Abstract
Objective
To report post hoc results on how adjustments to baseline antiseizure medications (ASMs) in a subset of study sites (10 US sites) from a long‐term, open‐label phase 3 study of adjunctive cenobamate affected tolerability, efficacy, and retention.
Methods
Patients with uncontrolled focal seizures taking stable doses of one to three ASMs were administered increasing doses of cenobamate (12.5, 25, 50, 100, 150, 200 mg/day) over 12 weeks at 2‐week intervals (target dose = 200 mg/day). Further increases to 400 mg/day by 50 mg/day biweekly increments were allowed during maintenance phase. Dose adjustments of cenobamate and concomitant ASMs were allowed. Data were assessed until last visit, at data cut‐off, on or after September 1, 2019.
Results
A total of 240 patients meeting eligibility criteria were assessed (median [max] exposure 30.2 [43.0] months), with 177 patients continuing cenobamate at data cut‐off. Most common baseline concomitant ASMs were lacosamide, levetiracetam, lamotrigine, zonisamide, and clobazam. For most baseline concomitant ASMs, ~70% of patients taking that ASM were continuing cenobamate at data cut‐off. Patients continuing cenobamate had greater mean ASM dose reductions and percent dose changes from baseline vs those who discontinued. Of patients continuing cenobamate, 24.6% discontinued one or more concomitant ASMs completely. Dose decreases for all concomitant ASMs generally occurred during titration or early maintenance phases and were mostly due to central nervous system (CNS)–related adverse events such as somnolence, dizziness, unsteady gait, and fatigue. Responder rates from ≥50% through 100% for patients continuing cenobamate were generally similar regardless of concomitant ASMs (of those most commonly taken), with ~81% being ≥50% responders and ~12% achieving 100% seizure reduction in the maintenance phase, which lasted up to 40.2 (median = 29.5) months.
Significance
Concomitant ASM dose reductions were associated with more patients remaining on cenobamate. This is likely due to efficacy and improved tolerability, with overall reduced concomitant drug burden in patients with uncontrolled seizures despite taking one to three baseline concomitant ASMs.
Keywords: antiepileptics, antiseizure medications, cenobamate, concomitant medications, focal epilepsy
Key Points.
Patients who continued cenobamate tended to have greater reductions in concomitant antiseizure medication (ASM) doses than patients who discontinued cenobamate.
Concomitant phenytoin, phenobarbital, clobazam, valproate, and lacosamide were decreased earliest in the clinical study.
Cenobamate's efficacy was demonstrated by many patients remaining seizure‐free for long periods and high retention rates even after reducing or completely discontinuing one or more of their concomitant ASMs.
As experience was accumulated and physicians learned about the potential interactions during this study, concomitant ASM doses were reduced for many patients very early on in cenobamate titration.
1. INTRODUCTION
The ultimate goal of epilepsy treatment is to achieve seizure freedom (100% seizure reduction) with minimal adverse events (AEs). For drug‐refractory patients, polytherapy has become widespread, even though the probability of additional efficacy diminishes with each successive antiseizure medication (ASM) regimen tried. 1 , 2 Moreover, evidence indicates that reducing treatment with one or more ASMs can occur in drug‐resistant patients who are receiving polytherapy, with no increases in seizure frequency. 3 Despite this, many patients continue to experience uncontrolled seizures and increased side effects due to polypharmacy that provides little additional therapeutic value.
Taking multiple concomitant ASMs can cause drug interactions that may lead to changes in efficacy and safety, including increases in AEs. 4 , 5 Adjustments in dosing and/or medications in patients taking multiple drugs can help optimize efficacy while minimizing side effects. However, with numerous possible drug combinations and the individualized nature of epilepsy treatment, physicians may find themselves in a continual cycle of transitional polytherapy with refractory patients.
For new medications, it is difficult to assess dose adjustments and achieve optimized efficacy and safety in the customary double‐blind, fixed‐dose, adjunctive therapy clinical trials. 6 , 7 This can confound determination of the true efficacy and tolerability of an adjunctive ASM, given the variable effects of concomitant medications and treatment combinations. Medication management during transitional therapy with new drugs thus requires close monitoring of a patient's AEs and efficacy along with an understanding of possible drug interactions and their effects on plasma concentrations of multiple ASMs.
Flexible‐dose open‐label studies allow for medication adjustments, including dose adjustments of concomitant ASMs. Data from these studies provide insights into the efficacy and tolerability of a newly introduced ASM, given that they measure all reasons for discontinuation, including lack of efficacy and AEs. 7 Moreover, retention data from these open‐label studies can serve as a surrogate for efficacy and provide information about which AEs patients are able to tolerate vs those they cannot.
Cenobamate (XCOPRI) is an ASM that is approved by the US Food and Drug Administration (FDA) for the treatment of adults with focal (partial‐onset) seizures. 8 Although cenobamate's mechanism of action is not fully understood, it has been shown to preferentially inhibit the persistent sodium current and to be a positive allosteric modulator of the γ‐aminobutyric acid A (GABAA) ion channel, through a non‐benzodiazepine GABAA receptor site. 8 , 9 , 10 This dual mechanism of action suggests that cenobamate may have the potential to both prevent seizure initiation and limit seizure spread. 11 , 12 , 13 , 14 , 15 , 16 , 17
Two phase 2, placebo‐controlled, randomized, double‐blind clinical studies (NCT01397968 [C013; Chung et al. 2020 18 ] and NCT01866111 [C017; Krauss et al. 2020 19 ]), in adults with uncontrolled focal epilepsy who had previously failed multiple ASMs, demonstrated substantial reductions in seizure frequency and high responder rates, including seizure freedom, with adjunctive cenobamate at 200 mg/day (C013) and 100, 200, and 400 mg/day (C017). 18 , 19 Cenobamate was generally safe and well tolerated across studies, and the most common AEs were central nervous system (CNS) related. The two adequate and well‐controlled studies (C013 and C017) along with a large (N = 1340) long‐term, open‐label, phase 3 study that focused on safety (NCT02535091 [C021; Sperling et al. 2020 20 ]) supported the FDA approval of the efficacy and safety of cenobamate.
The C021 study differed from the randomized placebo‐controlled trials in that dose adjustments to concomitant ASMs were allowed and a titration scheme was used that started with a low dose of cenobamate (12.5 mg/day), and the dose was increased slowly (titrated up every other week). 20 Although seizure outcomes were not assessed in the C021 study, given the utility of data on long‐term efficacy, a protocol amendment allowed post hoc collection of seizure data. The current post hoc analysis examined how dose adjustments to baseline concomitant ASMs from 10 US study sites of the C021 study affected tolerability, efficacy, and retention.
2. METHODS
2.1. Study design and patients
2.1.1. Full C021 study
Detailed study design and patient eligibility for the C021 study have been published previously. 20 Briefly, C021 was a multicenter, open‐label safety study in adults with uncontrolled focal seizures despite taking stable doses of one to three ASMs. Focal epilepsy was defined by International League Against Epilepsy (ILAE) seizure classification criteria. 20 , 21 , 22
A screening period of up to 21 days was followed by an open‐label treatment period consisting of a 12‐week titration phase, followed by an open‐label maintenance phase. Patients initiated cenobamate treatment at 12.5 mg/day for 2 weeks, followed by 25 mg/day for 2 weeks and 50 mg/day for 2 weeks; doses were then increased by 50 mg/day biweekly to a target dose of 200 mg/day (Figure S1). 20 Further increases up to 400 mg/day using biweekly increments of 50 mg/day were allowed during the maintenance phase. Reductions below 200 mg were allowed by investigators’ judgment, with a minimum allowed dose of 50 mg/day. Cenobamate monotherapy was not allowed. Patient visits occurred biweekly for 16 weeks and then every 1 to 3 months.
2.1.2. Post hoc analysis
Data for this post hoc analysis were collected from US sites in which ≥11 patients had started treatment with cenobamate. Eligible patients were required to have experienced one or more focal aware motor (FAM), focal impaired awareness (FIA), or focal to bilateral tonic‐clonic (FBTC) seizure per 13 weeks prior to screening visit; to have seizure data of FAM, FIA, or FBTC seizures while on treatment; and to have consistent documentation of raw seizure data. In addition, patients were required to have high‐quality seizure data for ≥85% of the total length of the study.
Concomitant ASMs (except phenobarbital and phenytoin) could be removed, added, or adjusted throughout the trial, and cenobamate doses could be adjusted during the titration phase, as clinically needed. For patients taking concomitant phenobarbital and phenytoin, no other concomitant ASMs could be lowered and no adjustments to the cenobamate titration schedule could be made during the titration phase. Phenobarbital and phenytoin dose adjustments were permitted and anticipated to be necessary, with dose reductions of up to ~50% of the original dose for phenytoin. 8 Once the maintenance phase was reached, adjustments to any concomitant ASM doses were allowed in patients taking concomitant phenobarbital or phenytoin.
Numbers of baseline concomitant ASMs, dose adjustments to concomitant ASMs, cenobamate dose at initiation of concomitant ASM dose reduction, and efficacy (using responder rates) by concomitant ASMs were compared among the following four patient outcome groups: all analysis patients (n = 240; “all patients”), all analysis patients in the maintenance phase (n = 214; “maintenance population”), patients continuing cenobamate at data cut‐off (ie, still taking cenobamate, n = 177), and patients who discontinued cenobamate (n = 63).
Duration of 100% seizure reduction was assessed moving backward from the last clinic visit (ie, interval includes last clinic visit). Patients with any missing seizure frequency data could not be counted as having 100% seizure reduction. For ≥50%, ≥75%, and ≥90% responder rates, the seizure frequency was analyzed as observed with imputation for missing seizure data during the assessed interval. Baseline demographics, exposure, reasons for discontinuation, rates of retention, treatment‐emergent adverse events (TEAEs), and efficacy by responder rates were summarized using descriptive statistics.
The C021 study was conducted in accordance with the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice guidelines. 20 , 23 An independent ethics committee or institutional review board reviewed and approved the study protocol, amendments, and post hoc analysis at each site in agreement with local requirements. Written informed consent was obtained from each patient prior to study participation.
3. RESULTS
3.1. Baseline demographics, exposure, and baseline ASMs
Among the 1340 patients within the larger C021 study, 12 US study sites (n = 299) were eligible for inclusion in the post hoc study. Two sites were unable to participate, resulting in 10 sites that were included in the post hoc analysis, with a total of 255 patients. Of these patients, 15 did not meet the post hoc inclusion criteria, leaving 240 patients who could be evaluated. The first of these patients was enrolled on July 20, 2016, and the last patient was enrolled on January 19, 2018.
Patients included in the current post hoc analysis had a mean age of 41.8 years, with a somewhat higher percentage of male patients (Table S1). The median duration of cenobamate exposure was 30.2 months (range: 0.10–43.0 months) including the titration phase, and 29.5 months (range: 0.80–40.2) for the maintenance phase alone. Of the analysis population (all patients, n = 240), 177 (73.8%) were still taking cenobamate (patients continuing cenobamate) at the data cut‐off visit on or after September 1, 2019 (median duration of exposure 32.9 months [range: 22.1–43.0 months]). Mean baseline seizure frequency/28 days was 18.1 (median 2.8) for all patients, 88% of whom (211/240) were taking two or three other ASMs prior to receiving the first cenobamate dose (Table S1). Demographic and disease characteristics of the 240 patients were generally similar to the remaining patient population from the primary study, except that there was a greater preponderance of concomitant lacosamide, clobazam, and zonisamide.
3.2. Patients continuing cenobamate vs patients who discontinued cenobamate, by concomitant ASM
Baseline concomitant ASMs, number of patients who continued or discontinued cenobamate by concomitant ASM, mean doses, dose reductions, and percentage dose change for each ASM are shown in Table 1. Greater percentages of patients remained on cenobamate than discontinued cenobamate for all concomitant ASMs taken at baseline, except for patients on phenobarbital (n = 7) and rufinamide (n = 2). For most baseline concomitant ASMs, ~70% of patients continued cenobamate treatment. In addition, the ASM dose reductions were greater in patients continuing cenobamate compared with those who discontinued cenobamate. The most common concomitant ASMs taken at baseline were lacosamide, levetiracetam, lamotrigine, zonisamide, and clobazam (Table 1). For these ASMs, mean dose reductions and percentage change in dose from baseline to last dose were greater in patients continuing cenobamate vs those who discontinued (Figure 1A–E). The greater study retention (for the five most common baseline ASMs, retention range: 71.9% to 78.9% for those continuing cenobamate; 21.1% to 28.1% for those who discontinued cenobamate) observed among patients with larger concomitant ASM dose reductions (for the five most common baseline ASMs, % dose change range: −65.8% to −23.4% for those continuing cenobamate; −7.8% to −25.0% for those who discontinued cenobamate) suggests that cenobamate efficacy was not impaired by decreasing the dose of concomitant ASMs, and that cenobamate tolerability may have improved with greater concomitant ASM dose reductions.
TABLE 1.
Patients taking concomitant ASMs at baseline, patients continuing cenobamate at data cut‐off, or patients who discontinued cenobamate, and mean dose adjustments by ASM at time of post hoc analysis
| Concomitant ASM | Taking at baseline, n (%) a | Patients continuing cenobamate, n (%) b | Patients who discontinued cenobamate, n (%) b | Mean dose at baseline in patients continuing cenobamate, mg | Mean last dose in patients continuing cenobamate, mg | Mean dose adjustment in patients continuing cenobamate, change in mg (% dose change) | Mean dose adjustment in patients who discontinued cenobamate, change in mg (% dose change) |
|---|---|---|---|---|---|---|---|
| Lacosamide | 98 (40.8) | 77 (78.6) | 21 (21.4) | 513.3 | 336.7 | −176.6 (−34.9) | −98.8 (−18.6) |
| Levetiracetam | 89 (37.1) | 64 (71.9) | 25 (28.1) | 2746.1 | 1898.4 | −847.7 (−30.9) | −350.0 (−11.5) |
| Lamotrigine | 66 (27.5) | 48 (72.7) | 18 (27.3) | 487.0 | 377.6 | −109.4 (−23.4) | −101.4 (−21.6) |
| Zonisamide | 39 (16.3) | 30 (76.9) | 9 (23.1) | 473.3 | 333.3 | −140.0 (−32.6) | −33.3 (−7.8) |
| Clobazam | 38 (15.8) | 30 (78.9) | 8 (21.1) | 38.0 | 12.7 | −25.3 (−65.8) | −11.2 (−25.0) |
| Carbamazepine | 24 (10.0) | 16 (66.7) | 8 (33.3) | 906.3 | 443.8 | −462.5 (−46.3) | −287.5 (−29.8) |
| Topiramate | 23 (9.6) | 15 (65.2) | 8 (34.8) | 336.7 | 253.3 | −83.4 (−21.7) | −193.8 (−35.3) |
| Valproate c | 21 (8.8) | 15 (71.4) | 6 (28.6) | 1400.0 | 950.0 | −450.0 (−30.0) | −125.0 (−16.7) |
| Oxcarbazepine | 20 (8.3) | 15 (75.0) | 5 (25.0) | 1870.0 | 1330.0 | −540.0 (−34.6) | −360.0 (−31.5) |
| Eslicarbazepine | 20 (8.3) | 13 (65.0) | 7 (35.0) | 1307.7 | 600.0 | −707.7 (−50.6) | 0.0 (0.0) |
| Perampanel | 19 (7.9) | 19 (100.0) | 0 (0.0) | 8.0 | 3.4 | −4.6 (−62.3) | 0.0 (0.0) |
| Phenytoin | 18 (7.5) | 12 (66.7) | 6 (33.3) | 390.8 | 139.2 | −251.6 (−60.8) | −238.3 (−50.6) |
| Brivaracetam | 14 (5.8) | 11 (78.6) | 3 (21.4) | 195.5 | 109.1 | −86.4 (−42.4) | −12.5 (−8.3) |
| Pregabalin | 12 (5.0) | 11 (91.7) | 1 (8.3) | 472.7 | 259.1 | −213.6 (−50.8) | −300.0 (−100.0) |
| Clonazepam | 7 (2.9) | 5 (71.4) | 2 (28.6) | 0.8 | 0.9 | +0.1 (20.0) | 0.0 (0.0) |
| Phenobarbital | 7 (2.9) | 3 (42.9) | 4 (57.1) | 116.4 | 75.6 | −40.8 (−40.0) | −101.7 (−58.3) |
| Gabapentin | 4 (1.7) | 3 (75.0) | 1 (25.0) | 1566.7 | 300.0 | −1266.7 (−75.0) | 0.0 (0.0) |
| Clorazepate | 4 (1.7) | 3 (75.0) | 1 (25.0) | 27.5 | 18.8 | −8.7 (−31.9) | −7.5 (−40.0) |
| Felbamate | 3 (1.3) | 3 (100.0) | 0 (0.0) | 2733.3 | 2200.0 | −533.3 (−33.3) | 0.0 (0.0) |
| Rufinamide | 2 (0.8) | 1 (50.0) | 1 (50.0) | 800.0 | 0.0 | −800.0 (−100.0) | 0.0 (0.0) |
Abbreviation: ASMs, antiseizure medications.
Most patients were taking >1 concomitant ASM. Percentages calculated from a total of 240 patients.
Percentages calculated from total number of patients taking the specific concomitant drug at baseline.
Includes valproic acid, sodium valproate, and divalproex sodium.
FIGURE 1.
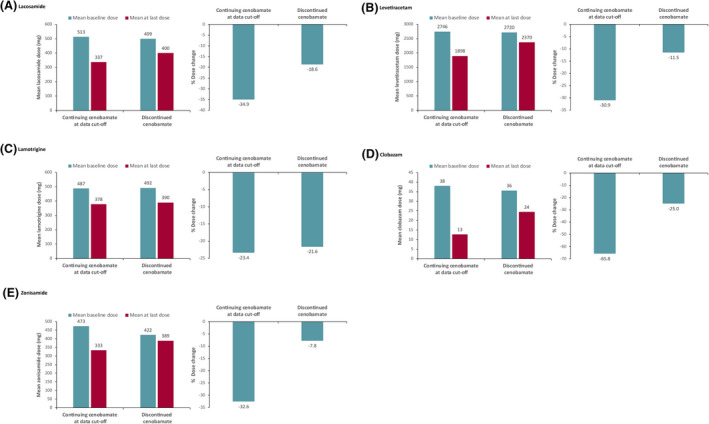
Mean dose at baseline vs at last dose and percent dose adjustment from baseline to last dose in patients continuing cenobamate vs patients who discontinued cenobamate by concomitant (A) lacosamide, (B) levetiracetam, (C) lamotrigine, (D) clobazam, and (E) zonisamide
Among patients continuing cenobamate, 97/395 (24.6%; most were taking more than one concomitant ASM) completely discontinued one or more of their concomitant ASMs (Table 2). Lacosamide, levetiracetam, and perampanel were the ASMs discontinued in the greatest number of patients overall, with n = 18, n = 13, and n = 11 patients, respectively, discontinuing these concomitant ASMs completely.
TABLE 2.
Patients continuing cenobamate at data cut‐off who discontinued their baseline concomitant ASMs
| Concomitant ASM a , n/N (%) | Patients continuing cenobamate (n = 177) |
|---|---|
| Lacosamide | 18/77 (23.4) |
| Levetiracetam | 13/64 (20.3) |
| Perampanel | 11/19 (57.9) |
| Clobazam | 8/30 (26.7) |
| Lamotrigine | 7/48 (14.6) |
| Zonisamide | 6/30 (20.0) |
| Carbamazepine | 5/16 (31.3) |
| Pregabalin | 5/11 (45.5) |
| Valproate b | 4/15 (26.7) |
| Oxcarbazepine | 4/15 (26.7) |
| Phenytoin | 4/12 (33.3) |
| Brivaracetam | 4/11 (36.4) |
| Eslicarbazepine | 3/13 (23.1) |
| Gabapentin | 2/3 (66.7) |
| Felbamate | 1/3 (33.3) |
| Phenobarbital | 1/3 (33.3) |
| Topiramate | 1/15 (7.0) |
| Clonazepam | 0/5 (0.0) |
| Clorazepate | 0/3 (0.0) |
Abbreviation: ASM, antiseizure medication.
Most patients were taking >1 concomitant ASM.
Includes valproic acid, sodium valproate, and divalproex sodium.
3.3. Timing and reason for dose adjustments to concomitant ASMs
Doses of concomitant phenytoin, phenobarbital, clobazam, valproate, and lacosamide were reduced earliest for all patients (Figure 2). Concomitant ASM doses were generally reduced during titration or early maintenance phases of the study. The study visits at which these concomitant ASMs were reduced and the mean cenobamate dose at the time of the dose reduction were generally similar for all patients and those continuing cenobamate (data not shown).
FIGURE 2.
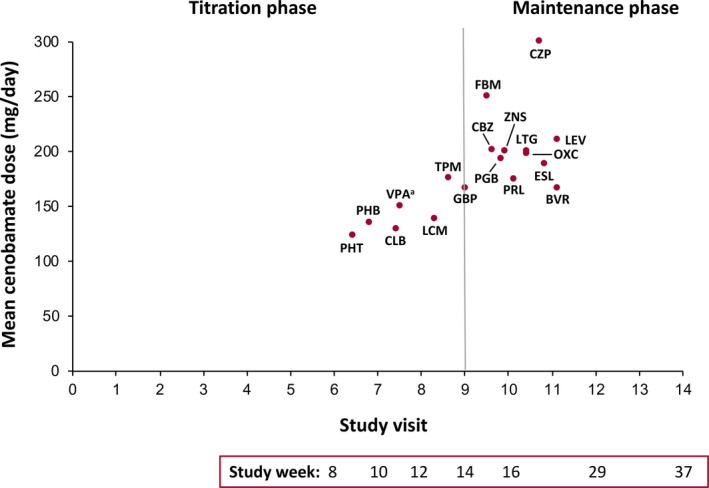
Mean cenobamate dose at initiation of concomitant ASM dose reduction by study visit (all patients, n = 240). aVPA includes valproic acid, sodium valproate, and divalproex sodium. ASM, antiseizure medication; BVR, brivaracetam; CBZ, carbamazepine; CLB, clobazam; CZP, clorazepate; ESL, eslicarbazepine; FBM, felbamate; GBP, gabapentin; LCM, lacosamide; LEV, levetiracetam; LTG, lamotrigine; OXC, oxcarbazepine; PGB, pregabalin; PHB, phenobarbital; PHT, phenytoin; PRL, perampanel; TPM, topiramate; VPA, valproate; ZNS, zonisamide
Doses of concomitant ASMs were most often lowered due to TEAEs. CNS‐related TEAEs such as somnolence, dizziness, and ataxia, as well as fatigue, were cited most often as the reasons for dose decreases in patients continuing cenobamate (Table 3). Other than TEAEs, reasons for dose decreases cited by investigators included reduction of polypharmacy, patient doing well, patient request, consolidation of treatment, and decreased seizure frequency. The TEAEs observed overall, those reported as leading to dose reductions of concomitant ASMs, and the safety profile seen for all patients were generally consistent with those that are known to occur with cenobamate and with the concomitant ASMs taken at baseline (Table 3 and Table S2). The most commonly reported TEAEs in ≥10% of patients were fatigue (34.6%), dizziness (32.1%), and somnolence (29.6%).
TABLE 3.
Adverse events leading to first dose reduction of concomitant ASMs for which ≥11 patients experienced AEs leading to dose reductions of that drug (all patients)
| Concomitant ASM | |
| Adverse event | n/total (%) a |
| Lacosamide, n = 56 | |
| Ataxia | 15/56 (26.8) |
| Dizziness | 12/56 (21.4) |
| Somnolence | 12/56 (21.4) |
| Physician/patient choice (decreased medication load) | 12/56 (21.4) |
| Fatigue | 8/56 (14.3) |
| Lamotrigine, n = 39 | |
| Ataxia | 9/39 (23.1) |
| Fatigue | 9/39 (23.1) |
| Dizziness | 7/39 (17.9) |
| Somnolence | 7/39 (17.9) |
| Levetiracetam, n = 38 | |
| Somnolence | 10/38 (26.3) |
| Physician/patient choice (decreased medication load) | 7/38 (18.4) |
| No decreased reason reported | 6/38 (15.8) |
| Cognitive dysfunction | 4/38 (10.5) |
| Clobazam, n = 28 | |
| Somnolence | 14/28 (50.0) |
| Fatigue | 5/28 (17.9) |
| Ataxia | 5/28 (17.9) |
| Dysarthria | 4/28 (14.3) |
| Zonisamide, n = 18 | |
| Fatigue | 7/18 (38.9) |
| No decreased reason reported | 3/18 (16.7) |
| Somnolence | 2/18 (11.1) |
| Physician/patient choice (decreased medication load) | 2/18 (11.1) |
| Carbamazepine, n = 16 | |
| Dizziness | 4/16 (25.0) |
| Physician/patient choice (decreased medication load) | 4/16 (25.0) |
| Somnolence | 3/16 (18.8) |
| Phenytoin, n = 16 | |
| Somnolence | 5/16 (31.3) |
| Elevated phenytoin levels | 4/16 (25.0) |
| Dizziness | 3/16 (18.8) |
| Ataxia | 3/16 (18.8) |
| Fatigue | 3/16 (18.8) |
| Physician/patient choice (decreased medication load) | 2/16 (12.5) |
| Perampanel, n = 15 | |
| Physician/patient choice (decreased medication load) | 5/15 (33.3) |
| Somnolence | 3/15 (20.0) |
| Dizziness | 2/15 (13.3) |
| Ataxia | 2/15 (13.3) |
| Fatigue | 2/15 (13.3) |
| Dysarthria | 2/15 (13.3) |
| No decreased reason reported | 2/15 (13.3) |
| Oxcarbazepine, n = 13 | |
| Dizziness | 8/13 (61.5) |
| Ataxia | 2/13 (15.4) |
| Diplopia | 2/13 (15.4) |
| Somnolence | 2/13 (15.4) |
| Physician/patient choice (decreased medication load) | 2/13 (15.4) |
| Topiramate, n = 12 | |
| Physician/patient choice (decreased medication load) | 4/12 (33.3) |
| Difficulty finding words | 2/12 (16.7) |
| Cognitive dysfunction | 2/12 (16.7) |
| Fatigue | 2/12 (16.7) |
| Eslicarbazepine, n = 11 | |
| Dizziness | 3/11 (27.3) |
| Ataxia | 2/11 (18.2) |
| Fatigue | 2/11 (18.2) |
Abbreviations: AEs, adverse events; ASM, antiseizure medication.
AEs occurring in ≥10% of patients.
Other ASMs such as carbamazepine, oxcarbazepine, eslicarbazepine, and lamotrigine, which enhance rapid inactivation of sodium channels, usually did not require dose reductions until the cenobamate maintenance phase (range of mean visit number at first dose reduction: 9.5–11.0). Given that cenobamate induces the cytochrome P450 (CYP)3A4 enzyme, 8 which metabolizes these drugs, plasma levels of these ASMs would likely be decreased, resulting in possible reduced TEAEs. Despite these reductions, mean percentage dose changes from study start to endpoint for these sodium channel drugs ranged from −23.4% (lamotrigine) to −50.6% (eslicarbazepine) in patients who received dose reductions (Table 1), and efficacy was generally maintained (data not shown).
3.4. Efficacy
No particular concomitant ASMs were associated with patients achieving seizure freedom when treated with adjunctive cenobamate. Among patients who were seizure‐free for ≥3, ≥6, or ≥12 months (data not shown), the proportion of patients taking any particular concomitant ASM was similar to that of the baseline population (Table 1). The similarity in these percentages suggests that these numbers reflect the proportion of patients on that particular concomitant ASM, and that the observed seizure reduction was due to treatment with cenobamate rather than an effect of the specific baseline ASM taken. Moreover, these seizure‐freedom rates were observed even as doses of concomitant ASMs were being decreased in patients continuing cenobamate, regardless of the specific ASM, further suggesting that cenobamate was effective in reducing seizures.
Responder rates from ≥50% through 100% for patients continuing cenobamate were generally similar regardless of whether patients were taking concomitant lacosamide, levetiracetam, lamotrigine, clobazam, or zonisamide (Figure 3A,B), except that fewer patients on concomitant lamotrigine reached 100% seizure reduction. Percentages of responders by concomitant ASM in the maintenance population (n = 214; median [max] 29.5 [40.2] months) ranged from ~75% of patients for ≥50% response to ~13% for 100% response. For patients continuing cenobamate at data cut‐off by concomitant ASM, ~81% were ≥50% responders and ~12% achieved 100% reduction in seizures for the entire maintenance phase, the duration of which was much longer than what is typically reported in open‐label long‐term extension studies that provide 100% seizure reduction rates.
FIGURE 3.
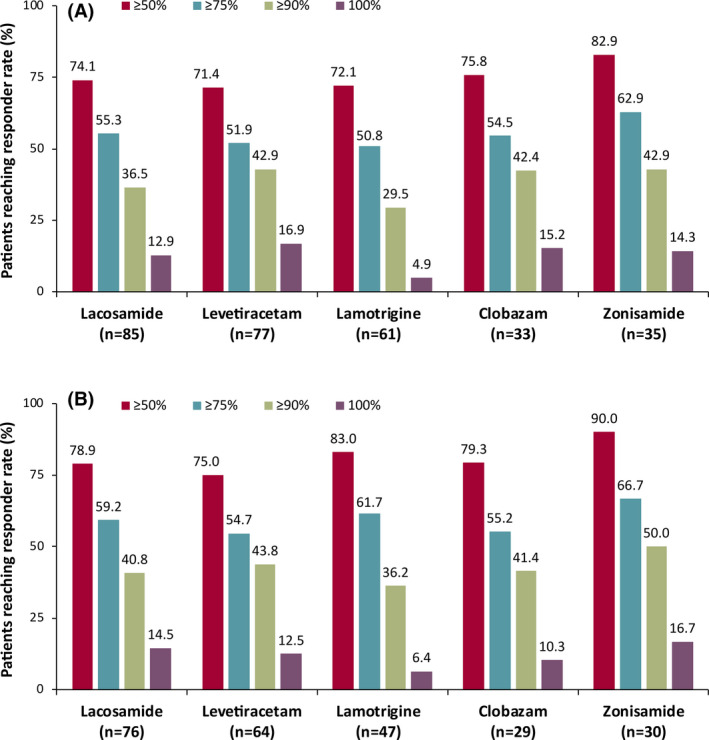
(A) Responder rates during the entire maintenance phase by concomitant ASM among all patients in the maintenance phase (maintenance population, n = 214), and (B) patients continuing cenobamate at data cut‐off (n = 177). Note: The median treatment duration for all patients in the maintenance phase was 29.5 months. The median treatment duration for patients continuing cenobamate at data cut‐off was 30.2 months. ASM, antiseizure medication
Temporary increases in seizures occurred at an individual visit in up to ~12% of patients; however, retention rates overall were high and 33.9% of patients continuing cenobamate at data cut‐off were seizure‐free for a mean 23.5 months at last visit (range: 11.6–40.1 months), indicating that efficacy was very good overall.
4. DISCUSSION
The flexible‐dose design of the C021 trial allowed investigators to make changes to concomitant ASMs early, while up‐titrating adjunctive cenobamate, which is more reflective of actual clinical practice. These data may be useful in informing physicians on the appropriate adjustment of concomitant ASMs while adding cenobamate in practice. As physicians in this trial became more experienced and learned about possible interactions and how to minimize them, concomitant ASM doses were reduced earlier in cenobamate titration. Thus for general neurologic practice, ASM dose reductions may occur sooner than seen here, depending on the early efficacy and tolerability observed while titrating patients onto cenobamate.
Doses of phenytoin, phenobarbital, clobazam, valproate, and lacosamide were decreased earliest in the current study (see Figure 2), while patients were still in the titration phase of cenobamate (as low as 12.5–50 mg/day cenobamate). In contrast, dosages of carbamazepine, oxcarbazepine, and eslicarbazepine were decreased later, during the maintenance phase (mean cenobamate dose range: 188.6–197.5 mg/day). These differences in timing of dose reductions across varying concomitant ASMs may be attributed to pharmacokinetic interactions that could cause changes in drug exposures and alterations in tolerability. Doses of concomitant ASMs were reduced most often due to TEAEs. Both the TEAEs leading to dose reductions and the overall safety profile seen throughout were consistent with those known to occur with these medications, and no new safety issues were observed. Some concomitant ASM doses were reduced for reasons other than AEs; for example, the concomitant ASMs reduced latest in the maintenance phase (levetiracetam and brivaracetam) may have been adjusted by investigators to reduce the overall drug load rather than because of specific issues with tolerability.
The specific adjustments made to concomitant ASMs throughout this study provide information on practical dosing changes that can improve tolerability and reduce drug‐drug interactions (DDIs) in patients receiving adjunctive cenobamate. As indicated, these data suggest that some concomitant ASMs may be lowered relatively early in cenobamate titration, with most dose adjustments recommended to mitigate pharmacokinetic interactions. For example, phenobarbital and phenytoin are both metabolized, in part, by the CYP2C19 isoenzyme. Cenobamate inhibits CYP2C19 and data have shown that plasma exposures of phenobarbital and phenytoin increase by a mean 37% and 84%, respectively, following multiple doses of adjunctive cenobamate. 8 , 24 Elevation of phenytoin levels may lead to increased risk of dizziness/ataxia, thus physicians should be aware that dose alterations may be necessary if these symptoms occur, or proactive reduction of phenytoin dose may be required. In this study, investigators reduced phenytoin doses in patients continuing cenobamate by a mean 60.8%, starting at a mean (min) cenobamate dose of 142.0 (0.0) mg/day (mean visit 7). Similarly, investigators reduced phenobarbital doses by a mean 40.0% starting early in titration (mean visit 6.5) at a mean (min) cenobamate dose of 131.3 (12.5) mg/day. Patients and caregivers should be aware that early reduction of phenobarbital doses may be required if drowsiness/somnolence occurs, or doses may be proactively decreased.
The active metabolite of clobazam, N‐desmethylclobazam, is also mainly metabolized by the CYP2C19 enzyme. Data on interactions between cannabidiol (another CYP2C19 inhibitor) and clobazam suggest that during coadministration of a CYP2C19 inhibitor, such as cenobamate, 8 , 24 N‐desmethylclobazam levels may increase by between 2 and 6 times, which can result in increased TEAEs. 25 Patients and caregivers should be aware that adjunctive cenobamate with clobazam may lead to increased somnolence. In the current analysis, for those continuing cenobamate, investigators started reducing doses of clobazam at a mean (min) cenobamate dose of 135.0 (0.0) mg/day, with most patients (~65%) receiving dose reductions at 50–150 mg/day cenobamate (data not shown). Due to the rate of somnolence (50.0%) leading to the first dose reduction of concomitant clobazam, as well as probable elevations in plasma N‐desmethylclobazam when coadministered with cenobamate, early reduction of clobazam is recommended when initiating cenobamate, which should often be proactively reduced.
Investigators made the above dose alterations based on probable pharmacokinetic interactions leading to alterations in blood levels. However, pharmacodynamic interactions may also arise and could require dose adjustments. For example, dizziness and somnolence were reported in some patients receiving lacosamide and cenobamate. At lower doses of cenobamate, there are no apparent pharmacokinetic interactions between these drugs; however, at 400 mg/day cenobamate, there can be up to 50% inhibition of CYP2C19 and possible small elevations of total lacosamide levels. This suggests that these side effects could be due to a pharmacodynamic interaction at lower cenobamate dosages and a possible minimal pharmacokinetic CYP2C19 interaction at 400 mg/day.
Both cenobamate and lacosamide exert their therapeutic effects through sodium channels but with different mechanisms of action. 10 Clinically, patients on higher doses of lacosamide, particularly at >500 mg/day range, will be more likely to require lacosamide dose decreases when adding cenobamate. In this analysis of patients continuing cenobamate who had their lacosamide dose reduced, ~70% of those taking >500 mg/day lacosamide at baseline had their dose reduced (mean lacosamide reduction: 229.3 mg/day), whereas ~40% of those receiving <500 mg/day lacosamide at baseline had their dose reduced (mean lacosamide reduction: 98.4 mg/day; data not shown).
Other blocking ASMs that alter sodium channel conductance, including carbamazepine, oxcarbazepine, eslicarbazepine, and lamotrigine, usually did not require dose reductions until the cenobamate maintenance phase. Because these ASMs are largely metabolized by CYP3A4, and cenobamate enhances this effect, blood levels for these concomitant drugs may be decreased. 8 These decreases may lead to a reduction in TEAEs that would allow cenobamate to be successfully up‐titrated. Once in the maintenance phase, some further reductions of these sodium channel drugs may be necessary.
For patients continuing cenobamate, valproate was lowered in the late titration phase (mean visit: 8.0) at a mean (minimum) dose of 167.9 (0.0) mg/day cenobamate, but no major pharmacokinetic interactions were noted. Drugs such as levetiracetam have few or no known interactions with cenobamate and lowering levetiracetam usually did not occur until the maintenance phase (mean visit: 11.5) at a mean (minimum) cenobamate dose of 217.0 (12.5) mg/day (see Figure 2).
The current post hoc analysis indicates that patients who remained on cenobamate had greater dose reductions of concomitant ASMs than patients who discontinued, suggesting that reducing the doses of these drugs may lead to greater retention over a long maintenance phase. This retention benefit could have been due to improved tolerability as a result of the reduced overall drug burden.
Because retention can serve as both a surrogate for efficacy and an indication of tolerability, the current analysis offers key insights into the clinical use of various concomitant ASMs with adjunctive cenobamate. The high retention rate observed here, even after reducing doses of or completely discontinuing concomitant ASMs, supports the efficacy of cenobamate. The high percentages of responders observed in patients continuing cenobamate, regardless of their concomitant ASM, further suggest that cenobamate treatment was effective in reducing seizures in these patients whose seizures were previously uncontrolled despite treatment with up to three other ASMs. Moreover, these responses were sustained over a lengthy maintenance phase. In most studies, the maintenance phase represents a 6‐ or 12‐week portion of the double‐blind studies; in open‐label studies, intervals of 3 months, 6 months, or a year are typically described for 100% reduction in seizures, or are not described at all. However, in this open‐label study, the maintenance phase represents the entire length of the study except the 12‐week titration phase (ie, up to 40.2 months of data), and sustained seizure reductions over this extensive period were still observed.
The current findings must be interpreted in context with the limitations of both the post hoc analyses and the larger C021 study. Because this was a retrospective analysis of a population from a subset of clinical sites, selection bias may exist, although the present cohort resembled the broader C021 sample. In addition, the C021 study was open label and was not originally designed to assess effects of changes to concomitant ASMs; therefore it was analyzed post hoc. As previously indicated, the open‐label study design allowed clinicians to make changes to cenobamate doses and concomitant ASMs, which may better reflect actual clinical practice.
Because patients with refractory epilepsy often experience tolerability issues due to polypharmacy, reducing concomitant medications during the addition of new therapy is frequently necessary. Many patients with uncontrolled epilepsy have excessive drug load, most commonly through use of combination therapy, which can result in significant TEAEs, DDIs, and reduced quality of life. 26 , 27 , 28 The ultimate goal of treatment is to reach 100% seizure reduction with the fewest possible TEAEs. When initiating treatment, slowly reducing the concomitant ASM dose in patients who experience TEAEs while adding another drug may aid in reducing drug burden while allowing for optimal efficacy. 26 Proper treatment with a combination of many ASMs requires transitional polytherapy during titration of a new drug, with a goal of monotherapy or reduced ASM polytherapy. 29 Titration allows for dose adjustment and a more personalized treatment regimen because some patients may experience efficacy at lower than recommended target doses. This strategy also provides greater opportunities to evaluate patients who may be able to transition to monotherapy. The information presented here offers physicians a data‐driven clinical approach to adjusting concomitant ASMs while up‐titrating adjunctive cenobamate in patients with uncontrolled focal seizures.
5. CONCLUSIONS
In the current study of adjunctive cenobamate, reducing doses of concomitant medications likely led to greater patient retention due to the combination of appropriate levels of efficacy and reduced concomitant drug burden in a population who were largely taking two to three concomitant ASMs at baseline. This post hoc analysis of a subset of the C021 study provides some direction to physicians on adjusting concomitant ASMs when adding on cenobamate treatment. As investigators gained experience and learned about potential interactions, concomitant ASM doses were reduced for many patients very early in cenobamate titration. Doses of concomitant phenytoin, phenobarbital, clobazam, valproate, and lacosamide, in particular, may be gradually reduced early on when adding cenobamate treatment to avoid possible tolerability issues. The number of patients who remained seizure‐free for prolonged periods and the high retention rates, even after reducing or discontinuing concomitant ASMs, suggest that cenobamate was efficacious. Post hoc efficacy analyses from the C021 safety study are also presented in a companion paper (Sperling et al. 2021), 30 and together, these analyses allow for a broader view of the effects of cenobamate on seizure reduction over the long term.
CONFLICTS OF INTEREST
WER: Consultant/advisor: SK Life Science, Inc.; Speaker: Eisai, Greenwich Biosciences (GW Pharmaceuticals), SK Life Science, Inc., Sunovion, and UCB Pharma; and Research support: Greenwich Biosciences, Marinus, Medtronic, Neurelis, Ovid, SK Life Science, Inc., Takeda, UCB Pharma, and Upsher‐Smith.
BAK: Research support: Cerevel, Otsuka, SK Life Science, Inc., UCB Pharma, and Xenon.
SA: Consultant/advisor: Eisai, SK Life Science, Inc.; and Speaker: Eisai, Sunovion.
PB: Research support: SK Life Science, Inc.
VB: Research support: SK Life Science, Inc.
GLK: Consultant/advisor: Adamas, Eisai, Otsuka, and Shire; and Research support: Biogen, SK Life Science, Inc., UCB Pharma, and Upsher‐Smith.
MRS: Consultant/advisor: Medtronic and Neurelis; Speaker: Eisai, International Medical Press, Medscape, NeurologyLive, Projects in Knowledge, and UCB Pharma; Research support: Cavion, Cerevel, Eisai, Engage, Medtronic, Neurelis, SK Life Science, Inc., Takeda, UCB Pharma, and Xenon; and Royalty: Oxford University Press.
DGV: Consultant/advisor: Otsuka and SK Life Science, Inc.; Speaker: Greenwich Biosciences, Neurelis, SK Life Science, Inc., and UCB Pharma; and Research support: Biogen, Eisai, SK Life Science, Inc., UCB Pharma, and Xenon.
PK: Consultant/advisor: Abbott, Aquestive, Arvelle, Eisai, Engage, Neurelis, SK Life Science, Inc., and UCB Pharma; Speaker: Aquestive, Eisai, Neurelis, Sunovion, and UCB Pharma; Research support: Eisai and Lundbeck; Member, Medical Advisory Board for Alliance‐Stratus and Scientific Advisory Board for OB Pharma; and CEO, PrevEp, LLC.
RW: Consultant/advisor: Brain Sentinel, Eisai, Engage, Greenwich Biosciences, Lundbeck, SK Life Science, Inc., Sunovion, and UCB Pharma; Speaker: Aquestive, Eisai, Greenwich Biosciences, LivaNova, Sunovion, and UCB Pharma; and Research support: Aquestive, Biogen, Eisai, Engage, Greenwich Biosciences, Lundbeck, Pfizer, SK Life Science, Inc., Sunovion, UCB Pharma, Xenon, and Zogenix.
ETHICAL PUBLICATION STATEMENT
The authors confirm that they have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
DATA SHARING
The data for the analyses described in this manuscript are available by request from the corresponding author or SK Life Science, Inc., the company sponsoring the clinical development of cenobamate for the treatment of focal epilepsy.
PREVIOUS PRESENTATION
Rosenfeld WE, et al. Dose adjustments to concomitant antiseizure medications: post‐hoc analysis of a phase 3, open‐label study of cenobamate for treatment of uncontrolled focal seizures. Presented at the American Epilepsy Society Virtual Annual Meeting, December 4–8, 2020, poster 336, and encored at the American Academy of Neurology Virtual Annual Meeting 2021.
Supporting information
Figure S1
Table S1‐S2
ACKNOWLEDGEMENTS
This study was funded by SK Life Science, Inc. Medical writing and editorial assistance were provided by Debika Chatterjea, PhD, and Don Fallon, ELS, of MedVal Scientific Information Services, LLC (Princeton, NJ, USA), and were funded by SK Life Science, Inc. This manuscript was prepared according to the International Society for Medical Publication Professionals’ “Good Publication Practice for Communicating Company‐Sponsored Medical Research: GPP3.”
Rosenfeld WE, Abou‐Khalil B, Aboumatar S, Bhatia P, Biton V, Krauss GL, et al. Post hoc analysis of a phase 3, multicenter, open‐label study of cenobamate for treatment of uncontrolled focal seizures: Effects of dose adjustments of concomitant antiseizure medications. Epilepsia. 2021;62:3016–3028. 10.1111/epi.17092
REFERENCES
- 1. Brodie MJ, Sills GJ. Combining antiepileptic drugs–rational polytherapy? Seizure. 2011;20(5):369–75. [DOI] [PubMed] [Google Scholar]
- 2. Chen Z, Brodie MJ, Liew D, Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30‐year longitudinal cohort study. JAMA Neurol. 2018;75(3):279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dash D, Aggarwal V, Joshi R, Padma MV, Tripathi M. Effect of reduction of antiepileptic drugs in patients with drug‐refractory epilepsy. Seizure. 2015;27:25–9. [DOI] [PubMed] [Google Scholar]
- 4. Patsalos PN, Perucca E. Clinically important drug interactions in epilepsy: interactions between antiepileptic drugs and other drugs. Lancet Neurol. 2003;2(8):473–81. [DOI] [PubMed] [Google Scholar]
- 5. Perucca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol. 2006;61(3):246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perucca E. From clinical trials of antiepileptic drugs to treatment. Epilepsia Open. 2018;3(s2):220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chung S, Wang N, Hank N. Comparative retention rates and long‐term tolerability of new antiepileptic drugs. Seizure. 2007;16(4):296–304. [DOI] [PubMed] [Google Scholar]
- 8. XCOPRI® (cenobamate tablets), for oral use, CV [prescribing information].. Paramus, NJ: SK Life Science, Inc.; 2021. [Google Scholar]
- 9. Sharma R, Nakamura M, Neupane C, Jeon BH, Shin H, Melnick SM, et al. Positive allosteric modulation of GABAA receptors by a novel antiepileptic drug cenobamate. Eur J Pharmacol. 2020;879:173117. [DOI] [PubMed] [Google Scholar]
- 10. Nakamura M, Cho JH, Shin H, Jang IS. Effects of cenobamate (YKP3089), a newly developed anti‐epileptic drug, on voltage‐gated sodium channels in rat hippocampal CA3 neurons. Eur J Pharmacol. 2019;855:175–82. [DOI] [PubMed] [Google Scholar]
- 11. Piredda SG, Woodhead JH, Swinyard EA. Effect of stimulus intensity on the profile of anticonvulsant activity of phenytoin, ethosuximide and valproate. J Pharmacol Exp Ther. 1985;232(3):741–5. [PubMed] [Google Scholar]
- 12. White HS. Clinical significance of animal seizure models and mechanism of action studies of potential antiepileptic drugs. Epilepsia. 1997;38(Suppl 1):S9–S17. [DOI] [PubMed] [Google Scholar]
- 13. Anderson LL, Thompson CH, Hawkins NA, Nath RD, Petersohn AA, Rajamani S, et al. Antiepileptic activity of preferential inhibitors of persistent sodium current. Epilepsia. 2014;55(8):1274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stafstrom CE. Persistent sodium current and its role in epilepsy. Epilepsy Curr. 2007;7(1):15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vreugdenhil M, Hoogland G, van Veelen CW, Wadman WJ. Persistent sodium current in subicular neurons isolated from patients with temporal lobe epilepsy. Eur J Neurosci. 2004;19(10):2769–78. [DOI] [PubMed] [Google Scholar]
- 16. Guignet M, Campbell A, White HS. Cenobamate (XCOPRI): can preclinical and clinical evidence provide insight into its mechanism of action? Epilepsia. 2020;61(11):2329. [DOI] [PubMed] [Google Scholar]
- 17. Löscher W, Sills GJ, White HS. The ups and downs of alkyl‐carbamates in epilepsy therapy: how does cenobamate differ? Epilepsia. 2021;62(3):596–614. [DOI] [PubMed] [Google Scholar]
- 18. Chung SS, French JA, Kowalski J, Krauss GL, Lee SK, Maciejowski M, et al. Randomized phase 2 study of adjunctive cenobamate in patients with uncontrolled focal seizures. Neurology. 2020;94(22):e2311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krauss GL, Klein P, Brandt C, Lee SK, Milanov I, Milovanovic M, et al. Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double‐blind, randomised, placebo‐controlled, dose‐response trial. Lancet Neurol. 2020;19(1):38–48. [DOI] [PubMed] [Google Scholar]
- 20. Sperling MR, Klein P, Aboumatar S, Gelfand M, Halford JJ, Krauss GL, et al. Cenobamate (YKP3089) as adjunctive treatment for uncontrolled focal seizures in a large, phase 3, multicenter, open‐label safety study. Epilepsia. 2020;61(6):1099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51(4):676–85. [DOI] [PubMed] [Google Scholar]
- 22. International League Against Epilepsy . Proposal for revised clinical and electroencephalographic classification of epileptic seizures. From the Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1981;22(4):489–501. [DOI] [PubMed] [Google Scholar]
- 23. International Council for Harmonisation . Integrated Addendum to ICH E6(R1): Guideline For Good Clinical Practice E6(R2). https://database.ich.org/sites/default/files/E6_R2_Addendum.pdf. (2016). Accessed 25 Feb, 2020.
- 24. Greene S, Kwak C, Kamin M, Vernillet L. The effect of cenobamate on the single dose pharmacokinetics of multiple cytochrome P450 probes using a cocktail approach in healthy subjects [poster]. Presented at American Society for Clinical Pharmacology & Therapeutics Annual Meeting, March 13‐16, 2019, Washington, DC. [DOI] [PMC free article] [PubMed]
- 25. Geffrey AL, Pollack SF, Bruno PL, Thiele EA. Drug‐drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia. 2015;56(8):1246–51. [DOI] [PubMed] [Google Scholar]
- 26. Schmidt D. Strategies to prevent overtreatment with antiepileptic drugs in patients with epilepsy. Epilepsy Res. 2002;52(1):61–9. [DOI] [PubMed] [Google Scholar]
- 27. Perucca E. Overtreatment in epilepsy: adverse consequences and mechanisms. Epilepsy Res. 2002;52(1):25–33. [DOI] [PubMed] [Google Scholar]
- 28. St Louis EK. Minimizing the adverse effects of epilepsy therapies: principles and practice. In: St. Louis EK, Ficker DM, O'Brien TJ, editors. Epilepsy and the interictal state: co‐morbidities and quality of life. Chichester, UK: John Wiley & Sons, Ltd.; 2015. p. 120–6. [Google Scholar]
- 29. St Louis EK. Truly "rational" polytherapy: maximizing efficacy and minimizing drug interactions, drug load, and adverse effects. Curr Neuropharmacol. 2009;7(2):96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sperling MR, Abou‐Khalil B, Aboumatar S, Bhatia P, Biton V, Klein P, et al. Efficacy of cenobamate for uncontrolled focal seizures: post‐hoc analysis of a phase 3, multicenter, open‐label study. Epilepsia. 2021. (in press); 10.1111/epi.17091 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1‐S2


