Abstract
Evolutionary developmental biology (evo‐devo) is the study of the evolution of developmental mechanisms. Here, I review some of the theories, models, and laws in evo‐devo, past and present. Nineteenth‐century evo‐devo was dominated by recapitulation theory and archetypes. It also gave us germ layer theory, the vertebral theory of the skull, floral organs as modified leaves, and the “inverted invertebrate” theory, among others. Newer theories and models include the frameshift theory, the genetic toolkit for development, the ABC model of flower development, the developmental hourglass, the zootype, Urbilateria, and the hox code. Some of these new theories show the influence of archetypes and recapitulation. Interestingly, recent studies support the old “primordial leaf,” “inverted invertebrate,” and “segmented head” theories. Furthermore, von Baer's first three laws may now need to be rehabilitated, and the hourglass model modified, in view of what Abzhanov has pointed out about the maternal‐zygotic transition. There are many supposed “laws” of evo‐devo but I argue that these are merely generalizations about trends in particular lineages. I argue that the “body plan” is an archetype, and is often used in such a way that it lacks any scientific meaning. Looking to the future, one challenge for evo‐devo will be to develop new theories and models to accommodate the wealth of new data from high‐throughput sequencing, including single‐cell sequencing. One step in this direction is the use of sophisticated in silico analyses, as in the “transcriptomic hourglass” models.
Keywords: evo‐devo, hourglass, phylotypic period, phylotypic stage, recapitulation, regulatory evolution, zootype
Evolutionary developmental biologists have long been fascinated by how the embryos of different animals develop along different pathways so as to lead to adults with different phenotypes. The “hourglass” model is one of the key models used to describe this divergence. Recent work suggests that this model may need to be modified due to maternal influences on the early stages of development (shaded in red).

Research highlights
Laws and other universal concepts, past and present, are reviewed.
I show that many concepts focus on conserved aspects of development.
The puzzle remains as to how embryonic phenotype, natural selection, and developmental mechanisms can be aligned to give an integrated view of evolution and development.
1. INTRODUCTION
1.1. Evolutionary developmental biology (evo‐devo)
Evolutionary developmental biology (evo‐devo) is a new name for an old discipline. Gould traces its roots to pre‐Socratics (Gould, 1977). However, evo‐devo did not become evolutionary, in the Darwinian sense, until 1859 when Origin of Species was published (C. Darwin, 1859). Evo‐devo flourished in the 19th and early 20th centuries (Gould, 1977; Hertwig, 1906b; Rádl, 1909; Rieppel, 1988; Russell, 1916). In Germany, one can recognize it in the discipline of systematic morphology, a “synthesis of comparative anatomy, palaeomorphology, embryology and systematics” (Naef, 1917, p. 69).
Interest in evolutionary developmental biology waned with the rise of experimental embryology or Entwicklungsmechanik, and genetics (Gilbert et al., 1996). Interestingly, Roux predicted that a new discipline of “comparative developmental mechanisms” might lie in the future (Roux, 1895a, p. 441). In 1977, Gould examined the history of recapitulation theory and heterochrony in his book Ontogeny and Phylogeny. The book, which set a high standard of scholarship, stimulated interest in evolutionary developmental biology. Soon afterward, a Dahlem Workshop on evolution and development, and a symposium in Sussex, were held (Bonner et al., 1982; Goodwin et al., 1983).
Homeobox genes were cloned in 1984, first in Drosophila, then in mammals (discussed by Duboule, 2009). The confluence of these and other events led to the establishment of a new discipline “in the interstices between molecular biology, geology, and evolution” (Davidson, quoted in Roush & Pennisi, 1997). This new discipline acquired the nickname “evo‐devo” (C. S. Goodman & Coughlin, 2000).
Before I consider some of the theories, laws, and models in evo‐devo, I begin with a brief explanation of what these scientific statements are.
1.2. Scientific statements
According to philosophers of science, laws and theories are examples of scientific statements (Popper, 1962, p. 54, 281). Scientific laws are simple statements about phenomena. They are generalizations in the sense that they ignore data peculiar to individual instances of the phenomenon (Hoyningen‐Huene, 2013, p. 47).
A theory is a set of scientific statements relating to a phenomenon (Hull, 1974, p. 2). Theories explain phenomena, often in terms of a mechanism (Rosenberg, 1985, p. 161). In the physical sciences, a theory may be a set of laws, whereas in evolutionary biology a theory can be a set of models (Beckner, 1959 p. 161; Lloyd, 1988 pp. 8–9, 37). A model is a bridge between a theory on one hand, and data on the other (discussed in Costa & French, 2000; Fraassen, 1989).
There are other ways of thinking about theories, laws, and models (for the many and varied perspectives on these issues, see Achinstein, 1971; Armstrong, 2016; Cartwright, 1983; Costa & French, 2000; Dhar & Giuliani, 2010; Dupré, 1993; Elgin, 2006; Feynman, 1992; Hanson, 1965; Lloyd, 1988; Lorenzano, 2006; Mayr, 1982, 2004; Murray, 2001; Pickover, 2008; Pigliucci & Muller, 2010; Rosenberg, 1985; Ruse, 1973; Smart, 1963; Fraassen, 1989; Waters, 1998; Weinert, 1995). See also the Stanford Encyclopedia of Philosophy for discussions of models (Frigg & Hartmann, 2020) and theories (Winther, 2020).
I now discuss a selection of scientific statements in evo‐devo, and a few from evolutionary biology and developmental biology for comparison. Most relate to vertebrates.
2. DEVELOPMENTAL STAGES
Developmental staging is a method that segments development into a series of stages, where each stage represents a temporal cluster of morphological character states. Because of developmental variation (polymorphism) within species, each stage is a generalization. Thus, embryos of the same stage may not share all of the characteristics of that stage. In other words, stages are polythetic. Because development involves continuous change, the author of a staging series makes entirely arbitrary decisions on how to divide it into stages. Evo‐devo research often requires stages to be compared in different species, a task that poses major problems. One of these problems, in the vertebrates at least, is heterochrony (Figure 1; Richardson, 1995). Developmental timing polymorphism within a species is another (Figure 2; Fischel, 1896; Jong et al., 2009). Yet another difficulty in comparing stages between species is character homology. For example, the Hamburger Hamilton stages for the chick (Hamburger & Hamilton, 1992) include feather tracts and the beak among its staging characters, making it difficult to apply, say, to the mouse. To address these and other issues, Werneburg (2009) developed the standard event system, a universal scheme for staging vertebrate embryos.
Figure 1.

Abacus model showing sequence heterochrony in the phylotypic period. Fig. 1 in Jeffery et al., 2002, based on data in Richardson (1995). Developmental sequences in four species (a–d) are shown as abacus diagrams and the resultant developmental sequences are aligned in (e). The horizontal axis represents developmental stage and the position of the colored beads indicates the stage at which various developmental characters (i–vii) first appear. Any vertical line drawn on the abacus represents a developmental stage. No such stage can intersect all the characters in all species. The only way to achieve that is with a window or period
Figure 2.

Polymorphism in the development of the cichlid Haplochromis piceatus shows a developmental funnel pattern. Fig. 1 in Jong et al. (2009)
3. ARCHETYPES
In evolutionary biology, an archetype is a model which represents a set of synapomorphic character states or taxic homologies (Richardson, Minelli, et al., 1998). In other words, archetypes represent homologous morphological features of a phylum, or comparable higher taxon. von Baer's types are much the same thing (von Baer, 1828 pp. vii–xxii). Archetypes are generalizations because they represent all species under consideration—but none of them in particular. And, because they represent phenotypes, archetypes are often presented visually, as diagrams or illustrations.
3.1. Body plans
Since the rise of evo‐devo as an independent field of study, the body plan concept has formed the backbone upon which much of the current research is anchored. (Willmore, 2012, p. 219).
A body plan (Ger. Bauplan, pl. Baupläne) is an archetype that supposedly represents the morphology of a single species (as in “the body plan of the zebrafish”) or a group of species (as in “the vertebrate body plan”). Isaac Newton gave a list of characters to illustrate his concept of a body plan.
Such a wonderful Uniformity in the Planetary System must be allowed the Effect of Choice. And so must the Uniformity in the Bodies of Animals, they having generally a right and a left side shaped alike, and on either side of their Bodies two Legs behind, and either two Arms … [continues list of characters] (Newton, 1718, p. 378).
More recently, some scientists have given detailed descriptions of body plans and placed them in a clear phylogenetic context (e.g., Brusca & Brusca, 1990). However, in evo‐devo, the term “body plan” is often used without any real definition, as though it were more a metaphysical concept than a scientific one. And adjectives such as fundamental, basic, or general are sometimes prefixed to “body plan” without adding any scientific meaning (as in “the basic vertebrate body plan”).
Some notion of a body plan emerged as soon as people started grouping species according to morphological and other similarities. Aristotle made many such groupings (Historia Animalium, book II, 497b, 4‐6 transl. Thompson, 1910). Two of them were his “animals with blood,” and “bloodless animals.” Lamarck, in his 1794 lectures, divided animals into those with vertebrae and those without vertebrae (as he later recalled in Lamarck, 1809, p. 118).
Buffon thought that animals shared a ‘primitive and general design' representing “an original plan on the basis of which they had all been conceived” (Buffon, 1753, p. 379). Appel suggests that Buffon's primitive plan influenced Geoffroy Saint‐Hilaire and his vertebrate type of organization (Appel, 1987; É. Geoffroy Saint‐Hilaire, 1818, p. xxxi–xxxii). In species that differ from the type of organization, Geoffroy used his principle of connections to reveal their homologies (his “analogies”; É. Geoffroy Saint‐Hilaire, 1807). Geoffroy later extended his “unity of organic composition” to all animals (É. Geoffroy Saint‐Hilaire, 1820a, 1820b). He argued, for example, that insects formed an additional class of vertebrates, their exoskeleton being analogous to the vertebral column (É. Geoffroy Saint‐Hilaire, 1822a, p. 99 et seq.).
This suggestion led to a clash with Cuvier (reviewed by Appel, 1987) who recognized four separate branches (embranchements) of the animal kingdom: Animalia vertebrosa, mollusca, articulata, and zoophyta (syn. radiata; Cuvier, 1812, pp. 83–84). The types of von Baer are equivalent to these branches (Gould, 1977, p. 57).
To some scientists, morphological similarities among animals were evidence that they were created by the same supernatural entity. Owen's archetype vertebrate skeleton (Figure 3), which he considered to be a “law” (Owen, 1847, p. 93), represented to him the common plan that pleased the Creator (Owen, 1847, p. 295). Owen defined his archetype as follows: “The archetype skeleton represents the idea of a series of essentially similar segments succeeding each other in the axis of the body; such segments being composed of parts similar in number and arrangement” (Owen, 1866, pp. xii–xiii).
Figure 3.

Owen's archetype vertebrate skeleton. (Owen, 1866, p. 30)
Darwin, by contrast, explained unity of type or body plan by unity of descent (C. Darwin, 1859 p. 206). According to Mayr, “Darwin thus replaced the archetype of idealistic morphology by the common ancestor” (Mayr, 1982, p. 465). This is not entirely true because Darwin did refer to ancestors as “archetypes” (C. Darwin, 1859, p. 435). Müller and Haeckel argued that similar embryonic morphology in different species is due to the repetition of ancestral adult stages during development. With this argument, they injected archetypes into recapitulation theory (see Section 6.5).
3.2. Developmental stages as archetypes
Many scientists have proposed that certain developmental stages are conserved among all members some or other higher taxon. These stages may even take on a life of their own, as when the Gastrula stage was thought to represent a real animal, the Gastraea. Haeckel proposed the Gastrula as a common stage in the development of many metazoans. The Gastrula, he said, was a hollow sac with a single opening and a wall consisting of two single‐cell layers: an inner entoderm and an outer exoderm (Haeckel, 1873, p. 254–255). Haeckel modestly wrote: “I regard the Gastrula as the most important and significant embryonic form in the whole animal kingdom.” (Haeckel, 1873, p. 254). The identical form of the gastrula in different animals suggested to Haeckel that they evolved from a common ancestor, the Gastraea (Haeckel, 1872, pp. 466–467; Engl. transl. in Haeckel, 1873, pp. 254–255; Levit et al., 2021). De Robertis and Sasai have commented: “Although simplistic, the Gastrea theory historically was very useful because it proposed that all multicellular animals were monophyletic” (De Robertis & Sasai, 1996, p. 40).
Sander defined the phylotypic stage as that stage in insect development when the Körpergrundgestalt appears (Sander, 1996, fig. 1). The Körpergrundgestalt, which translates as basic body shape or plan (of the phylum), was a concept used by Seidel (1953, p. 5); it may have originated with Alfred Kühn (according to Niklas et al., 2016). Seidel claimed that the body plan appears during vertebrate development after gastrulation (Seidel, 1953, p. 89).
The pharyngula was said to be a conserved stage in vertebrate development when the pharyngeal arches and pouches are present (Ballard, 1981 p. 392; Kimmel et al., 1995). It is difficult to identify this conserved stage precisely, at least in amniotes, because the pharyngeal arches develop in rostrocaudal sequence. Therefore, they do not appear in a single embryo simultaneously, in the same, undifferentiated state (Richardson, Allen, et al., 1998).
Another reason why it is difficult to identify conserved developmental stages in vertebrates is the rampant heterochrony in that taxon. Structures develop at different times in different species (reviewed by Richardson, 1995). This is why Duboule argued that there was no discrete phylotypic stage in the vertebrates but rather a series of stages that he called the phylotypic progression (Duboule, 1994 p. 139). My analysis confirmed this hypothesis and I used the term phylotypic period as a synonym for phylotypic progression (Richardson, 1995; see also Irie & Kuratani, 2014).
3.3. Molecular archetypes
Urbilateria is “The hypothetical ancestral animal… (primitive bilateral animal), from which the arthropod and the chordate lineages diverged 600 million years ago” (De Robertis & Sasai, 1996; De Robertis, 2008 p. 40). It is a reconstruction of an ancestral state based on molecular and other data. The zootype (Figure 4) represents a conserved pattern of gene expression seen in most metazoans at the tailbud stage (Slack et al., 1993). This stage was said to correspond to Sander's phylotypic stage.
Figure 4.

Updated zootype. From (Slack, 2012). Courtesy Prof. Jonathan Slack, permission from Wiley. The zootype is an important landmark in evo‐devo and a valuable aid to understanding complex data
The proto‐gnathostome pharyngeal skeleton is a model based on the expression pattern and function of Dlx and Hox genes (Depew et al., 2002; Graham, 2001). These molecular data are projected onto a proto‐gnathostome head skeleton to produce a combined morphological‐molecular archetype (Figure 5). While the authors acknowledge that the scheme is hypothetical, it is certainly a valuable heuristic scheme for understanding the evolution and development of a complex region.
Figure 5.

Proto‐gnathostome head skeleton showing genes important in patterning of the pharyngeal arches. Fig. 1A in Depew et al. (2002). Courtesy of Prof. John Rubenstein, and with permission of the American Association for the Advancement of Science. Abbreviations are given in the original paper
3.4. The archipterygium
Gegenbaur's archipterygium (Figure 6) is the hypothetical ancestral form of the endoskeleton in sarcopterygian paired appendages (Gegenbaur, 1870a, 1870b). The archipterygium has an axial skeletal element with a series of ray‐like cartilages arranged along its length (Gegenbaur, 1870b p. 416). An archipterygium‐like morphology is seen in lungfish, although this is no longer thought to represent the primitive condition for sarcopterygians. Instead, the archipterygium has been replaced with the metapterygium model (Ahlberg, 1989; Coates, 1994; Friedman et al., 2007; Stephens & Strecker, 1985).
Figure 6.

Gegenbaur's archipterygium. On the right (1) is the archipterygium with an unsegmented, cartilaginous stem (B) to which are attached a series of unsegmented cartilaginous rays. From this, a fin skeleton can be derived by segmentation of the stem and rays (2). From Gegenbaur (1870b, p. 416). Key: B, Basale of the axial element; b, successive divisions of the axial element; R, Randradius (protopterygium); r, successive fin rays
Before leaving archetypes, it is worth noting that the word phylotype was introduced as a synonym for the phylotypic stage (Minelli & Schram, 1994; Richardson, Minelli, et al., 1998 p. 158). I now think we probably introduced an unnecessary neologism into the literature. Nonetheless, the word phylotype has taken on a life of its own, evolving into a synonym for body plan (Kuratani et al., 2020; Leroi, 1998).
4. CONCEPTS
According to Mayr, one thing that sets biology apart from the physical sciences is that it deals with concepts (principles) rather than laws (Mayr, 1982). Mayr does not define “concept” (even in his glossary) but he does give examples, including homology, species, selection, and fitness.
4.1. Homology
Owen defined a homologue as “The same organ in different animals under every variety of form and function” (Owen, 1843, p. 379). Serial homology was Owen's term for the similarity between repeating structures or segments in one individual (Owen, 1847 p. 175). Redefining homology in evolutionary terms, we can say that it is the correspondence between characters in different species, that reflects their origin from the same character in a common ancestor. The character in question can be any structure or attribute (Hall, 1999b). Today, a correspondence in gene expression patterns in different species is often used to address homology questions, such as the homology of avian wing digits (Vargas et al., 2008). For more on homology see Hall, (1999a) and Mayr (1982), and for the history of the term homolog, see Owen (1847 pp. 173–175).
5. LAWS AND RULES
Laws are sometimes called “rules” or “principles,” but the choice of name is arbitrary. They are empirical generalizations. In some respects, laws can be thought of as scientific one‐liners, in which case Roux's epic statement that “development is change” (Roux, 1894, p. 3) could be a law (I think it is probably just a definition). Most laws of evo‐devo were coined in the 19th century. At that time, many scientists believed that biology, if it were to be regarded as a proper science, needed to come up with universal, mathematically based laws, like those used in physics and chemistry. Some biologists, motivated by physics envy, did try to reform biology in the image of the physical sciences. Examples include Carl Ludwig, with his “new physiology,” and Wilhelm His Sr. with his “physiological embryology” (Richardson & Keuck, 2021).
5.1. Laws of limb evo‐devo
Owen argued that distal skeletal elements are more subject to evolutionary variation than are proximal ones (Owen, 1835, p. 353). One could call this a law of variability of distal limb structures. Owen formulated it for vertebrates and it is, therefore, a lineage‐specific generalization. One obvious explanation for Owen's law is that the distal limb has more skeletal elements than the proximal limb, and therefore more degrees of freedom to vary.
Morse's law states that “when the number of fingers or toes is reduced in Mammalia and Reptilia, they are always taken away from the sides of the member, the thumb first disappearing and then the little finger” (Morse, 1874, p. 153). I have argued elsewhere (Richardson, 2012a) that this generalization, first suggested by Flower (1870, p. 255), has a number of exceptions and may therefore apply to fewer taxa than Morse suggested. In any case, it is really just a lineage‐specific generalization.
The primary axis of the limb (not to be confused with the primary body axis) is an empirical generalization about the sequence and spatial pattern of cartilage differentiation in the amniote limb. In whole mounts stained for cartilage, an inverted Y‐shaped tissue mass is seen, with its postaxial branch passing through the fourth digit (Burke & Alberch, 1985). This pattern can be used to support hypotheses about the homology of digits in the avian wing (Wagner & Gauthier, 1999, p. 5113).
5.2. von Baer's laws
These laws (von Baer, 1828, p. 224) describe patterns of phenotypic divergence between species during development (reviewed by Gould, 1977, pp. 52–63, 486; Abzhanov, 2013; Ospovat, 1976). They are as follows:
-
(1)
General features of a large animal group develop earlier than specific features.
-
(2)
From the most general morphological relations, less general ones develop, and so on until ultimately the special ones appear.
-
(3)
An embryo, instead of passing through the stages of other animals (during development) becomes increasingly different from them.
-
(4)
The embryo of a higher animal never resembles another animal; it only resembles its embryo.
von Baer also proposed a law of differentiation (von Baer, 1828, pp. 153–155) essentially his second law. It states that the homogeneous early embryo gradually becomes heterogeneous as a result of germ‐layer formation, histogenesis, and morphogenesis. Laws (1)–(3) reflect von Baer's view that there is an early, identical stage, or original form (Urform) in all animals: a hollow vesicle (von Baer, 1828, pp. 223–224). This view is in conflict with the hourglass pattern described by later embryologists (Section 8.4), which describes considerable diversity in early developmental stages. With its divergence from the same starting point in different species, von Baer's pattern of divergence is more of a developmental funnel than an hourglass (Section 8.3). Law 4 is an argument against recapitulation. For more on the supposed conflict between von Baer's laws and the developmental hourglass, see Section 9.4.
5.3. Law of embryonic resemblance
This law was discussed by Darwin (1859, p. 440), who says that other scientists before him had also observed it. The law states that the embryos of different species are more alike than their respective adults (Hall, 1997; Irie & Kuratani, 2014; Irie, 2017; Richardson & Keuck, 2002). By way of illustration, Darwin quotes von Baer's anecdote (and misattributes it to Agassiz): “I have two small embryos in spirits of wine, whose details I omitted to write down, and I am now completely unable to determine which class they belong to. They might be lizards, small birds, or very young mammals” (von Baer, 1828, p. 221). I have often wondered whether von Baer's failure to distinguish between avian and mammalian embryos might be explained by his myopia (although he claimed that it actually helped him see small objects in fine detail; see Baer and Oppenheimer (1986, p. 222).
5.4. Biogenetic law
Haeckel's version of recapitulation theory was embodied in his biogenetic law. It states that development is a rapid rerun of evolutionary history, with inheritance and adaptation as the underlying drivers (Haeckel, 1876a, p. 33; Gould, 1977; Olsson et al., 2017; see Section 6.5). Haeckel modestly adds that his law represents “the first law of development, a fundamental biogenetic law which stands or falls together with Darwinian theory itself” (Haeckel, 1872, p. 471).
5.5. His's growth law
Wilhelm His Sr. proposed that: “the entire development of the organism can be derived from a growth law which originates as a relatively simple function of space and time” (His, 1868, p. 220). The growth law was based on the differential growth of organ‐forming regions in the embryo (His, 1868, 1875). His suggested that the growth parameters of organ primordia vary between species, and that this is the mechanism underlying morphological evolution. The growth law is probably the first comprehensive model of morphogenesis and morphological evolution (Richardson & Keuck, 2021). Unfortunately, it was vaguely formulated and failed to gain traction among biologists.
5.6. Other laws of evo‐devo
The study of abnormal embryos led Geoffroy Saint‐Hilaire (the elder) to the principle of the balance of organs; he called it a general law of biology (É. Geoffroy Saint‐Hilaire, 1822b, p. 244 et seq.). His argument was that when an organ develops to an abnormally large size, other organs in that embryo will be smaller. Aristotle had made a similar statement in De Partibus Animalium (Ogle, 1911, book II 658a lines 35–36; Russell, 1916, p. 11). Geoffroy Saint‐Hilaire (the younger) also investigated this law, citing the example of a malformed fetus in which the kidney on one side was abnormally small, and its adrenal gland abnormally large (I. Geoffroy Saint‐Hilaire, 1832, p. 276).
Darwin wrote: “hence, if man goes on selecting, and thus augmenting, any peculiarity, he will almost certainly unconsciously modify other parts of the structure, owing to the mysterious laws of the correlation of growth” (C. Darwin, 1859, pp. 11–12). Haeckel discusses this concept under the name of the law of correlative adaptation: “According to this important law, actual adaptation not only changes those parts of the organism which are directly affected by its influence, but other parts also not directly affected by it” (Haeckel, 1876b, pp. 241–242). These phenomena might today be explained in terms of compensatory growth and pleiotropy (see Sections 6.12 and 7.7).
Louis Agassiz and Augustus Gould proposed what might be called a law of function in determining developmental sequences. They wrote: “the organs of the body are successively formed in the order of their organic importance, the most essential being always the earliest to appear” (Agassiz et al., 1867, p. 336). Müller gave a persuasive counter‐argument: “This proposition might be characterised à priori as undemonstrable, since it is impossible … to establish a sequence of importance amongst equally indispensable parts. Which is the more important, the lung or the heart?—the liver or the kidney?—the artery or the vein?” (Müller & Dallas, 1869, p. 102).
A size‐complexity rule was formulated by Bonner (2004). It states that an evolutionary increase in the body size of animals is accompanied by an increase in complexity, as the animal becomes subfunctionalized into more specialized cell types. Interestingly, something analogous happens in development: as Roux put it, development is “the coming into existence of visible complexity” (Roux, 1895b, p. 4).
Cuvier's principle of correlation referred to a correspondence in the form of different parts, whereby they function co‐operatively to meet the trophic needs of the organism (Cuvier, 1826, p. 59; Cuvier, 1829); an organism is, therefore, a functional “ensemble” of parts (Cuvier, 1826, p. 47). As an example, he cites carnivorous land mammals in which a powerful bite needs a powerful masseter muscle, and this, in turn, requires a robust zygomatic arch for its attachment, and so forth (Cuvier, 1826, p. 48).
Serres proposed a law of symmetry (E. R. A. Serres, 1830, p. 20). He noted that the embryonic blood vessels are initially formed in symmetrical pairs. He also suggested that the organism consists of primordial right and left halves that become fused during development (E. R. A. Serres, 1860, pp. 80, 205–220). Russell argued that this law has some similarities with the concrescence theory of Wilhelm His (Russell, 1916, p. 206 n. 2; for concrescence, see Section 7.4). For more recent discussions of symmetry, see Desgrange et al. (2018), Grimes (2019), Lobikin et al. (2012), Monsoro‐Burq and Levin (2018), and Smyth (2018).
There are various centripetal and centrifugal laws of development, stating that organs develop in a gradient toward, or away from, the center of the embryo, respectively. Serres argued that embryonic blood vessels develop from the periphery to the center (loi de formation de la circonférence au centre (E. R. A. Serres, 1830, p. 20). By contrast, von Baer held the centrifugal view (von Baer, 1828, pp. 158–159, 172), as did Wilhelm His Sr. (His, 1875, pp. 119–129).
Darwin (1859, pp. 131–170) discusses several laws of variation and gives a new explanation for them in terms of natural selection. Among them was the law that structures “developed in an extraordinary degree or manner” tend to be unusually variable (C. Darwin, 1859, p. 150). He got the idea from Waterhouse (1848, p. 452 n. 1; see also P. C. Darwin et al., 2009, p. 150). Darwin noted the variability of rudimentary structures (between individuals). He suggests a mechanism for this law: “their variability seems to be owing to their uselessness, and therefore to natural selection having no power to check deviations in their structure” (C. Darwin, 1859, pp. 149–150; Section 6.14; Figure 7).
Figure 7.

Phenotypic variation in the kiwi wing as an example of developmental instability in a rudimentary structure. Adult skeletal preparations. Detail of plate 17 in Parker (1891). Apteryx bulleri is shown in figs. 245–249. Note the variation in the size and presence of a claw, and the number of phalanges (which he details on p. 112)
6. THEORIES
Theories are compound scientific statements, consisting of a set of generalizations about a phenomenon. In evolutionary biology, a theory is often a set of models (Lloyd, 1988). However, the theory of natural selection has been characterized as a complex hierarchy of subtheories (Tuomi, 1981). Incidentally, critics of evolution who say that “evolution is only a theory” imply that scientific theories are, by definition, speculative. They are not. In scientific usage, a theory can be proven or unproven (Dawkins, 2009).
6.1. Preformation and epigenesis
Preformation theory holds that an organized individual is present in the early embryo, or in the gametes. Epigenesis is the theory that the embryo is formed de novo out of homogeneous matter (reviewed by Rádl, 1909). These two theories are usually thought to be mutually exclusive. However, Roux argues that there is no dichotomy, because neither theory is correct. He says that development is the production of visible complexity (Roux, 1895b, pp. 4–5). At earlier stages, that complexity may not yet be evident phenotypically but is there in terms of cell specification.
Preformation theory produced an icon of biology: Nicolaas Hartsoeker's illustration of a homunculus or miniature person (Figure 8). Hartsoeker never claimed to have seen such a thing; his drawing was purely hypothetical. He wrote: “if we could see the little animal [homunculus] through the skin that hides it, we would perhaps see it as represented in this figure” (Hartsoeker, 1694, pp. 229–230; see also Corcos, 1972; Hill, 1985). van Leeuwenhoek also depicted homunculi (Leeuwenhoek, 1699, figs. between pp. 266 and 267). They were his copies of satirical illustrations by Dalenpatius (1699); van Leeuwenhoek reproduced them only to ridicule them (Corcos, 1972; Hill, 1985; Leeuwenhoek, 1699).
Figure 8.

Drawings of a homunculus, one hypothetical, one satirical. Hartsoeker's hypothetical homunculus; (a) p. 230; (b) detail. From Hartsoeker (1694). The image was a speculation on what a homunculus might look like. (c,d) van Leeuwenhoek's reproductions of what he considered to be the bogus homunculus of Dalenpatius. (a,b) Courtesy Sophia Rare Books, Denmark. (c) Copyright, The Royal Society, London
6.2. Vertebral theory of the skull
This was Oken's theory, and it was based on his observation that some skull bones have the characteristics of vertebrae. In fact, he thought “the entire human is but a vertebra” (Oken, 1807, p. 5; see also Richards, 2002). Oken's epiphany on finding a deer skull is a great story:
In August 1806 I made a journey across the Harz Mountains with two students … I slipped down the southern side through the forest ‐ and lo and behold: the most beautiful skull of a female deer, bleached by exposure, lay at my feet. I picked it up, turned it around, looked at it, and that was it. It's a vertebral column! (Oken, 1818, p. 511)
Goethe claimed to have had a similar idea—when he looked at a piece of animal skull in the Jewish cemetery in Venice (Goethe, 2013 p. 126). Richards thinks this was perhaps a faulty recollection on Goethe's part (Richards, 2002). For other discussions of the vertebral skull theory and head segmentation, see Section 8.2, and Mitgutsch and Maienschein (2003) and Richards (2002).
6.3. The leaf as the fundamental organ of plants
Wolff argued that organ development in animals is comparable to “vegetation”—the development of leaves and floral organs in flowering plants (Wolff, 1768, 1769; transl. in Wolff, 1812, pp. 68–69). He also suggested that the calyx, petals, and fruit capsule of flowering plants are all modified leaves (Wolff, 1812, pp. 59–60). Two decades later, Goethe suggested something similar (Richards, 2002). “The organ that expanded on the stem as a leaf, assuming a variety of forms, is the same organ that now contracts in the calyx, expands again in the petal, contracts in the reproductive apparatus, only to expand finally as the fruit” (Goethe & Miller, 2009, Eng. transl. of Goethe, 1790, pp. 81–82, whence the italics). Goethe does not cite Wolff's earlier work, perhaps because he was unaware of it (Roe, 1979, p. 6, n. 12).
These old theories have recently found some support. Molecular‐genetic studies suggest that an ancestral patterning system for vegetative shoots was co‐opted during angiosperm evolution in order to pattern flowers (Lohmann et al., 2001). Furthermore, misexpression of MADS‐domain transcription factors can transform leaves into floral organs (Honma & Goto, 2001). The putative homologies that are now proposed are between leaves and floral organs; and between vegetative shoots and flowers (Detlef Weigel, personal communication).
6.4. Germ layer theory
This is the theory that three layers of cells are formed in the vertebrate embryo, that give rise to all tissues and organs (Hertwig, 1906a; Oppenheimer & Hamburger, 1976). Germ layers are often said to have been hinted at by Wolff, although that is a bit of a stretch. Wolff did suggest that the gut was formed by the folding of a membrane (Wolff, 1768, p. 448), but did not notice that the blastoderm was layered (Baer & Oppenheimer, 1986, p. 209). That fact was discovered by Pander who wrote of the blastodermal layers (Keimhautblätter; Pander, 1817, p. 22). von Baer thought of the germ layers as primitive organs that develop into definitive organs by folding or rolling‐up (von Baer, 1828, pp. 168–169). Remak gave them the name germ layers (Keimblätter; Remak, 1855, pp. 2–3). An interesting development in germ layer theory is the suggestion that the neural crest might constitute a fourth germ layer, which would mean that vertebrates are not triploblastic but quadroblastic (Hall, 2000).
6.5. Recapitulation theory
This theory holds that development is a re‐run (recapitulation) of evolutionary history (Haeckel, 1866a, p. 7; de Beer, 1951; Gould, 1977; Kuratani et al., 2020; Levit et al., 2021; Olsson et al., 2017; Rádl, 1909; Richardson & Keuck, 2002; Russell, 1916). Meckel and Serres were early proponents of recapitulation (Meckel, 1811, p. 3; M. Serres, 1827, p. 82–85; E. R. A. Serres, 1860, p. 401; reviewed by Gould, 1977; Jahn, 2002). One consequence of recapitulation is the supposed parallel between the data of embryology, comparative anatomy, and paleontology, noted by Agassiz (1857, pp. 135–136) and called the three‐fold parallelism by Gould (1977).
Müller framed recapitulation in Darwinian terms (F. Müller, 1864, transl. in Müller, 1869) as did Haeckel (1866a, 1866b). Recapitulation was rejected by von Baer (Section 5.2). Recently, Abzhanov (2013, fig. 2) has suggested that there is indeed a parallelism between the evolution and development of at least some characters. A good example is the apparent recapitulation of an ancestral heterocercal stage during caudal fin development in teleosts (reviewed by Gould, 1977, p. 67).
6.6. Inverted invertebrate theory
This theory of chordate origins has a long history. Geoffroy argued that a crayfish, turned on its back, has the same arrangement of organs as higher vertebrates (Appel, 1987; É. Geoffroy Saint‐Hilaire, 1822a, p. 113). He says that, from the animal's point of view, dorsal and ventral surfaces are not defined anatomically, but according to which surface the animal habitually faces toward the sky or the soil (É. Geoffroy Saint‐Hilaire, 1822a, p. 107). Semper suggested that the ventral structures of annelids correspond to the dorsal structures of vertebrates. (Semper, 1874, p. 547). And Dohrn wrote: “… I mostly agree with Geoffroy Saint‐Hilaire the elder [Étienne] when he refers to insects as vertebrates that walk on their backs — to the extent that his statement expresses the morphological agreement between the dorsum of vertebrates and the ventral structures of arthropods” (Dohrn, 1875, p. iii). More recently, developmental gene expression patterns have provided support for a dorsoventral inversion early in chordate evolution (Figure 9; Arendt & Nübler‐Jung, 1994, 1997; see also De Robertis & Sasai, 1996; Holland, 2016).
Figure 9.

The inverted invertebrate theory of vertebrate origins (Holland, 2016, fig. 1). Courtesy Prof. Nicholas Holland. See original for key. (a) Dohrn's annelid‐like ancestor; (b) transitional stage; (c) vertebrate stage; (d) Kleinenberg's scenario; (e) Beard's scenario; (f) Minot's scenario
6.7. Parablast theory
This theory of evolution and development was proposed by Wilhelm His Sr. (His, 1875, pp. 41–44). He proposed that the embryo has a dual genetic origin, part maternal, part zygotic. The maternal contribution consists of “parablast” cells that migrate from the ovary into the embryo via the yolk. Those cells make a major contribution to the mesoderm. The parablast theory drew much criticism and was difficult to reconcile with new ideas about gastrulation (Richardson & Keuck, 2021). For example, Kölliker's theory of chicken gastrulation, the basis of the modern view, was that the mesoderm was a purely zygotic tissue derived from the primitive streak (Kölliker, 1880, pp. 22–23).
6.8. Theories of gastrulation in the telolecithal egg
Balfour coined the term telolecithal for eggs that have a massive accumulation of nutrient yolk at one pole (Balfour, 1880, p. 90). These eggs undergo partial or meroblastic cleavage because, he argued, cytokinetic forces are too weak to split the massive yolk (Balfour, 1880, pp. 84–90). Roux suggested an alternative explanation: meroblastic cleavage may allow the embryo to grow unimpeded while the remaining yolk is kept aside as a nutrient reserve (Roux, 1895a, p. 31).
6.9. Germ plasm theory
Weismann proposed that there is a continuous lineage of living, hereditary substance, the germ plasm, that persists down the generations, giving rise to somatic cells during development (Weismann, 1892). According to this theory, information never flows from somatic cells to the germ plasm; the Lamarckian inheritance of acquired characters is therefore impossible (Weismann, 1893, p. 395). The current view is that, in some species, part of the oocyte cytoplasm, the germ plasm, becomes localized in a few blastomeres that become the primordial germ cells; in other species, the primordial germ cells are specified from somatic cells by induction (Nguyen et al., 2019).
6.10. Theories of vertebrate limb evo‐devo
According to the fin‐fold theory, the paired limbs are remnants of ancestral, continuous lateral fin folds (Balfour, 1878, p. 102; Mivart, 1879, p. 480; Thacher, 1877, p. 298). A related theory was based on gene expression in catfish and lamprey embryos (Freitas et al., 2006). It proposes that the potential for fin and limb development evolved first in the midline, and was then co‐opted or redeployed to the lateral plate. For reviews of the fin‐fold theory, see Diogo (2020) and Don et al. (2013).
Gegenbaur noted similarities in chondrichthyans between the branchial skeleton and the primordial fin skeleton or archipterygium (Section 3.4). He wrote: “By this approach one can imagine the formation of the skeleton of the limbs from a formation similar to the skeleton of the gill arches” (Gegenbaur, 1872, p. 181). This branchial theory of limb evolution was only a tentative suggestion that Gegenbaur relegated to a footnote (Thacher, 1877, p. 296; see also Diogo, 2020; Don et al., 2013).
Theories of limb‐genital developmental homology suggest a link between limb buds and the genital primordia, partly because they are both patterned by posterior hox genes (see Kondo et al., 1997). It has been suggested that in snakes, evolutionary shifts in the position of the cloaca, an inducer of the genital tubercle, recruited new cell populations to genital development (Tschopp et al., 2014, p. 394; see also Sanger et al., 2015). Interestingly, there is evidence of the shared cis‐regulation of some developmental genes in the limb buds and genital primordia (Infante et al., 2015).
The most anterior digit (MAD) theory states that the transcriptional landscape of digit I and the wrist and ankle constitutes a single, low‐hox zone (Woltering & Duboule, 2010). The MAD may have evolved from low‐hox tissue which gained Hoxa13 expression, and reinforced hoxd13 expression, as a result of evolutionary changes in gene regulation (Woltering & Duboule, 2010). The model was in part an alternative to the frameshift hypothesis (Wagner & Gauthier, 1999). The frameshift is a postulated change in positional values in the lineage leading to birds, that transformed digit “identities” in the avian wing from I‐II‐III to II‐III‐IV. A more limited frameshift has been proposed (Stewart et al., 2019) as has a model of digit homology that denies the existence of a frameshift entirely (Bakker et al., 2021, in press).
Sordino and colleagues suggested that the tetrapod digital plate evolved through changes in hox gene regulation, that led to prolonged cell proliferation in the fin bud (Sordino et al., 1995). A new, distal territory, the digital pate, thereby evolved as a neomorph. In line with this scenario, tetrapods show one or more late phases of hox gene expression not seen in the zebrafish.
6.11. Positional information theory
This is Wolpert's theory of what he called pattern formation—the spatial organization of cell differentiation (Wolpert, 1969). Cells in the embryo, he argued, become specified according to their position, and this specification constitutes positional information. The specification takes place in a positional field, typically 1 mm in extent, which can be modeled as a coordinate system (Wolpert, 1969). One important but overlooked consequence of positional information is non‐equivalence: “cells that look alike to the histologist but are in different positions in the body may have different intrinsic characters; they may have positional information, making them non‐equivalent” (J. H. Lewis & Wolpert, 1976, p. 479).
Wolpert also used positional information to explain what he called the French flag problem, a model of size‐independent pattern regulation (illustrated in Richardson, 2009). One of Wolpert's mechanisms of positional information involved gradients of diffusible morphogens. Evidence for gradients includes the role of sonic hedgehog in patterning the anteroposterior axis of the chicken limb (Riddle et al., 1993), and of bicoid in patterning the anteroposterior axis in the Drosophila egg (Driever & Nusslein‐Volhard, 1988). Wolpert later questioned whether diffusible gradients were actually sufficient to explain pattern formation (Richardson, 2009).
6.12. Modularity theory
The concepts of modularity and integration are central to the discourse on evolution and development. Modules are a subset of features of a given organism that are more integrated with each other than they are with the rest of the features of that organism. (Sánchez, 2012, p. 40).
Sánchez argues that integration(a mechanistic coupling between the development of different primordia) is closely related to various laws of the correlation of organs (Section 5.6). One mechanism for integration is pleiotropy, whereby functional disruption of one gene affects more than one organ system. Another is colinearity (Section 7.6), which might lead to integration because it locks the transcriptional activation of multiple patterning genes into a dependent sequence (Duboule, 1994).
The tooth‐jaw module (reviewed by A. S. Tucker & Fraser, 2014) is a concept relating to the evolution of dentitional phenotypes under the influence of a set of patterning genes. The expression patterns of these genes constitute a combinatorial odontogenic homeobox code in the dental lamina (A. Tucker & Sharpe, 2004). An important consequence of modularity is that it may facilitate an increase in complexity (Carroll, 2001, p. 1102; see also: Esteve‐Altava, 2017; Gilbert et al., 1996; Goswami, 2007; Redies & Puelles, 2001; Rucklin & Donoghue, 2015; Wagner, 1996).
6.13. Privileged embryo
The idea that midembryonic stages are not open to natural selection has been one of the dominant concepts in evo‐devo since 1859. This idea is implicit in the hourglass and phylotypic stage/period/progression theories. Darwin wrote:
… I shall attempt to show that the adult differs from its embryo, owing to variations supervening at a not early age, and being inherited at a corresponding age. This process, whilst it leaves the embryo almost unaltered, continually adds, in the course of successive generations, more and more difference to the adult (C. Darwin, 1859, p. 338, my italics).
Wolpert's take on this was that the embryo is evolutionarily privileged because “It need not, for example, seek food or mate, and so is not in direct competition for ecological niches. Its primary function is to develop reliably and this reliability is the main feature on which selection will act” (Wolpert, 1994, p. 83). The privileged embryo concept is also an explanation of the law of embryonic resemblance (Section 5.6).
6.14. Developmental stability
Developmental stability is a state reached when “the evolved or adaptive developmental trajectory is achieved despite environmental and genetic perturbations during development” (Thornhill & Furlow, 1998, p. 321; see also Beardmore, 1960; Juarez‐Carreño et al., 2018; Mather, 1953). A good example of developmental instability is the wide individual variation in the skeleton of the vestigial wing of the flightless kiwi (Figure 7).
Another example of instability is fluctuating asymmetry (reviewed by Lens et al., 2002) which consists of “nondirectional deviations from bilateral symmetry” (Palmer & Strobeck, 1986, p. 391), and which may reflect “the inability of individuals to buffer their development against small, random perturbations of cellular processes ('developmental noise')” (Lens et al., 2002, p. 28). Developmental instability may lead to a reduction in robustness and precision. Robustness is the stability of a patterning mechanism in the face of disturbance, and precision is the ability to specify accurately the boundary between different cell types (Kerszberg & Wolpert, 2007).
6.15. Complexity theory
There are many kinds of complexity. Morphological complexity is a function of the number of different parts (Carroll, 2001, p. 1104). Béclard talked about ‘the multitude of varieties or of degrees of complication' of organs (Béclard & Togno, 1830, p. 32). Carpenter noted that evolution and development both involve an increase in complexity or “complication” of form and function (Carpenter, 1839, pp. 170, 357). Many others have commented on the increase in complexity during development (Bonner, 2004; Maynard Smith, 1983, p. 33; M. Serres, 1827, pp. 82–85; Roux, 1895b, p. 4).
6.16. Constraints
Constraints are factors that limit the scope of evolutionary change, by canalizing that change along certain trajectories (reviewed by Furusawa & Irie, 2020; Richardson & Chipman, 2003). Constraints are implicit in the “why only five fingers?” question of C. J. Tabin (1992). Tabin's answer to that question was that pentadactyly is linked to the existence of five (and only five) expression domains of posterior HoxD genes in the limb bud (Izpisua‐Belmonte, Tickle, Dolle, et al., 1991). More recently, Tabin (2009, p. 730) has concluded that “Hox genes don't work like that.”
Raff expressed skepticism about constraints (Raff, 1996, pp. 295–303). I share this skepticism, at least when the logic of constraints is as follows: (i) some phenotype (a forbidden morphology) does not exist; (ii) therefore, there must be something (a constraint) preventing it from coming into existence. To me, this scenario is untestable and metaphysical, and depends on an expectation that a hypothetical phenotype ought to be able to exist. On the other hand, the concept of constraints is meaningful when it considers known phenotypes. For example, it is reasonable to ask “why only five fingers,” given that some fossil species did have more than five (Coates & Clack, 1990).
7. MECHANISMS
The explanatory element of a theory is usually a mechanism. In developmental biology, a mechanism is a sequence (pathway) of causal events (Sander, 1991, p. 2).
7.1. Heterochrony
7.1.1.
Heterochrony is an evolutionary change in developmental timing. One manifestation of heterochrony is a change in the sequence of developmental events during evolution, a phenomenon known as sequence heterochrony (See Abacus Model, Section 8.7). Heterochrony is the subject of another review in this special issue; see also (Keyte & Smith, 2014; Klingenberg, 1998; Maxwell & Harrison, 2009).
7.2. Resegmentation
Remak proposed that the somites in the chicken embryo undergo a resegmentation [neue Gliederung] as they develop into vertebrae (Remak, 1855, p. 42). As a result, the adult vertebra is derived from the adjacent halves of two neighboring somites. This means that the relationship between somites and adult vertebrae is not segmental but parasegmental. A revised “re‐segmentation‐shift” model for somites has been proposed by (Ward et al., 2017).
7.3. Terminal addition
One mechanism of recapitulation is terminal addition, whereby new developmental stages are added to the end of the ancestral sequence (reviewed by Olsson et al., 2017). Müller wrote: “Descendants therefore reach a new goal, either by deviating sooner or later whilst still on the way towards the form of their parents, or by passing along this course without deviation, but then, instead of standing still, advance still farther” (F. D. Müller & Dallas, 1869). The neomorph theory of tetrapod digits (Section 6.10) is an example of terminal addition.
7.4. Concrescence
Wilhelm His Sr. proposed a developmental mechanism that became known as concrescence (His, 1875, p. 198; Richardson & Keuck, 2021). It involves the fusion of left and right halves of the embryonic germ ring (in teleosts and chondrichthyans) so that the trunk is formed by something like the zipping‐up of the two halves in the midline. This mechanism is reminiscent of Serres' view that the left and right halves of the body have separate developmental origins (Section 5.6). Concrescence was found also in some “invertebrates” (reviewed by Kopsch, 1904).
Rabl described concrescence as “one of the most important developmental theories about the structure of the vertebrate body…” (Rabl, 1897, p. xii); and even Kopsch, who was skeptical about concrescence, argued that: “the epoch–making significance of His's concept consists in the extraordinarily simple way in which the embryonic development of vertebrates and invertebrates can now be explained by the same processes” (Kopsch, 1904, p. 4). Unfortunately, subsequent cell‐marking studies were not consistent with concrescence and the theory fell out of favor (Richardson & Keuck, 2021).
7.5. Clock mechanisms
Several clock mechanisms have been identified in embryos. Evolutionary changes in the parameters of such clocks might, in principle, underlie some examples of phenotypic evolution (Richardson & Oelschlager, 2002; Richardson et al., 2004). The progress zone mechanism was proposed to explain proximodistal patterning in the tetrapod limb. It involved cells counting cell divisions: the more cell divisions, the more distal the positional information of the cell (Summerbell & Lewis, 1975; Summerbell et al., 1973). The cyclical expression of hairy2 in the limb is consistent with a clock model (Pascoal et al., 2007). The original experiments supporting the progress zone model may also suggest a quite different model, one in which skeletal elements are specified early, then differentiate later (Dudley et al., 2002).
The segmentation clock model of somite development is based on a molecular oscillator that drives the cyclical expression of FGF, Notch, and Wnt genes (Gomez et al., 2008). These findings were interpreted on the basis of the earlier clock‐and‐wavefront model (Cooke & Zeeman, 1976) and can be used to explain the evolution of serpentiform animals (Gomez et al., 2008; Vonk & Richardson, 2008). The term hox clock describes the sequential activation of hox genes according to their location along the chromosome (Noordermeer et al., 2014). It has been suggested that the hox and segmentation clocks are mechanistically linked, and that both clocks are activated during the phylotypic period (Duboule, 1994). For more on clock models, see Andrade et al. (2005).
7.6. Other mechanisms involving homeobox genes
One way in which Hox genes may influence pattern formation is through combinatorial codes. Such codes are based on multiple, partially overlapping domains of developmental gene expression, and provide a series of discrete positional values (E. B. Lewis, 1978). Examples include the hox code in the vertebrate limb (Izpisua‐Belmonte, Tickle, Doll, et al., 1991, p. 588; C. J. Tabin, 1992), the ontogenic homeobox code in the dental lamina (see: Modularity), and the Dlx code that patterns the pharyngeal apparatus (Figure 5). However, expression domains may be a lot more complex than those implied by a simple code. For example, single‐cell sequencing of limb bud mesenchyme shows a mosaic expression pattern of HoxD genes within the same expression domain (Fabre et al., 2018). An interesting suggestion about the mechanism of hox gene function is that each hox cluster functions as a metagene, such that a set of information is encoded as a single unit (Duboule, 1994).
Related to these mechanisms is the concept of spatial colinearity. This is the phenomenon whereby the order of homeotic genes along the chromosome correlates with their spatial expression along the primary body axis: “The wild‐type and mutant segmentation patterns are consistent with an anteroposterior gradient in repressor concentration along the [Drosophila] embryo and a proximo‐distal gradient along the chromosome in the affinities for repressor of each gene's cis‐regulatory element” (E. B. Lewis, 1978, p. 565).
Another correlation is between the chromosomal location of homeotic genes and the timing of their activation during development—both along the primary body axis, and along the proximodistal and anteroposterior axes of the tetrapod limb (Dollé et al., 1989; Izpisua‐Belmonte, Falkenstein, et al., 1991, p. 2280). These phenomena are known as temporal colinearity.
One mechanism of colinearity involves competition among posterior hox genes for a remote enhancer (Kmita et al., 2002). For the history of colinearity, see Gaunt (2015). The Einbahnstraße (one‐way street) concept states that “the patterning system [in the limb] progresses from early to late, from anterior to posterior, from proximal to distal” (Duboule, 1994, p. 137).
7.7. Regulatory evolution
Mutations in the coding sequences of developmental genes may affect not one but several organ systems. For example, mutations in HOXA13 may produce malformations in both the urogenital tract and in the limbs (F. R. Goodman et al., 2000). In such examples, pleiotropy may act as a constraint because it multiplies the potential fitness costs of the mutation. Tissue‐specific regulation can break that constraint by limiting the impact of a mutation to a single organ or region. Evidence for tissue‐specific regulation comes, for example, from studies of the regulatory changes in shh expression that underlie limb loss in snakes (Kvon et al., 2016; Leal & Cohn, 2016).
Some have argued (Prud'homme et al., 2007) that regulatory changes are the dominant mechanism of morphological evolution because they can produce relatively small or spatially localized changes with few or no fitness costs:
regulatory evolution: (i) uses available genetic components in the form of preexisting and active transcription factors and [cis‐regulatory elements] to generate novelty; (ii) minimizes the penalty to overall fitness by introducing discrete changes in gene expression; and (iii) allows interactions to arise among any transcription factor and downstream CRE [cis‐regulatory element] (Prud'homme et al., 2007, p. 8605).
7.8. Genetic toolkit
The genetic toolkit for development is the set of genes involved in pattern formation (Carroll et al., 2005; Knoll & Carroll, 1999). Changes in the expression or functioning of these genes can lead to phenotypic change. The toolkit comprises transcription factors and secreted, diffusible peptides and proteins that function as short‐range signalling molecules, and other genes.
8. MODELS
One of the most memorable models in evo‐devo shows the conservation of homeobox gene expression and function between Drosophila and mammals (Figure 10). Like other models, it forms a link between theories on one hand, and the phenomena that they address on the other.
Figure 10.

Scheme of functional and expression domains of hox genes in the embryos of Drosophila and the mouse. From Martinez Arias and Stewart (2002). Courtesy of Professor Alfonso Martinez Arias; permission of Oxford University Press
8.1. ABC model of flower development
This is a model (Figure 11) of the development of organs from the floral meristem (Bowman et al., 2012). The letters ABC come from: “… the three overlapping fields, named A (APETALA2 gene function, AP2), B (APETALA3 and PISTILLATA, AP3/PI), and C (AGAMOUS, AG). AP2 was proposed to function in whorl 1 to define sepals, and AG in whorl 4 to control carpel identity” (Bowman et al., 2012, p. 4095). The model is based on the study of mutant alleles in the flowering plant Arabidopsis thaliana (Bowman et al., 1991; J.L. Bowman et al., 2012; Coen & Meyerowitz, 1991). In these studies, different parts of the flower were found to be affected by mutations in different MADS box genes. See Smyth (2018) for a review of floral patterning.
Figure 11.

The ABC model of floral development. From fig. 9 in Bowman et al. (1991). Courtesy of Professor Elliot Meyerowitz, and with permission of The Company of Biologists, Ltd. See text Section 8.1 for details
8.2. Head segmentation models
The idea that the head and the brain have a segmental (metameric) plan was implicit in Oken's vertebral theory of the skull (Section 6.2). There have been many efforts to understand head and brain segmentation, including the Cranioscopie of Carus (Figure 12). The prosomeric model of the brain (Section 8.9) envisages distinct segmental domains of gene expression and morphology common to all vertebrates (Figure 13).
Figure 12.

Carus's segmented skull hypothesis. Carus, 1841. Plate I detail (fig. III) in Carus (1841). From the library of the Royal College of Surgeons of England, with permission
Figure 13.
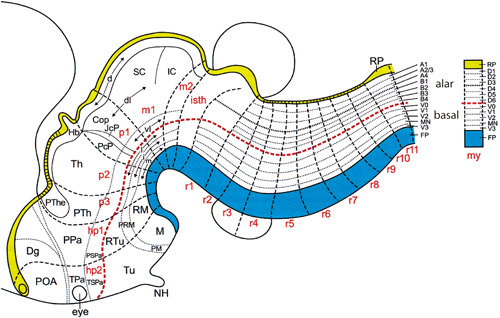
The updated prosomeric model of brain development (Puelles, 2018, fig. 8; Nieuwenhuys and Puelles (2016, fig. 9). Courtesy of Professors Luis Puelles and Rudolf Nieuwenhuys
The branchial region in vertebrates embryos has a segmental organization (Kuratani, 2005) as do the rhombomeres (Depew et al., 2002; Kiecker & Lumsden, 2005). The segmentation of the pharyngeal arches is known as branchiomery (Graham, 2001). The head cavities of elasmobranch embryos, and the somitomeres, have been described as pseudo‐segmental (reviewed by Kuratani, 2005; Mitgutsch & Maienschein, 2003).
8.3. Developmental funnel
von Baer argued that the embryos of all animals start their development as a hollow vesicle that is identical in all species. From this identical starting point, the embryos of different species becomes more divergent in phenotype as they develop (von Baer, 1828, pp. 223–224). This early stage or vesicle was characterized by von Baer as a primitive form. Haeckel later equated this vesicle with his “blastula” (Haeckel, 1877, p. 153). The pattern of divergence in animal development described by Baer (early similarity followed by progressive divergence) has been called the developmental funnel (Abzhanov, 2013; Figure 14c). A funnel‐like pattern has been described in the development of the cichlid Haplochromis piceatus (Figure 2, here; Jong et al., 2009). In that study, we were looking at polymorphisms in developmental sequences and found an invariant early developmental sequence, with a progressive increase in polymorphisms.
Figure 14.

The developmental hourglass or phylotypic egg‐timer. The horizontal gray rectangle represents the phylotypic period. (a) Fig. 3 in Duboule (1994). Transcription of hox genes is sequentially activated over an extended period, the phylotypic progression, in the middle of the hourglass. Image courtesy Prof. Denis Duboule, and with permission from the Company of Biologists, Ltd. (b) Model suggesting that the earliest stages of development are part of the maternal phenotype (red) not that of the embryo (blue). Original idea in Abzhanov (2013). (c) Purely hypothetical developmental funnel which ignores early divergences belonging to the maternal phenotype. In this model, the phylotypic period already shows some phenotypic divergence, consistent with (Richardson, 1995, 1999; Richardson, Allen, et al., 1998; Richardson et al., 1997)
8.4. Developmental hourglass or phylotypic egg‐timer
This is a model of phenotypic divergence between species during development (Figure 14a; Raff, 1996, p. 208–210; Duboule, 1994; Irie, 2017). The hourglass pattern was described by His, and by Keibel, in the vertebrates (His, 1875, pp. 190–191; Keibel, 1906, p. 172); and by Müller in arthropods:
… it sometimes happens that the greatest similarity occurs in the middle of the development. The most striking example of this is furnished by the Cirripedia and Rhizocephala, whether we compare the two orders or the members of each with one another; from a segmentation [cleavage] quite different in its course … proceed different forms of Nauplius, these become converted into exceedingly similar pupae, and from the pupae again proceed sexually mature animals, differing from each other toto coelo. (F. D. Müller & Dallas, 1869, p. 105).
The hourglass is supposed to reflect the stage‐specific action of natural selection. It is related conceptually to the law of embryonic resemblance (Section 5.3) and the privileged embryo theory (Section 6.13). In the middle of the hourglass sits the phylotypic period (Section 3.2). Interestingly, Abzhanov (2013) has suggested that stages of development before the maternal‐zygotic transition are not relevant to the hourglass model because they are part of the maternal phenotype (Figure 14b).
8.5. Inverted hourglass
Some animals show a pattern of development opposite to the hourglass model:
In other instances, the courses which lead from a similar starting‐point to a similar goal, separate widely in the middle of the development, as in the Prawns with Nauplius [larvae] already described (F. D. Müller & Dallas, 1869, p. 105).
Our quantitative analysis of developmental sequences showed that timing shifts reached a maximum in midembryonic stages. We called this pattern the inverted hourglass or spinning top (Figure 15). I invoked an inverted hourglass pattern to suggest that midembryonic stages are likely to be accessible to natural selection because these are stages when pattern formation in underway in numerous developmental fields (Richardson, 1999). An inverted (inverse) hourglass has also been used to summarize interphylum transcriptional divergence: “Comparing a set of species of distinct phyla, the mid‐developmental transition shows the most gene expression changes” (Yanai, 2018; Levin et al., 2016, fig. 4; see also Hejnol & Dunn, 2016 for a critique of that model).
Figure 15.

The inverted hourglass or spinning top. (a) The developmental hourglass; (b) the inverted (inverse) hourglass or spinning top, based on (c), a quantitative analysis of phenotypic divergence (PD) based on sequence variation in vertebrates, plotted against developmental time (Bininda‐Emonds et al., 2003, fig. 2C). Sequence variation is maximal in mid‐embryonic stages
8.6. Transcriptional (phylotranscriptomic) hourglass
As the body plan is largely laid down during the middle phases of embryo development in plants and animals, then it is perhaps not surprising this stage represents the narrow waist of the hourglass where the gene regulatory networks are the oldest and most robust and integrated, limiting species diversity and constraining morphological space (Cridge et al., 2016, p. 833).
Several recent transcriptomic analyses support the developmental hourglass model. They show that conserved developmental genes are relatively highly expressed during mid‐embryonic stages compared to earlier and later stages. This pattern (the phylotranscriptomic hourglass) has been confirmed in several plant and animal taxa (Cridge et al., 2016; Drost et al., 2015; Marlétaz et al., 2018; Quint et al., 2012; Tena et al., 2014; Yanai, 2018; reviewed by Richardson, 2012b). In plants, a transcriptional hourglass is repeated in post‐embryonic stages (Drost et al., 2016). The existence of transcriptomic hourglass patterns in both animals and plants suggests that those patterns evolved convergently (Quint et al., 2012).
8.7. Abacus model
This model (Figure 1) compares developmental sequences and shows how they can change during evolution—a phenomenon known as sequence heterochrony. The first incarnation of the abacus model (Richardson, 1995) aimed to show how heterochrony makes it impossible to define a vertebrate phylotypic stage, but possible to define a more extended phylotypic period (Richardson, 2012b). The abacus model is universal in the sense that developmental sequences from any species can be compared, provided the characters are homologous.
8.8. Prosomeric model of the vertebrate brain
The rhombencephalon (hindbrain) is an obviously segmental region of the head: “Compartition, together with segmentally reiterative neuronal architecture and the nested expression of Hox genes, indicates that the hindbrain has a truly metameric organization” (Kiecker & Lumsden, 2005, p. 553). Gene expression, fate mapping, and morphological analysis have led to the updated prosomeric model of brain organization (Nieuwenhuys & Puelles, 2016; Puelles, 2018; Rubenstein et al., 1994). This model (Figure 13) sees the brain as divided into longitudinal zones and a number of transverse segments or neuromeres in all vertebrates.
8.9. Maternal‐zygotic transition
This transition is a period (Figure 14b) during which maternal transcription is downregulated, maternal proteins are degraded, and development comes under the control of the zygotic genome, when zygotic transcription is activated by maternal transcription factors (reviewed by Rengaraj et al., 2020). In the zebrafish, this takes place gradually, beginning at cleavage stages, although maternal transcripts continue to exert a significant influence on gastrulation events (Solnica‐Krezel, 2020). In the chicken, and at least some mammals, there are successive waves of zygotic transcriptional activation beginning at cleavage (Rengaraj et al., 2020).
9. ANALYSIS
In this section, I pick up on some of the key points that emerged in this review article.
9.1. Scientific statements in evo‐devo
Some of the scientific statements used in evo‐devo are difficult to classify according to the various schemes proposed by philosophers of biology (Section 1.2). What, for example, is a developmental stage? It is not a theory, model, or law. It is perhaps one of Mayr's “concepts.” I suspect that most biologists do not worry too much about such questions, and take instead a pragmatic view: laws, theories, models, and concepts are tools for solving scientific problems (Azzouni, 2014).
9.2. Thinking in archetypes
Why are evo‐devo researchers so inordinately fond of archetypes such as the body plan and phylotypic stage? One explanation may lie in the sheer multidimensional complexity of evo‐devo questions. Such questions address the three spatial dimensions of the embryo, the expression patterns and functions of multiple developmental genes, how these parameters change over developmental time, and how they differ between species. Given this complexity, archetypes such as the zootype and the proto‐gnathostome pharyngeal skeleton are helpful heuristic devices that provide a simplified view of the data. The danger, of course, is that one loses sight of the actual data completely.
Another reason why we think in archetypes is that it comes naturally to us. Cognitive psychology tells us that humans and some other animals show perceptual categorization. This is the tendency to partition the environment into smaller sets of objects sharing certain features. These categories may be structured around “natural prototypes” (Rosch, 1973).
9.3. The problem with embryonic resemblance
There have always been widely divergent opinions on the extent of embryonic resemblance among vertebrates. Advocates of strong embryonic resemblance include Chambers (Anon, 1844, p. 212), Darwin (1859, p. 440), and Haeckel (1879, p.18). Scientists who have emphasized significant differences among vertebrate embryos include Vogt (1858, p. 169, n. 1.) Wilhelm His Sr. (His, 1875, p. 201; Sedgwick, 1894, p. 36) and Richardson et al. (1997).
How can scientists hold such divergent views? My answer is that we have often done little more than eyeball a group of embryos, then make a subjective judgment about their degree of similarity or dissimilarity. This is akin to a phenetic comparison—the grouping of organisms based on their overall similarity (Cain & Harrison, 1958, 1960; Rossello‐Mora & Amann, 2001; Sneath & Sokal, 1962; Vernon, 1988). This kind of comparison is just as problematic as the discredited “feature comparison” techniques used in some forensic sciences (Holdren & Lander, 2016). The way to address embryonic resemblance more scientifically may be to use character‐based or quantitative analyses.
9.4. A new light on von Baer and the hourglass
A common narrative is that von Baer's first three laws were incredibly interesting and important—but false. And they were false because von Baer thought that all animals had a common early stage, a vesicle, from which they diverged as they developed. That pattern of divergence looks like a funnel, and not like our current hourglass model.
However, Abzhanov (2013) notes that the earliest stages, before the maternal‐zygotic transition, are actually part of the maternal phenotype (Figure 14). If so, then the earliest stages are no more divergent than the corresponding zygote stripped of its maternal characters. If this is the case, then the true pattern of phenotypic divergence might indeed be more like a funnel than an hourglass (Figure 14c). This might be an interesting area of inquiry in the future.
9.5. Laws
… Zoology [has] its dogmas, which are as universally acknowledged, as they are disregarded in practice (F. D. Müller & Dallas, 1869, pp. 105–106).
Van Valen argued that it is valid to propose laws of evolutionary biology; he even proposed one of his own, relating to extinctions (Van Valen, 1973). Rensch also proposed evolutionary laws—quite a large number of them. He admitted that most of his laws of evolution were valid only in certain lineages (Rensch, 1960, p. 103), and I think that this caveat applies also to the laws of evo‐devo I have covered in this review.
Van Valen and Rensch were both confident in the validity of their laws. Today, however, a common view is that biological systems cannot be described using laws because: (i) they are too complex; (ii) they show emergent properties; and (iii) they are subject to evolutionary change. This last fact means that any laws proposed by biologists can potentially be broken by natural selection (Beatty, 1995, pp. 51–52, p. 61).
In any case, biologists no longer need to feel that they have to formulate laws to prove that biology is proper science. The truth is, science is not defined by its laws. Nor is it defined by its formulation of falsifiable hypotheses or its use of a hypothetico‐deductive method. In fact, the most compelling recent definition of science is that science is highly systematic when compared to other bodies of knowledge on the same subject (Hoyningen‐Huene, 2013). This is the “systematicity” definition of science. If we accept this definition, we can finally lay “physics envy” to rest.
9.6. Universality
In the physical sciences, laws are universal when they are assumed to apply everywhere in time and space (Smart, 1963, p. 53; Lloyd, 1988, p. 5). This is presumably what Newton had in mind when he wrote: “the Proposition will hold good of all bodies universally. Q.E.D.” (Newton, 1729, p. 294).
In biology, the word universal does not have that meaning at all. In molecular biology, for example, a universal statement can be one that applies to all molecules of a particular class (Mannige, 2017). In evolutionary biology, the word universal has a phylogenetic meaning, as in the universal (standard) genetic code, supposedly used by all organisms. But there is a problem here: the universal genetic code is not universal: codon reassignments have evolved in several lineages (Table 2 in Sengupta & Higgs, 2015, table 2; Koonin & Novozhilov, 2017, fig. 1; Osawa et al., 1990).
Lewis Wolpert's positional information theory was intended to be universal in its application to all species (Wolpert, 1969, p. 44). The word “universal” appears 22 times in Wolpert's foundational paper (Wolpert, 1969). I remember that when I was a PhD student with Wolpert, I wrote a manuscript for submission to Nature, entitled “A universal positional field in the feathers of birds.” It was based on transplant experiments that Amata Hornbruch and I had done, and which suggested that quail neural crest cells could reproduce a quail‐like pigment pattern in chicken feathers. Unfortunately, closer inspection showed that this was not the case (Richardson et al., 1989, 1991). Lewis, therefore, abandoned the idea of submitting the manuscript to Nature—much to my disappointment at the time.
The idea of universal developmental mechanisms is attractive because it provides common ground for scientists studying different model animals; it also gives legitimacy to the use of animal models in studying human disorders ( Richardson et al., 2001). But the reality is that organisms evolve, and so nothing in biology is universal—except those things which constitute your definition of “life” (e.g., cellularity, DNA, RNA, proteins, accessibility to natural selection, metabolism, etc.).
9.7. The future of evo‐devo
In the future, a major challenge will be to develop new evo‐devo models and theories to accommodate the wealth of new data from high‐throughput technologies. One example of these technologies is single‐cell sequencing, which is beginning to be applied to evo‐devo (Fabre et al., 2018; Post et al., 2020). To cope with massive datasets, evo‐devo researchers are using in silico analyses, such as those that underlie the “transcriptomic hourglass” model (Section 8.6). Whether the old archetypes and models of evo‐devo will survive in this new era of “big data” remains to be seen.
CONFLICT OF INTERESTS
The author declares that there are no conflict of interests.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/jez.b.23096
ACKNOWLEDGMENTS
This article is dedicated to the memory of Lewis Wolpert. He and Amata Hornbruch kindly gave me permission to relay the anecdote of the universal positional field. Gerhard Keuck advised on some of the German translations. In addition to colleagues acknowledged in the figure legends, I have had valuable discussions with Per Ahlberg, Toby Appel, Carel ten Cate, Michael Coates, Denis Duboule, Barbara Gravendeel, Paul Hoyningen‐Huene, Daan Noordermeer, Luis Puelles, Robert Richards, Martin Rücklin, C Kenneth Waters, Detlef Weigel and Ingmar Werneburg; this paper does not necessarily reflect their views.
& Richardson, M. K. (2022). Theories, laws, and models in evo‐devo. J Exp Zool B (Mol Dev Evol), 338, 36–61. 10.1002/jez.b.23096
[Correction added on 10 November 2021, after first online publication: Peer review history statement has been added.]
REFERENCES
- Abzhanov, A. (2013). von Baer's law for the ages: Lost and found principles of developmental evolution. Trends in Genetics, 29(12), 712–722. [DOI] [PubMed] [Google Scholar]
- Achinstein, P. (1971). Law and explanation: An essay in the philosophy of science. Oxford University Press. [Google Scholar]
- Agassiz, L. (1857). Contributions to the natural history of the United States of America (Vol. 1). Little, Brown and Company. [Google Scholar]
- Agassiz, L. , Gould, A. A. , & Wright, T. (1867). Outlines of comparative physiology: touching the structure and development of the races of animals, living and extinct, for the use of schools and colleges. Bell & Daldy. [Google Scholar]
- Ahlberg, P. E. (1989). Paired fin skeletons and relationships of the fossil group Porolepiformes (Osteichthyes: Sarcopterygii). Zoological Journal of the Linnean Society, 96, 119–166. [Google Scholar]
- Andrade, R. P. , Pascoal, S. , & Palmeirim, I. (2005). Thinking clockwise. Brain Research, 49(2), 114–119. [DOI] [PubMed] [Google Scholar]
- Anon (1844). Vestiges of the natural history of creation. Churchill. [Google Scholar]
- Appel, T. A. (1987). The Cuvier‐Geoffrey debate: French biology in the decades before Darwin. Oxford University Press. [Google Scholar]
- Arendt, D. , & Nübler‐Jung, K. (1994). Inversion of dorsoventral axis? Nature, 371(6492), 26. [DOI] [PubMed] [Google Scholar]
- Arendt, D. , & Nübler‐Jung, K. (1997). Dorsal or ventral: Similarities in fate maps and gastrulation patterns in annelids, arthropods and chordates. Mechanisms of Development, 61(1‐2), 7–21. [DOI] [PubMed] [Google Scholar]
- Armstrong, D. M. (2016). What is a law of nature?. Cambridge University Press. [Google Scholar]
- Azzouni, J. (2014). A new characterization of scientific theories. Synthese, 191(13), 2993–3008. [Google Scholar]
- Bakker, M. A. G. , van der Vos, W. , & de Jager, K. (2021, in press). Selection on phalanx development in the evolution of the bird wing. Molecular Biology and Evolution. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfour, F. M. (1878). A monograph on the development of elasmobranch fishes. Macmillan. [Google Scholar]
- Balfour, F. M. (1880). A treatise on comparative embryology (Vol. 1). Macmillan and Co. [Google Scholar]
- Ballard, W. W. (1981). Morphogenetic movements and fate maps of vertebrates. American Zoologist, 21(2), 391–399. [Google Scholar]
- Beardmore, J. A. (1960). Developmental stability in constant and fluctuating temperatures. Heredity, 14(3‐4), 411–422. [Google Scholar]
- Beatty, J. (1995). The evolutionary contingency thesis. Pittsburgh University Press. [Google Scholar]
- Beckner, M. (1959). The biological way of thought. Columbia University Press. [Google Scholar]
- Béclard, P. A. , & Togno, J. (1830). In Olivier M. D. (Ed.), Elements of general anatomy: or, A description of every kind of organs composing the human body/by P.A. Béclard; preceded by a critical and biographical memoir of the life and writings of the author. Translated from the French, with notes, by Joseph Togno. Carey and Lea. [Google Scholar]
- Beer, G. d (1951). Embryos and ancestors. Oxford University Press. [Google Scholar]
- Bininda‐Emonds, O. R. , Jeffery, J. E. , & Richardson, M. K. (2003). Inverting the hourglass: quantitative evidence against the phylotypic stage in vertebrate development. Proceedings of the Royal Society B: Biological Sciences, 270(1513), 341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner, J. T. (2004). Perspective: The size‐complexity rule. Evolution, 58, 1883–1890. [DOI] [PubMed] [Google Scholar]
- Bonner, J. T. , Dawid, I. , Gerhart, J. C. , Maderson, P. F. A. , Davidson, E. H. , Freeman, G. L. , & Sauer, H. W. (1982). Evolution and development: Report of the Dahlem Workshop on Evolution and Development.
- Bowman, J. L. , Smyth, D. R. , & Meyerowitz, E. M. (1991). Genetic interactions among floral homeotic genes of arabidopsis. Development, 112(1), 1–20. [DOI] [PubMed] [Google Scholar]
- Bowman, J. L. , Smyth, D. R. , & Meyerowitz, E. M. (2012). The ABC model of flower development: then and now. Development, 139(22), 4095–4098. [DOI] [PubMed] [Google Scholar]
- Brusca, R. C. , & Brusca, G. J. (1990). Invertebrates. Sinauer. [Google Scholar]
- Buffon, G. L. L.d (1753). Histoire naturelle, générale et particuliére (Vol. 4). L'Imprimerie Royale. [Google Scholar]
- Burke, A. C. , & Alberch, P. (1985). The development and homology of the chelonian carpus. J.morphol, 186, 119–131. [DOI] [PubMed] [Google Scholar]
- Cain, A. J. , & Harrison, G. A. (1958). An analysis of the taxonomist's judgment of affinity. Proceedings of the Zoological Society of London, 131(1), 85–98. [Google Scholar]
- Cain, A. J. , & Harrison, G. A. (1960). Phyletic weighting. Proceedings of the Zoological Society of London, 135(1), 1–31. [Google Scholar]
- Carpenter, W. B. (1839). Principles of general and comparative physiology. Churchill. [Google Scholar]
- Carroll, S. B. (2001). Chance and necessity: The evolution of morphological complexity and diversity. Nature, 409(6823), 1102–1109. [DOI] [PubMed] [Google Scholar]
- Carroll, S. B. , Grenier, J. K. , & Weatherbee, S. D. (2005). The genetic toolkit for development, From DNA to diversity: Molecular genetics and the evolution of animal design (pp. 17–53). Blackwell. [Google Scholar]
- Cartwright, N. (1983). How the laws of physics lie. Clarendon Press. [Google Scholar]
- Carus, C. G. (1841). Grundzüge einer neuen und wissenschaftlich begründeten cranio scopie (schädellehre). Balz. [Google Scholar]
- Coates, M. I. (1994). The origin of vertebrate limbs. Development, Suppl., 169–180. [PubMed] [Google Scholar]
- Coates, M. I. , & Clack, J. A. (1990). Polydactyly in the earliest tetrapod limbs. Nature, 347, 66–69. [Google Scholar]
- Coen, E. S. , & Meyerowitz, E. M. (1991). The war of the whorls: Genetic interactions controlling flower development. Nature, 353(6339), 31–37. [DOI] [PubMed] [Google Scholar]
- Cooke, J. , & Zeeman, E. C. (1976). A clock and wavefront model for control of the number of repeated structures during animal morphogenesis. J Theor.Biol, 58(2), 455–476. [DOI] [PubMed] [Google Scholar]
- Corcos, A. (1972). The little man who wasn't there. The American Biology Teacher, 34(9), 503–526. [Google Scholar]
- Costa, N. d , & French, S. (2000). Models, theories, and structures: Thirty years on. Philosophy of Science, 67, S116–S127. [Google Scholar]
- Cridge, A. G. , Dearden, P. K. , & Brownfield, L. R. (2016). Convergent occurrence of the developmental hourglass in plant and animal embryogenesis? Annali di Botanica, 117(5), 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuvier, G. (1812). Sur un nouveau rapprochement à établir entre les classes qui composent le règne animal. Annales du Muséum d'Histoire Naturelle, 19, 73–84. [Google Scholar]
- Cuvier, G. (1826). Discours sur les révolutions de la surface du globe. Paris: Dufour & d'Ocagne. [Google Scholar]
- Cuvier, G. (1829). A discourse on the revolutions of the surface of the globe, and the changes thereby produced in the animal kingdom. Translated from the French, with illustrations and a glossary. Whittaker, Treacher and Arnot. [Google Scholar]
- Darwin, C. (1859). On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. Murray. [PMC free article] [PubMed] [Google Scholar]
- Darwin, P. C. , Darwin, C. , & Costa, J. T. (2009). The annotated origin: A facsimile of the first edition of on the origin of species. Belknap Press of Harvard University Press. [Google Scholar]
- Dawkins, R. (2009). The greatest show on earth: The evidence for evolution. Transworld. [Google Scholar]
- Depew, M. J. , Lufkin, T. , & Rubenstein, J. L. R. (2002). Specification of jaw subdivisions by Dlx genes. Science, 298(5592), 381–385. [DOI] [PubMed] [Google Scholar]
- Desgrange, A. , Le Garrec, J. F. , & Meilhac, S. M. (2018). Left‐right asymmetry in heart development and disease: Forming the right loop. Development, 145(22). [DOI] [PubMed] [Google Scholar]
- Dhar, P. K. , & Giuliani, A. (2010). Laws of biology: Why so few? Systems and Synthetic Biology, 4(1), 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogo, R. (2020). Cranial or postcranial—Dual origin of the pectoral appendage of vertebrates combining the fin‐fold and gill‐arch theories? Developmental Dynamics, 249, 1182–1200. [DOI] [PubMed] [Google Scholar]
- Dohrn, A. (1875). Der ursprung der wirbelthiere und das princip des functionswechsels: Genealogische skizzen. Engelmann. [DOI] [PubMed] [Google Scholar]
- Dollé, P. , Izpisúa‐Belmonte, J. C. , Falkenstein, H. , Renucci, A. , & Duboule, D. (1989). Coordinate expression of the murine Hox‐5 complex homoeobox‐containing genes during limb pattern formation. Nature, 342(6251), 767–772. [DOI] [PubMed] [Google Scholar]
- Don, E. K. , Currie, P. D. , & Cole, N. J. (2013). The evolutionary history of the development of the pelvic fin/hindlimb. Journal of Anatomy, 222(1), 114–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driever, W. , & Nusslein‐Volhard, C. (1988). The bicoid protein determines position in the Drosophila embryo in a concentration‐dependent manner. Cell, 54(1), 95–104. [DOI] [PubMed] [Google Scholar]
- Drost, H. G. , Gabel, A. , Grosse, I. , & Quint, M. (2015). Evidence for active maintenance of phylotranscriptomic hourglass patterns in animal and plant embryogenesis. Molecular Biology and Evolution, 32(5), 1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drost, H. G. , Bellstädt, J. , Ó'maoiléidigh, D. S. , Silva, A. T. , Gabel, A. , Weinholdt, C. , Ryan, P. T. , Dekkers, B. J. , Bentsink, L. , Hilhorst, H. W. , Ligterink, W. , Wellmer, F. , Grosse, I. , & Quint, M. (2016). Post‐embryonic hourglass patterns mark ontogenetic transitions in plant development. Molecular Biology and Evolution, 33(5), 1158–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duboule, D. (1994). Temporal colinearity and the phylotypic progression: A basis for the stability of a vertebrate Bauplan and the evolution of morphologies through heterochrony. Development, Suppl., 135–142. [PubMed] [Google Scholar]
- Duboule, D. (2009). The Hox complex ‐ An interview with Denis Duboule. Int.J.Dev.Biol. 53, 717–723. [DOI] [PubMed] [Google Scholar]
- Dudley, A. T. , Ros, M. A. , & Tabin, C. J. (2002). A re‐examination of proximodistal patterning during vertebrate limb development. Nature, 418(6897), 539–544. [DOI] [PubMed] [Google Scholar]
- Dupré, J. (1993). The disorder of things: Metaphysical foundations of the disunity of science. Harvard University Press. [Google Scholar]
- Elgin, M. (2006). There may be strict empirical laws in biology, after all. Biology and Philosophy, 21(1), 119–134. [Google Scholar]
- Esteve‐Altava, B. (2017). In search of morphological modules: A systematic review. Biological Reviews of the Cambridge Philosophical Society, 92(3), 1332–1347. [DOI] [PubMed] [Google Scholar]
- Fabre, P. J. , Leleu, M. , Mascrez, B. , Lo Giudice, Q. , Cobb, J. , & Duboule, D. (2018). Heterogeneous combinatorial expression of Hoxd genes in single cells during limb development. BMC Biology, 16(1), 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feynman, R. P. (1992). The character of physical law. Penguin. [Google Scholar]
- Fischel, A. (1896). Uber variabilität und wachsthum des embryonalen körpers. Morphologisches Jahrbuch, 24, 369–404. [Google Scholar]
- Flower, W. H. (1870). Osteology of the mammalia. Macmillan. [Google Scholar]
- Fraassen, B. C. V. (1989). Laws and symmetry. Oxford University Press. [Google Scholar]
- Freitas, R. , Zhang, G. , & Cohn, M. J. (2006). Evidence that mechanisms of fin development evolved in the midline of early vertebrates. Nature, 442(7106), 1033–1037. [DOI] [PubMed] [Google Scholar]
- Friedman, M. , Coates, M. I. , & Anderson, P. (2007). First discovery of a primitive coelacanth fin fills a major gap in the evolution of lobed fins and limbs. Evolution & Development, 9(4), 329–337. [DOI] [PubMed] [Google Scholar]
- Frigg, R. , & Hartmann, S. (2020). Models in science. Stanford encyclopedia of philosophy. https://plato.stanford.edu/entries/models‐science/
- Furusawa, C. , & Irie, N. (2020). Toward understanding of evolutionary constraints: Experimental and theoretical approaches. Biophys Rev. [DOI] [PMC free article] [PubMed]
- Gaunt, S. J. (2015). The significance of Hox gene collinearity. International Journal of Developmental Biology, 59(4‐6), 159–170. [DOI] [PubMed] [Google Scholar]
- Gegenbaur, C. (1870a). Ueber das gliedmaassenskelet der enaliosaurier. Jenaische Zeitschrift fur Medicin und Naturwissenschaften, 5, 332–349. [Google Scholar]
- Gegenbaur, C. (1870b). Ueber das skelet der gliedmaassen der wirbelthiere im allgemeinen und der hinterrgliedmaassen der selachier insbesondere. Jenaische Zeitschrift fur Medicin und Naturwissenschaften, 5, 397–447. [Google Scholar]
- Gegenbaur, C. (1872). Untersuchungen zur vergleichenden anatomie der wirbelthiere: Das kopfskelet der selachier: Ein beitrag zur erkenntniss der genese des kopfskeletes der wirbelthiere (Vol. 3). Engelmann. [Google Scholar]
- Geoffroy Saint‐Hilaire, É. (1807). Considérations sur les pièces de la tête osseuse des animaux vertébrés, et particulièrement sur celles du 'crâne des oiseaux. Annales du Muséum d'Histoire Naturelle, 10, 342–365. [Google Scholar]
- Geoffroy Saint‐Hilaire, É. (1818). Philosophie anatomique (Vol. 1). Baillière. [Google Scholar]
- Geoffroy Saint‐Hilaire, É. (1820a). Mémoires sur l'organisation des insectes. Second mémoire, sur quelques règles fondamentales en philosophie naturelle; lu à l'Académie des sciences, le 17 janvier 1820, Par m. Geoffroy Saint‐Hilaire. Journal Complémentaire du Dictionnaire des Sciences Médicales, 6, 31–36. [Google Scholar]
- Geoffroy Saint‐Hilaire, É. (1820b). Rapport lu à Académiedes sciences, dans sa séance du 7 février 1820, sur un Mémoire de M. Audouill concernant l'organisation des insectes. Journal Complémentaire du Dictionnaire des Sciences Médicales, 6, 36–168. [Google Scholar]
- Geoffroy Saint‐Hilaire, É. (1822a). Considérations générales sur la vertèbre. Mémoires du Muséum d'Histoire Naturelle, 9, 89–119. [Google Scholar]
- Geoffroy Saint‐Hilaire, É. (1822b). Philosophie anatomique (Vol. 2). Baillière. [Google Scholar]
- Geoffroy Saint‐Hilaire, I. (1832). Histoire générale et particulière des anomalies de l'organisation chez l'homme et les animaux (Vol. 1). J.B. Baillière. [Google Scholar]
- Gilbert, S. F. , Opitz, J. M. , & Raff, R. A. (1996). Resynthesizing evolutionary and developmental biology. Developmental Biology 173, 357–372. [DOI] [PubMed] [Google Scholar]
- Goethe, J. W. (1790). Versuch die metamorphose der pflanzen zu erklären. Gotha. [Google Scholar]
- Goethe, J. W. (2013). Goethe, Johann Wolfgang von. Goethe's letters and letters to Goethe Vol. 2: Letters from 1786‐1805. C.H.Beck. [Google Scholar]
- Goethe, J. W. , & Miller, G. L. (2009). The metamorphosis of plants (transl. D. Miller). MIT Press. [Google Scholar]
- Gomez, C. , Ozbudak, E. M. , Wunderlich, J. , Baumann, D. , Lewis, J. , & Pourquié, O. (2008). Control of segment number in vertebrate embryos. Nature, 454(7202), 335–339. [DOI] [PubMed] [Google Scholar]
- Goodman, C. S. , & Coughlin, B. C. (2000). Introduction. The evolution of evo‐devo biology. Proceedings of the National Academy of Sciences of the United States of America, 97(9), 4424–4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman, F. R. , Bacchelli, C. , Brady, A. F. , Brueton, L. A. , Fryns, J. P. , Mortlock, D. P. , & Scambler, P. J. (2000). Novel HOXA13 mutations and the phenotypic spectrum of hand‐foot‐genital syndrome. American Journal of Human Genetics, 67(1), 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin, B. C. , Holder, N. , & Wylie, C. C. (1983). Development and evolution: the sixth symposium of the British Society for Developmental Biology. Cambridge University Press. [Google Scholar]
- Goswami, A. (2007). Cranial modularity and sequence heterochrony in mammals. Evolution & Development, 9(3), 290–298. [DOI] [PubMed] [Google Scholar]
- Gould, S. J. (1977). Ontogeny and phylogeny. Belknap Press. [Google Scholar]
- Graham, A. (2001). The development and evolution of the pharyngeal arches. Journal of Anatomy, 199(Pt 1‐2), 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes, D. T. (2019). Making and breaking symmetry in development, growth and disease. Development, 146(16):dev170985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeckel, E. (1866a). Generelle morphologie der organismen (Vol. 2). Reimer. [Google Scholar]
- Haeckel, E. (1866b). Generelle morphologie der organismen (Vol. 1). Reimer. [Google Scholar]
- Haeckel, E. (1872). Die kalkschwämme. Eine monographie. Volume 1 (genereller theil): Biologie der kalkschwämme (Vol. 1). Reimer. [Google Scholar]
- Haeckel, E. (1873). On the calcispongiae, their position in the animal kingdom, and their relation to the theory of descendence (transl. W. S. Dallas). Annals and Magazine of Natural History, 11, 241–430. [Google Scholar]
- Haeckel, E. (1876a). The history of creation (Engl. transl. by R.E. Lankester) (Vol. 2). Henry S. King. [Google Scholar]
- Haeckel, E. (1876b). The history of creation (Engl. transl. by R.E. Lankester) (Vol. 1). Henry S. King. [Google Scholar]
- Haeckel, E. (1877). Biologische studien, Studien zur gastraea‐theorie (Vol. 2). Dufft. [Google Scholar]
- Haeckel, E. (1879). The evolution of man. A popular exposition of the principal points of human ontogeny and phylogeny. (Eng. transl. of 3rd German Ed. of Anthropogenie). (Vol. 1). Appleton. [Google Scholar]
- Hall, B. K. (1997). Phylotypic stage or phantom: is there a highly conserved embryonic stage in vertebrates? Trends in Ecology & Evolution 12, 461–463. [DOI] [PubMed] [Google Scholar]
- Hall, B. K. (1999a). Homology (Novartis Foundation Symposium 222). Wiley. [PubMed] [Google Scholar]
- Hall, B. K. (1999b). Introduction. In Hall B. K. (Ed.), Homology (Novartis Foundation Symposium 222) (pp. 1–4). Wiley. [PubMed] [Google Scholar]
- Hall, B. K. (2000). The neural crest as a fourth germ layer and vertebrates as quadroblastic not triploblastic. Evolution & Development, 2(1), 3–5. [DOI] [PubMed] [Google Scholar]
- Hamburger, V. , & Hamilton, H. L. (1992). A series of normal stages in the development of the chick embryo. 1951. Developmental Dynamics, 195(4), 231–272. [DOI] [PubMed] [Google Scholar]
- Hanson, N. R. (1965). Patterns of discovery: An inquiry into the conceptual foundations of science. Cambridge University Press. [Google Scholar]
- Hartsoeker, N. (1694). Essay de dioptrique. Paris. [Google Scholar]
- Hejnol, A. , & Dunn, C. W. (2016). Animal evolution: Are phyla real? Current Biology, 26(10), R424–R426. [DOI] [PubMed] [Google Scholar]
- Hertwig, O. (1906a). Die lehre von den keimblättern. In Hertwig O. (Ed.), Handbuch der vergleichenden und experimentellen entwickelungslehre der wirbeltiere (Vol. 1, pp. 699–966). Fischer. [Google Scholar]
- Hertwig, O. (1906b). Handbuch der vergleichenden und experimentellen Entwicklungslehre der wirbeltiere. Fischer. [Google Scholar]
- Hill, K. A. (1985). Hartsoeker's homunculus: A corrective note. Journal of the History of the Behavioral Sciences, 21(2), 178–179. [DOI] [PubMed] [Google Scholar]
- His, W. (1868). Untersuchungen über die erste anlage des wirbelthierleibes. Die erste entwickelung des hühnchens im ei. Vogel. [Google Scholar]
- His, W. (1875). Unsere Körperform und das physiologische Problem ihrer Entstehung. Vogel. [Google Scholar]
- Holdren, J. P. , & Lander, E. S. (2016). Forensic science in criminal courts: Ensuring scientific validity of feature‐comparison methods: The President's Council of Advisors on Science and Technology.
- Holland, N. D. (2016). Nervous systems and scenarios for the invertebrate‐to‐vertebrate transition. Philosophical Transactions of the Royal Society, B: Biological Sciences, 371, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma, T. , & Goto, K. (2001). Complexes of MADS‐box proteins are sufficient to convert leaves into floral organs. Nature, 409(6819), 525–529. [DOI] [PubMed] [Google Scholar]
- Hoyningen‐Huene, P. (2013). Systematicity: The nature of science. Oxford University Press. [Google Scholar]
- Hull, D. L. (1974). Philosophy of biological science. Prentice‐Hall. [Google Scholar]
- Infante, C. R. , Mihala, A. G. , Park, S. , Wang, J. S. , Johnson, K. K. , Lauderdale, J. D. , & Menke, D. B. (2015). Shared enhancer activity in the limbs and phallus and functional divergence of a limb‐genital cis‐regulatory element in snakes. Developmental Cell, 35(1), 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie, N. (2017). Remaining questions related to the hourglass model in vertebrate evolution. Current Opinion in Genetics & Development, 45, 103–107. [DOI] [PubMed] [Google Scholar]
- Irie, N. , & Kuratani, S. (2014). The developmental hourglass model: A predictor of the basic body plan? Development, 141(24), 4649–4655. [DOI] [PubMed] [Google Scholar]
- Izpisua‐Belmonte, J. C. , Falkenstein, H. , Doll, P. , Renucci, A. , & Duboule, D. (1991). Murine genes related to the Drosophila Abd‐B homeotic genes are sequentially expressed during development of the posterior part of the body. EMBO Journal, 10(8), 2279–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izpisua‐Belmonte, J. C. , Tickle, C. , Dolle, P. , Wolpert, L. , & Duboule, D. (1991). Expression of the homeobox Hox‐4 genes and the specification of position in chick wing development. Nature, 350(6319), 585–589. [DOI] [PubMed] [Google Scholar]
- Izpisua‐Belmonte, J. C. , Tickle, C. , Doll, P. , Wolpert, L. , & Duboule, D. (1991). Expression of the homeobox Hox‐4 genes and the specification of position in chick wing development. Nature, 350, 585–589. [DOI] [PubMed] [Google Scholar]
- Jahn, I. (2002). The "Meckel‐Serres Statute", its origin and relation to evolutionary theories of the 19th Century. Annals of Anatomy, 184(6), 509–517. [DOI] [PubMed] [Google Scholar]
- Jeffery, J. E. , Bininda‐Emonds, O. R. , Coates, M. I. , & Richardson, M. K. (2002). Analyzing evolutionary patterns in amniote embryonic development. Evolution and Development, 4, 292–302. [DOI] [PubMed] [Google Scholar]
- Jong, I. L. M. d. , Colbert, M. W. , Witte, F. , & Richardson, M. K. (2009). Polymorphism in developmental timing: Intraspecific heterochrony in a Lake Victoria cichlid. Evolution and Development, 11(6), 625–635. [DOI] [PubMed] [Google Scholar]
- Juarez‐Carreño, S. , Morante, J. , & Dominguez, M. (2018). Systemic signalling and local effectors in developmental stability, body symmetry, and size. Cell stress, 2(12), 340–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keibel, F. (1906). Die Entwickelung der äusseren Körperform der Wirbeltierembryonen, insbesondere der menschlichen Embryonen aus den ersten 2 Monaten. In Hertwig O. (Ed.), Handbuch der vergleichenden und experimentellen Entwickelungslehre der Wirbelthiere (Vol. 1, pp. 1–176). Gustav Fischer. [Google Scholar]
- Kerszberg, M. , & Wolpert, L. (2007). Specifying positional information in the embryo: looking beyond morphogens. Cell, 130(2), 205–209. [DOI] [PubMed] [Google Scholar]
- Keyte, A. L. , & Smith, K. K. (2014). Heterochrony and developmental timing mechanisms: Changing ontogenies in evolution. Seminars in Cell & Developmental Biology, 34, 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecker, C. , & Lumsden, A. (2005). Compartments and their boundaries in vertebrate brain development. Nature Reviews Neuroscience, 6(7), 553–564. [DOI] [PubMed] [Google Scholar]
- Kimmel, C. B. , Ballard, W. W. , Kimmel, S. R. , Ullmann, B. , & Schilling, T. F. (1995). Stages of embryonic development of the zebrafish. Dev.Dyn. 203(3), 253–310. [DOI] [PubMed] [Google Scholar]
- Klingenberg, C. P. (1998). Heterochrony and allometry: The analysis of evolutionary change in ontogeny. Biological Reviews, 73(1), 79–123. [DOI] [PubMed] [Google Scholar]
- Kmita, M. , Fraudeau, N. , Herault, Y. , & Duboule, D. (2002). Serial deletions and duplications suggest a mechanism for the collinearity of Hoxd genes in limbs. Nature, 420(6912), 145–150. [DOI] [PubMed] [Google Scholar]
- Knoll, A. H. , & Carroll, S. B. (1999). Early animal evolution: Emerging views from comparative biology and geology. Science, 284(5423), 2129–2137. [DOI] [PubMed] [Google Scholar]
- Kondo, T. , Zákány, J. , Innis, J. W. , & Duboule, D. (1997). Of fingers, toes and penises. Nature, 390(6655), 29. [DOI] [PubMed] [Google Scholar]
- Koonin, E. V. , & Novozhilov, A. S. (2017). Origin and evolution of the universal genetic code. Annual Review of Genetics, 51, 45–62. [DOI] [PubMed] [Google Scholar]
- Kopsch, F. (1904). Untersuchungen über gastrulation and embryobildung bei den chordaten I, Die morphologische bedeutung des keimhautrandes und die embryobildung bei der forelle. Thieme. [Google Scholar]
- Kuratani, S. (2005). Craniofacial development and the evolution of the vertebrates: The old problems on a new background. Zoological Science, 22(1), 1–19. [DOI] [PubMed] [Google Scholar]
- Kuratani, S. , Uesaka, M. , & Irie, N. (2020). How can recapitulation be reconciled with modern concepts of evolution? Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. Advance online publication. 1–8. 10.1002/jez.b.23020 [DOI] [PubMed] [Google Scholar]
- Kvon, E. Z. , Kamneva, O. K. , Melo, U. S. , Barozzi, I. , Osterwalder, M. , Mannion, B. J. , & Visel, A. (2016). Progressive loss of function in a limb enhancer during snake evolution. Cell, 167(3), 633–642e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarck, J. B. (1809). Philosophie zoologique, ou exposition des considérations relatives à l'histoire naturelle des animaux. Paris: Dentu. [Google Scholar]
- Leal, F. , & Cohn, M. J. (2016). Loss and re‐emergence of legs in snakes by modular evolution of sonic hedgehog and HOXD enhancers. Current Biology, 26(21), 2966–2973. [DOI] [PubMed] [Google Scholar]
- Leeuwenhoek, A. v (1699). Part of a letter from Mr. Leuvenhook, dated June 9th, 1699, concerning the animalcula in semine humano, etc. Philosophical Transactions of the Royal Society, 21, 301–308. [Google Scholar]
- Lens, L. , Van Dongen, S. , Kark, S. , & Matthysen, E. (2002). Fluctuating asymmetry as an indicator of fitness: can we bridge the gap between studies? Biological Reviews of the Cambridge Philosophical Society, 77(1), 27–38. [DOI] [PubMed] [Google Scholar]
- Leroi, A. M. (1998). The burden of the bauplan. Trends in Ecology & Evolution, 13, 82–83. [DOI] [PubMed] [Google Scholar]
- Levin, M. , Anavy, L. , Cole, A. G. , Winter, E. , Mostov, N. , Khair, S. , & Yanai, I. (2016). The mid‐developmental transition and the evolution of animal body plans. Nature, 531(7596), 637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levit, G. S. , Hoßfeld, U. , Naumann, B. , Lukas, P. , & Olsson, L. (2021). The biogenetic law and the Gastraea theory: From Ernst Haeckel's discoveries to contemporary views. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. [DOI] [PubMed] [Google Scholar]
- Lewis, E. B. (1978). A gene complex controlling segmentation in Drosophila. Nature, 276(5688), 565–570. [DOI] [PubMed] [Google Scholar]
- Lewis, J. H. , & Wolpert, L. (1976). The principle of non‐equivalence in development. Journal of Theoretical Biology, 62, 479–490. [DOI] [PubMed] [Google Scholar]
- Lloyd, E. A. (1988). The structure and confirmation of evolutionary theory. Greenwood Press. [Google Scholar]
- Lobikin, M. , Wang, G. , Xu, J. , Hsieh, Y. ‐W. , Chuang, C. ‐F. , Lemire, J. M. , & Levin, M. (2012). Early, nonciliary role for microtubule proteins in left‐‐right patterning is conserved across kingdoms. Proceedings of the National Academy of Sciences of the United States, 109(31), 12586–12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann, J. U. , Hong, R. L. , Hobe, M. , Busch, M. A. , Parcy, F. , Simon, R. , & Weigel, D. (2001). A molecular link between stem cell regulation and floral patterning in Arabidopsis. Cell, 105(6), 793–803. [DOI] [PubMed] [Google Scholar]
- Lorenzano, P. (2006). Fundamental laws and laws of biology. In Ernst G., & Niebergall K.‐G. (Eds.), Philosophie der Wissenschaft – Wissenschaft der Philosophie. Festschrift für C. Ulises Moulines zum 60. Geburtstag (pp. 129–155). Mentis. [Google Scholar]
- Mannige, R. V. (2017). An exhaustive survey of regular peptide conformations using a new metric for backbone handedness. PeerJ, 5, e3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlétaz, F. , Firbas, P. N. , Maeso, I. , Tena, J. J. , Bogdanovic, O. , & Perry, M. (2018). Amphioxus functional genomics and the origins of vertebrate gene regulation. Nature, 564(7734), 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez Arias, A. , & Stewart, A. (2002). Molecular principles of animal development. Oxford University Press. [Google Scholar]
- Mather, K. (1953). Genetical control of stability in development. Heredity, 7(3), 297–336. [Google Scholar]
- Maxwell, E. E. , & Harrison, L. B. (2009). Methods for the analysis of developmental sequence data. Evolution & Development, 11(1), 109–119. [DOI] [PubMed] [Google Scholar]
- Maynard Smith, J (1983). Evolution and development. Cambridge University Press. [Google Scholar]
- Mayr, E. (1982). The growth of biological thought: Diversity, evolution, and inheritance. Belknap Press. [Google Scholar]
- Mayr, E. (2004). What makes biology unique?, Considerations on the autonomy of a scientific discipline. Cambridge University Press. [Google Scholar]
- Meckel, J. F. (1811). Beyträge zur vergleichenden Anatomie (Vol. 2). Reclam. [Google Scholar]
- Minelli, A. , & Schram, F. R. (1994). Owen revisited: A reappraisal of morphology in evolutionary biology. Bijdr.Dierk, 64, 65–74. [Google Scholar]
- Mitgutsch, C. , & Maienschein, J. (2003). On Carl Gegenbaur's theory on head metamerism and the selection of taxa for comparisons. Theory in Biosciences, 122(2), 204–229. [Google Scholar]
- Mivart, S. G. (1879). On the fins of Elasmobranchii. Transactions of the Zoological Society of London, 10, 439–484. [Google Scholar]
- Monsoro‐Burq, A. H. , & Levin, M. (2018). Avian models and the study of invariant asymmetry: How the chicken and the egg taught us to tell right from left. International Journal of Developmental Biology, 62(1‐2‐3), 63–77. [DOI] [PubMed] [Google Scholar]
- Morse, E. S. (1874). On the tarsus and carpus of birds. Annals of the Lyceum of Natural History of New York, 10, 141–158. [Google Scholar]
- Müller, F. (1864). Für Darwin. Engelmann. [Google Scholar]
- Müller, F. D. , & Dallas, W. S. (1869). Facts and arguments for Darwin. Murray. [Google Scholar]
- Murray, B. G. (2001). Are ecological and evolutionary theories scientific? Biological Reviews, 76, 255–289. [DOI] [PubMed] [Google Scholar]
- Naef, A. (1917). Die individuelle entwicklung organischer formen als urkunde ihrer stammesgeschichte: (Kritische betrachtungen ber das sogenannte "biogenetische grundgesetz"). Verlag von Gustav Fischer. [Google Scholar]
- Newton, I. (1718). Opticks: or, a treatise of the reflections, inflections and colours of light. Dover. [Google Scholar]
- Newton, I. (1729). The mathematical principles of natural philosophy (Eng. transl.). Motte. [Google Scholar]
- Nguyen, D. H. , Jaszczak, R. G. , & Laird, D. J. (2019). Heterogeneity of primordial germ cells. In R. Lehmann (Ed.), Current Topics in Developmental Biology (135, pp. 155–201). Academic Press; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys, R. , & Puelles, L. (2016). Towards a new neuromorphology. In R. N., & L. P. (Eds.), The Natural Coordinate System of the CNS (pp. 132‐140). Springer. [Google Scholar]
- Niklas, K. J. , Cobb, E. D. , & Kutschera, U. (2016). Haeckel's biogenetic law and the land plant phylotypic stage. BioScience, 66(6), 510–519. [Google Scholar]
- Noordermeer, D. , Leleu, M. , Schorderet, P. , Joye, E. , Chabaud, F. , & Duboule, D. (2014). Temporal dynamics and developmental memory of 3D chromatin architecture at Hox gene loci. eLife, 3, e02557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogle, W. (1911). De partibus animalium. Clarendon Press. [Google Scholar]
- Oken, L. (1807). Über die bedeutung der schädelknochen. Isis. [Google Scholar]
- Oken, L. (1818). Isis, oder, Encyclopädische Zeitung von Oken (Vol. Bd.2‐3=Jahrgang 1818:Bd.1‐2:Heft.1‐12. Isis. [Google Scholar]
- Olsson, L. , Levit, G. S. , & Hossfeld, U. (2017). The "Biogenetic Law" in zoology: From Ernst Haeckel's formulation to current approaches. Theory in Biosciences, 136(1‐2), 19–29. [DOI] [PubMed] [Google Scholar]
- Oppenheimer, J. M. , & Hamburger, V. (1976). The non‐specificity of the germ‐layers. The Quarterly Review of Biology, 51, 96–124. [Google Scholar]
- Osawa, S. , Muto, A. , Jukes, T. H. , Ohama, T. , & Clarke, B. C. (1990). Evolutionary changes in the genetic code. Proceedings of the Royal Society of London. Series B: Biological Sciences, 241(1300), 19–28. [DOI] [PubMed] [Google Scholar]
- Ospovat, D. (1976). The influence of Karl Ernst von Baer's embryology, 1828‐1859: A reappraisal in light of Richard Owen's and William B. Carpenter's "palaeontological application of 'Von Baer's Law". Journal of the History of Biology, 9(1), 1–28. [DOI] [PubMed] [Google Scholar]
- Owen, R. (1835). On the osteology of the chimpanzee and orang utan. The Transactions of the Zoological Society of London, 1, 343–379. [Google Scholar]
- Owen, R. (1843). Lectures on comparative anatomy, Hunterian lectures (p. 392). Longman, Brown. [Google Scholar]
- Owen, R. (1847). Report on the archetype and homologies of the vertebrate skeleton. Report of the British Association for the Advancement of Science (for 1846).
- Owen, R. (1866). On the anatomy of vertebrates. Vol I: Fishes and reptiles. Longmans, Green, & Co. [Google Scholar]
- Palmer, A. R. , & Strobeck, C. (1986). Fluctuating asymmetry: Measurement, analysis, patterns. Annual Review of Ecology and Systematics, 17, 391–421. [Google Scholar]
- Pander, C. H. (1817). Beiträge zur Entwickelungsgeschichte des Hühnchens im Eye. Würzburg: [s.n.].
- Parker, T. J. (1891). Observations on the anatomy and development of Apteryx. Phil Trans Roy Soc Lond (B). 182, 25–134. [Google Scholar]
- Pascoal, S. , Carvalho, C. R. , Rodriguez‐León, J. , Delfini, M. C. , Duprez, D. , Thorsteinsdóttir, S. , & Palmeirim, I. (2007). A molecular clock operates during chick autopod proximal‐distal outgrowth. Journal of Molecular Biology, 368(2), 303–309. [DOI] [PubMed] [Google Scholar]
- DALENPATIUS . (1699). Extraite d'une lettre de M. Dalenpatius l'auteur de ces Nouvelles, contenant une decouverte curieuse, faite par le moyen du microscope. Nouvelles de la Reipublique des Lettres, 552–554. [Google Scholar]
- Pickover, C. A. (2008). Archimedes to Hawking laws of science and the great minds behind them. Oxford University Press. [Google Scholar]
- Pigliucci, M. , & Muller, G. (2010). Evolution: The extended synthesis. MIT Press; [Google Scholar]
- Popper, K. R. (1962). Conjectures and refutations: The growth of scientific knowledge. Basic Books. [Google Scholar]
- Post, Y. , Puschhof, J. , Beumer, J. , Kerkkamp, H. M. , de Bakker, M. A. G. , Slagboom, J. , & Clevers, H. (2020). Snake venom gland organoids. Cell, 180(2), 233–247e221. [DOI] [PubMed] [Google Scholar]
- Prud'homme, B. , Gompel, N. , & Carroll, S. B. (2007). Emerging principles of regulatory evolution. Proceedings of the National Academy of Sciences, 104(Suppl 1), 8605–8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles, L. (2018). Developmental studies of avian brain organization. International Journal of Developmental Biology, 62(1–3), 207–224. [DOI] [PubMed] [Google Scholar]
- Quint, M. , Drost, H. G. , Gabel, A. , Ullrich, K. K. , Bonn, M. , & Grosse, I. (2012). A transcriptomic hourglass in plant embryogenesis. Nature, 490(7418), 98–101. [DOI] [PubMed] [Google Scholar]
- Rabl, C. (1897). Theorie des Mesoderms. Erster Band (Vol. 1). Engelmann. [Google Scholar]
- Rádl, E. (1909). Geschichte der biologischen Theorien in der Neuzeit. II. Teil: Geschichte der Entwicklungstheorien in der Biologie des XIX. Jahrhunderts. Engelmann. [Google Scholar]
- Raff, R. A. (1996). The shape of life: Genes, development and the evolution of animal form.. University of Chicago Press. [Google Scholar]
- Redies, C. , & Puelles, L. (2001). Modularity in vertebrate brain development and evolution. BioEssays, 23, 1100–1111. [DOI] [PubMed] [Google Scholar]
- Remak, R. (1855). Untersuchungen über die entwicklung der wirbelthiere. Reimer. [Google Scholar]
- Rengaraj, D. , Hwang, Y. S. , Lee, H. C. , & Han, J. Y. (2020). Zygotic genome activation in the chicken: A comparative review. Cellular and Molecular Life Sciences, 77(10), 1879–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensch, B. (1960). The Laws of Evolution. In (Ed.) Tax, S. , Evolution after Darwin. The University of Chicago Centennial. Volume 1. The evolution of life, its origin, history and future (pp. 95–116). University of Chicago Press; [Google Scholar]
- Richards, R. J. (2002). The romantic conception of life: Science and philosophy in the age of Goethe. University of Chicago Press. [Google Scholar]
- Richardson, M. K. (1995). Heterochrony and the phylotypic period. Developmental Biology, 172(2), 412–421. [DOI] [PubMed] [Google Scholar]
- Richardson, M. K. (1999). Vertebrate evolution: The developmental origins of adult variation. BioEssays, 21(7), 604–613. [DOI] [PubMed] [Google Scholar]
- Richardson, M. K. (2009). Diffusible gradients are out ‐ An interview with Lewis Wolpert. International Journal of Developmental Biology 53, 659–662. [DOI] [PubMed] [Google Scholar]
- Richardson, M. K. (2012a). Manus horribilis: The chicken wing skeleton. In Asher R. J., & Mller J. (Eds.), From clone to bone ‐ The synergy of morphological and molecular tools in palaeobiology. Cambridge University Press. [Google Scholar]
- Richardson, M. K. (2012b). A phylotypic stage for all animals? Developmental Cell, 22, 903–904. [DOI] [PubMed] [Google Scholar]
- Richardson, M. K. , & Keuck, G. (2002). Haeckel's ABC of evolution and development. Biological Reviews, 77(4), 495–528. [DOI] [PubMed] [Google Scholar]
- Richardson, M. K. , & Oelschlager, H. H. (2002). Time, pattern, and heterochrony: A study of hyperphalangy in the dolphin embryo flipper. Evol.Dev, 4(6), 435–444. [DOI] [PubMed] [Google Scholar]
- Richardson, M. K. , & Chipman, A. D. (2003). Developmental constraints in a comparative framework: A test case using variations in phalanx number during amniote evolution. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution, 296(1), 8–22. [DOI] [PubMed] [Google Scholar]
- Richardson, M. K. , & Keuck, G. (2021). The revolutionary developmental biology of Wilhelm His Sr. Biological Reviews. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, M. K. , Hornbruch, A. , & Wolpert, L. (1989). Pigment pattern expression in the plumage of the quail embryo and the quail‐chick chimera. Development, 107, 805–818. [Google Scholar]
- Richardson, M. K. , Hornbruch, A. , & Wolpert, L. (1991). Pigment patterns in neural crest chimeras constructed from quail and guinea fowl embryos. Developmental Biology, 143(2), 309–319. [DOI] [PubMed] [Google Scholar]
- Richardson, M. K. , Jeffery, J. E. , & Tabin, C. J. (2004). Proximodistal patterning of the limb: insights from evolutionary morphology. Evolution and Development, 6(1), 1–5. [DOI] [PubMed] [Google Scholar]
- Richardson, M. K. , Minelli, A. , Coates, M. , & Hanken, J. (1998). Phylotypic stage theory. Trends in Ecology & Evolution 13, 158. [DOI] [PubMed] [Google Scholar]
- Richardson, M. K. , Jeffery, J. E. , Coates, M. I. , & Bininda‐Emonds, O. R. (2001). Comparative methods in developmental biology. Zoology.(Jena), 104(3‐4), 278–283. [DOI] [PubMed] [Google Scholar]
- Richardson, M. K. , Allen, S. P. , Wright, G. M. , Raynaud, A. , & Hanken, J. (1998). Somite number and vertebrate evolution. Development, 125(2), 151–160. [DOI] [PubMed] [Google Scholar]
- Richardson, M. K. , Hanken, J. , Gooneratne, M. L. , Pieau, C. , Raynaud, A. , Selwood, L. , & Wright, G. M. (1997). There is no highly conserved embryonic stage in the vertebrates: implications for current theories of evolution and development. Anatomy and Embryology, 196(2), 91–106. [DOI] [PubMed] [Google Scholar]
- Riddle, R. D. , Johnson, R. L. , Laufer, E. , & Tabin, C. (1993). Sonic hedgehog mediates the polarizing activity of the ZPA. Cell, 75(7), 1401–1416. [DOI] [PubMed] [Google Scholar]
- Rieppel, O. C. (1988). Fundamentals of comparative biology. Birkhäuser. [Google Scholar]
- Robertis, E. M. D. (2008). Evo‐devo: Variations on ancestral themes. Cell, 132(2), 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertis, E. M. D. , & Sasai, Y. (1996). A common plan for dorsoventral patterning in Bilateria. Nature, 380(6569), 37–40. [DOI] [PubMed] [Google Scholar]
- Roe, S. A. (1979). Rationalism and embryology: Caspar Friedrich Wolff's theory of epigenesis. Journal of the History of Biology, 12(1), 1–43. [DOI] [PubMed] [Google Scholar]
- Rosch, E. H. (1973). Natural categories. Cognitive Psychology, 4(3), 328–350. [Google Scholar]
- Rosenberg, A. (1985). The structure of biological science. Cambridge University Press. [Google Scholar]
- Rossello‐Mora, R. , & Amann, R. (2001). The species concept for prokaryotes. FEMS Microbiology Reviews, 25(1), 39–67. [DOI] [PubMed] [Google Scholar]
- Roush, W. , & Pennisi, E. (1997). Growing pains: Evo‐devo researchers straddle cultures. Science, 277(5322), 38–39. [DOI] [PubMed] [Google Scholar]
- Roux, W. (1894). Einleitung. Archiv für Entwicklungsmechanik der Organismen, 1, 1–42. [Google Scholar]
- Roux, W. (1895a). Gesammelte abhandlungen über entwickelungsmechanik der organismen, Abhandlung XIII‐XXXIII: Über entwickelungsmechanik des embryo (Vol. 2). Wilhelm Engelmann. [Google Scholar]
- Roux, W. (1895b). Gesammelte abhandlungen über entwickelungsmechanik der organismen. Volume 2. Abhandlung XIII‐XXXIII: Über entwickelungsmechanik des embryo. Nr. 13. Einleitung zu den Beiträgen zur Entwickelungsmechanik des Embryo. Repr from Zeitschrift für Biologie Bd. XXI. 1885. (Vol. 2, pp. 2–23). Leipzig, Engelmann. [Google Scholar]
- Rubenstein, J. L. , Martinez, S. , Shimamura, K. , & Puelles, L. (1994). The embryonic vertebrate forebrain: The prosomeric model. Science, 266(5185), 578–580. [DOI] [PubMed] [Google Scholar]
- Rucklin, M. , & Donoghue, P. C. (2015). Romundina and the evolutionary origin of teeth. Biology Letters, 11(6), 20150326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruse, M. (1973). The philosophy of biology. Hutchinson University Library. [Google Scholar]
- Russell, E. S. (1916). Form and function: A contribution to the history of animal morphology. John Murray. [Google Scholar]
- Sánchez, M. R. (2012). Embryos in Deep Time: the Rock Record of Biological Development.
- Sander, K. (1991). Landmarks in developmental biology. Wilhelm Roux and his programme for developmental biology. Roux's Archives of Developmental Biology, 200(1), 1–3. [DOI] [PubMed] [Google Scholar]
- Sander, K. (1996). Pattern formation in insect embryogenesis: The evolution of concepts and mechanisms. International Journal of Insect Morphology and Embryology, 25(4), 349–367. [Google Scholar]
- Sanger, T. J. , Gredler, M. L. , & Cohn, M. J. (2015). Resurrecting embryos of the tuatara, Sphenodon punctatus, to resolve vertebrate phallus evolution. Biology Letters, 11(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick, A. (1894). On the law of development commonly known as von Baer's law; and on the significance of ancestral rudiments in embryonic development. Quarterly Journal of Microscopical Science, 36, 35–52. [Google Scholar]
- Seidel, F. (1953). Körpergrundgestalt und organbildung. De Gruyter. [Google Scholar]
- Semper, C. (1874). Ueber die Stammverwandtschaft der Wirbelthiere und Anneliden. Centralblatt fur die Medicinischen Wissenschaften, 12, 545–547. [Google Scholar]
- Sengupta, S. , & Higgs, P. G. (2015). Pathways of genetic code evolution in ancient and modern organisms. Journal of Molecular Evolution, 80(5‐6), 229–243. [DOI] [PubMed] [Google Scholar]
- Serres, E. R. A. (1830). Anatomie transcendante. Quatrième Mémoire. Loi de symétrie et de conjugaison du système sanguin. Annales des Sciences Naturelles, 21, 5–49. [Google Scholar]
- Serres, E. R. A. (1860). Principes d'embryogénie, de zoogénie et de tératogénie. Mémoires de l'Académie des Sciences de l'institut Impérial de France, 25, 942. [Google Scholar]
- Serres, M. (1827). Théorie des formations organiques. Annales des Sciences Naturelles, 12, 82. [Google Scholar]
- Slack, J. M. W. (2012). Essential developmental biology (3 ed.). Wiley. [Google Scholar]
- Slack, J. M., Holland, P. W. & Graham, C. F. (1993). The zootype and the phylotypic stage. Nature, 361, 490–492. [DOI] [PubMed] [Google Scholar]
- Smart, J. J. C. (1963). Philosophy and scientific realism. Routledge & Kegan Paul. [Google Scholar]
- Smyth, D. R. (2018). Evolution and genetic control of the floral ground plan. New Phytologist, 220(1), 70–86. [DOI] [PubMed] [Google Scholar]
- Sneath, P. H. , & Sokal, R. R. (1962). Numerical taxonomy. Nature, 193, 855–860. [DOI] [PubMed] [Google Scholar]
- Solnica‐Krezel, L. (2020). Maternal contributions to gastrulation in zebrafish. Current Topics in Developmental Biology, 140, 391–427. [DOI] [PubMed] [Google Scholar]
- Sordino, P. , van der Hoeven, F. , & Duboule, D. (1995). Hox gene expression in teleost fins and the origin of vertebrate digits. Nature, 375(6533), 678–681. [DOI] [PubMed] [Google Scholar]
- Stephens, T. D. , & Strecker, T. R. (1985). Radial condensation in the axis of the evolving limb. Evolution, 39(5), 1159–1163. [DOI] [PubMed] [Google Scholar]
- Stewart, T. A. , Liang, C. , Cotney, J. L. , Noonan, J. P. , Sanger, T. J. , & Wagner, G. P. (2019). Evidence against tetrapod‐wide digit identities and for a limited frame shift in bird wings. Nature Communications, 10(1), 3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerbell, D. , & Lewis, J. H. (1975). Time, place and positional value in the chick limb‐bud. Journal of Embryology and Experimental Morphology, 33(3), 621–643. [PubMed] [Google Scholar]
- Summerbell, D. , Lewis, J. H. , & Wolpert, L. (1973). Positional information in chick limb morphogenesis. Nature, 244, 492–496. [DOI] [PubMed] [Google Scholar]
- Tabin, C. (2009). Molecular tools, classic questions–An interview with Clifford Tabin. Interviewed by Richardson, Michael K. International Journal of Developmental Biology 53(5–6), 725–731. [DOI] [PubMed] [Google Scholar]
- Tabin, C. J. (1992). Why we have (only) five fingers per hand: Hox genes and the evolution of paired limbs. Development, 116(2), 289–296. [DOI] [PubMed] [Google Scholar]
- Tena, J. J. , González‐Aguilera, C. , Fernández‐Miñán, A. , Vázquez‐Marín, J. , Parra‐Acero, H. , Cross, J. W. , Rigby, P. W. , Carvajal, J. J. , Wittbrodt, J. , Gómez‐Skarmeta, J. L. , & Martínez‐Morales, J. R. (2014). Comparative epigenomics in distantly related teleost species identifies conserved cis‐regulatory nodes active during the vertebrate phylotypic period. Genome Research, 24(7), 1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacher, J. K. (1877). Median and paired fins, a contribution to the history of vertebrate limbs. Transactions of the Connecticut Academy of Arts and Sciences, 3, 281–310. [Google Scholar]
- Thompson, D. W. (1910). Historia animalium (Vol. 4). Clarendon Press. [Google Scholar]
- Thornhill, R. , & Furlow, B. (1998). Stress and human reproductive behavior: Attractiveness, women's sexual development, postpartum depression, and baby's cry. In (Eds.) Møller, A. P. , Milinski, M. & Slater, P. J. B. , Advances in the study of behavior (27, pp. 319–369). Academic Press; [Google Scholar]
- Tschopp, P. , Sherratt, E. , Sanger, T. J. , Groner, A. C. , Aspiras, A. C. , Hu, J. K. , Pourquié, O. , Gros, J. , & Tabin, C. J. (2014). A relative shift in cloacal location repositions external genitalia in amniote evolution. Nature, 516(7531), 391–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker, A. , & Sharpe, P. (2004). The cutting‐edge of mammalian development; how the embryo makes teeth. Nature Reviews Genetics, 5(7), 499–508. [DOI] [PubMed] [Google Scholar]
- Tucker, A. S. , & Fraser, G. J. (2014). Evolution and developmental diversity of tooth regeneration. Seminars in Cell & Developmental Biology, 25‐26, 71–80. [DOI] [PubMed] [Google Scholar]
- Tuomi, J. (1981). Structure and dynamics of Darwinian evolutionary theory. Systematic Biology, 30(1), 22–31. [Google Scholar]
- Van Valen, L. (1973). A new evolutionary law. Evolutionary Theory, 1, 1–30. [Google Scholar]
- Vargas, A. O. , Kohlsdorf, T. , Fallon, J. F. , Vandenbrooks, J. , & Wagner, G. P. (2008). The evolution of HoxD‐11 expression in the bird wing: Insights from Alligator mississippiensis. PLoS.ONE. 3(10), e3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon, K. (1988). The founding of numerical taxonomy. The British Journal for the History of Science, 21(2), 143–159. [Google Scholar]
- Vogt, C. T. (1858). Natürliche Geschichte Der Schöpfung Des Weltalls, Der Erde Und Der Auf Ihr Befindlichen Organismen. Vieweg. [Google Scholar]
- von Baer, K. E. (1828). Über entwickelungsgeschichte der thiere: Beobachtung und reflexion. Erster Theil. Bornträger. [Google Scholar]
- von Baer, K. E. , & Oppenheimer, J. M. (1986). Autobiography of Karl Ernst von Baer. Science History Publications. [Google Scholar]
- Vonk, F. J. , & Richardson, M. K. (2008). Developmental biology: Serpent clocks tick faster. Nature, 454(7202), 282–283. [DOI] [PubMed] [Google Scholar]
- Wagner, G. P. (1996). Homologues, natural kinds and the evolution of modularity. American Zoologist, 36, 36–43. [Google Scholar]
- Wagner, G. P. , & Gauthier, J. A. (1999). 1,2,3 = 2,3,4: A solution to the problem of the homology of the digits in the avian hand. Proceedings of the National Academy of Sciences of the United States of America, 96, 5111–5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, L. , Evans, S. E. , & Stern, C. D. (2017). A resegmentation‐shift model for vertebral patterning. Journal of Anatomy, 230(2), 290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse, G. R. (1848). A natural history of the mammalia. Hippolyte Baillière, 2. [Google Scholar]
- Waters, C. K. (1998). Causal regularities in the biological world of contingent distributions. Biology & Philosophy, 13, 5–36. [Google Scholar]
- Weinert, F. (1995). Laws of nature: Essays on the philosophical, scientific and historical dimensions. De Gruyter. [Google Scholar]
- Weismann, A. (1892). Die Continuität des Keimplasmas als grundlage einer Theorie der Vererbung: ein Vortrag (2 ed.). Fischer. [Google Scholar]
- Weismann, A. (1893). The germ‐plasm: A theory of heredity. Charles Scribner's Sons. [Google Scholar]
- Werneburg, I. (2009). A standard system to study vertebrate embryos. PLoS.ONE. 4(6), e5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmore, K. E. (2012). The body plan concept and its centrality in evo‐devo. Evolution: Education and Outreach, 5(2), 219–230. [Google Scholar]
- Winther, R. G. (2020). The structure of scientific theories. Stanford Encyclopedia of Philosophy. https://plato.stanford.edu/entries/structure‐scientific‐theories/
- Wolff, C. F. (1768). De formatione intestinorum (parts 1 and 2). Novi commentari academiae scientiarum imperialis petropolitanae, 12, 403–507. [Google Scholar]
- Wolff, C. F. (1769). De formatione intestinorum (part 3). Novi commentari academiae scientiarum imperialis petropolitanae, 13, 478–530. [Google Scholar]
- Wolff, C. F. (1812). Über die Bildung des Darmkanals im bebrüteten Hühnchen. Transl. J. F. Rengersche Buchhandlung. [Google Scholar]
- Wolpert, L. (1969). Positional information and the spatial pattern of cellular differentiation. Journal of Theoretical Biology, 25(1), 1–47. [DOI] [PubMed] [Google Scholar]
- Wolpert, L. (1994). The evolutionary origin of development: Cycles, patterning, privilege and continuity. Development. Supplement, 79–84. [PubMed] [Google Scholar]
- Woltering, J. M. , & Duboule, D. (2010). The origin of digits: Expression patterns versus regulatory mechanisms. Developmental Cell, 18(4), 526–532. [DOI] [PubMed] [Google Scholar]
- Yanai, I. (2018). Development and evolution through the lens of global gene regulation. Trends in Genetics, 34(1), 11–20. [DOI] [PubMed] [Google Scholar]


