Abstract
Background
The advantage of up‐front neck dissection (UFND) followed by chemoradiotherapy (CRT) for hypopharyngeal cancer (HPC) with advanced neck involvement remains controversial. We aimed to determine the indications.
Methods
The data of 41 and 14 patients with stage IVA/B (T1–T3 and ≥N2a) HPC who underwent UFND followed by CRT and received CRT, respectively, were retrospectively analyzed and compared.
Results
The 5‐year overall survival (OS) and disease‐specific survival rates for the UFND and CRT groups were 61% and 52% (p = 0.1019), and 89% and 74% (p = 0.2333), respectively. Moreover, patients aged ≥70 years or those with a pulmonary disease history had a significantly poorer prognosis due to aspiration pneumonia in the UFND group. The 5‐year regional control (RC) for the UFND and CRT groups were 92% and 57%, respectively (p = 0.0001).
Conclusions
UFND followed by CRT was feasible with satisfactory RC. To further improve OS, aspiration pneumonia prevention is essential.
Keywords: chemoradiotherapy, dysphagia, hypopharyngeal cancer, neck dissection, nodal involvement
1. INTRODUCTION
Head and neck squamous cell carcinomas (HNSCCs) frequently involve nodal metastasis, one of the most detrimental prognostic factors. 1 , 2 Owing to recent advances and accumulated evidence in the treatment of HNSCCs, chemoradiotherapy (CRT) is increasingly being chosen as the primary therapy. 3 Since metastatic lymph nodes are less sensitive to CRT than primary tumors, their control is key to improving prognosis. 4 , 5 In particular, neck dissection (ND) should be recommended in cases of bulky or multiple lymphadenopathies, extracapsular extension (ECE) of nodal disease, and necrotic nodes due to further lowering of the control rate; however, the timing remains controversial. 6
Up‐front neck dissection (UFND) followed by CRT, planned ND after CRT, and salvage ND for positron emission tomography (PET)‐evident persistence after definitive CRT are some of the management strategies for advanced node‐positive primaries. Among these, in nonrandomized trials, UFND was reported to result in higher nodal control; however, the lack of randomization makes it difficult to assess the role of UFND in an organ‐preserving CRT setting for head and neck cancer. 7 As long as the primary tumor and the stage are selected carefully, this combined treatment has the potential to be more effective. 8
Hypopharyngeal (HPC), supraglottic, and p16‐negative oropharyngeal cancers with advanced nodal disease are among the candidate diseases. Although analyses of the treatment of HNSCCs, including these diseases, have been performed, many studies have included several primary sites or p16‐positive oropharyngeal cancer, leading to unsatisfactory evaluations. 9 , 10 To demonstrate the advantage of UFND, we focused on HPC, which has a high incidence of nodal metastasis. 11 With respect to efficacy of CRT on the primary disease and delay of the treatment, clinical T1–T3 classification was considered to be suitable. Regarding the N classification, a classification ≥N2a appeared to be appropriate according to some studies. 12 Previous studies limited to HPC treated by UFND are scarce; therefore, the assessments of the indications are inadequate. 12 , 13
In this study, we retrospectively analyzed the data of patients with stage IVA/B (T1–T3 and ≥N2a) HPC treated with UFND followed by CRT and compared them with those of patients with stage IVA/B HPC treated with concurrent CRT. We aimed to demonstrate the feasibility of this combined treatment.
2. MATERIALS AND METHODS
This study conforms to the World Medical Association Declaration of Helsinki (version 2002) and was approved by the institutional review board of Kindai University Hospital.
Between April 2007 and March 2020, 41 patients with untreated T1–T3 and ≥N2a squamous cell carcinoma of the hypopharynx underwent UFND followed by RT with or without chemotherapy (UFND group), and 14 patients with T1–T3 and ≥N2a HPC received CRT (CRT group) at Kindai University Hospital. Their characteristics, including T and N classifications, based on the 2016 eighth Union for International Cancer Control TNM classification, are presented in Table 1. 14 All patients underwent blood tests, endoscopy, computed tomography (CT), or magnetic resonance imaging (MRI) before treatment. The advantages and disadvantages of both treatments were explained to the patients, and those who declined the UFND received definitive CRT. Patients with unresectable nodal lesions, such as encasement of the carotid artery, did not undergo UFND. Our treatment strategy for patients with HPC during the study period is shown in Table 2.
TABLE 1.
Patient demographics
| Characteristics (n [UFND] = 41; n (neck dissection) = 46; n [CRT] = 14) | |||
|---|---|---|---|
| Group | UFND | CRT | |
| Age, years | Mean (range) | 66 (45–84) | 65 (41–75) |
| Sex, no. (%) | Male | 39 (95) | 14 (100) |
| Female | 2 (5) | 0 | |
| Subsite, no. (%) | PS | 32 (78) | 9 (64) |
| PC | 4 (10) | 0 | |
| PW | 5 (12) | 5 (36) | |
| cT status, no. (%) | T1 | 15 (36) | 4 (29) |
| T2 | 20 (49) | 7 (50) | |
| T3 | 6 (15) | 3 (21) | |
| cN status, no. (%) | N2a | 0 | 0 |
| N2b | 10 (24) | 6 (43) | |
| N2c | 2 (5) | 3 (21) | |
| N3b | 29 (71) | 5 (36) | |
| Clinical stage, no. (%) | IVA | 12 (29) | 9 (64) |
| IVB | 29 (71) | 5 (36) | |
| cECE | Yes | 29 (71) | 5 (36) |
| No | 12 (29) | 9 (64) | |
| Neck dissection, no. (%) | Selective | 41 (89) | |
| Comprehensive | 5 (11) | ||
| Time (first visit to treatment), days | Median (range) | 22 (8–48) | 28 (15–47) |
| Time (surgery to radiotherapy), days | Median (range) | 20 (10–36) | |
| Radiation (IMRT), no. (%) | Yes | 30 (73) | 10 (71) |
| No | 11 (27) | 4 (29) | |
| Chemotherapy, no. (%) | Yes | 32 (78) | 14 (100) |
| No | 9 (22) | 0 | |
| Follow‐up, months | Median (range) | 67 (9–158) | 23 (11–41) |
Abbreviations: CRT, chemoradiotherapy; cECE, clinical extracapsular extension; UFND, up‐front neck dissection.
TABLE 2.
Primary treatment for hypopharyngeal cancer in our facility
| T stage | N stage | Treatment |
|---|---|---|
| T1 | N0 | Surgery or RT |
| T2 | Surgery or CRT | |
| T3 | ||
| T1 | N1 | CRT |
| T2 | ||
| T3 | ||
| T1 | ≥N2a | UFND + CRT or CRT |
| T2 | ||
| T3 | ||
| T4a | Any N | Surgery |
| T4b | IC + CRT |
Abbreviations: CRT, chemoradiotherapy; IC, induction chemotherapy; UFND, up‐front neck dissection.
Selective ND (level II–V) was routinely performed prior to RT with or without chemotherapy. Comprehensive ND (level I–V) was performed in cases of lymphadenopathies adhesive to the submandibular gland. Each of the sternocleidomastoid muscles, the internal jugular vein, and cranial nerve XI was dissected when invaded. Bilateral ND was performed when N2c classification was suspected.
RT with concurrent chemotherapy was usually applied to patients with ≥T2 classification or pathological ECE (pECE) of nodal lesions provided that they were medically suitable. Intensity‐modulated radiation therapy was utilized, except for cases where it was unavailable. Boosted RT was performed up to 70 Gy in areas with pathologically positive nodes; the total dose was 66–70 Gy. Dose reduction toward the cervical region was not performed in patients except for those with pathological N0 classification. Nine patients received RT alone after UFND.
Chemotherapy comprised triweekly cisplatin 100 mg/m2, although the total dose was subject to change depending on the patient's condition. Patients considered unfit for cisplatin (e.g., those aged ≥80 years or those with renal dysfunction [glomerular filtration rate <60 mL/min]) were administered weekly cetuximab (first dose: 400 mg/mm2; second and subsequent doses: 250 mg/mm2) up to seven times by the end of RT. In the UFND and CRT groups, 26 and 10 patients received CRT, respectively. In these groups, six and three patients received bioradiation therapy (BRT), respectively. In the CRT group, one patient received induction chemotherapy followed by BRT due to comorbidity of esophageal cancer.
Dysphagia, an adverse event of these treatments, was evaluated using the Common Terminology Criteria for Adverse Events version 5, for 3–5 months after completing the treatment. Larynx preservation was defined as follows: no local relapse and tracheostoma at the last visit, oral intake of adequate nutrition, and survival. The larynx preservation rate was calculated using the Kaplan–Meier method. In this method, local relapse, tracheostomy, requirement for a feeding tube, and death were regarded as events. 15
Regional control (RC), local control (LC), distant metastasis‐free survival (DMFS), disease‐free survival (DFS), disease‐specific survival (DSS), and overall survival (OS) rates were also determined using the Kaplan–Meier method. A comparative analysis of the aforementioned rates between the UFND and CRT groups was performed using the log‐rank test. Furthermore, the analysis of OS was performed between the following items in each group: age ≥70 versus <70 years; those with a history of lung diseases (i.e., chronic obstructive pulmonary disease, interstitial pneumonia, and pneumothorax) versus those without lung disease; and body mass index (BMI) ≥18.5 versus <18.5 kg/m2. The impact of ECE on the OS, DSS, and DFS rates and on surgical complications was analyzed using the log‐rank test and the Fisher's exact test, respectively, in the UFND group. A p‐value <0.05 was considered statistically significant. GraphPad Prism 8 (GraphPad Software, San Diego, CA) was used for all statistical analyses.
3. RESULTS
3.1. Patient demographics
Forty‐six NDs in 41 patients with HPC in the UFND group and 14 patients in the CRT group were included in the analysis. The mean ages were 66 (range, 45–84) and 65 (41–75) years in the UFND and CRT groups, respectively. Men accounted for ≥95% of both populations. The subsite of the hypopharynx in the UFND and CRT groups included the pyriform sinus (78% and 64% patients, respectively), the postcricoid region (10% and 0% patients, respectively), and the posterior wall (12% and 36% patients, respectively). Patient demographics are presented in Table 1. In T classification, the majority of patients had clinical T2 classifications in both groups, whereas the most common N classifications were N3b (71%) and N2b (43%) in the UFND and CRT groups, respectively. The mean size of the largest lymph node was also similar (30 [range, 11–56] mm in the UFND group and 26.6 [range, 13–75] mm in the CRT group). The median periods between the first visit and treatment were 22 (range, 8–48) and 28 (range, 15–47) days in the UFND and CRT groups, respectively. In the UFND group, selective ND was undertaken in 41 neck regions (89%) with a median time of 20 (range, 10–36) days prior to RT with or without chemotherapy. Postoperative CRT was performed in 32 patients (78%). No patient experienced clinical progression in the T classification until the start of CRT.
3.2. Pathological findings
The pathological status is displayed in Table 3. Thirty‐six and five patients underwent unilateral ND and bilateral ND, respectively. Two patients with clinical N2b and N2c were finally diagnosed with pathological N1. The other patient with clinical N2c was pathological N3b. The nodal levels involved in the 46 NDs were levels II, III, IV, and V in 42%, 33%, 18%, and 5% of cases on the ipsilateral side, respectively, and level II in 2% of cases on the contralateral side. Thirty‐four cases (83%) were associated with pECE, all of which were on the ipsilateral side. This was higher than the number of clinical ECE (N3b) cases (28 [71%]).
TABLE 3.
Pathological data
| Pathological findings | No. (%) | |
|---|---|---|
| pN status | N1 | 2 (5) |
| N2a | 0 | |
| N2b | 5 (12) | |
| N2c | 0 | |
| N3b | 34 (83) | |
| Ipsilateral levels involved | Level I | 0 |
| Level II | 28 (42) | |
| Level III | 22 (33) | |
| Level IV | 12 (18) | |
| Level V | 3 (5) | |
| Contralateral level involved | Level II | 1 (2) |
| pECE | Yes | 34 (83) |
| No | 7 (17) | |
Abbreviation: pECE, pathological extracapsular extension.
3.3. Locoregional and distant relapse control
The median follow‐up duration for the censored cases was 67 (range, 9–158) months in the UFND group and 23 (range, 11–41) months in the CRT group. In total, 13 cases of relapse in the UFND group were observed (i.e., two, seven, and four cases of regional, local, and distant relapse, respectively). Furthermore, of the seven patients who relapsed, three received RT alone, and four received CRT. Conversely, the CRT group had a higher frequency of relapse, while a partial response in the primary tumor and in nodal involvement was found in one and four cases, respectively. The comparative analysis demonstrated statistically significant differences in the RC, LC, and DFS between the UFND and CRT groups: the 5‐year rates were 92% versus 57%, 84% versus 51%, and 67% versus 39% for the RC (p = 0.0001), LC (p = 0.0118), and DFS (p = 0.0169), respectively (Figure 1). In contrast, no statistical difference was found in the DMFS between both groups (89% vs. 84%, p = 0.6711). In the UFND and CRT groups, the mean times until relapse were 32 months and 4 months, 25 months and 10 months, and 9 months and 11 months in the RC, LC, and DMFS arms, respectively. Regarding regional relapse in two cases of the UFND group, one patient had a level II lesion with ECE and two level IV lesions; the other patient had both level II and III lesions with ECE. In the CRT group, four out of six cases were associated with level II and III lesions with ECE. The nodal status in all of the patients with distant metastasis in both groups also represented ECE, although levels IV and V were involved in only one case of the UFND group and in no case of the CRT group. Regarding local recurrence through the follow‐up period, five out of seven cases in the UFND group were classified as T2, and the remaining cases were classified as T1 and T3; similarly, among the six cases in the CRT group, one, two, and three cases were classified as T1, T2, and T3, respectively.
FIGURE 1.
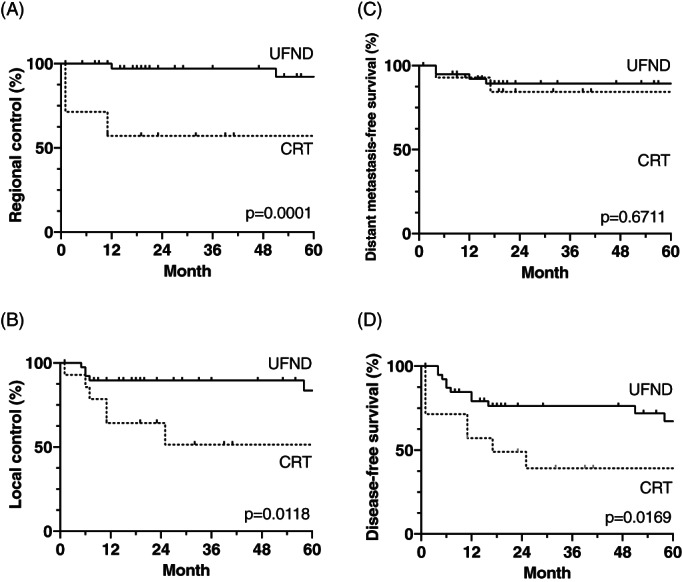
Kaplan–Meier curves of regional control (A), local control (B), DMFS (C), and disease‐free survival (D) were compared between the UFND and CRT groups. All these rates except for the DMFS rates of the UFND group were significantly superior to those of the CRT group. CRT, chemoradiotherapy; DMFS, distant metastasis‐free survival; UFND, up‐front neck dissection
3.4. Survival outcomes
Fourteen and six deaths in the UFND and CRT groups, respectively, occurred during the 5‐year follow‐up period. The comparative analysis demonstrated no statistically significant differences in the OS rates between the UFND and CRT groups: the 5‐year OS and DSS rates were 61% versus 52% (p = 0.1019) and 89% versus 74% (p = 0.2333) (Figure 2), respectively. In particular, cases with clinical ECE showed significantly worse OS rates (P = 0.0064), while the DSS and DFS rates did not differ significantly (Figure 3). The causes of death were primary disease, aspiration pneumonia, ventilator‐associated pneumonia, and others at 5 years (21% and 50%, 36% and 17%, 7% and 0%, and 36% and 33% cases, respectively). Aspiration pneumonia was more common than any other disease in the UFND group, and all patients died within 2 years after the treatment. To identify the preoperative risk factors of UFND, we examined the differences in the OS rates between the following groups: age ≥70 versus <70 years; those with a history of lung diseases versus those without lung disease; and BMI ≥18.5 versus <18.5 kg/m2. Age ≥70 years and lung disease history resulted in significantly shorter OS rates (p = 0.0002 and p = 0.0036, respectively; Figure 4).
FIGURE 2.
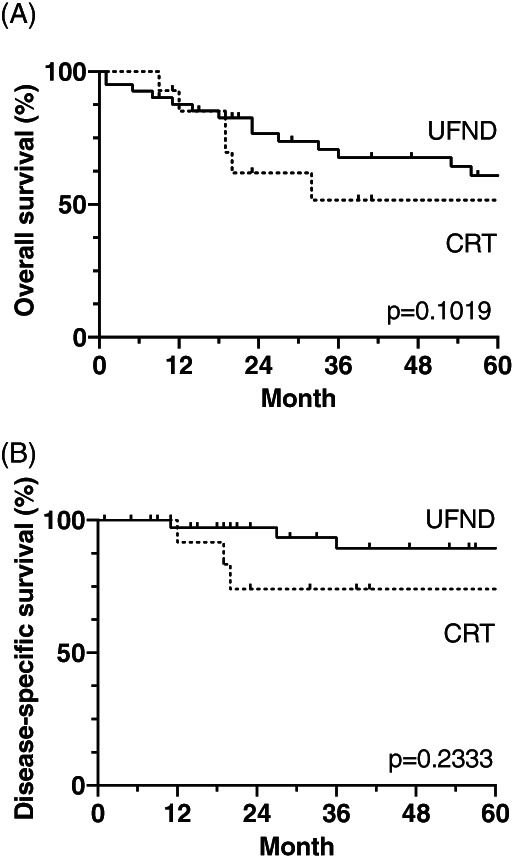
Kaplan–Meier curves of OS (A) and DSS (B) were analyzed in the UFND and CRT groups. There was not significant difference between both groups. Based on OS and DSS in the UFND group, diseases except for the primary disease had a worse impact on survival. CRT, chemoradiotherapy; DMFS, distant metastasis‐free survival; DSS, disease‐specific survival; OS, overall survival; UFND, up‐front neck dissection
FIGURE 3.
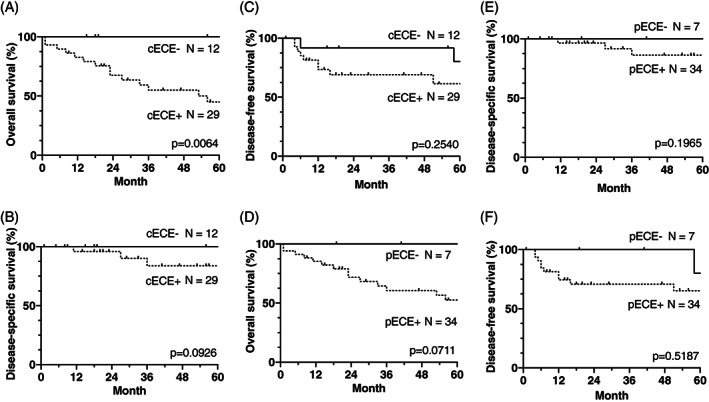
The impact of cECE and pECE on the OS, DSS, and DFS rates (A–C and D–F, respectively). Only cECE had a significantly poorer impact on OS. cECE, clinical extracapsular extension; pECE, pathological extracapsular extension; DMFS, distant metastasis‐free survival; DSS, disease‐specific survival; OS, overall survival
FIGURE 4.
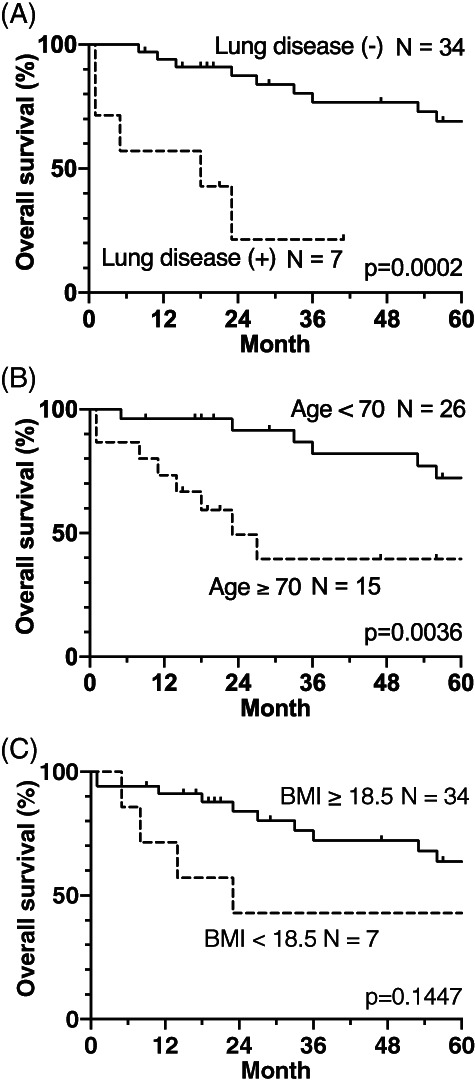
Influence of lung disease (A), age (B), and BMI (C) on the OS rate in the UFND group. Lung disease and age had a significantly poorer influence on OS. BMI, body mass index; OS, overall survival; UFND, up‐front neck dissection
3.5. Complications
Surgical complications occurred in six NDs (13%), as shown in Table 4. Four cases had chylous fistulas; CRT initiation was delayed in one case. Two patients who were suffering from damage to the hypoglossal nerve or the vagus nerve eventually died of aspiration pneumonia. Regarding dysphagia in the UFND and CRT groups, although the rates of grade <2 were 81% and 71%, respectively, and the oral intake rates were 76% and 57%, respectively, the aspiration pneumonia rates were similar in each group (24% and 21% patients, respectively). Five and two patients in each group underwent pharyngo‐laryngo‐esophagectomy; however, one out of five patients in the UFND group underwent surgery for metachronous esophageal cancer. The larynx preservation rates in the UFND and CRT groups were 60% versus 49% and 41% versus 29% (p = 0.3013) after 2 and 5 years, respectively (Figure 5). Local relapse, tracheostomy, requirement for a feeding tube, and death affected the rates in seven, four, six, and nine patients in the UFND group, as well as in four, zero, three, and two patients in the CRT group.
TABLE 4.
Post‐treatment conditions
| Post‐treatment events | No. (%) | No. (%) | |
|---|---|---|---|
| Group | UFND | CRT | |
| ND complications | None | 40 (87) | |
| Chylous fistula | 4 (9) | ||
| Hypoglossal nerve disorder | 1 (2) | ||
| Vagus nerve disorder | 1 (2) | ||
| Post‐CRT dysphagia | Grade <2 | 33 (81) | 10 (71) |
| Grade ≥2 | 5 (12) | 3 (21) | |
| Unknown | 3 (7) | 1 (7) | |
| Aspiration pneumonia | Yes | 10 (24) | 3 (21) |
| No | 31 (76) | 11 (79) | |
| Nutrition | Oral | 31 (76) | 8 (57) |
| Tube | 10 (24) | 6 (43) | |
| Tracheotomy | Yes | 4 (10) | 3 (21) |
| Laryngectomy | 5 (12) | 2 (14) | |
| No | 32 (78) | 9 (64) | |
Abbreviations: CRT, chemoradiotherapy; ND, neck dissection; UFND, up‐front neck dissection.
FIGURE 5.
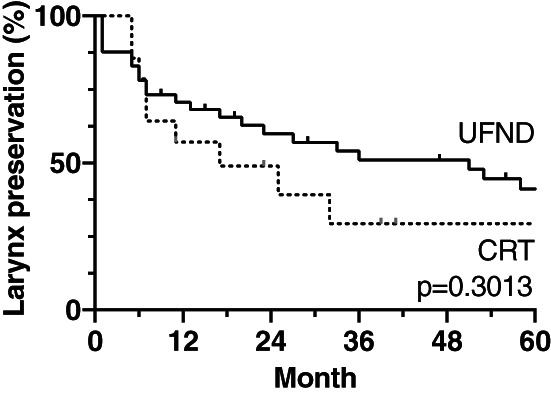
Kaplan–Meier curves of the larynx preservation rate were compared between the UFND and CRT groups. There was not statistically significant difference in the larynx preservation rates between both groups. Local relapse, tracheostomy, requirement for a feeding tube, and death were regarded as events. CRT, chemoradiotherapy; UFND, up‐front neck dissection
4. DISCUSSION
In this study, we focused on HPC cases considering the following viewpoints: supraglottic cancer frequently requires bilateral ND, thereby causing more complications; moreover, p16‐negative oropharyngeal cancers are rarely applied to the TN classification. Few studies have been conducted on HPC cases treated with UFND followed by CRT. In 2008, Prades et al. 12 first reported on the 2‐year RC and OS rates of 76 patients with pyriform sinus carcinoma. This study, which included only T3–T4 classification with N0–N1 cases accounting for more than half of the population, could not appropriately assess the benefit of this treatment. In 2012, Al‐Mamgani et al. 13 compared 32 patients undergoing UFND (Group 1), with 103 patients receiving definitive CRT (Group 2). However, 20% of the patients in Group 1 had N1 classification, for which ND is considered unnecessary, leading to inadequate analysis. Nevertheless, it is noteworthy that in both studies, UFND followed by CRT showed a better RC at a rate >90%. In particular, the latter report indicated superior OS rates in Group 1, suggesting that this combined treatment is more effective. In the present study, we analyzed the data of 41 and 14 patients who underwent UFND followed by CRT and received concurrent CRT for stage IVA/B (T1–T3 and ≥N2a) HPC, respectively. We aimed to evaluate the indications and efficacy of UFND. The 5‐year RC, LC, DMFS, DSS, and OS rates in the UFND and CRT groups were 92% versus 57% (p = 0.0001), 84% versus 51% (p = 0.0118), 89% versus 84% (p = 0.6711), 89% versus 74% (p = 0.2333), and 61% versus 52% (p = 0.0378), respectively. The outcomes in the UFND group were entirely superior to those in the CRT group, which can be primarily explained by the inclusion of appropriate samples. In the UFND group, the median periods between the first visit and surgery and between surgery and RT initiation were 22 and 20 days, respectively, relatively shorter than those in previous studies. 10 , 16 Postoperative complications delaying RT >20 days occurred in only two (5%) cases, indicating a low risk of T classification development during the treatment. Regarding distant metastasis, all four cases occurred within 2 years but had pECE and received CRT after ND. Furthermore, both clinical ECE and pECE showed poorer impact on the OS and DSS rates (Figure 3). Given that the nodal involvement was managed properly, local and distant relapse could be problematic. Henceforth, we anticipate that new chemotherapy regimens will include immune checkpoint inhibitors as first‐line therapy.
Aspiration pneumonia was the most common cause of death, followed by the primary disease at 5 years (36% versus 21%) in the UFND group, whereas one patient (17%) died of aspiration pneumonia in the CRT group. Accordingly, to improve the OS rate, more attention should be paid to ND techniques with swallowing functions preserved as much as possible (for instance, the cervical ansa is kept intact) and to putative preoperative risk factors of aspiration pneumonia. To determine the risk factors, we assessed whether age, BMI, or a history of pulmonary diseases, such as chronic obstructive pulmonary disease, interstitial pneumonia, or pneumothorax, affected the OS rate. Patients aged ≥70 years and those with pulmonary disease history showed significantly shorter OS rates. Therefore, a keen awareness of swallowing function is necessary when performing UFND followed by CRT in patients aged ≥70 years and/or in those with pulmonary diseases. Prophylactic swallowing exercises may help improve swallowing function. 17 Conversely, in the CRT group, the aforementioned factors did not have poor impact on OS.
Since the survival rate was similar between patients who underwent PET‐CT surveillance with salvage ND as necessary after CRT and those who underwent planned ND after CRT, the former appeared to be a superior treatment procedure. 18 Furthermore, this strategy resulted in the prevention of unnecessary ND in many cases and was more cost‐effective. These benefits were derived from a high negative predictive value ranging from 95% to 100% for PET surveillance after CRT 19 ; however, definitive CRT for HNSCCs, which consists primarily of oropharyngeal cancers with N2–N3 classification, reportedly resulted in a 5‐year RC rate of approximately 80%. Additionally, more postoperative complications occurred in salvage ND. 20 Moreover, as it is likely that nodal disease with ECE indicated for ND is more poorly controlled because of a lower sensitivity to CRT, CRT should be selected carefully depending on nodal conditions. According to a previous report, short‐ and long‐axis diameters of nodal metastases exceeding 17 mm also represented higher recurrence. 21 In our study, patients in the UFND and CRT groups with lymphadenopathy showed pECE in 34 (83%) and 5 cases (36%), respectively, and exceeded 17 mm in 30 (73%) and 6 cases (43%), respectively; nevertheless, the 5‐year RC was 92% in the UFND group, significantly superior to that observed in the CRT group (57%).
Surgical complications occur in <10% of up‐front ND cases. 8 In this study, these occurred in 6 out of 46 ND cases (i.e., chylous fistula, hypoglossal nerve disorder, and vagus nerve disorder in four, one, and one cases, respectively). Among them, CRT was delayed in only one case with chylous fistula. Regarding the correlation with ECE, 5 out of 34 patients with pECE+ experienced surgical complications, whereas only one out of seven patients with pECE− experienced chylous fistula, which was not significantly different (p > 0.9999). Better surgical techniques performed with care are necessary to maintain the quality of UFND. However, it is difficult to prevent dysphagia during this treatment. 22 This was emphasized in our findings as only 76% of the patients received sufficient nutrition by oral intake, and 5 out of the 41 patients died from aspiration pneumonia. The primary reason seemed to result from the high rate of pECE indicated for CRT after ND (83%) in our study. Since pECE itself did not affect the postoperative oral intake (p > 0.9999), ND with CRT could affect swallowing functions. Moreover, another reason was that concomitant sternocleidomastoid muscle, accessory nerve, and internal jugular vein resections in ND were required in 14 cases (30%) because of large lymphadenopathies. Since each of the patients experiencing impairment of the hypoglossal and vagus nerves died of aspiration pneumonia, it is vital to preoperatively evaluate the probability of nerve impairment related to swallowing dysfunction with thorough imaging.
ND is considered to be unnecessary for N1 classification because of a 90% control rate by definitive RT. 23 Thus, classification ≥N2 was considered to be a suitable candidate. In particular, cases with conventional adverse prognostic factors, such as a lesion size >3 cm, classification ≥N2c, contralateral lymphadenopathies (N2c or bilateral N3), extension to levels IV and V, fixed lymphadenopathies, and ECE, were more suitable. 24 Therefore, the assessment of ECE is important to select UFND. In our study, the chance that clinical ECE determined by palpation, CT, and MRI corresponded to pECE was 83%. Pathological ECE appears to be difficult to predict from clinical ECE 25 , 26 ; therefore, we recommended using UFND when clinical ECE is suspected.
One of the limitations of this study was its retrospective design, which led to selection bias. Also, the number of patients was small, and the study was conducted at a single facility. Furthermore, the CRT group included three patients with unresectable nodal disease. Since it is essential to accurately assess swallowing function to prevent aspiration pneumonia, future studies should include both video endoscopy and videofluoroscopic examination pre‐ and postoperatively. These objective examinations are also associated with better selection of diet texture and appropriate swallowing rehabilitation.
5. CONCLUSIONS
UFND followed by CRT in patients with stage IVA/B (T1–T3, ≥N2a) HPC is strongly recommended because of better RC. To further improve the OS rates, it is necessary to prevent dysphagia; in particular, more attention should be paid to patients aged ≥70 years with pulmonary disease. Furthermore, a future large‐scale study is required to demonstrate the benefits of UFND.
ACKNOWLEDGMENTS
This study was supported by the following grant: Grant‐in‐Aid for Young Scientists 19K18824 (to M.P.S). We would like to thank Editage (www.editage.com) for English language editing.
Sato MP, Otsuki N, Kitano M, et al. Up‐front neck dissection followed by chemoradiotherapy for T1–T3 hypopharyngeal cancer with advanced nodal involvement. Head & Neck. 2021;43(12):3810‐3819. doi: 10.1002/hed.26881
Section Editor: Mark Wax
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions.
REFERENCES
- 1. Paleri V, Urbano TG, Mehanna H, et al. Management of neck metastases in head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130(S2):S161‐S169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thariat J, Hamoir M, Garrel R, et al. Management of the neck in the setting of definitive chemoradiation: is there a consensus? A GETTEC study. Ann Surg Oncol. 2012;19:2311‐2319. [DOI] [PubMed] [Google Scholar]
- 3. Haussmann J, Tamaskovics B, Bölke E, et al. Addition of chemotherapy to hyperfractionated radiotherapy in advanced head and neck cancer—a meta‐analysis. Strahlenther Onkol. 2019;195(12):1041‐1049. [DOI] [PubMed] [Google Scholar]
- 4. Bataini JP, Bernier J, Jaulerry C, Brunin F, Pontvert D, Lave C. Impact of neck node radioresponsiveness on the regional control probability in patients with oropharynx and pharyngolarynx cancers managed by definitive radiotherapy. Inl J Radiat Oncol Biol Phys. 1987;13(6):817‐824. [DOI] [PubMed] [Google Scholar]
- 5. Strasser MD, Gleich LL, Miller MA, Saavedra HI, Gluckman JL. Management implications of evaluating the N2 and N3 neck after organ preservation therapy. Laryngoscope. 1999;109(11):1776‐1780. [DOI] [PubMed] [Google Scholar]
- 6. Huang SH, O'Sullivan B, Xu W, et al. Temporal nodal regression and regional control after primary radiation therapy for N2–N3 head‐and‐neck cancer stratified by HPV status. Int J Radiat Oncol Biol Phys. 2013;87:1078‐1085. [DOI] [PubMed] [Google Scholar]
- 7. Elicin O, Nisa L, Dal Pra A, et al. Up‐front neck dissection followed by definitive (chemo)‐radiotherapy in head and neck squamous cell carcinoma: rationale, complications, toxicity rates, and oncological outcomes—a systematic review. Radiother Oncol. 2016;119(2):185‐193. [DOI] [PubMed] [Google Scholar]
- 8. D'cruz AK, Pantvaidya GH, Agarwal JP, et al. Split therapy: planned neck dissection followed by definitive radiotherapy for a T1, T2 pharyngolaryngeal primary cancer with operable N2, N3 nodal metastases—a prospective study. J Surg Oncol. 2006;93(1):56‐61. [DOI] [PubMed] [Google Scholar]
- 9. Liu XK, Li Q, Zhang Q, et al. Planned neck dissection before combined chemoradiation in organ preservation protocol for N2‐N3 of supraglottic or hypopharyngeal carcinoma. ORL J Otorhinolaryngol Relat Spec. 2012;74(2):64‐69. [DOI] [PubMed] [Google Scholar]
- 10. Paximadis PA, Christensen ME, Dyson G, et al. Up‐front neck dissection followed by concurrent chemoradiation in patients with regionally advanced head and neck cancer. Head Neck. 2012;34(12):1798‐1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Riviere D, Mancini J, Santini L, et al. Nodal metastases distribution in laryngeal cancer requiring total laryngectomy: therapeutic implications for the N0 neck. Eur Ann Otorhinolaryngol Head Neck Dis. 2019;136(3S):S35‐S38. [DOI] [PubMed] [Google Scholar]
- 12. Prades JM, Timoshenko AP, Schmitt TH, et al. Planned neck dissection before combined chemoradiation for pyriform sinus carcinoma. Acta Otolaryngol. 2008;128(3):324‐328. [DOI] [PubMed] [Google Scholar]
- 13. Al‐Mamgani A, Meeuwis CA, van Rooij PH, et al. Node‐positive hypopharyngeal cancer treated by (chemo)radiotherapy: impact of up‐front neck dissection on outcome, toxicity, and quality of life. Head Neck. 2013;35(9):1278‐1286. [DOI] [PubMed] [Google Scholar]
- 14. Brierley JD, Gospodarowicz MK, Ch W, eds. UICC International Union against Cancer. TNM Classification of Malignant Tumours. 8th ed. Wiley‐Blackwell; 2016. [Google Scholar]
- 15. Lefebvre JL, Ang KK. Larynx preservation consensus panel. Larynx preservation clinical trial design: key issues and recommendations—a consensus panel summary. Head Neck. 2009;31(4):429‐441. [DOI] [PubMed] [Google Scholar]
- 16. Byers RM, Clayman GL, Guillamondequi OM, Peters LJ, Goepfert H. Resection of advanced cervical metastasis prior to definitive radiotherapy for primary squamous carcinomas of the upper aerodigestive tract. Head Neck. 1992;14(2):133‐138. [DOI] [PubMed] [Google Scholar]
- 17. Messing BP, Ward EC, Lazarus CL, et al. Prophylactic swallow therapy for patients with head and neck cancer undergoing chemoradiotherapy: a randomized trial. Dysphagia. 2017;32(4):487‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mehanna H, Wong WL, McConkey CC, et al. PET‐CT surveillance versus neck dissection in advanced head and neck cancer. N Engl J Med. 2016;374(15):1444‐1454. [DOI] [PubMed] [Google Scholar]
- 19. Nayak JV, Walvekar RR, Andrade RS, et al. Deferring planned neck dissection following chemoradiation for stage IV head and neck cancer: the utility of PET–CT. Laryngoscope. 2007;117:2129‐2134. [DOI] [PubMed] [Google Scholar]
- 20. Adelstein DJ, Lavertu P, Saxton JP, et al. Mature results of a phase III randomized trial comparing concurrent chemoradiotherapy with radiation therapy alone in patients with stage III and IV squamous cell carcinoma of the head and neck. Cancer. 2000;88:876‐883. [DOI] [PubMed] [Google Scholar]
- 21. van den Bosch S, Dijkema T, Verhoef LCG, Zwijnenburg EM, Janssens GO, Kaanders JHAM. Patterns of recurrence in electively irradiated lymph node regions after definitive accelerated intensity modulated radiation therapy for head and neck squamous cell carcinoma. Int J Radiat Oncol. 2016;94:766‐774. [DOI] [PubMed] [Google Scholar]
- 22. Lango MN, Egleston B, Ende K, et al. Impact of neck dissection on long‐term feeding tube dependence in patients with head and neck cancer treated with primary radiation or chemoradiation. Head Neck. 2010;32(3):341‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Horiot JC, Le Fur R, N'Guyen T, et al. Hyperfractionation versus conventional fractionation in oropharyngeal carcinoma: final analysis of a randomized trial of the EORTC Cooperative Group of Radiotherapy. Radiother Oncol J Eur Soc Ther Radiol Oncol. 1992;25:231‐241. [DOI] [PubMed] [Google Scholar]
- 24. Kharytaniuk N, Molony P, Boyle S, et al. Association of extracapsular spread with survival according to human papillomavirus status in oropharynx squamous cell carcinoma and carcinoma of unknown primary site. JAMA Otolaryngol Head Neck Surg. 2016;142:683‐690. [DOI] [PubMed] [Google Scholar]
- 25. Chai RL, Rath TJ, Johnson JT, et al. Accuracy of computed tomography in the prediction of extracapsular spread of lymph node metastases in squamous cell carcinoma of the head and neck. JAMA Otolaryngol Head Neck Surg. 2013;139:1187‐1194. [DOI] [PubMed] [Google Scholar]
- 26. Ghadjar P, Schreiber‐Facklam H, Gräter R, et al. Quantitative analysis of extracapsular extension of metastatic lymph nodes and its significance in radiotherapy planning in head and neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2010;76:1127‐1132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request due to privacy/ethical restrictions.


