Abstract
Objective
We investigated (1) the associations of pre‐stroke aspirin use with thrombus burden, infarct volume, hemorrhagic transformation, early neurological deterioration (END), and functional outcome, and (2) whether stroke subtypes modify these associations in first‐ever ischemic stroke.
Methods
This multicenter magnetic resonance imaging (MRI)‐based study included 5,700 consecutive patients with acute first‐ever ischemic stroke, who did not undergo intravenous thrombolysis or endovascular thrombectomy, from May 2011 through February 2014. Propensity score‐based augmented inverse probability weighting was performed to estimate adjusted effects of pre‐stroke aspirin use.
Results
The mean age was 67 years (41% women), and 15.9% (n = 907) were taking aspirin before stroke. Pre‐stroke aspirin use (vs nonuse) was significantly related to a reduced infarct volume (by 30%), particularly in large artery atherosclerosis stroke (by 45%). In cardioembolic stroke, pre‐stroke aspirin use was associated with a ~50% lower incidence of END (adjusted difference = −5.4%, 95% confidence interval [CI] = −8.9 to −1.9). Thus, pre‐stroke aspirin use was associated with ~30% higher likelihood of favorable outcome (3‐month modified Rankin Scale score < 3), particularly in large artery atherosclerosis stroke and cardioembolic stroke (adjusted difference = 7.2%, 95% CI = 1.8 to 12.5 and adjusted difference = 6.4%, 95% CI = 1.7 to 11.1, respectively). Pre‐stroke aspirin use (vs nonuse) was associated with 85% less frequent cerebral thrombus‐related susceptibility vessel sign (SVS) in large artery atherosclerosis stroke (adjusted difference = −1.4%, 95% CI = −2.1 to −0.8, p < 0.001) and was associated with ~40% lower SVS volumes, particularly in cardioembolic stroke (adjusted difference = −0.16 cm3, 95% CI = −0.29 to −0.02, p = 0.03). Moreover, pre‐stroke aspirin use was not significantly associated with hemorrhagic transformation (adjusted difference = −1.1%, p = 0.09).
Interpretation
Pre‐stroke aspirin use associates with improved functional independence in patients with first‐ever ischemic large arterial stroke by reducing infarct volume and/or END, likely by decreasing thrombus burden, without increased risk of hemorrhagic transformation. ANN NEUROL 2021;90:763–776
Recent advances in intra‐arterial intervention have substantially expanded the time window for revascularization therapy in patients with large cerebral vessel occlusion. 1 , 2 , 3 Nevertheless, revascularization therapy is still only appropriate for fewer than 20% of patients with ischemic stroke. 4 Despite successful revascularization, a substantial proportion of patients do not regain functional independence, 5 , 6 indicating that prevention may be a better strategy than treatment.
Aspirin is effective in secondary stroke prevention due to its antithrombotic activity. However, aspirin has been shown to have neutral effects on primary stroke prevention, 7 , 8 , 9 although it might be beneficial in preventing atherothrombotic stroke in individuals with high cardiovascular risk, such as those with large artery atherosclerosis. 10 We previously demonstrated that pre‐stroke aspirin was associated with less severe stroke and better functional outcome at discharge, especially for large artery atherosclerosis (LAA) stroke as opposed to small vessel occlusion (SVO) and cardioembolic (CE) stroke. 11 However, this previous study did not investigate (a) how pre‐stroke aspirin affected stroke severity and outcomes and (b) whether pre‐stroke aspirin was associated with 3‐month functional outcome. Moreover, there has been no large‐scale quantitative neuroimaging study that investigated the effect of pre‐stroke aspirin use on both imaging and functional outcomes.
In the present magnetic resonance imaging (MRI)‐based multicenter study of 5,700 consecutive patients with first‐ever acute ischemic stroke, we hypothesized that pre‐stroke aspirin use associates with reduced cerebral thrombus, smaller infarcts, less frequent early neurological deterioration, and thus with better functional outcome at 3 months.
Methods
Study Population
A total of 14,497 patients with acute ischemic strokes were admitted to the hospital within 7 days of symptom onset between May 2011 and Feb 2014 at 11 participating institutions. All patients were enrolled in our prospective multicenter registry, 12 , 13 , 14 , 15 , 16 , 17 and 8,797 were excluded sequentially for the following reasons: (1) 1,249 patients did not undergo MRI; (2) 8 had diffusion‐weighted images (DWIs) that were technically inadequate for measuring infarct volume; (3) 2,645 had a history of stroke; (4) 2,610 had other determined or undetermined stroke etiologies; (5) 1,032 were taking any anticoagulants or any antiplatelet agents other than aspirin before stroke; (6) 134 were lacking an infarct on initial DWIs, and (7) 1,119 were receiving intravenous tissue plasminogen activator treatment or intra‐arterial mechanical thrombectomy, as MRI scans obtained at different time points either before or after revascularization therapy likely introduce bias in assessing treatment effects with imaging outcomes, such as thrombus burden and infarct volume. The remaining 5,700 patients constituted the study cohort; 2,873 had been also included in a previous study. 11 The institutional review boards of all participating centers approved this study, and patients or their legally authorized representatives provided written informed consent.
Clinical Data Collection
Using a standardized protocol, 18 we collected demographic data, medication histories, and details about vascular risk factors. Stroke subtypes were determined by consensus among experienced neurologists in each participating center, using a validated MRI‐based algorithm 19 built on Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria. 20 Pre‐stroke aspirin users were defined as patients who had taken aspirin within the 7 days preceding the index stroke and nonusers as those who had not taken any antithrombotic medication during this period. Admission National Institutes of Health Stroke Scale (NIHSS) scores, pre‐stroke modified Rankin Scale (mRS) scores, and 3‐month mRS scores were collected prospectively. 15 , 17 The mRS measures degree of disability and functional outcomes, ranging from 0 (no symptoms) to 6 (death). An mRS score of 0 to 2 indicates functional independence after stroke.
Attending neurologists assessed neurological status for each patient daily. Early neurological deterioration was defined as any new neurological symptoms or signs or neurological worsening occurring within 3 days after stroke onset, using the following criteria: (1) an increment in total NIHSS score of ≥2 points, (2) an increment in NIHSS consciousness score (1a–1c) of ≥1, (3) an increment in NIHSS motor score (5a–6b) of ≥1, or (4) any new neurological deficit (even if not reflected by the NIHSS). 15 , 17 , 18
Magnetic Resonance Image Registration and Analysis
Brain MRI was performed using 1.5 Tesla (n = 4,705) or 3.0 Tesla (n = 995) systems. DWI protocols were: b‐values of 0 and 1,000 s/mm2, repetition time 2,400 to 9,000 ms, echo time 50 to 99 ms, voxel size 1 × 1 × 3 to 1 × 1 × 5 mm3, and interslice gap 0 to 2 mm. Fluid‐attenuated inversion recovery (FLAIR) image protocols were: repetition time 6,000 to 11,000 ms, echo time 76 ms, voxel size 1 × 1 × 3 to 1 × 1 × 7 mm3, and interslice gap 0 to 2.25 mm. Gradient‐echo (GRE) image protocols were: echo time 13 to 26 ms, repetition time 400 to 1,197 ms, voxel size 1 × 1 × 3 to 1 × 1 × 5 mm3, and interslice gap 0 to 2.25 mm. Susceptibility image protocols were: echo time 20 ms, repetition time 27 ms, voxel size 1 × 1 × 1.5 mm3, and interslice gap 0 mm. All images were stored at the Korean Brain MRI Data Center for quantitative analysis. As previously described, 12 , 13 , 15 , 16 , 17 we quantified infarct volumes on DWIs and white matter hyperintensity (WMH) volumes on FLAIR images. Briefly, after normalizing images, high signal intensity lesions on DWI and FLAIR images were semi‐automatically segmented and registered onto brain templates under the close guidance of a vascular neurologist (author W.‐S.R.). Infarct volumes on DWIs and WMH volumes on FLAIR images were converted to brain volume percentages by expressing the number of voxels in lesions as percentages of total brain voxels in the template images, followed by log‐transformation: log‐transformed percentage of brain parenchymal volume. While segmenting and registering FLAIR WMHs, only chronic lesions were included, and high signal lesions caused by acute infarcts were excluded. Inter‐ and intra‐observer correlation coefficients were high for quantified lesion volume, with coefficients ranging from 0.836 to 0.977 for infarct volumes on DWIs and 0.987 to 0.995 for WMH volumes on FLAIR images. 16 Gradient‐echo (n = 5,041) or susceptibility‐weighted images (n = 13) were reviewed by an investigator (author W.‐S.R.) blinded to patients’ data except for information about the infarction hemispheric side. Hemorrhagic transformation was defined as any degree of hypointensity on GRE or susceptibility‐weighted images within an infarcted area. 21 Symptomatic hemorrhagic transformation was defined as any hemorrhagic transformation that worsened the NIHSS score by ≥4 points. 22 As a highly specific sign of occlusive thrombus in association with large arterial infarction, 23 the susceptibility vessel sign (SVS) on GRE or susceptibility‐weighted images was defined as (a) hypointense signal within the vascular cisterns corresponding to the symptomatic middle cerebral artery 24 and (b) signal diameter exceeding contralateral vessel diameter (ie, the susceptibility signal was more than the expected flow void in the vessel). 24 The total volume of thrombus‐related SVS‐positive voxels (SVS volume, cm3) was quantified, as previously reported. 25
Statistical Analysis
Data were analyzed using STATA software 16.0 (STATA Corp., College Station, TX, USA), and a p value < 0.05 was considered statistically significant. Baseline characteristics of included versus excluded patients and of aspirin users versus nonusers were compared using the Student's t test, the rank‐sum test, or the chi‐square test according to variable types, as appropriate. Time intervals from stroke onset to arrival for different stroke subtypes were compared using the Kruskal‐Wallis test.
Pre‐stroke aspirin users and nonusers may differ systematically due to “confounding by indication” for aspirin. Therefore, propensity score‐based inverse probability weighting (IPW) was used to adjust for measured confounding and thus create a balanced covariate distribution between pre‐stroke aspirin users and nonusers. 26 Propensity scores were calculated to estimate the probability of individual patients’ aspirin use before the index stroke by using multivariable logistic regression with pre‐stroke aspirin use (vs nonuse) as the dependent variable and with the following covariates that were chosen a priori as the independent variables: age, sex, stroke subtype, hypertension, diabetes, hyperlipidemia, smoking, atrial fibrillation, coronary artery disease, pre‐stroke mRS score, pre‐stroke statin use, pre‐stroke antihypertensive use, pre‐stroke antidiabetic use, and the time from stroke onset to MRI (C‐statistics = 0.79). To create an inverse probability weighted data (pseudo‐data) with the distribution of measured covariates being independent of pre‐stroke aspirin use, observations were weighted according to the inverse of the calculated probability of receiving the exposure that each patient actually received (ie, aspirin use vs nonuse). Post‐weighting covariate balance between aspirin users and nonusers was assessed using standardized mean differences.
In the inverse probability weighted data, potential outcome means 27 of aspirin users and nonusers and subsequently intergroup differences were estimated for (1) infarct volume, (2) the incidence of hemorrhagic transformation, (3) the incidence of early neurological deterioration, and (4) the proportion of favorable functional outcome at 3 months (mRS scores 0–2) by using the teffects aipw function in STATA software to perform augmented IPW: a “doubly robust” method 27 combining IPW with outcome regression. In the outcome regression of the augmented IPW, predefined variables that have been reported to be associated with infarct volume or stroke outcomes 28 , 29 , 30 , 31 , 32 , 33 , 34 were used. In the analysis of the relationship between pre‐stroke aspirin use and infarct volume, WMH volume was included as a covariate in the augmented IPW analysis to explore the association between pre‐stroke aspirin use and infarct volume independently of WMH burden, which has been shown to associate with larger infarct volume 35 and more frequent infarct growth. 36 For hemorrhagic transformation, early neurological deterioration, and favorable 3‐month outcome, adjusted odds ratios (95% confidence interval [CI]) of pre‐stroke aspirin use (vs nonuse) were also estimated by using multivariable logistic regression analysis in the inverse probability weighted data with adjustment for the covariates that were used for the augmented IPW. 28 , 29 , 30 , 31 , 32 , 33 , 34 As a priori subgroup analyses, effect modification by stroke subtypes was assessed using multiple linear regression analysis (for infarct volume) or multivariable logistic regression analysis (for hemorrhagic transformation, early neurological deterioration, and favorable 3‐month outcome) in the inverse probability weighted data, with adjustment for the covariates that were used for the IPW. Moreover, a sensitivity analysis of the relationship between aspirin use and infarct volume was performed using augmented IPW in patients who underwent MRI within 24 hours of stroke onset.
The association between pre‐stroke aspirin use and the presence of SVS was assessed using IPW. Odds ratios for this association and the effect modification by stroke subtypes were examined using unadjusted logistic regression analysis in the inverse probability weighted data. In the patients who were SVS‐positive, the association between pre‐stroke aspirin use and the SVS volume was assessed using IPW to estimate the adjusted difference between the groups. The effect modification by stroke subtypes in relation to SVS volume was estimated using unadjusted linear regression in the inverse probability weighted data.
To complement the aforementioned analyses, the following analyses using the inverse probability weighted data were also performed. Quantile regressions were performed to determine how the association between pre‐stroke aspirin use and infarct volume changes as infarct volumes increase for each infarct volume quantile (ie, decile). 37 Quantile regression relaxes the normality assumption of the residuals required in multiple linear regression 38 and allowed us to investigate whether the relationship between pre‐stroke aspirin and infarct volumes was more obvious in large infarcts than in small infarcts, where pre‐stroke aspirin use would hardly make a difference. 38 The covariates that were used in the multiple linear regression analysis (for the assessment of effect modification by stroke subtypes in the inverse probability weighted data) were entered into the quantile regression model. Consequently, the model could provide coefficients of pre‐stroke aspirin use (vs nonuse), which represent the covariate‐adjusted differences of infarct volumes (percentage of brain parenchymal volume) between the groups, at 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, and 0.9 quantiles of all infarct volumes. In addition to the nonparametric quantile regression analysis, as a parametric analysis, multiple linear regression analysis was performed using the inverse probability weighted data to obtain an ordinal least square estimate of pre‐stroke aspirin use (vs nonuse) in relation with infarct volume.
Infarct volume, incidence of early neurological deterioration, and proportion of favorable outcome were compared between the patients who were SVS‐positive and negative by using the rank‐sum test and the chi‐squared test as appropriate. In the patients who were SVS‐positive, the association between SVS volume and infarct volume was examined using linear regression analysis. SVS volumes stratified by early neurological deterioration, and favorable 3‐month outcome were compared using the rank‐sum test.
Results
Characteristics of Pre‐Stroke Aspirin Users Versus Nonusers
Compared with the patients who met the inclusion criteria (n = 5,700), those who were excluded (n = 8,797) were older and had more risk factors (Table S1 in Online Supplementary Material). In addition, the excluded patients had more severe strokes, a greater infarct volume, a higher rate of early neurological deterioration, and a lower proportion of favorable outcome. Mean age of the 5,700 included patients was 67.2 years (SD = 12.9), 41.0% (n = 2,337) were women, and 15.9% (n = 907) were taking aspirin before the index stroke. Important baseline differences between the pre‐stroke aspirin users and nonusers were noted on several characteristics (Table 1). Pre‐stroke aspirin users were older (71.1 vs 66.4 years), more likely to be women (46.5% vs 40.0%), and had more risk factors compared with nonusers. In addition, pre‐stroke aspirin users took more medications to control risk factors, had higher median WMH volumes (0.85% vs 0.73%), and were more likely to have CE stroke compared with nonusers (35.9% vs 17.7%). Stroke severity and the time from onset to MRI, however, were comparable between the 2 groups. The application of the propensity score‐based IPW balanced the baseline characteristics between the aspirin group and the non‐aspirin group (see Table 1; Fig 1). When stratified by stroke subtypes, the time from symptom onset to MRI was shortest for CE stroke (median = 12.9 hours; p < 0.001), followed by SVO stroke (median = 20.9 hours) and LAA stroke (median = 22.6 hours).
TABLE 1.
Baseline Characteristics by Pre‐Stroke Aspirin Use in 5,700 Patients With First‐Ever Ischemic Stroke Before and After Inverse Probability Weighting a
| Characteristics | Original observed data | Inverse probability weighted data b | ||
|---|---|---|---|---|
| Aspirin users (n = 907) | Nonusers (n = 4,793) | Aspirin users (n = 2,727.1) | Nonusers (n = 2,972.9) | |
| Age, mean (SD), yr | 71.1 (10.8) | 66.4 (13.1) | 68.7 (12.3) | 67.2 (12.9) |
| Sex, women | 422 (46.5) | 1,915 (40.0) | 1,180.9 (43.4) | 1,224.9 (41.2) |
| Hypertension | 786 (86.7) | 2,940 (61.3) | 1,901.4 (69.7) | 1,947.7 (65.5) |
| Diabetes | 382 (42.1) | 1,532 (31.9) | 1,063.1 (39.0) | 1,009.0 (33.9) |
| Hyperlipidemia | 344 (37.9) | 1,337 (27.9) | 791.6 (29.0) | 879.3 (29.6) |
| Current or recent c smoking | 314 (34.6) | 2,139 (44.6) | 1,113.3 (40.8) | 1,272.0 (42.8) |
| Atrial fibrillation | 278 (30.7) | 573 (12.0) | 471.6 (17.3) | 451.3 (15.2) |
| Coronary artery disease | 177 (19.5) | 263 (5.5) | 235.7 (8.6) | 230.1 (7.7) |
| Pre‐stroke mRS of 0 or 1 | 792 (87.3) | 4,383 (91.5) | 2,444.5 (89.6) | 2,712.1 (91.3) |
| Pre‐stroke statin use | 241 (26.6) | 308 (6.4) | 308.6 (11.3) | 294.0 (9.9) |
| Pre‐stroke antihypertensive use | 723 (79.7) | 1,930 (40.3) | 1,435.2 (52.6) | 1,391.9 (46.8) |
| Pre‐stroke antidiabetic use | 325 (35.8) | 1,033 (21.6) | 827.7 (30.4) | 717.8 (24.2) |
| Admission NIHSS scores | ||||
| Mean (SD) | 4.9 (5.7) | 4.4 (4.8) | 4.3 (5.2) | 4.6 (5.1) |
| Median (IQR) | 3 (1 to 6) | 3 (1 to 5) | ||
| Time from onset to MRI, h | ||||
| Mean (SD) | 34.2 (37.1) | 34.2 (35.6) | 34.6 (37.3) | 34.1 (35.7) |
| Median (IQR) | 18.6 (8.4–46.1) | 20.2 (9.2–47.4) | ||
| Stroke subtype | ||||
| Large artery atherosclerosis | 371 (40.9) | 2,607 (54.4) | 1,379.6 (50.1) | 1,559.9 (52.5) |
| Small vessel occlusion | 210 (23.2) | 1,338 (27.9) | 775.2 (28.4) | 785.5 (26.4) |
| Cardioembolism | 326 (35.9) | 848 (17.7) | 572.4 (21.0) | 627.4 (21.1) |
| WMH volume, percentage of brain parenchymal volume d | ||||
| Mean (SD) | 1.24 (1.17) | 1.11 (1.16) | 1.22 (1.17) | 1.13 (1.16) |
| Median (IQR) | 0.85 (0.45–1.76) | 0.73 (0.40–1.44) | ||
All values are reported as no. (%) unless otherwise specified.
The inverse probability weighted data represent propensity score‐weighted data.
Quit smoking within 5 yr of stroke onset.
Excluding 5.2% without fluid‐attenuated inversion recovery image.
IQR = interquartile range; MRI = magnetic resonance imaging; mRS = modified Rankin Scale; NIHSS = National Institute of Health Stroke Scale; WMH = white matter hyperintensity.
FIGURE 1.
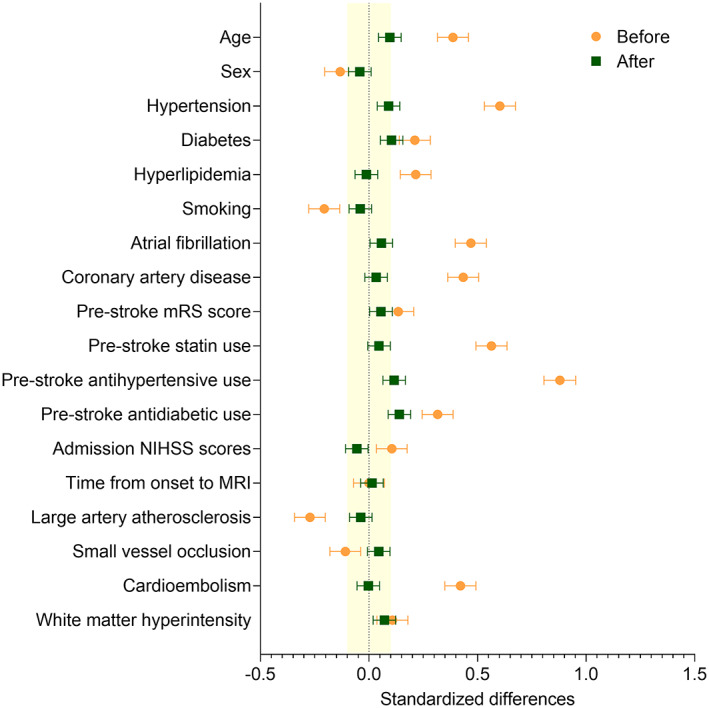
Standardized differences before versus after augmented inverse‐probability weighting. Orange circles and green squares indicate standardized differences before and after the inverse probability weighting, respectively. Horizontal error bars indicate 95% confidence intervals. The yellow shaded area indicates the area of the absolute standardized difference <0.1. MRI = magnetic resonance imaging; mRS = modified Rankin Scale; NIHSS = National Institute of Health Stroke Scale.
Association between Pre‐stroke Aspirin Use and Infarct Volume
Without the propensity score‐based adjustment, aspirin users and nonusers appeared to have similar infarct volumes: −1.96 and −2.02 (log‐transformed percentage of brain parenchymal volume), which, in the absence of log‐transformation, correspond to 0.14 and 0.13 {percentage of brain parenchymal volume}, respectively. However, the augmented IPW showed that adjusted infarct volumes (ie, potential outcome means) were lower in pre‐stroke aspirin users (−2.30, {0.10}; Table 2) than in nonusers (−1.94, {0.14}), with statistically significant (p < 0.001) adjusted difference = −0.36, 95% CI = −0.53 to −0.19; {0.70 [0.59 to 0.83]}. This finding suggests that pre‐stroke aspirin use (vs nonuse) reduced infarct volume by about one third, as indicated by the following calculation: “(0.14–0.10)/0.14” or “1.00–0.70.” It is notable that 0.10/0.14 is approximately 0.70 and that the difference between the logarithms of 2 values (log0.10–log0.14) corresponds to the logarithm of their ratio: log (0.10/0.14).
TABLE 2.
Association Between Pre‐Stroke Aspirin Use and Log‐Transformed Infarct Volume a
| Aspirin users (n = 2,576.3) POM b (SD) {non‐logarithmic value of POM} c | Aspirin nonusers (n = 2,828.7) POM b (SD) {non‐logarithmic value of POM} c | Mean difference of infarct volume (95% CI) d {non‐logarithmic infarct volume ratio [95% CI]} c , e | |
|---|---|---|---|
| All patients | −2.30 (1.98) {0.10} | −1.94 (2.00) {0.14} | −0.36 (−0.53 to −0.19) {0.70 [0.59 to 0.83]} |
| p value | <0.001 | ||
| Subtypes | |||
| LAA | −2.39 (1.88) {0.09} | −1.78 (1.82) {0.17} | −0.59 (−0.88 to −0.31) {0.55 [0.41 to 0.73]} |
| p value | <0.001 | ||
| SVO | −3.51 (1.07) {0.03} | −3.40 (1.08) {0.03} | −0.12 (−0.32 to 0.09) {0.89 [0.73 to 1.09]} |
| p value | 0.26 | ||
| CE | −0.68 (2.07) {0.51} | −0.57 (2.08) {0.57} | −0.11 (−0.42 to 0.19) {0.90 [0.65 to 1.21]} |
| p value | 0.48 | ||
| p value f | 0.02 |
Measured by using diffusion‐weighted magnetic resonance images.
Adjusted results obtained from “doubly robust” 27 inverse probability weighting based on propensity scores with adjustment for hypertension, diabetes, hyperlipidemia, smoking, atrial fibrillation, coronary artery disease, pre‐stroke mRS score, pre‐stroke statin use, pre‐stroke antihypertensive use, pre‐stroke antidiabetic use, time from onset to MRI, and log‐transformed white matter hyperintensity volume. Five thousand four hundred five patients were included in the model (2,802 large artery atherosclerosis, 1,502 small vessel occlusion, and 1,101 cardioembolism).
Values are calculated using back‐transformation from log‐transformed estimates.
Mean differences are for pre‐stroke aspirin users relative to pre‐stroke aspirin nonusers.
Ratios are for pre‐stroke aspirin users relative to pre‐stroke aspirin nonusers.
The p value for interaction between pre‐stroke aspirin use and stroke subtypes and from a multiple linear regression analysis in the inverse probability weighted data with adjustment for covariates.
CE = cardioembolism; CI = confidence interval; LAA = larger artery atherosclerosis; MRI = magnetic resonance imaging; mRS = modified Rankin Scale; POM = potential outcome mean; SD = standard deviation; SVO = small vessel occlusion.
The association of pre‐stroke aspirin use with a lower infarct volume was significant in LAA stroke (adjusted difference = −0.59, 95% CI = −0.88 to −0.31, p < 0.001), but not in SVO or CE strokes (p for interaction = 0.02; see Table 2). When the analysis was confined to patients who underwent MRI within 24 hours of onset (n = 3,075), the association between pre‐stroke aspirin use (vs nonuse) and infarct volume remained significant (adjusted difference − 0.35, 95% CI = −0.56 to −0.14), as did modifications of this association by stroke subtypes (p for interaction = 0.005).
The quantile regression analysis showed that the estimated infarct volume difference between pre‐stroke aspirin users and nonusers grew as infarct volumes increased (Fig 2A). Stratification by stroke subtypes revealed that the association between pre‐stroke aspirin use and greater estimated infarct volume difference in larger infarcts was more prominent in LAA strokes than in SVO and CE strokes (Fig 2B−D).
FIGURE 2.
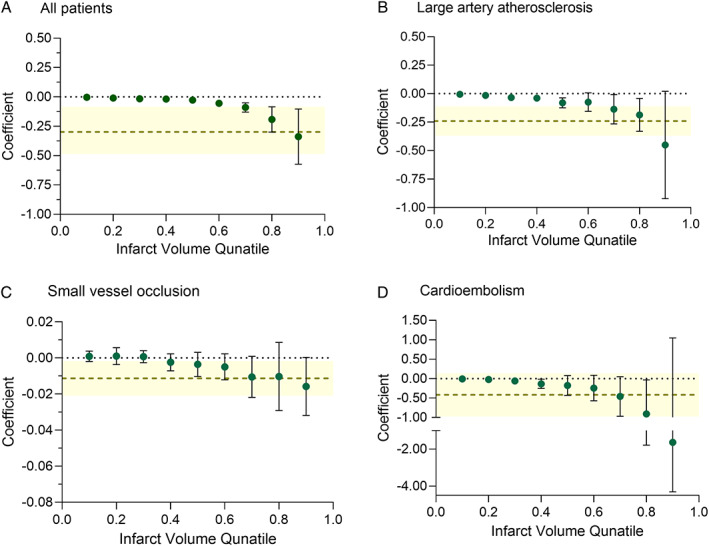
Association between pre‐stroke aspirin use and infarct volume. Quantile regression analysis adjusts for age, sex, stroke subtype, hypertension, diabetes, hyperlipidemia, smoking, atrial fibrillation, coronary artery disease, pre‐stroke modified Rankin Scale score, pre‐stroke statin use, pre‐stroke antihypertensive use, pre‐stroke antidiabetic use, and the time from symptom onset to magnetic resonance imaging (MRI) in the inverse probability weighted data. Green dots indicate the (nonparametric) coefficients of pre‐stroke aspirin use (vs nonuse), which represent the covariate‐adjusted differences of infarct volumes (percentage of brain parenchymal volume) between the groups, at 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, and 0.9 deciles of all infarct volumes. Black error bars indicate 95% confidence intervals. In addition, light olive‐colored horizontal dashed lines indicate ordinal least square estimates (parametric coefficients: mean infarct volume differences between aspirin users and nonusers) obtained using multiple linear regression in the inverse probability weighted data, with the yellow shaded areas showing their 95% confidence intervals. p for interaction <0.001.
Associations of Pre‐stroke Aspirin Use with Thrombus‐Related SVS, Hemorrhagic Transformation, and Early Neurological Deterioration
SVS was observed in 90 of the 5,054 subjects (1.8%), with similar frequency in pre‐stroke aspirin users and nonusers (1.8% vs 1.8%; Table S2 in Online Supplementary Material) in the original observed data without IPW. The prevalence of SVS was highest in CE stroke (4.9%), followed by LAA stroke (1.5%), and no patient with SVO stroke had the sign. SVS volume and the presence of SVS sign were associated positively with infarct volume and negatively with favorable outcome, but not with early neurological deterioration (Fig 3).
FIGURE 3.
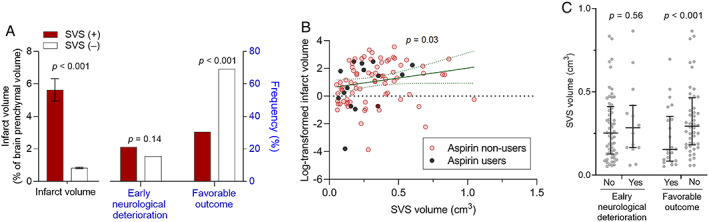
Associations of thrombus‐related susceptibility vessel sign (presence and volume) with infarct volume, early neurological deterioration, and favorable 3‐month outcome. (A) Association of the presence (+) versus absence (−) of the susceptibility vessel sign (SVS) with the infarct volume (left y‐axis) and the frequencies of early neurological deterioration and favorable 3‐month outcome (right y‐axis). Infarct volume is expressed as mean ± standard error, and favorable outcome is defined as 3‐month modified Rankin Scale score of 0–2. (B) Association between SVS‐positive lesion volume and log‐transformed infarct volume. Each dot indicates a log‐transformed infarct volume (percentage of brain parenchymal volume) in each patient. Green line (with green dot lines) indicates a linear fit (with a 95% confidence interval) between the thrombus‐related susceptibility vessel sign‐positive lesion volumes (thrombus volumes) and the infarct volumes. (C) Associations of SVS‐positive lesion volume with early neurological deterioration and favorable 3‐month outcome. Each dot indicates the thrombus volume of each patient. Horizontal lines and vertical bars indicate medians and interquartile ranges, respectively.
After IPW, the prevalence of SVS did not differ significantly between pre‐stroke aspirin users and nonusers (1.5% vs 1.9%, adjusted odds ratio = 0.78, 95% CI = 0.50 to 1.20; Table S2 in Online Supplementary Material). However, pre‐stroke aspirin use was associated with a lower SVS rate in LAA stroke (0.2% vs 1.6%, adjusted odds ratio = 0.15, 95% CI = 0.04 to 0.48), which was not the case in CE stroke (5.8% vs 4.8%, adjusted odds ratio = 1.33, 95% CI = 0.79 to 2.24; p for interaction <0.001). In patients who were SVS‐positive (n = 90), pre‐stroke aspirin use was associated with a lower adjusted SVS volume (0.22 cm3, 95% CI = 0.13 to 0.30 cm3 vs 0.36 cm3, 95% CI = 0.30 to 0.41 cm3, p = 0.03), particularly in CE stroke.
Hemorrhagic transformation was observed in 234 of the 5,054 (4.6%) patients. Pre‐stroke aspirin users tended to experience hemorrhagic transformation more often than nonusers (5.7% vs 4.4%, p = 0.10). Symptomatic hemorrhagic transformation was observed in 5 (0.6%) of pre‐stroke aspirin users and 20 (0.4%) of nonusers. After IPW and multivariable adjustment, pre‐stroke aspirin use tended to be inversely associated with hemorrhagic transformation (adjusted difference = −1.1%, 95% CI = −2.6 to 0.4; adjusted odds ratio = 0.68, 95% CI = 0.56 to 1.01, p = 0.06; Table 3), and stroke subtypes did not modify this result. In addition, pre‐stroke aspirin use was not associated with symptomatic hemorrhagic transformation.
TABLE 3.
Association between Pre‐stroke Aspirin Use and Incidence of Hemorrhagic Transformation a
| Aspirin users POM, % (95% CI) b (n = 2,411.8) | Aspirin nonusers POM, % (95% CI) b (n = 2,642.2) | Risk difference, % (95% CI) b , c | Odds ratio (95% CI) d | |
|---|---|---|---|---|
| All patients | 3.7 (2.3 to 5.1) | 4.8 (4.1 to 5.4) | −1.1 (−2.6 to 0.4) | 0.68 (0.56 to 1.01) |
| p value | 0.09 | 0.06 | ||
| Subtypes | ||||
| LAA | 1.8 (0.3 to 3.3) | 2.8 (2.1 to 3.4) | −1.0 (−2.5 to 0.5) | 0.67 (0.39 to 1.18) |
| p value | 0.28 | 0.16 | ||
| SVO | 0.6 (0.0 to 1.6) | 0.4 (0.0 to 0.7) | 0.3 (0.0 to 1.4) | 0.37 (0.05 to 2.69) |
| p value | 0.62 | 0.32 | ||
| CE | 11.3 (7.5 to 15.1) | 15.5 (12.9 to 18.1) | −4.2 (−8.8 to 0.4) | 0.71 (0.49 to 1.00) |
| p value | 0.08 | 0.055 | ||
| p value e | 0.83 |
Hemorrhagic transformation was defined as any degree of hypointensity on gradient‐echo or susceptibility‐weighted images within an infarcted area. Five thousand fifty‐four patients were included (2,607 large artery atherosclerosis, 1,275 small vessel occlusion, and 1,039 cardioembolism).
Potential outcome means (95% CI) and risk differences (95% CI) are from “doubly robust” 27 inverse probability weighting analysis adjusted for age, sex, stroke subtypes, admission NIHSS score, hypertension, diabetes, hyperlipidemia, smoking, atrial fibrillation, coronary artery disease, pre‐stroke mRS score, pre‐stroke statin use, pre‐stroke antihypertensive use, pre‐stroke antidiabetic use, log‐transformed infarct volume, and time from onset to MRI.
Risk differences are for pre‐stroke aspirin users relative to pre‐stroke aspirin non‐users.
Adjusted odds ratios and 95% CIs are from multivariable logistic regression analysis in the inverse probability weighted data adjusted for age, sex, stroke subtypes, admission NIHSS score, hypertension, diabetes, hyperlipidemia, smoking, atrial fibrillation, coronary artery disease, pre‐stroke mRS score, pre‐stroke statin use, pre‐stroke antihypertensive use, pre‐stroke antidiabetic use, log‐transformed infarct volume, and time from onset to MRI.
The p value for interaction between pre‐stroke aspirin use and stroke subtypes and from a multivariable logistic regression analysis in the inverse probability weighted data with adjustment for covariates.
CE = cardioembolism; CI = confidence interval; LAA = larger artery atherosclerosis; MRI = magnetic resonance imaging; mRS = modified Rankin Scale; NIHSS = National Institute of Health Stroke Scale; POM = potential outcome mean; SVO = small vessel occlusion.
Early neurological deterioration was noted in 645 (11.3%) patients, and its frequency was not significantly different between pre‐stroke aspirin users and nonusers (10.8% vs 11.4%). Yet, after IPW and multivariable adjustment, the effect of pre‐stroke aspirin use on early neurological deterioration was significantly modified by stroke subtypes (p for interaction = 0.009; Table 4); pre‐stroke aspirin use was associated with a significantly lower risk of early neurological deterioration in CE stroke (adjusted risk difference = −5.4%, 95% CI = −8.9 to −1.9; adjusted odds ratio = 0.52, 95% CI = 0.34 to 0.78, p = 0.002) only.
TABLE 4.
Association Between Pre‐stroke Aspirin Use and Early Neurological Deterioration a
| Aspirin users POM, % (95% CI) b (n = 2,727.1) | Aspirin nonusers POM, % (95% CI) b (n = 2,972.9) | Risk difference, % (95% CI) b , c | Odds ratio (95% CI) d | |
|---|---|---|---|---|
| All patients | 9.9 (7.3 to 12.5) | 11.3 (10.4 to 12.2) | −1.4 (−4.1 to 1.4) | 0.86 (0.72 to 1.03) |
| p value | 0.33 | 0.10 | ||
| Subtypes | ||||
| LAA | 12.5 (8.6 to 16.4) | 12.2 (10.9 to 13.5) | 0.3 (−3.9 to 4.5) | 1.07 (0.85 to 1.34) |
| p value | 0.89 | 0.59 | ||
| SVO | 7.6 (2.4 to 12.8) | 8.7 (7.1 to 10.2) | −1.1 (−6.5 to 4.3) | 0.79 (0.55 to 1.14) |
| p value | 0.70 | 0.21 | ||
| CE | 7.1 (4.3 to 9.9) | 12.5 (10.3 to 14.8) | −5.4 (−8.9 to −1.9) | 0.52 (0.34 to 0.78) |
| p value | 0.002 | 0.002 | ||
| p value e | 0.009 |
Five thousand seven hundred patients were included (2,987 large artery atherosclerosis, 1,548 small vessel occlusion, and 1,174 cardioembolism).
Potential outcome means (95% CI) and risk differences (95% CI) are from “doubly robust” 27 inverse probability weighting analysis adjusted for age, sex, stroke subtypes, admission NIHSS score, hypertension, diabetes, hyperlipidemia, smoking, atrial fibrillation, coronary artery disease, pre‐stroke mRS score, pre‐stroke statin use, pre‐stroke antihypertensive use, pre‐stroke antidiabetic use, log‐transformed infarct volume, and onset to MRI.
Risk differences are for pre‐stroke aspirin users relative to pre‐stroke aspirin nonusers.
Adjusted odds ratios and 95% CIs are from multivariable logistic regression analysis in the inverse probability weighted data adjusted for age, sex, stroke subtypes, admission NI HSS score, hypertension, diabetes, hyperlipidemia, smoking, atrial fibrillation, coronary artery disease, pre‐stroke mRS score, pre‐stroke statin use, pre‐stroke antihypertensive use, pre‐stroke antidiabetic use, log‐transformed infarct volume, and time from onset to MRI.
The p value for interaction between pre‐stroke aspirin use and stroke subtypes and from a multivariable logistic regression analysis in the inverse probability weighted data with adjustment for covariates.
CE = cardioembolism; CI = confidence interval; LAA = larger artery atherosclerosis; MRI = magnetic resonance imaging; mRS = modified Rankin Scale; NIHSS = National Institute of Health Stroke Scale; POM = potential outcome mean; SVO = small vessel occlusion.
Association between Pre‐stroke Aspirin Use and Functional Independence at 3 Months after Stroke
Data for mRS scores at 3 months were missing for 154 (2.7%) patients, and 3,794 (68.4%) had favorable outcome (mRS scores of 0–2). After IPW and multivariable adjustment, pre‐stroke aspirin use was associated with 32% higher likelihood of favorable outcome (adjusted difference = 3.5%, 95% CI = 0.2–6.7%; adjusted odds ratio = 1.32, 95% CI = 1.13–1.54; Table 5). In addition, this association was modified by stroke subtypes (p for interaction <0.001). The adjusted odds ratios of a favorable outcome by pre‐stroke aspirin use (vs nonuse) were 1.54 (95% CI = 1.26–1.90) for LAA stroke and 1.82 for CE stroke (95% CI = 1.29–2.57), demonstrating statistical significance, but 0.74 (0.55–1.00) for SVO stroke.
TABLE 5.
Association Between Pre‐Stroke Aspirin Use and Favorable Outcome at 3 Months a
| Aspirin users POM, % (95% CI) b (n = 2,658.7) | Aspirin nonusers POM, % (95% CI) b (n = 2,887.3) | Favorable outcome difference, % (95% CI) b , c | Odds ratio (95% CI) d | |
|---|---|---|---|---|
| All patients | 71.3 (68.1 to 74.5) | 67.8 (66.5 to 69.2) | 3.5 (0.2 to 6.7) | 1.32 (1.13 to 1.54) |
| p value | 0.037 | <0.001 | ||
| Subtypes | ||||
| LAA | 72.7 (67.5 to 77.9) | 65.6 (63.7 to 67.4) | 7.2 (1.8 to 12.5) | 1.54 (1.26 to 1.90) |
| p value | 0.009 | < 0.001 | ||
| SVO | 82.0 (76.5 to 87.6) | 84.0 (82.0 to 86.0) | −1.9 (−7.6 to 3.8) | 0.74 (0.55 to 1.00) |
| p value | 0.51 | 0.052 | ||
| CE | 58.2 (53.7 to 62.7) | 51.8 (48.5 to 55.0) | 6.4 (1.7 to 11.1) | 1.82 (1.29 to 2.57) |
| p value | 0.007 | 0.001 | ||
| p value e | <0.001 |
Favorable outcome was defined as 3‐mo mRS scores of 0–2. 3‐mo mRS scores were missing in 2.7% of patients, who were then excluded (5,546 patients were included; 2,890 large artery atherosclerosis, 1,523 small vessel occlusion, and 1,133 cardioembolism).
Potential outcome means (95% CI) and risk differences (95% CI) are from “doubly robust” 27 inverse probability weighting analysis adjusted for age, sex, stroke subtypes, admission NIHSS score, hypertension, diabetes, hyperlipidemia, smoking, atrial fibrillation, coronary artery disease, pre‐stroke mRS score, pre‐stroke statin use, pre‐stroke antihypertensive use, pre‐stroke antidiabetic use, log‐transformed infarct volume, and time from onset to MRI.
Favorable outcome differences are for pre‐stroke aspirin users relative to pre‐stroke aspirin nonusers.
Adjusted odds ratios and 95% CIs are from multivariable logistic regression analysis in the inverse probability weighted data adjusted for age, sex, stroke subtypes, admission NIHSS score, hypertension, diabetes, hyperlipidemia, smoking, atrial fibrillation, coronary artery disease, pre‐stroke mRS score, pre‐stroke statin use, pre‐stroke antihypertensive use, pre‐stroke antidiabetic use, log‐transformed infarct volume, and time from onset to MRI.
The p value for interaction between pre‐stroke aspirin use and stroke subtypes and from a multivariable logistic regression analysis in the inverse probability weighted data with adjustment for covariates.
CE = cardioembolism; CI = confidence interval; LAA = larger artery atherosclerosis; MRI = magnetic resonance imaging; mRS = modified Rankin Scale; NIHSS = National Institute of Health Stroke Scale; POM = potential outcome mean; SVO = small vessel occlusion.
Discussion
In the present study of 5,700 consecutive patients with first‐ever acute ischemic stroke, pre‐stroke aspirin use was associated with 30% lower infarct volume on DWI, particularly in LAA stroke, more so in patients with larger infarcts. In CE stroke, pre‐stroke aspirin use was ~50% less frequently associated with early neurological deterioration, which was not the case for LAA or SVO stroke. The incidence of hemorrhagic transformation did not differ significantly between the aspirin group and non‐aspirin group. Taken together with the finding that aspirin users (particularly, patients who had LAA and CE stroke) had lower thrombus burden, the results of this multicenter study support the notion that pre‐stroke aspirin use (vs nonuse) could lead to more favorable post‐stroke outcomes (~30% higher likelihood in this study), particularly in large arterial strokes.
Previously, we reported that pre‐stroke aspirin use was associated with less severe LAA stroke and better functional outcomes at discharge after ischemic stroke. 11 , 39 This stroke severity‐related analysis is supported by the present study demonstrating the association between aspirin use before LAA stroke and infarct volume, which is a major determinant of initial stroke severity. 40 In addition, the work we performed using a large‐scale imaging dataset aligns with a previous study (n = 310) showing an inverse association between aspirin resistance and infarct volume in pre‐stroke aspirin users, which was significant in LAA stroke but not in SVO or CE stroke. 41 Furthermore, although our previous work used discharge as a meaningful time‐point, the present study reports that the associations between pre‐stroke aspirin use and good functional outcome in LAA stroke persists to at least 3 months, which is the most widely used time point in acute stroke research.
Our current study shows that pre‐stroke aspirin use is associated with better functional outcome in CE stroke at 3 months; our earlier work, however, did not show functional improvement at discharge. The previous study included patients who received revascularization therapy, which may have masked the effect of aspirin pretreatment on functional improvement in CE strokes, unlike in LAA strokes. In the present study, pre‐stroke aspirin use was associated with significantly lower odds of early neurological deterioration in CE stroke (by 44%) but not LAA stroke. Patients who had a CE stroke underwent MRI earlier than patients with other subtypes (with a median difference of 8–10 hours) due to shorter onset‐to‐hospital arrival times and/or more obvious symptoms. Thus, patients who had a CE stroke may have been more prone to developing early neurological deterioration or having early neurological deterioration detected during admission and thus were more likely to benefit (or show benefit) from aspirin pretreatment. We speculate that patients with LAA stroke probably have less severe initial symptoms, visit the hospital later, and more frequently exhibit early neurological deterioration before admission, compared with patients with CE stroke.
There are conflicting reports regarding the association between pre‐stroke aspirin use and hemorrhagic transformation. 11 , 42 , 43 In the present study, multivariable analysis showed that pre‐stroke aspirin use was not significantly associated with hemorrhagic transformation. Considering that infarct volume is a key factor in hemorrhagic transformation, 44 we theorize that pre‐stroke aspirin use may have reduced infarct growth and thus prevented early neurological deterioration and offset the antiplatelet drug‐related risk of hemorrhagic transformation.
Antiplatelet therapy is known to preferentially reduce the incidence of LAA stroke rather than CE or SVO stroke. 45 Yet, previous research has shown that platelet activation plays important roles in CE as well as in LAA stroke, 46 , 47 which may accord with results on aspirin‐related lower thrombus burden (as was also demonstrated in our recent preclinical study on carotid artery thrombosis 48 ) and less frequent early neurological deterioration in CE stroke. On the other hand, platelet activation appears to be less important in SVO stroke, which mainly results from chronic hypertension‐mediated lipohyalinosis of small arterioles. 49 Moreover, because infarct volume is already small in SVO stroke, aspirin may not have an effect.
That pre‐stroke aspirin use did not significantly associate with infarct volume in CE stroke appears to conflict with a previous study that reported an inverse relation between pre‐stroke antiplatelet use and stroke severity in CE stroke. 50 This discrepancy remains to be clarified and might be partly attributed to differences in the study population (Korean patients with first‐ever ischemic stroke vs Canadian patients with first‐ever or recurrent ischemic stroke) and excluding versus including patients who received revascularization therapy.
Recent clinical trials failed to demonstrate the efficacy of aspirin for primary stroke prevention, despite enrolling participants with high‐risk profiles, 8 , 51 whereas our study shows that aspirin pretreatment was associated with lower infarct volume, less frequent early neurological deterioration, and better 3‐month functional independence in a large number of patients with first‐ever ischemic stroke. Further investigation is required to test if the aspirin‐related beneficial effects might hold true in primary stroke prevention for high‐risk individuals.
The strengths of this study include its large sample size, consecutive patient enrollment, prospective systematic data collection, regular data audits, and rigorous adjustments for covariates. We also acknowledge, however, that this study has some limitations. First, we did not have aspirin resistance data, which may have improved interpretation. Second, we only included Korean patients with stroke with or without pre‐stroke aspirin use; thus extrapolating our findings to other ethnicities or other antiplatelet medications should be undertaken with caution. Third, we excluded patients who received recanalization therapy, which could potentially increase the risk of aspirin pretreatment‐related hemorrhagic transformation. Excluding these patients was unavoidable, because MRI was performed before recanalization in some patients and after in others. However, pre‐stroke aspirin did not appear to increase the risk of symptomatic hemorrhagic transformation after intravenous thrombolysis or mechanical thrombectomy in these patients with first‐ever ischemic stroke (aspirin use 3.4% [25 / 885] vs aspirin nonuser 2.8% [8 / 234], p = 0.63). Fourth, we did not obtain the information regarding dose and duration of aspirin use. Fifth, unmeasured confounders that could influence the use of aspirin – such as risk of gastrointestinal bleeding, annual household income, health behavior beliefs, and social environment – may have affected study results. 52 , 53 Last, compared with age‐specific brain templates, the Montreal Neurological Institute (MNI) template shows less age‐related atrophy, potentially leading to underestimation of lesion volumes (percentage of the template brain parenchymal volume) in elderly patients, 54 although our semi‐automatic method for the registration of ischemic lesions accounts for cerebral atrophy and distortion. 55
In conclusion, we found that pre‐stroke aspirin use associated with better functional outcomes in patients with first‐ever ischemic large arterial stroke, probably by reducing infarct volume or the incidence of early neurological deterioration. These effects may be partly due to aspirin‐mediated decrease in thrombus burden and were achieved without increasing the risk of hemorrhagic transformation. These data motivate prospective clinical trials to investigate if aspirin improves stroke outcomes in addition to preventing stroke, particularly for individuals with higher risk of developing large arterial cerebral infarction.
Author Contributions
W.‐S.R. and D.‐E.K. contributed to the conception and design of the study. All authors contributed to the acquisition, analyzing, and interpretation of data. W.‐S.R., D.W., M.N., and D.‐E.K. contributed to drafting the text and preparing the figures. All authors contributed to the critical revisions of the manuscript for important intellectual content.
Potential Conflicts of Interest
The authors declared no conflict of interest.
Supporting information
Appendix S1. Supporting information
Acknowledgements
This study was supported by the Global Research Lab program (NRF‐2015K1A1A2028228), the National Priority Research Center program (NRF‐2021R1A6A1A03038865), and individual researcher grants (NRF‐2018R1C1B5086249 and NRF‐2020R1A2C3008295) of the National Research Foundation, the Korea Medical Device Development Fund grant (Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety, Project Number: 9991006771, KMDF_PR_202012B07‐0), and the National Center for Standard Reference Data, funded by the Korean government, Republic of Korea. [Correction added on October 20, 2021, after first online publication: acknowledgement details were updated.]
References
- 1. Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378:708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jovin TG, Nogueira RG, Investigators D. Thrombectomy 6 to 24 hours after stroke. N Engl J Med 2018;378:1161–1162. [DOI] [PubMed] [Google Scholar]
- 3. Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11–21. [DOI] [PubMed] [Google Scholar]
- 4. Lee KJ, Kim BJ, Kim DE, et al. Nationwide estimation of eligibility for endovascular Thrombectomy based on the DAWN trial. J Stroke 2018;20:277–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296–2306. [DOI] [PubMed] [Google Scholar]
- 6. Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20. [DOI] [PubMed] [Google Scholar]
- 7. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med 2018;379:1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Group ASC , Bowman L, Mafham M, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018;379:1529–1539. [DOI] [PubMed] [Google Scholar]
- 9. Ridker PM. Should aspirin be used for primary prevention in the post‐statin era? N Engl J Med 2018;379:1572–1574. [DOI] [PubMed] [Google Scholar]
- 10. Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke 2009;40:e573–e583. [DOI] [PubMed] [Google Scholar]
- 11. Park JM, Kang K, Cho YJ, et al. Comparative effectiveness of prestroke aspirin on stroke severity and outcome. Ann Neurol 2016;79:560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim DE, Park JH, Schellingerhout D, et al. Mapping the supratentorial cerebral arterial territories using 1160 large artery infarcts. JAMA Neurol 2019;76:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim DE, Ryu WS, Schellingerhout D, et al. Estimation of acute infarct volume with reference maps: a simple visual tool for decision making in thrombectomy cases. J Stroke 2019;21:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ryu WS, Schellingerhout D, Ahn HS, et al. Hemispheric asymmetry of white matter hyperintensity in association with lacunar infarction. J Am Heart Assoc 2018;7:e010653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ryu WS, Schellingerhout D, Hong KS, et al. White matter hyperintensity load on stroke recurrence and mortality at 1 year after ischemic stroke. Neurology 2019;93:e578–e589. [DOI] [PubMed] [Google Scholar]
- 16. Ryu WS, Woo SH, Schellingerhout D, et al. Grading and interpretation of white matter hyperintensities using statistical maps. Stroke 2014;45:3567–3575. [DOI] [PubMed] [Google Scholar]
- 17. Ryu WS, Woo SH, Schellingerhout D, et al. Stroke outcomes are worse with larger leukoaraiosis volumes. Brain 2017;140:158–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim BJ, Park JM, Kang K, et al. Case characteristics, hyperacute treatment, and outcome information from the clinical research center for stroke‐fifth division registry in South Korea. J Stroke 2015;17:38–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ko Y, Lee S, Chung JW, et al. MRI‐based algorithm for acute ischemic stroke subtype classification. J Stroke 2014;16:161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 21. Paciaroni M, Agnelli G, Corea F, et al. Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome: results of a prospective multicenter study. Stroke 2008;39:2249–2256. [DOI] [PubMed] [Google Scholar]
- 22. Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke‐monitoring study (SITS‐MOST): an observational study. Lancet 2007;369:275–282. [DOI] [PubMed] [Google Scholar]
- 23. Darcourt J, Withayasuk P, Vukasinovic I, et al. Predictive value of susceptibility vessel sign for arterial recanalization and clinical improvement in ischemic stroke. Stroke 2019;50:512–515. [DOI] [PubMed] [Google Scholar]
- 24. Flacke S, Urbach H, Keller E, et al. Middle cerebral artery (MCA) susceptibility sign at susceptibility‐based perfusion MR imaging: clinical importance and comparison with hyperdense MCA sign at CT. Radiology 2000;215:476–482. [DOI] [PubMed] [Google Scholar]
- 25. Cho YH, Park HS, Choi JH, et al. Diagnostic value of thrombus size on T2(*)‐weighted gradient Echo imaging in acute middle cerebral artery occlusion. J Cerebrovasc Endovasc Neurosurg 2014;16:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rose S, van der Laan M. A double robust approach to causal effects in case‐control studies. Am J Epidemiol 2014;179:663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Funk MJ, Westreich D, Wiesen C, et al. Doubly robust estimation of causal effects. Am J Epidemiol 2011;173:761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shimoyama T, Kimura K, Uemura J, et al. Elevated glucose level adversely affects infarct volume growth and neurological deterioration in non‐diabetic stroke patients, but not diabetic stroke patients. Eur J Neurol 2014;21:402–410. [DOI] [PubMed] [Google Scholar]
- 29. Yaghi S, Elkind MS. Lipids and cerebrovascular disease: research and practice. Stroke 2015;46:3322–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gokcay F, Arsava EM, Baykaner T, et al. Age‐dependent susceptibility to infarct growth in women. Stroke 2011;42:947–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kufner A, Ali HF, Ebinger M, et al. The smoking paradox in ischemic stroke patients treated with intra‐arterial thrombolysis in combination with mechanical thrombectomy‐VISTA‐endovascular. PLoS One 2021;16:e0251888. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32. Goldhoorn RB, Verhagen M, Dippel DWJ, et al. Safety and outcome of endovascular treatment in Prestroke‐dependent patients. Stroke 2018;49:2406–2414. [DOI] [PubMed] [Google Scholar]
- 33. Hong KS, Lee JS. Statins in acute ischemic stroke: a systematic review. J Stroke 2015;17:282–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Woodhouse LJ, Manning L, Potter JF, et al. Continuing or temporarily stopping prestroke antihypertensive medication in acute stroke: an individual patient data meta‐analysis. Hypertension 2017;69:933–941. [DOI] [PubMed] [Google Scholar]
- 35. Henninger N, Lin E, Haussen DC, et al. Leukoaraiosis and sex predict the hyperacute ischemic core volume. Stroke 2013;44:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ay H, Arsava EM, Rosand J, et al. Severity of leukoaraiosis and susceptibility to infarct growth in acute stroke. Stroke 2008;39:1409–1413. [DOI] [PubMed] [Google Scholar]
- 37. Hao L, Naiman DQ. Quantile Regression. Thousand Oaks, CA: Sage Publications, 2007. [Google Scholar]
- 38. Koenker R, Chernozhukov V, He X, et al. Handbook of Quantile Regression. Boca Raton, FL: CRC Press, Taylor & Francis Group, 2018. [Google Scholar]
- 39. Kim WJ, Ko Y, Yang MH, et al. Differential effect of previous antiplatelet use on stroke severity according to stroke mechanism. Stroke 2010;41:1200–1204. [DOI] [PubMed] [Google Scholar]
- 40. Saver JL, Johnston KC, Homer D, et al. Infarct volume as a surrogate or auxiliary outcome measure in ischemic stroke clinical trials. The RANTTAS Investigators. Stroke 1999;30:293–298. [DOI] [PubMed] [Google Scholar]
- 41. Oh MS, Yu KH, Lee JH, et al. Aspirin resistance is associated with increased stroke severity and infarct volume. Neurology 2016;86:1808–1817. [DOI] [PubMed] [Google Scholar]
- 42. Jaillard A, Cornu C, Durieux A, et al. Hemorrhagic transformation in acute ischemic stroke. The MAST‐E study MAST‐E Group. Stroke 1999;30:1326–1332. [DOI] [PubMed] [Google Scholar]
- 43. Cho BH, Kim JT, Chang J, et al. Prediction of hemorrhagic transformation in acute ischaemic stroke by micro‐ and macroalbuminuria after intravenous thrombolysis. Eur J Neurol 2013;20:1145–1152. [DOI] [PubMed] [Google Scholar]
- 44. Larrue V, von Kummer R, del Zoppo G, et al. Hemorrhagic transformation in acute ischemic stroke. Potential contributing factors in the European Cooperative Acute Stroke Study. Stroke 1997;28:957–960. [DOI] [PubMed] [Google Scholar]
- 45. Rajkumar CA, Floyd CN, Ferro A. Antiplatelet therapy as a modulator of stroke aetiology: a meta‐analysis. Br J Clin Pharmacol 2015;80:331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sato Y, Ishibashi‐Ueda H, Iwakiri T, et al. Thrombus components in cardioembolic and atherothrombotic strokes. Thromb Res 2012;130:278–280. [DOI] [PubMed] [Google Scholar]
- 47. Marder VJ, Chute DJ, Starkman S, et al. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke 2006;37:2086–2093. [DOI] [PubMed] [Google Scholar]
- 48. Kim J, Jang HJ, Schellingerhout D, et al. Short‐term cessation of dabigatran causes a paradoxical prothrombotic state. Ann Neurol 2020;89:444–458. [DOI] [PubMed] [Google Scholar]
- 49. Fisher CM. Lacunar strokes and infarcts: a review. Neurology 1982;32:871–876. [DOI] [PubMed] [Google Scholar]
- 50. O'Donnell M, Oczkowski W, Fang J, et al. Preadmission antithrombotic treatment and stroke severity in patients with atrial fibrillation and acute ischaemic stroke: an observational study. Lancet Neurol 2006;5:749–754. [DOI] [PubMed] [Google Scholar]
- 51. Zheng AS, Churilov L, Colley RE, et al. Association of aspirin resistance with increased stroke severity and infarct size. JAMA Neurol 2013;70:208–213. [DOI] [PubMed] [Google Scholar]
- 52. Fernandez‐Jimenez R, Wang TJ, Fuster V, et al. Low‐dose aspirin for primary prevention of cardiovascular disease: use patterns and impact across race and ethnicity in the Southern Community Cohort Study. J Am Heart Assoc 2019;8:e013404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mainous AG, Tanner RJ, Shorr RI, et al. Use of aspirin for primary and secondary cardiovascular disease prevention in the United States, 2011‐2012. J Am Heart Assoc 2014;3:e000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rorden C, Bonilha L, Fridriksson J, et al. Age‐specific CT and MRI templates for spatial normalization. Neuroimage 2012;61:957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kim DE, Park KJ, Schellingerhout D, et al. A new image‐based stroke registry containing quantitative magnetic resonance imaging data. Cerebrovasc Dis 2011;32:567–576. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information


