Abstract
Hemin, a substrate of heme oxygenase (HO)‐1, induces HO‐1 expression on a variety of cells to exert anti‐oxidant and anti‐inflammatory roles. However, the role of HO‐1 in allergic diseases for dendritic cells (DCs) is not fully understood. Here, we report that HO‐1 modulates asthmatic airway inflammation by hemin‐treated DC‐released extracellular vesicles (DCEVs). Following induction of bone marrow‐derived DCs by hemin and then by house dust mite (HDM) in vitro, mouse CD4+ naïve T cells were cocultured with DCEVs to determine T helper (h) cell differentiation. C57BL/6 mice were sensitized by different stimuli‐induced DCEVs and challenged with HDM to analyze the changes of inflammatory cells and cytokines in the lung and bronchoalveolar lavage fluid. The results showed that hemin‐treated DCEVs (hemin‐DCEVs) express phosphatidylserine (PS), CD81, heat shock protein 70, and HO‐1, which facilitates regulatory T (Treg) cells differentiation in vitro and in vivo. In HDM‐induced asthmatic mouse model, hemin‐DCEVs inhalation reduced eosinophils infiltration and mucus secretion in the airway, decreased the levels of IL‐4, IL‐5, and IL‐13 in the lung and the number of Th2 cells in mediastinal lymph nodes (MLNs), and increased the number of Treg cells in MLNs. Thus, our study demonstrated, for the first time, that EVs from HO‐1‐overexpressing DCs alleviate allergic airway inflammation of eosinophilic asthma by potentiating Treg cells differentiation and limiting proinflammatory cytokine secretion, which expands our understanding of HO‐1 function, opening the door for HO‐1 inducer‐like hemin as a novel therapeutic strategy for asthma or other allergic diseases.
Keywords: asthma, dendritic cells, extracellular vesicles, heme oxygenase‐1, regulatory T cells
Graphical Abstract
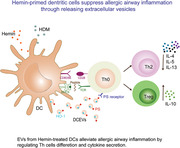
Hemin‐induced heme oxygenase (HO)‐1 alleviates asthmatic airway inflammation by dendritic cells (DCs)‐released extracellular vesicles (DCEVs).
Abbreviations
- (HDM + hemin)‐DCEVs
both HDM and hemin treated dendritic cells‐released extracellular vesicles
- BALF
bronchoalveolar lavage fluid
- BM
Bone marrow
- BMDCs
bone marrow‐derived DCs
- Cy7
Cyanine
- DCEVs
dendritic cells‐released extracellular vesicles
- DCs
dendritic cells
- EGFP
enhanced green fluorescent protein
- EVs
extracellular vesicles
- EXO
exosome
- FCM
flow cytometry
- Foxp
forkhead box P
- HDM
house dust mite
- HDM‐DCEVs
HDM‐treated dendritic cells‐released extracellular vesicles
- Hemin‐DCEVs
hemin treated dendritic cells‐released extracellular vesicles
- HO‐1
heme oxygenase‐1
- HSP
heat shock protein
- MLN
mediastinal lymph node
- NTA
nanoparticle microscopy tracking analysis
- PE
phycoerythrin
- PS
Phosphatidylserine
- SEM
scanning electron microscope
- Th
T helper cell
- Treg
regulatory T cell
- WT
Wild‐type
1. INTRODUCTION
Bronchial asthma is a chronic inflammatory airway disease characterized by chronic airway inflammation, bronchial hyper‐responsiveness, and reversible airway obstruction. 1 As the most important antigen‐presenting cells, dendritic cells (DCs) bridge the innate and adaptive immune responses and play a pivotal role in the induction of allergic airway inflammation. 2 By capturing allergens and migrating into the draining lymph nodes, DCs present antigen‐MHC to naïve T cells and promote polarization of naïve T cells to T helper (Th) cells, ultimately leading to airway inflammation. 3 , 4 On the other hand, DCs also maintain immune homeostasis by induction of apoptosis or anergy without costimulatory signals required for promoting the differentiation of interacting T cells into regulatory T (Treg) cells. 5
In addition to direct cell‐to‐cell contact, DCs manipulate adaptive immunity through secretion of extracellular vesicles (EVs). 6 , 7 EVs are nano‐sized membranous vesicles secreted by a variety of cell types including epithelial cells, macrophages, T cells, platelets, and DCs. 8 , 9 , 10 , 11 EVs not only play a role in physiologic processes, but also participate in pathophysiologic process, such as viral infection and cancer. 12 , 13 , 14 , 15 In addition, EVs transport antioxidant and anti‐inflammatory substances to protect cells and organs, and EVs from macrophages are reported to carry suppressor of cytokine signaling 3 and exert anti‐inflammation by inhibiting JAK‐STAT3 signaling. 10 EVs are currently classified into two categories: exosomes (EXOs, 30–150 nm) and particles (ectosome, microparticles, 100 nm to >1 μm). 16 , 17 Immunomodulatory function of EVs is dependent on types of inclusions. 18 For instance, perivascular DCs transfer antigen‐bearing EVs to neighboring mast cells and DCs, thereby potentiating inflammatory and immune response to blood‐borne antigens. 6 In contrast, induction of immune tolerance is also reported by DC‐derived EVs from a previous study showing that immature DC‐derived EXOs induce Treg cells differentiation by negatively regulating rho‐associated protein kinase 2 in renal allograft model mice. 19 Further investigating anti‐inflammatory function of EVs could unveil potential therapeutic targets to control allergic airway inflammation.
Heme oxygenase (HO) is a rate‐limiting enzyme that degrades heme into biliverdin, free divalent iron, and carbon oxidant. HO contains three subtypes, HO‐1, HO‐2, and HO‐3. 20 HO‐1 is induced by a variety of stimuli including heme, heavy metals, and inflammatory cytokines, and exerts anti‐inflammatory, anti‐oxidative stress, and anti‐smooth muscle proliferation functions. 21 , 22 Our previous studies demonstrated that HO‐1 inhibits Th2 and Th17 responses, 23 , 24 promotes Treg cells formation and IL‐10 release, 25 and alleviates allergic airway inflammation. Studies from several groups have found that the expression of HO‐1 determines the maturity of DCs and affects DC‐primed inflammation. 26 , 27 , 28 However, whether and how DCs exert anti‐inflammatory function in a cell‐dependent or cell‐independent manner is unclear. In this study, we explored the role and mechanism of DC‐released EVs (DCEVs) in maintaining lung immune homeostasis through hemin‐induced HO‐1 expression.
2. MATERIALS AND METHODS
2.1. Sorting and treatment of bone marrow‐derived DCs (BMDCs)
BM cells of femur and tibia of C57BL/6 (Shanghai Laboratory Animal Center, Shanghai, China) and enhanced green fluorescent protein (EGFP+) mice (Model Animal Research Center of Nanjing University, Nanjing, China) at 6–8 wk of age were passed through a 70 μm cell strainer and centrifuged for 5 min at 400 ×g. After RBC lysis (Beyotime, Shanghai, China), cells were incubated with recombinant murine IL‐4 (1 ng/ml) (R&D Systems, Minneapolis, MN, USA) and GM‐CSF (10 ng/ml) (R&D Systems) for 48 h. Suspended cells were gently removed and adherent cells were further cultured. On day 6 of culture, the CD11C+ BMDCs isolated by negative selection with anti‐mouse CD11C magnetic beads (Miltenyi, Teterow, Germany) were collected and further pretreated with or without hemin (7.5 μmol/L, Sigma‐Aldrich, St Louis, MO, USA) for 2 h to induce HO‐1 expression and then stimulated with house dust mite (HDM; 50 μg/ml, Greer lab, Boston, MA, USA) or treated with PBS as a control for 24 h.
2.2. Isolation of EVs
Bovine EV‐depleted FBS was obtained by overnight ultracentrifugation of RPMI 1640 supplemented with 50% FBS (Gibco, Grand Island, NY, USA) at 100,000 ×g for 20 h. On day 6 of DC differentiation, cells were washed in PBS and further cultured in RPMI 1640 with 20% EV‐depleted FBS for 48 h. EVs were isolated as described in the following text. Briefly, conditioned medium was centrifuged at 350 ×g for 15 min at 4°C to pellet cells. Supernatant was centrifuged at 3000 ×g for 20 min at 4°C, and finally centrifuged for 1 h at 17,500 ×g at 4°C. Pellets were washed in 40 ml PBS and re‐centrifuged at the same speed before being resuspended in 200 μl sterile PBS and stored at −80°C. Protein concentrations of EVs were determined by bicinchoninic acid assay (Beyotime).
2.3. Western blot
Whole‐cell extracts from BMDCs or DCEVs were obtained utilizing ice‐cold radioimmune precipitation assay buffer (Beyotime) containing protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific, Waltham, MA, USA). The extracts containing 30–50 μg proteins were separated on 10% SDS‐PAGE and then transferred to polyvinylidene fluoride membranes. The membrane was blocked with Tris‐buffered saline Tween 20 buffer containing 5% skim milk and incubated with the following primary antibodies: antigen‐MHC II (Abcam, Cambridge, UK), CD81 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), heat shock protein (HSP)70 (Abcam), HO‐1 (StressMarq Bioscience, Victoria, CA). The samples were incubated overnight followed by the addition of their corresponding horseradish peroxidase‐conjugated anti‐rabbit or anti‐mouse IgG secondary antibodies (Cell Signaling Technology, Danvers, MA, USA). The signals were visualized via enhanced chemiluminescence using a Thermo ECL kit (Thermo Fisher Scientific) in accordance with the manufacturer's instructions.
2.4. Nanoparticle tracking analysis
The size and concentration of EVs were measured using nanoparticle tracking analysis (NTA) at VivaCell Biosceinces with ZetaView PMX 110 (Particle Metrix, Meerbusch, Germany) and corresponding software ZetaView 8.04.02. Isolated EVs samples were appropriately diluted using PBS buffer (Biological Industries, Kibbutz, Israel) to measure the particle size and concentration. NTA measurement was recorded and analyzed at 11 positions. The ZetaView system was calibrated using 110 nm polystyrene particles. Temperature was maintained around 23°C and 30°C.
2.5. Scanning electron microscopy (SEM)
For SEM, EVs were loaded on a poly‐L‐lysine‐coated coverslip and fixed with 2% glutaraldehyde in PBS at room temperature for 1 h. Samples were vacuum‐dried and dehydrated by critical‐point drying with CO2. The specimens were mounted on metallic supports with carbon tape and ion sputtered with cathodic gold. Analysis of samples was performed using a Zeiss Ultra 55 with high‐resolution Schottky thermal field emission gun (Schottky SEM‐FEG).
2.6. The detection of EVs by flow cytometry (FCM)
To detect the expression of phosphatidylserine (PS) in EVs, EVs recovered were labeled with Annexin‐V‐FITC (BD Biosciences, San Jose, CA, USA) for 15 min. After rinsing, all labeled EVs were detected using FCM on the CytExpert Flow Analyzer. Pacific blue 450 nm laser channel was used to circle the cell size. In addition, CD4+ naïve T (1.5 × 105 cells/well) isolated by positive selection with anti‐mouse CD4 magnetic beads (R&D Systems) were collected and further cultured with EGFP+DC‐derived EVs (secreted by 1.2 × 107 cells) for 48 h in 100 μl of T cell medium per well (96‐well plate), and analyzed by FCM. Cells were stained for the surface marker anti‐CD4‐APC (Biolegend, San Diego, CA, USA). After rinsing, all labeled cells were detected using FCM on the CytExpert Flow Analyzer. Data were analyzed with CytExpert.
2.7. T cell differentiation and FCM analysis
CD4+ naïve T (1.5 × 105 cells/well) isolated by positive selection with anti‐mouse CD4 magnetic beads (R&D Systems) were collected and further cultured with different stimuli‐induced DC‐derived EVs (secreted by 1.2 × 107 cells) for 6 d in 100 μl of T cell medium per well (96‐well plate), and analyzed by FCM. For intracellular cytokine staining, cells were stimulated for 4 h with 1 μg/ml PMA (Sigma‐Aldrich) and 1 μg/ml ionomycin (Calbiochem, Germany) in the presence of 10 μg/ml brefeldin A (Sigma‐Aldrich). Cells were stained for the surface marker anti‐CD4‐V450 (BD Bioscience), anti‐CD4‐FITC (eBioscience, Waltham, MA, USA), and anti‐CD25‐APC (Biolegend). Cells were then fixed, permeabilized and labelled with intracellular and intranuclear staining reagents according to the manufacturer's instructions (eBioscience), and further stained with anti‐IL‐17A‐phycoerythrin (PE)‐cyanine (Cy7), anti‐forkhead box P (Foxp)3‐PE (Biolegend), anti‐IFN‐γ‐APC, or anti‐IL‐13‐PE (Thermo Fisher Scientific). All labeled cells were detected using FCM on the CytExpert Flow Analyzer. Data were analyzed with FlowJo software.
2.8. Preparation of asthmatic mouse model
Wild‐type (WT) C57BL/6 mice at 6–8 wk of age were utilized in this study and were housed in the specified pathogen‐free facilities in the Research Center for Experimental Medicine of Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine. All animal experiments were approved by the Ruijin Hospital Animal Ethics Committee. The asthmatic mouse model induced by HDM was performed using previously described methods. 29 To establish hemin stimulated (hemin‐DCEVs) pretreatment model, mice were treated with 100 μg hemin‐DCEVs in 50 μl of sterile PBS intratracheally at day 1 before sensitization and challenged with HDM. DCEVs induced by HDM, HDM + hemin, and hemin stimulation were obtained in vitro. C57BL/6 mice were sensitized with 100 μg DCEVs in 50 μl of sterile PBS on day 0, and were intranasally challenged with 10 μg HDM in 50 μl sterile PBS on days 7, 8, 9, 10, and 11. The control group received equivalent amount of PBS or HDM on days 0, 7, 8, 9, 10, and 11. On day 14, all mice were anesthetized with isoflurane and sacrificed, the inferior lobe of the right lung was fixed in 4% formaldehyde solution for histology analysis, and the other parts were used for ELISA detection.
2.9. Lung histology
The lungs were fixed in 4% formaldehyde overnight and embedded in paraffin. Lung sections of 5 μm were stained with H&E, periodic acid‐Schiff stain and evaluated by light microscope (Olympus, Tokyo, Japan).
2.10. Bronchoalveolar lavage fluid (BALF)
Seventy‐two hours after the final challenge (day 14), all mice were anesthetized with isoflurane and sacrificed. After blunt dissection of the tracheas, the lungs were lavaged three times with ice‐cold saline (0.6 ml each) using a 22‐gauge i.v. catheter, and BALF was collected. A total of 80–90% of the infused fluid can be recovered by this procedure. The collected BALF was centrifuged at 460 ×g at 4°C for 5 min. The cells were then resuspended in 1 ml PBS, and the total cell numbers were counted with a hemocytometer.
2.11. ELISA
The concentrations of IL‐6, IL‐23, IFN‐γ, IL‐4, IL‐5, IL‐13, IL‐17A, and IL‐10 (Biolegend) in supernatants of lung tissue homogenate or cell culture supernatant were analyzed, respectively, with ELISA kits in accordance with the manufacturer's instructions.
2.12. The detection of cells in BALF and mediastinal lymph node (MLN) by FCM
BALF cells recovered were labeled with anti‐CD11c‐APC, anti‐Ly6G‐FITC, anti‐CD11b‐PE‐Cy7 (Biolegend), anti‐CD45‐APC‐Cy7, and anti‐Siglec‐F‐PE (BD Biosciences) for 45 min. MLN cells were isolated. The cell clumps were disaggregated into single‐cell suspensions using nylon mesh (70 μm pore size) filtration, and erythrocytes were lysed with RBC lysis. For detection of Th1, Th2, Th17, and Treg cells, the MLN cells isolated above were stimulated with lymphocyte activator mixture for 5 h and labeled with surface markers anti‐CD4‐V450 (BD Biosciences), anti‐CD4‐FITC (eBioscience), or anti‐CD25‐APC (Biolegend). After washing, fixing, and permeabilizing according to the manufacturer's instructions, cells were labeled intracellularly with anti‐IL‐17A‐PE‐Cy7, anti‐Foxp3‐PE (Biolegend), anti‐IFN‐γ‐APC, and anti‐IL‐13‐PE (Thermo Fisher Scientific), and then were incubated for 45 min. After rinsing, all labeled cells were detected using FCM on the FACScan Flow Analyzer. Data were analyzed with FlowJo software.
2.13. Statistical analysis
Data are presented as mean ± sd. The P‐value was calculated with ordinary 1‐way ANOVA with Tukey's multiple comparisons test (Graph Pad Prism version 8.0). Differences between samples were considered to have significance when P < 0.05.
3. RESULTS
3.1. HO‐1 reduces expression of costimulatory molecules in BMDCs
To elucidate the effects of HO‐1 on DCs function, BMDCs were stimulated by HDM in the presence or absence of hemin. Results showed that HDM caused a slight but statistical increase in HO‐1 expression in BMDCs. However, hemin combined with HDM stimulation significantly increased HO‐1 expression (Fig. 1A). In addition, costimulatory molecules including CD40/CD80/CD86/OX40L/MHC II (Fig. 1B) as well as IL‐23 (Fig. 1C) and IL‐6 (Fig. 1D) were significantly up‐regulated in the HDM group when compared to that in the PBS group. IL‐10 showed mild but not statistically significant increase (Fig. 1E). However, hemin treatment reversed the phenomena induced by HDM challenge (Fig. 1C–E). Hence, our results suggest that induced HO‐1 may significantly decrease the production of costimulatory molecules, such as CD40 and CD86, and in turn reducing cytokines including IL‐23 and IL‐6.
FIGURE 1.
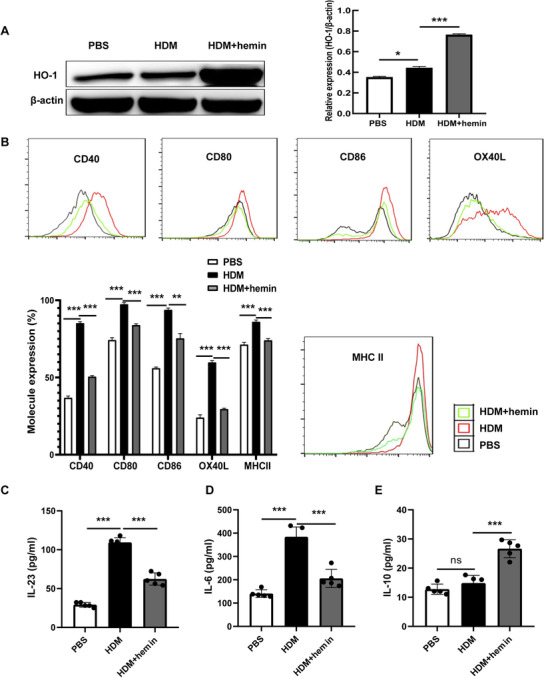
Heme oxygenase (HO)‐1 reduces the expression of costimulatory molecules in bone marrow‐derived dendritic cells (BMDCs). BMDCs were stimulated with house dust mite (HDM) or HDM followed by hemin (HDM + hemin), PBS‐stimulated BMDCs were as control (PBS). A: Expression of HO‐1 and β‐actin in each group was examined by Western blot analysis. B: Flow cytometry (FCM) of expression of costimulatory molecules in BMDCs. C–E: The levels of IL‐23, IL‐6, and IL‐10 in cell culture supernatant were detected with ELISA. For statistical analysis of Western blot, FCM and ELISA, data are pooled from three independent experiments. The data are shown as mean ± sd, *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant
3.2. Identification and effect of mouse DCEVs on Th cell differentiation
Morphologically, DC‐secreted EVs have a shape of spherical vesicles and the diameter ranges from 0.1 and 1 μm (Fig. 2A, B). According to recommendations by the International Society for Extracellular Vesicles (ISEV), 30 characterization of EVs include general characterization (two positive protein markers, CD81 and HSP70) and characterization of single vesicles (FCM, NTA, SEM). Our results showed that all of EVs ubiquitously express CD81 and HSP70. Interestingly, the expression of HO‐1 but not MHC II from hemin‐treated DCEVs was significantly increased whereas increased expression of MHC II only was observed in PBS‐ or HDM‐treated DCEVs (Fig. 2C). In addition, these EVs can express PS (Annexin V+) (Fig. 2D). To explore whether EVs communicate with T cells, EGFP+DC‐derived EVs were cocultured with CD4+ naïve T cells isolated from spleen for 2 d. Results of FCM showed that these T cells expressed EGFP (Fig. 2E), which indicates EVs directly contact with CD4+ naïve T cells.
FIGURE 2.
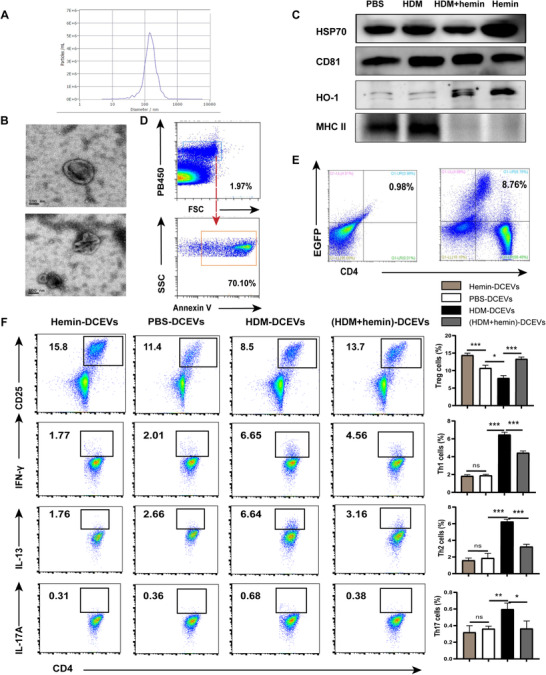
Mouse dendritic cell (DC)‐released extracellular vesicles (DCEVs) identification and effect on Th cell subsets differentiation. EVs were obtained from cell culture supernatants and identified by scanning electron microscopy (SEM), Western blot, flow cytometry (FCM), and nanoparticle microscopy tracking analysis (NTA), respectively. A: NTA; B: SEA; C: expression of heme oxygenase (HO)‐1, MHC II, heat shock protein (HSP)70, and CD81 were examined by Western blot; and D: expression of phosphatidylserine (PS; Annexin V+) in EVs was detected by FCM. Mouse CD4+ naïve T cells were cocultured in vitro with enhanced green fluorescent protein (EGFP)+DC‐derived EVs for 2 d. E: Proportion of EGPF+T cells was detected by FCM. Mouse CD4+ naïve T cells were cocultured in vitro with different stimulus‐induced DCEVs for 6 d. F: Proportion of regulatory T cells (Treg; CD4+ CD25+ forkhead box P [Foxp]3+), Th1 (CD4+ IFN‐ γ+), Th2 (CD4+ IL‐13+), and Th17 cells (CD4+ IL‐17A+) were detected by FCM. For statistical analysis of FCM, data are pooled from three independent experiments. The data are shown as mean ± sd, *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant
To investigate the effects of DCEVs on Th cells differentiation, CD4+ naïve T cells were co‐incubated with different stimuli‐induced DCEVs. The results showed that the proportion of Treg (CD4+ CD25+ Foxp3+) cells in the hemin group was significantly higher than that in the PBS group. In comparison, the proportions of Th1 (CD4+ IFN‐ γ+), Th2 (CD4+ IL‐13+), and Th17 (CD4+ IL‐17A+) cells showed slight but not significant variation. In contrast, the proportion of Treg cells in HDM group was significantly decreased, accompanying increased numbers of Th1, Th2, and Th17 cells. After hemin treatment, the proportion of Treg cells was significantly increased, following lower numbers of Th1, Th2, and Th17 cells in the HDM + hemin group compared to that in the HDM group (Fig. 2F). Further, the expression of HO‐1 was significantly increased whereas the expression of MHC II was significantly decreased (Fig. 2C) in hemin‐induced DCEVs compared to PBS or HDM group, suggesting that the expression of HO‐1 and MHC II may be impacted by the function of DCEVs.
3.3. The role of DCEVs in HDM‐induced allergic airway inflammation
We further investigated the role of DCEVs in airway inflammation in an asthmatic mouse model, which was established by DCEVs or HDM sensitization followed by HDM challenge (Fig. 3A). Mice were randomly divided into five groups: HDM/HDM, HDM‐DCEVs/HDM, (HDM + hemin)‐DCEVs/HDM, hemin‐DCEVs/HDM, and PBS/PBS groups. We observed a significant increase in MLN size (Fig. 3B), cells infiltration around airways (Fig. 3C), and the levels of inflammatory cytokines (IL‐4, IL‐5, IL‐13, and IL‐17A) in lung tissue (Fig. 3D) in HDM/HDM and HDM‐DCEVs/HDM groups compared to those in PBS/PBS groups; however, there was a remarkable decrease in all three measures in (HDM + hemin)‐DCEVs/HDM and hemin‐DCEVs/HDM groups compared to those in HDM/HDM and HDM‐DCEVs/HDM groups. These findings demonstrate that HDM‐stimulated DCEVs may carry antigenic components and successfully induce allergic airway inflammation although the specific component is unknown. However, hemin‐stimulated DCEVs may have beneficial effects on reducing HDM‐induced airway allergic features.
FIGURE 3.
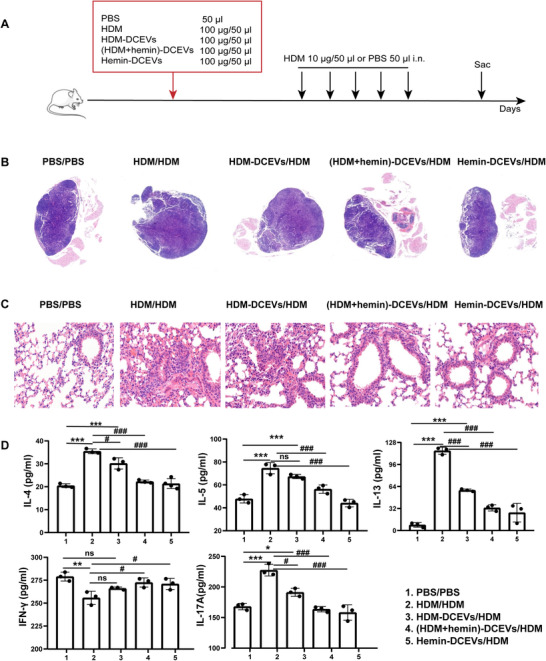
The role of dendritic cell (DC)‐released extracellular vesicles (DCEVs) in house dust mite (HDM)‐induced allergic airway inflammation. Mice were sensitized with different stimuli‐induced DCEVs (HDM‐DCEVs/HDM group, (HDM + hemin)‐DCEVs/HDM group, hemin‐DCEVs/HDM group), and challenged with HDM. HDM or PBS‐sensitized and challenged mice were as a control group (HDM/HDM, PBS/PBS). A: illustration of the model; B: the pathologic changes of mouse mediastinal lymph nodes (MLNs) were observed using H&E staining; C: the pathological changes of mouse lung tissue were observed using H&E staining; and D: the levels of cytokines in lung tissue homogenate were detected with ELISA. For analysis of ELISA, data are pooled from three independent experiments with three mice each group. The data are shown as mean ± sd. Data for compared with the PBS/PBS group, *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant. Data for compared with the HDM/HDM group, # p < 0.05; ### p < 0.001; i.n., intranasally
3.4. Hemin‐treated DCEVs alleviate HDM‐induced allergic airway inflammation
To further determine the role of HO‐1‐overexpressing DCEVs in vivo, we established an HDM‐induced mouse model of eosinophilic asthma and administrated hemin‐DCEVs (Fig. 4A). HDM administration led to a significant increase in inflammatory cell infiltration around airway (Fig. 4B) and cytokine levels (IL‐4, IL‐5, IL‐13, and IL17A) (Fig. 4C), accompanying a decreasing trend of IFN‐γ (Fig. 4C); however, these earlier mentioned results were substantially reversed by hemin‐DCEVs when compared to that in HDM‐induced mice (Fig. 4B, C). In addition, recruitment of peribronchial eosinophils and neutrophils and hypersecretion of mucus were alleviated in the lungs in HDM + hemin‐DCEVs group mice compared to that in HDM‐challenged mice (Fig. 4D). Altogether, our observations unveil that hemin‐stimulated DCEVs may be able to prevent airway remodeling induced by HDM.
FIGURE 4.
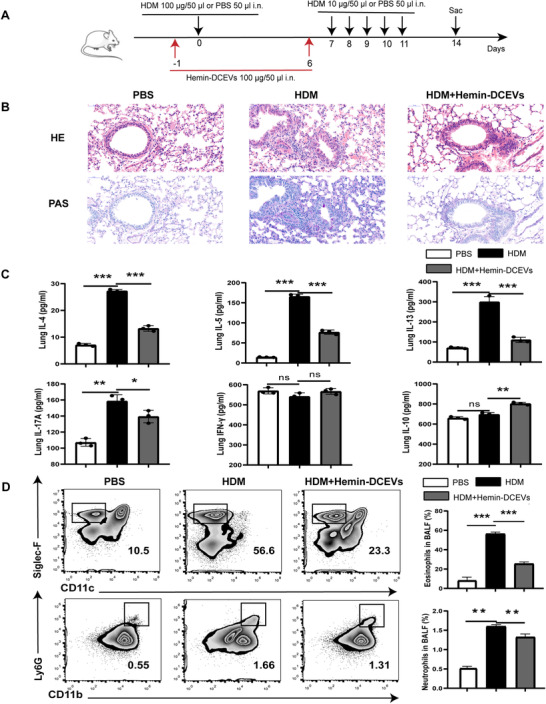
Hemin‐treated dendritic cell (DC)‐released extracellular vesicles (DCEVs) alleviate house dust mite (HDM)‐induced allergic airway inflammation. HDM‐sensitized and challenged mice were instilled intranasally with 100 μg hemin‐DCEVs 1 d before sensitization and challenge (HDM + hemin‐DCEVs group). A: illustration of the model; B: representative images of proximal airway showing inflamed lung areas; C: the levels of IL‐4/IL‐5/IL‐13/IL‐17‐A/IFN‐γ/IL‐10 in lung homogenates; and D: flow cytometry (FCM) of eosinophils (CD45+ Siglec‐F+ CD11c−) and neutrophils (CD45+ Ly6G+ CD11b+) in bronchoalveolar lavage fluid (BALF). For analysis of ELISA and FCM, data are pooled from three independent experiments with three mice each group. The data are shown as mean ± sd, *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant; i.n., intranasally
3.5. Hemin‐treated DCEVs affect proportion of Th cells in mouse MLNs
To gain insight into the in vivo biologic relevance of hemin‐DCEVs, we attempt to characterize the populations of various T cell subsets, including Th1 (CD4+ IFN‐ γ+), Th2 (CD4+ IL‐13+), Th17 (CD4+ IL‐17A+), and Treg (CD4+ CD25+ Foxp3+) cells in MLNs. The results showed that Treg cells were markedly decreased in the HDM group but were increased by intervention with hemin‐DCEVs (Fig. 5A). In addition, no significant difference was observed in Th1 cells (Fig. 5B). In contrast, both Th2 cells (Fig. 5C) and Th17 cells (Fig. 5D) showed a significant increase in HDM group but a decrease in mice with hemin‐DCEVs pretreatment. Collectively, our studies delineate that different T subsets may have distinct responses toward the hemin‐stimulated DCEVs as a mechanism of altering the airway allergic reactions.
FIGURE 5.
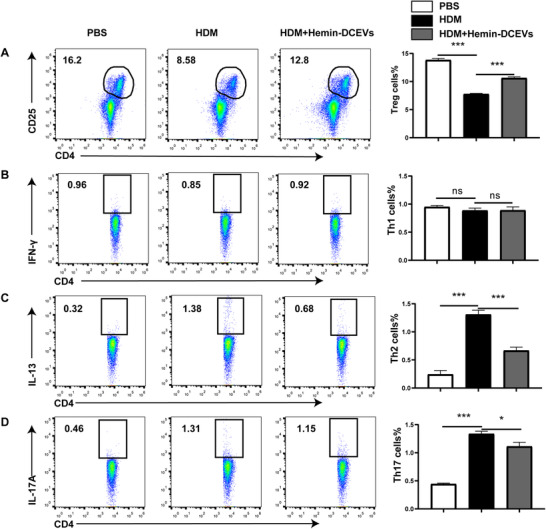
Hemin‐treated dendritic cell (DC)‐released extracellular vesicles (DCEVs) affect proportion of Th cells in mouse mediastinal lymph node (MLNs). House dust mite (HDM)‐sensitized and challenged mice were instilled intranasally with 100 μg hemin‐DCEVs at 1 d before sensitization and challenge (HDM + hemin‐DCEVs group). A: flow cytometry (FCM) of regulatory T cells (Tregs; CD4+ CD25+ forkhead box P [Foxp]3+) in MLNs; B: FCM of Th1 (CD4+ IFN‐ γ+) in MLNs; C: FCM of Th2 (CD4+ IL‐13+) in MLNs; and D: FCM of Th17 (CD4+ IL‐17A+) in MLNs. For statistical analysis of FCM, data are pooled from three independent experiments with three mice each group. The data are shown as mean ± sd, *P < 0.05; ***P < 0.001; ns, not significant
4. DISCUSSION
Although amenities of HO‐1 in DCs‐related immune tolerance have been reported by several studies, 31 , 32 our current study goes beyond to show that not only DCs, but also DC‐derived EVs alleviate allergic airway inflammation, and this function may depend on the packed HO‐1 in the EVs. Furthermore, our results indicated that direct inhibition of Th cells proliferation and promotion of Treg cells differentiation by these EVs may explain their immunosuppressive function and thus alleviate allergic airway inflammation. These findings redefine the understanding of HO‐1, have vast implications for the manipulation of “healthy and unhealthy” DCs, and open avenues for HO‐1 inducer‐like hemin as a new therapeutic strategy for asthma or potentially other allergic diseases.
It is reported that DC‐derived EXOs contribute to allergic airway inflammation by presenting allergens and direct contact with CD4+ T cells. 33 , 34 Our findings timely demonstrated the presence and immunosuppressive role of DC‐derived EVs in asthmatic mice, which in part supports one previous study reporting increased circulating platelet EVs in asthma. 35 EVs were obtained by differential ultracentrifugation, and protein concentrations of EVs were determined by bicinchoninic acid assay. The results showed that these EVs ubiquitously expressed CD81, a transmembrane protein associated with plasma membrane, and HSP70, a cytosolic protein, and were vesicle‐like structure with a diameter of 0.1–1 μm through SEM, NTA, and FCM, and with expression of a nonprotein component PS (Annexin V+) as a marker of EVs. 36 , 37 Our results ascertain that the vesicle structures obtained by ultracentrifugation were EVs. More importantly, concentrated EVs of HDM‐stimulated DCs can express MHC II, bear the capacity to induce Th2 cells differentiation, and successfully promote allergic airway inflammation. These results prompt us to speculate that DCEVs may carry HDM antigenic components, consistent with our previous study demonstrating that EXOs derived from HDM‐treated airway epithelial cells contain some peptides and proteins specific to mites and promote allergic airway inflammation in mice models of asthma. 38 In comparison, sensitization with DCEVs stimulated with both HDM and hemin failed to induce allergic airway inflammation. This may be explained by the presence of by‐products of hemin in these EVs, especially HO‐1 and carbon oxidant, all of which are reported to be anti‐inflammatory and have the therapeutic applications in disease treatment. 39 , 40 Furthermore, in contrast to the proinflammatory role of EVs from HDM‐stimulated DCs, alleviated lung inflammation after treatment with EVs from hemin‐stimulated DCs not only confirmed the role of DCs but also exhibited a new anti‐inflammatory function of DC‐shed EVs. Further exploration revealed that this anti‐inflammatory role depended on manipulating Th17/Treg balance and Th cells proliferation. These findings indicate a novel way for HO‐1 in the metabolic regulation of adaptive immunity thresholds. It would be interesting to discover whether additional regulatory targets and cells exhibit similar effects via HO‐1.
An important finding is that HO‐1 presence in DC‐derived EVs critically reduces HDM‐induced asthmatic disease, which is in line with previous studies showing HO‐1 expression links DCs dysfunction and suppressed proinflammatory function. 26 Critically, we observed a brake‐like, negative regulator effect of EVs on Th cells differentiation in cells culture and asthmatic mice model, which are consistent with previous studies. 41 EVs drive naïve T cells to Treg cells differentiation by currently unknown mechanisms, possibly direct EV‐to‐cell contact or phagocytosis, even without DCs. Mechanistically, the communication ways between EVs and T cells appear to have common ground. PS externalization on EVs as an eat me signal increases interaction with phagocytes such as macrophages and endothelial cells, 42 leading to phagocyte function change, but less is known whether EVs are phagocytosed by CD4+ T cells. Theoretically, this phagocytosis is the most efficient way to receive the whole content of EVs by T cells. In this study, DCEVs express high levels of PS (Annexin V+), and coculture of WT CD4+ T cells with EGFP+ DC‐derived EVs for 2 d showed that WT CD4+ T cells could have engulfed EGFP+ DCEVs, which suggests that EVs containing HO‐1 may be delivered to T cells. This process was possibly mediated in a PS‐dependent manner as PS expression is a strong “eat me” signal. Nevertheless, future studies are needed to confirm HO‐1‐associated activity in EVs compared to that in EV‐depleted cell cultures according to recommendations by ISEV. 30
Careful examination reveals that not only Th2 but also Th17 cells were decreased in vivo and in vitro after sensitization by EVs from hemin‐stimulated DCs. This is a quite surprising finding as we and others previously demonstrated, 43 , 44 , 45 that not only Th17 but also Th2 cell differentiation depends on STAT3 signaling. 46 , 47 Nevertheless, a decrease in Th2 and Th17 cells in our data may not necessarily indicate a direct role of EV‐carrying HO‐1 in the inhibition of STAT3; instead it may indicate an inhibitory role of hemin by‐products, including HO‐1, bilirubin, and carbon oxidant. Also, we could not demonstrate if the Treg/Th cells balance maintenance benefits from HO‐1 or T cells’‐produced IL‐10 because Treg cells are reported to release IL‐10. 48 In light of this, we consider that lack of comparison of EVs from carbon oxidant‐ or bilirubin‐stimulated DCs, analysis of Th cells differentiation by stimulation of IL‐10, and so on, may represent a limitation of our work and therefore deserves further studies.
In conclusion, our data suggest that EVs derived from DCs participate in the occurrence and development of asthma and EVs from hemin‐activated DCs exhibited a trigger‐like role for the balance between Treg and Th cells. Our study leaves unanswered the level of DC‐derived EVs in BALF and circulation of patients with asthma. Also, it will be interesting to further explore the anti‐inflammatory effects of HO‐1 for control of asthma, and the potential role of HO‐1 in asthma treatment.
AUTHORSHIP
Y.W. performed the experiments, and was responsible for data acquisition, analysis, and interpretation, and the original draft. Q.Y. and M.Z. contributed substantially to acquisition, analysis, and interpretation of the data. Y.Z. perform some experiments. X.S. participated in the analysis of the data. Z.X., with inputs from J.L. and M.W., designed the project, revised the article critically for important intellectual content, and provided final approval of the version to be published.
Yujiao Wu, Qianying Yu, and Meng Zhang contributed equally to this article.
DISCLOSURES
The authors declare no conflicts of interest.
DATA STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
ACKNOWLEDGMENTS
The authors thank the staff of the Research Center for Experimental Medicine of Ruijin Hospital affiliated with Shanghai Jiao Tong University School of Medicine and the flow cytometry core facility at Shanghai Jiao Tong University School. This study was supported by the National Natural Science Foundation of China (grant nos. 91542202 and 81970021 to Z.X.; grant nos. 91942305 and 81730001 to X.S.; and grant no. 81900015 to J.L.) and the Innovative Research Team of High‐Level Local Universities in Shanghai (grant to Z.X.).
Wu Yu, Yu Q, Zhang M, et al. Hemin‐primed dendritic cells suppress allergic airway inflammation through releasing extracellular vesicles. J Leukoc Biol. 2022;111:837–848. 10.1002/JLB.3A0321-175R
Contributor Information
Min Wu, Email: min.wu@und.edu.
Jiajia Lv, Email: 19880316j@163.com.
Zhenwei Xia, Email: xzw10484@rjh.com.cn.
REFERENCES
- 1. Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716‐725. [DOI] [PubMed] [Google Scholar]
- 2. Lambrecht BN, Hammad H. Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annu Rev Immunol. 2012;30:243‐270. [DOI] [PubMed] [Google Scholar]
- 3. Kumar S, Jeong Y, Ashraf MU, Bae YS. Dendritic cell‐mediated Th2 immunity and immune disorders. Int J Mol Sci. 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krishnamoorthy N, Oriss TB, Paglia M, et al. Activation of c‐kit in dendritic cells regulates T helper cell differentiation and allergic asthma. Nat Med. 2008;14:565‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coquet JM, Ribot JC, Babala N, et al. Epithelial and dendritic cells in the thymic medulla promote CD4+Foxp3+ regulatory T cell development via the CD27‐CD70 pathway. J Exp Med. 2013;210:715‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choi HW, Suwanpradid J, Kim IH, et al. Perivascular dendritic cells elicit anaphylaxis by relaying allergens to mast cells via microvesicles. Science. 2018:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lindenbergh MFS, Stoorvogel W. Antigen presentation by extracellular vesicles from professional antigen‐presenting cells. Annu Rev Immunol. 2018;36:435‐459. [DOI] [PubMed] [Google Scholar]
- 8. Sjöqvist S, Ishikawa T, Shimura D, et al. Exosomes derived from clinical‐grade oral mucosal epithelial cell sheets promote wound healing. J Extracell Vesicles. 2019;8:1565264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maugeri N, Capobianco A, Rovere‐Querini P, et al. Platelet microparticles sustain autophagy‐associated activation of neutrophils in systemic sclerosis. Sci Transl Med. 2018;10. [DOI] [PubMed] [Google Scholar]
- 10. Bourdonnay E, Zasłona Z, Penke LR, et al. Transcellular delivery of vesicular SOCS proteins from macrophages to epithelial cells blunts inflammatory signaling. J Exp Med. 2015;212:729‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shefler I, Salamon P, Levi‐Schaffer F, et al. MicroRNA‐4443 regulates mast cell activation by T cell‐derived microvesicles. J Allergy Clin Immunol. 2018;141:2132‐2141.e2134. [DOI] [PubMed] [Google Scholar]
- 12. ELA S, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347‐357. [DOI] [PubMed] [Google Scholar]
- 13. Al‐Nedawi K, Meehan B, Micallef J, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619‐624. [DOI] [PubMed] [Google Scholar]
- 14. Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581‐593. [DOI] [PubMed] [Google Scholar]
- 15. Yanez‐Mo M, Siljander PR, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tricarico C, Clancy J, D'Souza‐Schorey C. Biology and biogenesis of shed microvesicles. Small GTPases. 2017;8:220‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113:E968‐E977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kowal J, Tkach M. Dendritic cell extracellular vesicles. Int Rev Cell Mol Biol. 2019;349:213‐249. [DOI] [PubMed] [Google Scholar]
- 19. Pang XL, Wang ZG, Liu L, et al. Immature dendritic cells derived exosomes promotes immune tolerance by regulating T cell differentiation in renal transplantation. Aging. 2019;11:8911‐8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bakhautdin B, Das D, Mandal P, et al. Protective role of HO‐1 and carbon monoxide in ethanol‐induced hepatocyte cell death and liver injury in mice. J Hepatol. 2014;61:1029‐1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase‐1. Annu Rev Pharmacol Toxicol. 2010;50:323‐354. [DOI] [PubMed] [Google Scholar]
- 22. Konrad FM, Braun S, Ngamsri KC, Vollmer I, Reutershan J. Heme oxygenase‐1 attenuates acute pulmonary inflammation by decreasing the release of segmented neutrophils from the bone marrow. Am J Physiol Lung Cell Mol Physiol. 2014;307:L707‐L717. [DOI] [PubMed] [Google Scholar]
- 23. Lv J, Su W, Yu Q, et al. Heme oxygenase‐1 protects airway epithelium against apoptosis by targeting the proinflammatory NLRP3‐RXR axis in asthma. J Biol Chem. 2018;293:18454‐18465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin XL, Lv JJ, Lv J, et al. Heme oxygenase‐1 directly binds STAT3 to control the generation of pathogenic Th17 cells during neutrophilic airway inflammation. Allergy. 2017;72:1972‐1987. [DOI] [PubMed] [Google Scholar]
- 25. Xia ZW, Xu LQ, Zhong WW, et al. Heme oxygenase‐1 attenuates ovalbumin‐induced airway inflammation by up‐regulation of foxp3 T‐regulatory cells, interleukin‐10, and membrane‐bound transforming growth factor‐ 1. Am J Pathol. 2007;171:1904‐1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chauveau C, Rémy S, Royer PJ, et al. Heme oxygenase‐1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL‐10 expression. Blood. 2005;106:1694‐1702. [DOI] [PubMed] [Google Scholar]
- 27. Cheng C, Noorderloos M, van Deel ED, et al. Dendritic cell function in transplantation arteriosclerosis is regulated by heme oxygenase 1. Circ Res. 2010;106:1656‐1666. [DOI] [PubMed] [Google Scholar]
- 28. Campbell NK, Fitzgerald HK, Fletcher JM, Dunne A. Plant‐derived polyphenols modulate human dendritic cell metabolism and immune function via AMPK‐dependent induction of heme oxygenase‐1. Front Immunol. 2019;10:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Machiels B, Dourcy M, Xiao X, et al. A gammaherpesvirus provides protection against allergic asthma by inducing the replacement of resident alveolar macrophages with regulatory monocytes. Nat Immunol. 2017;18:1310‐1320. [DOI] [PubMed] [Google Scholar]
- 30. Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Campbell NK, Fitzgerald HK, Malara A, et al. Naturally derived heme‐oxygenase 1 inducers attenuate inflammatory responses in human dendritic cells and T cells: relevance for psoriasis treatment. Sci Rep. 2018;8:10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Al‐Huseini LM, Aw Yeang HX, Hamdam JM, et al. Heme oxygenase‐1 regulates dendritic cell function through modulation of p38 MAPK‐CREB/ATF1 signaling. J Biol Chem. 2014;289:16442‐16451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Näslund TI, Gehrmann U, Qazi KR, Karlsson MC, Gabrielsson S. Dendritic cell‐derived exosomes need to activate both T and B cells to induce antitumor immunity. J Immunol. 2013;190:2712‐2719. [DOI] [PubMed] [Google Scholar]
- 34. Tkach M, Kowal J, Zucchetti AE, et al. Qualitative differences in T‐cell activation by dendritic cell‐derived extracellular vesicle subtypes. EMBO J. 2017;36:3012‐3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Duarte D, Taveira‐Gomes T, Sokhatska O, et al. Increased circulating platelet microparticles as a potential biomarker in asthma. Allergy. 2013;68:1073‐1075. [DOI] [PubMed] [Google Scholar]
- 36. Lai RC, Tan SS, Yeo RW, et al. MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J Extracell Vesicles. 2016;5:29828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Atkin‐Smith GK, Tixeira R, Paone S, et al. A novel mechanism of generating extracellular vesicles during apoptosis via a beads‐on‐a‐string membrane structure. Nat Commun. 2015;6:7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang M, Yu Q, Tang W, et al. Epithelial exosomal contactin‐1 promotes monocyte‐derived dendritic cells‐dominant T cell responses in asthma. J Allergy Clin Immunol. 2021. [DOI] [PubMed] [Google Scholar]
- 39. Goebel U, Wollborn J. Carbon monoxide in intensive care medicine‐time to start the therapeutic application? Intensive Care Med Exp. 2020;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gomperts E, Belcher JD, Otterbein LE, et al. The role of carbon monoxide and heme oxygenase in the prevention of sickle cell disease vaso‐occlusive crises. Am J Hematol. 2017;92:569‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maybruck BT, Pfannenstiel LW, Diaz‐Montero M, Gastman BR. Tumor‐derived exosomes induce CD8(+) T cell suppressors. J Immunother Cancer. 2017;5:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kumar S, Calianese D, Birge RB. Efferocytosis of dying cells differentially modulate immunological outcomes in tumor microenvironment. Immunol Rev. 2017;280:149‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lin X, Lv J, Ge D, et al. Heme oxygenase‐1 alleviates eosinophilic inflammation by inhibiting STAT3‐SOCS3 signaling. Pediatr Pulmonol. 2020;55:1440‐1447. [DOI] [PubMed] [Google Scholar]
- 44. Irvin C, Zafar I, Good J, et al. Increased frequency of dual‐positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol. 2014;134:1175‐1186.e1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Na H, Lim H, Choi G, et al. Concomitant suppression of T(H)2 and T(H)17 cell responses in allergic asthma by targeting retinoic acid receptor‐related orphan receptor γt. J Allergy Clin Immunol. 2018;141:2061‐2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mari N, Hercor M, Denanglaire S, Leo O, Andris F. The capacity of Th2 lymphocytes to deliver B‐cell help requires expression of the transcription factor STAT3. Eur J Immunol. 2013;43:1489‐1498. [DOI] [PubMed] [Google Scholar]
- 47. Gavino AC, Nahmod K, Bharadwaj U, Makedonas G, Tweardy DJ. STAT3 inhibition prevents lung inflammation, remodeling, and accumulation of Th2 and Th17 cells in a murine asthma model. Allergy. 2016;71:1684‐1692. [DOI] [PubMed] [Google Scholar]
- 48. Lewkowicz N, Mycko MP, Przygodzka P, et al. Induction of human IL‐10‐producing neutrophils by LPS‐stimulated Treg cells and IL‐10. Mucosal Immunol. 2016;9:364‐378. [DOI] [PubMed] [Google Scholar]


