Abstract
Aims
Irisin is a hormone cleaved from fibronectin type‐III domain‐containing protein 5 in response to exercise and may be therapeutic in Alzheimer's disease (AD). Irisin is shown to repair damage caused by midlife cardiometabolic risk factors for AD (i.e., diabetes mellitus; hypertension), prevent neural amyloid beta aggregation and reduce neuroinflammation. However, there are no investigations of irisin's effect on AD‐associated tauopathy in the brain. This study begins to address this gap in knowledge.
Methods
Transgenic htau mice that selectively develop age‐related tauopathy were treated with recombinant irisin (100 µg/kg weekly i.p.) beginning at a pre‐symptomatic age (4 months) to determine if irisin could prevent emergence of early neuropathology. One month later, mice were sacrificed to collect brain tissue and serum. Protein levels of ptau (serine 202), inflammatory cytokine tumour necrosis factor alpha (TNFα) and FNDC5 were quantified using capillary‐based western blotting (Wes).
Results
Our data show that irisin treatment significantly reduced ptau and TNFα in the hippocampus and serum of female htau mice compared to vehicle‐treated controls. Irisin treatment did not alter ptau levels in male htau hippocampus and appeared to enhance both neural and systemic TNFα levels.
Conclusions
This study provides the first evidence that enhancing the endogenous hormone irisin may be therapeutic against emerging neuropathology in a tauopathy‐selective AD model. This is important because there are currently no disease‐modifying therapeutics available for AD, and few agents in development address the multiple disease targets irisin appears to—making irisin an intriguing therapeutic candidate for further investigation.
Keywords: Alzheimer's disease, anti‐inflammatory agents, dementia, metabolic diseases, neurofibrillary tangles, physical exertion, tauopathies
This study provides the first evidence that exogenous administration of the exercise‐evoked hormone irisin reduces early accumulation of phosphorylated tau and inflammation in the brains of htau mice–a model that develops tauopathy similar to what presents in Alzheimer's disease.
![]()
INTRODUCTION
Neurodegenerative disease and metabolic disorders share many pathophysiological features, including disruption of cellular energy metabolism and inflammation. As such, midlife occurrence of cardiometabolic disorders such as type II diabetes mellitus, obesity, dyslipidaemia, hypertension, atherosclerosis and cardiovascular disease significantly increase risk for future diagnosis of Alzheimer's disease (AD) at ages 65+. 1 , 2 However, these risk factors are modifiable—and therapeutic management of them is shown to reduce the increased risk they confer. 3 Therefore, uncovering the mechanistic links between cardiometabolic disorders and neurodegenerative disease may illuminate new, desperately needed strategies to combat the escalating incidence of AD. 4
While cardiometabolic diseases typically originate in peripheral tissues/organ systems, their chronic effects eventually affect the central nervous system. 5 , 6 Neurons are among the most metabolically demanding cells of the body and require extensive energy resources for communication and survival; therefore, deficits in cellular energy metabolism profoundly affect brain function and structure. 7 Compounding these problems, chronic peripheral inflammation associated with cardiometabolic disorders contributes to inflammatory changes in the brain, promoting protein misfolding and accumulation of extracellular amyloid beta (Aβ) 8 and intracellular neurofibrillary tau tangles. 9 In turn, these hallmark proteinopathies of AD trigger immune‐mediated responses from microglia and astrocytes, exacerbating neuroinflammation and neurodegeneration. 10 , 11 , 12
Irisin, a hormone released by myocytes and other cells (including neurons), may be capable of therapeutically addressing metabolic dysfunction, inflammation and neuropathology. 13 Discovered in 2012, the glycoprotein irisin is a cleaved fragment of the transmembrane fibronectin type III domain‐containing protein 5 (FNDC5). 13 When the skeletal muscle is subject to exercise, FNDC5 is cleaved into a soluble irisin fragment, which is then secreted into the bloodstream. 13 , 14 , 15 FNDC5 and irisin are also constitutively expressed in other oxidative tissues/organs such as bone, liver, heart and brain. 13 , 16 , 17 , 18 , 19 Peripherally, irisin is shown to restore energy homeostasis and optimise cellular energy utilisation by ‘browning’ white adipose tissue and enhancing thermogenesis 13 , resolving insulin resistance and improving glucose utilisation in diabetic models 20 , 21 , and reducing inflammation 22 and expression of inflammatory genes. 23 Irisin is thought to mediate the beneficial effects of exercise on the brain. Exercise‐induced FNDC5/irisin release enhances expression of brain‐derived neurotrophic factor (BDNF) in the hippocampus, promoting synaptic plasticity, hippocampal neurogenesis and memory formation. 24 , 25 , 26 Compelling data show that irisin may provide neuroprotection against ischaemic damage after stroke 27 and neurodegenerative diseases such as multiple sclerosis 28 and AD. 29 Irisin treatment has been used to rescue memory deficits, restore synaptic impairments, and ameliorate Aβ‐induced suppression of BDNF in APP/PS1dE9 AD‐model mice 30 ; reduce soluble Aβ oligomer formation in cultured APP/PS1 neurons 30 , 31 ; prevent cleavage of amyloid‐precursor protein (APP) into aggregation‐prone Aβ42 fragments 31 ; and ameliorate neuroinflammation. 32 Notably, no studies have yet investigated how irisin affects the hyperphosphorylation of tau (ptau) and its subsequent assembly into damaging intraneuronal neurofibrillary tangles (collectively known as tauopathy), another major neuropathological hallmark of AD and other forms of dementia. The goal of this study was to determine whether pre‐treatment with exogenous irisin protein could reduce the emergence of early ptau accumulation and inflammation in the brains of transgenic htau mice, a selective model of age‐related tauopathy.
MATERIALS AND METHODS
Subjects
Male and female htau mice (B6.Cg‐Mapt tm1(GFP) Tg(MAPT)8cPdav/J; Jax stock# 005491) on a C57BL/6 J background were used for the primary analyses of this study. Htau mice are a tau overexpression model that was created by random insertion of the human microtubule‐associated protein tau (MAPT) gene into ‘tau knockout’ mice that have a neomycin cassette‐induced disruption at exon 1 of the endogenous mouse Mapt. 33 , 34 The human MAPT transgene is driven by a tau promoter that causes these mice to overexpress all six isoforms (including 3R/4R) of human MAPT in the absence of endogenous mouse Mapt. 33 , 34 Phenotypically, htau mice form age‐related accumulation of hyperphosphorylated tau (ptau) that leads to microtubule destabilisation, ptau aggregation and formation of neurofibrillary tangles in the hippocampus and frontal cortices, among other brain areas. 33 , 34 This tauopathy coincides with significant neuroinflammation and contributes to age‐related cognitive deficits in htau mice. 33 , 35 Htau mice have been characterised for studying dementia‐related tauopathy analogous to late‐onset AD and frontotemporal dementia. 36 However, two caveats when considering the external validity of data obtained from this model are that htau mice produce tau isoforms in different ratios than humans, 37 and the genomic integration site and conformation of the human MAPT/vector transgene array in this model have not yet been determined. Age‐ and sex‐matched C57BL/6 J (C57) mice, the background strain of htau mice, were used as non‐transgenic healthy controls in this study. For secondary analyses, an additional group of age‐ and sex‐matched ‘tau null’ mice (littermates of htau) were included in the study. While these mice function as genetic and negative controls for their htau littermates, tau null mice lack both the humanised MAPT transgene and endogenous Mapt gene and are thus tau deficient—exhibiting phenotypic abnormalities across neural, metabolic and behavioural parameters 38 , 39 that make them inadequate as primary healthy controls.
In total, 16 htau (8 per sex) and 14 C57 (7 per sex) mice were used for the primary analyses of this study. Data from female and male mice were not combined for analysis but evaluated in separate data sets 40 because of anticipated sex differences in htau phenotype based on our previous work, 38 , 41 as well as an increasing number of studies describing inherent sex differences in other AD models. 42 , 43 , 44 Study treatment was initiated in mice at the ‘pre‐symptomatic’ age of 4 months, where early isoforms of ptau and indicators of neuroinflammation are minimally present in in non‐cognitive brain regions 38 , 41 and are just emerging in the hippocampus and frontal cortex. 35 This pre‐symptomatic staging was used to approximate the clinical relevance of mitigating early metabolic risk factors associated with AD as a preventative measure rather than a restorative treatment. Mice were 5 months old at the terminal endpoint of the study when tissue was collected for histology. A separate replicative cohort of age‐ and sex‐matched tau null (n = 15) and htau (n = 13) mice were used to optimise irisin delivery and provide control data for relevant assays (see Figure S1).
All mice were bred at the Northeast Ohio Medical University (NEOMED) from original stocks obtained at Jax Mice; all groups were housed under identical conditions. All experimental procedures were approved by the NEOMED Institutional Animal Care and Use Committee and conducted in accordance with the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health.
Treatment
Female and male mice were stratified into treatment groups by body weight to ensure overall uniformity of subjects. Htau (n = 8/sex) and C57 (n = 7/sex) mice in the experimental treatment group received one weekly intraperitoneal (i.p.) injection of 100 µg/kg recombinant human irisin protein (r‐irisin; #11451: Cayman Chemicals; Ann Arbor, MI) reconstituted in sterile saline at a concentration of 10 µg/ml. Dosage, frequency and route of administration for r‐irisin were based on previous studies that demonstrated reproducible treatment effects. 18 , 45 Htau (n = 8/sex) and C57 (n = 7/sex) mice in the vehicle control group received i.p. injections of sterile saline. Prior to injection, mice were weighed and individually coded syringes were pre‐loaded with a weight‐based volume of r‐irisin or vehicle to keep experimenters blind to treatment during the actual injection procedure. Mice received a total of 4 weekly injections across one month's time, with injections being delivered at the same time on the same day each week. A timeline for treatment administration, experimental groups and ages is presented in Figure 1. Group numbers and experimental parameters for the secondary control replicative cohort of tau null and htau mice are provided in Figure S1.
FIGURE 1.
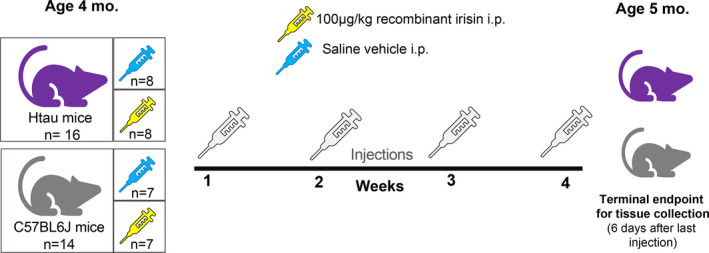
Illustration of timeline and experimental groups in study. Four‐month‐old 'pre‐symptomatic’ female and male htau and matched non‐AD control (C57BL/6 J) mice were assigned to irisin and vehicle treatment groups. Mice received weekly injections of either 100 µg/kg recombinant human irisin or equivalent volume of saline vehicle for the duration of 4 weeks. Six days after the last treatment injection, mice (at 5 months of age) were sacrificed for fresh tissue collection. Thirty mice were used for the primary analyses of this study with the strain/treatment group breakdown as follows: 16 htau mice: 8 per treatment group; 14 C57BL/6 J mice: 7 per treatment group. All groups had equivalent numbers of each sex so that female and male data could be analysed separately
Fresh tissue collection and analysis for protein
Six days after the last treatment injection, mice were sacrificed via decapitation under 4% inhaled isoflurane administered via anaesthetic vaporiser. The hippocampus, prefrontal cortices, brainstem and hypothalamus were freshly dissected, immediately frozen on dry ice and then stored at −80°C until further analysis. Trunk blood was collected and centrifuged for 10 min at 4°C (at 2000 g) for separation/extraction of serum which was then stored at −80°C until use. The freshly dissected hippocampus, brainstem, hypothalamus and prefrontal cortex were prepared for automated capillary‐based western blotting on a Wes platform (Protein Simple). The hippocampus was targeted as the primary region of interest, because it is a major area impacted by neuropathology in htau mice and is also shown to express FNDC5. 26 Secondary regions assessed included: brainstem, in which 4‐month‐old htau mice exhibited elevated ptau (serine 202) in serotonergic neurons of the dorsal raphe nucleus 38 ; hypothalamus, a structure involved in metabolic regulatory functions that has been shown to express FNDC5/irisin 45 ; and prefrontal cortex, a common target of tauopathy in older htau mice. 35
Tissue preparation for Wes occurred as described previously, 38 but briefly, brain tissue was homogenised in ice‐cold T‐PER buffer containing protease and (where relevant) phosphatase inhibitors (Thermo Scientific #78440) via sonification (10% amplitude for three 2‐s pulses), and centrifuged at 14,000 rpm for 20 min at 4°C. The Wes platform detects and provides quantitative size‐based measurement of targeted proteins by computing spectra of chemiluminescence signals versus the apparent molecular weight of protein, which are determined by mapping ladder peaks to capillary positions based on fluorescent signalling of labelled protein standards of known molecular weight. 46 Wes was used to quantify tau (ptau ser202; Cell Signaling #11834; 1:75 and total tau; Tau5 Abcam#ab80579; 1:25), FNDC5 (ProteinTech #23995‐1‐AP; 1:25) and TNFα (Rockland Scientific #210‐401‐321S; 1:10) in brain homogenates. For quantification of ptau levels, we measured tau phosphorylated at serine 202 because this form is shown to emerge in the pre‐symptomatic pathological stage we were targeting in htau mice, 38 as well as in the incipient pathology stage in human clinical samples. 35 , 38 , 47 , 48 We chose TNFα as our surrogate marker of inflammation in both brain and peripheral circulation because it is a pro‐inflammatory cytokine commonly observed in htau mice and shown to be mitigated by irisin. 49 All antibodies were diluted (ProteinSimple antibody diluent 2; #042‐195) prior to use. Tissue homogenates were loaded into 12–230 kDa (ptau, tau, FNDC5) and 2–40 kDa (TNFα) Wes plates for automated assay. To permit comparison with tau data from our previously published studies, 38 GAPDH (Sigma #G9545; 1:5000) was used as the loading control for ptau and total tau assays; for other analytes (FNDC5 and TNFα), a total protein detection module (ProteinSimple, #DM‐TP01) was used to control for the amount of protein loaded into the assay. Tissue homogenates from tau null mice were used as negative control samples for ptau plates. Recombinant irisin protein was loaded as a positive control for FNDC5/irisin plates. Compass software (ProteinSimple, v3.1.7) was used to perform peak area calculations using the default Gaussian method and generate artificial lane view images from the spectra for graphic representation. Compass provided molecular weight, signal intensity (area), percent area, and signal‐to‐noise readout from each sample in units of normalised chemiluminescence. ‘Virtual’ bands were generated by Compass software using chemiluminescence values to graphically represent signal molecular weight and intensity for each analyte and are provided in Figure S2.
Enzyme‐Linked immunosorbent assay for measurement of irisin in serum
Irisin was measured in serum samples from mice using a validated enzyme‐linked immunosorbent assay (ELISA) kit (Phoenix Pharmaceuticals #EK‐067–29: Burlingame) shown to measure the 12 kDA form of cleaved irisin. 28 , 50 , 51 Serum was diluted 10‐fold in assay buffer and both samples and positive controls were ran in duplicate per manufacturer instructions. Assay sensitivity was 1.84 ng/ml with a dynamic range of 0.1–1000 mg/ml. Intra‐ and inter‐assay coefficients of variation were <10% and <15%, respectively. Assay absorbance was read at 450 nm on a Molecular Devices SpectraMax M5 plate reader and reported in ng/ml.
Statistical analysis
Group sizes were calculated with a priori power analysis using G*Power software 52 based on our previously published effect sizes in htau mice. 38 , 41 Statistical analysis was performed using IBM SPSS 26 Software. Separate datasets were created for female and male mice so that each sex could be analysed independently of each other. This was done to increase the sensitivity of detecting treatment effects within each sex strata, 40 , 53 as the design of the study was not intended to directly compare the sexes by including sex as an independent variable. Prior to analysis, data were screened for outliers and normalcy using boxplots and frequency distributions in SPSS. Outliers that extended more than 2.5 standard deviations from subgroup mean were evaluated and in two cases where measurement error was obvious, data were removed. Factorial analysis of variance (ANOVA) models were built for each dataset (female and male) and were used to test for treatment (irisin; vehicle) x strain (htau; control) effects and interactions. To follow‐up significant interactions in each data set, data were stratified (using the SPLIT function in SPSS) by treatment to test for strain differences and then by strain to test for treatment effects within these strata. Bonferroni‐corrected post hoc ANOVA were used to determine subgroup differences. In the absence of significant interactions, simple main effects were used to describe group differences. Pearson correlations were used to describe relationships between continuous variables to provide additional information. Data from the replicative cohort with tau null and htau mice were analysed separately; statistics and results are provided in legend of Figure S1.
RESULTS
Statistical results for omnibus factorial ANOVAs are provided in Table 1.
TABLE 1.
Main effect and interaction statistics for omnibus ANOVAs conducted on sex‐stratified datasets
| Factorial ANOVA F, p and eta2 values | |||||
|---|---|---|---|---|---|
| Effect | Ptau HC | TNFα HC | TNFα serum | FNDC5 HC | Irisin serum |
| Females (n = 15) | |||||
| df | 1, 7 | 1, 7 | 1, 10 | 1, 7 | 1, 11 |
| Strain | 59.44, p < 0.001* | 5.187, p = 0.042* | 74.54, p < 0.001* | 3.332, p = ns | 0.005, p = ns |
| Treatment | 9.03, p = 0.02*, eta2 = 0.099 | 8.273, p = 0.014*, eta2 = 0.233 | 11.06, p < 0.001*, eta2 = 0.051 | 0.262, p = ns | 21.16, p = 0.001*, eta2 = 0.656 |
| Interaction | 11.30, p = 0.012*, eta2 = 0.124 | 6.458, p = 0.026*, eta2 = 0.182 | 86.36, p < 0.001*, eta2 = 0.40 | 0.165, p = ns | 0.059, p = ns |
| Males (n = 15) | |||||
| df | 1, 7 | 1, 7 | 1, 10 | 1, 7 | 1, 11 |
| Strain | 21.87, p = 0.002* | 106.89, p < 0.001* | 22.66, p < 0.001* | 14.44, p = 0.007* | 0.072, p = ns |
| Treatment | 0.061, p = ns | 9.016, p = 0.017*, eta2 = 0.066 | 0.255, p = ns | 1.517, p = ns | 34.11, p < 0.000*, eta2=0.747 |
| Interaction | 0.056, p = ns | 13.585, p = 0.006*, eta2 = 0.099 | 0.187, p = ns | 0.091, p = ns | 0.077, p = ns |
Strain = htau or C57 control; treatment = r‐irisin or vehicle control; interaction = strain by treatment comparison. For sex, F = female; M = male; HC = hippocampus. FNDC5 HC analyses reported above based on composite variable created from sum of 20 and 37kDa forms. Degrees of freedom (df), ANOVA statistic (F), significance value (p) and effect sizes (eta2) are provided for treatment main effects and interactions.
Indicates significant difference of p < 0.05.
Hippocampal ptau levels are significantly reduced in female htau mice after 4 weeks of exogenous irisin treatment
Figure 2 summarises protein assay data for ptau and total tau measured from brain samples of htau mice via automated western blotting. Female htau mice treated with r‐irisin exhibited a significant reduction in hippocampal ptau (F1,8 = 9.82, p = 0.01; Figure 2A). This finding represented a 30% difference in ptau levels between vehicle‐treated and r‐irisin treated htau mice after only 4 weeks of injections. Total tau was not affected by treatment (Figure 2B). Ptau did not significantly differ as a function of treatment group in brainstem, hypothalamus, or prefrontal cortex (Figure 2C).
FIGURE 2.
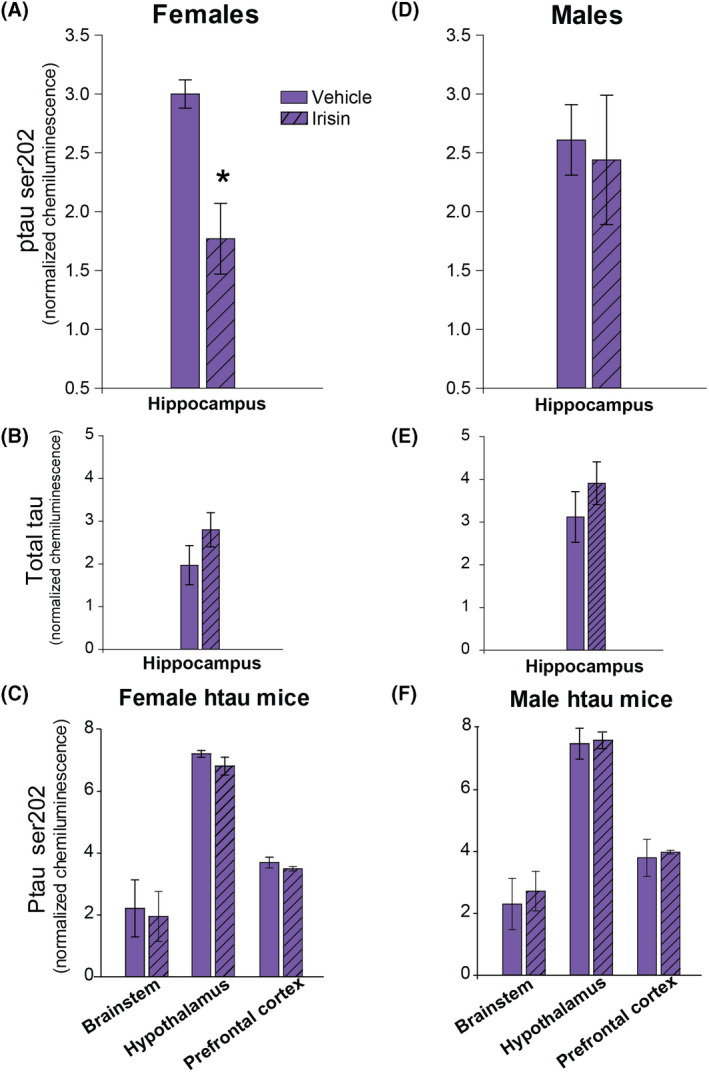
Mean tau (ptau serine 202 and total tau) chemiluminescence in brain regions of htau mice as a function of irisin treatment. (A) Female htau mice treated with r‐irisin (striped bars) had a significant reduction in hippocampal ptau202 compared to vehicle‐treated (solid bars) conspecifics. (B) Irisin treatment did not have an effect on total tau levels in female htau hippocampus. (C) Ptau in female htau prefrontal cortex, brainstem and hypothalamus did not statistically differ as a function of treatment. (D) In male htau mice, r‐irisin treatment had no effect on ptau. (E) Irisin treatment did not have an effect on total tau levels in male htau hippocampus. (F) Ptau level did not vary by treatment in any other brain regions of male htau mice. Both ptau202 and total tau data were calibrated for the amount protein loaded per sample using peak intensity chemiluminescence data for GAPDH. Error bars show s.e.m.; * indicates significant treatment group difference. p < 0.05 in all significant comparisons. n = 16 htau mice (4/sex/treatment group)
Recombinant irisin treatment does not reduce neural ptau load in male htau mice
Irisin treatment had no effect on ptau levels in male htau mice in any brain region (Figure 2D,F), nor did it affect total tau (Figure 2E).
Irisin treatment significantly reduces neuroinflammation in female htau mice
Irisin‐treated female htau mice had significantly reduced hippocampal TNFα relative to their vehicle‐treated counterparts (F1,5 = 12.97, p < 0.05; Figure 3A). Notably, TNFα and ptau levels in the hippocampus were significantly correlated in female htau mice (Figure 3B). While not statistically significant, TNFα levels in female htau brainstem and prefrontal cortex showed a trend towards similar irisin‐related reductions (Figure 3B). Hippocampal TNFα levels were significantly greater in untreated female htau mice compared to C57 controls (Figure 3A) and tau null controls (Figure S1), but this difference was eliminated in irisin‐treated htau mice.
FIGURE 3.
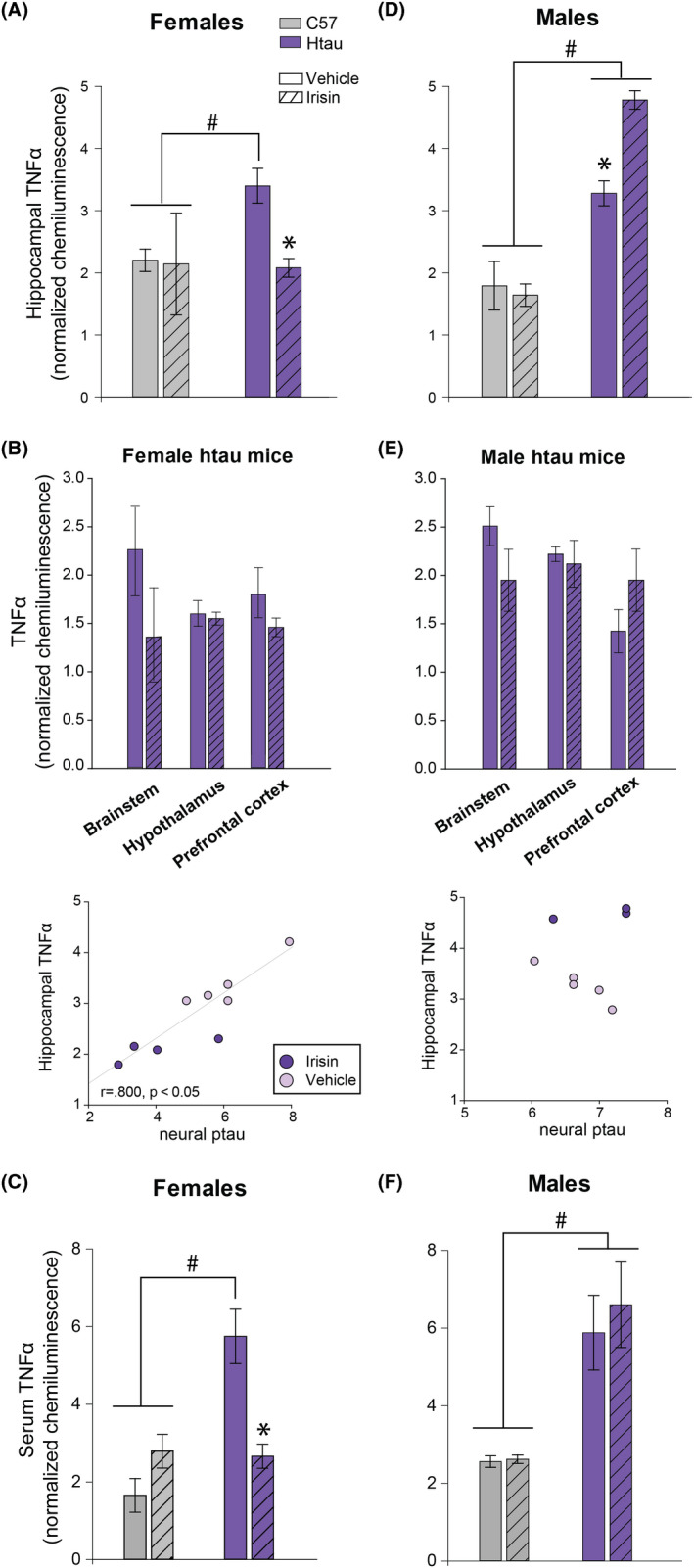
TNFα as a surrogate marker of inflammation in brain and serum of mice. Mean TNFα chemiluminescence in brain and serum of htau (purple) and control C57BL/6 J (grey) mice as a function of treatment (striped bars = r‐irisin treated mice; solid bars = vehicle‐treated mice). (A) Treatment with r‐irisin significantly reduced elevated levels of pro‐inflammatory cytokine TNFα in hippocampus of female htau mice. TNFα in C57 control mice did not vary as a function of treatment. (B) Brainstem and prefrontal cortex ptau levels appeared to be reduced in irisin‐treated female htau mice, but this was not statistically significant. Scatterplots depict strong correlation (indicated by Pearson's r value, p < 0.05) between hippocampal TNFα and ptau measurements for individual female htau mice. (C) Mean serum measurements of TNFα were significantly reduced in irisin‐treated female htau mice. (D) Irisin‐treated male htau mice showed significantly enhanced hippocampal inflammation compared to vehicle‐treated htau mice. (E) TNFα also appeared to be elevated in irisin‐treated male htau prefrontal cortex data but this was not statistically significant. Scatterplots illustrate the lack of correlation between hippocampal TNFα and ptau measurements for individual male htau mice (p = ns). (F) Mean serum measurements of TNFα did not differ as a function of irisin treatment in male htau mice, however they trended towards an irisin‐induced increase. Results are calibrated by total protein loaded per sample. Error bars show s.e.m.; * indicates significant difference between treatment groups. # indicates significant strain difference between htau and C57 mice of same treatment group. p < 0.05 in all significant comparisons. n = 16 htau mice (4/sex/treatment group); 14 C57 mice (3‐4/sex/treatment group)
Irisin treatment exacerbated neuroinflammation in male htau mice
Irisin treatment produced different results in male htau mice; it enhanced hippocampal TNFα levels compared to male htau mice given saline vehicle, (F1,5 = 30.63, p = 0.003; Figure 3D). Unlike female htau mice, male htau hippocampal TNFα levels were not correlated with overall neural ptau load (Figure 3E). TNFα levels in the prefrontal cortex of r‐irisin‐treated male htau mice also appeared to be slightly elevated relative to vehicle‐treated mice (Figure 3E), but this was not statistically supported. Hippocampal TNFα levels were significantly greater in male htau mice compared to C57 controls (Figure 3D) and tau null controls (Figure S1) regardless of treatment condition.
Irisin treatment effects on htau peripheral inflammation were consistent with effects on htau neuroinflammation
Since r‐irisin was administered peripherally via intraperitoneal injection, we measured serum levels of TNFα to determine whether this treatment affected systemic inflammation. Consistent with our findings in neural tissue, r‐irisin treatment reduced serum TNFα levels in female htau mice, (F1,6 = 16.25, p = 0.007; Figure 3C), but failed to do so in male htau mice (Figure 3F). While not meeting criteria for statistical significance, it was noted that serum TNFα levels showed a trend of being higher among r‐irisin treated males compared to those that received the saline vehicle, (F1,4 = 5.8, p = 0.07, ns).
FNDC5/irisin levels in the brain did not change as a function of irisin treatment
Neural levels of FNDC5/irisin were measured to determine whether peripherally administered irisin directly increased these protein levels in the brain. We detected several bands of FNDC5/irisin at different molecular weights (Figure S2)—a finding consistent with previously published literature that identified multiple FNDC5/irisin bands associated with the significant amount of post‐translational modification/glycosylation of this protein that occurs in the brain. 17 , 30 , 54 , 55 Therefore, we quantified and reported data from two bands (20 kDA and 37 kDA) that were consistent with those reported by Varela‐Rodríguez et al. 45 that represented un‐glycosylated and glycosylated forms of FNDC5, respectively. Post‐translational modification also causes the molecular weight range to overlap between the full‐length FNDC5 membrane‐bound protein and truncated irisin. 54 , 56 Both are shown to colocalise in parenchymal brain tissue, preventing specific determination of the cleaved protein (irisin) from its parent (FNDC5) in our Wes assays. Therefore, to preserve accuracy, we refer to these measurements in brain tissue as ‘FNDC5/irisin’ as was done by Lourenco and colleagues. 30 In contrast, our ELISA measurements in serum are presumed to contain only the cleaved, secreted irisin protein and not membrane‐bound FNDC5, thus we refer to these as ‘irisin’. 30 , 57
For initial comparisons, we used a composite‐dependent variable for FNDC5/irisin created from the sum of both 20 and 37 kDa values for each brain region. The results did not indicate any significant treatment‐related changes in composite FNDC5/irisin (Figure 4A,B), but in male mice, there was a significant strain effect for the hippocampus (Table 1), prefrontal cortex (F1,8 = 8.48, p = 0.02) and brainstem (F1,8 = 18.28, p = 0.004). To further evaluate these findings, we analysed the 20 and 37 kDa bands separately. Data from vehicle‐control mice showed that male htau had intrinsically elevated neural 37kDA FNDC5/irisin compared to male C57 mice in the hippocampus (F1,3 = 83.79, p = 0.003; Figure 4B), prefrontal cortex (F1,4 = 11.65, p = 0.03), hypothalamus (F1,4 = 12.24, p = 0.03) and brainstem (F1,3 = 23.31, p = 0.02)—a region which also showed significantly increased measurements of the 20 kDA form (F1,3 = 36.88, p = 0.009). Data are summarised in Figure 4C.
FIGURE 4.
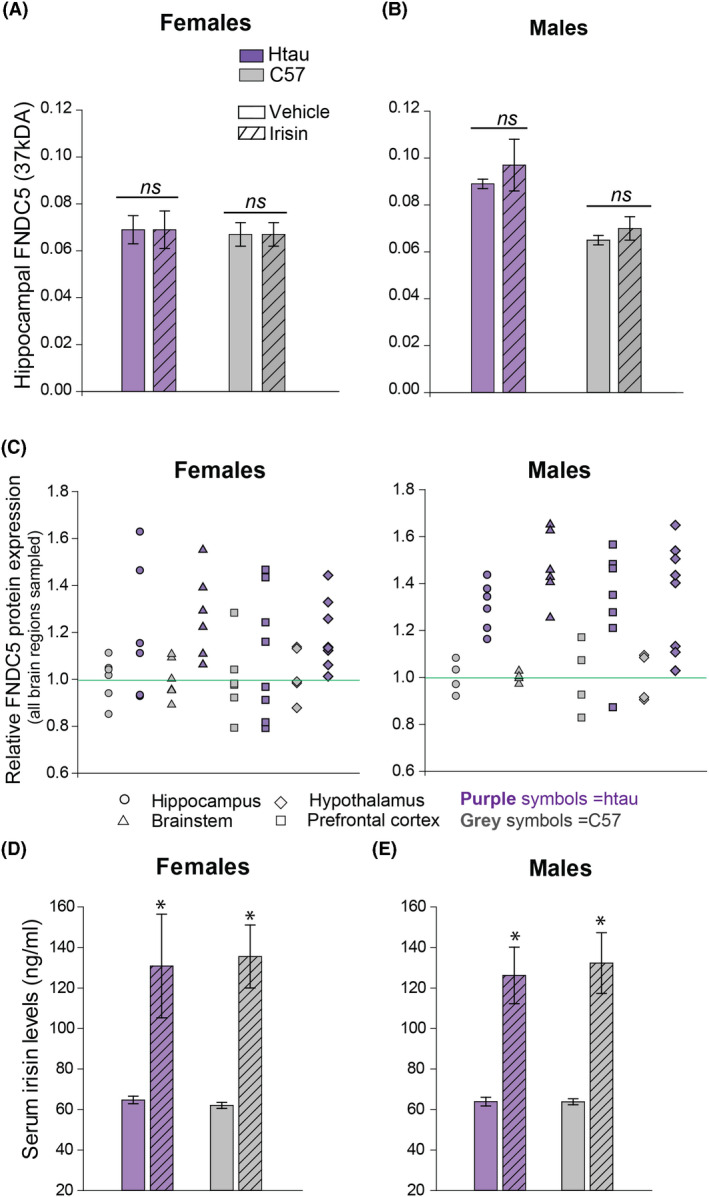
Irisin levels in mice. Mean FNDC5/irisin levels (37kDA band) in the hippocampus of htau (purple) and control C57BL/6 J (grey) mice as a function of treatment (striped bars = r‐irisin treated mice; solid bars = vehicle‐treated mice). (A) Female brain FNDC5/irisin levels did not differ as a function of treatment or strain. (B) Male brain FNDC5/irisin levels did not differ as a function of treatment, but vehicle‐treated male htau mice exhibited significantly higher FNDC5/irisin levels than vehicle‐treated C57 males. (C) Dot density scatterplots show individual composite measurements summed across 20kDA and 37kDA bands for FNDC5/irisin protein in the hippocampus, brainstem, hypothalamus and prefrontal cortex for female and male htau and C57 mice. These summed composite values were normalised across all brain regions for depiction. (D&E) Serum levels of irisin are elevated in female and male mice treated with r‐irisin regardless of strain. * indicates significant treatment group differences, significance = p < 0.05. n = 16 htau mice (4/sex/treatment group); 14 C57 mice (3–4/sex/treatment group)
Serum irisin levels were elevated in response to treatment condition
Contrary to measurements in brain tissue, serum irisin levels were significantly enhanced in both female and male mice receiving r‐irisin treatment regardless of strain (F1,22 = 51.39, p < 0.01; Figure 4D,E), confirming that peripheral administration of 100 µg/kg r‐irisin effectively doubled the amount of circulating irisin in mice. This finding also showed that blood levels of irisin were sustained for a relatively long duration post‐injection, as serum samples were collected from mice six days after the last treatment. No intrinsic differences in circulating irisin levels were shown between mouse strains nor did these levels appear to differ between females and males.
DISCUSSION
Previous studies support a protective role for irisin against AD‐associated amyloid pathology and inflammation. 30 , 31 Here, we expand this knowledge on irisin's therapeutic potential with evidence that one month of peripherally delivered irisin significantly reduced hippocampal ptau and neuroinflammation in female htau mice—a model that approximates (but is not identical) to tauopathy in clinical dementia. This reduction was observed in pre‐symptomatic female mice at an age where they only exhibit ‘pre‐tangle’ and emerging pathology—therefore, it remains to be determined how irisin impacts more advanced aspects of tauopathy including neurofibrillary tangle formation and human pathology. However, these findings provide important insights to irisin's potential as an early intervention in the disease process.
It is notable that the therapeutic effects of irisin were specific to female htau mice. Our data showed that irisin treatment did not alter ptau levels in male htau brains and actually seemed to enhance inflammation in these mice. While we anticipated some degree of sex differences in our results, this large divergence in findings was unexpected. AD disproportionately affects women 4 by reasons that are not fully understood; a lack of direct, sex‐based characterisations of both AD clinical presentations and experimental model phenotypes has likely contributed to this. 58 While an increasing number of studies include subjects of both sexes, sex is not always analysed as a variable and data are often pooled for analysis. This is problematic for the field; if we had pooled data across sexes in this study, we would have obscured the intriguingly dichotomous sex‐based treatment effects of irisin (recall Figures 2 & 3).
As such, clinical and experimental studies that analyse sex‐stratified data or directly test for sex effects are beginning to identify large sex differences in pathological phenotype. 41 , 42 , 43 , 44 , 59 Sex differences in irisin levels have been shown in human serum and cerebrospinal fluid samples, although the functional significance of this remains unknown. 51 , 60 , 61 FNDC5 gene polymorphisms have been linked to cardiovascular complications in diabetic women but not men, 62 suggesting that alterations in basal levels of FNDC5/irisin may confer sex‐based vulnerability to metabolic dysfunction. Sex differences in circulating gonadal hormones may also influence response to irisin. For example, gonadectomy was shown to alter serum irisin levels in female but not male mice. 61 In our study, brain levels of FNDC5/irisin were intrinsically elevated in male htau mice compared to male controls and all females. Similar elevations in irisin have been reported in the context of obesity, hypoglycaemia, hypertension, and even normal aging; this elevated irisin is postulated to occur as a compensatory response to challenged energy homeostasis. 63 , 64 , 65 , 66 There are also data showing that these compensatory increases in irisin may confer insensitivity to effects of the hormone. 67 This ‘irisin‐resistance’ could explain our observed lack of treatment effect on ptau levels in male htau mice. However, our TNFα data do not support this because they show that irisin treatment increased both neural and systemic inflammation in male htau mice. A recent study found that glutaminergic receptors in the brains of female and male mice exhibit strikingly different pharmacological responses to pathological AD proteins. 68 These authors suggest that such intrinsic neurobiological differences between females and males may cause certain therapeutics to be effective in one sex but not the other. This is a critically important concept for the field to recognise and may account for the current data showing sex‐based divergence of treatment response to irisin in htau mice. Much more work is needed focusing on the underlying mechanisms and identification of sex differences in response to therapeutic treatment.
As previously indicated, irisin‐induced reduction of hippocampal ptau in female htau mice coincided with reduced pro‐inflammatory TNFα levels—in both brain and serum—supporting the premise that irisin's anti‐inflammatory properties may partially account for its therapeutic effect in reducing neural ptau. 69 , 70 In female htau mice, we observed a significant correlation between peripheral and neural TNFα levels, as well as between neuroinflammation and overall neural ptau load. While TNFα was used heuristically because it is a robust inflammatory cytokine that maximised our chance of detecting treatment‐related changes in overall inflammatory load, these results are limited by the fact that this is only a single marker within an incredibly complex inflammatory landscape that irisin may impact. In brain and peripheral tissue, irisin has been found to inhibit pro‐inflammatory p38/MAPK, STAT3 32 , 70 and toll‐like receptor/MYD88/NFκB signalling pathways, 22 , 69 , 71 as well as NL3R inflammasome signalling. 72 , 73 In AD, inflammatory signalling that arises either acutely in the brain or chronically from the periphery is shown to facilitate Aβ and ptau accumulation. 74 , 75 Current studies in our laboratory are underway to conduct a more comprehensive screening of how irisin treatment impacts various pro‐ and anti‐ inflammatory cytokines and chemokines in CNS and periphery of htau mice, and future work will need to further refine the mechanisms irisin exerts in this and other inflammatory models.
Another mechanism by which irisin may provide neuroprotection involves its role in mediating insulin signalling. 6 , 76 In obesity and T2DM models, irisin treatment can correct insulin resistance and increase glucose uptake by cells. 20 , 77 While insulin is not required for glucose uptake in neurons, 78 it is critical for regulating neuronal growth, protein synthesis, and autophagy, 6 , 76 , 79 and insulin receptor signalling activates MAPK and PI3/Akt pathways. 79 This is important because PI3/Akt regulates glycogen synthase kinase 3β (GSK3β), a stress kinase that contributes to tau hyperphosphorylation in AD. 80 , 81 Insulin signalling enhances PI3/Akt‐mediated inhibition of GSK3β, which prevents this kinase from phosphorylating tau 82 ; thus, chronic inflammation and/or insulin deficiency resulting from cardiometabolic disease can disrupt insulin‐PI3/Akt‐mediated inhibition of GSK3β in the brain. 83 While other kinase pathways also contribute to tauopathy in AD, this insulin‐dependent regulation of the GSK3 pathway is a potential mechanism by which irisin could exert its neuroprotective effects. Future studies will need to explore how irisin impacts both insulin‐dependent and ‐independent (e.g., Wnt/β‐catenin 84 ) GSK3β regulation in AD models.
We did not observe any increases in neural FNDC5/irisin levels in mice as a result of exogenous treatment with recombinant irisin protein; however, serum levels of irisin were doubled in all animals receiving this treatment. This provokes a question of how peripherally delivered irisin drives therapeutic effects in female htau mouse brain. Serum irisin is presumed to only contain the cleaved, secreted irisin protein and not membrane‐bound FNDC5. 30 , 56 , 85 The molecular weights of FNDC5 we detected in brain tissue were higher than serum measurements and the recombinant irisin control protein (12 kDa). However, this was consistent with previous literature reporting that FNDC5/irisin undergoes significant post‐translational modification in the brain that changes the molecular weight of the protein. 17 , 30 , 54 , 55 It is possible that these changes alter protein function, 54 uncoupling FNDC5 and irisin levels and obscure accurate detection of these proteins in the brain. 56
Another issue to consider is that while several studies 26 , 30 , 51 , 86 indirectly support the premise that irisin crosses the blood–brain barrier (BBB), there have been no direct assessments of irisin's BBB permeability and/or membrane transport mechanism. With r‐irisin treatment doubling serum irisin levels in mice, we would have expected to see some change in brain FNDC5/irisin levels in our mice if irisin were independently BBB permeable. It is possible that circulating irisin may indirectly affect the CNS by interacting with an unknown, intermediate mechanism that has greater membrane portability (e.g., lactate, as described in the study by El Hayek et al. 87 ). Future work will need to determine the specific attributes of irisin's BBB permeability.
Much remains to be learned about irisin. Although this work is in its infancy, we are beginning to see the importance of this ‘exercise hormone’ in normal health and aging, and how it may hold promise for combatting AD, which is unceasingly fatal and currently untreatable.
ETHICS STATEMENT
All experimental procedures were approved by the NEOMED Institutional Animal Care and Use Committee and conducted in accordance with the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health, USA.
AUTHOR CONTRIBUTIONS
Katie Bretland: Investigation; Project Administration; Writing—original draft; Writing—review and editing; Li Lin: Investigation; Methodology; Validation; Kimberly Bretland: Investigation; Validation; Matthew Smith: Methodology; Writing—review and editing; Sheila Fleming: Conceptualisation, Supervision; Christine Dengler‐Crish: Conceptualisation; Formal Analysis; Funding Acquisition; Supervision; Writing—review and editing.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/nan.12711.
Supporting information
Fig S1‐S2
ACKNOWLEDGEMENTS
This work was supported by a philanthropic donation provided by the Shah Family Foundation, a philanthropic fellowship from the Alan and Janice Woll Foundation to K. A. Bretland, and institutional research start‐up funds (Northeast Ohio Medical University) to C. M. Dengler‐Crish. We are grateful to Dr Sam Crish and Dr Sarah Terrill for proofreading the manuscript. A subset of data from this manuscript were presented as a senior undergraduate honours thesis by co‐author K. M. Bretland that is published here: Bretland, K. (2020). Treating Neuroinflammation with the Novel Hormone Irisin. (Electronic Thesis). Retrieved from http://rave.ohiolink.edu/etdc/view?acc_num=ksuhonors1588335342425119.
Bretland KA, Lin L, Bretland KM, Smith MA, Fleming SM, Dengler‐Crish CM. Irisin treatment lowers levels of phosphorylated tau in the hippocampus of pre‐symptomatic female but not male htau mice. Neuropathol Appl Neurobiol. 2021;47(7):967-978. 10.1111/nan.12711
Contributor Information
Katie A. Bretland, Email: kbretland@neomed.edu
Li Lin, Email: llin@neomed.edu.
Kimberly M. Bretland, Email: kbretlan@kent.edu
Matthew A. Smith, Email: msmith13@neomed.edu
Sheila M. Fleming, Email: sfleming1@neomed.edu
Christine M. Dengler‐Crish, Email: ccrish@neomed.edu.
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Tortelli R, Lozupone M, Guerra V, et al. Midlife metabolic profile and the risk of late‐life cognitive decline. J Alzheimer's Dis. 2017;59(1):121‐130. [DOI] [PubMed] [Google Scholar]
- 2. Lee S‐H, Han K, Cho H, et al. Variability in metabolic parameters and risk of dementia: a nationwide population‐based study 11 Medical and health sciences 1117 public health and health services. Alzheimer’s Res Ther. 2018;10(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yashkin AP, Akushevich I, Ukraintseva S, Yashin A. The effect of adherence to screening guidelines on the risk of Alzheimer’s disease in elderly individuals newly diagnosed with type 2 diabetes mellitus. Gerontol Geriatr Med. 2018;4:233372141881120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alzheimer’s Association . Alzheimer's disease facts and figures. Alzheimer's Dement J Alzheimer's Assoc. 2018;14(3):367‐429. [Google Scholar]
- 5. Baulch JE, Acharya MM, Agrawal S, Apodaca LA, Monteiro C, Agrawal A. Immune and inflammatory determinants underlying Alzheimer's disease pathology. J Neuroimmune Pharmacol. 2020;15:852‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Macklin L, Griffith CM, Cai Y, Rose GM, Yan X‐X, Patrylo PR. Glucose tolerance and insulin sensitivity are impaired in APP/PS1 transgenic mice prior to amyloid plaque pathogenesis and cognitive decline. Exp Gerontol. 2017;88:9‐18. [DOI] [PubMed] [Google Scholar]
- 7. Chow H‐M, Shi M, Cheng A, et al. Age‐related hyperinsulinemia leads to insulin resistance in neurons and cell‐cycle‐induced senescence. Nat Neurosci. 2019;22(11):1806‐1819. [DOI] [PubMed] [Google Scholar]
- 8. Webers A, Heneka MT, Gleeson PA. . The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer's disease. Immunol Cell Biol. 2020;98(1):28‐41. [DOI] [PubMed] [Google Scholar]
- 9. Liu Y, Zhang S, Li X, et al. Peripheral inflammation promotes brain tau transmission via disrupting blood‐brain barrier. Biosci Rep. 2020;40(2):BSR20193629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dionisio‐Santos DA, Olschowka JA, O’Banion MK. Exploiting microglial and peripheral immune cell crosstalk to treat Alzheimer’s disease. J Neuroinflammation. 2019;16(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Apelt J, Schliebs R. Beta‐amyloid‐induced glial expression of both pro‐ and anti‐inflammatory cytokines in cerebral cortex of aged transgenic Tg2576 mice with Alzheimer plaque pathology. Brain Res. 2001;894(1):21‐30. [DOI] [PubMed] [Google Scholar]
- 12. Littlefield A, Kohman RA. Differential response to intrahippocampal interleukin‐4/interleukin‐13 in aged and exercise mice. Neuroscience [Internet]. 2017;343:106‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boström P, Wu J, Jedrychowski MP, et al. A PGC1‐α‐dependent myokine that drives brown‐fat‐like development of white fat and thermogenesis. Nature. 2012;481(7382):463‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jin Y, Sumsuzzman DM, Choi J, Kang H, Lee SR, Hong Y. Molecular and functional interaction of the myokine irisin with physical exercise and Alzheimer's disease. Molecules. 2018;23(12):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang J, Valverde P, Zhu X, et al. Exercise‐induced irisin in bone and systemic irisin administration reveal new regulatory mechanisms of bone metabolism. Bone Res. 2017;5:16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aydin S, Kuloglu T, Aydin S, et al. A comprehensive immunohistochemical examination of the distribution of the fat‐burning protein irisin in biological tissues. Peptides. 2014;61:130‐136. [DOI] [PubMed] [Google Scholar]
- 17. Panati K, Narala VR, Narasimha VR, Derangula M, Arva Tatireddigari VRR, Yeguvapalli S. Expression, purification and biological characterisation of recombinant human irisin (12.5 kDa). J Genet Eng Biotechnol. 2018;16(2):459‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Colaianni G, Cuscito C, Mongelli T, et al. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci. 2015;112(39):12157‐12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Forouzanfar M, Rabiee F, Ghaedi K, et al. Fndc5 overexpression facilitated neural differentiation of mouse embryonic stem cells. Cell Biol Int. 2015;39(5):629‐637. [DOI] [PubMed] [Google Scholar]
- 20. Ye X, Shen YiMin, Ni C, et al. Irisin reverses insulin resistance in C2C12 cells via the p38‐MAPK‐PGC‐1α pathway. Peptides. 2019;119:170120. [DOI] [PubMed] [Google Scholar]
- 21. Liu T‐Y, Shi C‐X, Gao R, et al. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin Sci. 2015;129(10):839‐850. [DOI] [PubMed] [Google Scholar]
- 22. Mazur‐Bialy AI, Bilski J, Pochec E, Brzozowski T. New insight into the direct anti‐inflammatory activity of a myokine irisin against proinflammatory activation of adipocytes. implication for exercise in obesity. J Physiol Pharmacol. 2017;68(2):243‐251. [PubMed] [Google Scholar]
- 23. de Oliveira M, De Sibio MT, Mathias LS, Rodrigues BM, Sakalem ME, Nogueira CR. Irisin modulates genes associated with severe coronavirus disease (COVID‐19) outcome in human subcutaneous adipocytes cell culture. Mol Cell Endocrinol. 2020;515:110917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Choi SH, Bylykbashi E, Chatila ZK, et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science (80‐) [Internet]. 2018;361(6406):eaan8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moreno‐Jiménez EP, Flor‐García M, Terreros‐Roncal J, et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer's disease. Nat Med. 2019;25(4):554‐560. [DOI] [PubMed] [Google Scholar]
- 26. Wrann CD, White JPP, Salogiannnis J, et al. Exercise induces hippocampal BDNF through a PGC‐1α/FNDC5 pathway. Cell Metab. 2013;18(5):649‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Asadi Y, Gorjipour F, Behrouzifar S, Vakili A. Peptide protects brain against ischemic injury through reducing apoptosis and enhancing BDNF in a Rodent model of stroke. Neurochem Res. 2018;43(8):1549‐1560. [DOI] [PubMed] [Google Scholar]
- 28. Briken S, Rosenkranz SC, Keminer O, et al. Effects of exercise on Irisin, BDNF and IL‐6 serum levels in patients with progressive multiple sclerosis. J Neuroimmunol. 2016;299:53‐58. [DOI] [PubMed] [Google Scholar]
- 29. Azimi M, Gharakhanlou R, Naghdi N, Khodadadi D, Heysieattalab S. Moderate treadmill exercise ameliorates amyloid‐β‐induced learning and memory impairment, possibly via increasing AMPK activity and up‐regulation of the PGC‐1α/FNDC5/BDNF pathway. Peptides. 2018;102:78‐88. [DOI] [PubMed] [Google Scholar]
- 30. Lourenco MV, Frozza RL, de Freitas GB, et al. Exercise‐linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat Med. 2019;25(1):165‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Noda Y, Kuzuya A, Tanigawa K, et al. Fibronectin type III domain‐containing protein 5 interacts with APP and decreases amyloid β production in Alzheimer's disease. Mol Brain. 2018;11:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang K, Li H, Wang H, Wang J, Song F, Sun Y. Irisin exerts neuroprotective effects on cultured neurons by regulating astrocytes. Mediators Inflamm. 2018;2018:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Andorfer C, Kress Y, Espinoza M, et al. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem. 2003;86(3):582‐590. [DOI] [PubMed] [Google Scholar]
- 34. Andorfer C, Acker CM, Kress Y, Hof PR, Duff K, Davies P. Cell‐cycle reentry and cell death in transgenic mice expressing nonmutant human tau isoforms. J Neurosci. 2005;25(22):5446‐5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Polydoro M, Acker CM, Duff K, Castillo PE, Davies P. Age‐dependent impairment of cognitive and synaptic function in the htau mouse model of tau pathology. J Neurosci. 2009;29(34):10741‐10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Panda D, Samuel JC, Massie M, Feinstein SC, Wilson L. Differential regulation of microtubule dynamics by three‐ and four‐repeat tau: implications for the onset of neurodegenerative disease. Proc Natl Acad Sci U S A. 2003;100(16):9548‐9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Duff K, Knight H, Refolo LM, et al. Characterization of pathology in transgenic mice over‐expressing human genomic and cDNA tau transgenes. Neurobiol Dis. 2000;7(2):87‐98. [DOI] [PubMed] [Google Scholar]
- 38. Dengler‐Crish CM, Smith MA, Wilson GN. Early evidence of low bone density and decreased serotonergic synthesis in the dorsal raphe of a Tauopathy model of Alzheimer’s disease. J Alzheimer’s Dis. 2017;55(4): 1605‐1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Geiszler PC, Barron MR, Pardon M‐C. Impaired burrowing is the most prominent behavioral deficit of aging htau mice. Neuroscience. 2016;329:98‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Beery AK. Inclusion of females does not increase variability in rodent research studies. Curr Opin Behav Sci. 2018;23:143‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dengler‐Crish CM, Ball HC, Lin L, Novak KM, Cooper LN. Evidence of Wnt/β‐catenin alterations in brain and bone of a tauopathy mouse model of Alzheimer's disease. Neurobiol Aging. 2018;67:148‐158. [DOI] [PubMed] [Google Scholar]
- 42. Kane AE, Shin S, Wong AA, et al. Sex differences in healthspan predict lifespan in the 3xTg‐AD mouse model of Alzheimer’s disease. Front Aging Neurosci. 2018;10:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Carroll JC, Rosario ER, Kreimer S, et al. Sex differences in beta‐amyloid accumulation in 3xTg‐AD mice: role of neonatal sex steroid hormone exposure. Brain Res. 2010;1366:233‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang JT, Wang ZJ, Cai HY, et al. Sex differences in neuropathology and cognitive behavior in APP/PS1/tau triple‐transgenic mouse model of Alzheimer’s disease. Neurosci Bulletin. 2018;34(5):736‐746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Varela‐Rodríguez BM, Pena‐Bello L, Juiz‐Valiña P, Vidal‐Bretal B, Cordido F, Sangiao‐Alvarellos S. FNDC5 expression and circulating irisin levels are modified by diet and hormonal conditions in hypothalamus, adipose tissue and muscle. Sci Rep. 2016;6(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mishra M, Tiwari S, Gomes AV. Protein purification and analysis: next generation Western blotting techniques. Expert Review of Proteomics. 2017;14(11):1037‐1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Komuro Y, Xu G, Bhaskar K, Lamb BT. Human tau expression reduces adult neurogenesis in a mouse model of tauopathy. Neurobiol Aging. 2015;36(6):2034‐2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stratmann K, Heinsen H, Korf H‐W, et al. Precortical phase of Alzheimer's disease (AD)‐related tau cytoskeletal pathology. Brain Pathol. 2016;26(3):371‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li D‐J, Li Y‐H, Yuan H‐B, Qu L‐F, Wang P. The novel exercise‐induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metabolism. 2017;68:31‐42. [DOI] [PubMed] [Google Scholar]
- 50. Wang S, Pan J. Irisin ameliorates depressive‐like behaviors in rats by regulating energy metabolism. Biochem Biophys Res Commun. 2016;474(1):22‐28. [DOI] [PubMed] [Google Scholar]
- 51. Ruan Q, Huang Y, Yang L, et al. The effects of both age and sex on irisin levels in paired plasma and cerebrospinal fluid in healthy humans. Peptides. 2019;113:41‐51. [DOI] [PubMed] [Google Scholar]
- 52. Faul F, Erdfelder E, Lang A‐G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175‐191. [DOI] [PubMed] [Google Scholar]
- 53. Becker JB, Arnold AP, Berkley KJ, et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinol. 2005;146(4):1650‐1673. [DOI] [PubMed] [Google Scholar]
- 54. Nie Y, Liu D. N‐Glycosylation is required for FDNC5 stabilization and irisin secretion. Biochem J. 2017;474(18):3167‐3177. [DOI] [PubMed] [Google Scholar]
- 55. Albrecht E, Schering L, Buck F, et al. Irisin: still chasing shadows. Mol Metab. 2020;1(34):124‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mazur‐Bialy AI. Superiority of the non‐glycosylated form over the glycosylated form of irisin in the attenuation of adipocytic meta‐inflammation: a potential factor in the fight against insulin resistance. Biomolecules. 2019;9(9):394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Huang L, Yan S, Luo L, Yang L. Irisin regulates the expression of BDNF and glycometabolism in diabetic rats. Mol Med Rep. 2019;19(2):1074–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ullah MF, Ahmad A, Bhat SH, Abu‐Duhier FM, Barreto GE, Ashraf GM. Impact of sex differences and gender specificity on behavioral characteristics and pathophysiology of neurodegenerative disorders. Neurosci Biobehav Rev. 2019;102:95‐105. [DOI] [PubMed] [Google Scholar]
- 59. Kapadia M, Mian MF, Michalski B, et al. Sex‐dependent differences in spontaneous autoimmunity in adult 3xTg‐AD mice. J Alzheimer's Dis. 2018;63(3):1191‐1205. [DOI] [PubMed] [Google Scholar]
- 60. Conti E, Grana D, Stefanoni G, et al. Irisin and BDNF serum levels and behavioral disturbances in Alzheimer's disease. Neurol Sci. 2019;40(6):1145‐1150. [DOI] [PubMed] [Google Scholar]
- 61. Zügel M, Qiu S, Laszlo R, et al. The role of sex, adiposity, and gonadectomy in the regulation of irisin secretion. Endocrine. 2016;54(1):101‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Brondani LA, Boelter G, Assmann TS, Leitão CB, Canani LH, Crispim D. Irisin‐encoding gene (FNDC5) variant is associated with changes in blood pressure and lipid profile in type 2 diabetic women but not in men. Metabolism. 2015;64(9):952‐957. [DOI] [PubMed] [Google Scholar]
- 63. Campolo J, Corradi E, Rizzardi A, et al. Irisin and markers of metabolic derangement in non‐diabetic Caucasian subjects with stage I‐II obesity during early aging. PLoS One. 2020;15(2):e0229152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yu Q, Kou W, Xu X, et al. FNDC5/Irisin inhibits pathological cardiac hypertrophy. Clinical Science. 2019;133(5):611–627. [DOI] [PubMed] [Google Scholar]
- 65. Chen K, Xu Z, Liu Y, et al. Irisin protects mitochondria function during pulmonary ischemia/reperfusion injury. Sci Transl Med. 2017;9:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lunetta C, Lizio A, Tremolizzo L, et al. Serum irisin is upregulated in patients affected by amyotrophic lateral sclerosis and correlates with functional and metabolic status. J Neurol. 2018;265(12):3001‐3008 [DOI] [PubMed] [Google Scholar]
- 67. De Meneck F, Victorino de Souza L, Oliveira V, do Franco MC. High irisin levels in overweight/obese children and its positive correlation with metabolic profile, blood pressure, and endothelial progenitor cells. Nutr Metab Cardiovasc Dis. 2018;28(7):756‐764. [DOI] [PubMed] [Google Scholar]
- 68. Abd‐Elrahman KS, Albaker A, de Souza JM, et al. Aβ oligomers induce pathophysiological mGluR5 signaling in Alzheimer's disease model mice in a sex‐selective manner. Sci Signal. 2020;13(662):eabd2494. [DOI] [PubMed] [Google Scholar]
- 69. Mazur‐Bialy AI, Pocheć E, Zarawski M. Anti‐inflammatory properties of irisin, mediator of physical activity, are connected with TLR4/Myd88 signaling pathway activation. Int J Mol Sci. 2017;18(4):701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang K, Song F, Xu K, et al. Irisin attenuates neuroinflammation and prevents the memory and cognitive deterioration in streptozotocin‐induced diabetic mice. Mediators Inflamm. 2019;2019:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang D, Xie T, Leung PS. Irisin ameliorates glucolipotoxicity‐associated β‐cell dysfunction and apoptosis via AMPK signaling and anti‐inflammatory actions. Cell Physiol Biochem. 2018;51(2):924‐937. [DOI] [PubMed] [Google Scholar]
- 72. Deng X, Huang W, Peng J, et al. Irisin alleviates advanced glycation end products‐induced inflammation and endothelial dysfunction via Inhibiting ROS‐NLRP3 inflammasome signaling. Inflammation. 2018;41(1):260‐275. [DOI] [PubMed] [Google Scholar]
- 73. Shao L, Meng D, Yang F, Song H, Tang D. Irisin‐mediated protective effect on LPS‐induced acute lung injury via suppressing inflammation and apoptosis of alveolar epithelial cells. Biochem Biophys Res Commun. 2017;487(2):194‐200. [DOI] [PubMed] [Google Scholar]
- 74. Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer's disease. Alzheimer's Dement Transl Res Clin Interv. 2018;4(1):575‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Esen N, Kielian T. Central role for MyD88 in the responses of microglia to pathogen‐associated molecular patterns. J Immunol. 2006;176(11):6802‐6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Griffith CM, Macklin LN, Cai Y, et al. Impaired glucose tolerance and reduced plasma insulin precede decreased AKT phosphorylation and GLUT3 translocation in the hippocampus of Old 3xTg‐AD Mice. J Alzheimer's Dis. 2019;68(2):809‐837. [DOI] [PubMed] [Google Scholar]
- 77. Xiong X‐Q, Chen D, Sun H‐J, et al. FNDC5 overexpression and irisin ameliorate glucose/lipid metabolic derangements and enhance lipolysis in obesity. Biochim Biophys Acta ‐ Mol Basis Dis. 2015;1852(9):1867‐1875. [DOI] [PubMed] [Google Scholar]
- 78. Kim B, Feldman EL. Insulin resistance in the nervous system. Trends Endocrinol Metab. 2012;23(3):133‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gabbouj S, Natunen T, Koivisto H, et al. Intranasal insulin activates Akt2 signaling pathway in the hippocampus of wild‐type but not in APP/PS1 Alzheimer model mice. Neurobiol Aging. 2019;75:98‐108. [DOI] [PubMed] [Google Scholar]
- 80. Fang X, Xia T, Xu F, et al. Isoflurane aggravates peripheral and central insulin resistance in high‐fat diet/streptozocin‐induced type 2 diabetic mice. Brain Res. 2020;1727:146511. [DOI] [PubMed] [Google Scholar]
- 81. Pláteník J, Fišar Z, Buchal R, et al. GSK3β, CREB, and BDNF in peripheral blood of patients with Alzheimer's disease and depression. Prog Neuro‐Psychopharmacology Biol Psychiatry. 2014;3(50):83‐93. [DOI] [PubMed] [Google Scholar]
- 82. Gabbouj S, Ryhänen S, Marttinen M, et al. Altered insulin signaling in Alzheimer's disease brain – special emphasis on PI3K‐Akt pathway. Front Neurosci. 2019;13:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Perry VH. Contribution of systemic inflammation to chronic neurodegeneration. Acta Neuropathol. 2010;120:277‐286. [DOI] [PubMed] [Google Scholar]
- 84. Ma EB, Sahar NE, Jeong M, Huh JY. Irisin exerts inhibitory effect on adipogenesis through regulation of Wnt signaling. Front Physiol. 2019;10:1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Schumacher MA, Chinnam N, Ohashi T, Shah RS, Erickson HP. The structure of irisin reveals a Novel Intersubunit β‐sheet fibronectin type III (FNIII) dimer. J Biol Chem. 2013;288(47):33738‐33744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ruan Q, Zhang L, Ruan J, et al. Detection and quantitation of irisin in human cerebrospinal fluid by tandem mass spectrometry. Peptides. 2018;1(103):60‐64. [DOI] [PubMed] [Google Scholar]
- 87. El Hayek L, Khalifeh M, Zibara V, et al. Lactate mediates the effects of exercise on learning and memory through sirt1‐dependent activation of hippocampal brain‐derived neurotrophic factor (BDNF). J Neurosci. 2019;39(13):2369‐2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S2
Data Availability Statement
Data available on request from the authors.


