Abstract
Purpose
This review aims to systematically evaluate the effects of Paro on older adults and provide a stronger basis for the rational application of Paro in aged care facilities.
Methods
Articles published between January 2003 and January 2020 via five databases (PubMed, Web of Science, EMBASE, Cochrane Library, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Chinese database SinoMed) were searched. The Cochrane collaboration tool for randomized controlled trials was used to assess the quality of all included studies.
Results
Nine articles were included in this systematic review. All articles were summarized according to three themes: quality of life, and biopsychological conditions, and drug usage.
Conclusions
The review demonstrated that interaction with Paro can be beneficial for improving quality of life (QOL), biopsychological conditions, and reducing psychotropic and pain medical usage. Since the differences of the study design and low to moderate quality of these studies, however, we should be cautious to make positive comments on the role of Paro.
Implications of nursing practice
The implications of Paro in aged care facilities have positive effects on nursing outcomes. This review helps caregivers understand the advantages and disadvantages of care robots, and promotes the integration of intelligent technology and manual services in nursing practice.
Keywords: Paro, aged care facilities
INTRODUCTION
The number of people over 65 years old exceeded the number of people under 5 years old in 2018. It was estimated that people over 65 years old will account for 16% of the total population (Nations, 2019). Following the growing aging population, the number of older adults living in varying aged care facilities is increasing worldwide (Wu, 2019). However, changes in older adults’ actual or desired need for social relationships, such as physical separation from loved ones when they are living in aged care facilities can precipitate loneliness, depression, and other negative emotions (Ross, 1997). In addition, the lack of nursing resources in these kinds of institutions makes it difficult to meet residents’ spiritual and emotional needs, which may even affect older adults’ physical health (Fahy & Livingston, 2001). Given that the contradiction between the gradual expansion of spiritual and emotional needs and insufficient allocation of nursing staffs is intensified, new approaches are needed for older adults living in long‐term care facilities to maintain their physical and mental health.
As technology advances, the use of social robots is supporting caregivers with a broad range of capabilities in helping older adults relieve emotional problems (Pineau et al., 2003). The most popular companion robot for older adults is Paro, which has been variously described as companion robot, social robot, and seal robot (Moyle et al., 2013; Robinson et al., 2013; Valentí Soler et al., 2015). Designed in Japan by Takanori Shibata, Paro is a therapeutic, pet‐type robot with the appearance of a baby harp seal (Moyle et al., 2013). Compared with other social robot shaped like cats and dogs, this unfamiliar seal‐like appearance allows it to be more easily accepted as older adults are less likely to have preexisting notions about its behavior (Shibata et al., 2012). Since Paro has five types of sensors: light, tactile, posture, temperature, and audio, it can move its tail and flippers and open its eyes when people pet it. It can also respond to sounds and show emotions as it interacts with older adults. In this case, Paro might be able to provide a sense of social connectedness and emotional support for older adults living in aged care facilities (McGlynn et al., 2017).
Studies have reported that Paro could increase residents’ social interaction, decrease stress and loneliness, and even increase immune system response (Broekens et al., 2009; Shibata & Wada, 2011). Paro can also promote psychological and physiological well‐being and improve quality of life (Sharkey & Wood, 2014). These findings suggest that Paro can yield health benefits for older adults. The potential for Paro to impact positively on older adults’ health may be evidenced by Animal‐assisted therapy (AAT) (Majić et al., 2013), which aims to incorporate animals into human services, health, and education for the therapeutic benefit to humans. Thus, people attempted to replicate these effects by using pet‐type robots. However, AAT is not always available since animals are often not allowed in aged care facilities (Kimura et al., 2010), due to the risk of injury to patients, staff or visitors, the possibility of allergic reactions, and the potential nuisance of cleaning up after animals (Velde et al., 2005). Older adults or staffs may be reluctant to interact with animals. The costs for taking care of animals such as space, time, and money could also be the extra burdens to both care staffs and facilities. Thus, pet robots have been suggested as a potential viable alternative to real animals (Shibata, 2012)
Most previous individual studies, however, had varying methodological problems, including small groups, no or inappropriate control groups and limited outcome measures, which make the results of these studies unreliable and unpersuasive (Moyle et al., 2013). Furthermore, previous systematic reviews (Pu et al., 2018, 2019) tended to investigate the role of Paro in integrated environment including hospitals, homes, long‐term care homes and other usage scenarios. How can Paro help older adults in aged care facilities remains unclear. In addition, though existing reviews (Mordoch et al., 2013; Rabbitt et al., 2015) based on nonrandomized trials or observational studies have contributed to the knowledge basis of potential application of Paro, a systematic review of randomized controlled trials can provide stronger evidence for rational application of Paro.
In general, the effects of Paro on older adults in varying aged care facilities require further exploration. Thus, we performed this systematic review of randomized controlled trials (RCTs) to comprehensively explore the roles of Paro in assisting older adults living in the aged care facilities to promote more rational application of Paro.
METHOD
This systematic review was based on the preferred reporting items in the systematic review and meta‐analyses (PRISMA) guidelines (Moher et al., 2009).
Literature search strategy
PubMed, Web of Science, EMBASE, Cochrane Library, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Chinese database SinoMed were searched for relevant articles in March 2020. As the Paro robot was commercialized in 2003, studies conducted from January 1st, 2003 to January 31st, 2020 were searched for the review. The search terms are sought in the publication titles, keywords, abstracts and full texts. For each database, key words followed the PICOS principles (see Supporting Information for detailed search strategy), including:
Population: elder OR elderly OR “elderly people” OR older OR “older adults” OR “older people” OR aged OR geriatric OR senior.
Intervention: robot OR Paro OR “seal robot” OR “socially interactive robot*” OR “socially assistive robot*” OR “socially commitment robot*” OR “assistive robot*” OR “companion robot*” OR “personal assistive robot*” OR “personal robot*” OR “therapeutic robot*” OR “therapeutic seal robot*” OR “robot* therapy” OR “robot interaction.”
Study design: “randomized controlled trial” OR “clinical controlled trial” OR “randomized” OR “trial” OR “randomly” OR “groups.”
Criteria for inclusion and exclusion
Studies were included in this review if they met the following criteria: (1) Study population: older adults in aged care facilities; (2) Intervention method: Paro robot assisted activities; (3) Outcomes: the study reported available detailed data about the effects of the intervention; (4) Study type: random controlled trials; (5) published in English or Chinese.
Excluded articles included: (1) studies where subjects were children or younger adults; (2) nonrandomized studies, pre‐post studies without control group, case studies, observational studies, cross‐sectional studies, qualitative studies, study protocols, reviews; (3) studies without full text; (4) studies’ main purposes were not to explore the effects of Paro on the health of older adults, such as the exploration of the quality and function improvement of Paro.
Study selection and data extraction
All of the searched records were imported into EndNote X9 to eliminate duplicated studies and to reduce the number of papers by screening titles, abstracts, and full‐text articles that did not meet the criteria. From each included study, we extracted data including author, publication year, country, participants’ mean age, intervention details (e.g., type, frequency, duration, and total number of sessions), follow‐up times and outcome. Fig. 1 shows a summary of the study selection process.
Figure 1.
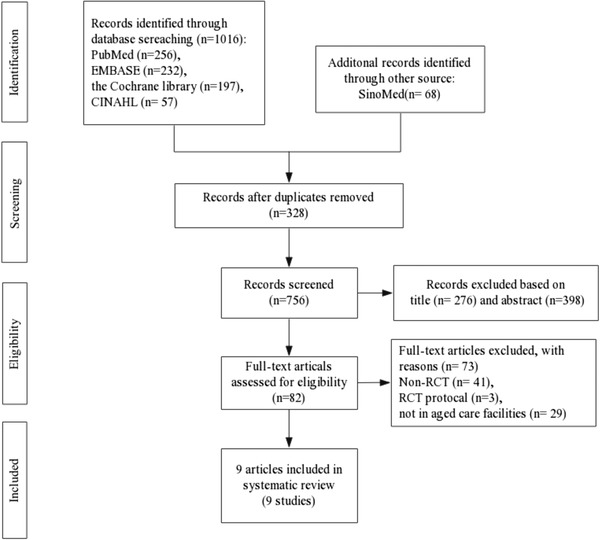
Flow chart of study selection
Quality assessment
The nine articles were examined for potential bias by two independent reviewers prior to inclusion in the systematic review. The quality appraisal of the studies in this review was conducted using the Cochrane Collaboration tool (Higgins et al., 2011) for randomized controlled trials and conflicting results were resolved within discussions.
Data synthesis
This meta‐analysis was based on the data from the scales used in the respective studies. We used the standardized mean difference (SMD) with 95% CI in most cases. The SMD were interpreted according to Cohen's definitions: 0.2–0.5 represented a small effect size, 0.5–0.8 represented a moderate effect size, and > 0.8 represented a large effect size (Cohen, 1962). The level of heterogeneity was evaluated by the I 2 method, and a value of I 2 > 50% was regarded as significant heterogeneity (Higgins, Thompson, Deeks, & Altman, 2003). Missing data were obtained from study authors if possible. We used the fixed‐effects model to calculate the pooled effect size if the data were not significantly heterogeneity. Otherwise, the random‐effects model was used. RevMan 5.4 provided by Cochrane Collaboration was used for statistical calculations, and p < 0.05 was considered statistically significant. A narrative summary was also employed to present the results that were not suitable to be included in the meta‐analysis.
RESULTS
Overview of the included studies
A total of 1084 records were identified from the electronic databases in the final search. After removal of duplicates, 674 articles were removed based on title and abstract. Therefore, we retrieved 82 full‐text publications to further evaluate their eligibility. Seventy‐three publications were excluded because they did not meet the inclusion criteria. Ultimately, a total of nine publications (Jøranson et al., 2015, 2016; Liang et al., 2017; Moyle et al., 2013, 2017, 2018; Petersen et al., 2017; Robinson et al., 2013; Valentí Soler et al., 2015) met the criteria eligibility criteria and were included in this systematic review. The main characteristics of the included studies are shown in Tables 1 and 2.
Table 1.
Participants characteristics of included studies (n = 9)
| Study(year) | Country | Participants (n) | Mean age (IG/CG) (years) | Setting |
|---|---|---|---|---|
| Moyle et al. (2013) | Australia | Older adults with dementia (n = 18) | 85.3 ± 8.4 | 1 residential care facility |
| Robinson et al. (2013) | New Zealand | Older adults with and without dementia (n = 40) | NA | a retirement home |
| Jøranson et al. (2015) | Norway | Older adults with and without dementia (n = 53) | 83.9 ± 7.2 / 84.1 ± 6.7 | 3 nursing homes |
| Soler et al. (2015) | Spain | Older people with dementia phase 1(n=101) phase 2 (n = 110) | 84.68 | a nursing home |
| Jøranson et al. (2016) | Norway | Older adults with and without dementia (n = 53) | 83.9 ± 7.2/84.1 ± 6.7 | nursing homes |
| Liang et al. (2017) | New Zealand | Older adults with dementia (n = 24) | NA | 2 dementia day care centers |
| Moyle et al. (2017) | Australia | Older adults with dementia (n = 415) | 84.0 ± 8.4/86 ± 7.6 | 28 long‐term care homes |
| Petersen et al. (2017) | USA | Older adults with dementia (n = 53) | 83.3 ± 6.0/83.5 ± 5.8 | 5 urban secure dementia units |
| Moyle et al. (2018) | Australia | Older adults with dementia (n = 455) | 84.0 ± 8.8/86 ± 7.6 | 28 long‐term care homes |
NA. Not Available
Table 2.
Characteristics of interventions of included studies (n = 9)
| Study(year) | Intervention types | Intervention methods | Frequency, duration of intervention | Intervention format | Follow‐ups | Measures/Outcomes |
|---|---|---|---|---|---|---|
| Moyle et al. (2013) | IG: companion robot intervention CG: reading | Activities around the concepts of discovery, engaging an emotional response, social interaction in the group through discussion about PARO, and touching PARO | 3 times per week 45 min per session 5 weeks | Group | Baseline, 5 weeks, 10 weeks | QOL‐AD/RAID/AES/ GDS/AWS/OERS |
| Robinson et al. (2013) | IG: companion robot intervention CG: bus trips, crafts, movies, bingo, and other alternative activities | Discussion groups were held, and all residents had a chance to interact with the robot | 2 weekday afternoon for 12 weeks | Group | Baseline, 12 weeks | UCLA loneliness scale (version 3)/GDS/QOL‐AD/behavior |
| Jøranson et al. (2015) | IG:robot‐assisted intervention CG: treatment as usual | A protocol for the Paro program: interact with Paro | 30 min per session, twice a week, 12 weeks | Group | Baseline, 12 weeks, 6 months | BARS/CSDD |
| Soler et al. (2015) | IG: animal‐shaped robot therapy CG: conventional therapy | Identifying numbers, words, and colors using flash cards and several other activities | 2 days a week for 3 months | Group | Baseline, 3 months | GDS/MMSE/sMMSE/NPI/ APADEM‐NH/QUALID |
| Jøranson et al. (2016) | IG: companion robot intervention CG: usual care | Paro‐activity protocol | 30 min per session, twice a week, 12 weeks | Group | Baseline, 12 weeks, 6 months | EARS/CDR/QUALID/medical uses |
| Liang et al. (2017) | IG: companion robot intervention CG: standard activities | INTERACT with Paro, such as stroking Paro's flippers | 30 min per session, 2–3 times a week, 6 weeks | Group | Baseline, 6 weeks | Agitated behavior/facial expressions/social interactions/Addenbrooke's cognitive examination/CMAI‐SF/NPI‐Q/CSDD/blood pressure/salivary cortisol/hair cortisol |
| Moyle et al. (2017) | IG: Paro intervention CG: usual care TG: Plush toy intervention | Participants interacted with PARO as they like | 15 min per session, 3 times per week, 10 weeks | Individual | Baseline, 1 week, 5 weeks, 10 weeks, 15 weeks | CMAI‐SF/engagement/mood states/agitation |
| Petersen et al. (2017) | IG: PARO treatment CG: standard care, which includes music, physical activity, and mental stimulation | Interactions with PARO | 20 min per day, 3 days per week, 3 months | Group | Baseline, 3 months | RAID/CSDD/GDS/GSR/pulse oximeter/pulse rate |
| Moyle et al. (2018) | IG: Paro intervention CG: usual care TG: Plush toy intervention | Participants interacted with PARO as they like | 15 min per session, 3 times per week, 10 weeks | Individual | Baseline, 5 weeks, 10 weeks, 15 weeks | Motor activity/sleep patterns |
Abbreviations: AES, Apathy Evaluation Scale; APADEM‐NH, Apathy Scale for Institutionalized Patients with Dementia Nursing Home version; AWS, Revised Wandering Scale; BARS, Brief Agitation Rating Scale; CG, control group; CMAI‐SF, Cohen‐Mansfield Agitation Inventory‐Short Form; CSDD, Cornell Scale for Depression in Dementia; GDS, Geriatric Depression Scale; IG, intervention group; MMSE, Mini Mental State Examination; NPI, Neuropsychiatric Inventory; NPI‐Q, Neuropsychiatric Inventory Brief Questionnaire Form; QOL‐AD, Quality of Life in Alzheimer's Disease scale; RAID, Rating Anxiety in Dementia Scale; QUALID, Quality of life in Late‐Stage Dementia scale; TG, toy group; .
All of the included studies reported clear inclusion and exclusion criteria for their participants. The risk of bias included studies were shown in Figures 2 and 3. All included studies reported randomized allocation. Five of the studies specifically reported that the randomization sequence was generated by random number generator on a computer (Liang et al., 2017; Moyle et al., 2013, 2017, 2018; Robinson et al., 2013), a coin toss (Petersen et al., 2017), or a six‐sided die (Valentí Soler et al., 2015). Three other studies performed random allocation using an external research center (Jøranson et al., 2016; Moyle et al., 2017, 2018). However, only four studies (Moyle et al., 2013, 2017, 2018; Valentí Soler et al., 2015) were considered as low risk of blinding outcome assessors and one study (Moyle et al., 2013) were judged as high risk of incomplete outcome data because of advanced cognitive impairment of participants. Figures 2 and 3 shows the risk of bias of included studies.
Figure 2.
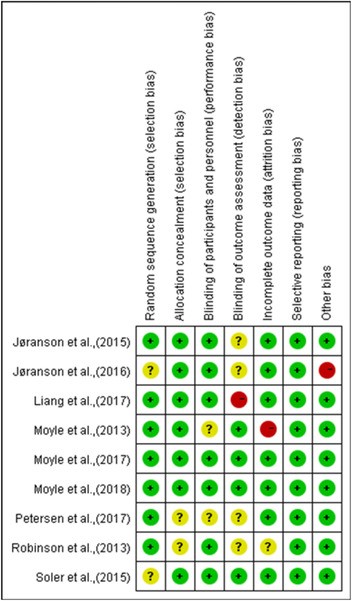
Risk of bias summary
Figure 3.
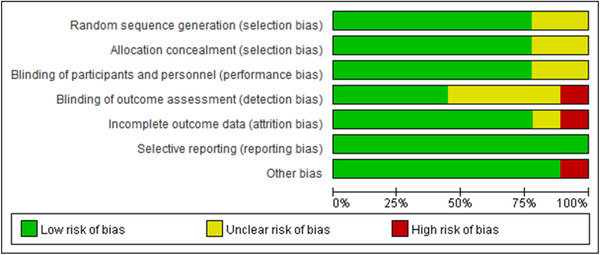
Risk of bias graph
Functions of Paro in helping older adults in aged care facilities
Improving quality of life
Four studies (Jøranson et al., 2015; Moyle et al., 2013; Robinson et al., 2013; Valentí Soler et al., 2015) assessed participants’ QOL by either the QOL‐Alzheimer's Disease scale or the QOL in late‐stage dementia (QUALID) scale. Significant improvement in QOL was reported in all these four studies. The result of the meta‐analysis showed that compared with the control participants, the participants in the intervention group experienced significant amelioration of QOL (n = 250, SMD = 0.35, 95% CI [0.10, 0.60], p = 0.007, I 2 = 0, the fixed‐effects model; Figure 4).
Figure 4.
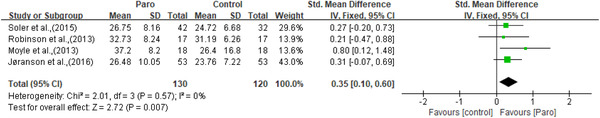
The effect of Paro on participants’ quality of life
Improving biopsychological conditions
Since all studies included the participants with dementia, neuropsychiatric symptoms, such as apathy, depression, anxiety, agitation, and wandering were considered to be biopsychological conditions.
Apathy was measured by the apathy in dementia nursing home version scale (APADEM‐NH) (Valentí Soler et al., 2015) and the apathy evaluation scale (AES) (Moyle et al., 2013). Apathy was significantly improved in one study, only in phase one (Valentí Soler et al., 2015). Depression was measured by the geriatric depression scale (GDS) (Moyle et al., 2013; Robinson et al., 2013) and the Cornell scale for depression in dementia (CSDD) (Jøranson et al., 2015; Liang et al., 2017; Petersen et al., 2017). Though two of the three studies using the CSDD reported significant improvement in depression (Jøranson et al., 2015; Petersen et al., 2017), meta‐analysis showed no statistically significant effects for depressive symptoms (n = 208, SMD = 0.08, 95% CI [‐0.19,0.35], p = 0.57, I 2 = 0, the fixed‐effects model; Figure 5). Anxiety was measured by the rating for anxiety in dementia (RAID) scale (Moyle et al., 2013; Petersen et al., 2017) and indicated improvement in the study of Petersen et al. (2017). However, no significant results were observed on anxiety through meta‐analysis (n = 97, SMD = ‐0.08, 95% CI [‐0.48,0.32], p = 0.71, I 2 = 0, the fixed‐effects model; Figure 6). Agitation was measured by the brief agitation rating scale (BARS) (Jøranson et al., 2015) and the Cohen Mansfield agitation inventory‐short form (CMAI‐SF) (Liang et al., 2017; Moyle et al., 2017). All three studies (Jøranson et al., 2015; Liang et al., 2017; Moyle et al., 2017) showed a significant decrease in agitation. Although the SMD for agitation of older adults was ‐0.23 [95% CI: ‐0.53, 0.07], but not at a statistically significant level (n = 173, p = 0.14, I 2 = 27%, the fixed‐effects model; Figure 7). Wandering was measured using the revised Algase wandering scale‐nursing home version and a clinically improvement was observed in this pilot study (Moyle et al., 2013).
Figure 5.
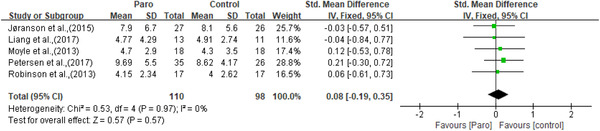
The effect of Paro on depression
Figure 6.
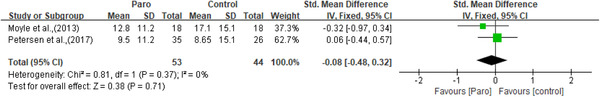
The effect of Paro on anxiety
Figure 7.
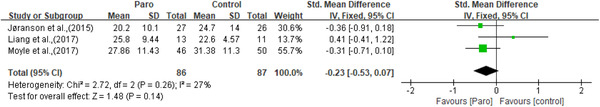
The effect of Paro on agitation
Two of the nine studies used physiological and biochemical indexes as the indicators of biopsychological conditions. In one study (Liang et al., 2017), participants’ blood pressure, pulse rate, and salivary levels were measured before and after the session. Hair cortisol, which reflected cumulative cortisol secretion two months prior to the sampling, was also obtained at baseline and at six weeks. No significant differences in any of the three indicators were reported. While in the other study (Petersen et al., 2017), galvanic skin response, pulse rate, and pulse oximetry readings as indicators of stress and anxiety were examined before and after each 20‐min exposure to the robotic pets. Researchers found significant differences in pulse rates and pulse oximetry readings.
One study concentrated on the effect of Paro on the motor activity and sleep patterns of older adults, which was measured by wearable technology (Moyle et al., 2018). The device was placed on participants’ upper nondominant arm for 24 h during the baseline period, at weeks 5 and 10 of intervention. The intervention group showed a greater reduction in daytime step count than usual care. The Paro group also had a greater reduction in nighttime physical activity than the usual‐care group. But there was no difference in sleep patterns.
Reducing drug usage
Medication usage among participants were reported in two studies. It was checked for psychoactive medications, pain medications, sleep medications and behavioral medications. One study (Jøranson et al., 2016) found that there was a significant decrease in the use of psychotropic medications among participants with advanced dementia. However, there is no difference in medication usage for participants with mild or moderate dementia. The other study (Petersen et al., 2017) reported that the dosage of medications for pain and behavior decreased significantly with a Paro intervention after 3 months, while there is no difference in the use of sleep medications or depression medications.
DISCUSSION
Social robots have developed at an astounding pace in recent years as increasing studies suggested that social robots could improve older adults’ well‐being, physical, and mental health. Even though, roles of the most popular social robot—Paro in assisting long‐term care for older adults living in aged care facilities were not well clarified. Stronger and more comprehensive evidence were provided for investigating the effects of Paro on older adults through this systematic review of RCTs. Three domains were identified: Paro could improve the QOL, biopsychological conditions, and reducing medical usage of older adults.
Quality of evidence
Most of included studies were of low to moderate quality and only two studies (Moyle et al., 2017, 2018) were regarded as low risk in all seven criteria for RCTs. All studies reported the method of randomization but two of them (Jøranson et al., 2016; Valentí Soler et al., 2015) did not give detailed statement. The concealment of allocation in two studies (Petersen et al., 2017; Robinson et al., 2013) were not clear. Though it is hard to blind the subjects, seven studies were at low risk of performance bias. Blinding outcome assessors was comparatively practical, however, only four studies were at low risk of detection bias. One study (Moyle et al., 2013) was at high risk of incomplete outcome data because the participants of this study included older adults with advanced dementia. Overall, more rigorously designed RCTs were needed to interpret the roles of Paro scientifically.
Participants and intervention examined
Six studies involved participants with varying levels of dementia and three studies included participants with and without dementia. Because studies reported their results generally, it seemed like both types of subjects benefited from the Paro intervention, particularly for those with advanced dementia (Takayanagi et al., 2014). Researchers were more willing to explore the roles of Paro for the older adults with intensive need of spiritual and emotional care, such as older adults with cognitive impairment or disability due to their poor ability to control their behavior and emotions and these kinds of older adults cannot express their own spiritual needs effectively (Hirakawa et al., 2020), even though Paro was designed to improve positive feelings for all individuals (McGlynn et al., 2017). However, in a quasi‐experimental study (Bemelmans et al., 2015), researchers using Paro in intramural care as the intervention method found that therapeutic outcomes had no relationships with dementia severity. Thus, on one hand more studies should focus on exploring the potential of Paro for healthy older adults. On the other hand, more RCTs are needed to verify the effectiveness of Paro on older adults with dementia.
Intervention formats (individual or group) and group size varied largely in this systematic review. Previous studies have shown that interacting with Paro in a group format can enhance cooperation and communication with other participants, therapists, and staff, which could be useful in improving older adults’ social connectedness and enlarging social networks (Jøranson et al., 2015; Wada et al., 2004). However, interacting with Paro individually can better meet the needs of each participant and it is more suitable for relieving individual negative mental state and emotional problems, as this allows older adults more time to interact with Paro (Kang et a., 2020). Meanwhile, it should be noted that the results of the study may be affected because participants had their preferred scale of intervention formats (De Graaf & Allouch, 2013). We need further research to explore the optimal intervention formats based on users’ needs and desires to maximize the beneficial of Paro.
Similar to intervention formats, intervention frequency and duration was also different in each study. Due to lack of relevant studies, we cannot determine the dose–response effects of Paro intervention. However, since Paro may be used as a substitute for real animals, clues from AAT studies may shine a light on this question. A meta‐analysis (Nimer & Lundahl, 2007) showed that as the number of AAT sessions increase, there could be an improvement in subjects’ behavioral outcomes, such as communication or social interaction, but their sense of well‐being could be weakened. The result of this study indicates that further research are needed to examine appropriate intervention frequency and duration as well as the effects of dosage of Paro intervention.
Roles of Paro in improving quality of life
Our meta‐analysis indicated that Paro could improve quality of life of older adults, and this finding is consistent with another literature review (Leng et al., 2019). This may because Paro could help older adults combat loneliness and stimulate social interaction (Cohen‐Mansfield, 2013) which could contribute to the promotion in mood state, social engagement, and well‐being (Bemelmans et al., 2015). However, Jøranson et al. (2016) found no significant improvement in quality of life of older adults within mild‐to‐moderate dementia even though they received Paro intervention. This may be explained by the fact that this group of participants had higher remaining psychological and physical functions, so they could carry out some daily activities and maintain meaningful social interactions freely and independently (Engedal & Haugen, 2004). In this case, the effects of Paro to improve the quality of life of older adults in mild‐to‐moderate stage of dementia were very weak.
Roles of Paro in improving biopsychological conditions
Results of this review showed that Paro could impose positive impacts on neuropsychiatric symptoms such as apathy, depression, agitation, anxiety, and wandering of older adults with dementia. Meanwhile, physiological indexes related to stress and anxiety including blood pressure, pulse oximetry and pulse also changed as these symptoms improved. Previous studies (Banks et al., 2008; Libin & Cohen‐Mansfield, 2004) have found that interacting with social robotic pets had similar benefits as AAT. Several studies (Friedmann et al., 2015; Majić et al., 2013; Menna et al., 2016) have confirmed the beneficial effects of AAT. The underlying mechanisms may be explained by changes in hormone levels (Handlin et al., 2012), social support (Barker et al., 2003), or attachment (Miesen, 1993) that occur when older adults are in contact with animals. Similar to AAT, Paro may exert positive effects on older adults through similar mechanisms (Leng et al., 2019). When older adults interact with Paro including stroking, hugging, and petting the soft fur of pet robots, stress‐relieving hormones will be released that alter stress responses and reduce depression and agitation (Remington, 2002). These stress‐relieving hormones would also lower blood pressure and reduce cortisol levels. In brief, the role of Paro in improving the health of older adults may result in reducing hormone responses and physical interactions with pet robots.
Roles of Paro in reducing drug usage
This review found that interacting with Paro can reduce the use of psychoactive medications, behavioral medications, and pain medications. Due to the functions and mechanisms of Paro in relieving neuropsychiatric symptoms which were mentioned earlier, symptoms‐related behaviors could be alleviated and improved. Accordingly, the dosage of psychoactive and behavioral drugs would also be reduced. In addition, unmanaged pain could also be a stressor which contributed to people's stress and depression (Brecher & West, 2016). When older adults interacted with Paro, older adults could be distracted from pain (Lane et al., 2016). Meanwhile, stress‐relieving hormones would release in order to alleviate the bad emotional experience caused by pain to a certain extent (Remington, 2002). Thus, the dosage of pain medications would be reduced. Paro might become a nonpharmacological tool with great development significance.
Limitations and future research
Our findings suggested that Paro may be a suitable tool to improve the well‐being and the quality of life for older adults living in the aged care facilities. However, this research also has some limitations. First, only nine articles were included in this study. Though each trial provided detailed data, the limited number of included trials limits the strength of the evidence and resulted in a poor interpretation of the findings of some of these studies (Leng et al., 2019). For example, only two included studies mentioned the roles of Paro in medication usage, so the results of these studies may not be convincing enough. Second, the included studies used varying experimental design methods, such as group and individual interventions, cluster and parallel methods, and varying durations of interventions. Thus, these differences would affect the results of this meta‐analysis. Third, most subjects included in this systematic review were older adults with dementia. This may make our study lack of universality among older adults without intellectual obstacles who also need mental and spiritual care. Last, most of the included studies were of low to moderate quality, which could also be a potential risk of bias.
Besides, several issues require attention to further investigation. First, in addition to determine the proper intervention formats of Paro to meet varying needs of older adults, it is also very important training for facilitators, staffs, and potential informants for optimizing the use of Paro. Facilitators and staffs as well as older adults in the aged care facilities should receive the education program from certified Paro trainer and education materials to help the residents of aged care facilities have more efficient interaction with Paro. The articles (Jøranson et al., 2015; Jøranson et al., 2016; Moyle et al., 2013; Valentí Soler et al., 2015) we reviewed in this paper also reported the facilitator training with protocol and assessors though information of training protocol is limited. Moreover, as older adults with dementia lost part or all of their autonomy, ethical issues should be considered during the application of Paro as intervention methods. How to protect the rights of older adults when they interact with Paro is worth thinking deeply about.
CONCLUSIONS
A total of nine articles were included in our systematic review. The results of the meta‐analysis showed that Paro have positive effects on the quality of life, biopsychological conditions, and medical usage of the older adults living in the aged care facilities. However, the potential beneficial effects on daytime and nighttime physical activities and sleep patterns remain unclear. Considering that most of the included studies were of low to moderate quality and that only included limited number of studies with varying study design, there were potential risk of bias which limited the strength of evidence. Thus, we should be cautious to make positive comments on the role of Paro. More RCTs should be established for exploring the role of Paro in older adults without intellectual obstacle and the optimal intervention methods in helping older adults interacting with Paro.
FUNDING SOURCES
This research received the funding from the Technology Innovation and application development special general project of Chongqing, China.
CONFLICT OF INTEREST
The authors declare that there if no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
AUTHOR CONTRIBUTIONS
Xinxia Wang and Jun Shen were involved in the study design. Searching strategies were designed by Xinxia Wang and Jun Shen. Xinxia Wang and Qiu Chen searched five databases and examined potential bias of included nine articles. This manuscript was drafted by Xinxia Wang. All authors revised and approved the final version of this manuscript.
Wang, X. , Shen, J. , & Chen, Q. (2022). How PARO can help older people in elderly care facilities: A systematic review of RCT. Int J Nurs Terminol Knowledge, 33: 29–39. 10.1111/2047-3095.12327
REFERENCES
- Banks, M. R. , Willoughby, L. M. , & Banks, W. A. (2008). Animal‐assisted therapy and loneliness in nursing homes: Use of robotic versus living dogs. Journal of the American Medical Directors Association, 9(3), 173–177. 10.1016/j.jamda.2007.11.007 [DOI] [PubMed] [Google Scholar]
- Barker, S. B. , Pandurangi, A. K. , & Best, A. M. (2003). Effects of animal‐assisted therapy on patients' anxiety, fear, and depression before ECT. The Journal of ECT, 19(1), 38–44.Retrieved from https://journals.lww.com/ectjournal/Fulltext/2003/03000/Effects_of_Animal_Assisted_Therapy_on_Patients_.8.aspx [DOI] [PubMed] [Google Scholar]
- Bemelmans, R. , Gelderblom, G. J. , Jonker, P. , & de Witte, L. (2015). Effectiveness of robot Paro in intramural psychogeriatric care: A multicenter quasi‐experimental study. Journal of the American Medical Directors Association, 16(11), 946–950. 10.1016/j.jamda.2015.05.007 [DOI] [PubMed] [Google Scholar]
- Brecher, D. B. , & West, T. L. (2016). Underrecognition and undertreatment of pain and behavioral symptoms in end‐stage dementia. American Journal of Hospice and Palliative Care, 33(3), 276–280. 10.1177/1049909114559069 [DOI] [PubMed] [Google Scholar]
- Broekens, J. , Heerink, M. , & Rosendal, H. (2009). Assistive social robots in elderly care: A review. Gerontechnology, 8(2), 94–103. [Google Scholar]
- Cohen‐Mansfield, J. (2013). Nonpharmacologic treatment of behavioral disorders in dementia. Current Treatment Options in Neurology, 15(6), 765–785. [DOI] [PubMed] [Google Scholar]
- Cohen, J. (1962). The statistical power of abnormal‐social psychological research: A review. The Journal of Abnormal and Social Psychology, 65(3), 145–153. 10.1037/h0045186 [DOI] [PubMed] [Google Scholar]
- De Graaf, M. M. , & Allouch, S. B. (2013). Exploring influencing variables for the acceptance of social robots. Robotics and Autonomous Systems, 61(12), 1476–1486. [Google Scholar]
- Engedal, K. , & Haugen, P. K. (2004). Demens: Fakta og utfordringer: En lærebok. Nasjonalt kompetansesenter for aldersdemens. [Google Scholar]
- Fahy, M. A. , & Livingston, G. A. (2001). The needs and mental health of older people in 24‐hour care residential placements. Aging & Mental Health, 5(3), 253–257. 10.1080/13607860120065050 [DOI] [PubMed] [Google Scholar]
- Friedmann, E. , Galik, E. , Thomas, S. A. , Hall, P. S. , Chung, S. Y. , & McCune, S. (2015). Evaluation of a pet‐assisted living intervention for improving functional status in assisted living residents with mild to moderate cognitive impairment: A pilot study. American Journal of Alzheimer's Disease & Other Dementiasr, 30(3), 276–289. 10.1177/1533317514545477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handlin, L. , Nilsson, A. , Ejdebäck, M. , Hydbring‐Sandberg, E. , & Uvnäs‐Moberg, K. (2012). Associations between the psychological characteristics of the human–dog relationship and oxytocin and cortisol levels. Anthrozoos, 25(2), 215–228. 10.2752/175303712X13316289505468 [DOI] [Google Scholar]
- Higgins, J. P. T. , Thompson, S. G. , Deeks, J. J. , & Altman, D. (2003). Measuring inconsistency in meta‐analyses. BMJ, 327(7414), 557–560. 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. P. , Altman, D. G. , Gøtzsche, P. C. , Jüni, P. , Moher, D. , Oxman, A. D. , Savović, J. , Schulz, K. F. , Weeks, L. , & Sterne, J. A. (2011). The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical Research Ed.), 343, d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa, Y. , Yajima, K. , Chiang, C. , & Aoyama, A. (2020). Meaning and practices of spiritual care for older people with dementia: Experiences of nurses and care workers. Psychogeriatrics, 20(1), 44–49. 10.1111/psyg.12454 [DOI] [PubMed] [Google Scholar]
- Jøranson, N. , Pedersen, I. , Rokstad, A. M. , & Ihlebæk, C. (2015). Effects on symptoms of agitation and depression in persons with dementia participating in robot‐assisted activity: A cluster‐randomized controlled trial. Journal of the American Medical Directors Association, 16(10), 867–873. 10.1016/j.jamda.2015.05.002 [DOI] [PubMed] [Google Scholar]
- Jøranson, N. , Pedersen, I. , Rokstad, A. M. , & Ihlebaek, C. (2016). Change in quality of life in older people with dementia participating in Paro‐activity: A cluster‐randomized controlled trial. Journal of Advanced Nursing, 72(12), 3020–3033. 10.1111/jan.13076 [DOI] [PubMed] [Google Scholar]
- Kang, H. S. , Makimoto, K. , Konno, R. , & Koh, I. S. (2020). Review of outcome measures in PARO robot intervention studies for dementia care. Geriatric Nursing, 41(3), 207–214. 10.1016/j.gerinurse.2019.09.003 [DOI] [PubMed] [Google Scholar]
- Kimura, R. , Miura, K. , Murata, H. , Yokoyama, A. , & Naganuma, M. (2010). Consideration of physiological effect of robot assisted activity on dementia elderly by electroencephalogram (EEG): Estimation of positive effect of RAA by neuroactivity diagram. Proceedings of SICE Annual Conference 2010, August 18–21, Taipei, Taiwan. 1418–1422.
- Lane, G. W. , Noronha, D. , Rivera, A. , Craig, K. , Yee, C. , Mills, B. , & Villanueva, E. (2016). Effectiveness of a social robot, “Paro,” in a VA long‐term care setting. Psychological Services, 13(3), 292–299. 10.1037/ser0000080 [DOI] [PubMed] [Google Scholar]
- Leng, M. , Liu, P. , Zhang, P. , Hu, M. , Zhou, H. , Li, G. , Yin, H. , & Chen, L. (2019). Pet robot intervention for people with dementia: A systematic review and meta‐analysis of randomized controlled trials. Psychiatry Research, 271, 516–525. 10.1016/j.psychres.2018.12.032 [DOI] [PubMed] [Google Scholar]
- Liang, A. , Piroth, I. , Robinson, H. , MacDonald, B. , Fisher, M. , Nater, U. M. , Skoluda, N. , & Broadbent, E. (2017). A pilot randomized trial of a companion robot for people with dementia living in the community. Journal of the American Medical Directors Association, 18(10), 871–878. 10.1016/j.jamda.2017.05.019 [DOI] [PubMed] [Google Scholar]
- Libin, A. , & Cohen‐Mansfield, J. (2004). Therapeutic robocat for nursing home residents with dementia: Preliminary inquiry. American Journal of Alzheimers Disease and Other Dementias, 19(2), 111–116. 10.1177/153331750401900209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majić, T. , Gutzmann, H. , Heinz, A. , Lang, U. E. , & Rapp, M. A. (2013). Animal‐assisted therapy and agitation and depression in nursing home residents with dementia: A matched case–control trial. The American Journal of Geriatric Psychiatry, 21(11), 1052–1059. 10.1016/j.jagp.2013.03.004 [DOI] [PubMed] [Google Scholar]
- McGlynn, S. A. , Kemple, S. , Mitzner, T. L. , King, C.‐H. A. , & Rogers, W. A. (2017). Understanding the potential of Paro for healthy older adults. International Journal of Human‐Computer Studies, 100, 33–47. 10.1016/j.ijhcs.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menna, L. F. , Santaniello, A. , Gerardi, F. , Di Maggio, A. , & Milan, G. (2016). Evaluation of the efficacy of animal‐assisted therapy based on the reality orientation therapy protocol in Alzheimer's disease patients: A pilot study. Psychogeriatrics, 16(4), 240–246. 10.1111/psyg.12145 [DOI] [PubMed] [Google Scholar]
- Miesen, B. M. L. (1993). Alzheimer's disease, the phenomenon of parent fixation and Bowlby's attachment theory. International Journal of Geriatric Psychiatry, 8(2), 147–153. 10.1002/gps.930080207 [DOI] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , Altman, D. G. , & The, P. G. (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. PLOS Medicine, 6(7), e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordoch, E. , Osterreicher, A. , Guse, L. , Roger, K. , & Thompson, G. (2013). Use of social commitment robots in the care of elderly people with dementia: A literature review. Maturitas, 74(1), 14–20. 10.1016/j.maturitas.2012.10.015 [DOI] [PubMed] [Google Scholar]
- Moyle, W. , Cooke, M. , Beattie, E. , Jones, C. , Klein, B. , Cook, G. , & Gray, C. (2013). Exploring the effect of companion robots on emotional expression in older adults with dementia: A pilot randomized controlled trial. Journal of Gerontological Nursing, 39(5), 46–53. 10.3928/00989134-20130313-03 [DOI] [PubMed] [Google Scholar]
- Moyle, W. , Jones, C. , Murfield, J. , Thalib, L. , Beattie, E. , Shum, D. , O'Dwyer, S. , Mervin, M. C. , & Draper, B. (2018). Effect of a robotic seal on the motor activity and sleep patterns of older people with dementia, as measured by wearable technology: A cluster‐randomised controlled trial. Maturitas, 110, 10–17. 10.1016/j.maturitas.2018.01.007 [DOI] [PubMed] [Google Scholar]
- Moyle, W. , Jones, C. J. , Murfield, J. E. , Thalib, L. , Beattie, E. R. A. , Shum, D. K. H. , O'Dwyer, S. , Mervin, M. C. , & Draper, B. M. (2017). Use of a robotic seal as a therapeutic tool to improve dementia symptoms: A cluster‐randomized controlled trial. Journal of the American Medical Directors Association, 18(9), 766–773. 10.1016/j.jamda.2017.03.018 [DOI] [PubMed] [Google Scholar]
- Nations, U. (2019). Population division: World population prospects 2019. Retrieved from https://www.un.org/development/desa/publications/world‐population‐prospects‐2019‐highlights.html
- Nimer, J. , & Lundahl, B. (2007). Animal‐assisted therapy: A meta‐analysis. Anthrozoos, 20(3), 225–238. [Google Scholar]
- Petersen, S. , Houston, S. , Qin, H. , Tague, C. , & Studley, J. (2017). The utilization of robotic pets in dementia care. Journal of Alzheimers Disease, 55(2), 569–574. 10.3233/jad-160703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineau, J. , Montemerlo, M. , Pollack, M. , Roy, N. , & Thrun, S. (2003). Towards robotic assistants in nursing homes: Challenges and results. Robotics and Autonomous Systems, 42(3), 271–281. 10.1016/S0921-8890(02)00381-0 [DOI] [Google Scholar]
- Pu, L. , Moyle, W. , Jones, C. , & Todorovic, M. (2018). The effectiveness of social robots for older adults: A systematic review and meta‐analysis of randomized controlled studies. The Gerontologist, 59(1), e37–e51. 10.1093/geront/gny046 [DOI] [PubMed] [Google Scholar]
- Pu, L. , Moyle, W. , Jones, C. , & Todorovic, M. (2019). The effectiveness of social robots for older adults: A systematic review and meta‐analysis of randomized controlled studies. The Gerontologist, 59(1), e37–e51. 10.1093/geront/gny046 [DOI] [PubMed] [Google Scholar]
- Rabbitt, S. M. , Kazdin, A. E. , & Scassellati, B. (2015). Integrating socially assistive robotics into mental healthcare interventions: Applications and recommendations for expanded use. Clinical Psychology Review, 35, 35–46. 10.1016/j.cpr.2014.07.001 [DOI] [PubMed] [Google Scholar]
- Remington, R. (2002). Calming music and hand massage with agitated elderly. Nursing Research, 51(5), 317–323.Retrieved from https://journals.lww.com/nursingresearchonline/Fulltext/2002/09000/Calming_Music_and_Hand_Massage_With_Agitated.8.aspx [DOI] [PubMed] [Google Scholar]
- Robinson, H. , Macdonald, B. , Kerse, N. , & Broadbent, E. (2013). The psychosocial effects of a companion robot: A randomized controlled trial. Journal of the American Medical Directors Association, 14(9), 661–667. 10.1016/j.jamda.2013.02.007 [DOI] [PubMed] [Google Scholar]
- Ross, L. A. (1997). Elderly patients' perceptions of their spiritual needs and care: A pilot study. Journal of Advanced Nursing, 26(4), 710–715. 10.1046/j.1365-2648.1997.00393.x [DOI] [PubMed] [Google Scholar]
- Sharkey, A. , & Wood, N. (2014). The Paro seal robot: Demeaning or enabling. Proceedings of AISB.
- Shibata, T. (2012). Therapeutic seal robot as biofeedback medical device: Qualitative and quantitative evaluations of robot therapy in dementia care. Proceedings of the IEEE, 100(8), 2527–2538. 10.1109/JPROC.2012.2200559 [DOI] [Google Scholar]
- Shibata, T. , Kawaguchi, Y. , & Wada, K. (2012). Investigation on people living with seal robot at home. International Journal of Social Robotics, 4(1), 53–63. [Google Scholar]
- Shibata, T. , & Wada, K. (2011). Robot therapy: A new approach for mental healthcare of the elderly – a mini‐review. Gerontology, 57(4), 378–386. 10.1159/000319015 [DOI] [PubMed] [Google Scholar]
- Takayanagi, K. , Kirita, T. , & Shibata, T. (2014). Comparison of verbal and emotional responses of elderly people with mild/moderate dementia and those with severe dementia in responses to seal robot, PARO. Frontiers in Aging Neuroscience, 6, 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentí Soler, M. , Agüera‐Ortiz, L. , Olazarán Rodríguez, J. , Mendoza Rebolledo, C. , Pérez Muñoz, A. , Rodríguez Pérez, I. , … Martínez Martín, P. (2015). Social robots in advanced dementia. Frontiers in Aging Neuroscience, 7, 133–133. 10.3389/fnagi.2015.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velde, B. P. , Cipriani, J. , & Fisher, G. (2005). Resident and therapist views of animal‐assisted therapy: Implications for occupational therapy practice. Australian Occupational Therapy Journal, 52(1), 43–50. 10.1111/j.1440-1630.2004.00442.x [DOI] [Google Scholar]
- Wada, K. , Shibata, T. , Saito, T. , & Tanie, K. (2004). Effects of robot‐assisted activity for elderly people and nurses at a day service center. Proceedings of the IEEE, 92(11), 1780–1788. [Google Scholar]
- Wu, J. (2019). Aging population spurs integrated elderly care services . Retrieved from http://www.china.org.cn/china/2019‐08/07/content_75076267.htm


