Abstract
Background
Burnpatients characteristically have increased energy, glucose, and protein requirements. Glutamine supplementation is strongly recommended during early‐phase treatment and is associated with improved immunity, wound healing, and reduced mortality. This study evaluated if early burn exudative losses might contribute to higher supplementation needs.
Methods
Patients admitted to the burn intensive care unit (ICU) had exudate collection from tight bandages applied to arms or legs during the first week (exudate aliquot twice daily). Seven amino acids (alanine, arginine, cystEine, glutamine, leucine, lysine, and methionine) were quantified by liquid chromatography–mass spectrometry. Descriptive analysis of all results is provided as median and interquartile range or in value ranges.
Results
Eleven patients aged 19–77 years, presenting with burns on 18%–70% of the body surface, with a median simplified acute physiology score II of 33 (range, 16–56) were included during the study period. The highest amino acid losses were observed during the first 3 days with an important interpatient and intrapatient variability. Glutamine and alanine losses were highest, followed by leucine and lysine in all patients; amino acid exudate concentrations were in the range of normal plasma concentrations and were stable over time. Total glutamine losses were correlated to the burned surface (r2 = 0.552, P = .012), but not to enteral glutamine supplements.
Conclusions
The study shows significant exudative losses during early‐stage burn recovery and particularly for glutamine and alanine. Glutamine loss generally decreased with wound closure, the subsequent decline of exudation, and the evolving size of burn surfaces.
Keywords: amino acid, metabolism, nutrition, protein, wound healing
CLINICAL RELEVANCY STATEMENT
Randomized studies have confirmed glutamine supplementation benefits after a major burn injury. The present study shows substantial burn wound exudative losses that may explain the higher glutamine needs observed in major burn patients.
INTRODUCTION
Burn injury represents a significant public health problem worldwide. 1 , 2 In recent decades, burn resuscitation and associated patient survival has enhanced the need for efficient roles of metabolic management, 3 surgical technique innovation with associated cell therapies, and long‐term rehabilitation. Overall, nutrition status is essential for recovery after major burns. Furthermore, intense endocrine changes and inflammatory responses, immune defense depression, and increased nutrition needs represent major challenges. 3 , 4 Decreased immune response, which begins within the first 12 h postinjury and persists for months, 5 is associated with increased infection risk, organ failure risk, and mortality. 3 , 6 Energy, protein, and micronutrient requirements are increased much beyond the needs of other critically ill patients. Following burn injury, protein needs are as high as 2 g/kg/day in adults and 3 g/kg/day in children. 7 , 8
Since the 1990s, low blood glutamine (GLN) concentrations have been associated with low immunity and poor outcome in critically ill patients. Supplements providing doses 0.2–0.3 g/kg/day have been shown to improve outcomes by enhancing immune system function, antioxidant status, glucose metabolism, and heat shock protein responses. 9 , 10 Thus, “conditionally essential” amino acid supplementation during critical illness has been proven particularly important in major burns. Persistent low‐GLN blood levels in major burns may result either from insufficient synthesis or excessive consumption. Alternatively, it might also be lost simply by passive excretion. GLN is a substrate for rapidly proliferating cells and for maintenance of an adequate immune function, providing fuel for rapidly multiplying cells, including intestinal mucosa, immune‐related, and all cell types involved in wound healing. 11 In burns, enteral supplementation with either GLN 12 , 13 , 14 , 15 or its precursor, ornithine alpha‐ketoglutarate, 16 has been shown to improve clinical outcome. Enteral GLN in doses 0.3–0.5 g/kg/day 17 has been associated with significant reductions of infectious complications and mortality. 4 , 18 Hence, the European nutrition recommendations for major burns have recommended delivering enteral GLN with an evidence level A to B. 7
A previous study conducted in the Lausanne Burn Intensive Care Unit (ICU), which aimed at defining the chemical composition of burn wound exudates, 19 found unexpected high GLN concentrations that were higher than the GLN concentrations observed in blood. 19 The present study aimed at quantifying and measuring the kinetics of GLN and other amino acid losses in burn wound exudates collected over time with a novel system in patients immediately following burn injury. 20
METHODS
The study was designed as a prospective, noninterventional observational quality study using waste material. After approval by the State Ethics Commission (Commission Cantonale d’éthique de la recherche sur l’être humain; CER‐VD Ethics # 488/2013), the study was conducted with individual post hoc consent.
Patients
ICU admission to the Lausanne Burn ICU between February 2014 and April 2015, age >18 years, and burns exceeding 10% of total body surface area (BSA) involving at least one arm or leg were the inclusion criteria for the study and are related to management of pain, airway monitoring (not necessarily intubation), and subsequent surgical management. Exclusion criteria were age <18 years; the presence of a comorbidity, such as local wound infection, diabetes, or HIV; and burns that did not involve the arms or legs. Severity of clinical condition was assessed by the simplified acute physiology score II (SAPS II) score and burn scores (Ryan score, abbreviated burn severity index score, and modified Baux score). 21
Clinical management
The Parkland formula (2–4 ml/kg/% BSA, depending on burn <50% BSA or >50% BSA) was oriented for fluid resuscitation in burns >20% BSA, with subsequent adaptation based on hemodynamic patient response. The room temperatures were maintained at 28 °C.
By standardized protocol, enteral nutrition (EN) was initiated in mechanically ventilated patients within 12 h postinjury (Promote Fiber Plus, Abbott, Baar, Switzerland). In patients burned on >20% BSA, an enteral GLN‐containing product (30 g GLN, 300 mcg selenium (Se), 20 mg zinc (Zn), 1500 mg vitamin C; Intestamin, Fresenius Kabi, Stanz, Switzerland) was initiated with EN and delivered for 10 days (20%–60% BSA) or 30 days (>60% BSA). The total protein goal was 1.5–2.0 g/kg/day. Trace elements were provided as a combination of one vial Decan (Laboratoires Aguettant, Lyon, France), an intravenous additional dose of copper‐Se‐Zn and enteral Intestamin. 22 , 23 Indirect calorimetry or the Toronto equation guided the energy goals. For patients receiving Intestamin, the daily GLN dose delivered to the patient was extracted from the ICU clinical information system (MetaVision, iMDsoft, Tel Aviv, Israel).
Burn wound exudate and blood collection
The exudate was collected from second‐degree superficial or deep burns on the arms or legs on surfaces ranging between 4% and 12% BSA each. Sample collection began with the initiation of wound treatment upon admission to the burn center, using a negative pressure dressing that collected the fluid into a reservoir bottle as described previously (Figure S1) 20 and visualized at http://links.lww.com/PRSGO/A285. Exudate sampleswere collected twice daily (morning and evening) during the first week after trauma by changing the reservoir bottle. All samples were prepared in aliquots in sterile tubes and kept at −80 °C until analysis. Sample collection was discontinued upon grafting of the wound site or natural arrest of exudation. There was no GLN blood sampling during the first week.
GLN and other amino acid analysis
Free amino acids in exudate samples were derivatized according to a modified protocol of the (Waters Corp, Milford, MA, USA) AccQ Tag derivatization kit and analyzed by liquid chromatography–mass spectrometry (LC‐MS) on a Waters Quadrupole Dalton mass spectrometer coupled with an H‐class ultraperformance liquid chromatography (UPLC) system. In brief, exudate samples were protein precipitated via the addition of a 10% m/v sulfosalicylic acid solution containing 20 13C and, for most of them, also 15N isotopically labeled amino acids as internal standards. After centrifugation, supernatants were mixed with an alkaline borate buffer (AccQ Tag Ultra Borate buffer, Waters) and the reaction buffer (AccQ Tag Ultra Reagent, Waters, according to the manufacturer preparation protocol) and incubated at 55 °C for 10 min. Two microliters of the obtained solution were injected on the LC‐MS system, using an auto‐addition of 10 µl of analytical‐grade water. Separation of amino acids was performed in 12 min on a Cortex UPLC C18 (1.6‐µm particle size, 2.1 × 150 mm) column (Waters Corp), maintained at 55 °C, with a 0.1% formic acid containing Water: Acetonitrile mobile‐phase gradient. Data acquisition was performed by electrospray ionization in positive mode using time‐constrained selected ion recording functions for derivatized amino acids as well as their corresponding internal standards. Quantitation was performed with TargetLynx (Waters Corp). Seven amino acids were analyzed: alanine (ALA), arginine (ARG), cystEine (CYS), GLN, leucine (LEU), lysine (LYS), and methionine (MET).
Calculations
Total amino acid loss during a 24‐h period was extrapolated as the measured concentration × 24‐h exudate volume × % BSA/% surface collection.
Statistical analysis
Data are presented as median and interquartile range (IQR, 25–75). Two‐way analysis of variance was used to analyze the changes over time according to enteral GLN supplements (or not) or burn size below or superior to 20% BSA. Pearson correlation coefficients were calculated between amino acid losses and burn size. A value of P < .05 was considered significant. Statistical package utilized was JMP V14.2 (SAS Institute, Cary, NC, USA).
RESULTS
Patients
There were a total of 44 patients admitted to the burn center ICU during the study period. Of these patients, 20 who met the inclusion criteria were enrolled in the study. A total of 15 patients completed the study, and only 11 patients provided enough exudate for amino acid analysis over the appropriate time periods. The patients' age was 19 to 77 years (10 men, 1 woman) and burned surface ranged from 18% to 70% BSA (Table 1). The three patients with the lowest percentage of burn surface were not intubated and received one multimicronutrient table daily. Their ICU stay was also shorter (3.9, 1.6, and 9.6 days total, respectively). There was no wound infection during the study nor any episode of fever >39 °C. Eight out of 11 patients received a median dose of 30 g enteral GLN supplement per day. GLN from standard feeds was not calculated.
TABLE 1.
Patient characteristics
| No. | Age, years | Gender | SAPS II | Preadm weight, kg | BMI, kg/m2 | Burned BSA, % | Surface collected, % | Enteral GLN | Ryan score | Baux modified score | ABSI score | Length ICU stay, days | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 39 | M | 37 | 71 | 21.9 | 60 | 10 | Yes | 2 | 115 | 10 | 113.8 | Alive |
| 4 | 86 | M | 56 | 110 | 34.0 | 42 | 6 | Yes | 2 | 128 | 11 | 4.9 | Dead |
| 5 | 40 | M | 40 | 73 | 23.8 | 60 | 12 | Yes | 2 | 123 | 11 | 36.8 | Alive |
| 6 | 19 | M | 26 | 132 | 38.9 | 40 | 9 | Yes | 2 | 76 | 7 | 39.8 | Alive |
| 7 | 57 | M | 50 | 86 | 29.8 | 25 | 4 | Yes | 1 | 99 | 8 | 18.7 | Alive |
| 9 | 24 | M | 16 | 67 | 21.9 | 35 | 8 | Yes | 0 | 76 | 8 | 19.8 | Alive |
| 11 | 55 | M | 19 | 68 | 23.5 | 18 | 4 | No | 0 | 75 | 6 | 3.8 | Alive |
| 12 | 52 | M | 25 | 90 | 26.9 | 27 | 5 | Yes | 0 | 95 | 8 | 7.9 | Alive |
| 18 | 77 | M | 20 | 70 | 24.2 | 18 | 9 | No | 0 | 39 | 4 | 1.6 | Alive |
| 20 | 24 | F | 33 | 75 | 26.0 | 20 | 5 | No | 1 | 86 | 8 | 9.6 | Alive |
| 22 | 24 | M | 45 | 83 | 24.8 | 70 | 5 | Yes | 2 | 112 | 11 | 61.4 | Alive |
| Median | 40 | 33 | 75 | 24.8 | 35 | 8 | 1 | 95 | 8 | 18.7 | |||
| Count | 10/1 | 8/3 | 10/1 | ||||||||||
| IQR | 24, 57 | 20, 45 | 70, 90 | 23.5, 29.8 | 20, 60 | 5, 9 | 2, 2 | 75, 115 | 7, 11 | 4.9, 39.8 |
Note: Reported IQR values are presented as the 25th and 75th percentiles.
Abbreviations: ABSI, abbreviated burn severity index; BMI, body mass index; BSA, body surface area; F, female; GLN, glutamine; ICU, intensive care unit; IQR, interquartile range; M, male; Preadm, preadmission; SAPS II, simplified acute physiology score II.
Exudative losses
The total median volume of exudate collection was 42 (25–70) ml/day with a high intrapatient and interpatient variability. Exudation was highest during the first 3 days, and declining thereafter. No correlation was found with fluid resuscitation.
The median amino acid concentrations in the exudate fluid were (µmol/L) ALA, 34 (263–458); ARG, 18 (8–35); CYS, 55 (41–65); GLN, 468 (408–584); LYS, 187 (146–231); and LEU, 151 (124–215). The exudate concentrations were in the range of normal plasma values in our laboratory (Table A1) and generally corresponded to normal plasma concentrations, except for GLN and LEU being in the upper ranges. The calculated amino acid losses were highest during the first 3 days, declining thereafter but persisting in several patients; individual patterns are shown in Figure 1 and the mean daily losses in Figure 2. The GLN losses were the most important in all patients, followed by ALA, LEU, and LYS, and ARG, CYS, MET having lower recorded losses. The highest cumulated GLN losses were observed in the largest burns (r2 = 0.522, P = .012) (Figure 3). The amino acid concentrations were not correlated to the exudate volume (R2 < 0.01). The GLN losses were similar both in concentrations and in calculated amounts in patients whether they had or had not received supplemental enteral GLN.
FIGURE 1.
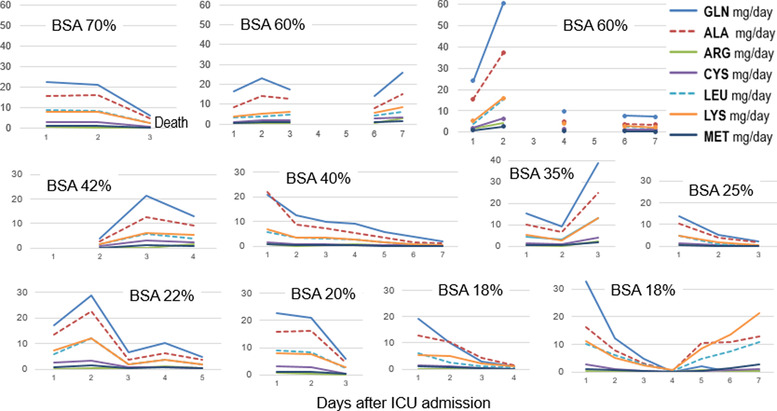
Individual amino acid losses in mg/day (smallest burns on the top, largest at the bottom) showing that GLN and ALA are the amino acids with the largest losses. Days with no values result from the absence of samples. ALA, alanine; ARG, arginine; BSA, body surface area; CYS, cysteine; GLN, glutamine; ICU, intensive care unit; LEU, leucine; LYS, lysine; MET, methionine
FIGURE 2.
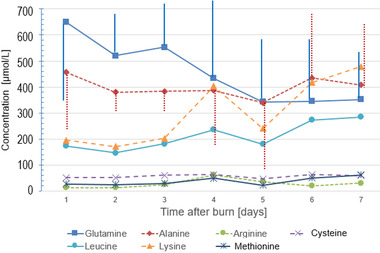
Mean daily amino acid exudate concentrations during the first week. (Vertical bars represent SD values for glutamine and alanine)
FIGURE 3.
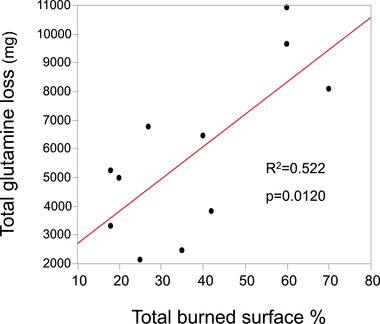
Cumulated glutamine loss during the study period related to burned surface area
DISCUSSION
The present study is the first, to our knowledge, to analyze the amino acid losses in the exudates of burn patients. The volumes collected from arms and legs were highly variable, ranging between 4 and 230 ml. The largest losses were observed during the first 3 days, but probably persisted beyond day 8 in patients with larger burns. Amino acids could be measured in all available exudate samples. GLN and other amino acids were found in significant quantities, but the GLN and ALA losses were the most abundant. There was no relationship between the exudative losses and the enteral GLNsupplementation. This absence of relationship may indicate that the quantities in the exudate resulted from plasma extravasation or from a local wound‐healing process. The comparison of the different amino acids does confirm the initial observation 19 that GLN losses were the largest among amino acids analyzed in the wound exudates.
The lostquantity is not impressive when compared with the nutrition doses (0.2–0.3 g/kg/day). Such a difference between the dose (g) required to maintain blood concentrations and the blood concentration (µmol) are well known in metabolic diseases. The focus on these seven amino acids was motivated by their special involvement in immunity, wound healing, and protein metabolism. LEU, GLN, and MET have been shown to exert direct regulatory properties in body protein turnover. 24 ARG regulates interorgan metabolism of energy substrates and the function of multiple organs and has been shown to improve wound healing. 25 Cysteine is one of the two key sulfur‐containing amino acids with important functions in redox homeostasis, protein functionality, and metabolism. 26
Veterinary medicine studies show that when one amino acid is not provided in adequate quantities, protein synthesis is limited to the rate at which the specific deficient amino acid is available, which takes a role as the limiting amino acid. 27 One might hypothesize that with the exudative GLN losses, a local deficit may occur that would compromise wound healing but could be restored by high protein and additional doses of GLN. The development of the dipeptide GLN‐ALA by Fürst and Stehle in the 1980s provided the possibility that GLN was finally available for parenteral nutrition. 28 This intravenous form enabled the testing of the specific effects of GLN. In critically ill patients, GLN administration by the parenteral route has been shown to be safe and beneficial: three systematic reviews and a meta‐analysis have provided significant evidence on this point, 29 , 30 , 31 and studies in major burns provided even stronger positive results. 18
EN generally contains 8%–12% of the amino acids as GLN, 32 which corresponds to the normal proportion of GLN in omnivore food. 33 With higher needs caused by enhanced metabolism and specific losses, such as shown here, this proportion of GLN may not be sufficient for major burns, which would explain why additional GLN has proven beneficial.Based on actual data, blood GLN is monitored by protocol in our center after 3 weeks in patients with burns >50% BSA to determine the necessity of continuing, or not, the administration of additional GLN whenever the GLN concentrations are <400 µmol/L.
The proportion of GLN in food is variable. A study using gene sequencing found similar proportions of GLN in protein from meat and casein‐based formulas, 33 and the proportion of GLN was found to be 4.8 vs 4.4%. By protocol, feeding is progressed to energy and protein goals (1.5 g/kg) over 3–4 days, reaching 100–110 g/day of protein on day 4 in patients weighing 75 kg. The ICU feeding solution (Promote Energy/Fiber, Abbott) contains protein from casein and soy, which delivers 8%–14% of amino acids as GLN/glutamic acid, which is the usual indication on feeding products. GLN is then formed in the body from ammonia and glutamic acid. 34 We could reasonably assume that the feeding provided 8–17 g GLN/day (normal nutrition intake) on full feeding, which is additive (in patients with burns >20% BSA) to the 30 g from the 500 ml Intestamin solution (which is always entirely delivered).
The ongoing RE‐ENERGIZE study 35 will be the first major international, multicenter study testing the role of additional GLN in burns; it is likely to have positive results and provide data as to the monitoring of blood concentrations.
The very low comparative exudative losses of ARG might reflect its more limited role in wound healing, compared with its immune‐enhancing effects. 36 However, trials have provided indecisive results, and enteral ARG supplementation does not improve wound healing of skin donor sites in rodents. 37
Limitations
The study includes a small number of patients with variable burn severity, but this is characteristic of burn studies. Further, the high interday variability volume of the exudate collected reflects an important limitation of the collection method in severe burn patients. Indeed, to achieve a tight bandage, the suction device was placed on limbs (either arms or legs), which tend to have less exudation than the trunk. Therefore, the calculated loss is likely to be an underestimation, particularly in the severe burn patient. The different volumes collected resulted in highly variable amino acid quantities on days with low volume, despite a similar concentration. Further, the amino acid losses were extrapolated as the measured concentration multiplied by the 24‐h exudate volume and the total BSA, which is an oversimplification because we assumed that the entire burned surface produces exudation in a similar manner. Finally, no blood samples were collected, as the study was based on the exclusive use of noninvasive samples and the least interference with routine nursing care. Therefore, exudates (a waste material) were eligible for multiple collection time points within the study. Therefore, no comparison between blood and exudate concentrations could be accomplished.
In conclusion
This study shows that exudative GLN losses probably contribute to the increased GLN requirements after major burns, as this amino acid is lost in significant amounts in the exudates derived from the burn injury. The amino acid losses decline over time with the subsequent decrease of the exudate but persist in burn injuries with larger surfaces.
FUNDING INFORMATION
None declared.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Mette M. Berger, Wassim Raffoul, and Lee Ann Applegate equally contributed to the conception and design of the research; Mette M. Berger, Mélanie Charrière, Corinne Scaletta, Pierre‐Alain Binz, Clothilde Roux, Olivier Pantet, and Wassim Raffoul contributed to the acquisition and analysis of the data; Mette M. Berger, Pierre‐Alain Binz, Clothilde Roux, and Olivier Pantet contributed to the interpretation of the data; and Mette M. Berger drafted the manuscript. All authors critically revised the manuscript, agreed to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Supporting information
Figure S1. Method of burn wound exudate collection.
ACKNOWLEDGMENTS
We would like to thank the B5 platform members (Swiss National Platform for development of biological bandages for burn wounds) and the Lausanne Burn Center for their collaboration. A special thank you to Mme Murielle Michetti for assistance for illustrations. Open access funding provided by Universite de Lausanne.
TABLE A1.
Local Lausanne University Hospital blood reference ranges of the study amino acids
| Plasmatic reference intervals in adults, µmol/L | ||
|---|---|---|
| Amino acid | Female | Male |
| Alanine | 200–550 | 240–600 |
| Arginine | 25–125 | 35–140 |
| Cysteine | 30–80 | 40–75 |
| Glutamine | 440–810 | 550–830 |
| Leucine | 75–170 | 105–215 |
| Lysine | 115–250 | 135–260 |
| Methionine | 20–40 | 20–45 |
Berger MM, Binz PA, Roux C, et al. Exudative glutamine losses contribute to high needs after burn injury. JPEN J Parenter Enteral Nutr. 2022;46:782–788 10.1002/jpen.2227.
REFERENCES
- 1. Forjuoh SN. Burns in low‐ and middle‐income countries: a review of available literature on descriptive epidemiology, risk factors, treatment, and prevention. Burns. 2006;32(5):529‐537. [DOI] [PubMed] [Google Scholar]
- 2. Peck M, Pressman MA. The correlation between burn mortality rates from fire and flame and economic status of countries. Burns. 2013;39(6):1054‐1059. [DOI] [PubMed] [Google Scholar]
- 3. Berger MM, Pantet O. Nutrition in burn injury: any recent changes? Curr Opin Crit Care. 2016;22(4):285‐291. [DOI] [PubMed] [Google Scholar]
- 4. Wischmeyer PE. Glutamine in burn injury. Nutr Clin Pract. 2019;34(5):681‐687. [DOI] [PubMed] [Google Scholar]
- 5. Barrett LW, Fear VS, Waithman JC, Wood FM, Fear MW. Understanding acute burn injury as a chronic disease. Burns Trauma. 2019;7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wischmeyer PE. Glutamine: mode of action in critical illness. Crit Care Med. 2007;35(9 Suppl):S541‐S44. [DOI] [PubMed] [Google Scholar]
- 7. Rousseau AF, Losser MR, Ichai C, Berger MM. ESPEN endorsed recommendations: nutritional therapy in major burns. Clin Nutr. 2013;32(4):497‐502. [DOI] [PubMed] [Google Scholar]
- 8. Pantet O, Stoecklin P, Vernay A, Berger MM. Impact of decreasing energy intakes in major burn patients: a 15 year retrospective cohort study. Clin Nutr. 2018;36:818‐824. [DOI] [PubMed] [Google Scholar]
- 9. Griffiths RD, Allen KD, Andrews FJ, Jones C. Infection, multiple organ failure, and survival in the intensive care unit: influence of glutamine‐supplemented parenteral nutrition on acquired infection. Nutrition. 2002;18:546‐552. [DOI] [PubMed] [Google Scholar]
- 10. Bongers T, Griffiths RD, McArdle A. Exogenous glutamine: the clinical evidence. Crit Care Med. 2007;35(9):S545‐S552. [DOI] [PubMed] [Google Scholar]
- 11. Moore FA, Phillips SM, McClain CJ, Patel JJ, Martindale RG. Nutrition support for persistent inflammation, immunosuppression, and catabolism syndrome. Nutr Clin Pract. 2017;32(1):121S‐127S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Zanten AR, Dhaliwal R, Garrel D, Heyland DK. Enteral glutamine supplementation in critically ill patients: a systematic review and meta‐analysis. Crit Care. 2015;19(1):294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou YP, Jiang ZM, Sun YH, Wang XR, Ma EL, Wilmore D. The effect of supplemental enteral glutamine on plasma levels, gut function, and outcome in severe burns: a randomized, double‐blind, controlled clinical trial. JPEN J Parenter Enteral Nutr. 2003;27(4):241‐245. [DOI] [PubMed] [Google Scholar]
- 14. Garrel D, Patenaude J, Nedelec B, et al. Decreased mortality and infectious morbidity in adult burn patients given enteral glutamine supplements: a prospective, controlled, randomized clinical trial. Crit Care Med. 2003;31(10):2444‐2449. [DOI] [PubMed] [Google Scholar]
- 15. Pattanshetti VM, Powar RS, Godhi AS, Metgud SC. Enteral glutamine supplementation reducing infectious morbidity in burns patients: a randomised controlled trial. Indian J Surg. 2009;71:193‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coudray‐Lucas C, LeBever H, Cynober L, DeBandt JP, Carsin H. Ornithine α‐ketoglutarate improves wound healing in severe burn patients: a prospective randomized double‐blind trial versus isonitrogenous controls. Crit Care Med. 2000;28(6):1772‐1776. [DOI] [PubMed] [Google Scholar]
- 17. Singer P, Reintam‐Blaser A, Berger MM, et al. ESPEN guidelines: nutrition in the ICU. Clin Nutr. 2019;38(1):48‐79. [DOI] [PubMed] [Google Scholar]
- 18. Lin JJ, Chung XJ, Yang CY, Lau HL. A meta‐analysis of trials using the intention to treat principle for glutamine supplementation in critically ill patients with burn. Burns. 2013;39(4):565‐570. [DOI] [PubMed] [Google Scholar]
- 19. Gonzalez MR, Fleuchot B, Lauciello L, et al. Effect of human burn wound exudate on Pseudomonas aeruginosa virulence. mSphere. 2016;1(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baudoin J, Jafari P, Meuli J, Applegate LA, Raffoul W. Topical negative pressure on burns: an innovative method for wound exudate collection. Plast Reconstr Surg Glob Open. 2016;4:e1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pantet O, Faouzi M, Brusselaers N, Vernay A, Berger MM. Comparison of mortality prediction models and validation of SAPS II in critically ill burns patients. Ann Burns Fire Disasters. 2016;29(2):123‐129. [PMC free article] [PubMed] [Google Scholar]
- 22. Jafari P, Thomas A, Haselbach D, et al. Trace element intakes should be revisited in burn nutrition protocols: a cohort study. Clin Nutr. 2018;37(3):958‐964. [DOI] [PubMed] [Google Scholar]
- 23. Pantet O, Stoecklin P, Charriere M, Voirol P, Vernay A, Berger MM. Trace element repletion following severe burn injury: a dose‐finding cohort study. Clin Nutr. 2019;38(1):246‐251. [DOI] [PubMed] [Google Scholar]
- 24. Kadowaki M, Kanazawa T. Amino acids as regulators of proteolysis. J Nutr. 2003;133(6 suppl 1):2052S‐2056S. [DOI] [PubMed] [Google Scholar]
- 25. Wu G, Bazer FW, Davis TA, et al. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2009;37(1):153‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kohl JB, Mellis AT, Schwarz G. Homeostatic impact of sulfite and hydrogen sulfide on cysteine catabolism. Br J Pharmacol. 2019;176:554‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Berry TH, Becker DE, Rasmussen OG, Jensen AH, Norton H W. The limiting amino acids in soybean protein. J Animal Science. 1962;21(3):558‐561. 10.2527/jas1962.213558x [DOI] [Google Scholar]
- 28. Fürst P, Stehle P. Are intravenous amino acid solutions unbalanced? New Horizons. 1994;2:215‐223. [PubMed] [Google Scholar]
- 29. Bollhalder L, Pfeil AM, Tomonaga Y, Schwenkglenks M. A systematic literature review and meta‐analysis of randomized clinical trials of parenteral glutamine supplementation. Clin Nutr. 2013;32(2):213‐223. [DOI] [PubMed] [Google Scholar]
- 30. Wischmeyer PE, Dhaliwal R, McCall M, Ziegler TR, Heyland DK. Parenteral glutamine supplementation in critical illness: a systematic review. Crit Care. 2014;18(2):R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stehle P, Ellger B, Kojic D, et al. Glutamine dipeptide‐supplemented parenteral nutrition improves the clinical outcomes of critically ill patients: a systematic evaluation of randomised controlled trials. Clin Nutr ESPEN. 2017;17(Feb):75‐85. [DOI] [PubMed] [Google Scholar]
- 32. Kuhn KS, Stehle P, Furst P. Glutamine content of protein and peptide‐based enteral products. JPEN J Parenter Enteral Nutr. 1996;20(4):292‐295. [DOI] [PubMed] [Google Scholar]
- 33. Lenders CM, Liu S, Wilmore DW, et al. Evaluation of a novel food composition database that includes glutamine and other amino acids derived from gene sequencing data. Eur J Clin Nutr. 2009;63(12):1433‐1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Newsholme P, Procopio J, Lima MM, Pithon‐Curi TC, Curi R. Glutamine and glutamate–their central role in cell metabolism and function. Cell Biochem Funct. 2003;21:1‐9. [DOI] [PubMed] [Google Scholar]
- 35. Heyland DK, Wischmeyer P, Jeschke MG, et al. A RandomizEd trial of ENtERal glutamine to minimIZE thermal injury (The RE‐ENERGIZE trial): a clinical trial protocol. Scars Burn Heal. 2017;3:2059513117745241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wijnands KA, Castermans TM, Hommen MP, Meesters DM, Poeze M. Arginine and citrulline and the immune response in sepsis. Nutrients. 2015;7(3):1426‐1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Debats IB, Booi DI, Wehrens KM, et al. Oral arginine supplementation and the effect on skin graft donor sites: a randomized clinical pilot study. J Burn Care Res. 2009;30(3):417‐426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Method of burn wound exudate collection.


