Abstract
Understanding carbon (C) dynamics from ecosystem to global scales remains a challenge. Although expansion of global carbon dioxide (CO2) observatories makes it possible to estimate C‐cycle processes from ecosystem to global scales, these estimates do not necessarily agree. At the continental US scale, only 5% of C fixed through photosynthesis remains as net ecosystem exchange (NEE), but ecosystem measurements indicate that only 2% of fixed C remains in grasslands, whereas as much as 30% remains in needleleaf forests. The wet and warm Southeast has the highest gross primary productivity and the relatively wet and cool Midwest has the highest NEE, indicating important spatial mismatches. Newly available satellite and atmospheric data can be combined in innovative ways to identify potential C loss pathways to reconcile these spatial mismatches. Independent datasets compiled from terrestrial and aquatic environments can now be combined to advance C‐cycle science across the land–water interface.
In a nutshell:
-
•
From a societal perspective, there has never been a more urgent time to advance our understanding of the carbon (C) cycle, given that the atmospheric growth rate of carbon dioxide (CO2) has reached record levels
-
•
From a scientific perspective, however, there has never been a better time to be a global ecologist, because global C observing systems are becoming more expansive and intensive, allowing scientists to make innovative insights at ecosystem, macrosystem, and global scales
-
•
A fundamental goal of macrosystems research is to reconcile important processes from ecosystem to continental scales, which is now achievable using long‐term and consistent measurements of C‐cycle dynamics
-
•
Comparisons across scales also reveal many CO2 loss pathways other than respiration that may not be included in ecosystem‐process models
Carbon (C) is the building block of life. Global photosyn‐thesis generates approximately 100 terawatts (TW) of energy each year by converting solar radiation into stored chemical energy (Barber 2009). Photosynthesis also represents the largest global annual C flux, of ~125 petagrams (Pg; where 1 Pg equals 1015 grams [g] and 1 Pg C is roughly equivalent to 0.47 parts per million [ppm] of CO2), with the second greatest flux consisting of the subsequent release of CO2 via respiration (~122 Pg C/year). Both of these fluxes are an order of magnitude greater than fossil‐fuel emissions (Ballantyne et al. 2015). The atmospheric CO2 that is fixed during photosynthesis is subsequently stored and transferred as chemical energy, which in turn fuels the metabolic reactions of most autotrophs and heterotrophs. Although C is the most common element in the terrestrial biosphere, representing approximately 50 parts per hundred (%) of all organic matter, CO2 represents only a very small fraction of the atmosphere and is therefore measured in ppm (~415 ppm in 2020). Given the abundance of C in the terrestrial biosphere and the massive fluxes of C occurring between the biosphere and the atmosphere, it is no surprise that scientists have developed a myriad of innovative ways for measuring and simulating C‐cycle processes across a range of scales in time and space. For example, chloroplast CO2 fluxes are estimated over millimeters per second, whereas biome CO2 fluxes may be estimated over thousands of kilometers per year. There have been many advances in C‐cycle science over the past 60 years at leaf, plant, ecosystem, and global scales, but both challenges to and opportunities for scientific advancement remain. Progress is necessary, however, especially at the macrosystem scale, where human management and ecological processes are often at odds and create interesting interactions of C dynamics.
One of the greatest impediments to accurate predictions of future climate is the uncertain response of the terrestrial C cycle to impending changes in temperature, precipitation, and atmospheric CO2 concentrations (Friedlingstein et al. 2013). Even though land‐surface models have become increasingly realistic in their mechanistic representation of C‐cycle processes by including nutrient limitation (Thornton et al. 2007), surface hydrology (Wang et al. 2013), and microbial processes (Wieder et al. 2013), this increased complexity does not necessarily reduce the range of uncertainty in projections of C uptake among models (eg see Friedlingstein et al. [2006] compared to Friedlingstein et al. [2013]). In parallel, there is now a globally nested CO2 observation network that allows for unprecedented measurements of changes in CO2 concentrations and fluxes (Schimel et al. 2015). These continuous measurements allow estimates to be made of net CO2 exchange from ecosystem to global scales, but not necessarily the underlying processes that regulate this net exchange (Ciais et al. 2019). In contrast, land‐surface models simulate the underlying processes that result in net CO2 exchange, but these are difficult to benchmark due to a lack of process‐level data at the appropriate scale (Luo et al. 2012; Anav et al. 2013).
Although enhanced net C accumulation in the terrestrial biosphere can be inferred from the global C budget, identifying the ecosystems in which C is accumulating is still difficult. For example, at the global scale, it can be concluded with confidence that ~25% of CO2 emitted to the atmosphere from fossil‐fuel and land‐use emissions has been taken up by the terrestrial biosphere (Ballantyne et al. 2015; Le Quéré et al. 2016), but biomass datasets are too sparse in extent or too short in duration to document which ecosystems continue to accumulate C. More detailed ocean and land measurements now make it possible to identify specific processes affecting the net CO2 atmospheric exchange between the marine biosphere (Landschützer et al. 2015) and terrestrial biosphere (Anderegg et al. 2015), in some instances at regional scales (Ciais et al. 2019). However, partitioning net C fluxes into their component gross fluxes of photosynthesis and respiration remains a challenge (Wehr et al. 2016).
Another vexing problem in global C‐cycle research is that top‐down global estimates of net terrestrial C uptake do not agree with bottom‐up ecosystem estimates when integrated globally. For instance, top‐down estimates of global net terrestrial C uptake in 2010 are an order of magnitude less (2.2 ± 2.1 Pg C/year; Ballantyne et al. 2015) than eddy covariance estimates up‐scaled globally (22 ± 5 Pg C/year; Jung et al. 2011). Although some of the discrepancy between top‐down and bottom‐up estimates of net terrestrial C uptake may be due to issues associated with eddy covariance methods (Keenan et al. 2019) – particularly regarding measurement of nighttime respiration, which often violates eddy covariance requirements of turbulent flux and biases in the sampling network – a portion can also be explained by non‐respiratory CO2 loss pathways (~7 Pg C/year; Randerson et al. 2002). This suggests that there are many C transformation and transport pathways that ultimately lead to a loss of CO2 from ecosystems back to the atmosphere. Approximately 90% of inland lakes and streams are net sources of CO2 to the atmosphere (Cole et al. 1994), and at a global scale approximately 2 Pg C/year is returned to the atmosphere via CO2 loss from rivers and lakes (Raymond et al. 2013). Although this estimate of CO2 loss from aquatic ecosystems is comparable to the magnitude of net C uptake by terrestrial ecosystems, it is less than 2% of total inferred CO2 respiration from the terrestrial biosphere back to the atmosphere (Ballantyne et al. 2017). As such, characterizing the C balance at the macrosystem scale for direct comparison with different biomes in Earth system models remains difficult (Peylin et al. 2013).
Although from a societal perspective there has never been a more urgent time to study the C cycle and its sensitivity to climate change (Obama 2017), from a scientific perspective there has never been a more exciting time to study C‐cycle processes. The global C observation network supports innovative analyses and syntheses across scales from ecosystems to the entire planet. Currently, there are over 800 eddy covariance sites operating around the world that contribute measurements of net CO2 exchange, as well as estimates of primary productivity and total respiration across a wide array of ecosystems (Chu et al. 2017). However, in the US, fewer than half of the ecosystem functional types are represented in the combined core sites of the AmeriFlux Network and the National Ecological Observatory Network (NEON) (Villarreal et al. 2018), and many ecosystems remain underrepresented, especially in climate‐sensitive Arctic tundra and tropical rainforests. Other C flux databases have continued to expand, such as a recently updated database on soil respiration that has been used to identify the climate sensitivity of soil respiration over time (Bond‐Lamberty and Thomson 2010), which is critical for evaluating how C supply, soil temperature, and moisture interact to regulate soil respiration (Hursh et al. 2017).
Global measurement networks and satellite observations of atmospheric CO2 now allow for the characterization of biome‐scale C fluxes at greater temporal and spatial resolutions (Figure 1). The global greenhouse observation network has grown sporadically, with approximately 90 in situ sites now in operation worldwide (GLOBALVIEW‐CO2 1999). Several of these sites also provide atmospheric profile measurements that are essential for estimating latitudinal differences in CO2 exchange (Stephens et al. 2007), in addition to seasonal differences in regional uptake (Gatti et al. 2014). Regional atmospheric CO2 monitoring networks often engage in intensive atmospheric campaigns to better define regional C fluxes in urban continental settings (Corbin et al. 2010) or to determine recent changes in the C balance of ecosystems in climate sensitive regions, such as the Arctic (Commane et al. 2017). When combined with three‐dimensional atmospheric transport modeling and estimates of surface fossil‐fuel emissions, these so‐called “atmospheric inversions” deliver critical information about the net exchange of CO2 at biome scales (Peylin et al. 2013). The array of Earth observing satellites has also grown tremendously, providing better spatiotemporal coverage of vegetation indices that are useful for assessing patterns and trends of global productivity since ~1982 (Pinzon and Tucker 2010), as well as valuable information on changes in vegetation cover (Song et al. 2018) and ecosystem stress (Anderegg et al. 2018). Recent advances in satellite observations facilitate quantification of concentration estimates integrated over the entire total atmospheric column for CO2 (ie XCO2) and CH4 (ie XCH4). Although potentially less precise than those relying on surface measurements using infrared gas analyzers, these estimates provide more continuous global coverage, improving characterization of regional flux anomalies and attribution to specific C‐cycle processes (Liu et al. 2017).
Figure 1.

Image of airborne observations combined with eddy flux observations of carbon (C) fluxes to measure ecosystem–atmosphere exchanges of carbon dioxide (CO2).
A Varlagin/imaggeo.egu.eu
Innovative ways to combine ecosystem measurements with satellite observations have made it possible to quantify how different ecosystems are responding to concomitant changes in atmospheric composition, including CO2 concentration, surface temperatures, and regional precipitation. Moreover, these top‐down and bottom‐up observations are helping researchers to disentangle net C exchange into its component processes of photosynthesis and respiration across various scales, which provides important diagnostics for models that are designed to simulate the concurrent ecological processes and not just net CO2 exchange. For instance, combined satellite and meteorological observations have been used in a machine‐learning framework to up‐scale eddy covariance measurements to provide spatially and temporally continuous estimates of global primary productivity (Jung et al. 2011). Likewise, global atmospheric CO2 measurements have been used to constrain net CO2 exchange in combination with satellite data to constrain primary productivity to infer the uncoupling of photosynthesis and respiration on decadal timescales (Ballantyne et al. 2017). The challenge for the scientific community is figuring out ways in which emergent patterns of net CO2 exchange can be used (Cox et al. 2013) to identify underlying mechanistic processes that can be diagnosed in models (Anderegg et al. 2015). Ultimately, this will lead to scientific advances and societal benefits through improved Earth system models with less uncertainty in future climate predictions.
Theoretical representation of C‐cycle processes
Although the global C observing system has been greatly expanded and advanced over the past six decades, the theoretical and conceptual framework for understanding C‐cycle dynamics has not necessarily kept pace (Figure 2). There has been extensive discussion over the past several decades concerning how the biosphere–atmosphere C exchange can best be defined. The challenges in defining C exchange lie across several axes, including time, space, and C form. Additional issues arise from the different processes occurring in and the transfer of C between aquatic and terrestrial ecosystems. Although we focus solely on terrestrial processes occurring from the ecosystem to biome scale here, we acknowledge the importance of the aquatic interface (Butman et al. 2018). The evolution of C‐cycle measurements and key issues regarding terminology was described by Chapin et al. (2006), who defined net ecosystem C balance (NECB) simply as the change in C per unit time, but then broke this measure down into its component fluxes:
| ((Equation 1).) |
Figure 2.
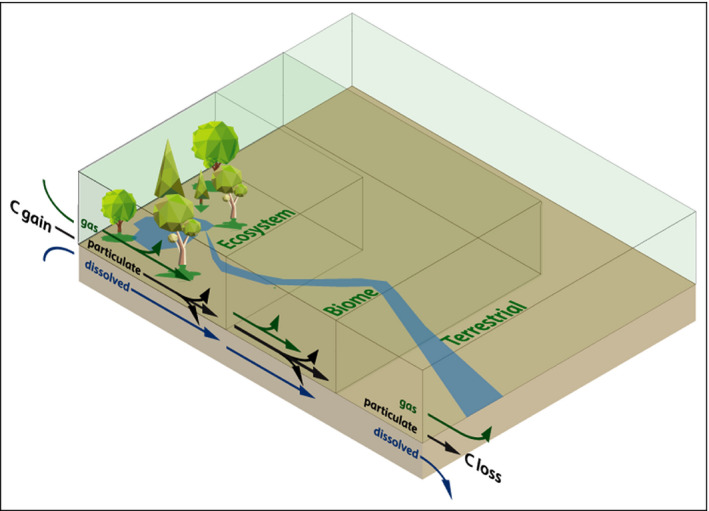
Conceptual figure showing pathways of C gain and loss from ecosystem to biome to terrestrial scales within the biosphere. Although it is often assumed that very little change occurs among the gas, particulate, and dissolved phases of C, ecosystems are very effective at transforming C, such that C gain pathways may not correspond with C loss pathways, leading to an apparent C imbalance across scales. Furthermore, C can be transported across scales via either advection through the atmosphere or fluvial processes in aquatic ecosystems.
In this formulation, net ecosystem exchange (NEE) is a measure of the net ecosystem CO2 exchange as the difference between gross primary productivity (GPP) and total ecosystem respiration (TER), and at the ecosystem scale is typically measured using eddy covariance techniques (Wofsy et al. 1993). Although the CO2 flux associated with NEE is usually the dominant form of net C exchange in many ecosystems, it cannot be assumed that transformations of C do not occur as a result of ecosystem processes. For instance, fluxes of carbon monoxide (F CO), volatile organic compounds (F VOC), methane (F CH4), dissolved inorganic C (F DIC), dissolved organic C (F DOC), and particulate C (F PC) all represent C loss pathways that may affect the net C balance over time. Although NEE is sometimes used synonymously with NECB, it is an approximation that can, under certain circumstances, leave out quantitatively important non‐respiratory processes that contribute to ecosystem C balance.
A second key issue in C balance terminology emerges at larger spatial or temporal scales when other factors can become major contributors to C balance. Notably, large disturbances like wildfire, landslides, and insect infestations can cause large or punctuated redistributions of C. In human managed ecosystems, activities such as logging, harvest, and other forms of C transfer can result in C taken up at the ecosystem scale being lost at the biome scale, and this transfer can actually cause NECB to shift from a net C sink to a net C source. The net biome productivity (NBP) concept was first introduced by Schulze et al. (2000) to account for C transfer and subsequent loss at regional scales. In Chapin et al. (2006), NECB represents NBP integrated over fixed space and time domains, with the assumption that additional processes analogous to those shown in Equation (1) may need to be added to account for C fluxes driven by periodic events. At the global scale, we can assume that CO2 mass is conserved in the atmosphere and thus, given fossil‐fuel emissions to the atmosphere and estimates of net CO2 uptake by the oceans, net CO2 uptake by the terrestrial biosphere can be inferred (Le Quéré et al. 2016). More importantly, the atmosphere and oceans provide constraints on global C exchange because these are relatively well‐mixed homogenous reservoirs as compared to ecosystem C pools and fluxes that tend to be much more spatially and temporally heterogeneous. Resolving C‐cycle processes from ecosystem to global scales may therefore require an update to C‐cycle nomenclature (see WebPanel 1).
Spatial scale differences in C balance
The “C exchange efficiency” (CEE = NEE/GPP) may provide a useful framework (see WebFigure 1) for comparing relative fluxes across ecosystem to global scales. At the global scale, only ~2% of C fixed annually through GPP remains in the biosphere as a result of NEE (2.5/125 Pg C/year), suggesting that CEE of the terrestrial biosphere is remarkably low. At the scale of the continental US, approximately 5% of C fixed annually through photosynthesis remains in the terrestrial biosphere (Figure 3). However, estimates of CEE derived from eddy covariance methods reveal very large differences among terrestrial ecosystems. Ecosystems with lower levels of GPP tend to fall on the CEE line at the continental scale, whereas more productive ecosystems tend to deviate from the CEE line. For example, grasslands have very low CEE (~2%), a level consistent with the global CEE, whereas evergreen needleleaf forests and deciduous broadleaf forests appear to have quite high CEE values (~31% and ~24%, respectively). Therefore, our ecosystem‐scale measurements suggest that these forests are strong C sinks, whereas our global‐scale measurements suggest that much of this apparent forest C uptake is lost, indicating that these forests may be acting more like “C sieves”. Moreover, croplands vary considerably, with less productive croplands falling on the continental CEE line and more productive croplands deviating considerably, with an overall CEE of 23%. It should be noted, however, that according to mass balance, the integral of NEE across all ecosystems (aquatic and terrestrial) should be equal to global NEE; in other words, CEE estimates from the different ecosystems shown in Figure 3 should all fall on the continental CEE line (Chapin et al. 2006). Therefore, measurements of net CO2 exchange at the ecosystem scale are biased, or CO2 loss pathways at the continental scale are offsetting the apparent net uptake of CO2 by certain ecosystems.
Figure 3.
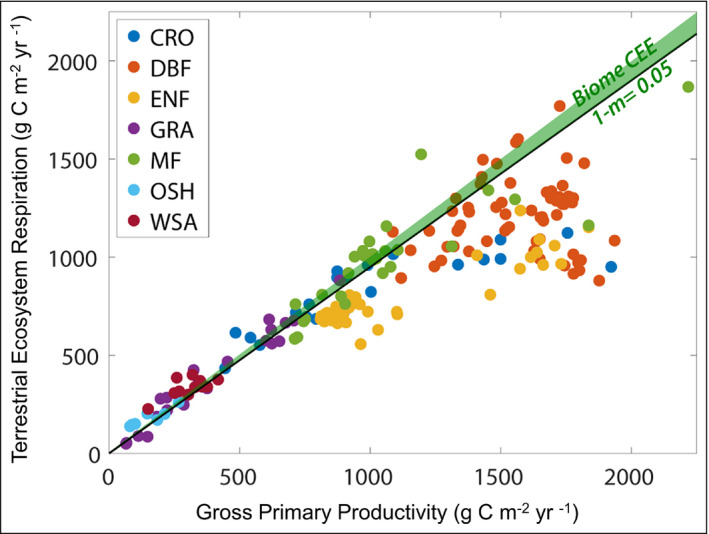
Comparison of C exchange efficiency (CEE) at ecosystem to biome scales across the continental US. Each point represents the mean annual gross ecosystem productivity and total ecosystem respiration for cropland (CRO), deciduous broadleaf forests (DBF), evergreen needleleaf (ENF), grassland (GRA), mixed forest (MF), open shrubland (OSH), and woody savanna (WSA) eddy covariance sites across the US. The diagonal line was derived from satellite estimates of gross primary productivity (GPP) and atmospheric estimates of net CO2 exchange at the scale of the continental US and indicates that 95% of C fixed during photosynthesis is lost to the atmosphere through respiration, or that CEE is only 5% (1 – 0.95 = 0.05), represented by the green wedge.
Measurement biases of ecosystem C fluxes stem from the location of eddy flux sites or systematic biases in NEE measurements. In the US, this network bias should be reduced with the addition of more observation sites, such as NEON sites; however, there remain notable gaps in the intermountain west, north‐central plains, and parts of the Southeast. Also noteworthy is that eddy flux sites are often situated in rapidly regenerating ecosystems and as such may not capture the full trajectory of ecosystem C dynamics (Luyssaert et al. 2008). Furthermore, the eddy flux approach only measures the net ecosystem CO2 exchange (ie NEE) directly, whereas photosynthetic fluxes and total ecosystem respiration fluxes are estimated, resulting in the potential for systematic biases to occur in these measurements. Eddy covariance methods are inherently challenging in ecosystems with dense canopies (Thomas et al. 2013), which can lead to nocturnal C storage within the canopy (Fu et al. 2018) and decoupling of the canopy and the atmosphere that may vary seasonally (Jocher et al. 2017). This may help explain the strong divergence between both deciduous broadleaf and evergreen needleleaf forests and the CEE line at the continental scale (Figure 3). If daytime respiration is reduced with respect to nighttime respiration, large overestimates of both photosynthetic gains and respiration losses at the ecosystem scale may result, which would increase relative ecosystem CEE (Keenan et al. 2019).
The discrepancy between CEE at the biome scale and the ecosystem scale can also be explained by the lack of measurements of non‐respiratory loss pathways of CO2 back to the atmosphere (Figure 2). For example, aquatic ecosystems, which are effective at transporting dissolved and particulate forms of inorganic and organic C and transforming it to CO2 such that it may be lost to the atmosphere (Neff and Asner 2001; Hotchkiss et al. 2015), were not plotted on our diagnostic CEE plot (Figure 3). There are many measurements of the partial pressure of CO2 in aquatic environments, which determine whether CO2 is diffusing in or out of aquatic ecosystems, but these are not always combined with productivity estimates (albeit see Hotchkiss et al. 2015; Bernhardt et al. 2018). Volatile organic C (VOC) compounds are another major source of C loss from ecosystems, which may help to reconcile the discrepancy between ecosystem‐ and biome‐scale C exchange efficiencies. Estimates of VOC production are tightly coupled to primary productivity and range around 450 teragrams (Tg) C/year, making them a very small fraction of terrestrial GPP (less than 0.4%) but an appreciable fraction of NEE (~15%), assuming that VOCs are rapidly oxidized to form CO2 (Unger et al. 2013). Finally, the only ecosystem‐scale C loss pathways that can help reconcile ecosystem‐ and global‐scale estimates of CEE are oxidative pathways that ultimately lead to atmospheric CO2 (eg CO2 emissions from wildfires), meaning that other loss pathways leading to reduced C (eg CH4 emissions) will not help reconcile these discrepancies of scale.
We can also look at the spatial distribution of CEE from the biome to ecosystem scale (Figure 4). At the continental scale in the US, it is apparent that high CEE in the midwestern region near the Great Lakes is driven primarily by high mean annual NEE, and very high CEE in the intermountain west is driven by low GPP and modest NEE. In contrast, highly productive regions, such as the Pacific Northwest and the Southeast, do not necessarily retain a large fraction of GPP as NEE, as reflected in their relatively low CEE values. These regional differences in CEE seem to be corroborated by eddy covariance sites in certain biomes, such as the Northeast and parts of the Southwest, but less so in other regions. There appears to be a strong mismatch in CEE near the Great Lakes, with regional estimates suggesting a relatively high CEE, whereas eddy flux sites indicate a much lower CEE. This may be due to the specific locations of eddy flux sites that may not capture the diverse array of midwestern ecosystems. A similar mismatch is evident in the Pacific Northwest, where regional CEE values are extremely low – and in some instances negative – due to an apparent net source of CO2 to the atmosphere, while eddy flux measurements from central Oregon suggest high CEE.
Figure 4.
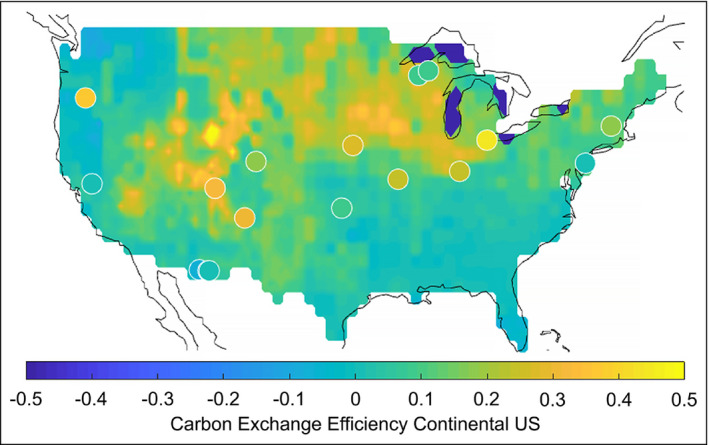
CEE for the continental US. Spatially continuous estimates of GPP derived from Moderate Resolution Imaging Spectroradiometer (MODIS) satellite estimates and continuous estimates of net ecosystem exchange derived from atmospheric inversions over the continental US (Peters et al. 2007) are compared with in situ ecosystem‐scale measurements made at 16 different eddy covariance core sites within the AmeriFlux Network (circle points). Positive values indicate regions where ecosystems are a net sink of C from the atmosphere, whereas negative values indicate regions where ecosystems are a net source of C to the atmosphere.
Climate sensitivity of C‐cycle processes
At the continental scale in the US, mean annual primary productivity and net CO2 exchange do not necessarily covary spatially and appear to occupy different climate spaces at regional scales (Figure 5; Liu et al. 2018). GPP is highest in the relatively warm and wet Southeast (Figure 5a), corresponding with high levels of mean annual precipitation (MAP, >1200 mm) across a range of mean annual temperatures (MAT, ~10–20°C; Figure 5c). In contrast, NEE is more variable, with the highest values in the Midwest (Figure 5b) at intermediate to high levels of MAP (~750–1200 mm) and lower MAT (<10°C) (Figure 5d). The spatial covariance of GPP and NEE becomes decoupled as water availability increases. We found a strong precipitation threshold of ~700 mm/year over the continental US, below which NEE is regulated by photosynthetic gains and above which NEE is regulated to a greater degree by respiration losses (Liu et al. 2018). This result is consistent with ecosystem‐scale studies that show the greatest response in productivity to precipitation anomalies in semi‐arid grassland and shrubland ecosystems (Knapp and Smith 2001). However, the lateral transport of C through river flow and human harvest may also be important in uncoupling GPP from NEE at continental scales. This spatial mismatch is an important finding because it is often assumed that anomalies in photosynthesis directly result in anomalies in net CO2 exchange. In fact, it is impossible in standard eddy covariance approaches for partitioning fluxes to have increases in net exchange without increases in photosynthesis (Reichstein et al. 2005), and net C exchange in land‐surface models is dominated by photosynthetic inputs (Liu et al. 2018).
Figure 5.
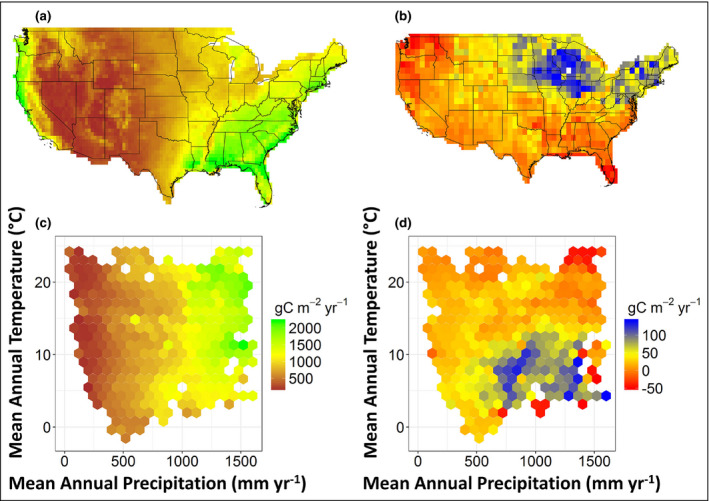
Continental‐scale estimates of mean annual GPP and net CO2 exchange (ie NEE) and their sensitivities to climate factors. (a) Continental‐scale estimates of GPP from MODIS satellite observations plotted within (c) their climate space of mean annual precipitation and mean annual temperature. (b) Continental‐scale estimates of NEE from atmospheric CO2 inversion methods plotted within (d) their climate space. All flux estimates are reported as g C/m2/year and have been projected to ecosystem area (modified from Liu et al. [2018]; see WebTable 1).
At the global scale, the relationship between the interannual variability of the atmospheric CO2 growth rate and tropical land‐surface temperatures has been identified as an emergent constraint, such that higher surface temperatures diminish NEE (Cox et al. 2013). However, identifying the processes associated with this diminished NEE is difficult because increased tropical temperatures suppress photosynthesis and/or promote respiration, both of which lead to reduced net C exchange. It has been suggested that total respiration is the most likely mechanism explaining the emergent relationship between interannual variability in the atmospheric growth rate and tropical surface temperature (Anderegg et al. 2015) and that water limitation is important in regulating net CO2 exchange at the local scale, whereas temperature becomes more important at global scales (Jung et al. 2017). Recent satellite evidence suggests that terrestrial water availability that integrates temperature and precipitation variability may be the ultimate mechanism regulating interannual NEE at the global scale (Humphrey et al. 2018). However, evidence derived from satellite estimates of XCO2 and solar induced fluorescence during the recent 2015/2016 El Niño event suggest that net tropical C uptake was reduced by different processes in different tropical regions – such as reduced photosynthesis in South America, increased respiration in Africa, and increased fire emissions in Southeast Asia (Liu et al. 2017). Therefore, even though we are gaining new insight on the climate sensitivity of important C‐cycle processes, ecosystem‐scale observations are still lacking in certain regions to help reconcile different C‐cycle processes operating at different spatial scales.
Conclusions and frontiers in C‐cycle research
A central goal of ecology at the macrosystem scale is to understand biosphere processes and their complex interactions with climate, land use, and changes in species distribution at regional to continental scales This has also been a central challenge of C‐cycle research because there is a long history of atmospheric CO2 observations that have enabled a better understanding of the C cycle at the global scale and a network of eddy covariance measurements of CO2 exchange at the ecosystem scale. However, reconciling differences in net CO2 exchange measured at these different scales continues to be difficult. We are now acquiring data from aircraft and satellites that allow important C‐cycle processes to be resolved at the biome scale. The terrestrial and aquatic ecological research communities are also compiling databases to elucidate important C‐cycle processes that may be merged to provide an integrated understanding of C transport and transformations across watersheds. Collectively, we are identifying missing pieces of the global C puzzle that now make it possible to reconcile and understand processes that help to explain discrepancies in C dynamics across scales.
Supporting information
Fig S1
Panel S1
Table S1
Acknowledgements
Publication of this Special Issue was funded by the US National Science Foundation (NSF award number DEB 1928375). APB was supported by the NSF Macrosystems Biology Program (1802810). ZL acknowledges support from the National Natural Science Foundation of China (41922006) and the KC Wong Education Foundation. PS acknowledges support from NSF (1552976, 1632810, 1702029) and the US Department of Agriculture National Institute of Food and Agriculture (USDA NIFA) Hatch project 228396. WRLA was supported by the David and Lucille Packard Foundation, NSF grants 1714972 and 1802880, and the USDA NIFA Agricultural and Food Research Initiative Competitive Program, Ecosystem Services and Agro‐ecosystem Management (2018‐67019‐27850). BP acknowledges support from the National Aeronautics and Space Administration Terrestrial Ecology Program.
Front Ecol Environ. 2021; 19(1): 57–65, doi: 10.1002/fee.2296
References
- Anav A, Friedlingstein P, Kidston M, et al. 2013. Evaluating the land and ocean components of the global carbon cycle in the CMIP5 Earth system models. J Climate 26: 6801–43. [Google Scholar]
- Anderegg WRL, Ballantyne AP, Smith WK, et al. 2015. Tropical nighttime warming as a dominant driver of variability in the terrestrial carbon sink. P Natl Acad Sci USA 112: 15591–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderegg WRL, Konings AG, Trugman AT, et al. 2018. Hydraulic diversity of forests regulates ecosystem resilience during drought. Nature 561: 538–41. [DOI] [PubMed] [Google Scholar]
- Ballantyne AP, Andres R, and Houghton R. 2015. Audit of the global carbon budget: estimate errors and their impact on uptake uncertainty. Biogeosciences 12: 2565–84. [Google Scholar]
- Ballantyne A, Smith W, Anderegg W, et al. 2017. Accelerating net terrestrial carbon uptake during the warming hiatus due to reduced respiration. Nat Clim Change 7: 148. [Google Scholar]
- Barber J. 2009. Photosynthetic energy conversion: natural and artificial. Chem Soc Rev 38: 185–96. [DOI] [PubMed] [Google Scholar]
- Bernhardt ES, Heffernan JB, Grimm NB, et al. 2018. The metabolic regimes of flowing waters. Limnol Oceanogr 63: S99–118. [Google Scholar]
- Bond‐Lamberty B and Thomson A. 2010. Temperature‐associated increases in the global soil respiration record. Nature 464: 579–82. [DOI] [PubMed] [Google Scholar]
- Butman DE, Striegl RG, Stackpoole SM, et al. 2018. Inland waters. In: Cavallaro N, Shrestha G, Birdsey R, et al. (Eds). Second State of the Carbon Cycle Report (SOCCR2): a sustained assessment report. Washington, DC: US Global Change Research Program. [Google Scholar]
- Chapin FS, Woodwell GM, Randerson JT, et al. 2006. Reconciling carbon‐cycle concepts, terminology, and methods. Ecosystems 9: 1041–50. [Google Scholar]
- Chu H, Baldocchi DD, John R, et al. 2017. Fluxes all of the time? A primer on the temporal representativeness of FLUXNET. J Geophys Res-Biogeo 122: 289–307. [Google Scholar]
- Ciais P, Tan J, Wang X, et al. 2019. Five decades of northern land carbon uptake revealed by the interhemispheric CO2 gradient. Nature 568: 221–25. [DOI] [PubMed] [Google Scholar]
- Cole JJ, Caraco NF, Kling GW, and Kratz TK. 1994. Carbon dioxide supersaturation in the surface waters of lakes. Science 265: 1568–70. [DOI] [PubMed] [Google Scholar]
- Commane R, Lindaas J, Benmergui J, et al. 2017. Carbon dioxide sources from Alaska driven by increasing early winter respiration from Arctic tundra. P Natl Acad Sci USA 114: 5361–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin KD, Denning AS, Lokupitiya EY, et al. 2010. Assessing the impact of crops on regional CO2 fluxes and atmospheric concentrations. Tellus B 62: 521–32. [Google Scholar]
- Cox PM, Pearson D, Booth BB, et al. 2013. Sensitivity of tropical carbon to climate change constrained by carbon dioxide variability. Nature 494: 341–44. [DOI] [PubMed] [Google Scholar]
- Friedlingstein P, Cox P, Betts R, et al. 2006. Climate–carbon cycle feedback analysis: results from the C4MIP model intercomparison. J Climate 19: 3337–53. [Google Scholar]
- Friedlingstein P, Meinshausen M, Arora VK, et al. 2013. Uncertainties in CMIP5 climate projections due to carbon cycle feedbacks. J Climate 27: 511–26. [Google Scholar]
- Fu Z, Gerken T, Bromley G, et al. 2018. The surface–atmosphere exchange of carbon dioxide in tropical rainforests: sensitivity to environmental drivers and flux measurement methodology. Agr Forest Meteorol 263: 292–307. [Google Scholar]
- Gatti LV, Gloor M, Miller JB, et al. 2014. Drought sensitivity of Amazonian carbon balance revealed by atmospheric measurements. Nature 506: 76–80. [DOI] [PubMed] [Google Scholar]
- GLOBALVIEW‐CO 2 . 1999. Cooperative atmospheric data integration project – carbon dioxide. Boulder, CO: National Oceanic and Atmospheric Administration. [Google Scholar]
- Hotchkiss ER, Hall RO Jr, Sponseller RA, et al. 2015. Sources of and processes controlling CO2 emissions change with the size of streams and rivers. Nat Geosci 8: 696. [Google Scholar]
- Humphrey V, Zscheischler J, Ciais P, et al. 2018. Sensitivity of atmospheric CO2 growth rate to observed changes in terrestrial water storage. Nature 560: 628–31. [DOI] [PubMed] [Google Scholar]
- Hursh A, Ballantyne A, Cooper L, et al. 2017. The sensitivity of soil respiration to soil temperature, moisture, and carbon supply at the global scale. Glob Change Biol 23: 2090–103. [DOI] [PubMed] [Google Scholar]
- Jocher G, Ottosson Löfvenius M, De Simon G, et al. 2017. Apparent winter CO2 uptake by a boreal forest due to decoupling. Agr Forest Meteorol 232: 23–34. [Google Scholar]
- Jung M, Reichstein M, Margolis HA, et al. 2011. Global patterns of land–atmosphere fluxes of carbon dioxide, latent heat, and sensible heat derived from eddy covariance, satellite, and meteorological observations. J Geophys Res-Biogeo 116: G00J07. [Google Scholar]
- Jung M, Reichstein M, Schwalm CR, et al. 2017. Compensatory water effects link yearly global land CO2 sink changes to temperature. Nature 541: 516–20. [DOI] [PubMed] [Google Scholar]
- Keenan TF, Migliavacca M, Papale D, et al. 2019. Widespread inhibition of daytime ecosystem respiration. Nature Ecol Evol 3: 407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp AK and Smith MD. 2001. Variation among biomes in temporal dynamics of aboveground primary production. Science 291: 481–84. [DOI] [PubMed] [Google Scholar]
- Landschützer P, Gruber N, Haumann FA, et al. 2015. The reinvigoration of the Southern Ocean carbon sink. Science 349: 1221–24. [DOI] [PubMed] [Google Scholar]
- Le Quéré C, Andrew RM, Canadell JG, et al. 2016. Global carbon budget 2016. Earth Syst Sci Data 8: 605–49. [Google Scholar]
- Liu J, Bowman KW, Schimel DS, et al. 2017. Contrasting carbon cycle responses of the tropical continents to the 2015–2016 El Niño. Science 358: eaam5690. [DOI] [PubMed] [Google Scholar]
- Liu Z, Ballantyne AP, Poulter B, et al. 2018. Precipitation thresholds regulate net carbon exchange at the continental scale. Nat Commun 9: 3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo YQ, Randerson JT, Friedlingstein P, et al. 2012. A framework for benchmarking land models. Biogeosciences 9: 3857–74. [Google Scholar]
- Luyssaert S, Schulze E‐D, Börner A, et al. 2008. Old‐growth forests as global carbon sinks. Nature 455: 213–15. [DOI] [PubMed] [Google Scholar]
- Neff JC and Asner GP. 2001. Dissolved organic carbon in terrestrial ecosystems: synthesis and a model. Ecosystems 4: 29–48. [Google Scholar]
- Obama B. 2017. The irreversible momentum of clean energy. Science 355: 126–29. [DOI] [PubMed] [Google Scholar]
- Peters W, Jacobson AR, Sweeney C, et al. 2007. An atmospheric perspective on North American carbon dioxide exchange: CarbonTracker. P Natl Acad Sci USA 104: 18925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peylin P, Law RM, Gurney KR, et al. 2013. Global atmospheric carbon budget: results from an ensemble of atmospheric CO2 inversions. Biogeosciences 10: 6699–720. [Google Scholar]
- Pinzon JE and Tucker CJ. 2010. GIMMS 3g NDVI set and global NDVI trends. In: Second Yamal Land‐Cover Land‐Use Change Workshop; 8–10 Mar 2010; Rovaniemi, Finland. Fairbanks, AK: University of Alaska.
- Randerson JT, Chapin FS III, Harden JW, et al. 2002. Net ecosystem production: a comprehensive measure of net carbon accumulation by ecosystems. Ecol Appl 12: 937–47. [Google Scholar]
- Raymond PA, Hartmann J, Lauerwald R, et al. 2013. Global carbon dioxide emissions from inland waters. Nature 503: 355–59. [DOI] [PubMed] [Google Scholar]
- Reichstein M, Falge E, Baldocchi D, et al. 2005. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: review and improved algorithm. Glob Change Biol 11: 1424–39. [Google Scholar]
- Schimel D, Pavlick R, Fisher JB, et al. 2015. Observing terrestrial ecosystems and the carbon cycle from space. Glob Change Biol 21: 1762–76. [DOI] [PubMed] [Google Scholar]
- Schulze E‐D, Wirth C, and Heimann M. 2000. Managing forests after Kyoto. Science 289: 2058–59. [DOI] [PubMed] [Google Scholar]
- Song X‐P, Hansen MC, Stehman SV, et al. 2018. Global land change from 1982 to 2016. Nature 560: 639–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens BB, Gurney KR, Tans PP, et al. 2007. Weak northern and strong tropical land carbon uptake from vertical profiles of atmospheric CO2 . Science 316: 1732–35. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Martin JG, Law BE, and Davis K. 2013. Toward biologically meaningful net carbon exchange estimates for tall, dense canopies: multi‐level eddy covariance observations and canopy coupling regimes in a mature Douglas‐fir forest in Oregon. Agr Forest Meteorol 173: 14–27. [Google Scholar]
- Thornton PE, Lamarque J‐F, Rosenbloom NA, and Mahowald NM. 2007. Influence of carbon–nitrogen cycle coupling on land model response to CO2 fertilization and climate variability. Global Biogeochem Cy 21: GB4018. [Google Scholar]
- Unger N, Harper K, Zheng Y, et al. 2013. Photosynthesis‐dependent isoprene emission from leaf to planet in a global carbon–chemistry–climate model. Atmos Chem Phys 13: 10243–69. [Google Scholar]
- Villarreal S, Guevara M, Alcaraz‐Segura D, et al. 2018. Ecosystem functional diversity and the representativeness of environmental networks across the conterminous United States. Agr Forest Meteorol 262: 423–33. [Google Scholar]
- Wang T, Ottlé C, Boone A, et al. 2013. Evaluation of an improved intermediate complexity snow scheme in the ORCHIDEE land surface model. J Geophys Res-Atmos 118: 6064–79. [Google Scholar]
- Wehr R, Munger JW, McManus JB, et al. 2016. Seasonality of temperate forest photosynthesis and daytime respiration. Nature 534: 680–83. [DOI] [PubMed] [Google Scholar]
- Wieder WR, Bonan GB, and Allison SD. 2013. Global soil carbon projections are improved by modelling microbial processes. Nat Clim Change 3: 909. [Google Scholar]
- Wofsy SC, Goulden ML, Munger JW, et al. 1993. Net exchange of CO2 in a mid‐latitude forest. Science 260: 1314–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Panel S1
Table S1


