 16. Casparie M, Tiebosch AT, Burger G, et al. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. 2007;29:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sanden GD, Coebergh J‐W, Schouten LJ, Visser O, Leeuwen FEvan. Cancer incidence in the Netherlands in 1989 and 1990: first results of the nationwide Netherlands cancer registry. Eur J Cancer. 1995;31:1822–1829. [DOI] [PubMed] [Google Scholar]
- 18. Obrocea FL, Sajin M, Marinescu EC, Stoica D. Colorectal cancer and the 7th revision of the TNM staging system: review of changes and suggestions for uniform pathologic reporting. Rom J Morphol Embryol. 2011;52:537–544. [PubMed] [Google Scholar]
- 19. Willauer AN, Liu Y, Pereira AAL, et al. Clinical and molecular characterization of early‐onset colorectal cancer. Cancer. 2019;125:2002–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang R, Wang MJ, Ping J. Clinicopathological features and survival outcomes of colorectal cancer in young versus elderly: a population‐based cohort study of SEER 9 registries data (1988–2011). Medicine (Baltimore). 2015;94:e1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murphy CC, Wallace K, Sandler RS, Baron JA. Racial disparities in incidence of young‐onset colorectal cancer and patient survival. Gastroenterology. 2019;156:958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Montminy EM, Zhou M, Maniscalco L, et al. Contributions of adenocarcinoma and carcinoid tumors to early‐onset colorectal cancer incidence rates in the United States. Ann Intern Med. 2021;174:157–166. [DOI] [PubMed] [Google Scholar]
- 23. Murphy G, Devesa SS, Cross AJ, Inskip PD, McGlynn KA, Cook MB. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer. 2011;128:1668–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zahnd WE, Gomez SL, Steck SE, et al. Rural‐urban and racial/ethnic trends and disparities in early‐onset and average‐onset colorectal cancer. Cancer. 2021;127:239–248. [DOI] [PubMed] [Google Scholar]
- 25. Kim S‐E, Paik HY, Yoon H, Lee JE, Kim N, Sung M‐K. Sex‐ and gender‐specific disparities in colorectal cancer risk. World J Gastroenterol. 2015;21:5167–5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chang GJ, Rodriguez‐Bigas MA, Skibber JM, Moyer VA. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. 2007;99:433–441. [DOI] [PubMed] [Google Scholar]
- 27. Cianchi F, Palomba A, Boddi V, et al. Lymph node recovery from colorectal tumor specimens: recommendation for a minimum number of lymph nodes to be examined. World J Surg. 2002;26:384–389. [DOI] [PubMed] [Google Scholar]
- 28. Haigis KM, Kendall KR, Wang Y, et al. Differential effects of oncogenic K‐Ras and N‐Ras on proliferation, differentiation and tumor progression in the colon. Nat Genet. 2008;40:600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Watson R, Liu TC, Ruzinova MB. High frequency of KRAS mutation in early onset colorectal adenocarcinoma: implications for pathogenesis. Hum Pathol. 2016;56:163–170. [DOI] [PubMed] [Google Scholar]
- 30. Sanz‐Garcia E, Argiles G, Elez E, Tabernero J. BRAF mutant colorectal cancer: prognosis, treatment, and new perspectives. Ann Oncol. 2017;28:2648–2657. [DOI] [PubMed] [Google Scholar]
- 31. Perea J, Arriba M, Rodríguez Y, et al. Frequency and impact of KRAS mutation in early onset colorectal cancer. Hum Pathol. 2017;61:221–222. [DOI] [PubMed] [Google Scholar]
- 32. Kaplan M, Ozaydin S, Yerlikaya H, et al. Clinicopathologic and prognostic differences between three different age groups (child/adolescent, young adults, and adults) of colorectal cancer patients: a multicentre study. Oncol Res Treat. 2019;42:516–522. [DOI] [PubMed] [Google Scholar]
- 33. Vatandoust S, Price TJ, Ullah S, et al. Metastatic colorectal cancer in young adults: a study from the south Australian population‐based registry. Clin Colorectal Cancer. 2016;15:32–36. [DOI] [PubMed] [Google Scholar]
- 34. Rodriguez L, Brennan K, Karim S, Nanji S, Patel SV, Booth CM. Disease characteristics, clinical management, and outcomes of young patients with colon cancer: a population‐based study. Clin Colorectal Cancer. 2018;17:e651–e661. [DOI] [PubMed] [Google Scholar]
- 35. Burnett‐Hartman AN, Powers JD, Chubak J, et al. Treatment patterns and survival differ between early‐onset and late‐onset colorectal cancer patients: the patient outcomes to advance learning network. Cancer Causes Control. 2019;30:747–755. [DOI] [PubMed] [Google Scholar]
- 36. Johdi NA, Sukor NF. Colorectal cancer immunotherapy: options and strategies. Front Immunol. 2020;11:1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Strum WB, Boland CR. Characterization and identification of colorectal cancer in persons younger than 50 years. Clin Gastroenterol Hepatol. 2019;17:2600–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yeo H, Betel D, Abelson JS, Zheng XE, Yantiss R, Shah MA. Early‐onset colorectal cancer is distinct from traditional colorectal cancer. Clin Colorectal Cancer. 2017;16:293–299.e6. [DOI] [PubMed] [Google Scholar]
- 39. Murphy CC, Singal AG, Baron JA, Sandler RS. Decrease in incidence of young‐onset colorectal cancer before recent increase. Gastroenterology. 2018;155:1716–1719.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pearlman R, Frankel WL, Swanson B, et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early‐onset colorectal cancer. JAMA Oncol. 2017;3:464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Archambault AN, Su Y‐R, Jeon J, et al. Cumulative burden of colorectal cancer‐associated genetic variants is more strongly associated with early‐onset vs late‐onset cancer. Gastroenterology. 2020;158:1274–1286.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1‐S2
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


