Abstract
Food‐safety measures are recommended to solid organ transplant (SOT) recipients. However, the burden of foodborne infections in SOT recipients has not been established. We describe the epidemiology and outcomes of bacterial foodborne infections in a nationwide cohort including 4405 SOT recipients in Switzerland between 2008 and 2018. Participants were prospectively followed for a median of 4.2 years with systematic collection of data on infections, and patient and graft‐related outcomes. We identified 151 episodes of microbiologically confirmed bacterial foodborne infections occurring in median 1.6 years (IQR 0.58–3.40) after transplantation (131 [88%] Campylobacter spp. and 15 [10%] non‐typhoidal Salmonella). The cumulative incidence of bacterial foodborne infections was 4% (95% CI 3.4–4.8). Standardized incidence rates were 7.4 (95% CI 6.2–8.7) and 4.6 (95% CI 2.6–7.5) for Campylobacter and Salmonella infections, respectively. Invasive infection was more common with Salmonella (33.3% [5/15]) compared to Campylobacter (3.2% [4/125]; p = .001). Hospital and ICU admission rates were 47.7% (69/145) and 4.1% (6/145), respectively. A composite endpoint of acute rejection, graft loss, or death occurred within 30 days in 3.3% (5/151) of cases. In conclusion, in our cohort bacterial foodborne infections were late post‐transplant infections and were associated with significant morbidity, supporting the need for implementation of food‐safety recommendations.
Keywords: clinical research/practice, complication: infectious, epidemiology, infection and infectious agents ‐ bacterial, infectious disease, patient safety
Implementation of food‐safety recommendations is supported by data from a large, nationwide, prospective cohort of solid organ transplant recipients that shows that bacterial foodborne infections occur late after transplant and are associated with significant morbidity such as high hospitalization rates.

Abbreviations
- CI
confidence interval
- EHEC
enterohemorrhagic E. coli
- EIEC
enteroinvasive E.coli
- EPEC
enteropathogenic E. coli
- ETEC
enterotoxigenic E. coli
- ICU
intensive care unit
- IQR
interquartile range
- MMF
mycophenolate mofetil
- MPA
mycophenolic acid
- mTOR
mammalian target of rapamycine
- NAT
nucleic acid amplification test
- OR
odds ratio
- SIR
standardized incidence rate
- SOT
solid organ transplant
- STCS
Swiss Transplant Cohort Study
- TMP‐SMX
trimethoprim‐sulfamethoxazole
1. INTRODUCTION
Foodborne illnesses are frequent community‐acquired infections, accounting for up to 9 million episodes per year in the United States. 1 Campylobacter, Salmonella, Shigella, enteropathogenic (EPEC), enterotoxigenic (ETEC), enteroinvasive (EIEC), or enterohemorrhagic (EHEC) Escherichia coli, Yersinia, and Listeria monocytogenes may be transmitted by consumption of specific food products including undercooked or raw meat or eggs, or unpasteurized cheese. Campylobacter and non‐typhoidal Salmonella are the bacteria most commonly causing clinically significant foodborne infections. 1 , 2
While bacterial foodborne infections result in mild and self‐resolving gastroenteritis in the general population, invasive infections and severe diarrhea may occur with advanced age, underlying diseases, and immunosuppression. 3 , 4 , 5 In particular, solid organ transplant (SOT) recipients are at risk for invasive infections because of underlying immunosuppression. 6 , 7 In addition, diarrhea in SOT recipients may influence patient and allograft survival. 8 , 9 Accordingly, SOT recipients are generally recommended to avoid foods associated with an increased risk of infection and to apply rigorous hygiene measures to reduce epidemiological exposure and therefore potential complications from foodborne infections. 10 However, recent data suggest that adherence to food‐safety recommendations by SOT recipients is heterogeneous in real‐life. 11
Despite potentially significant epidemiological exposure and impact on patients and allografts related outcomes, the epidemiology and the consequences of bacterial foodborne infections in SOT recipients remain largely unexplored. In this study, we aimed to assess the epidemiology, clinical presentation, and outcomes of bacterial foodborne infections in a large cohort of SOT recipients prospectively followed after transplantation.
2. METHODS
2.1. Study design and participants
The Swiss transplant cohort study (STCS) is a prospective, observational, nationwide cohort enrolling consecutive patients undergoing solid organ transplantation at one of the six transplant centers in Switzerland (Basel, Bern, Geneva, Lausanne, St. Gallen, and Zurich) since May 2008. 12 Patients enrolled from May 2008 until April 30, 2018 were included in this study. We considered a follow‐up until December 31, 2018 (censoring), dropout, first graft‐loss, or death, whichever came first. Patients without written informed consent at enrollment or with subsequent consent withdrawal were excluded. The local ethics committees at each participating center approved the STCS. The local ethics committee at the Lausanne University Hospital approved the protocol of the present study (Protocol number 2019‐01750).
2.2. Data collection, definitions, and endpoints
In the STCS, clinical and laboratory data (including comorbidities, graft related outcomes, immunosuppression drugs, antimicrobial prophylaxis, and infections) are prospectively collected from hospital medical charts and referral documentations of outpatient events in an electronic case report form (eCRF) for all participants at transplantation, 6 and 12 months after transplantation, and yearly thereafter. 12 Infections are diagnosed as part of routine clinical practice by treating physicians at each center according to local guidelines; thus microbiological tests are chosen and performed at each center when deemed indicated. Nucleic acid amplification tests (NATs) and panel tests for the detection of enteropathogens in stool samples are available in Switzerland since 2016, and widely used in all participating centers since 2017–2018. Infections diagnosed during follow‐up are reviewed and collected in the STCS database using a standardized eCRF by transplant infectious diseases specialists, according to the definitions developed by the STCS infectious diseases working group. 13 , 14
We identified episodes of microbiologically confirmed bacterial foodborne infection through search in the STCS database during the study period for Campylobacter spp., Salmonella spp., Shigella spp., Yersinia spp., Listeria monocytogenes, EPEC, ETEC, EIEC, and EHEC E. coli infections. We verified all episodes of bacterial foodborne infection by chart review at participating centers. For each episode, we retrospectively collected additional variables not included in the STCS database (detailed clinical presentation, need for hospital or intensive care unit [ICU] admission because of bacterial foodborne infection, microbiological test used for identification of enteropathogens in stool samples, antibiotic therapy, and international travel before infection).
We defined bacterial foodborne infection as the detection of Campylobacter spp., Salmonella spp., Shigella spp., Yersinia spp., Listeria monocytogenes, EPEC, ETEC, EIEC, or EHEC in stool, blood, or other primary sterile samples (i.e., joint aspirate, bone). Invasive infection was defined as bacteremia, mycotic aneurysm, or detection of foodborne bacteria in a primary sterile sample with or without concomitant gastrointestinal infection. Infection was considered severe when hospital admission was required because of bacterial foodborne infection. Graft failure was defined as re‐transplantation; death due to cardiac, respiratory, hepatic or renal failure according to the type of transplant; return to dialysis for kidney transplant recipients; or recurrence of insulin‐dependent diabetes for pancreas and islets transplant recipients. In the STCS, immunosuppression reduction is defined as withdrawal or at least 50% dose reduction of one drug.
The primary endpoint of the study was the incidence of bacterial foodborne infections after solid organ transplantation. Secondary endpoints were the occurrence of invasive infection, of severe infection with a need for hospital admission, and the occurrence of adverse patient and graft outcomes (a composite endpoint including treated acute rejection, graft failure, and death) within 30 days after bacterial foodborne infection.
2.3. Statistics
We used descriptive statistics to illustrate baseline characteristics of study participants with and without bacterial foodborne infection, and characteristics of bacterial foodborne infections. Categorical variables were represented as numbers and percentages, and continuous variables as medians and interquartile ranges (IQR) and compared using two‐sided Fisher exact test and Mann–Whitney U test, respectively. We calculated cumulative incidence of first bacterial foodborne infection and of first Campylobacter and first Salmonella infections, treating death and graft failure before infection as competing risks, as well as incidence rates of bacterial foodborne infection and of each specific infection per 1000 patient‐years. We calculated age‐adjusted, and overall standardized incidence rates (SIR) by comparing the incidence of Campylobacter and Salmonella infections in SOT recipients between 2010 and 2018 to their incidence in the general Swiss population, using yearly incidence per 100 000 people in Switzerland from mandatory laboratory notification (public available at www.bag.admin.ch). We used multivariable logistic regression to identify risk factors for severe bacterial foodborne infection. In addition to age and sex, risk factors that were statistically significant in the univariate analysis were included in the multivariable model. The statistical softwares R version 3.6.1 (R Foundation for Statistical Computing) and STATA version 14 (StataCorp) were used for statistical analyses.
3. RESULTS
3.1. Study population
Over the 10‐year study period, 4781 patients underwent solid organ transplantation in Switzerland, of whom 92% (4405; 2467 [56%] kidney, 944 [21%] liver, 335 [8%] heart, 403 [9.2%] lung, 30 [0.7%] islets, 13 [0.3%] pancreas, 1 small‐bowel, and 212 [4.8%] combined transplant recipients) were included in this study. Median age at enrolment was 53 years (IQR 41–61). Most of the participants received induction immunosuppression with basiliximab (70.2% [3093/4405]) and were on an initial immunosuppression regimen consisting of tacrolimus (70.2% [3269/4405]), mycophenolate (89.5% [3943/4405]) and prednisone (90.9% [4005/4405]). Characteristics of the study population are detailed in Table 1. The median follow‐up was 4.2 years (IQR 1.9–7.0).
TABLE 1.
Characteristics of study population
|
All SOT (n = 4405) |
No bacterial foodborne infection (n = 4268) |
Bacterial foodborne infection (n = 137) |
|
|---|---|---|---|
| Age at enrollment, median (IQR) | 53 (41–61) | 54 (42–61) | 51 (37–60) |
| Sex (female), n (%) | 1599 (36.3%) | 1553 (36.4%) | 46 (33.6%) |
| Type of transplantation, n (%) | |||
| Kidney | 2467 (56.0%) | 2375 (55.7%) | 92 (67.2%) |
| Liver | 944 (21.4%) | 925 (21.7%) | 19 (13.9%) |
| Heart | 335 (7.6%) | 321 (7.5%) | 14 (10.2%) |
| Lung | 403 (9.2%) | 397 (9.3%) | 6 (4.4%) |
| Combined a | 212 (4.8%) | 207 (4.9%) | 5 (3.7%) |
| Other b | 44 (1.0%) | 43 (1.0%) | 1 (0.7%) |
| Living donor, n (%) | 1075 (24.4%) | 1028 (24.1%) | 47 (34.3%) |
| Re or second transplantation at enrollment, n (%) | 470 (10.7%) | 446 (13.3%) | 24 (17.5%) |
| Induction immunosuppression, n (%) | |||
| Basiliximab | 3093 (70.2%) | 3000 (70.3%) | 93 (67.9%) |
| Anti‐lymphocyte globulin | 1007 (22.9%) | 974 (22.8%) | 33 (24.1%) |
| Maintenance immunosuppression, c n (%) | |||
| Tacrolimus | 3269 (74.2%) | 3166 (74.2%) | 103 (75.2%) |
| Cyclosporin | 944 (21.4%) | 911 (21.3%) | 33 (24.1%) |
| Mycophenolate (MMF or MPA) | 3943 (89.5%) | 3812 (89.3%) | 131 (95.6%) |
| Azathioprine | 76 (1.7%) | 74 (1.7%) | 2 (1.5%) |
| mTOR inhibitors | 143 (3.3%) | 140 (3.3%) | 3 (2.2%) |
| Prednisone | 4005 (90.9%) | 3878 (91.0%) | 127 (92.7%) |
| TMP‐SMX prophylaxis, n (%) | 3880 (88.1%) | 3755 (88.0%) | 125 (91.2%) |
| Months of TMP‐SMX, median (IQR) | 6.1 (4.9–12.4) | 6.1 (4.9–12.4) | 6.3 (5.3–13.4) |
| Follow‐up in years, median (IQR) | 4.2 (1.9–7.0) | 4.2 (1.9–7.0) | 5.7 (3.8–7.9) |
| Outcome at end of follow‐up, n (%) | |||
| Lost to follow‐up | 46 (1.0%) | 44 (1.0%) | 2 (1.5%) |
| Patients with graft failure d , e | 452 (10.3%) | 441 (13.2%) | 11 (8.0%) |
| Death e | 538 (12.2%) | 529 (15.8%) | 9 (6.6%) |
| Death with functioning allograft | 444 (10.1%) | 435 (13.0%) | 9 (6.6%) |
Abbreviations: IQR, interquartile range; MMF, mycophenolate mofetil; MPA, mycophenolic acid; mTOR, mammalian target of rapamycin; SOT, solid organ transplant; TMP‐SMX, trimethoprim‐sulfamethoxasole.
Including kidney‐pancreas (93), kidney‐kidney (43), kidney‐liver (38), kidney‐islets (16), kidney‐heart (8), liver‐lung (4), pancreas‐small bowel (2) heart‐lung (1), kidney‐lung (1), islets‐liver (1), liver‐pancreas (1), kidney‐kidney‐pancreas (2), kidney‐islets‐liver (1), islets‐liver‐lung (1) transplant recipients.
Including islets (30), pancreas (13), and small bowel (1) transplant recipients.
Maintenance immunosuppression represent immunosuppression at day 30 after transplantation.
Including primary non‐function (50).
Death and graft failure occurred on the same day in 94 patients.
3.2. Epidemiology of bacterial foodborne infections in SOT
During the study period, 151 microbiologically confirmed bacterial foodborne infections (131 Campylobacter spp., 15 non‐typhoidal Salmonella, 1 Shigella spp., 2 Yersinia spp., and 2 Listeria monocytogenes) occurred in 137 (3.1%) of 4405 SOT recipients (92/2467 [3.7%] kidney, 19/944 [2%] liver, 14/335 [4.2%] heart, 6/403 [1.5%] lung, 5/212 [2.4%] combined, and 1/13 [7.7%] pancreas transplant recipients). Enteropathogenic E. coli was detected simultaneously in two episodes of Salmonella and one Campylobacter infections. There were no cases of concomitant detection of parasites or C. difficile. The median time from transplantation to infection was 1.57 years (IQR 0.58–3.40). Three infections occurred within the first week after liver transplantation (one Listeria monocytogenes bacteremia [5 days] and two Campylobacter gastroenteritis [6 days]) and were probably acquired before surgery. The overall cumulative incidence of bacterial foodborne infections was 4.0% (95% CI 3.4–4.8). Cumulative incidences were 3.5% (95% CI 3.0–4.3) for Campylobacter spp. infections and 0.4 (95% CI 0.2–0.7) for salmonellosis (Figure 1). The overall incidence rate of bacterial foodborne infections was 7.6 (95% CI 6.4–8.9) per 1000 person‐years. Specifically, incidence rates per 1000 person‐years were 6.6 (95% CI 5.5–7.8) for Campylobacter spp., 1.1 (95% CI 0.6–1.7) for Salmonella spp., 0.3 (95% CI 0.01–1.6) for Shigella spp., 0.5 (95% CI 0.06–1.7) for Yersinia spp., and 0.4 (95% CI 0.05–1.4) for Listeria monocytogenes infections. Of 131 Campylobacter infections, 32 (24%) occurred in January and December, and 56 (43%) in May to August. Standardized incidence rates (SIR) of Campylobacter and Salmonella infections compared to the Swiss general population were 7.4 (95% CI 6.2–8.7) and 4.6 (95% CI 2.6–7.5), respectively. SIRs adjusted by age are illustrated in Figure 2. International travel history was found in 10 (15%) of the 68 episodes of infection for whom this information was available, including travel to developing countries before six episodes of infection: one Campylobacter, four Salmonella, and the only episode of Shigella infection. Episodes of foodborne infection in our cohort were not linked to outbreaks.
FIGURE 1.
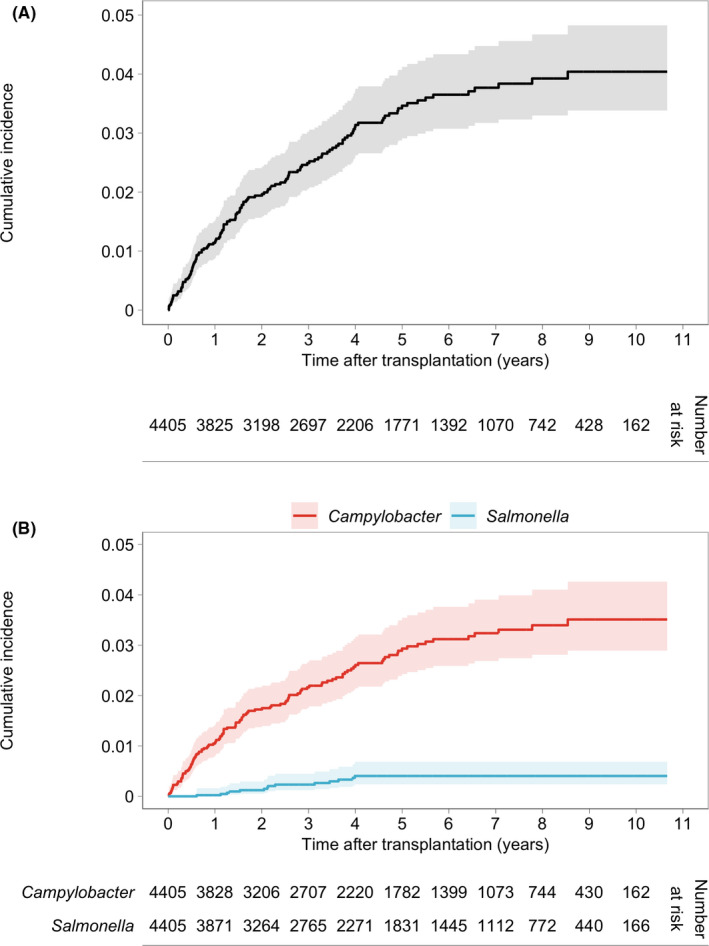
Cumulative incidence of bacterial foodborne infections in SOT recipients. Cumulative incidence and 95% confidence interval (black line and gray shading) of first bacterial foodborne infection, treating death and graft failure before infection as competing risks (A). Cumulative incidence and 95% confidence interval of first Campylobacter (red line and light red shading) and first Salmonella (blue line and light blue shading) infections, treating death and graft failure before infection as competing risks (B) [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 2.
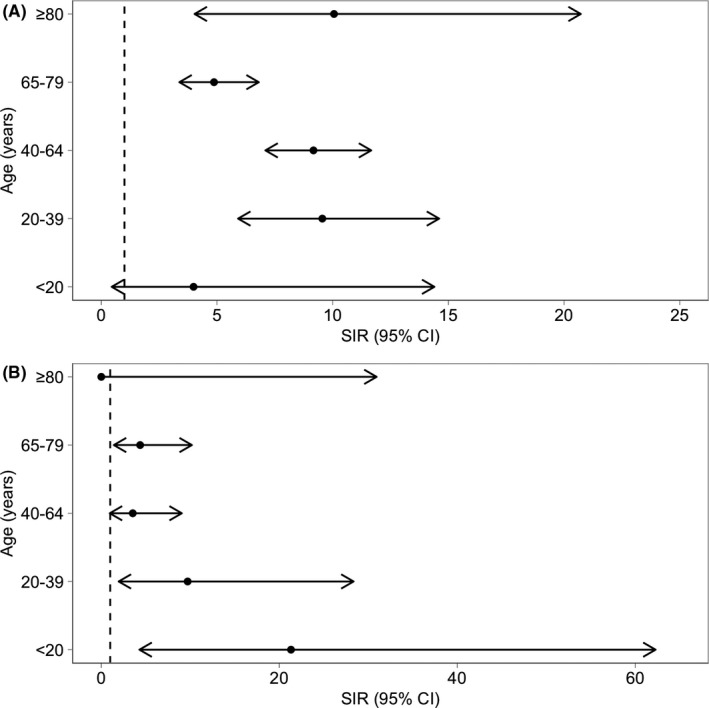
Standardized incidence rates of Campylobacter and Salmonella infections in SOT recipients. Forest plot representing standardized incidence rates and 95% confidence intervals of Campylobacter (A) and Salmonella (B) diagnosis in the Swiss Transplant Cohort Study compared to the Swiss general population from 2010 to 2018 according to age. Annual incidence of Campylobacter and Salmonella diagnosis per 100,000 patient‐years from 2010 to 2018 are available from the Swiss Federal Office of Public Health. CI, confidence interval; SIR, standardized incidence rate
3.3. Clinical characteristics and management of bacterial foodborne infections in SOT
Detailed clinical characteristics were available for 139 (90%) of 151 bacterial foodborne infections. At least one gastrointestinal symptom was present in 132 (95%) of 139 episodes. Diarrhea was the most frequent presenting symptom (93.5% [130/139]), followed by abdominal pain (39.6% [55/139]), fever (36.7% [51/139]), and nausea or vomiting (24.5% [34/139]). Blood in stool was present in only four (2.9%) of 139 episodes. Diarrhea was more common with Campylobacter when compared to Salmonella (97.5% vs. 73.3%; p = .003), while there was a trend for more frequent fever with salmonellosis (53.3% vs. 32.8%; p = .15; Figure 3).
FIGURE 3.
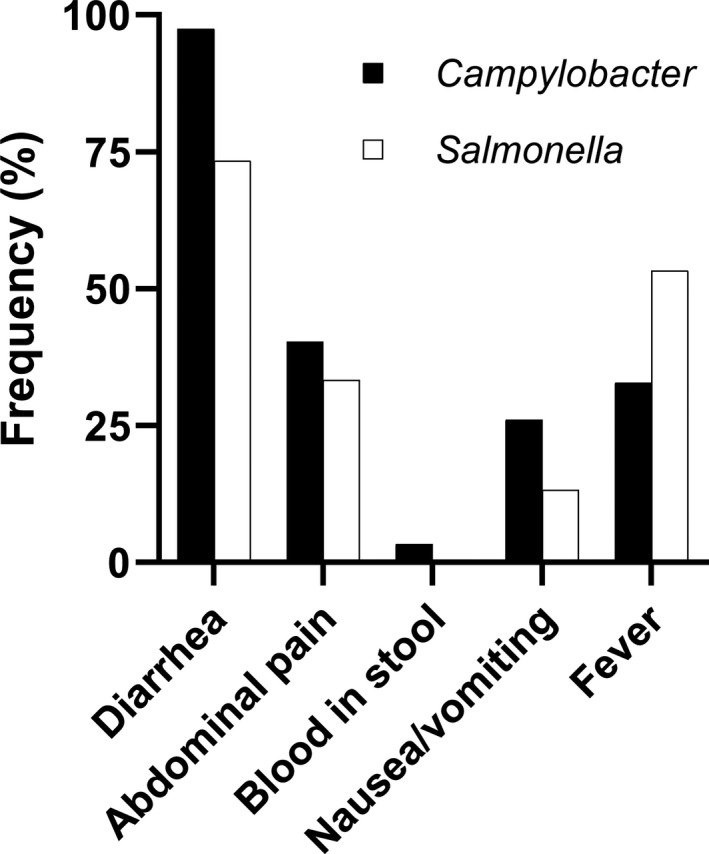
Clinical characteristics of Campylobacter and Salmonella infections in SOT recipients. Proportion of episodes with diarrhea, abdominal pain, blood in stool, nausea or vomiting, and fever in Campylobacter (black bars) and Salmonella (white bars) infections (p = .003 for diarrhea, p = .78 for abdominal pain, p = 1.0 for blood in stool, p = .36 for nausea/vomiting, and p = .15 for fever in Campylobacter vs. Salmonella)
Most of the bacterial foodborne gastrointestinal infections were diagnosed through the culture of stool (86.3%, 113 of 131 episodes with bacteria detected in a stool sample for whom the microbiological test used was known); while 7.6% (10/131) were diagnosed by nucleic acid amplification test (NAT), and 6.1% (8/131) by both NAT and culture.
Invasive infection occurred in 11 (7.6%) of 145 episodes (extra intestinal localization could not be assessed in six infections); there were three Campylobacter jejuni bacteremia, one Campylobacter fetus septic arthritis, three Salmonella bacteremia, one Salmonella bacteremia with septic arthritis, one Salmonella mycotic aneurysm, and two Listeria bacteremia (invasive and gastrointestinal infections according to the type of transplantation and pathogen are illustrated in Supplementary Table S1). Invasive infection was more common with Salmonella (33.3% [5/15]) compared to Campylobacter (3.2% [4/125]; p = .001). Treatment for acute rejection was administered during the previous 180 days in 27.3% (3/11) of the patients with invasive infection compared to 7.5% (10/134) of those with infection limited to the gastrointestinal tract (p = .021). In contrast, there were no differences in age, sex, time from transplantation, transplanted organ, immunosuppressive regimen, or past use of anti‐lymphocyte globulins in bacterial foodborne infections with and without extra intestinal dissemination (Table 2).
TABLE 2.
Risk factors for invasive infection
|
Gastroenteritis (n = 134) |
Invasive infection (n = 11) |
p | |
|---|---|---|---|
| Age at infection, median (IQR) | 54 (41–62) | 32 (21–66) | 0.113 |
| Sex (F), n (%) | 42 (31.3%) | 3 (27.3%) | 1.000 |
| Years after transplantation, median (IQR) | 1.6 (0.6–3.2) | 2.1 (1.3–4.0) | 0.424 |
| Transplanted organ, n (%) | |||
| Kidney | 93 (69.4%) | 6 (54.6%) | 0.326 |
| Liver | 17 (12.7%) | 2 (18.2%) | 0.638 |
| Heart | 12 (9.0%) | 2 (18.2%) | 0.287 |
| Lung | 6 (4.5%) | 1 (9.1%) | 0.431 |
| Combined | 5 (3.7%) | 0 | 1.000 |
| Other | 1 (0.8%) | 0 | 1.000 |
| Salmonella, n (%) | 10 (7.5%) | 5 (45.5%) | 0.002 |
| Maintenance immunosuppression, a n (%) | |||
| Tacrolimus | 103 (76.9%) | 8 (72.7%) | 0.720 |
| Mycophenolate (MMF or MPA) | 120 (89.6%) | 10 (90.9%) | 1.000 |
| Prednisone | 98 (73.1%) | 10 (90.9%) | 0.290 |
| Azathioprine | 4 (3.0%) | 1 (9.1%) | 0.330 |
| TMP‐SMX prophylaxis, a n (%) | 39 (29.1%) | 2 (18.2%) | 0.729 |
| Acute rejection, b n (%) | 6 (4.5%) | 3 (27.3%) | 0.021 |
| Use of anti‐lymphocyte globulin, c n (%) | 5 (3.7%) | 1 (9.1%) | 0.383 |
Two‐sided Fisher exact test and Mann–Whitney U test were used for comparison of categorical and continuous variables, respectively. Invasive infection could not be assessed for six episodes.
Abbreviations: F, female; IQR, interquartile range; MMF, mycophenolate mofetil; MPA, mycophenolic acid; TMP‐SMX, trimethoprim‐sulfamethoxazole.
Maintenance immunosuppression and TMP‐SMX prophylaxis at time of infection.
Treated acute rejection within 180 days before infection.
Use of anti‐lymphocyte globulin within 180 days before infection.
Overall, antibiotics were administered for 117 (86.7%) of 135 bacterial foodborne infections and immunosuppression was reduced in 12 (10%) of 121 bacterial foodborne infections for whom this information was available. Initial antibiotic treatment for Campylobacter infections consisted of a quinolone in 39.7% (46/116), a macrolide in 37.9% (44/116), and a carbapenem in 1.7% (2/116). In the 46 episodes treated initially with a quinolone, the regimen was modified for a macrolide in 10 episodes. In 14.7% of Campylobacter infections (17/116) no antibiotic were administered. In median antibiotics were administered for five days (IQR 3–10) for Campylobacter gastroenteritis. All the untreated episodes of Campylobacter gastroenteritis spontaneously resolved, without immunosuppression reduction. Initial treatment for Salmonella consisted in a cephalosporin in six, a quinolone in five and a macrolide in two episodes. One Salmonella infection did not receive an effective treatment.
3.4. Outcomes of bacterial foodborne infections in SOT
Hospital and intensive care unit admission were required respectively in 47.7% (69/145) and 4.1% (6/145) of the bacterial foodborne infections. The calculated incidence of hospital admission for bacterial foodborne infection was 3.45 (95% CI 2.69–4.37) per 1000 patient‐years. In a multivariable logistic regression model, a longer time after transplantation was significantly associated with an increased risk for severe infection with a need for hospital admission (adjusted odds ratio [OR] 1.35 per year, 95% CI 1.11–1.66; p = .003). The need for hospital admission was not found to be significantly associated with age, sex, type of transplantation or immunosuppression, infection with Salmonella, and treatment for acute rejection during the six months preceding infection (Table 3). Salmonella infections more frequently led to ICU admission (20% [3/15]) when compared to Campylobacter (2.4% [3/125]; p = .017).
TABLE 3.
Risk factors for hospital admission for bacterial foodborne infection
| OR (95% CI) | p | Adjusted OR (95% CI) | p | |
|---|---|---|---|---|
| Age at infection | 0.98 (0.96–1.00) | 0.078 | 0.98 (0.96–1.00) | 0.108 |
| Sex (F) | 0.78 (0.39–1.59) | 0.500 | 0.67 (0.32–1.44) | 0.307 |
| Years after transplantation | 1.32 (1.08–1.61) | 0.007 | 1.35 (1.11–1.66) | 0.003 |
| Transplanted organ | ||||
| Kidney | 0.48 (0.24–0.99) | 0.046 | 0.53 (0.25–1.13) | 0.099 |
| Liver | 1.44 (0.53–3.89) | 0.471 | ||
| Heart | 2.13 (0.68–6.70) | 0.196 | ||
| Lung | 2.89 (0.54–15.41) | 0.214 | ||
| Salmonella infection | 1.29 (0.44–3.77) | 0.639 | ||
| Maintenance immunosuppression a | ||||
| Tacrolimus | 0.54 (0.24–1.21) | 0.133 | ||
| Mycophenolate (MMF or MPA) | 0.42 (0.13–1.28) | 0.127 | ||
| Prednisone | 0.56 (0.26–1.21) | 0.139 | ||
| TMP‐SMX prophylaxis a | 0.81 (0.39–1.68) | 0.577 | ||
| Acute rejection b | 0.87 (0.22–3.39) | 0.846 |
Hospital admission was unknown in six episodes.
Abbreviations: CI, confidence interval; F, female; MMF, mycophenolate mofetil; MPA, mycophenolic acid; OR, odds ratio; TMP‐SMX, trimethoprim‐sulfamethoxazole.
At time of infection.
Treated acute rejection within 180 days before infection.
Within 30 days after infection, acute rejection, graft failure or death occurred in five patients corresponding to 3.3% (5/151) of bacterial foodborne infection episodes. One kidney transplant recipient developed acute humoral rejection following Campylobacter gastroenteritis, one liver transplant recipient had rejection concomitantly to Salmonella bacteremia, and one kidney–pancreas recipient had pancreas rejection after Campylobacter gastroenteritis, which ultimately led to pancreas graft failure. None of the patients with subsequent acute rejection had immunosuppression reduction because of bacterial foodborne infection. One additional patient had liver graft failure due to arterial thrombosis after Campylobacter infection. One kidney transplant recipient died because of ruptured Salmonella aortic aneurysm.
4. DISCUSSION
In this study, we present an updated epidemiology of bacterial foodborne infections in SOT recipients. Over a ten‐year period, we identified 151 episodes of medically attended and microbiologically confirmed bacterial foodborne infections in a nationwide cohort including 4405 SOT recipients with a median follow‐up of 4.2 years. The incidence rate of bacterial foodborne infections was 7.6 per 1000 patient‐years, resulting in 3.5 hospital admissions per 1000 patient‐years.
Although severe cases of Campylobacter and Salmonella infections in SOT recipients are described in the literature, 6 , 7 there are surprisingly few reliable data on the burden of bacterial foodborne infections in this population as, to the best of our knowledge, their epidemiology was never assessed in prospective cohorts. In studies evaluating SOT recipients presenting with diarrhea, the prevalence of bacterial foodborne infections ranged from 1% to 12%, 15 , 16 , 17 , 18 with a higher prevalence of bacterial pathogens when stool samples were tested by nucleic acid amplification tests. 19 However, all but one of these studies were limited to kidney transplant recipients, spanned a relatively short period of time (1 year), and, most importantly, did not allow an estimation of the incidence because of the lack of a denominator.
In our cohort, Campylobacter was by far the most common pathogen (87% of the infections, followed by Salmonella) and we observed a second winter peak of Campylobacter infections, mirroring the epidemiology in the Swiss general population. 20 , 21 While campylobacteriosis most commonly occur during warm months, infections occurring between December and January in Switzerland have been attributed to the consumption of chicken meat fondue by Swiss during Christmas and New Year. 20 , 21 Taking advantage of mandatory laboratory declarations of microbiologically confirmed bacterial foodborne infections in Switzerland, we found a significant increase in the incidence of Campylobacter (overall SIR 7.4) and Salmonella (overall SIR 4.6) in SOT recipients compared to the general population. Although data for salmonellosis need to be interpreted more cautiously because of low numbers, significantly higher incidence of Campylobacter infection is observed also in older SOT recipients. Underdiagnoses of mild and spontaneously resolving infections in the general population altogether with increased testing in SOT recipients might contribute to these findings. Nevertheless, 47% of the infections in our cohort required hospital admission, while reported hospital admission rates in the general population range from 14% to 20%, and from 22% to 28% for Campylobacter and Salmonella, respectively. 2 , 4 , 20 Although the threshold for hospital admission might be lower in SOT recipients, our results confirm the significant burden associated with bacterial foodborne infections in SOT recipients, since most of the infections detected in our cohort were clinically significant. For comparison purposes, the incidence of hospital admission for bacterial foodborne infection (3.5 per 1000 patient‐years) approach influenza, which is an important pathogen in the transplant population (5.9 hospital admissions per 1000 patient‐years in a previous study in our cohort). 22
Invasive infection (including bacteremia or infection of other normally sterile sites) occurred in up to one‐third of Salmonella compared to 3% of Campylobacter infections. In the general population, invasive infection occurs in 4% to 6% of nontyphoidal Salmonella and 1% of Campylobacter infections. 3 , 4 , 5 , 23 Highlighting the role of immunosuppression in facilitating invasive disease, we also found an association between previous treatment for acute rejection and development of extra intestinal dissemination. Overall, our data suggest an increased susceptibility to infection and to a more severe disease in SOT recipients. However, since bacteremia, long‐term vascular complications, and invasive infections remain rare, their incidence should be more precisely estimated in larger cohorts of SOT recipients.
Besides direct morbidity resulting from invasive infections, bacterial foodborne infections may impair allograft and patient outcomes by several mechanism. In particular, diarrhea, the most common presenting symptom in our cohort, may cause acute kidney failure, increased absorption of tacrolimus resulting in drug toxicity, or may lead to a reduction of immunosuppression or withdrawal of mycophenolate potentially leading to an increased risk of rejection. 8 , 24 , 25 In our cohort, episodes of acute rejection were not explained by the reduction of immunosuppression. Whether episodes of rejection result from immune activation triggered by bacterial foodborne infection is unknown. 26 Overall, early adverse outcomes (including death, graft failure, or acute rejection) only occurred in 3.3% of bacterial foodborne infections in our cohort. Because of the low number of events (five), we were not able to assess whether the bacterial foodborne infection is an independent risk factor for acute rejection, graft failure, or death, which will need to be tested in further studies.
We found bacterial foodborne infections to be a late post‐transplant infection occurring at a median of 1.6 years after transplantation, when most of the SOT recipients probably resumed their normal lifestyle. We previously demonstrated that SOT recipients less stringently followed food‐safety recommendations beyond the first year after transplantation. 11 Although food‐safety behavior of study participants is unknown, taken together, these results suggest a need for continuous specific patient educational programs on food‐safety measures, and vigilance and a high degree of clinical suspicion for the clinicians caring for those patients, particularly late after transplantation.
Notably and although early post‐transplant period is characterized by more intense immunosuppression and a higher risk for infection, 9 we found late‐onset to be associated with more severe infection. Changes in patients’ behavior, including less meticulous respect of food‐safety, but also delay in seeking for medical care in case of symptoms, and higher physicians’ threshold for testing late after transplantation potentially resulting in an overrepresentation of more severe infections, may partially explain this finding.
Most of the episodes of bacterial foodborne infections in our cohort were treated with antibiotics according to current guidelines. 27 , 28 Of note, quinolones were used as initial treatment in over one‐third of Campylobacter infections. High resistance rates for quinolones are reported in Switzerland (71.5% for Campylobacter coli and 60.8% for Campylobacter jejuni). 29 Although we do not know resistance patterns in our cohort and we therefore cannot assess appropriateness of antimicrobial therapy, alternative empirical treatment should be considered when Campylobacter gastroenteritis is suspected. Because of the small number of untreated infections, it is difficult to estimate the frequency of spontaneous resolution of Campylobacter gastroenteritis based on our data.
Some limitations of our study need to be acknowledged. First, we included only microbiologically confirmed bacterial foodborne infections potentially leading to an underrepresentation of milder episodes that might remain undiagnosed. The inclusion of viral pathogens (such as norovirus and hepatitis E) would have increased the observed burden of disease. We decided to focus our analysis only in bacterial foodborne infections since these viral pathogens may not be exclusively foodborne. Heterogeneity in testing across different centers and type of transplantations may result on differences in the incidence of foodborne infections. In particular, epidemiology of foodborne infections may vary over time according to the increased use of molecular methods (widely used in Switzerland since 2017–2018), which are associated with improved sensitivity as compared to stool culture. 19 Because the epidemiology of foodborne infections varies worldwide according to different cultural norms and food habits, as well as food processing procedures, across geographical areas, our results might not be extrapolated to all other countries. 20 The retrospective collection of clinical characteristics of foodborne infections and travel histories, led to some missing data. Despite the considerable number of patients included in our cohort, the relatively small number of events limited the analysis of risk factors for invasive infections. Finally, we could not assess the role of food‐safety measures, because behavior of participants is unknown, and this preclude the performance of a meaningful analysis of the risk factors for foodborne infection in our study. However, since more than 92% of the SOT recipients in Switzerland are included in our cohort and systematically followed, our study allows a representative estimation of the epidemiology and burden of bacterial foodborne infections, a relatively unexplored but potentially significant and preventable complication in SOT recipients, in our country.
In conclusion, in our cohort bacterial foodborne infections were a late post‐transplant infection and were associated with significant morbidity. This study provides the first estimation of the epidemiology and the burden represented by bacterial foodborne infections in SOT recipients, and a rationale supporting the implementation of food‐safety recommendations.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
AUTHOR CONTRIBUTIONS
MM, LvdB, and OM conceived and designed this study. LvdB collected additional variables not included in the STCS database. MM and BML performed statistical analysis. MM and LvdB wrote the first draft of the paper. All the authors contributed to data interpretation, participated in revision of the manuscript and approved his final version.
Supporting information
Table S1
ACKNOWLEDGMENTS
The authors thank all patients who participate in the Swiss Transplant Cohort Study, the study nurses, the central and local data managers and all the investigators involved in the STCS. Open Access Funding provided by Universite de Lausanne.
APPENDIX 1.
List of active members of the Swiss Transplant Cohort Study (STCS)
Patrizia Amico, Andres Axel, John‐David Aubert, Vanessa Banz, Beckmann Sonja, Guido Beldi, Christoph Berger, Isabelle Binet, Pierre‐Yves Bochud, Sanda Branca, Heiner Bucher, Thierry Carrel, Emmanuelle Catana, Yves Chalandon, Sabina de Geest, Olivier de Rougemont, Michael Dickenmann, Joëlle Lynn Dreifuss, Michel Duchosal, Thomas Fehr, Sylvie Ferrari‐Lacraz, Christian Garzoni, Paola Gasche Soccal, Christophe Gaudet, Déla Golshayan, Karine Hadaya, Jörg Halter, Dimitri Hauri, Dominik Heim, Christoph Hess, Sven Hillinger, Hans Hirsch, Patricia Hirt, Günther Hofbauer, Uyen Huynh‐Do, Franz Immer, Michael Koller (Head of the data center), Bettina Laesser, Brian Lang, Roger Lehmann, Alexander Leichtle, Christian Lovis, Oriol Manuel, Hans‐Peter Marti, Pierre Yves Martin, Michele Martinelli, Katell Mellac, Aurélia Merçay, Karin Mettler, Nicolas Mueller (Chairman Scientific Committee), Antonia Müller, Thomas Müller, Ulrike Müller‐Arndt, Beat Müllhaupt, Mirjam Nägeli, Manuel Pascual (Executive office), Klara Posfay‐Barbe, Juliane Rick, Anne Rosselet, Simona Rossi, Silvia Rothlin, Frank Ruschitzka, Urs Schanz, Stefan Schaub, Aurelia Schnyder, Macé Schuurmans, Federico Simonetta, Katharina Staufer, Susanne Stampf, Jürg Steiger (Head, Excecutive office), Guido Stirniman, Ueli Stürzinger, Christian Toso, Christian Van Delden (Executive office), Jean‐Pierre Venetz, Jean Villard, Julien Vionnet, Madeleine Wick (STCS coordinator), Markus Wilhlem, Patrick Yerly.
van den Bogaart L, Lang BM, Neofytos D, et al; Swiss Transplant Cohort Study . Epidemiology and outcomes of medically attended and microbiologically confirmed bacterial foodborne infections in solid organ transplant recipients. Am J Transplant. 2022;22:199–209. 10.1111/ajt.16831
Funding information
This study has been conducted in the framework of the STCS, supported by the Swiss National Science Foundation (grant number 33CS30_177522), Unimedsuisse and the Swiss Transplant Centers. OM is a recipient of the “Bourse Leenards de la relève.”
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Scallan E, Hoekstra RM, Angulo FJ, et al. Foodborne illness acquired in the United States–major pathogens. Emerg Infect Dis. 2011;17(1):7‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tack DM, Ray L, Griffin PM, et al. Preliminary incidence and trends of infections with pathogens transmitted commonly through food ‐ foodborne diseases active surveillance network, 10 U.S. Sites, 2016‐2019. MMWR Morb Mortal Wkly Rep. 2020;69(17):509‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pacanowski J, Lalande V, Lacombe K, et al. Campylobacter bacteremia: clinical features and factors associated with fatal outcome. Clin Infect Dis. 2008;47(6):790‐796. [DOI] [PubMed] [Google Scholar]
- 4. Kennedy M, Villar R, Vugia DJ, et al. Hospitalizations and deaths due to Salmonella infections, FoodNet, 1996–1999. Clin Infect Dis. 2004;38(Suppl 3):S142‐148. [DOI] [PubMed] [Google Scholar]
- 5. Barton Behravesh C, Jones TF, Vugia DJ, et al. Deaths associated with bacterial pathogens transmitted commonly through food: foodborne diseases active surveillance network (FoodNet), 1996–2005. J Infect Dis. 2011;204(2):263‐267. [DOI] [PubMed] [Google Scholar]
- 6. Gerada J, Ganeshanantham G, Dawwas MF, et al. Infectious aortitis in a liver transplant recipient. Am J Transplant. 2013;13(9):2479‐2482. [DOI] [PubMed] [Google Scholar]
- 7. Pereira L, Sampaio S, Tavares I, Bustorff M, Pestana M. Bacteremia due to Campylobacter in renal transplantation: a case report and review of literature. Transpl Infect Dis. 2014;16(6):1007‐1011. [DOI] [PubMed] [Google Scholar]
- 8. Bunnapradist S, Neri L, Wong W, et al. Incidence and risk factors for diarrhea following kidney transplantation and association with graft loss and mortality. Am J Kidney Dis. 2008;51(3):478‐486. [DOI] [PubMed] [Google Scholar]
- 9. Fishman JA. Infection in organ transplantation. Am J Transplant. 2017;17(4):856‐879. [DOI] [PubMed] [Google Scholar]
- 10. Avery RK, Michaels MG, AST Infectious Diseases Community of Practice . Strategies for safe living following solid organ transplantation‐Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13519. [DOI] [PubMed] [Google Scholar]
- 11. Lindup M, van den Bogaart L, Golshayan D, et al. Real‐life food‐safety behavior and incidence of foodborne infections in solid organ transplant recipients. Am J Transplant. 2020;20(5):1424‐1430. [DOI] [PubMed] [Google Scholar]
- 12. Koller MT, van Delden C, Muller NJ, et al. Design and methodology of the Swiss Transplant Cohort Study (STCS): a comprehensive prospective nationwide long‐term follow‐up cohort. Eur J Epidemiol. 2013;28(4):347‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Delden C, Stampf S, Hirsch HH, et al. Burden and timeline of infectious diseases in the first year after solid organ transplantation in the Swiss Transplant Cohort Study. Clin Infect Dis. 2020;71(7):e159‐e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Humar A, Michaels M, AST ID Working Group on Infectious Disease Monitoring . American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am J Transplant. 2006;6(2):262‐274. [DOI] [PubMed] [Google Scholar]
- 15. Arslan H, Inci EK, Azap OK, Karakayali H, Torgay A, Haberal M. Etiologic agents of diarrhea in solid organ recipients. Transpl Infect Dis. 2007;9(4):270‐275. [DOI] [PubMed] [Google Scholar]
- 16. Echenique IA, Penugonda S, Stosor V, Ison MG, Angarone MP. Diagnostic yields in solid organ transplant recipients admitted with diarrhea. Clin Infect Dis. 2015;60(5):729‐737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maes B, Hadaya K, de Moor B, et al. Severe diarrhea in renal transplant patients: results of the DIDACT study. Am J Transplant. 2006;6(6):1466‐1472. [DOI] [PubMed] [Google Scholar]
- 18. Roos‐Weil D, Ambert‐Balay K, Lanternier F, et al. Impact of norovirus/sapovirus‐related diarrhea in renal transplant recipients hospitalized for diarrhea. Transplantation. 2011;92(1):61‐69. [DOI] [PubMed] [Google Scholar]
- 19. Coste JF, Vuiblet V, Moustapha B, et al. Microbiological diagnosis of severe diarrhea in kidney transplant recipients by use of multiplex PCR assays. J Clin Microbiol. 2013;51(6):1841‐1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bless PJ, Schmutz C, Suter K, et al. A tradition and an epidemic: determinants of the campylobacteriosis winter peak in Switzerland. Eur J Epidemiol. 2014;29(7):527‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmutz C, Mausezahl D, Jost M, Baumgartner A, Mausezahl‐Feuz M. Inverse trends of Campylobacter and Salmonella in Swiss surveillance data, 1988‐2013. Euro Surveill. 2016;21(6):1988‐2013. [DOI] [PubMed] [Google Scholar]
- 22. Mombelli M, Lang BM, Neofytos D, et al. Burden, epidemiology, and outcomes of microbiologically confirmed respiratory viral infections in solid organ transplant recipients: a nationwide, multi‐season prospective cohort study. Am J Transplant. 2021;21(5):1789‐1800. [DOI] [PubMed] [Google Scholar]
- 23. Mughini‐Gras L, Pijnacker R, Duijster J, et al. Changing epidemiology of invasive non‐typhoid Salmonella infection: a nationwide population‐based registry study. Clin Microbiol Infect. 2020;26(7):941.e9‐941.e14. [DOI] [PubMed] [Google Scholar]
- 24. Bunnapradist S, Lentine KL, Burroughs TE, et al. Mycophenolate mofetil dose reductions and discontinuations after gastrointestinal complications are associated with renal transplant graft failure. Transplantation. 2006;82(1):102‐107. [DOI] [PubMed] [Google Scholar]
- 25. Lemahieu W, Maes B, Verbeke K, Rutgeerts P, Geboes K, Vanrenterghem Y. Cytochrome P450 3A4 and P‐glycoprotein activity and assimilation of tacrolimus in transplant patients with persistent diarrhea. Am J Transplant. 2005;5(6):1383‐1391. [DOI] [PubMed] [Google Scholar]
- 26. Chong AS, Alegre ML. The impact of infection and tissue damage in solid‐organ transplantation. Nat Rev Immunol. 2012;12(6):459‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Angarone M, Snydman DR, AST ID Community of Practice . Diagnosis and management of diarrhea in solid‐organ transplant recipients: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13550. [DOI] [PubMed] [Google Scholar]
- 28. Shane AL, Mody RK, Crump JA, et al. 2017 Infectious Diseases Society of America Clinical Practice Guidelines for the Diagnosis and Management of Infectious Diarrhea. Clin Infect Dis. 2017;65(12):e45‐e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Federal Office of Public Health and Federal Food Safety and Veterinary Office . Swiss Antibiotic Resistance Report 2020. Usage of Antibiotics and Occurrence of Antibiotic Resistance in Switzerland. November 2020. FOPH publication number: 2020‐OEG‐64.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


