Abstract
Background
During the Italian Phase‐2 of the coronavirus pandemic, it was possible to restart elective surgeries. Because hospitals were still burdened with coronavirus disease 2019 (COVID‐19) patients, it was focal to design a separate “clean path” for the surgical candidates and determine the possible effects of major surgery on previously infected patients.
Methods
From May to July 2020 (postpandemic peak), 259 consecutive patients were scheduled for elective cardiac surgery in three different centers. Our original roadmap with four screening steps included: a short item questionnaire (STEP‐1), nasopharyngeal swab (NP) (STEP‐2), computed tomography (CT)‐scan using COVID‐19 reporting and data system (CO‐RADS) scoring (STEP‐3), and final NP swab before discharge (STEP‐4).
Results
Two patients (0.8%) resulted positive at STEP‐2: one patient was discharged home for quarantine, the other performed a CT‐scan (CO‐RADS: <2), and underwent surgery for unstable angina. Chest‐CT was positive in 6.3% (15/237) with mean CO‐RADS of 2.93 ± 0.8. Mild‐moderate lung inflammation (CO‐RADS: 2–4) did not delay surgery. Perioperative mortality was 1.15% (3/259), and cumulative incidence of pulmonary complications was 14.6%. At multivariable analysis, only age and cardiopulmonary bypass (CPB) time were independently related to pulmonary complications composite outcome (age >75 years: odds ratio [OR]: 2.6; 95% confidence interval [CI]: 1.25–5.57; p = 0.011; CPB >90 min. OR: 4.3; 95% CI: 1.84–10.16; p = 0.001). At 30 days, no periprocedural contagion and rehospitalization for COVID‐19 infections were reported.
Conclusions
Our structured roadmap supports the safe restarting of an elective cardiac surgery list after a peak of a still ongoing COVID‐19 pandemic in an epicenter area. Mild to moderate CT residuals of coronavirus pneumonia do not justify elective cardiac surgery procrastination.
Keywords: cardiac surgery, coronavirus disease 2019, public health
1. INTRODUCTION
During coronavirus disease 2019 (COVID‐19) pandemic Phase‐1, the Italian government has been forced to timely reallocate resources to maximize hospital/intensive care unit (ICU) capacity to cope with the exponential increase in critically ill Sars‐CoV‐2 patients. 1 The Lombardia region was heavily hit by the pandemic, facing almost 40% of the total cases in the whole national territory. In particular, urban hospitals in Bergamo and Brescia recorded the highest incidence of infections and fatalities by May 1st 2020. 2 , 3 During the Phase‐1 total national lockdown lasting almost 10 weeks, surgeons have canceled elective cases, and hospital managers have reorganized hospitals into Hub and Spoke Center, according to the Lombardia Region guidelines, to ensure medical and surgical treatment for those emergent/urgent cases that could not be postponed. 1 However, suspension of elective activity to save ICU beds for COVID‐19 caused a backlog with patient accumulation in the waiting list, thus making recovery of an efficient cardiac surgery workflow critical.
Phase‐2 started when pandemic contagion decreased after a plateau stage. 2 At that point, ICU beds became available for elective surgical patients, even though some hospitals located in the middle of the “Red Zone” in the Lombardia region were still burdened with COVID‐19 patients. During Phase‐2, hospitals began planning how to provide high standards of care to COVID‐19 positive patients simultaneously and restart elective surgical activity limiting intra‐hospital contagion between COVID‐19 positive and COVID‐19 negative patients referred for elective surgery. Specific protocols and “clean paths” were created and applied to keep high safety standards and contain complications while surgical units were reopening for elective cases. Moreover, for patients scheduled to undergo complex surgical procedures requiring endotracheal intubation, mechanical ventilation, and, in the case of cardiac surgery, cardiopulmonary bypass (CPB), it was necessary to understand if and how a possible previous infection with Sars‐CoV‐2 may have impacted upon periprocedural outcomes, in particular upon respiratory complications.
In the present manuscript, developed within the premises of an Italian epicenter region (Lombardia), we: (a) propose a roadmap to restart elective cardiac surgery activity after COVID‐19 pandemic peaks safely, (b) present the results of our screening protocol and roadmap aimed at identifying and adequately managing COVID‐19 positive patients (active or previous infection) referred for elective cardiac surgery, and (c) investigate the impact of residual lung damage secondary to previous COVID‐19 disease in patients undergoing cardiac surgery.
2. MATERIALS AND METHODS
This retrospective multicenter study has been registered by the Ethics Committee at Spedali Civili di Brescia (SCBS), Brescia, Italy, under nasopharyngeal (NP) 4368, and waived for informed consent.
Three adult cardiac surgery departments located in urban hospitals within the Italian region of Lombardia (among the highest world rates of COVID‐19 infection prevalence, incidence, mortality, and lethality during winter 2020) have contributed to the ideation and application of the protocol presented in this manuscript.
Participating centers include: SCBS and Bergamo, Ospedale Papa Giovanni XXIII (PGXXIII), and Clinica Humanitas Gavazzeni (CHG).
Priority Classes A and B patients (surgical intervention mandatory within 30–60 days) were planned to undergo elective cardiac surgery during pandemic Phase‐2, from May 1st to July 15th 2020.
2.1. Screening and management protocol
Patients listed for elective cardiac surgery were screened according to the study protocol outlined in Figure 1. The protocol was designed during multidisciplinary discussions, including cardiac surgeons, cardiologists, anesthesiologists, intensive care specialists, radiologists, and infectious disease specialists.
Figure 1.
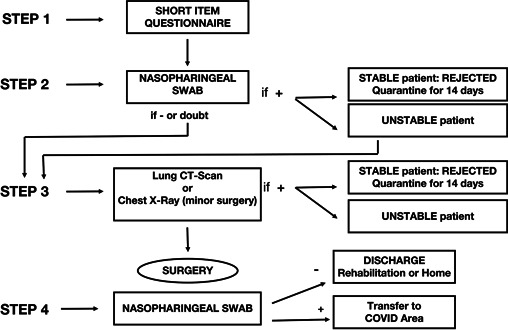
Chart of patients' flow at different screening time point
In detail, patients scheduled for elective cardiac surgery completed a preliminary short item COVID‐19 questionnaire administered by doctor residents or consultants (STEP‐1), where patients were asked to declare any contact with documented COVID‐19 positive people, and were interviewed about any symptoms/signs leading to high suspicion of active/recent Sars‐CoV‐2 infection.
Patients then proceeded to STEP‐2. The day before admission, patients were interviewed for possible changes in their symptoms and contact status. They were admitted to an isolated area to undergo NP swab and routine blood work and were then discharged home within 1 h. If NP swab was positive, the Heart Team, together with an infective disease specialist, reassessed the patient and decided to discharge her/him to home quarantine or, if surgery was deemed not deferrable, to plan hospitalization in a COVID‐19 area. Discharged patients were then reassessed after 14 days. Dubious NP swab interpretation was considered positive, and patients were discharged home unless clinical conditions required urgent management. Of note, PGXXIII and CHG performed the short item COVID‐19 questionnaire simultaneously as STEP‐2.
Once STEP‐2 was completed and NP swab resulted negative for COVID‐19, patients then proceeded to STEP‐3 the following day: chest‐computed tomography (CT) scan was performed to exclude an ongoing viral pneumonia and identify residual signs of a healed lung infection. A “green” COVID‐free path was created to move patients among different services inside the hospital (i.e., radiology, echocardiography lab, and operative theater), avoiding contacts with COVID‐19 patients running through a “red” infected path.
At the end of STEP‐2, patients with ongoing Sars‐CoV‐2 infection were not admitted for surgery and were instead put under home quarantine and reassessed 14 days later.
After surgery, NP swab and chest X‐Ray were repeated the day before discharge unless patients experienced fever or suspicious signs of COVID‐19 during hospitalization. In case of certified intra‐hospital infection, patients were isolated in a specific COVID‐19 area and treated for Sars‐CoV‐2 infection according to Infectious Disease protocols.
2.2. Diagnostic tests
According to World Health Organization laboratory testing guidelines for COVID‐19 suspected in human cases, 4 upper respiratory tract NP swab was performed using Dacron or Polyester flocked swabs, then transported into viral transport medium containing antifungal and antibiotic supplements. NP swabs were stored at a temperature of 2–8°C until processed (within 2 h) using real‐time reverse‐transcription polymerase chain reaction. Viral target genes included N (nucleocapsid), E (envelope), and RdRP (RNA dependent polymerase) genes.
NP swab was considered positive when at least one of those three genes was revealed.
Chest X‐ray and 1‐mm slice thickness chest‐CT were performed. 5 Chest‐CT images have been reviewed and scored according to the COVID‐19 reporting and data system (CO‐RADS) Classification. 6 , 7 This classification has shown excellent performances in diagnosing COVID‐19 patients, 8 and it encodes the level of suspicion for COVID‐19 based on Chest‐CT findings (1, very‐low suspicion; 2, low suspicion; 3, equivocal; 4, high suspicion; 5, very‐high suspicion). All scans were performed without intravenous contrast with the patient in the supine position during end‐inspiration. 9
Besides routine blood work for elective cardiac surgery, D‐dimer, fibrinogen, and ferritin were also analyzed as markers of the systemic hyper inflammation phase of COVID‐19. 10
2.3. Statistical analysis
The database was formatted through the Microsoft‐Excel® (Microsoft Corporation) and later imported from the IBM‐SPSS® ver.26.0.1 (IBM SPSS Inc.). The use of the Stata® ver.16.0 (Stata Corporation) was also considered for comparison or test output implementation. Continuous variables were presented as means and SD (in case of a normal distribution), or medians, interquartile range (IQR), and min/max in case of a nonparametric distribution and compared using Mann–Whitney U test. Distribution normality was assessed using the Kolmogorov–Smirnov test. Categorical variables were presented as frequencies or percentages and compared with the use of the χ 2 test or the Fisher's exact test, as appropriate. Univariable and multivariable (backward logistic regression) analysis was carried out with binary logistic regression using as dependent variable a composite end‐point (prolonged mechanical ventilation >48 h, tracheostomy, periprocedural pneumonia, and noninvasive ventilation). The variables included in the model were selected after literature review of variables considered to be significantly correlated to postoperative pulmonary complication after cardiac surgery. 11
3. RESULTS
During COVID‐19 pandemic Phase‐2, 259 consecutive patients not deferrable over 60 days were planned for cardiac surgery. After screening with a short item questionnaire (STEP‐1), no patient reported contacts with certified positive relatives or colleagues and all 259 patients were planned for NP swab (STEP‐2).
Among 259 patients undergoing NP swab (STEP‐2), two patients resulted positive (0.8%): one patient (CASE‐1) was discharged home for quarantine and reassessed after 14 days; one patient (CASE‐2) repeated a second NP swab that resulted again positive. Because he was symptomatic for low threshold unstable angina, a multidisciplinary discussion was called, and the patient underwent a lung‐CT scan that showed a CO‐RADS = 2, excluding extensive lung involvement.
NP swab resulted negative in 99.2% (257/259) of patients. Twenty‐two patients were scheduled for minor surgery without CPB (i.e., sternal debridement, pericardiocentesis, wound infection) and, underwent only chest X‐ray imaging before surgery.
Chest‐CT scan was performed in 237 patients: 15 patients (6.3%) showed signs of past Sars‐CoV‐2 lung involvement with a mean CO‐RADS score of 2.93 ± 0.8 (Figure 2) and, after anesthesiologic evaluation, proceeded to surgery. One patient (CASE‐3) (Figure 3) showed diffuse lung inflammation signs (CO‐RADS = 5) leading to a high anesthesiologic risk for invasive ventilation and CPB. Thus, surgery was postponed. This patient was sent home and reassessed 6 weeks later. A follow‐up Chest‐CT showed improvement of the previously described lung involvement (CO‐RADS = 2).
Figure 2.
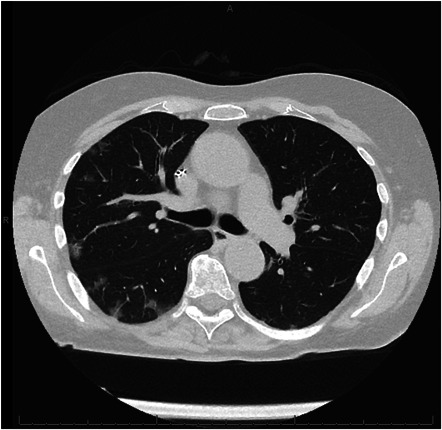
CT scan of a patient with previous COVID‐19 infection (CO‐RADS = 3). CO‐RADS, COVID‐19, coronavirus disease 2019; CT, computed tomography
Figure 3.
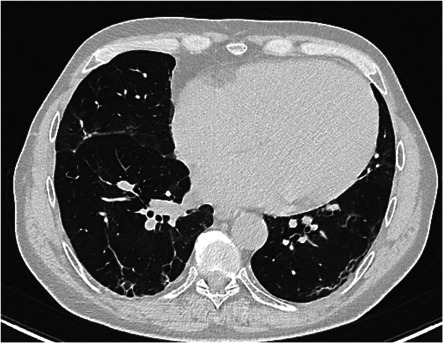
CT scan of CASE‐3 (CO‐RADS = 5). CO‐RADS, COVID‐19 reporting and data system; CT, computed tomography
Chest‐CT scan was negative for previous Sars‐CoV‐2 infection in 93.7% (222/237) of the patients.
Patient's baseline characteristics are listed in Table 1. Table 2 summarizes the clinical actions to manage four patients, resulting in positive for active COVID‐19 infection or having CT signs of extensive inflammatory lung damage post‐Sars‐CoV‐2 infection during perioperative screening. Table 3 summarizes CT findings in 15 patients with residual signs of previous Sars‐CoV‐2 lung infection as well as CO‐RADS classification for each patient.
Table 1.
Preoperative baseline characteristics
| Variables | Values (N = 259) |
|---|---|
| Age (years), mean (SD) | 67 (11.5) |
| Male sex, N (%) | 176 (68) |
| Body mass index (kg/m2), mean (SD) | 26.2 (4.1) |
| Hypertension, N (%) | 199 (76.8) |
| Dyslipidemia, N (%) | 132 (51) |
| History of smoking, N (%) | 96 (37.1) |
| COPD, N (%) | 31 (12) |
| Diabetes mellitus, N (%) | 55 (21.2) |
| NYHA classification, median (IR) | 2 (1–2) |
| EuroSCORE‐II, median (IR) | 2 (1–5) |
Abbreviations: COPD, chronic obstructive pulmonary disease; IR, interquartile range; NYHA, New York Heart Association.
Table 2.
Paths for specific cases
| Step‐1: Questionnaire | Step‐2: NP Swab | Step‐3: CT | Decision | Step‐4: NP Swab | Decision | |
|---|---|---|---|---|---|---|
| Case‐1 | − | + | Quarantine | |||
| − | + | Quarantine | ||||
| − | − | − | Surgery | − | Discharge | |
| Case‐2 | − | + | − | Surgery | − | Discharge |
| Case‐3 | − | − | + | Quarantine | ||
| / | − | − | Surgery | − | Discharge | |
| Case‐4 | − | − | − | Surgery | + | Covid path: false + |
Note: +, positive; −, negative; / , not performed.
Abbreviations: CT, computed tomography; NP, nasopharyngeal.
Table 3.
CT findings in 15 patients with residual signs of previous Sars‐CoV‐2 lung infection
| Patient | Ground glass opacities | Parenchymal fibrotic retraction | Bronchiectasis/atelectasis | Septal/pulmonary thickening | Alveolar infiltrate | Pleural effusion | Nodules/nodular calcification | CO‐RADS |
|---|---|---|---|---|---|---|---|---|
| 1 | x | x | ‐/x | 4 | ||||
| 2 | x | 3 | ||||||
| 3 | x | 2 | ||||||
| 4 | x | ‐/x | x | 3 | ||||
| 5 | x | 3 | ||||||
| 6 | x | x | 3 | |||||
| 7 | x | x | x/‐ | x/‐ | 5 | |||
| 8 | x | x/‐ | 2 | |||||
| 9 | ‐/x | 3 | ||||||
| 10 | x | ‐/x | x/‐ | x | 3 | |||
| 11 | x | ‐/x | x | 2 | ||||
| 12 | x | x/‐ | 2 | |||||
| 13 | ‐/x | x/‐ | x | 3 | ||||
| 14 | x | 3 | ||||||
| 15 | x | 3 |
Abbreviations: CO‐RADS, COVID‐19, coronavirus disease 2019; CT, computed tomography; NP, nasopharyngeal.
Operative details are shown in Table 4, as well as postoperative pulmonary complications. Of note, the median ICU stay was 2 (IQR: 1–4) days. The cumulative incidence of pulmonary complications was 14.6%. In particular, prolonged ventilation more than 48 h was required in 23 patients (8.9%), pneumonia occurred in 11 patients (4.2%), while tracheostomy was necessary for 4 patients (1.5%).
Table 4.
Operative and perioperative details
| Variables | Values (N = 259) |
|---|---|
| CABG, N (%) | 60 (23.2) |
| MV repair/replacement, N (%) | 45 (17.4) |
| AVR, N (%) | 26 (10) |
| TAVI, N (%) | 6 (2.3) |
| Ascending aorta surgery, N (%) | 7 (2.7) |
| 2 procedures, N (%) | 52 (20) |
| 3 or more procedures, N (%) | 18 (7) |
| Other procedures, N (%) | 23 (8.9) |
| Minor surgery, N (%) | 22 (8.5) |
| Non operated, N (%) | 7 (2.7) |
| Infective endocarditis, N (%) | 12 (4.6) |
| REDO, N (%) | 16 (6.2) |
| IOT time (hours), median (IR) | 11 (9–15) |
| ICU stay (days), mean (SD) | 3.4 (5.1) |
| Hospital stay (days), mean (SD) | 12.9 (9) |
| Pulmonary complications, N (%) | 38 (14.6) |
| Ventilation >48 h, N (%) | 23 (8.9) |
| Pneumonia, N (%) | 11 (4.2) |
| Tracheostomy, N (%) | 4 (1.5) |
| Mortality, N (%) | 4 (1.5) |
Abbreviations: AVR, aortic valve replacement; CABG, coronary artery bypass grafting; ICU, intensive care unit; IOT, orotracheal intubation; IR, interquartile range; MV, mitral valve; REDO, reoperative surgery; TAVI: transcatheter aortic valve implantation.
Results of univariable and multivariable analysis are summarized in Table 5. At univariate analysis age over 75 years (odds ratio [OR]: 2.06; 95% confidence interval [CI]: 1.02–4.17; p = 0.044) and CPB time longer than 90 min (OR: 3.65; CI 95%: 1.61–8.33; p = 0.002) were significantly related to the pulmonary complications' composite outcome. At multivariable analysis, both variables were confirmed to be independent determinants of pulmonary complications (age >75 years: OR: 2.6; 95% CI: 1.25–5.57; p = 0.011; CPB: 90 min. OR: 4.3; 95% CI: 1.84–10.16; p = 0.001).
Table 5.
Univariable and multivariable analysis for determinants of pulmonary complications
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age over 75 years | 2.06 | 1.02–4.17 | 0.044 | 2.6 | 1.25–5.57 | 0.011 |
| BMI over 30 | 0.95 | 0.34–2.65 | 0.927 | |||
| COPD > Grade II | 3.28 | 0.75–14.34 | 0.114 | |||
| CPB over 90 min | 3.65 | 1.61–8.33 | 0.002 | 4.3 | 1.84–10.16 | 0.001 |
| CO‐RADS > II | 0.38 | 0.49–3.01 | 0.361 | |||
Abbreviations: BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CO‐RADS, COVID‐19 reporting and data system; CPB, cardiopulmonary bypass; OR, odds ratio.
Perioperative mortality was 1.15% (3/259): two patients died for postoperative low cardiac output syndrome; one patient died for multiorgan failure. The median hospital length of stay was 7 (IQR: 5–9) days. During hospitalization, no patient developed signs and symptoms suggestive of acute Sars‐CoV‐2 lung infection. All patients were discharged home or to a rehabilitation hospital once NP swab was performed (STEP‐4).
No cases of intra‐hospital transmission of Sars‐Co‐2 infection were registered. Only one patient (CASE‐4) resulted in positive to one out of three antigens at the predischarge NP swab. Preoperative chest‐CT scan was consistent with mild postinflammatory alterations (CO‐RADS = 2). Preoperative and early postoperative NP swabs had resulted in negative as well. Two additional NP swabs were repeated while the patient was in isolation, and both resulted in negative. After multidisciplinary discussion, we considered this case as being a false positive/in‐lab contamination with radiographic signs of recent COVID‐19 lung infection at a healing stage. The patient was discharged to a cardiovascular rehabilitation unit. Finally, 1 month after surgery, no patient experienced Sars‐CoV‐2 infection symptoms.
4. DISCUSSION
Since Northern Italy was hit hard by Sars‐CoV‐2, hospital reorganization was imposed to react to this dramatic pandemic outbreak. The provinces of Brescia and Bergamo (Lombardia) were epicenter areas, having reported the highest COVID‐19 incidence in Italy. 2 , 3 During the national lockdown, elective cardiac surgery procedures were withheld. As reported by the Italian Society of Cardiac Surgery and the Lombardia Regional Government guidelines, a Hub and Spoke net was organized to manage emergent/urgent cases and reallocate resources to COVID‐19 patients. 1 For this reason, while the pandemic was at its highest peak, patients in the cardiac surgery institutional waiting list accumulated, with progressive worsening of their clinical conditions.
During pandemic Phase‐2, the Sars‐CoV‐2 infection rate inverted its trend (third decade of April 2020). Thus, the decision to cautiously restart elective cardiac surgery activity for patients not deferrable over 60 days was taken. 2 , 3
Following specific regional guidelines, different paths were created to separate clinical management of positive patients from non‐COVID‐19 patients and hospital workers. 1
Although the primary concern in this complex scenario was to create safe protocols to prevent intra‐hospital COVID‐19 dissemination in patients admitted for elective treatments, specific considerations concerning cardiac surgery candidates were raised.
The experience with our roadmap for restarting elective cardiac surgery activity can be summarized as follows: (1) Despite its invasiveness and its impact upon the immune system and pulmonary function, elective cardiac surgery can be resumed safely after COVID‐19 pandemic peaks, even in pandemic epicenter areas; (2) In the roadmap for restarting elective cardiac surgery, NP swab remains the primary method to rule out on‐going COVID‐19 infection; (3) Screening with chest‐CT detects promptly the residual consequences of COVID‐19 lung infection in patients planned for major surgery; (4) In an epicenter area for COVID‐19 infection, previously unsuspected residual lung lesions suggestive of possible past‐infection can be detected in more than 6% of the screened patients, documenting that rates of COVID‐19 unreported asymptomatic infections are possibly higher; (5) CT findings of residual diffuse lung damage (CO‐RADS = 5) secondary to previous and asymptomatic COVID‐19 infection are rare (<1%) and, when present, should justify postponing cardiac surgery procedures; (6) residual mild to moderate lung inflammation (CO‐RADS: 2–4) secondary to previous and asymptomatic COVID‐19 infection do not seem to impact upon the occurrence of pulmonary complications in the perioperative phases after cardiac surgery on CPB.
Our findings are encouraging because obtained within three leading urban hospitals that have experienced the highest density and turnover of COVID‐19 patients during the Italian pandemic Phase‐1 and Phase‐2, with more than 850 patients managed simultaneously in April 2020 at SCBS.
Different authors have investigated the global challenge of restarting elective surgery after the COVID‐19 pandemic. Mayol 12 and Tuech 13 have emphasized the importance of adapting the surgical system's organization to the different phases of the pandemic. They suggested minimizing elective surgeries during prepeaking, peak, and plateau stages and planning restarting surgical activities in the descending phases of the pandemic. Cardiac surgery entails a high level of invasiveness, including full narcosis, endotracheal intubation, and CPB, which harm the immune system and pulmonary function. For this reason, when planning our roadmap to resume elective cardiac surgery, we were faced with two major tasks: (1) identify, isolate, and postpone COVID‐19 positive patients, and (2) stratify perioperative risk of patients with residual signs of healed COVID‐19 lung infection. Chest‐CT has high sensitivity (97%) in COVID‐19 infection detection in symptomatic patients residing in endemic areas. 14 Knol et al. 7 have investigated the usefulness of chest‐CT for COVID‐19 preoperative screening in a contemporary cohort (107 patients) of asymptomatic patients undergoing cardiac surgery. The 7.3% observed rate of preoperative CT‐scan abnormalities was similar to the 8% rate previously found in unexposed historical controls (p > 0.999). RT‐PCR testing was performed only on patients with abnormal radiological findings, and, for this reason, a proper estimation of the diagnostic accuracy of Chest‐CT screening could not be established in Knol et al. 7 investigation. 15
In a recent meta‐analysis 16 the diagnostic performance of chest‐CT and RT‐PCR was investigated. The authors confirmed that in areas with a low‐prevalence of COVID‐19 (1%–22.9%), chest‐CT screening of patients with suspected disease had a low positive predictive value (1.5%–30.7%). It is challenging to determine the real infection prevalence in areas where testing is not systematically performed on most of the population, as was the case during Phase‐1 of the COVID‐19 pandemic. In this context, we have intentionally proceeded to screen every preoperative patient with NP swab and chest‐CT because the infection rate in Lombardia during Phase‐1 was the highest in Italy and possibly one of the highest in Europe. Many citizens had been possibly already infected without ever being diagnosed, and they could have still carried the consequences of COVID‐19 lung infection.
For this reason, CO‐RADS scores were calculated to determine the risk in patients undergoing major surgery on CPB. Even though the CO‐RADS classification was not created with this specific intention, we collaterally used this score to grade preoperative lungs' anatomical state before. It is well known how patients with on‐going Sars‐CoV‐2 infection showed a higher perioperative risk of complications or mortality. 17 However, less is known about perioperative risks of cardiac surgery in patients showing residual signs of past COVID‐19 pulmonary involvement. Among 15 patients with “abnormal” chest‐CT and CO‐RADS ≥2, subgroup analysis did not show differences in perioperative complications (including respiratory complications and mortality). It is a piece of vital information to consider considering the global reach of this pandemic. More and more patients who will have to undergo surgery may show extensive residual lung involvement.
Finally, a comment should be given to the hospitalization length of patients undergoing complex surgery during the COVID‐19 pandemic. In our personal experience, a slowing in the postoperative discharge process has been observed and was mainly due to latency in the patients' admission capacity of external rehabilitation units. Length of stay was reported as 12.9 ± 9 days, slightly longer when compared to the average of 2019 (9.8 ± 17.1 days). Most of the regional rehabilitation centers, usually supporting the postprocedural triage of patients undergoing major surgery, have been involved in managing COVID‐19 patients. For this reason, they are still struggling to resume a physiological bed turnover. In this light, a structured preprocedural roadmap may reduce the risk of “iatrogenic contagion” resulting from postsurgical prolonged hospitalization within hospital facilities having a high‐density of Sars‐CoV‐2 infection.
Last, we believe NP swabs performed at the end of hospitalization (STEP‐4) have the logic to avoid further spread of COVID‐19 in immune‐depressed patients after major surgery. In particular, all patients who had major or minor surgery were tested utilizing an NP swab 1 day before discharge. As they resulted negative, they were safely sent home or to rehabilitation units. This strategy allowed to trace patients' virological status and avoid transferring positive, potentially infectious patients to secondary care hospitals having a high density of fragile patients.
5. LIMITATIONS
This study's main limitations are its descriptive and retrospective nature and the lack of a comparison group. In light of the gravity and high mortality of the COVID‐19 outbreak, we thought a randomized study would not have been ethical. Although the studied sample could appear to be small, it represents three very active Institutions located in geographic areas with high concentrations of COVID‐19 patients and at the European pandemic epicenter.
Finally, the aim of the current study was not to analyze the economic and financial impact of performing the described roadmap, as this cost‐benefit analysis should be done by the appropriate specialists in the field.
6. CONCLUSION
To our knowledge, this is the first study to propose a structured roadmap to restart elective cardiac surgery after COVID‐19 pandemic peaks safely and to present data resulting from its successful application in hospitals that were more profoundly affected by the Sars‐CoV‐2 infection (epicenter area). Thanks to the development and testing of our structured roadmap and thanks to the vaccination campaign for the hospital personnel, the cardiac surgery schedules' organization can normalize even during the upcoming waves of the pandemic, containing the increased mortality observed on the long waiting list. In particular, when writing this manuscript, the Lombardia region faces Phase‐3 of the pandemic. Despite that, we have maintained an elective list of cardiac surgery procedures without experiencing intra‐hospital patients' infection and increased periprocedural complications.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Fabrizio Rosati: conceptualization, data curation, investigation, methodology, project administration, supervision, validation, writing—original draft and review‐editing. Claudio Muneretto: methodology, supervision, validation, writing—review and editing. Massimo Baudo: formal analysis, software, visualization, writing—review‐editing. Giuseppe D'Ancona: data curation, investigation, methodology, project administration, supervision, validation, writing—original draft and review‐editing. Samuele Bichi: data curation, investigation, supervision, writing—review and editing. Maurizio Merlo: conceptualization, supervision, validation. Besart Cuko: data curation, formal analysis, investigation, writing—original draft. Piersilvio Gerometta: supervision, methodology, validation. Valentina Grazioli: data curation, formal analysis, investigation, validation, writing—review and editing. Laura Giroletti: data curation, investigation. Lorenzo Di Bacco: software, formal analysis. Alberto Repossini: Data curation. Stefano Benussi: conceptualization, supervision, validation, writing—review and editing.
ACKNOWLEDGMENT
Open Access Funding provided by Universita degli Studi di Brescia within the CRUI‐CARE Agreement.
Rosati F, Muneretto C, Baudo M, et al. A multicentre roadmap to restart elective cardiac surgery after COVID‐19 peak in an Italian epicenter. J Card Surg. 2021;36:3308‐3316. 10.1111/jocs.15776
REFERENCES
- 1. Lombardia R, Deliberazione N. XI/2906[Internet]. [cited 2021. Mar 8]; Available from: https://www.regione.lombardia.it/wps/wcm/connect/5e0deec4-caca-409c-825b-25f781d8756c/DGR%2B2906%2B8%2Bmarzo%2B2020.pdf?MOD=AJPERES%26CACHEID=ROOTWORKSPACE-5e0deec4-caca-409c-825b-25f781d8756c-n7b4lOB
- 2.COVID‐19 ITALIA ‐ Desktop[Internet]. [cited 2021. Mar 8]. Available from: https://opendatadpc.maps.arcgis.com/apps/opsdashboard/index.html#/b0c68bce2cce478eaac82fe38d4138b1
- 3.pcm‐dpc/COVID‐19[Internet]. GitHub. [cited 2021 Mar 8]. Available from: https://github.com/pcm-dpc/COVID-19
- 4.Laboratory testing for 2019 novel coronavirus (2019‐nCoV) in suspected human cases [Internet]. [cited 2021. Mar 8]. Available from: https://www.who.int/publications/i/item/10665-331501
- 5. Rubin GD, Ryerson CJ, Haramati LB, et al. The role of chest imaging in patient management during the COVID‐19 pandemic: a multinational consensus statement from the fleischner society. Chest. 2020;158(1):106‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prokop M, van Everdingen W, van Rees Vellinga T, et al. CO‐RADS: a categorical CT assessment scheme for patients suspected of having COVID‐19‐definition and evaluation. Radiology. 2020;296(2):E97‐E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Knol WG, Thuijs DJFM, Odink AE, et al. Preoperative Chest computed tomography screening for coronavirus disease 2019 in asymptomatic patients undergoing cardiac surgery. Semin Thorac Cardiovasc Surg. 2020;33(3):417‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lieveld AWE, Azijli K, Teunissen BP, et al. Chest CT in COVID‐19 at the ED: validation of the COVID‐19 reporting and data system (CO‐RADS) and CT severity score: a prospective, multicenter, observational study. Chest. 2021;159(3):1126‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bernheim A, Mei X, Huang M, et al. Chest CT findings in coronavirus disease‐19 (COVID‐19): relationship to duration of infection. Radiology. 2020;295(3):200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang I, Pranata R, Lim MA, Oehadian A, Alisjahbana B. C‐reactive protein, procalcitonin, D‐dimer, and ferritin in severe coronavirus disease‐2019: a meta‐analysis. Ther Adv Respir Dis. 2020;14:1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weissman C. Pulmonary complications after cardiac surgery. Semin Cardiothorac Vasc Anesth. 2004;8(3):185‐211. [DOI] [PubMed] [Google Scholar]
- 12. Mayol J, Fernández, Pérez C. Elective surgery after the pandemic: waves beyond the horizon. Br J Surg. 2020;107(9):1091‐1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tuech JJ, Gangloff A, Schwarz L. Our challenge is to adapt the organization of our system to the six stages of the epidemic to go beyond the COVID‐19 crisis. Br J Surg. 2020;107(7):e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT‐PCR testing for coronavirus disease 2019 (COVID‐19) in China: a report of 1014 cases. Radiology. 2020;296(2):E32‐E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kamel MK. Commentary: do radiological findings play a role in the screening of COVID‐19 in patients undergoing cardiac surgery? Semin Thorac Cardiovasc Surg. 2020;33:427‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim H, Hong H, Yoon SH. Diagnostic performance of CT and reverse transcriptase polymerase chain reaction for coronavirus disease 2019: A meta‐analysis. Radiology. 2020;296(3):E145‐E155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Doglietto F, Vezzoli M, Gheza F, et al. Factors associated with surgical mortality and complications among patients with and without coronavirus disease 2019 (COVID‐19) in Italy. JAMA Surg. 2020;155(8):691‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]


