ABSTRACT
The Active‐Controlled Fracture Study in Postmenopausal Women With Osteoporosis at High Risk (ARCH) trial (NCT01631214; https://clinicaltrials.gov/ct2/show/NCT01631214) showed that romosozumab for 1 year followed by alendronate led to larger areal bone mineral density (aBMD) gains and superior fracture risk reduction versus alendronate alone. aBMD correlates with bone strength but does not capture all determinants of bone strength that might be differentially affected by various osteoporosis therapeutic agents. We therefore used quantitative computed tomography (QCT) and finite element analysis (FEA) to assess changes in lumbar spine volumetric bone mineral density (vBMD), bone volume, bone mineral content (BMC), and bone strength with romosozumab versus alendronate in a subset of ARCH patients. In ARCH, 4093 postmenopausal women with severe osteoporosis received monthly romosozumab 210 mg sc or weekly oral alendronate 70 mg for 12 months, followed by open‐label weekly oral alendronate 70 mg for ≥12 months. Of these, 90 (49 romosozumab, 41 alendronate) enrolled in the QCT/FEA imaging substudy. QCT scans at baseline and at months 6, 12, and 24 were assessed to determine changes in integral (total), cortical, and trabecular lumbar spine vBMD and corresponding bone strength by FEA. Additional outcomes assessed include changes in aBMD, bone volume, and BMC. Romosozumab caused greater gains in lumbar spine integral, cortical, and trabecular vBMD and BMC than alendronate at months 6 and 12, with the greater gains maintained upon transition to alendronate through month 24. These improvements were accompanied by significantly greater increases in FEA bone strength (p < 0.001 at all time points). Most newly formed bone was accrued in the cortical compartment, with romosozumab showing larger absolute BMC gains than alendronate (p < 0.001 at all time points). In conclusion, romosozumab significantly improved bone mass and bone strength parameters at the lumbar spine compared with alendronate. These results are consistent with greater vertebral fracture risk reduction observed with romosozumab versus alendronate in ARCH and provide insights into structural determinants of this differential treatment effect. © 2021 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Keywords: BONE STRENGTH, BONE MINERAL CONTENT, FINITE ELEMENT ANALYSIS, POSTMENOPAUSAL OSTEOPOROSIS, QUANTITATIVE COMPUTED TOMOGRAPHY
Introduction
Vertebral fractures are the consequence of compromised bone strength resulting from loss of bone mass and deteriorated bone microstructure.( 1 , 2 ) They are the most common osteoporotic fractures and are associated with the highest risk of subsequent fractures.( 1 , 3 , 4 ) Therapeutic agents that increase the density and strength of vertebrae and other sites prone to fragility fractures are a mainstay of osteoporosis management, and accumulating evidence supports treatment strategies that improve and then maintain bone mineral density (BMD) to desired goals in order to reduce fracture risk.( 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 ) Therapies that stimulate bone formation can quickly increase vertebral BMD, resulting in a rapid reduction in fracture risk.( 8 , 10 , 11 , 13 , 14 )
Ultimately, the efficacy of any therapeutic agent for the treatment of osteoporosis depends on its ability to increase bone strength and reduce fracture risk, especially at the hip and spine.( 12 ) However, direct measurement of bone strength in human subjects is not feasible.( 15 ) Areal BMD (aBMD), measured by dual‐energy x‐ray absorptiometry (DXA), is the most commonly used outcome measure for assessing treatment effects of osteoporosis therapeutic agents.( 12 ) Although aBMD correlates with bone strength,( 16 ) it does not capture all determinants of bone strength such as changes in microarchitecture and does not distinguish between cortical and trabecular compartments, which may be differentially affected by different therapeutic agents.( 2 , 17 , 18 , 19 , 20 )
An alternative and US Food and Drug Administration (FDA)‐cleared approach to evaluate treatment effects, including estimated changes in bone strength, is finite element analysis (FEA) of quantitative computed tomography (QCT) scans.( 2 , 15 , 21 ) This technique, also known as biomechanical computed tomography,( 21 ) provides robust estimates of bone strength and can also discriminate effects on cortical versus trabecular compartments, enabling a better understanding of specific effects of different osteoporosis therapies on each compartment.
Romosozumab (EVENITY®; romosozumab‐aqqg in the US),( 22 ) a bone‐forming agent with the dual effect of increasing bone formation and decreasing bone resorption,( 23 , 24 ) has been approved in several countries. Monthly romosozumab 210 mg sc for 12 months produced larger gains in lumbar spine and total hip aBMD by DXA( 23 , 25 ) and reduced the risk of fractures compared with placebo( 13 ) and alendronate.( 14 ) In the Active‐Controlled Fracture Study in Postmenopausal Women With Osteoporosis at High Risk (ARCH) trial,( 14 ) 1 year of romosozumab reduced the risk of vertebral, clinical, nonvertebral, and hip fractures compared with alendronate. Fracture risk reduction was maintained when patients were transitioned to alendronate.( 14 )
The ARCH trial included a protocol‐specified substudy that had an imaging component in which a subset of eligible patients had QCT scans of the lumbar spine taken at the specified time points for assessing volumetric bone mineral density (vBMD) and corresponding bone strength by FEA. The substudy was performed to characterize the effect of romosozumab and alendronate on vBMD and bone strength of different bone compartments, which may contribute to a better understanding of the greater vertebral fracture risk reduction observed with romosozumab versus alendronate in ARCH. In this article, we report results from the QCT/FEA imaging component of the ARCH substudy to determine the effect of romosozumab followed by alendronate on changes in aBMD, vBMD, and bone strength at the lumbar spine compared with alendronate alone. We also report results of additional outcomes from the substudy including effect on bone volume and bone mineral content (BMC) at the lumbar spine; relationships of changes in bone strength with changes in aBMD, vBMD, and BMC at the lumbar spine; and characteristics of patients who developed new vertebral fractures on study.
Patients and Methods
Study design and patient population
This protocol‐specified analysis was based on ARCH (NCT01631214; https://clinicaltrials.gov/ct2/show/NCT01631214), a phase 3, multicenter, international, randomized, active‐controlled, double‐blind study in postmenopausal women with osteoporosis and a prior fragility fracture. Details of the ARCH study have been published.( 14 ) Briefly, postmenopausal women with low BMD (T‐score ≤ −2.5) and a prior fragility fracture were randomized 1:1 to receive monthly romosozumab 210 mg sc or weekly oral alendronate 70 mg for 12 months (Supplemental Figure S1). After completion of the double‐blind study period, all patients received open‐label weekly oral alendronate 70 mg through end of study, blinded to initial treatment assignment. Patients received daily calcium and vitamin D.( 14 )
A subset of patients in ARCH at study sites with CT availability were enrolled in the preplanned ARCH imaging substudy. Patients were ineligible for the ARCH imaging substudy if they experienced a nonvertebral fracture or clinical vertebral fracture within 6 months before enrollment or had non‐evaluable vertebrae in the region of interest for spine QCT scans as assessed by the central imaging vendor at the time of screening, based on lateral spine x‐rays. This report is focused on results from the QCT/FEA analysis in the subset of patients enrolled in the imaging component of the ARCH substudy.
Study procedures
aBMD was measured by DXA (Lunar, GE Medical Systems, Madison, WI, USA or Hologic, Hologic, Inc., Bedford, MA, USA) at screening/baseline and every 12 months thereafter at the lumbar spine; in a subset of 167 patients enrolled in the substudy, aBMD was also measured at months 6 and 18. A central laboratory (BioClinica, previously known as Synarc, Newark, CA, USA) analyzed the scans blinded to treatment assignments and provided quality control of the individual scans and densitometers.
QCT scans of the lumbar spine were obtained using whole‐body spiral CT scanners (with at least six detector rows) from four CT manufacturers (Siemens, Munich, Germany; GE, Boston, MA, USA; Philips, Amsterdam, Netherlands; and Toshiba, Minato, Tokyo, Japan). Scans were performed at baseline and at months 6, 12, and 24. By default, the CT scan covered the range from mid‐T12 to mid‐L3 and vertebrae L1 and L2 were analyzed. However, if any of these two vertebrae were fractured at baseline, the scan range was adjusted to analyze a different set of two unfractured vertebrae; for example, if L2 was fractured, the scan was adjusted, covering the range from mid‐T11 to mid‐L4, to analyze T12 and L1, L1 and L3 (Supplemental Figure S2), or T12 and L3.
Lumbar spine scans were performed at 120 kV with a pitch of 1 using default 100 mAs. Automatic exposure control techniques integrated into the CT scanner acquisition software were used, if available, to reduce radiation exposure. Patients were scanned while lying supine on top of the bone density calibration (BDC) phantom (QRM GmbH, Möhrendorf, Germany). For each scan, two datasets were reconstructed: a large field of view (FOV) reconstruction of 360 mm, which was selected for the analysis of the calibration phantom; and a smaller FOV reconstruction of 120 mm centered on the vertebrae selected for the analysis of vBMD, bone volume, and BMC of the different bone compartments. A medium kernel and a slice thickness of 1 or 1.25 mm were used to reconstruct the CT datasets using the technique of filtered back projection.
For each subject scan, the BDC phantom was used to determine vBMD from the measured CT values. For cross‐calibration of BMD results among the different CT devices, a European Spine Phantom (ESP; QRM GmbH) was scanned on all CT scanners included in the study. The scanner‐specific differences between the measured and the known nominal vBMD values of the ESP were used to cross‐calibrate the vBMD results of the individual subjects. A QRM Spine Phantom (QSP) was used to monitor the longitudinal stability of the scanner at each CT facility (Supplemental Figure S3). Precision errors of the technique reported as root mean square coefficient of variation are 1.2%, 1.5%, and 2.3% for integral, trabecular, and cortical vBMD, respectively.( 26 )
BioClinica analyzed the QCT scans blinded to treatment assignments using Medical Imaging Analysis Framework (MIAF) Spine software (version 5.0.0MR) as described.( 27 ) Briefly, a three‐dimensional (3D) segmentation technique was used to establish the periosteal surface of each vertebral body, which defined the integral volume of interest (VOI) (Supplemental Figure S2). The endosteal surface together with the periosteal surface defined the cortical bone compartment (which appears as a thin shell of ≤0.5 mm in the human spine), as previously described.( 28 ) Then the outer 2 mm of the volume enclosed by the endosteal surface were “peeled off”; that is, removed. The remaining volume was defined as the trabecular bone compartment. The selection of a 2‐mm peeling distance was based on phantom measurements to account for blurring artifacts introduced by the limited spatial resolution of the CT scanners.( 29 ) In this study, the peeled volume termed subcortical VOI was not included in any analysis. vBMD and bone volume were measured for the integral bone as well as for the cortical shell and trabecular bone. BMC was determined from vBMD and bone volume of the respective integral bone, cortical shell, and trabecular bone.
To estimate bone strength at the lumbar spine, O.N. Diagnostics (Berkeley, CA, USA) analyzed the QCT large FOV scans, blinded to treatment assignments and MIAF measurements, using the VirtuOst software (version 2.1; O.N. Diagnostics), previously described.( 15 ) Briefly, following segmenting, calibrating, registering, and resampling the images, the scans from one vertebral level were converted into finite element models by converting image voxels into cube‐shaped finite elements (1.0 mm size to produce finite element models having approximately 40,000 elements).( 15 , 30 ) A thin layer of plastic was virtually applied over each endplate through which the vertebral body was loaded to simulate failure for a uniform compressive overload. The bones were virtually loaded to failure to estimate the breaking strength (defined from the resulting nonlinear force‐deformation curves as the force at 1.9% overall deformation). The precision error for these vertebral strength measurements is 0.6%.( 31 ) Additional controlled variations of the models were performed to provide estimates of the changes in overall strength associated with changes in only the cortical shell (thin outer 2 mm ring of bone) and trabecular bone. Following standard procedures, trabecular bone strength was computed using models that had the outer 2 mm of bone virtually removed and the strength of the cortical shell was calculated as the overall spine strength minus the spine trabecular strength. More QCT scans could be evaluated by VirtuOst than MIAF for reasons detailed in Supplemental Methods.
To provide mechanistic insight into the treatment effects, animations of the FEA‐based virtual stress testing were developed to illustrate changes in the predicted failure behavior within the vertebra at baseline and at months 6, 12, and 24. Internal bone failure was visualized for when the bone was virtually to the level of vertebral strength (4500 N). This force level equals the threshold vertebral strength value for “fragile bone strength,” below which clinical studies have shown women to be at high risk of an incident vertebral fracture.( 32 , 33 ) Thus, for the individual patient, there is presumably a high risk of a future vertebral fracture if the patient's vertebra is loaded to this force level and cannot withstand that level of force. Visualizing the predicted internal failure of bone tissue at this level of loading therefore provides unique mechanistic insight into how well the bone can resist such high‐risk loading, with greater amounts of failed tissue indicating lower resistance; changes to the distribution of such failed tissue also point to how the treatment is effective biomechanically in altering overall vertebral strength. To illustrate internal tissue failure during this virtual loading, two representative patients (one from each treatment group) with similar baseline characteristics (including baseline bone strength and density values) and showing a typical response to each therapy were selected. For comparison across the four visits at baseline and at months 6, 12, and 24, each patient's vertebra was virtually loaded to 2% strain or to 4500 N, whichever led to a lower force. After the models were computed, the internal regions of failed tissue were graphically displayed as a sectioned 3D model in a visualization program as the bone was virtually loaded to higher and higher levels of force. VirtuOst was used for all simulations.
Lateral radiographs of the lumbar spine taken at screening and at months 12 and 24 were assessed at a central imaging center to identify patients who developed new vertebral fractures during the study, as previously described.( 13 )
Study outcomes
Protocol‐specified outcomes for the QCT/FEA imaging component of the ARCH substudy were percentage change from baseline in lumbar spine aBMD by DXA, percentage change from baseline in lumbar spine (integral and trabecular) vBMD by QCT, and the corresponding percentage change from baseline in lumbar spine vertebral‐estimated bone strength by QCT‐derived FEA. Additional outcomes were percentage change from baseline in cortical lumbar spine vBMD by QCT; percentage change from baseline in lumbar spine bone volume (integral, cortical, and trabecular) by QCT; and absolute and percentage change from baseline in lumbar spine BMC (integral, cortical, trabecular) by QCT. We then used Spearman's correlation (R) to assess the relationships of postbaseline absolute changes in FEA bone strength with postbaseline absolute changes in integral DXA aBMD, integral QCT vBMD, and integral QCT BMC at the lumbar spine. We also assessed the characteristics of patients who developed new vertebral fractures during the study.
Statistical analysis
aBMD analyses included patients with aBMD values at baseline and one or more postbaseline DXA visits and with QCT values at baseline and one or more postbaseline QCT visits. Summary statistics (mean, standard deviation, and important percentiles) and box plots were provided for observed aBMD percentage change from baseline. Least squares (LS) mean aBMD percentage change from baseline was estimated by analysis of covariance (ANCOVA) model, adjusting for presence of severe vertebral fracture at baseline, baseline value, machine type, and baseline aBMD value‐by‐DXA scanner type interaction. Missing values were imputed by the last postbaseline observation carried forward method.
vBMD, bone volume, BMC, and bone strength analyses included patients with values of the outcome of interest at baseline and one or more postbaseline QCT visits derived from scans that could be analyzed by the MIAF or VirtuOst software. Summary statistics and box plots were provided for observed vBMD, bone volume, and bone strength percentage change from baseline. LS mean percentage change from baseline for these parameters were estimated by the ANCOVA model, adjusting for presence of severe vertebral fracture at baseline and corresponding baseline value of the outcome of interest. BMC absolute change from baseline was summarized by box plots and evaluated by the ANCOVA model. For these ANCOVA analyses, missing values were imputed by the last postbaseline observation carried forward method. Analyses of correlation coefficients included randomized patients with baseline and one or more postbaseline reported data for the outcomes of interest. In addition, multivariate linear regression was used to model the difference of the effect of integral DXA aBMD, integral QCT vBMD, and integral QCT BMC on integral FEA bone strength between the two treatment groups.
Results
Patients and baseline demographics
ARCH enrolled 4093 patients (2046 romosozumab, 2047 alendronate); 167 of these patients were enrolled in the ARCH imaging substudy (Supplemental Figure S4). Of the patients in the imaging substudy, 90 (49 romosozumab, 41 alendronate) participated in the QCT/FEA imaging component (Supplemental Figure S4). Months 6 and 12 assessments were during the double‐blind period when patients received monthly romosozumab 210 mg sc or weekly oral alendronate 70 mg for 12 months; months 18 and 24 assessments were during the open‐label period when all patients received open‐label weekly oral alendronate 70 mg through the end of the study. Most patients had both QCT vBMD/bone volume/BMC and FEA bone strength assessments: 76 (40 romosozumab, 36 alendronate) had baseline and one or more postbaseline scans that could be analyzed by MIAF to derive vBMD/bone volume/BMC assessments and 86 (47 romosozumab, 39 alendronate) had baseline and one or more postbaseline scans that could be analyzed by VirtuOst software to derive bone strength assessments (Supplemental Figure S4). For the ANCOVA analyses, missing values were imputed using last postbaseline observation carried forward, with ≥15% of the values being imputed (Supplemental Table S1).
Baseline demographic and clinical characteristics for the treatment groups in the QCT/FEA imaging component of the ARCH substudy did not differ significantly from each other and were similar to those of the overall ARCH study population (Table 1, Supplemental Table S2). Mean baseline DXA T‐scores for the romosozumab and alendronate groups at the lumbar spine were −2.8 and −3.4, respectively, for the QCT/FEA imaging component of the ARCH substudy population and −2.9 and −3.0, respectively, for the ARCH overall study population.( 14 )
TABLE 1.
Baseline demographic and clinical characteristics of patients in the ARCH QCT/FEA substudy and the ARCH overall study
| Patients included in the ARCH QCT/FEA substudy | Patients included in the ARCH overall study | |||
|---|---|---|---|---|
| Characteristic | Romosozumab/alendronate n = 49 | Alendronate/alendronate n = 41 | Romosozumab/alendronate n = 2046 | Alendronate/alendronate n = 2047 |
| Age (years), mean ± SD | 73.1 ± 7.1 | 72.8 ± 7.6 | 74.4 ± 7.5 | 74.2 ± 7.5 |
| aBMD T‐score, mean ± SD | ||||
| Lumbar spine | −2.8 ± 1.2 | −3.4 ± 1.0 | −2.9 ± 1.3 | −3.0 ± 1.2 |
| Total hip | −2.7 ± 0.7 | −2.8 ± 0.7 | −2.8 ± 0.7 | −2.8 ± 0.7 |
| Femoral neck | −2.8 ± 0.4 | −2.8 ± 0.5 | −2.9 ± 0.5 | −2.9 ± 0.5 |
| QCT lumbar spine vBMD (mg/cm3), mean ± SD | ||||
| Integral | 130.3 ± 25.0 | 120.5 ± 27.5 | ND | ND |
| Cortical | 284.6 ± 41.8 | 270.9 ± 51.4 | ND | ND |
| Trabecular | 60.1 ± 18.0 | 53.7 ± 20.6 | ND | ND |
| FEA vertebral strength (N), mean ± SD | ||||
| Integral | 3459 ± 906 | 3192 ± 775 | ND | ND |
| Cortical | 2105 ± 467 | 1988 ± 431 | ND | ND |
| Trabecular | 1354 ± 514 | 1203 ± 422 | ND | ND |
| Previous osteoporotic fracture at ≥45 years of age, n1 (%) | 48 (98.0) | 41 (100.0) | 2022 (98.8) | 2029 (99.1) |
| Prevalent vertebral fracture, n1 (%) | 46 (93.9) | 41 (100.0) | 1969 (96.2) | 1964 (95.9) |
| Severe | 27 (55.1) | 24 (58.5) | 1369 (66.9) | 1321 (64.5) |
| Previous nonvertebral fracture at ≥45 years of age, n1 (%) | 23 (46.9) | 16 (39.0) | 767 (37.5) | 770 (37.6) |
| History of hip fracture, n1 (%) | 3 (6.1) | 0 (0) | 175 (8.6) | 179 (8.7) |
Notes: Previous osteoporotic fractures include both nonvertebral and prevalent vertebral fractures, excluding high trauma and pathologic fractures. n = number of randomized patients enrolled in the QCT/FEA imaging component of the ARCH substudy and with values at baseline and ≥1 postbaseline QCT visit or number of randomized patients enrolled in the ARCH overall study. n1 = number of patients with the characteristic.
Abbreviations: aBMD, areal bone mineral density; ARCH, Active‐Controlled Fracture Study in Postmenopausal Women With Osteoporosis at High Risk; FEA, finite element analysis; ND, not determined; QCT, quantitative computed tomography; vBMD, volumetric bone mineral density.
Lumbar spine aBMD by DXA
Patients in both the romosozumab and alendronate treatment groups experienced significant gains in lumbar spine aBMD from baseline at months 6, 12, 18, and 24 (Figure 1 and Supplemental Figure S5). Significantly larger aBMD gains were observed with romosozumab compared with alendronate in the double‐blind period, with LS mean differences of 7.8%, p < 0.001 at month 6 and 10.3%, p < 0.001 at month 12 (Figure 1). These differences between treatment groups persisted upon transition to open‐label alendronate, such that significantly larger aBMD gains from baseline were observed with romosozumab‐to‐alendronate compared with alendronate‐to‐alendronate in the open‐label period through month 24, with LS mean differences of 9.6%, p < 0.001 at month 18 and 9.6%, p < 0.001 at month 24 (Figure 1).
FIGURE 1.
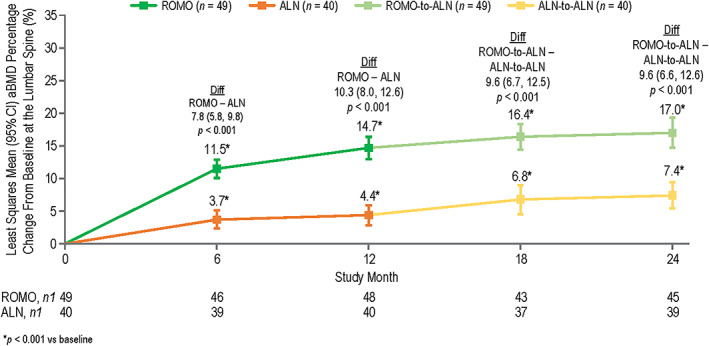
Least squares mean aBMD percentage change from baseline at the lumbar spine with romosozumab or alendronate treatment at months 6, 12, 18, and 24 by DXA. n = number of randomized patients enrolled in the QCT/FEA imaging component of the ARCH substudy with values at baseline and one or more postbaseline DXA visit at month 6 or month 18 and with values at baseline and one or more postbaseline QCT visit; n1 = number of patients with values at that time point. Month 6 and month 12 measurements were during the double‐blind period when patients received monthly romosozumab 210 mg sc or weekly oral alendronate 70 mg for 12 months; month 18 and month 24 measurements were during the open‐label period when patients received weekly oral open‐label alendronate 70 mg for 12 months. Data were based on ANCOVA model, adjusting for presence of severe vertebral fracture at baseline, baseline aBMD value, machine type, and baseline aBMD value‐by‐machine type interaction. Missing values were imputed by carrying forward the last nonmissing postbaseline value prior to the missing value and within the treatment period. Abbreviations: aBMD, areal bone mineral density; ALN, alendronate; ANCOVA, analysis of covariance; ARCH, Active‐Controlled Fracture Study in Postmenopausal Women With Osteoporosis at High Risk; Diff, difference between the treatment groups; DXA, dual‐energy x‐ray absorptiometry; FEA, finite element analysis; QCT, quantitative computed tomography; ROMO, romosozumab.
Lumbar spine vBMD, bone volume, and BMC by QCT
As noted in the Patients and Methods section, the peeled volume termed subcortical VOI was not included in any of the QCT analyses reported in this study. As a result, the sum of the gains in cortical plus trabecular vBMD, bone volume, or BMC were less than the gains in the total integral bone for each respective parameter for both romosozumab and alendronate (Figure 2; Supplemental Figures S6, S7, and S8; Table 2; Supplemental Tables S3 and S4).
FIGURE 2.
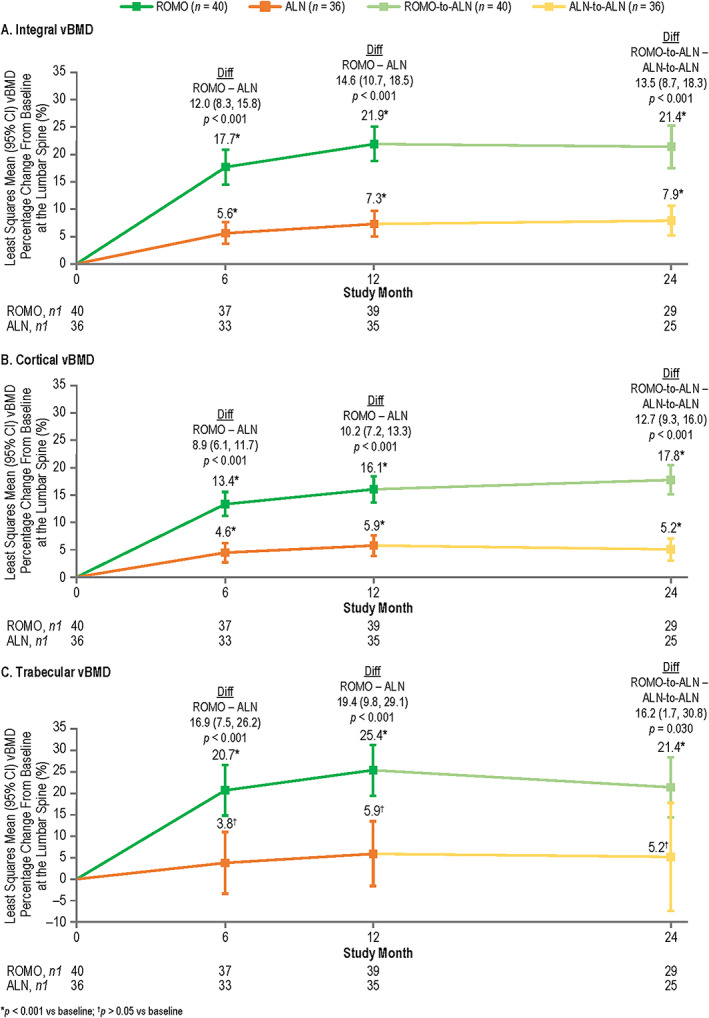
Least squares mean vBMD percentage change from baseline at the lumbar spine with romosozumab or alendronate treatment at months 6, 12, and 24 by QCT: integral vBMD (A), cortical vBMD (B), and trabecular vBMD (C). n = number of randomized patients enrolled in the QCT/FEA imaging component of the ARCH substudy with values at baseline and one or more postbaseline visits; n1 = number of patients with values at that time point. Month 6 and month 12 measurements were during the double‐blind period when patients received monthly romosozumab 210 mg sc or weekly oral alendronate 70 mg for 12 months; month 24 measurements were during the open‐label period when patients received open‐label weekly oral alendronate 70 mg for 12 months. Data were based on ANCOVA model, adjusting for presence of severe vertebral fracture at baseline and baseline vBMD value. Missing values were imputed by carrying forward the last nonmissing postbaseline value prior to the missing value and within the treatment period. Abbreviations: ALN, alendronate; ANCOVA, analysis of covariance; ARCH, Active‐Controlled Fracture Study in Postmenopausal Women With Osteoporosis at High Risk; Diff, difference between the treatment groups; FEA, finite element analysis; QCT, quantitative computed tomography; ROMO, romosozumab; vBMD, volumetric bone mineral density.
TABLE 2.
Least squares mean vBMD percentage change from baseline at the lumbar spine (integral, cortical, and trabecular) with romosozumab or alendronate treatment at months 6, 12, and 24
| Least squares mean vBMD percentage change from baseline | ||||
|---|---|---|---|---|
| Parameter |
Romosozumab/alendronate (n = 40) % (95% CI) |
Alendronate/alendronate (n = 36) % (95% CI) |
Difference (romosozumab − alendronate) % (95% CI) |
p for difference |
| Month 6 | ||||
| Integral | 17.7 (14.5, 20.8)* | 5.6 (3.7, 7.6)* | 12.0 (8.3, 15.8) | p < 0.001 |
| Cortical | 13.4 (11.3, 15.6)* | 4.6 (2.8, 6.4)* | 8.9 (6.1, 11.7) | p < 0.001 |
| Trabecular | 20.7 (14.8, 26.6)* | 3.8 (−3.3, 11.0) † | 16.9 (7.5, 26.2) | p < 0.001 |
| Difference (trabecular minus cortical) | 7.5 (3.0, 11.9); p = 0.001 | −1.0 (−8.0, 6.0); p = 0.780 | 8.5 (0.2, 16.7) | p = 0.045 |
| Month 12 | ||||
| Integral | 21.9 (18.8, 25.1)* | 7.3 (5.0, 9.7)* | 14.6 (10.7, 18.5) | p < 0.001 |
| Cortical | 16.1 (13.7, 18.5)* | 5.9 (4.0, 7.7)* | 10.2 (7.2, 13.3) | p < 0.001 |
| Trabecular | 25.4 (19.4, 31.3)* | 5.9 (−1.6, 13.5) † | 19.4 (9.8, 29.1) | p < 0.001 |
| Difference (trabecular minus cortical) | 9.3 (4.8, 13.9); p < 0.001 | 0.1 (−7.0, 7.0); p = 0.990 | 9.3 (0.9, 17.6) | p = 0.031 |
| Month 24 | ||||
| Integral | 21.4 (17.5, 25.3)* | 7.9 (5.2, 10.6)* | 13.5 (8.7, 18.3) | p < 0.001 |
| Cortical | 17.8 (15.2, 20.5)* | 5.2 (3.2, 7.2)* | 12.7 (9.3, 16.0) | p < 0.001 |
| Trabecular | 21.4 (14.4, 28.4)* | 5.2 (−7.4, 17.8) † | 16.2 (1.7, 30.8) | p = 0.030 |
| Difference (trabecular minus cortical) | 3.9 (−2.3, 10.0); p = 0.220 | −0.3 (−12.9, 12.2); p = 0.960 | 4.2 (−9.9, 18.2) | p = 0.55 |
Notes: n = number of randomized patients enrolled in the QCT/FEA imaging component of the ARCH substudy and with values at baseline and ≥1 postbaseline QCT visit. Month 6 and month 12 measurements were during the double‐blind period where patients received monthly romosozumab 210 mg sc or weekly oral alendronate 70 mg for 12 months; month 24 measurements were during the open‐label period when patients received open‐label weekly oral alendronate 70 mg for 12 months. Data were based on ANCOVA model adjusting for treatment, presence of severe vertebral fracture at baseline, and baseline vBMD value. Missing values were imputed by carrying forward the last nonmissing postbaseline value prior to the missing value and within the treatment period.
Abbreviations: ANCOVA, analysis of covariance; ARCH, Active‐Controlled Fracture Study in Postmenopausal Women With Osteoporosis at High Risk; CI, confidence interval; FEA, finite element analysis; QCT, quantitative computed tomography; vBMD, volumetric bone mineral density.
p < 0.001 versus baseline.
p > 0.05 versus baseline.
Patients in both the romosozumab and alendronate treatment groups experienced significant gains in lumbar spine vBMD from baseline at months 6, 12, and 24 (Figure 2, Supplemental Figure S6, Table 2). Significant gains in integral, cortical, and trabecular vBMD were observed with romosozumab at months 6 and 12, and with romosozumab‐to‐alendronate at month 24 (Figure 2, Table 2). Significant gains in integral and cortical vBMD were also observed with alendronate at months 6, 12, and 24 (Figure 2A,B; Table 2). Trabecular vBMD tended to increase with alendronate but the LS mean gains did not reach statistical significance at any time point (Figure 2 C, Table 2).
Similar to what was observed for DXA aBMD, significantly larger gains from baseline in integral vBMD were observed with romosozumab compared with alendronate at months 6 and 12 (LS mean difference of 12.0% and 14.6%, respectively), and with romosozumab‐to‐alendronate compared with alendronate‐to‐alendronate at month 24 (LS mean difference of 13.5%), with p < 0.001 for all comparisons (Figure 2 A, Table 2). Significantly larger gains in cortical and trabecular vBMD were also observed with romosozumab compared with alendronate at month 6 (LS mean difference of 8.9%, p < 0.001 and 16.9%, p < 0.001, respectively) and month 12 (LS mean difference of 10.2%, p < 0.001 and 19.4%, p < 0.001, respectively), and with romosozumab‐to‐alendronate compared with alendronate‐to‐alendronate at month 24 (LS mean difference of 12.7%, p < 0.001 and 16.2%, p = 0.030, respectively) (Figure 2B,C ; Table 2).
When expressed in percentage gains, treatment with romosozumab was associated with significantly larger changes in trabecular vBMD than in cortical vBMD at months 6 and 12 (LS mean difference [trabecular minus cortical] of 7.5%, p = 0.001 at month 6; 9.3%, p < 0.001 at month 12) and numerically larger gains at month 24 (3.9%, p = 0.220) (Table 2). Alendronate treatment resulted in similar percentage gains in cortical and trabecular vBMD at all time points (LS mean difference [trabecular minus cortical] of −1.0%, p = 0.780 at month 6; 0.1%, p = 0.990 at month 12; and − 0.3%, p = 0.960 at month 24) (Table 2).
Integral bone volume did not increase significantly at months 6, 12, and 24 relative to baseline levels for patients in both the romosozumab and alendronate groups (Supplemental Table S3). By treatment, romosozumab had significantly larger cortical bone volume gains and significantly larger reductions in trabecular VOI compared with alendronate at months 6, 12, and 24 (Supplemental Table S3).
For BMC, patients in both the romosozumab and alendronate treatment groups experienced significant integral, cortical, and trabecular absolute and percentage BMC gains at months 6, 12, and 24 (Supplemental Figures S7 and S8, Supplemental Table S4). BMC comparison by treatment showed that most of the newly formed bone is deposited in the cortical shell compared with the trabecular bone, with romosozumab showing larger absolute BMC gains compared with alendronate (Supplemental Table S4).
Lumbar spine bone strength by FEA
Patients in both treatment groups experienced significant gains in integral, cortical, and trabecular bone strength at all time points (except alendronate at month 24) (Figure 3, Supplemental Figure S9, Table 3). Significantly larger increases in integral, cortical, and trabecular bone strength were observed with romosozumab versus alendronate at all time points (Figure 3 A‐C, Table 3). Within each treatment group, improvements in bone strength were similar in the cortical and trabecular compartments at all time points (p > 0.050 for all comparisons) (Table 3).
FIGURE 3.
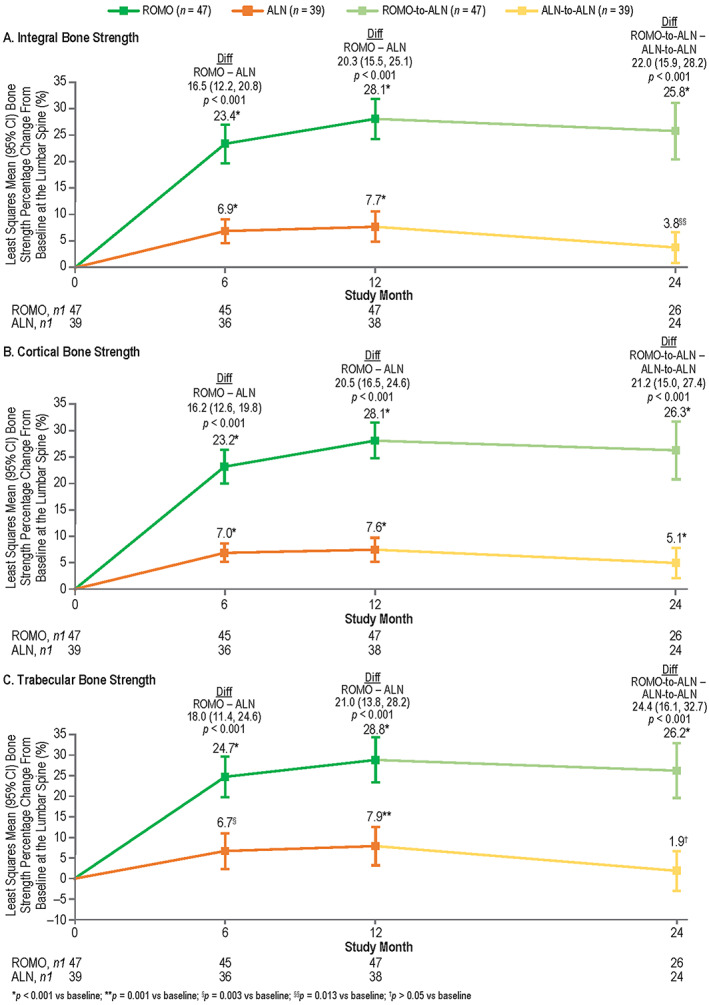
Least squares mean bone strength percentage change from baseline at the lumbar spine with romosozumab or alendronate treatment at months 6, 12, and 24 by FEA: integral bone strength (A), cortical bone strength (B), and trabecular bone strength (C). n = number of randomized patients enrolled in the QCT/FEA imaging component of the ARCH substudy with values at baseline and one or more postbaseline visits; n1 = number of patients with values at that time point. Month 6 and month 12 measurements were during the double‐blind period where patients received monthly romosozumab 210 mg sc or weekly oral alendronate 70 mg for 12 months; month 24 measurements were during the open‐label period when patients received weekly oral open‐label alendronate 70 mg for 12 months. Data were based on ANCOVA model, adjusting for presence of severe vertebral fracture at baseline and baseline FEA value. Missing values were imputed by carrying forward the last nonmissing postbaseline value prior to the missing value and within the treatment period. Abbreviations: ALN, alendronate; ANCOVA, analysis of covariance; ARCH, Active‐Controlled Fracture Study in Postmenopausal Women With Osteoporosis at High Risk; Diff, difference between the treatment groups; FEA, finite element analysis; QCT, quantitative computed tomography; ROMO, romosozumab.
TABLE 3.
Least squares mean bone strength percentage change from baseline at the lumbar spine (integral, cortical, and trabecular) with romosozumab or alendronate treatment at months 6, 12, and 24
| Least squares mean bone strength percentage change from baseline | ||||
|---|---|---|---|---|
| Parameter | Romosozumab/alendronate (n = 47) % (95% CI) | Alendronate/alendronate (n = 39) % (95% CI) | Difference (romosozumab – alendronate) % (95% CI) | p for difference |
| Month 6 | ||||
| Integral | 23.4 (19.7, 27.0)* | 6.9 (4.6, 9.1)* | 16.5 (12.2, 20.8) | p < 0.001 |
| Cortical | 23.2 (20.0, 26.4)* | 7.0 (5.3, 8.7)* | 16.2 (12.6, 19.8) | p < 0.001 |
| Trabecular | 24.7 (19.7, 29.6)* | 6.7 (2.3, 11.0) § | 18.0 (11.4, 24.6) | p < 0.001 |
| Difference (cortical minus trabecular) | −1.3 (−4.3, 1.7); p = 0.390 | 0.1 (−3.2, 3.4); p = 0.940 | −1.4 (−5.9, 3.0); p = 0.520 | |
| Month 12 | ||||
| Integral | 28.1 (24.3, 31.9)* | 7.7 (4.9, 10.6)* | 20.3 (15.5, 25.1) | p < 0.001 |
| Cortical | 28.1 (24.8, 31.5)* | 7.6 (5.4, 9.8)* | 20.5 (16.5, 24.6) | p < 0.001 |
| Trabecular | 28.8 (23.4, 34.3)* | 7.9 (3.2, 12.5)** | 21.0 (13.8, 28.2) | p < 0.001 |
| Difference (cortical minus trabecular) | −0.4 (−4.3, 3.4); p = 0.830 | −0.7 (−3.6, 2.3); p = 0.650 | 0.3 (−4.6, 5.1); p = 0.920 | |
| Month 24 | ||||
| Integral | 25.8 (20.5, 31.1)* | 3.8 (0.8, 6.7) §§ | 22.0 (15.9, 28.2) | p < 0.001 |
| Cortical | 26.3 (20.8, 31.7)* | 5.1 (2.3, 8.0)* | 21.2 (15.0, 27.4) | p < 0.001 |
| Trabecular | 26.2 (19.6, 32.9)* | 1.9 (−2.9, 6.7) † | 24.4 (16.1, 32.7) | p < 0.001 |
| Difference (cortical minus trabecular) | 0.1 (−4.9, 5.0); p = 0.970 | 3.2 (−1.4, 7.7); p = 0.160 | −3.1 (−9.8, 3.6); p = 0.360 | |
Notes: n = number of randomized patients enrolled in the QCT/FEA imaging component of the ARCH substudy and with values at baseline and ≥1 postbaseline QCT visit. Month 6 and month 12 measurements were during the double‐blind period where patients received monthly romosozumab 210 mg sc or weekly oral alendronate 70 mg for 12 months; month 24 measurements were during the open‐label period when patients received open‐label weekly oral alendronate 70 mg for 12 months. Data were based on ANCOVA model, adjusting for treatment, presence of severe vertebral fracture at baseline, and baseline FEA value. Missing values were imputed by carrying forward the last nonmissing postbaseline value prior to the missing value and within the treatment period.
Abbreviations: ANCOVA, analysis of covariance; ARCH, Active‐Controlled Fracture Study in Postmenopausal Women With Osteoporosis at High Risk; CI, confidence interval; FEA, finite element analysis; QCT, quantitative computed tomography.
p < 0.001 versus baseline.
p = 0.001 versus baseline.
p = 0.003 versus baseline.
p = 0.013 versus baseline.
p > 0.05 versus baseline.
We also performed virtual stress testing by applying simulated loads to model the vertebra's structural response over the course of treatment when loaded to a 4500‐N level of force that is associated with a high risk of fracture (Supplemental Figure S10, Supplemental Video S1). For a representative patient in each treatment group, appreciable failure occurred within the vertebra at baseline in both cases because the vertebral strengths at baseline of approximately 3700 N were less than the applied force of 4500 N. For the representative patient in the romosozumab‐to‐alendronate group (patient 1), by month 6, vertebral strength increased to 5010 N (36% gain from baseline), reflected by reduced failure regions at month 6 for a virtual loading of 4500 N; similarly, strength continued to increase and failure regions reduced at month 12 (vertebral strength of 5350 N, 45% gain from baseline), and again at month 24 (vertebral strength of 5560 N, 51% gain from baseline). By month 24, failure regions, which at baseline occurred in both cortical and trabecular regions, were restricted primarily to the trabecular region. By contrast, for the representative patient in the alendronate‐to‐alendronate group (patient 2), although vertebral strength increased significantly over time the strength remained below the applied force of 4500 N (strength of 4000 N at month 6, 8% gain from baseline; 4220 N at month 12, 14% gain from baseline; and 4170 N at month 24, 12% gain from baseline) and therefore the predicted failure regions within the bone did not change considerably over time.
Relationships between changes in bone strength with changes in aBMD, vBMD, and BMC at the lumbar spine
Significant correlations were observed between postbaseline absolute change in integral FEA bone strength and postbaseline absolute change in integral DXA aBMD at the lumbar spine for both romosozumab‐to‐alendronate (n1 = 115; R = 0.52, p < 0.001) and alendronate‐to‐alendronate groups (n1 = 96; R = 0.32, p = 0.002) (Figure 4A ); where n1 is the number of evaluable measurements with one or more measurements per patient. Relationships between integral bone strength and integral absolute aBMD change were significantly different between the two treatment groups. For a given observed absolute change in integral aBMD, there was generally a larger increase in bone strength per increase in integral aBMD for the romosozumab‐to‐alendronate group compared with the alendronate‐to‐alendronate group (slope difference: 4.74 N/mg/cm2, p < 0.001).
FIGURE 4.
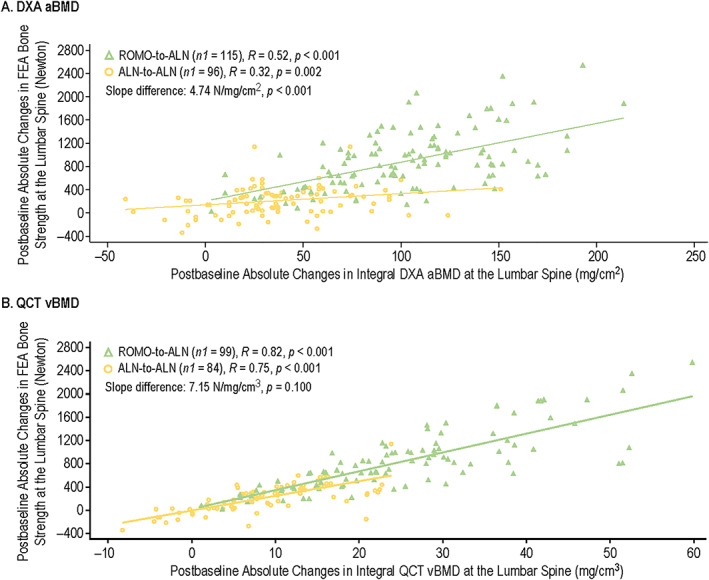
Correlation of postbaseline absolute changes in integral FEA bone strength and postbaseline absolute changes in integral DXA aBMD (A) and integral QCT vBMD (B) at the lumbar spine for romosozumab‐to‐alendronate and alendronate‐to‐alendronate groups through month 24. Includes randomized patients enrolled in the QCT/FEA imaging component of the ARCH substudy with baseline and one or more postbaseline reported results for the parameters of interest. n1 = number of evaluable measurements, with one or more measurements per patient. Month 6 and month 12 measurements were during the double‐blind period where patients received monthly romosozumab 210 mg sc or weekly oral alendronate 70 mg for 12 months; month 24 measurements were during the open‐label period when patients received open‐label weekly oral alendronate 70 mg for 12 months. Abbreviations: aBMD, areal bone mineral density; ALN, alendronate; ARCH, Active‐Controlled Fracture Study in Postmenopausal Women With Osteoporosis at High Risk; DXA, dual‐energy x‐ray absorptiometry; FEA, finite element analysis; QCT, quantitative computed tomography; R, Spearman's correlation coefficient; ROMO, romosozumab; vBMD, volumetric bone mineral density.
High correlations were observed between postbaseline absolute change in integral FEA bone strength and postbaseline absolute change in integral QCT vBMD at the lumbar spine for both romosozumab‐to‐alendronate (n1 = 99; R = 0.82, p < 0.001) and alendronate‐to‐alendronate groups (n1 = 84; R = 0.75, p < 0.001) (Figure 4B ). Relationships between integral bone strength and absolute vBMD change were similar between the two treatment groups. For a given observed absolute change in integral vBMD, there was no significant difference of increase in bone strength per increase in integral vBMD between the two treatment groups (slope difference: 7.15 N/mg/cm3, p = 0.100).
High correlations were also observed between postbaseline absolute change in integral bone strength and postbaseline absolute change in integral BMC for romosozumab‐to‐alendronate (n1 = 99, R = 0.87, p < 0.001) and alendronate‐to‐alendronate (n1 = 84, R = 0.72, p < 0.001) (Supplemental Figure S11). Relationships between integral bone strength and absolute BMC change were significantly different between the two treatment groups. For a given observed absolute change in integral BMC, there was generally a larger increase in bone strength per increase in integral BMC for the romosozumab‐to‐alendronate group compared with the alendronate‐to‐alendronate group (slope difference: 0.39 N/mg, p = 0.006).
Characteristics of patients who developed new vertebral fractures on study
By month 24, no patients in the romosozumab‐to‐alendronate group had experienced a confirmed fracture whereas two patients in the alendronate‐to‐alendronate group had experienced confirmed fractures (Supplemental Table S5). A new vertebral fracture occurred at L2 (selected for QCT and FEA scanning) for one patient and at T4 and T7 for the other patient. By month 36, no patients had experienced a fracture in the romosozumab‐to‐alendronate group and two additional patients in the alendronate‐to‐alendronate group experienced a new vertebral fracture, occurring at L1 for one patient and at T6 for the other patient. Available data for integral aBMD, vBMD, bone volume, BMC, and bone strength for these four patients are shown in Supplemental Table S5.
Discussion
In this study of postmenopausal women with osteoporosis and a prevalent fragility fracture, romosozumab was associated with larger gains in integral QCT vBMD and QCT BMC than alendronate at the lumbar spine. These larger gains were observed in both the cortical and trabecular regions and corresponded to larger gains in FEA‐estimated vertebral bone strength. Romosozumab was associated with greater improvements in vertebral strength, with vertebral strength gains appearing to be distributed equally between the cortical and trabecular compartment at all time points (p > 0.05 for the difference between cortical and trabecular for all comparisons). Romosozumab was also associated with larger gains in DXA aBMD than alendronate at the lumbar spine. In the Spearman correlations to assess the relationships between QCT, DXA, and FEA parameters, changes in vertebral strength showed high correlation with changes in integral aBMD, integral vBMD, and integral BMC for both treatment groups; however, the relationship between absolute changes in strength and absolute changes in aBMD or absolute changes in BMC differed between the treatment groups, suggesting that, for a given observed absolute change in overall aBMD or BMC, there was generally a larger increase in vertebral strength for the romosozumab group compared with the alendronate group.
Effective agents for the treatment of osteoporosis should increase bone mass, but ultimately bone strength must improve to counteract the effects of bone loss and microarchitectural deterioration that characterize osteoporosis and lead to increased fracture risk.( 2 ) Thus, these larger gains in BMD, BMC, and vertebral strength with romosozumab versus alendronate likely account for the greater fracture risk reduction with romosozumab demonstrated in the parent ARCH trial,( 14 ) where 12 months of romosozumab reduced the risk of new vertebral fractures by 37% compared with alendronate (p = 0.003). Importantly, this effect on fracture risk reduction was maintained when patients were transitioned to alendronate from month 12 to the end of study, with a 48% risk reduction in new vertebral fractures in the romosozumab‐to‐alendronate group compared with the alendronate‐to‐alendronate group at month 24 (p < 0.001) and beyond.( 14 )
Our data suggest that percentage changes in BMD, whether assessed by DXA or QCT, may underestimate the treatment's relative effect on overall vertebral strength when comparing across the two treatment groups, although the majority of change in strength can be explained by absolute change in BMD or BMC. For example, at 24 months, the percentage change from baseline in vertebral strength for the romosozumab‐to‐alendronate group was 25.8%, which was over sixfold greater than the 3.8% change for the alendronate‐to‐alendronate group. The respective changes in aBMD by DXA were 16.7% and 7.8% and in integral vBMD by QCT were 20.9% and 8.4%. Thus, on average, the percentage changes between treatment groups were further apart when assessed by vertebral strength than by BMD. Even so, the highly significant correlation between absolute change in vertebral strength and absolute change in either vBMD (R 2 = 0.56–0.67 for the two treatments) or BMC (R 2 = 0.52–0.76) indicates that an appreciable portion of the change in vertebral strength can be directly attributed to the underlying changes in vBMD and BMC.
The significant correlation between absolute change in vertebral strength and absolute change in aBMD shows that, compared to treatment with alendronate, treatment with romosozumab alters the relationship between changes in DXA aBMD and changes in bone strength. For the same absolute change in DXA aBMD, the change in vertebral strength is greater with romosozumab treatment than it is with alendronate treatment, and the effect is greater for greater changes in DXA aBMD. For example, for a change in DXA aBMD of 100 mg/cm2, bone strength changed twice as much with romosozumab (about 800 N) than with alendronate (about 400 N). The results imply that the change in BMD is not homogenous throughout the vertebra and, more importantly, that the relative BMD increase in the vertebral body is much higher with romosozumab than with alendronate. This finding argues in favor of FEA/QCT over DXA in estimating BMD changes with osteoporosis treatments; consistent with data from epidemiological studies showing that FEA and QCT can better predict vertebral fractures compared with DXA. QCT/FEA of the vertebral body may be more clinically useful than aBMD of the whole vertebra for assessing treatment effects. This would have to be further validated in large populations using fracture endpoints.
Although exploratory and qualitative, the virtual stress testing animations provide unique insight into possible biomechanical mechanisms of bone strengthening and fracture risk reduction during treatment. The virtual force of 4500 N applied in our analysis is equal to the threshold vertebral strength for “fragile bone strength,” below which clinical studies have shown women to be at high risk of an incident vertebral fracture.( 32 , 33 ) The ability to withstand that amount of applied force by a vertebra without sustaining meaningful internal tissue failure should translate into reduced likelihood of fracture. The simulations illustrated how the amount of failed tissue for patient 1 representing the romosozumab‐to‐alendronate group, was high at baseline but declined after 6 months of romosozumab treatment and remained low at 12 and 24 months. Further, by month 24 failure was confined to the trabecular region. By contrast, the bone for patient 2 representing the alendronate‐to‐alendronate group continued to sustain substantial damage despite slight strengthening over time, and its overall strength remained lower than the applied force in the virtual stress test.
Similar results were reported in a substudy of a phase 2 trial in postmenopausal women with low bone mass,( 23 , 27 ) where 12 months of romosozumab increased FEA bone strength at the lumbar spine compared with teriparatide (27.3% vs. 18.5%; p = 0.005) or placebo (27.3% vs. –3.9%; p < 0.001).( 30 ) DXA aBMD and QCT vBMD changes in that study were also shown to underestimate vertebral strength changes; but, similar to the current study, confidence intervals were also highest for vertebral strength.( 30 ) Other studies have evaluated improvements in vBMD/BMC and bone strength with other antiresorptive and bone‐forming agents. Once‐monthly oral ibandronate for 12 months in postmenopausal women significantly improved lumbar spine DXA aBMD, QCT vBMD, and FEA‐estimated bone strength compared with placebo.( 34 ) In the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) study,( 15 ) denosumab treatment every 6 months for 36 months in women with postmenopausal osteoporosis also increased lumbar spine FEA‐estimated bone strength compared with placebo, owing to positive treatment effects in both the cortical and trabecular bone compartments.
In our analysis, integral bone volume did not change during the study. Thus, the observed increase in cortical volume suggests a deposition of newly formed bone in the cortical region, in particular at the endocortical surface, although we did not investigate the spatial distribution of bone gains across the cortex and the resolution might not have allowed an accurate determination. Also, cortical results should be viewed in the context of the limited resolution of whole‐body clinical scanners to precisely measure the thin human cortical vertebral shell.( 35 , 36 ) The small increase of integral bone volume at month 24 for alendronate is probably a segmentation effect because periosteal bone increases for alendronate have not been previously observed. Segmentation of the vertebral bodies in this elderly osteoporotic population is difficult due to growing osteophytes and other degenerative changes. The exclusion of osteophytes often required user interaction to correct the automated segmentation, and although CT images of all three visits were analyzed at the same time, slight differences in integral volume may have occurred.
Our analysis revealed that the bone‐strengthening effects of romosozumab at the lumbar spine were associated with contributions from both the cortical and trabecular compartments. At month 24, percentage changes in strength were similar for the trabecular (26.2%) and cortical (26.3%) regions, whereas percentage changes in vBMD trended higher for the trabecular than for the cortical region (21.4% vs. 17.8%). Cortical thickness increased, and we found that more than half of the newly formed bone (492 mg vs. 178 mg) was deposited in the endocortical region. Realizing that the cortical and trabecular regions were defined in slightly different ways between the FEA and vBMD/BMC analyses, these findings nonetheless illustrate the biomechanical complexity of how treatment‐induced changes in the cortical and trabecular bone contribute to changes in overall vertebral strength. Some of this complexity likely results from the nonuniform load sharing between the cortical and trabecular bone that occurs along the cross‐section of the vertebral body.( 37 ) A more mechanistic explanation of the relative contributions of the cortical and trabecular changes to overall treatment effects remains a topic of ongoing research.
The BMC results also demonstrated that the difference between the BMC deposited in the cortical and trabecular compartments is much higher with romosozumab than with alendronate. Our findings show that romosozumab can rapidly improve BMD, BMC, and vertebral strength and therefore is likely to benefit patients at very high risk of fracture. This supports recent clinical guidelines that recommend romosozumab as first‐line therapy for patients in the very high‐risk category for fractures.( 38 , 39 , 40 )
A study in cynomolgus monkeys showed that FEA and direct mechanical testing could both reveal significant treatment effects of osteoporosis therapy on overall vertebral strength, which further validates FEA for the assessment of treatment effects, at least for vertebrae.( 41 ) That study also showed that both the cortical and trabecular increases were important for increasing overall vertebral strength.( 41 ) As suggested by the video animations of the virtual stress testing (Supplemental Figure S10, Supplemental Video S1), strengthening of the vertebral cortex with romosozumab may provide a protective effect for the otherwise weaker vertebral trabecular bone. This protective effect may explain why the relation between changes in BMC and changes in vertebral strength differed between the two treatment groups.
The strength of our analysis is that it allows the evaluation of the effect of romosozumab versus alendronate on bone mass and vertebral strength parameters in a subpopulation of the ARCH trial. Additionally, the patients in the treatment groups were overall well‐balanced in terms of baseline characteristics. QCT and FEA are validated techniques and strict quality control protocols were used; in addition, assessments were blinded to treatment. However, a number of study limitations must be taken into consideration. First, the assessments in the QCT/FEA imaging component of the ARCH substudy included only 90 patients, out of the 4093 enrolled in ARCH. Second, imaging sites were selected based on the availability of QCT. Therefore, this study may not be fully representative of the ARCH overall population. Additionally, as already discussed, it is important to note that the thin cortical shell (typically ≤0.5 mm) in the human spine cannot be precisely measured in vivo because of the limited resolution of current whole‐body clinical scanners,( 35 , 36 ) which introduces the risk of partial volume artifacts. However, the segmentation technique applied in our analysis was identical to other published QCT studies using the MIAF‐Spine analysis, allowing for comparability of results.( 15 , 30 , 34 ) Further, an in‐depth comparison of cortical and trabecular results obtained for vBMD/BMC and FEA is limited by different segmentation approaches used by MIAF and VirtuOst. Finally, p values were not adjusted for multiplicity in this post hoc exploratory analysis.
In conclusion, results from our analysis show that compared with alendronate, romosozumab significantly improved lumbar spine DXA aBMD and QCT vBMD and BMC. These changes were accompanied by improvements in FEA‐estimated vertebral strength, which highly correlated with increases in QCT vBMD and BMC. BMD/BMC and vertebral strength improvements occurred rapidly, as early as 6 months after initiation of treatment and were sustained through 12 months and beyond after transitioning to alendronate. Most newly formed bone was accrued in the cortical compartment, with romosozumab showing larger absolute BMC gains than alendronate. Such densitometric and structural improvements are consistent with greater fracture risk reduction observed in ARCH with the romosozumab‐to‐alendronate sequence compared with the alendronate‐to‐alendronate sequence. These results support the use of romosozumab as a first‐line therapy in treating patients at very high risk for fracture for rapid gains in BMD and bone strength.
Disclosures
Jacques P. Brown has received research support from Mereo BioPharma, Radius Health, and Servier; has served as a consultant for Amgen and Servier; and has served on speakers' bureaus for Amgen. Klaus Engelke is a part time employee of BioClinica. Tony M. Keaveny has served as a consultant for Amgen, O.N. Diagnostics, AgNovos Healthcare, and Bone Health Technologies and owns equity in O.N. Diagnostics. Arkadi Chines, Zhenxun Wang, and Mary K. Oates are employees of Amgen and own stock in Amgen. Roland Chapurlat has received research support from Amgen, UCB Pharma, Chugai, and MSD, and has served as a consultant for Amgen, UCB Pharma, Pfizer, PKMed, Sanofi, Arrow, and BMS. A. Joseph Foldes has nothing to disclose. Xavier Nogues has served as a consultant for Amgen, Eli Lilly, and STADA, and has served on speakers' bureaus for Amgen, Eli Lilly, Italfarmaco, and FAES. Roberto Civitelli has received research support from Mereo BioPharma. Tobias De Villiers has served as a consultant for Eli Lilly and has served on speakers' bureaus for Abbott, Pfizer, and Adcock Ingram. Fabio Massari has nothing to disclose. Cristiano A.F. Zerbini has received research support from Amgen, Eli Lilly, Pfizer, and Sanofi. Christopher Recknor has received grants/research support from Amgen, CytoDyn, Eli Lilly, and Roche, and has served as a consultant for Amgen and CytoDyn. Cesar Libanati is an employee of UCB Pharma and owns stock in UCB Pharma.
Author Contributions
Jacques P. Brown was involved in the study conceptualization, methodology, data curation, and supervision and also writing of the first draft and reviewing and editing of subsequent drafts of the manuscript. Klaus Engelke and Tony M. Keaveny were involved in the study conceptualization, methodology and analysis of DXA/QCT scans, and data curation and reviewing and editing drafts of the manuscript. Arkadi Chines and Mary K. Oates were involved in the study conceptualization and methodology and reviewing and editing drafts of the manuscript. Roland Chapurlat, A. Joseph Foldes, Xavier Nogues, Roberto Civitelli, Tobias De Villiers, Fabio Massari, Cristiano A.F. Zerbini, and Christopher Recknor were involved in the study conceptualization and methodology and reviewing and editing drafts of the manuscript. Zhenxun Wang was involved in the study conceptualization, methodology, formal data analysis and data curation, and reviewing and editing drafts of the manuscript. Cesar Libanati was involved in the study conceptualization, methodology, data curation, and reviewing and editing of drafts of the manuscript. All authors approved the final version of the manuscript and agreed to submit it to the Journal of Bone and Mineral Research for publication.
Supporting information
Appendix S1. Supporting Information
Appendix S2. Video for Figure S10.
Acknowledgments
The study was funded by Amgen Inc., Astellas Pharma, Inc., and UCB Pharma. Representatives of Amgen, Inc. designed the study. Martha Mutomba (on behalf of Amgen, Inc.) and Lisa Humphries (Amgen, Inc.) provided medical writing support.
[Correction added on 3 September 2021, after first online publication: Figure 3 and Supporting information PDF have been replaced]
Data Availability Statement
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: http://www.amgen.com/datasharing.
References
- 1. Morgan EF, Unnikrisnan GU, Hussein AI. Bone mechanical properties in healthy and diseased states. Annu Rev Biomed Eng. 2018;20:119‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bouxsein ML, Seeman E. Quantifying the material and structural determinants of bone strength. Best Pract Res Clin Rheumatol. 2009;23:741‐753. [DOI] [PubMed] [Google Scholar]
- 3. Warriner AH, Patkar NM, Curtis JR, et al. Which fractures are most attributable to osteoporosis? J Clin Epidemiol. 2011;64:46‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kendler DL, Bauer DC, Davison KS, et al. Vertebral fractures: clinical importance and management. Am J Med. 2016;129:221.e1‐‐e10. [DOI] [PubMed] [Google Scholar]
- 5. Austin M, Yang Y‐C, Vittinghoff E, et al. Relationship between bone mineral density changes with denosumab treatment and risk reduction for vertebral and nonvertebral fractures. J Bone Miner Res. 2012;27:687‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cosman F, Crittenden DB, Ferrari S, et al. FRAME study: the foundation effect of building bone with 1 year of romosozumab leads to continued lower fracture risk after transition to denosumab. J Bone Miner Res. 2018;33:1219‐1226. [DOI] [PubMed] [Google Scholar]
- 7. Ferrari S, Adachi JD, Lippuner K, et al. Further reductions in nonvertebral fracture rate with long‐term denosumab treatment in the FREEDOM open‐label extension and influence of hip bone mineral density after 3 years. Osteoporos Int. 2015;26:2763‐2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kendler DL, Marin F, Zerbini CAF, et al. Effects of teriparatide and risedronate on new fractures in post‐menopausal women with severe osteoporosis (VERO): a multicentre, double‐blind, double‐dummy, randomised controlled trial. Lancet. 2018;391:230‐240. [DOI] [PubMed] [Google Scholar]
- 9. Saag KG, Zanchetta JR, Devogelaer J‐P, et al. Effects of teriparatide versus alendronate for treating glucocorticoid‐induced osteoporosis: thirty‐six‐month results of a randomized, double‐blind, controlled trial. Arthritis Rheum. 2009;60:3346‐3355. [DOI] [PubMed] [Google Scholar]
- 10. Langdahl BL, Libanati C, Crittenden DB, et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open‐label, phase 3 trial. Lancet. 2017;390:1585‐1594. [DOI] [PubMed] [Google Scholar]
- 11. Cosman F, Miller PD, Williams GC, et al. Eighteen months of treatment with subcutaneous abaloparatide followed by 6 months of treatment with alendronate in postmenopausal women with osteoporosis: results of the ACTIVExtend trial. Mayo Clin Proc. 2017;92:200‐210. [DOI] [PubMed] [Google Scholar]
- 12. Bouxsein ML, Eastell R, Lui L‐Y, et al. Change in bone density and reduction in fracture risk: a meta‐regression of published trials. J Bone Miner Res. 2019;34:632‐642. [DOI] [PubMed] [Google Scholar]
- 13. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532‐1543. [DOI] [PubMed] [Google Scholar]
- 14. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417‐1427. [DOI] [PubMed] [Google Scholar]
- 15. Keaveny TM, McClung MR, Genant HK, et al. Femoral and vertebral strength improvements in postmenopausal women with osteoporosis treated with denosumab. J Bone Miner Res. 2014;29:158‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bauer JS, Kohlmann S, Eckstein F, et al. Structural analysis of trabecular bone of the proximal femur using multislice computed tomography: a comparison with dual x‐ray absorptiometry for predicting biomechanical strength in vitro. Calcif Tissue Int. 2006;78:78‐89. [DOI] [PubMed] [Google Scholar]
- 17. Nawathe S, Akhlaghpour H, Bouxsein ML, Keaveny TM. Microstructural failure mechanisms in the human proximal femur for sideways fall loading. J Bone Miner Res. 2014;29:507‐515. [DOI] [PubMed] [Google Scholar]
- 18. Fields AJ, Lee GL, Liu XS, et al. Influence of vertical trabeculae on the compressive strength of the human vertebra. J Bone Miner Res. 2011;26:263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ammann P, Rizzoli R. Bone strength and its determinants. Osteoporos Int. 2003;14:S13‐S18. [DOI] [PubMed] [Google Scholar]
- 20. Osterhoff G, Morgan EF, Shefelbine SJ, et al. Bone mechanical properties and changes with osteoporosis. Injury. 2016;47(Suppl 2):S11‐S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keaveny TM. Biomechanical computed tomography—noninvasive bone strength analysis using clinical computed tomography scans. Ann N Y Acad Sci. 2010;1192:57‐65. [DOI] [PubMed] [Google Scholar]
- 22. Amgen Inc. EVENITY® (romosozumab‐aqqg) US prescribing information. Amgen Inc., Thousand Oaks, CA, US; 2019. https://www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/evenity/evenity_pi_hcp_english.ashx. Accessed July 12, 2021.
- 23. McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412‐420. [DOI] [PubMed] [Google Scholar]
- 24. Padhi D, Jang G, Stouch B, Fang L, Posvar E. Single‐dose, placebo‐controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res. 2011;26:19‐26. [DOI] [PubMed] [Google Scholar]
- 25. McClung MR, Brown JP, Diez‐Perez A, et al. Effects of 24 months of treatment with romosozumab followed by 12 months of denosumab or placebo in postmenopausal women with low bone mineral density: a randomized, double‐blind, phase 2, parallel group study. J Bone Miner Res. 2018;33:1397‐1406. [DOI] [PubMed] [Google Scholar]
- 26. Engelke K, Mastmeyer A, Bousson V, et al. Reanalysis precision of 3D quantitative computed tomography (QCT) of the spine. Bone. 2009;44:566‐572. [DOI] [PubMed] [Google Scholar]
- 27. Genant HK, Engelke K, Bolognese MA, et al. Effects of romosozumab compared with teriparatide on bone density and mass at the spine and hip in postmenopausal women with low bone mass. J Bone Miner Res. 2017;32:181‐187. [DOI] [PubMed] [Google Scholar]
- 28. Mastmeyer A, Engelke K, Fuchs C, Kalender WA. A hierarchical 3D segmentation method and the definition of vertebral body coordinate systems for QCT of the lumbar spine. Med Image Anal. 2006;10:560‐577. [DOI] [PubMed] [Google Scholar]
- 29. Prevrhal S, Engelke K, Kalender WA. Accuracy limits for the determination of cortical width and density: the influence of object size and CT imaging parameters. Phys Med Biol. 1999;44:751‐764. [DOI] [PubMed] [Google Scholar]
- 30. Keaveny TM, Crittenden DB, Bolognese MA, et al. Greater gains in spine and hip strength for romosozumab compared with teriparatide in postmenopausal women with low bone mass. J Bone Miner Res. 2017;32:1956‐1962. [DOI] [PubMed] [Google Scholar]
- 31. Lee DC, Hoffmann PF, Kopperdahl DL, Keaveny TM. Phantomless calibration of CT scans for measurement of BMD and bone strength—inter‐operator reanalysis precision. Bone. 2017;103:325‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Allaire BT, Lu D, Johannesdottir F, et al. Prediction of incident vertebral fracture using CT‐based finite element analysis. Osteoporos Int. 2019;30:323‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kopperdahl DL, Aspelund T, Hoffmann PF, et al. Assessment of incident spine and hip fractures in women and men using finite element analysis of CT scans. J Bone Miner Res. 2014;29:570‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lewiecki EM, Keaveny TM, Kopperdahl DL, et al. Once‐monthly oral ibandronate improves biomechanical determinants of bone strength in women with postmenopausal osteoporosis. J Clin Endocrinol Metab. 2009;94:171‐180. [DOI] [PubMed] [Google Scholar]
- 35. Silva MJ, Wang C, Keaveny TM, Hayes WC. Direct and computed tomography thickness measurements of the human, lumbar vertebral shell and endplate. Bone. 1994;15:409‐414. [DOI] [PubMed] [Google Scholar]
- 36. Prevrhal S, Fox JC, Shepherd JA, Genant HK. Accuracy of CT‐based thickness measurement of thin structures: modeling of limited spatial resolution in all three dimensions. Med Phys. 2003;30:1‐8. [DOI] [PubMed] [Google Scholar]
- 37. Eswaran SK, Gupta A, Adams MF, Keaveny TM. Cortical and trabecular load sharing in the human vertebral body. J Bone Miner Res. 2006;21:307‐314. [DOI] [PubMed] [Google Scholar]
- 38. Eastell R, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1595‐1622. [DOI] [PubMed] [Google Scholar]
- 39. Shoback D, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020;105:587‐594. [DOI] [PubMed] [Google Scholar]
- 40. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis–2020 update–Executive Summary. Endocr Pract. 2020;26(5):564‐570. [DOI] [PubMed] [Google Scholar]
- 41. Lee DC, Varela A, Kostenuik PJ, Ominsky MS, Keaveny TM. Finite element analysis of denosumab treatment effects on vertebral strength in ovariectomized cynomolgus monkeys. J Bone Miner Res. 2016;31:1586‐1595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information
Appendix S2. Video for Figure S10.
Data Availability Statement
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: http://www.amgen.com/datasharing.


