Abstract
Aim of the study
Outcome of fetuses, prenatally diagnosed with sacrococcygeal teratoma (SCT), is still poorly documented. This study assesses the incidence and prenatal predictors of outcome in all fetuses prenatally diagnosed with SCT.
Methods
This is a retrospective study on all fetuses prenatally diagnosed with SCT from 1998 to 2018 in the Netherlands. Poor outcome was defined as terminations of pregnancy (TOP) because of expected unfavorable outcome, intrauterine fetal death, or early neonatal death. Potential risk factors for poor outcome were analyzed.
Main results
Eighty‐four fetuses were included. Sixteen (19.0%) TOPs were excluded from statistical analysis. Eleven of the remaining 68 fetuses had poor outcome. Overall mortality was 32.1%, with a mortality excluding TOPs of 13.1%. Thirteen fetal interventions were performed in 11 (13.1%) fetuses. Potential risk factors for poor outcome were the presence of fetal hydrops (OR: 21.0, CI: 2.6–275.1, p = 0.012) and cardiomegaly (OR: 10.3, CI: 1.9–55.8, p = 0.011).
Conclusions
The overall mortality of fetuses prenatally diagnosed with SCTs including tTOP was 32.1%. This high mortality rate was mainly due to termination of pregnancy. Mortality excluding TOP was 13.1%. Potential risk factors for poor outcome were fetal hydrops and cardiomegaly.
Key points
What's already known about the topic?
50%–82% of sacrococcygeal teratomas (SCTs) are currently diagnosed in the prenatal period
Perinatal mortality rate of prenatally diagnosed SCT varies between 13% and 50%
High tumor volume index (TVI), fast SCT growth rate, and predominantly solid, highly vascularized tumors are factors predictive for poor outcome
What does this study add?
The incidence of patients prenatally diagnosed with SCT is approximately one in 35,000 with a poor outcome in 13%
Fetal hydrops and cardiomegaly are both risk factors for poor outcome in fetuses prenatally diagnosed with SCT
1. INTRODUCTION
Sacrococcygeal teratoma (SCT) is the most common congenital neoplasm with an estimated incidence of 1 per 28,500 newborns in the Netherlands. 1 Children with postnatally diagnosed SCTs have a good prognosis after surgical resection, with only a small risk of demise due to perioperative hemorrhage during or soon after birth. 2 , 3 , 4 , 5
Malignant disease at the time of initial diagnosis occurs in 11%–35% of patients. 2 In many of these patients, the diagnosis SCT is only made several months or years after birth, due to a mainly internal position of the tumor. The likelihood of malignant transformation in SCT increases with age, resulting in malignancy rates up to 70% if SCT is diagnosed at the age of 1 year. 6
With the introduction of routine prenatal ultrasound, 50%–82% of SCTs are currently diagnosed in the prenatal period. 7 , 8 Fetuses with prenatally diagnosed SCTs have a high risk of death despite fetal interventions which may improve the outcome. 9 The perinatal mortality rate varies between 13% and 50%, and most of these deaths are attributed to cardiac failure, exsanguinating tumor hemorrhage, or both. 8 , 10 , 11 , 12 , 13 Moreover, 9%–24% of the parents choose termination of pregnancy (TOP). 9 , 14 , 15
Factors such as high tumor volume index (TVI), fast SCT growth rate, and predominantly solid, highly vascularized tumors are predictive for poor outcome, defined as prenatal or early neonatal death. 16 , 17 , 18 , 19 All these prognostic factors suggest cardiac overload from a high‐output status with fetal hydrops as the underlying pathophysiologic mechanism. Cardiac overload and hydrops are associated with poor outcome. 20 Most studies on the outcome of prenatally diagnosed SCT were conducted in single institutions with relatively small numbers of patients often with selection bias because some of the institutions were specialized centers for fetal treatment. 16 , 21 , 22
We aimed to determine the incidence and outcome of all cases with the prenatal diagnosis SCT in one of the seven Dutch Fetal Medicine Units (FMU) between January 1998 and December 2018 and to evaluate potential risk factors for poor outcome.
2. MATERIALS AND METHODS
2.1. Patients and study design
The medical records of all fetuses with the prenatal diagnosis SCT between January 1998 and December 2018 at the seven Fetal Medicine Units in the Netherlands (Amsterdam University Medical Centers, Erasmus Medical Center Rotterdam, Leiden University Medical Center, Maastricht University Medical Center, St. Radboud University Medical Center Nijmegen, University Medical Center Groningen, University Medical Center Utrecht) were studied retrospectively. In all centers, Astraia (Astraia Software GMBH, Munich), MososU (ICT Healthcare Technology Solutions, Houten), or HiX (Hospital information eXchange, Chipsoft, Amsterdam) software were used for patient registration. Clinical and outcome data were collected from the original medical records. Medical records contained information retrieved by systematically conducted ultrasound.
All patients with complete follow‐up until TOP, intrauterine fetal death (IUFD), or live birth were included in the study. TOPs were excluded from statistical analysis unless the medical record clearly stated that the parent's decision for TOP was based on expected unfavorable outcome. The patients included in statistical analysis were divided in poor outcome and good outcome groups.
2.2. Statistical analysis and definitions
The incidence of fetuses prenatally diagnosed with SCT was calculated per decade by dividing the number of fetuses by the number of births in the Netherlands. 23 Poor outcome was defined as IUFD, early neonatal death (ENND), or TOP, if parent's decision was based on expected non‐vital outcome. Intrauterine fetal death was defined as an infant delivered without signs of life after 24 weeks of gestation. 24 Univariate and multivariate relative risk analyses for outcome were carried out for variables measurable before birth, using logistic regression. Risk factors investigated, with their respective categories, were Altman classification (grade I and II vs. III and IV), 25 gestational age (GA) at diagnosis (<24, ≥24), morphology (cystic, solid), cardiomegaly (yes, no), polyhydramnios (yes, no), fetal hydrops (yes, no), and highly vascularized tumor (yes, no). Tumor location was categorized according to Altman's classification determined by prenatal ultrasound. Polyhydramnios was defined as a single deepest vertical amniotic pocket diameter of >8 cm. The presence of signs of fetal hydrops was defined as presence of fetal subcutaneous tissue edema accompanied by abnormal fluid collection in more than one other area of the fetal body. Vascularity was determined by Doppler ultrasound. TVI was calculated as the largest diameter of the tumor divided by the head circumference, to adjust for gestational age.
Categorical variables were compared with Fisher's exact test. Statistical analyses were performed using SPSS for Windows version 25.0 software (SPSS) and Graph Pad Prism 8 (GraphPad Sofware, Incl.). Unless stated otherwise, data are expressed as mean (SD). P < 0.05 was considered statistically significant.
According to the Medical Ethical Board of AMC, the Medical Research Involving Human Subjects Act (WMO) does not apply to the study and official approval of the committee was not required (reference number: W19_112 # 19.145).
3. RESULTS
3.1. Patient characteristics
From 1998 to 2018, 88 fetuses were suspected to have SCT in one of the seven Dutch Fetal Medicine Units (Figure 1). The calculated incidence was 1 in 69,174 fetuses in 1998–2008 and 1 in 34,355 in 2009–2018.
FIGURE 1.

The outcomes of prenatally diagnosed sacrococcygeal teratoma (1998–2018)
Four fetuses that had not been followed until TOP, IUFD, or live birth owing to maternal transfer were excluded from the study, so 84 fetuses with the prenatal diagnosis SCT were included in the study.
Eighteen were male (21.4%) and 62 were female (73.8%). In four (4.8%), sex was unknown. Two cases were multiple pregnancies: one trichorionic, triamniotic triplet and one monochorionic, diamniotic twin; in both cases, only one fetus was affected.
Eighteen out of 84 (21.4%) pregnancies were terminated before GA 23 weeks. In two cases, the parent's decision for termination was based on expected unfavorable outcome due to extensive tumor growth and were therefore included in statistical analysis. In the other 16 fetuses, the reason for the parent's decision of termination was unknown. These 16 fetuses were therefore excluded from statistical analyses. Two fetuses (2.4%) died in utero, both at GA 25 weeks. Sixty‐four fetuses (76.2%) were born alive.
Twenty fetuses (31.2%) were born before GA 37 weeks, 38 fetuses (59.4%) were delivered at term, and in 6 fetuses (9.4%) GA at birth was unknown. Twenty‐eight fetuses (43.7%) were born vaginally, 30 (46.9%) were born by cesarean section and in 6 (9.4%) mode of delivery was unknown. Their mean GA at diagnosis was 22.5 (SD 4.9) weeks and their mean GA at birth was 36.2 (SD 4.1), with a mean birth weight of 3.1 (SD 0.95) kg.
Eleven fetuses (13.1%) had associated anomalies which may be attributed to the SCT including genitourinary (n = 5; hydronephrosis and bladder dilation) and anorectal malformations (n = 3; anal atresia), musculoskeletal disorders (n = 2; clubfoot), and pulmonary disorder (n = 1; pulmonary hypoplasia). Furthermore, two (2.4%) fetuses had additional structural anomalies, neural (n = 1; spina bifida) and cardiovascular (n = 1; atrial and ventricular septal defect).
Fifty‐three fetuses (63.1%) had a predominantly external tumor (Altman I or II) (Figure 2) and 13 (15.5%) had a predominantly internal tumor (Altman III or IV) (Figure 3); in 7 fetuses (8.3%) distinction between predominantly external or internal tumor could not be made. In 11 (13.1%) fetuses, Altman classification was unknown.
FIGURE 2.
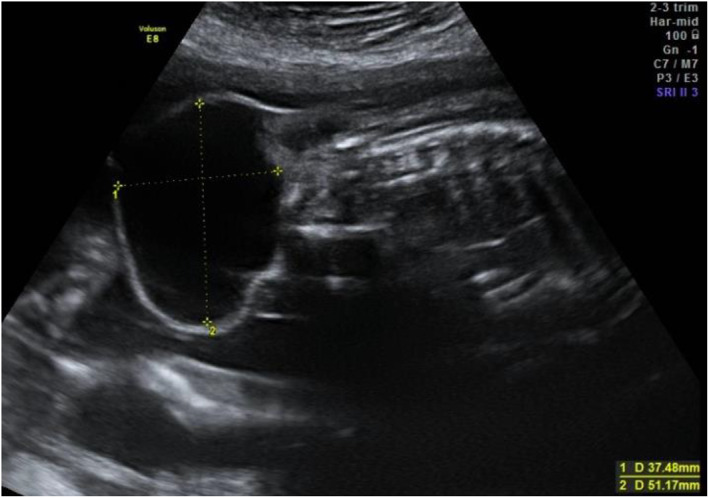
Ultrasound of a cystic type I sacrococcygeal teratoma at 22 weeks'’ gestation. The fetus was delivered with planned cesarean section at 38 weeks' gestation because of breech position. The baby survived
FIGURE 3.

Ultrasound of cystic Altman type III sacrococcygeal teratoma at 26 weeks' gestation. The fetus was born with a spontaneous vaginal delivery at 40 weeks' gestation. The baby survived
After resection and pathological evaluation, one fetus was diagnosed with infantile myofibroma and one fetus with lymphangioma instead of SCT. The fetus with lymphangioma had a good outcome and the fetus with infantile myofibroma died within the first week of life. So, two fetuses (2.4%) were prenatally misdiagnosed with SCT.
3.2. Fetal intervention
Thirteen fetal interventions were performed in 11 (13.1%) fetuses. In four, an intrauterine transfusion was performed because of anemia. Corresponding hemoglobin (Hb) values are described in Table 1. One fetus was transfused because of bleeding the tumor mass, two because of high‐output status from the vascular steal of the tumor, and in one fetus the reason for transfusion was unknown. Three of the four fetuses that received intrauterine transfusion had poor outcomes: one died in utero at GA 25 weeks and two fetuses died within 24 h after birth. The first fetus died due to intratumoral hemorrhage at GA 27 weeks (Figure 4) and the second fetus died at GA 31 weeks of an unspecified cause of death. In the last fetus, anemia was suspected; however, hemoglobin was 12.4 g/dl with a fetal middle cerebral arterial (MCA) peak systolic velocity (PSV) of 81 cm/s measured with Doppler. Furthermore, this fetus received amnioreduction of 2500 ml and drainage of a cystic SCT component. The fetus was born at GA 34 weeks and survived. One fetus received ultrasound guided interstitial laser coagulation at GA 25 weeks because of maternal mirror syndrome. Vascularization could be well visualized on ultrasound; however, the base of the vessels was located within the small pelvis. Therefore, not all vessels could be coagulated. The fetus was delivered at GA 27 weeks and died shortly after birth. The mother survived with no remaining symptoms. Six other fetuses (46%) survived: in the first fetus, the cystic SCT was punctured at GA 21 weeks. In the other three fetuses, cystic SCT was punctured prior to delivery at GA 31 weeks, GA 38 weeks, and GA 40 weeks, respectively. In the other two fetuses, amniodrainage of 1800–1850 ml was performed because of polyhydramnios.
TABLE 1.
Intrauterine transfusion
| GA | Transfusion | Hb‐prior (g/dl) | Hb‐post (g/dl) | Outcome |
|---|---|---|---|---|
| 25 + 0 | Unknown | Unknown | 7.6 | IUFD at GA 25 + 1 |
| 26 + 3 | 65 ml erythrocyte concentrate | 4.0 | 9.3 | ENND at GA 27 + 0 |
| 28 + 0 | 30 ml erythrocyte concentrate in hepatic vein, 30 ml intraperitoneal | 9.5 | 12.2 | ENND at GA 31 + 1 |
| 33 + 5 | 68 ml erythrocyte concentrate | 12.4 | 15.3 | Labor induction at GA 34 + 3, survived |
Abbreviations: ENND, early neonatal death; GA, gestational age; Hb, hemoglobin; IUFD, intrauterine fetal death.
FIGURE 4.

Ultrasound of type I hypervascular sacrococcygeal teratoma consisting of cystic and solid components at 21 weeks' gestation. This fetus received intrauterine transfusion at 26 weeks because of anemia. At 27 weeks, the fetus was delivered by cesarean section and died shortly after birth due to intratumor hemorrhage
3.3. Poor outcome
In two cases (2.4%), parents chose TOP because of expected unfavorable outcome: one because of fast tumor growth of a highly vascularized type I SCT with 22‐mm increase in diameter from 20 to 22 weeks gestation. In the other fetuses, the TOP was because of a high risk of fetal hydrops due to a highly vascularized tumor with a diameter of 57 mm at 19 weeks gestation. Two fetuses (2.4%) died in utero, both at GA 25 weeks because of heart failure, one of whom received an intrauterine transfusion because of anemia and died 1 day after it. Seven (8.3%) of 64 surviving neonates died in the first week after birth because of heart failure and hemorrhagic shock. Table 2 shows patient‐ and disease‐related characteristics of the 11 fetuses with poor outcome.
TABLE 2.
Characteristics of fetuses with poor outcome
| Sex | Year of diagnosis | GA diagnosis (weeks) | Altman type | Maximal tumor diameter (cm) (GA) | Estimated tumor volume (cm3) (GA) | Fetal intervention (GA) | GA at death (weeks) | Outcome | Cause of death |
|---|---|---|---|---|---|---|---|---|---|
| Female | 2001 | 19 | Type I/II | 5.2 (22 weeks) | 105 (22 weeks) | None | 22 | TOP | Rapid SCT growth |
| Unknown | 2001 | 19 | Type I/II | 5.7 (19 weeks) | Unknown | None | 19 | TOP | Highly vascularized SCT |
| Male | 2002 | 32 | Type I/II | 10.2 (32 weeks) | 734 (32 weeks) | None | Unknown | ENND | Heart failure: death during operation |
| Female | 2003 | 29 | Type I/II | Unknown | Unknown | None | 30 | ENND | Hemorrhagic shock |
| Female | 2007 | 21 | Type I/II | 13.2 (26 weeks) | 1932 (26 weeks) | Interstitial laser coagulation (25 weeks) | 27 | ENND | Heart failure |
| Female | 2008 | 21 | Type I/II | 12.5 (26 weeks) | 1460 (24 weeks) | None | 25 | IUFD | Heart failure |
| Male | 2012 | 20 | Type I/II | 9.8 (24 weeks) | Unknown | Transfusion because of anemia (25 weeks) | 25 | IUFD | Unknown |
| Female | 2012 | 20 | Type I/II | Unknown | Unknown | None | 26 | ENND | Unknown |
| Female | 2013 | 21 | Type I/II | 9.0 (26 weeks) | 486 (26 weeks) | Transfusion because of anemia (27 weeks) | 27 | ENND | Intratumor hemorrhage and fetal anemia |
| Female | 2016 | 19 | Type I/II | 8.6 (26 weeks) | 516 (26 weeks) | Transfusion because of anemia (26 weeks) | 31 | ENND | Unknown |
| Female | 2018 | 24 | Type I/II | Unknown | 205 (24 weeks) | None | 28 | ENND | Unknown |
Abbreviations: ENND, early neonatal death; GA, gestational age; SCT, sacrococcygeal teratoma; TOP, termination of pregnancy.
3.4. Risk factors for poor outcome
In 68 fetuses, risk factors for poor outcome were analyzed with statistical analysis. In a univariate analysis, fetuses with cardiomegaly (P = 0.011) and fetal hydrops (P = 0.012) were at increased risk of poor outcome. Both cardiomegaly and fetal hydrops remained significant when the statistical analysis was adjusted for other factors (Table 3) in multivariate analysis. Other factors, including Altman classification, ultrasound aspect of the SCT, GA at diagnosis, polyhydramnios, and a highly vascularized tumor were not predictive for poor outcome in fetuses with the prenatally diagnosis SCT.
TABLE 3.
Patient‐ and disease‐related characteristics associated with poor outcome
| Poor outcome rate | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| Odds ratio | p‐Value | Odds ratio | p‐Value | ||
| Altman classification | |||||
| I and II | 8 of 42 | ‐ | 0.176 | ||
| III and IV | 0 of 13 | 1 | |||
| Morphology | |||||
| Cystic | 5 of 28 | 1 | |||
| Solid | 2 of 3 | 3.152 (0.560, 17.758) | 0.176 | ||
| GA diagnosis (weeks) | |||||
| <24 | 9 of 46 | 2.311 (0.4871–11.40) | 0.481 | ||
| ≥24 | 2 of 21 | 1 | |||
| Cardiomegaly | |||||
| Yes | 4 of 7 | 10.29 (1.9–55.8) | 0.011* | 7.0 (1.1–0.45.5) | 0.039* |
| No | 7 of 61 | 1 | 1 | ||
| Fetal hydrops | |||||
| Yes | 3 of 4 | 21.00 (2.63–275.1) | 0.012* | 13.15 (1.0–166.7) | 0.048* |
| No | 8 of 64 | 1 | 1 | ||
| Polyhydramnios | |||||
| Yes | 5 of 25 | 1.542 (0.4466–5.246) | 0.5166 | ||
| No | 6 of 43 | 1 | |||
| Highly vascularized tumor | |||||
| Yes | 4 of 15 | 2.390 (0.6769–9.101) | 0.2426 | ||
| No | 7 of 53 | 1 | |||
Note: * Indicates statistical significance.
Abbreviation: GA, gestational age.
3.5. Tumor volume index
Tumor volume was measured throughout pregnancy in all patients included in the study. TVI of TOPs was excluded from TVI analysis. Figure 5 compares the TVI between the good and poor outcome groups. TVI was compared between the two groups at multiple gestational ages. In fetuses with poor outcome, TVI increased more during pregnancy compared to TVI of the good outcome group. TVI of the good outcome group remained around 0.3 during pregnancy.
FIGURE 5.

Tumor volume index per gestational age for good and poor outcomes
4. DISCUSSION
In this large cohort study, we found an incidence of fetuses prenatally diagnosed with SCT of one in 69,174 fetuses in the period 1998–2008 and one in 34,355 in 2009–2018. The overall mortality of fetuses with the prenatal diagnosis SCT in our study was 32.1% (27/84). After the exclusion of terminations of pregnancy, mortality was 13.1% (11/84). In the present study, cardiomegaly and fetal hydrops were significant risk factors for poor outcome. Both risk factors remained significant in multivariate analysis. Altman classification, polyhydramnios, highly vascularized tumor, morphology, and GA at diagnosis had no association with poor outcome. TVI increased more during pregnancy in the poor outcome group, compared to the good outcome group.
Others report a higher incidence of SCT, ranging from 1; 10,700 to 1; 27,500 births. 7 , 26 , 27 , 28 The incidence of SCT that was found is probably higher as SCT may be missed on prenatal ultrasound, mainly because some diagnoses may be overlooked, particularly in type IV SCT cases. Prevalence may be higher, owing to the fact that a few women decline ultrasound examination and the screening policy changed during the study. Before 2007, there was no routine ultrasound in the Netherlands and referrals were based on occasional findings. Since 2007 a routine 20‐week scan is offered with an uptake of about 98%. 29 Alternatively, the tumor may be less frequent in our population.
The mortality of fetuses prenatally diagnosed with SCT excluding TOP varies in different studies from 16% to 46%. 9 , 20 The relatively low mortality rate (16.2%) in our study may be influenced by advances made in maternal and fetal management over the last 20 years. 7 Fetal management options have improved and included intrauterine transfusion, interstitial laser coagulation, SCT cyst puncture, and amnioreduction in our study group. This may have prevented fetal death in some and has facilitated delivery in others. However, there were no strict criteria for fetal therapy.
Moreover, the effect of selection bias in some institutions could affect mortality rates, as some studies were conducted in highly specialized fetal medicine and surgery centers. 16 , 21 , 22 Last, more parents could have chosen TOP because of expected unfavorable outcome; however, these fetuses were not included in analyses because it was not clearly stated in the medical record.
Risk factors related to poor outcome in fetuses prenatally diagnosed with SCTs can be divided into tumor‐related and patient‐related factors. 30 Tumor size and tumor growth rate as well as tumor morphology (solid/cystic) may be risk factors correlated to poor outcome. 8 , 14 , 21 , 22 , 31 , 32 , 33 , 34 , 35 , 36
Cardiomegaly and fetal hydrops have also been reported as risk factors for poor fetal outcome. 9 , 37 These risk factors frequently occur in succession because both are an indication for heart failure in the fetus. 20 Other studies also found cardiomegaly and fetal hydrops as independent risk factors for poor outcome. 9 , 37
In our study, Altman classification, polyhydramnios, and GA at diagnosis had no association with poor outcome, which confirms data from smaller studies. 16 , 20 , 38 Tumor aspect was a risk factor in some studies 8 , 16 ; however, others found no correlation between tumor morphology and poor outcome. Also, the relationship between tumor size and outcome is not uniform. 20 This inconsistency may result from the unpredictable pattern of tumor growth with advancing gestation. To quantify tumor growth, several studies have tried to adjust it for fetal size by using fetal weight or head volume. 16 , 21 , 38 , 39 Tumor volume‐to‐abdominal circumference ratio, as in the frequently used Hadock formula, is less appropriate because of the variability of the abdominal circumference due to fetal hydrops or tumor size. 20 , 40 Therefore, head circumference was used in the present study to adjust for fetal size. Unfortunately, statistical analysis could not be performed due to small numbers. Other studies found tumor volume‐to‐fetal weight ratio (TFR) and validated TFR >0.12 prior to 24 weeks GA as a significant predictor for poor outcome or found a TVI larger than 60 cm3/cm as a risk factor for poor outcome. 16 , 21 , 38
The association of cardiomegaly, fetal hydrops, and poor outcome found in the present cohort and in other studies raises the question if the proposed management options should be offered in these fetuses. Needle drainage of a cystic SCT is likely unwarranted, except prior to delivery to overcome difficulties during delivery due to the tumor volume, to either allow vaginal delivery or to allow cesarean with a normal hysterotomy as opposed to a classical incision. Baumgarten et al. 41 advises open fetal surgery in fetuses with type I and II SCT with progressive evolution to high‐output cardiac failure before a GA of 27 weeks. After 27 weeks' GA, iatrogenic preterm birth with immediate SCT resection should be considered.
Some limitations inherent to the retrospective design of the present study require mentioning. First, some patients were lost during follow‐up or were followed in other hospitals than the Fetal Medicine Units or moved to a foreign country and could therefore not be included in the study. Furthermore, advances in imaging technology and neonatal care during the study period undoubtedly impacted some outcomes. This leads to an earlier and more accurate diagnosis of SCT which enables better prenatal care and treatment, and also to better care in the first days after birth reducing the risk of early death of the neonate.
Tumor volume was not documented in all fetuses. In most fetuses, tumor volume was not measured during each ultrasound. Therefore, exact statistical analyses could not be performed. Lastly, our data were based on a single measurement by an experienced perinatologist.
Further research with increased numbers and postnatal data is necessary to consider different counseling of parents in possible termination of pregnancy and determining which fetuses require fetal intervention and when early delivery is necessary. Furthermore, imaging should be performed at regular time points with equivalent intervals during pregnancy with special attention to tumor size, vascularization, growth, and tumor size compared to fetal size.
In conclusion, the current incidence of fetuses prenatally diagnosed with SCT in the Netherlands is approximately 1 in 35,000. Poor outcome was observed in 16.2% of the fetuses. Fetal hydrops and cardiomegaly are both risk factors for poor outcome in fetuses prenatally diagnosed with SCT.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The authors would like to thank dr. Ir. N. van Geloven for her help with the statistical analyses. The authors received no funding for this work.
van Heurn LJ, Coumans ABC, Derikx JPM, et al. Factors associated with poor outcome in fetuses prenatally diagnosed with sacrococcygeal teratoma. Prenat Diagn. 2021;41(11):1430‐1438. doi: 10.1002/pd.6026
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Derikx JP, De Backer A, van de Schoot L, et al. Long‐term functional sequelae of sacrococcygeal teratoma: a national study in the Netherlands. J Pediatr Surg. 2007;42(6):1122‐1126. [DOI] [PubMed] [Google Scholar]
- 2. Rescorla FJ, Sawin RS, Coran AG, Dillon PW, Azizkhan RG. Long‐term outcome for infants and children with sacrococcygeal teratoma: a report from the Childrens Cancer Group. J Pediatr Surg. 1998;33(2):171‐176. [DOI] [PubMed] [Google Scholar]
- 3. Huddart SN, Mann JR, Robinson K, et al. Sacrococcygeal teratomas: the UK Children's Cancer Study Group's experience. I. Neonatal. Pediatr Surg Int. 2003;19(1‐2):47‐51. [DOI] [PubMed] [Google Scholar]
- 4. Gabra HO, Jesudason EC, McDowell HP, Pizer BL, Losty PD. Sacrococcygeal teratoma ‐‐ a 25‐year experience in a UK regional center. J Pediatr Surg. 2006;41(9):1513‐1516. [DOI] [PubMed] [Google Scholar]
- 5. Kremer ME, Wellens LM, Derikx JP, et al. Hemorrhage is the most common cause of neonatal mortality in patients with sacrococcygeal teratoma. J Pediatr Surg. 2016;51(11):1826‐1829. [DOI] [PubMed] [Google Scholar]
- 6. De Backer T, Sandler A. Study of the factors associated with recurrence in children with sacrococcygeal teratoma – discussion. J Pediatr Surg. 2006;41(1):181. [DOI] [PubMed] [Google Scholar]
- 7. Swamy R, Embleton N, Hale J. Sacrococcygeal teratoma over two decades: birth prevalence, prenatal diagnosis and clinical outcomes. Prenat Diagn. 2008;28(11):1048‐1051. [DOI] [PubMed] [Google Scholar]
- 8. Usui N, Kitano Y, Sago H, et al. Outcomes of prenatally diagnosed sacrococcygeal teratomas: the results of a Japanese nationwide survey. J Pediatr Surg. 2012;47(3):441‐447. [DOI] [PubMed] [Google Scholar]
- 9. Hedrick HL, Flake AW, Crombleholme TM, et al. Sacrococcygeal teratoma: prenatal assessment, fetal intervention, and outcome. J Pediatr Surg. 2004;39(3):430‐438. discussion‐8. [DOI] [PubMed] [Google Scholar]
- 10. Murphy JJ, Blair GK, Fraser GC. Coagulopathy associated with large sacrococcygeal teratomas. J Pediatr Surg. 1992;27(10):1308‐1310. [DOI] [PubMed] [Google Scholar]
- 11. Sy ED, Lee H, Ball R, et al. Spontaneous rupture of fetal sacrococcygeal teratoma. Fetal Diagn Ther. 2006;21(5):424‐427. [DOI] [PubMed] [Google Scholar]
- 12. Flake AW, Harrison MR, Adzick NS, Laberge JM, Warsof SL. Fetal sacrococcygeal teratoma. J Pediatr Surg. 1986;21(7):563‐566. [DOI] [PubMed] [Google Scholar]
- 13. Kaneyama K, Yamataka A, Kobayashi H, et al. Giant, highly vascular sacrococcygeal teratoma: report of its excision using the LigaSure vessel sealing system. J Pediatr Surg. 2004;39(12):1791‐1793. [DOI] [PubMed] [Google Scholar]
- 14. Benachi A, Durin L, Vasseur Maurer S, et al. Prenatally diagnosed sacrococcygeal teratoma: a prognostic classification. J Pediatr Surg. 2006;41(9):1517‐1521. [DOI] [PubMed] [Google Scholar]
- 15. Bond SJ, Harrison MR, Schmidt KG, et al. Death due to high‐output cardiac failure in fetal sacrococcygeal teratoma. J Pediatr Surg. 1990;25(12):1287‐1291. [DOI] [PubMed] [Google Scholar]
- 16. Akinkuotu AC, Coleman A, Shue E, et al. Predictors of poor prognosis in prenatally diagnosed sacrococcygeal teratoma: a multiinstitutional review. J Pediatr Surg. 2015;50(5):771‐774. [DOI] [PubMed] [Google Scholar]
- 17. Coleman A, Shaaban A, Keswani S, Lim FY. Sacrococcygeal teratoma growth rate predicts adverse outcomes. J Pediatr Surg. 2014;49(6):985‐989. [DOI] [PubMed] [Google Scholar]
- 18. Westerburg B, Feldstein VA, Sandberg PL, Lopoo JB, Harrison MR, Albanese CT. Sonographic prognostic factors in fetuses with sacrococcygeal teratoma. J Pediatr Surg. 2000;35(2):322‐326. [DOI] [PubMed] [Google Scholar]
- 19. Holterman AX, Filiatrault D, Lallier M, Youssef S. The natural history of sacrococcygeal teratomas diagnosed through routine obstetric sonogram: a single institution experience. J Pediatr Surg. 1998;33(6):899‐903. [DOI] [PubMed] [Google Scholar]
- 20. Lee SM, Suh DH, Kim SY, et al. Antenatal prediction of neonatal survival in sacrococcygeal teratoma. J Ultrasound Med. 2018;37(8):2003‐2009. [DOI] [PubMed] [Google Scholar]
- 21. Shue E, Bolouri M, Jelin EB, et al. Tumor metrics and morphology predict poor prognosis in prenatally diagnosed sacrococcygeal teratoma: a 25‐year experience at a single institution. J Pediatr Surg. 2013;48(6):1225‐1231. [DOI] [PubMed] [Google Scholar]
- 22. Rodriguez MA, Cass DL, Lazar DA, et al. Tumor volume to fetal weight ratio as an early prognostic classification for fetal sacrococcygeal teratoma. J Pediatr Surg. 2011;46(6):1182‐1185. [DOI] [PubMed] [Google Scholar]
- 23. Centraal Bureau voor de Statistiek . Geboorte; kerncijfers. 2019. https://opendata.cbs.nl/statline/#/CBS/nl/dataset/37422ned/table?fromstatweb. Accessed January 14, 2020. [Google Scholar]
- 24. Nederlandse Vereniging voor Obstetrie en Gynaecologie Richtlijn . Begeleiding bij foetale sterfte en doodgeboorte. 2014. https://www.nvog.nl/wp‐content/uploads/2018/02/Begeleiding‐bij‐foetale‐sterfte‐en‐doodgeboorte‐1.0‐22‐05‐2014.pdf. Accessed February 2, 2020. [Google Scholar]
- 25. Altman RP, Randolph JG, Lilly JR. Sacrococcygeal teratoma: American Academy of Pediatrics Surgical Section Survey‐1973. J Pediatr Surg. 1974;9(3):389‐398. [DOI] [PubMed] [Google Scholar]
- 26. Forrester MB, Merz RD. Descriptive epidemiology of teratoma in infants, Hawaii, 1986‐2001. Paediatr Perinat Epidemiol. 2006;20(1):54‐58. [DOI] [PubMed] [Google Scholar]
- 27. Hambraeus M, Arnbjornsson E, Borjesson A, Salvesen K, Hagander L. Sacrococcygeal teratoma: a population‐based study of incidence and prenatal prognostic factors. J Pediatr Surg. 2016;51(3):481‐485. [DOI] [PubMed] [Google Scholar]
- 28. Pauniaho SL, Heikinheimo O, Vettenranta K, et al. High prevalence of sacrococcygeal teratoma in Finland – a nationwide population‐based study. Acta Paediatr. 1992;102(6):e251‐6. [DOI] [PubMed] [Google Scholar]
- 29. Rijkstinstituut voor Volksgezondheid en Milieu. Milieu RvVe . Informatie over de organisatie van het programma prenatale screening op down‐, edwards‐ en patausyndroom en het structureel echoscopisch onderzoek. https://www.pns.nl/down‐edwards‐patau‐en‐seo/professionals/organisatie. Accessed January 14, 2020.
- 30. Fumino S, Tajiri T, Usui N, et al. Japanese Clinical Practice Guidelines for Sacrococcygeal teratoma. Pediatr Int official J Jpn Pediatr Soc. 2017. [DOI] [PubMed] [Google Scholar]
- 31. Brace V, Grant SR, Brackley KJ, Kilby MD, Whittle MJ. Prenatal diagnosis and outcome in sacrococcygeal teratomas: a review of cases between 1992 and 1998. Prenat Diagn. 2000;20(1):51‐55. [PubMed] [Google Scholar]
- 32. Wilson RD, Hedrick H, Flake AW, et al. Sacrococcygeal teratomas: prenatal surveillance, growth and pregnancy outcome. Fetal Diagnosis Ther. 2009;25(1):15‐20. [DOI] [PubMed] [Google Scholar]
- 33. Perrelli L, D'Urzo C, Manzoni C, et al. Sacrococcygeal teratoma. Outcome and management. An analysis of 17 cases. J Perinat Med. 2002;30(2):179‐184. [DOI] [PubMed] [Google Scholar]
- 34. Yoneda A, Usui N, Taguchi T, et al. Impact of the histological type on the prognosis of patients with prenatally diagnosed sacrococcygeal teratomas: the results of a nationwide Japanese survey. Pediatr Surg Int. 2013;29(11):1119‐1125. [DOI] [PubMed] [Google Scholar]
- 35. De Backer A, Madern GC, Hakvoort‐Cammel FG, Haentjens P, Oosterhuis JW, Hazebroek FW. Study of the factors associated with recurrence in children with sacrococcygeal teratoma. J Pediatr Surg. 2006;41(1):173‐181. discussion‐81. [DOI] [PubMed] [Google Scholar]
- 36. Addeo R, Crisci S, D'Angelo V, et al. Bax mutation and overexpression inversely correlate with immature phenotype and prognosis of childhood germ cell tumors. Oncol Rep. 2007;17(5):1155‐1161. [PubMed] [Google Scholar]
- 37. Okada T, Sasaki F, Cho K, et al. Management and outcome in prenatally diagnosed sacrococcygeal teratomas. Pediatr Int. 2008;50(4):576‐580. [DOI] [PubMed] [Google Scholar]
- 38. Gebb JS, Khalek N, Qamar H, et al. High tumor volume to fetal weight ratio is associated with worse fetal outcomes and increased maternal risk in fetuses with sacrococcygeal teratoma. Fetal Diagn Ther. 2019;45(2):94‐101. [DOI] [PubMed] [Google Scholar]
- 39. Sy ED, Filly RA, Cheong ML, et al. Prognostic role of tumor‐head volume ratio in fetal sacrococcygeal teratoma. Fetal Diagn Ther. 2009;26(2):75‐80. [DOI] [PubMed] [Google Scholar]
- 40. Beutler GM, Kurmanavicius J, Hoffmann M, Welzl E, Huch R, Bajka M. New nomogram for foetal weight estimation based on Hadlock's two‐parameter formula. Ultraschall Med. 2004;25(1):58‐64. [DOI] [PubMed] [Google Scholar]
- 41. Baumgarten HD, Gebb JS, Khalek N, et al. Preemptive delivery and immediate resection for fetuses with high‐risk Sacrococcygeal teratomas. Fetal Diagn Ther. 2019;45(3):137‐144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


