Abstract
Kidney transplant recipients (KTR) may be at increased risk of adverse COVID-19 outcomes, due to prevalent comorbidities and immunosuppressed status. Given the global differences in COVID-19 policies and treatments, a robust assessment of all evidence is necessary to evaluate the clinical course of COVID-19 in KTR. Studies on mortality and acute kidney injury (AKI) in KTR in the World Health Organization COVID-19 database were systematically reviewed. We selected studies published between March 2020 and January 18th 2021, including at least five KTR with COVID-19. Random-effects meta-analyses were performed to calculate overall proportions, including 95% confidence intervals (95% CI). Subgroup analyses were performed on time of submission, geographical region, sex, age, time after transplantation, comorbidities, and treatments. We included 74 studies with 5559 KTR with COVID-19 (64.0% males, mean age 58.2 years, mean 73 months after transplantation) in total. The risk of mortality, 23% (95% CI: 21%–27%), and AKI, 50% (95% CI: 44%–56%), is high among KTR with COVID-19, regardless of sex, age and comorbidities, underlining the call to accelerate vaccination programs for KTR. Given the suboptimal reporting across the identified studies, we urge researchers to consistently report anthropometrics, kidney function at baseline and discharge, (changes in) immunosuppressive therapy, AKI, and renal outcome among KTR.
KEYWORDS: clinical research/practice, complication: infectious, immunosuppressive regimens, infection and infectious agents - viral, infectious disease, kidney transplantation/nephrology, meta-analysis, translational research/science
Abbreviations: AKI, acute kidney injury; CI, confidence interval; KTR, kidney transplant recipients; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; WHO, World Health Organization
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the associated coronavirus disease 2019 (COVID-19) have a severe impact on healthcare systems, including organ transplantation programs worldwide.1, 2, 3 Kidney transplant recipients (KTR) with COVID-19 may be at increased risk of adverse outcomes, due to the high prevalence of comorbidities such as reduced kidney function, hypertension and diabetes and the use of immunosuppressive drugs.4, 5, 6 As KTR are more vulnerable to infectious diseases,6, 7, 8, 9 it is likely that rates of mortality and AKI are higher among KTR, compared to the general population.
During the initial phase of the pandemic, knowledge regarding the treatment and outcomes of COVID-19 in KTR was mainly shared through case reports and case series. Based on these studies, several systematic reviews and meta-analyses were published that aimed to evaluate the clinical course and the effects of treatment modalities of COVID-19 in KTR.10 , 11 In the past months, significantly more evidence on this topic has become available through larger studies. Having an updated overview of the risks of KTR with COVID-19 is necessary to adequately weigh the risks and benefits of kidney transplantation during the current pandemic. In addition, many international differences regarding policies and the treatment of COVID-19 in KTR exist,12 and the described study populations are heterogeneous with regard to ethnicity, time after transplantation, and patient characteristics.10 In this systematic review, we therefore aimed to assess all current evidence, to provide an updated and robust insight into the clinical course and outcome of COVID-19 in KTR. In addition, we aimed to review the global use of treatment modalities, and modifications in immunosuppressive regimens among KTR with COVID-19. Finally, we aimed to identify an essential set of variables with regard to baseline characteristics, treatment, and outcome measures to be reported for studies on outcome and management of KTR in any potential future epidemics.
2. MATERIALS AND METHODS
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (Table S1),13 and was prospectively registered in PROSPERO (CRD42021235502).
2.1. Information sources and search strategy
We conducted an extensive systematic search in the World Health Organization (WHO) COVID-19 database for eligible studies. This database concerns the following 25 bibliographic and grey literature sources: Medline (Ovid and PubMed), PubMed Central, Embase, CAB Abstracts, Global Health, PsycInfo, Cochrane Library, Scopus, Academic Search Complete, Africa Wide Information, CINAHL, ProQuest Central, SciFinder, the Virtual Health Library, LitCovid, WHO COVID-19 website, CDC COVID-19 website, Eurosurveillance, China CDC Weekly, Homeland Security Digital Library, ClinicalTrials.gov, bioRxiv (preprints), medRxiv (preprints), chemRxiv (preprints), and SSRN (preprints). The systematic search was performed on January 18th 2021. We used the following search terms: “(kidney OR renal) AND (transplantation* OR transplant* OR graft*)”. No limitations regarding language, study type, or publication type were applied for the initial literature search phase. Database records were then uploaded to Rayyan QCRI, the Systematic Reviews web app (Qatar Computing Research Institute).14 After study selection, included articles were checked for retractions in the Retraction Watch Database.
2.2. Inclusion and exclusion criteria and process
The objective of this review was to assess etiological and therapeutic factors associated with patient outcomes in KTR with COVID-19. We therefore included all randomized controlled trials, cohort studies, registry studies, and case series that contained targeted clinical data in at least five kidney transplant recipients with COVID-19, as confirmed by reverse transcriptase-polymerase chain reaction (RT-PCR) or antibody testing. Poster abstracts including such data were also included. Animals studies were excluded. In addition, studies with no available English text or abstract were excluded.
All records were screened based on abstract and title by three contributors (D. K., T. T. P., and B. W. M. v. B), where assessments of the other contributors were blinded. In case of conflicts, studies were included for the next stage. Following the screening, studies were assessed for eligibility based on full texts by two contributors (assessment performed by D. K., T. T. P., and B. W. M. v. B.), where assessments of the other contributor were blinded. After unblinding, conflicts were discussed with the assessing authors, and the third assessor adjudicated in case of disagreement.
2.3. Data collection and analysis
For each included study, we independently extracted data using a standardized data-extraction form regarding the trial characteristics (study design, publication date, geographical region), patient characteristics (age, sex, ethnicities, comorbidities, weight, body mass index), transplant-related parameters (time after transplantation, maintenance immunosuppressive treatment), and treatment after the COVID-19 diagnosis (change in maintenance immunosuppressive treatment, use of dexamethasone, tocilizumab, remdesivir, and hydroxychloroquine). The outcomes of interest were hospitalization, duration of hospitalization, the occurrence of acute kidney injury (AKI), kidney function after discharge, the necessity of dialysis treatment, rejection or failure of the kidney graft, and mortality. For the dichotomous outcomes, we collected the number of participants who experienced the event and the total number of hospitalized participants. If the number of hospitalized patients for a study was unknown, we used the total number of study participants. For continuous outcomes, we used the mean or median and measure of variability. Corresponding authors were contacted in case clarification was necessary.
2.4. Risk of bias analyses
The risk of bias of the included studies was assessed using the Newcastle-Ottawa Scale (NOS) on: (1) representativeness of the exposed cohort; (2) ascertainment of exposure; (3) demonstration that the outcome of interest (i.e., AKI and mortality) was not present at the start of the study; (4) comparability of cohorts on the basis of the design or analyses; (5) assessment of outcome; (6) was follow-up long enough for outcomes to occur; (7) adequacy of follow-up of cohorts. Given that all included studies were single-arm studies, the item “selection of the non-exposed cohort” of NOS was not applicable to our risk of bias assessment.
2.5. Statistical analyses
We performed meta-analyses to derive pooled proportions of AKI and mortality of the number of hospitalized patients. To explore differences in outcomes, we performed subgroup analyses based on date of manuscript submission, geographical region, sex, age, comorbidities, time after transplantation, and treatment modalities. We used the metaprop package in R (version 3.5.1) to perform random-effect meta-analyses of binomial data of proportions, and calculated their respective 95% confidence intervals (95% CI) according to the Clopper-Pearson method.15 We visually inspected the presence of heterogeneity in our forest plots and calculated the I2 statistic, which quantifies the heterogeneity across studies.
2.6. Sensitivity analyses
Because many studies included a limited number of participants, we performed sensitivity analyses for estimated proportions of death and AKI in studies that included at least 100 participants, to decrease potential selection and publication bias.
3. RESULTS
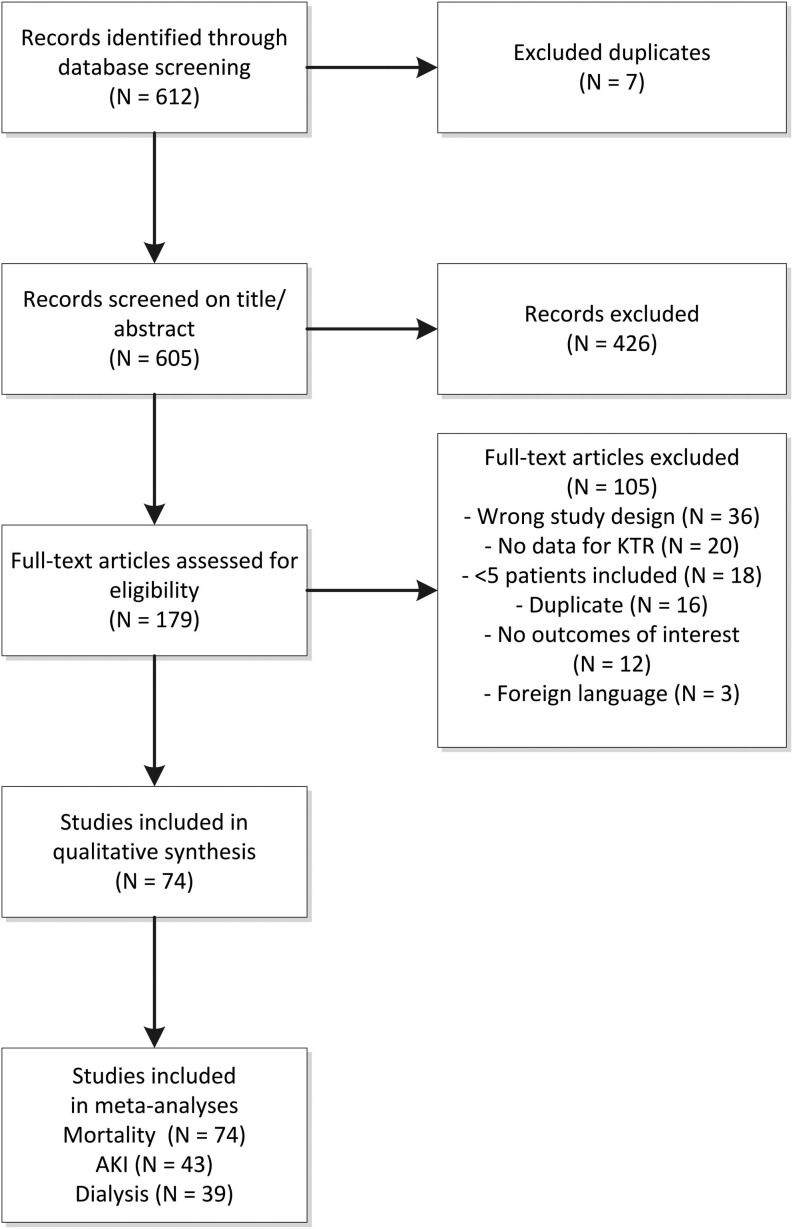
The study selection flow is visualized in Figure 1. In total, 605 records were identified in the database screening. Of these records, 426 (70.4%) were excluded based on title and abstract, as they did not meet the predefined eligibility criteria. Common reasons for exclusion at this stage were lack of targeted clinical data (e.g., editorials, perspectives, or opinion commentaries without new clinical data), or wrong study population (no targeted data in KTR). Consequently, we conducted a full-text review of 179 articles, of which 105 (59%) were excluded. Reasons for exclusion at this stage are shown in Table S2. As a result, 74 studies published between March 2020 and January 18th 2021, were included in the current systematic review, including a total of 5559 KTR (Table S3).
FIGURE 1.
PRISMA study selection flow. AKI, acute kidney injury; KTR, kidney transplant recipients
3.1. Study characteristics
In total, 38 studies (51%) were conducted in Europe, 25 (34%) in the United States, and 10 (14%) in Asia or the Pacific. One study (1%) contained combined data from Europe and the United States. Although a large proportion of included studies (22 studies, 30%) was first submitted in March, April, or May of 2020, these studies accounted for only 604 patients (11% of the total study population). In the first months, only case reports and relatively small case series were published. Studies with more than 100 included patients were first submitted in June 2020, and 80% of the included patients were reported in papers first submitted after August 1, 2020.
3.2. Population characteristics and incidence of adverse outcomes
Among patients with available data, 64% were male, mean age was 58 years (range: 43–74 years), and mean time after transplantation was 73 months (range: 1–161 months). Hypertension and diabetes were common (82% and 42%, respectively), and the majority of KTR were treated with calcineurin inhibitors (92%), proliferation inhibitors (76%), and corticosteroids (73%), as maintenance immunosuppression.
The majority of KTR reported in the included studies were admitted to the hospital (86%). Of these patients, 25% required intensive care treatment, and 23% (95% CI: 21%–27%) died. However, substantial heterogeneity was identified in this meta-analysis (I2: 72%). AKI was reported in 42 studies (57%) and based on various criteria, and was common in KTR with COVID-19 (50%, 95% CI: 44%–56%). Again, substantial heterogeneity was identified for this outcome (I2: 66%). The median duration of hospitalization was reported in only 27 studies (36%), and varied from 4 to 36 days, depending on the study. Rejection and graft failure were explicitly reported in only 9 and 48 cases (0.16% and 0.86%, respectively), but may have been subject to reporting bias. The necessity of dialysis treatment was reported in 280 patients (6%), but this outcome was explicitly mentioned in only 39 studies (53%) and may thus have been underreported.
3.2.1. Early and later phases of the pandemic
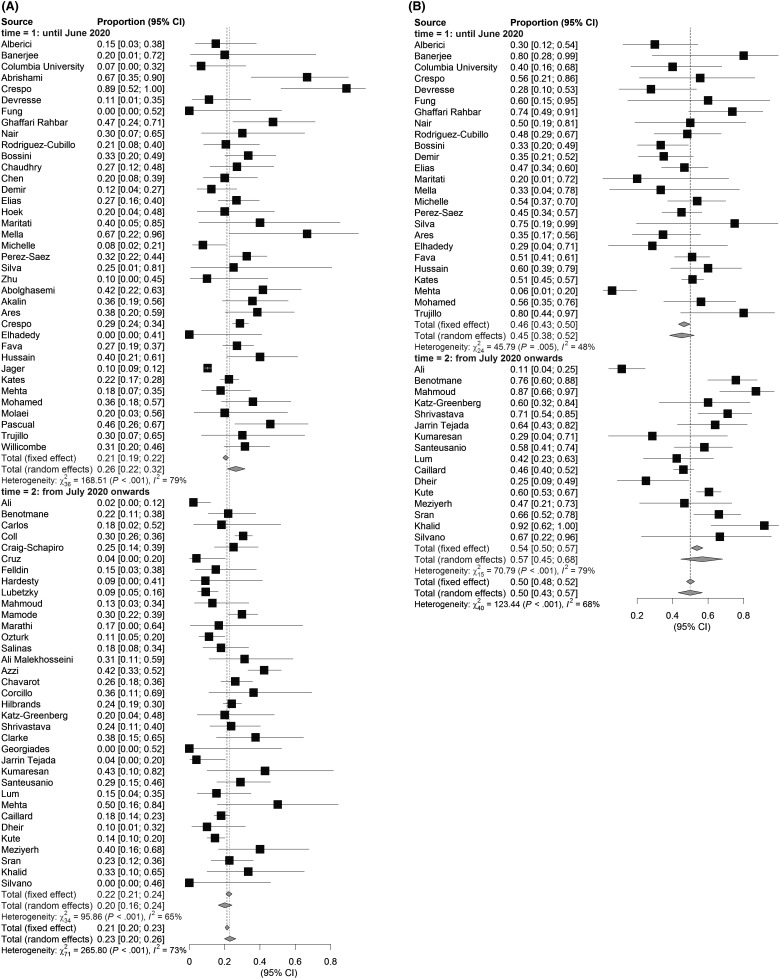
There was an insignificant trend towards higher mortality rates in the early phase of the pandemic (studies first submitted before July 2020; estimated proportion of deaths: 26%; 95% CI: 22%–32%), compared to the later phase (studies submitted from July 2020 onwards; estimated proportion of deaths: 23%; 95% CI: 20%–26%; Figure 2). The incidence of AKI was slightly lower in the earlier studies (estimated proportion of AKI: 45%; 95% CI: 38%–52%), compared to studies submitted from July 2020 onwards (estimated proportion of AKI: 57%; 95% CI: 45%–68%), although the differences between these subgroups were also not significant.
FIGURE 2.
Reported outcomes of KTR with COVID-19. Overall reported mortality of hospitalized KTR with COVID-19 (A) is 23% and appears higher in the first period. AKI (B) occurs in 50% of the cases and appears to be increased in the period after July 2020
3.2.2. Region
Mortality rates tended to be lower in the USA (estimated proportion of deaths: 18%; 95% CI: 14%–23%) than in Asia/Pacific and Europe (estimated proportion of deaths: 24%; 95% CI: 13%–40%, and 26%; 95% CI: 22%–30%, respectively, Figure S1), albeit that the difference was not significant. The incidence of AKI tended to be lower in the Asia/Pacific region (estimated proportion of AKI: 39%; 95% CI: 19%–64%) than in the USA (estimated proportion of AKI: 53%; 95% CI: 39%–66%) and Europe (estimated proportion of AKI: 50%; 95% CI: 43%–57%) although this difference was also not significant.
3.3. Potential risk factors for AKI and mortality
3.3.1. Sex
No clear differences between subgroups based on sex were identified with regard to either mortality rates or AKI incidence (Figure S2).
3.3.2. Age
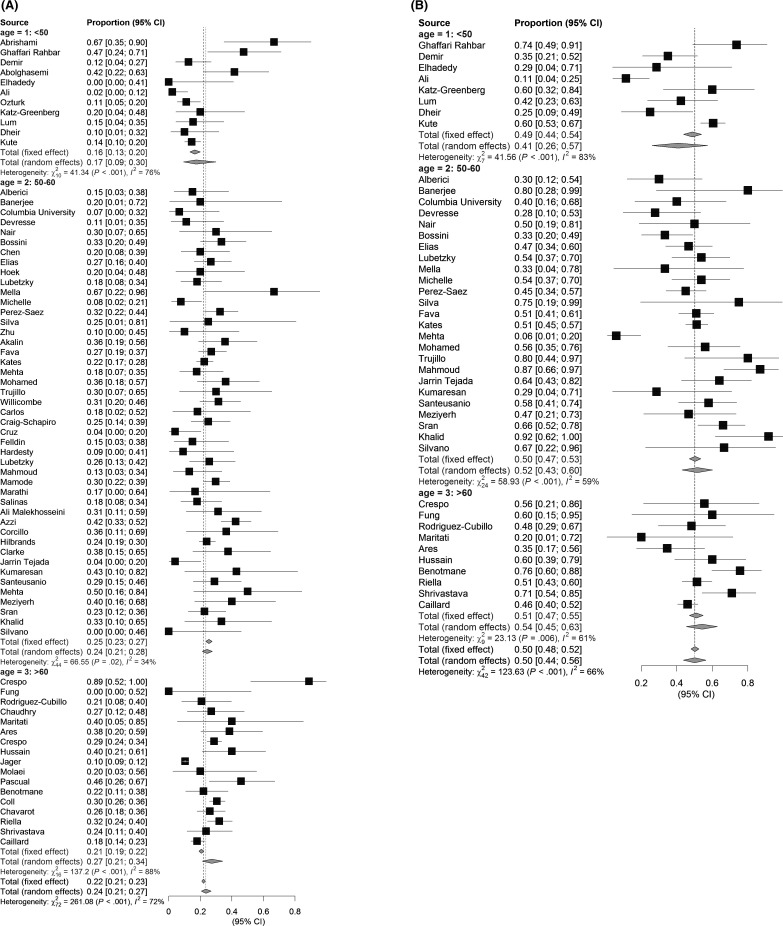
The estimated mortality rate increased with the reported mean age of included patients, and was lowest in studies with a mean age <50 years (estimated proportion of deaths: 17%, 95% CI: 9%–30%). Studies with a mean age between 50 and 60 years had higher mortality rates (estimated proportion of deaths: 24%, 95% CI: 21%–28%), and studies with a mean age of 60 years or older had the highest mortality rates (estimated proportion of deaths: 27%, 95% CI: 21%–34%; Figure 3). Similarly, the estimated incidence of AKI was lowest in studies with a mean age <50 years (estimated AKI incidence: 41%, 95% CI: 26%–57%), and highest in studies with a mean age >60 years (estimated AKI incidence: 54%, 95% CI: 45%–63%). However, both trends for increasing incidence of death and AKI were not statistically significant.
FIGURE 3.
Reported outcomes of KTR with COVID-19 among age groups. Overall reported mortality of hospitalized KTR with COVID-19 (A) is 17% in studies with a mean age lower than 50 years, 23% in the age group 50–60 years, and 27% in studies reporting on age groups older than 60 years. Occurrence of AKI (B) was lowest in the youngest age group (41%) and highest in the oldest groups (54%)
3.3.3. Anthropometrics
Notably, anthropometric measurements, including body mass index were described in only 25 studies (34%), including 1120 patients (20%). Meta-analyses were therefore not performed for this well-known risk factor.
3.3.4. Time after transplantation
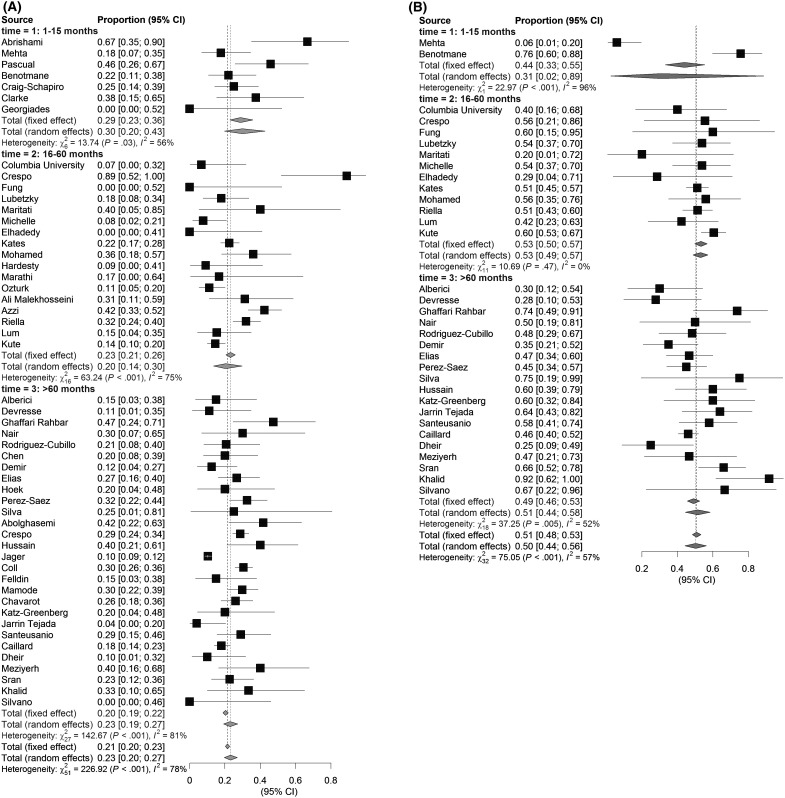
Studies including KTR with a mean or median of ≤15 months after transplantation had the highest mortality rates (estimated proportion of deaths: 30%; 95% CI: 20%–43%), compared with studies including KTR who received their kidney between 16 and 60 months (estimated proportion of deaths: 20%, 95% CI 14%–30%), or more than 60 months (estimated proportion of deaths: 23%, 95% CI 19%–27%) after transplantation, although the differences between the subgroups were not statistically significant ( Figure 4). The number of studies with a mean time after transplantation until 15 months that reported on AKI was too small to draw any conclusions, but incidence rates did not differ significantly between the other subgroups.
FIGURE 4.
Effect of time between transplantation and COVID-19 diagnosis on outcome. KTR who received their kidney 15 months or less before COVID-19 diagnosis appeared to have a (A) higher mortality risk compared to patients who received their kidney 16–60 or more than 60 months before COVID-19 diagnosis, whereas the occurrence of (B) AKI was similar in all groups
3.3.5. Comorbidities
We observed no clear trends with regard to mortality and AKI in subgroups of studies depending on the prevalence of hypertension and diabetes (Figure S3). Among the subgroups, mortality rates were lowest among studies with 50%–75% hypertension prevalence, but heterogeneity in this subgroup was high, and mortality rates did not differ significantly from the entire study population. Similarly, no clear trends for mortality or AKI incidence were observed between subgroups of studies depending on the prevalence of diabetes.
3.3.6. Treatment
Immunosuppressive maintenance therapy was reduced in the majority of patients. Reductions in proliferation inhibitors appeared most common, but reductions in CNI dosages were also commonly reported. The exact modifications in immunosuppressive regime after COVID-19 diagnosis were often not described, and we therefore did not perform meta-analyses in subgroups for immunosuppressive modifications.
Mortality rates did not differ significantly between subgroups of studies depending on the proportion of hydroxychloroquine use. Although insignificant, mortality rates were highest among studies with <25% and >75% hydroxychloroquine use (Figure S4). With regard to tocilizumab, there was a trend toward higher mortality rates among studies with the highest proportions of tocilizumab use. It must be noted that studies in this subgroup were all published in the earlier stages of the pandemic (May or June 2020).
3.4. Sensitivity analyses
Of studies including at least 100 KTR, 12 reported mortality rates, and five reported incidence of AKI. The proportion of deaths in these relatively large studies was similar to the population, including all studies (estimated proportion of deaths: 24%; 95% CI: 20%–30%). AKI incidence was also similar when including only the larger studies (52%; 95% CI: 47–56%). Notably, the study by De Jager et al.16 provided no information regarding the proportions of hospitalized patients, and the corresponding author has responded to us that there is no such data available. This study, accounting for a large group of patients, may therefore not be representative for the hospitalized KTR population, which may explain their significantly lower mortality rates, compared to the other large studies.
3.5. Risk of bias assessment
The risk of bias assessment is presented in Table S4. All studies were single-arm studies and therefore did not compare the outcomes with a non-exposed cohort. Common methodological shortcomings are identified in the representativeness of the exposed cohort (e.g., a selection of elderly patients). The outcomes of interests were commonly not adjusted for important confounders, such as age, gender, and comorbidities, leading to potentially biased effect estimates. In 49 studies (66%) the follow-up was considered long enough (i.e., 30 days) for the outcomes of interest to occur and nine studies (12%) had a high proportion of patients lost to follow-up (i.e., ≥10%).
4. DISCUSSION
To the best of our knowledge, this is the most comprehensive and up-to-date systematic review and meta-analysis of COVID-19 in KTR, including 74 studies with a total of 5559 KTR with COVID-19. The study population included 64% males, with a mean age of 58 years, and a mean time after transplantation of approximately 6 years. Immunosuppressive therapy was modified in most KTR after COVID-19 diagnosis. KTR who were hospitalized with COVID-19 had a poor prognosis compared to the general population regardless of sex, age, comorbidities, and treatment modality, with a mean mortality rate of approximately 23%. AKI affected up to half of the included patients, although it was inconsistently reported. There was a trend towards lower mortality rates, but not towards lower AKI incidence, in studies submitted from July 2020 onwards, compared to studies that were submitted until June 2020. No significant differences were observed in mortality rates and AKI incidence depending on geographical region.
Compared to an age-matched population, the mortality rate among KTR included in this meta-analysis was approximately 4–10 times higher.17 Additionally, mortality rates in our analyses were approximately 1.5 times higher compared to other hospitalized patients,18 , 19 which is a more adequate comparison since most included KTR in our meta-analysis were hospitalized. This finding is supported by studies directly comparing matched patients with similar comorbidities with and without kidney transplantation.20 , 21
Although studies including individual patient data indicate that age, poor baseline kidney graft function and other comorbidities are independently associated with an increased risk of mortality among KTR,22 we were limited in analyzing these factors, due to suboptimal reporting, and the fact that we performed the meta-analyses solely on mean effect estimates as opposed to individual patient data.
Recent studies have shown that mortality rates among hospitalized COVID-19 patients in England and the USA decreased drastically over time, especially in the second half of 2020, when the value of corticosteroids in COVID-19 became increasingly clear.18 , 19 A similar pattern of decreasing mortality rates was observed in our analyses, although the decrease was less striking and did not reach statistical significance. In contrast, the incidence of AKI appeared slightly higher in the subgroup of studies submitted after July 1st 2020, but the difference between the groups was not statistically significant. The inverse relation between the observed trends, suggesting decreased mortality rates and increased incidence of AKI, may well be explained by the overall improved care and associated outcomes for COVID-19 patients,18 , 19 providing the window for patients, including KTR, to develop COVID-19-induced AKI. No significant differences with regard to AKI or mortality were present between geographical regions, with the important notice that developing regions including South America, Africa, West and South East Asia were underrepresented in global literature on COVID-19 in KTR.
The incidence of AKI was described in less than 60% of included studies. Based on these studies, the incidence of AKI in KTR hospitalized with COVID-19 appeared high compared to estimates in hospitalized patients with COVID-19 in general.23, 24, 25, 26, 27, 28, 29 The high incidence of AKI in COVID-19 is in part attributed to direct pathological effects of SARS-CoV-2 on the kidneys, but also by the systemic immune response with hypercoagulation, and coexistent hypovolemia and hypoxia.30 Although the incidence of AKI in hospitalized patients with COVID-19 has been shown to drastically worsen prognosis,23 our study could not adequately assess AKI as a risk factor for COVID-19 mortality among KTR, because of the lack of individual patient data. However, the deleterious effects of AKI on both kidney and the patient outcomes may be even higher among KTR compared to the general population. Both short-term and long-term kidney graft outcomes should therefore be described in future studies.
The importance of studies into long-term kidney graft outcomes is highlighted by a recent study among approximately 4000 hospitalized (non-KTR) COVID-19 patients in the USA, which showed that less than one-third of the population had full recovery of kidney function by the time of discharge.26 In addition, accumulating evidence suggests that COVID-19-associated AKI may be more deleterious than non-COVID-19-associated AKI.31 These long-term effects on kidney function maybe even more detrimental among KTR, given their pre-existent limited kidney function, frequent comorbidities and possible detrimental effects of immunosuppression reduction during the infection. However, despite the clinical relevance, very few studies have reported kidney function at baseline and after recovery from COVID-19 in KTR ( Table 1). Future cohort and registry studies should report these figures, in order to more adequately assess the long-term kidney graft outcome among KTR after COVID-19.
TABLE 1.
Essential variables to be reported in studies on KTR
| Variable | Reported |
|---|---|
| Baseline kidney function | <10/74 |
| Kidney function at discharge | <10/74 |
| BMI | 28/74 |
| Specified immunosuppressive regimen | 63/74* |
| Changes in immunosuppressive regimen | 61/74* |
| Occurrence of AKI including definition according to KDIGO or RIFLE guidelines | 42/74 |
| Time between transplantation and event | 53/74 |
| Renal outcome (rejection/graft loss) | 16/74 |
Recommended variables related to kidney function and transplantation, and the illustrative occurrence of these variables in studies included in this systematic review (*: only partial information available in most studies).
In general, the studies included in this systematic review and meta-analysis provided limited data on anthropometrics, baseline kidney function, immunosuppressive (maintenance) therapy, changes in immunosuppressive therapy, and renal outcomes including AKI, the necessity of kidney replacement therapy, and graft failure. However, these measures and outcomes are essential to be able to compare KTR populations, and to robustly assess and compare treatment effects. In addition, transplant populations and transplant care vary per country, and even per hospital, which is further reflected by the high heterogeneity among the included studies. We therefore strongly urge scientists and scientific journals to consistently provide information regarding mentioned patient characteristics and immunosuppressive therapy. The use of online supplemental materials may be suitable for this purpose.
4.1. Strengths and limitations
With this systematic review and meta-analysis, we appraised all clinical evidence on COVID-19 in KTR until January 2021. This study provides robust evidence on the clinical course of COVID-19 in KTR. In contrast with our initial study protocol, we decided to exclude case reports and case series describing fewer than five patients to reduce publication and selection bias. However, this exclusion has slightly decreased the number of included patients. The sensitivity analyses that we performed in the larger studies with less risk of these sources of bias, showed similar rates of mortality and AKI, which is reassuring with regard to the validity of the other analyses. Nevertheless, selection and publication bias remains an important concern, also in the current meta-analysis. In addition, because of the lack of individual patient data and the observational nature of the included studies, only descriptive statistics but no causal evidence could be provided with this review. Also, the large heterogeneity in patient characteristics (e.g., age and comorbidities) and study design characteristics (e.g., sample size and follow-up) among the different studies highlights the need for prudence in the extrapolation of these results to specific populations. Given that we solely included KTR in our meta-analyses, we cannot formally compare our results with the risk of dying of COVID-19 in the general population or non-KTR patients with CKD. The question remains whether the general population, or a population with specific characteristics (age, comorbidities) would be the correct control population for COVID-19-infected KTR.32 Finally, our risk of bias analysis suggests that common methodological shortcomings are identified in the included studies and we stress the importance of adjusting effect estimates for important confounders and an adequate follow-up with good retention.
5. CONCLUSION
The risk of mortality is high among KTR, compared to the general population, regardless of sex, age and reported comorbidities, underlining the urge for accelerated vaccination programs for KTR. Evidence on long-term kidney graft function after COVID-19 recovery is scarce. Given the heterogeneity in the KTR population and global transplant care, researchers should consistently report anthropometrics, baseline kidney function, immunosuppressive therapy, and kidney graft outcomes including AKI and kidney function after recovery in the current pandemic, and in any potential future pandemics.
Acknowledgments
DISCLOSURE
The authors of this manuscript have no conflicts of interest todisclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
A data availability statement is not applicable for this article.
Footnotes
Robin W. M. Vernooij and Bas W. M. van Balkom equally contributed as last author.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
Supplementary Material
REFERENCES
- 1.Ahn C, Amer H, Anglicheau D, et al. Global transplantation COVID report. Transplant. 2020;2020:1974–1983. doi: 10.1097/TP.0000000000003258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelico R, Trapani S, Manzia TM, et al. The COVID-19 outbreak in Italy: initial implications for organ transplantation programs. Am J Transplant. 2020;20(7):1780–1784. doi: 10.1111/ajt.15904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Domínguez-Gil B, Coll E, Fernández-Ruiz M, et al. COVID-19 in Spain: transplantation in the midst of the pandemic. Am J Transplant. 2020;20(9):2593–2598. doi: 10.1111/ajt.15983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michaels MG, La Hoz RM, Danziger-Isakov L, et al. Coronavirus disease 2019: implications of emerging infections for transplantation. Am J Transplant. 2020;20(7):1768–1772. doi: 10.1111/ajt.15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357(25):2601–2614. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 7.Pruthi R, Steenkamp R, Feest T. UK renal registry 16th annual report: chapter 8 survival and cause of death of UK adult patients on renal replacement therapy in 2012: national and centre-specific analyses. Nephron - Clin Pract. 2014;125(1-4):139–169. doi: 10.1159/000360027. [DOI] [PubMed] [Google Scholar]
- 8.Howard RJ, Patton PR, Reed AI, et al. The changing causes of graft loss and death after kidney transplantation. Transplant. 2002;73(12):1923–1928. doi: 10.1097/00007890-200206270-00013. [DOI] [PubMed] [Google Scholar]
- 9.Kinnunen S, Karhapää P, Juutilainen A, et al. Secular trends in infection-related mortality after kidney transplantation. Clin J Am Soc Nephrol. 2018;13(5):755–762. doi: 10.2215/CJN.11511017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marinaki S, Tsiakas S, Korogiannou M, et al. A systematic review of COVID-19 infection in kidney transplant recipients: a universal effort to preserve patients’ lives and allografts. J Clin Med. 2020;9(9):2986. doi: 10.3390/jcm9092986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oltean M, Søfteland JM, Bagge J, et al. Covid-19 in kidney transplant recipients: a systematic review of the case series available three months into the pandemic. Infect Dis (Auckl). 2020;52(11):830–837. doi: 10.1080/23744235.2020.1792977. [DOI] [PubMed] [Google Scholar]
- 12.Anderson RM, Heesterbeek H, Klinkenberg D, et al. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020;395(10228):931–934. doi: 10.1016/S0140-6736(20)30567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26(4):404. doi: 10.2307/2331986. [DOI] [Google Scholar]
- 16.Jager KJ, Kramer A, Chesnaye NC, et al. Results from the ERA-EDTA registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98(6):1540–1548. doi: 10.1016/j.kint.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. WHO COVID-19 detailed surveillance data dashboard. Microsoft Power BI. https://app.powerbi.com/view?r=eyJrIjoiYWRiZWVkNWUtNmM0Ni00MDAwLTljYWMtN2EwNTM3YjQzYmRmIiwidCI6ImY2MTBjMGI3LWJkMjQtNGIzOS04MTBiLTNkYzI4MGFmYjU5MCIsImMiOjh9. 2021. Accessed March 28, 2021.
- 18.Asch DA, Sheils NE, Islam MN, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181(4):471. doi: 10.1001/jamainternmed.2020.8193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones S, Mason N, Palser T, et al. Trends in risk-adjusted 28-day mortality rates for patients hospitalized with COVID-19 in England. J Hosp Med. 2021;16(5):E1–E4. doi: 10.12788/jhm.3599. [DOI] [PubMed] [Google Scholar]
- 20.Ozturk S, Turgutalp K, Arici M, et al. Mortality analysis of COVID-19 infection in chronic kidney disease, haemodialysis and renal transplant patients compared with patients without kidney disease: a nationwide analysis from Turkey. Nephrol Dial Transpl. 2020;35(12):2083–2095. doi: 10.1093/ndt/gfaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chavarot N, Gueguen J, Bonnet G, et al. COVID-19 severity in kidney transplant recipients is similar to non-transplant patients with similar comorbidities. Am J Transpl. 2021;21(3):1285–1294. doi: 10.1111/ajt.16416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oto OA, Ozturk S, Turgutalp K, et al. Predicting the outcome of COVID-19 infection in kidney transplant recipients. BMC Nephrol. 2021;22(1):100. doi: 10.1186/s12882-021-02299-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically Ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1436. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohamed MMB, Lukitsch I, Torres-Ortiz AE, et al. Acute kidney injury associated with coronavirus disease 2019 in Urban New Orleans. Kidney360. 2020;1(7):614–622. doi: 10.34067/kid.0002652020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA - J Am Med Assoc. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan L, Chaudhary K, Saha A, et al. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. 2021;32(1):151–160. doi: 10.1681/ASN.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher M, Neugarten J, Bellin E, et al. AKI in hospitalized patients with and without COVID-19: a comparison study. J Am Soc Nephrol. 2020;31(9):2145–2157. doi: 10.1681/ASN.2020040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nadim MK, Forni LG, Mehta RL, et al. COVID-19-associated acute kidney injury: consensus report of the 25th acute disease quality Initiative (ADQI) workgroup. Nat Rev Nephrol. 2020;16(12):747–764. doi: 10.1038/s41581-020-00356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nugent J, Aklilu A, Yamamoto YU, et al. Assessment of acute kidney injury and longitudinal kidney function after hospital discharge among patients with and without COVID-19. JAMA Netw open. 2021;4(3):e211095. doi: 10.1001/jamanetworkopen.2021.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osmanodja B, Mayrdorfer M, Halleck F, et al. Undoubtedly, kidney transplant recipients have a higher mortality due to COVID-19 disease compared to the general population. Transpl Int. 2021;34(5):769–771. doi: 10.1111/tri.13881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
A data availability statement is not applicable for this article.