Abstract
Purpose
This study aimed to assess the safety risks associated with using nonsteroidal anti‐inflammatory drugs (NSAIDs) in elderly patients (≥65 years) compared with younger patients (<65 years) with osteoarthritis (OA) and/or chronic low back pain (CLBP).
Methods
A retrospective analysis was conducted on anonymized claims data of patients prescribed NSAIDs for OA and/or CLBP from 2009 to 2018 using hospital‐based administrative database—Medical Data Vision (MDV). The key outcome was the incidence of developing gastrointestinal (GI), renal, and acute myocardial infarction (AMI) that are well‐known events associated with NSAID use.
Results
Of 288,715 patients included, 23.7%, 60.5%, and 15.8% had OA, CLBP, or both, respectively. Elderly patients used non‐oral NSAIDs more frequently than oral NSAIDs (57.8% and 38.7%, respectively), whereas younger patients showed comparable use (50.7% and 52.8%, respectively). The incidence of events per 10,000 person‐years (95% CI) was higher in the elderly than in younger patients: GI, 29.68(27.67–31.68) vs. 16.61(14.60–18.63); renal, 124.77(120.56–128.99) vs. 39.88(36.72–43.03); and AMI, 27.41(25.48–29.35) vs. 10.90(9.27–12.53), respectively. After adjusting for covariates, the increase in risk for these events was seen in patients >70 years compared with younger patients (18–30 years) and was remarkable in patients >80 years with 2‐fold, 10‐fold, and 7‐fold higher risk for developing GI, renal, and AMI events, respectively.
Conclusion
Risk for developing NSAID‐associated events was higher in the elderly; particularly, renal and AMI events that remarkably increased in patients >80 years. To reduce them, NSAIDs should be prescribed at the lowest effective dose for the shortest duration possible.
Keywords: acute myocardial infarction, chronic low back pain, gastrointestinal events, nonsteroidal anti‐inflammatory drugs, osteoarthritis, renal events
INTRODUCTION
Musculoskeletal conditions such as osteoarthritis (OA) and chronic low back pain (CLBP) manifest as pain and disability and are a common health issue in aging populations worldwide. 1 , 2 These conditions exacerbate the economic and disease burden in countries whose populations comprise a high proportion of the elderly, for example, Japan with >25% of the population ≥65 years. 3 The average age of Japanese patients with OA or CLBP is reported to be 65–69 years. 4 Super‐elderly (≥80 years old) comprised approximately 9% of the Japanese general population in 2018, and this percentage is on the rise. 5 In Japan, ~25.3 million people >40 years have OA, and the prevalence of CLBP is ~24.8% in people >50 years. 6 , 7 Various age‐related mental, physical, and psychological changes (eg, dementia, age‐related changes in central pain processing, physical inactivity, and spinal degeneration) affect pain management in elderly patients. 8 Moreover, higher prevalence of comorbid conditions and resultant polypharmacy further complicates the management of musculoskeletal pain in this population. 8
Transdermal NSAIDs are recommended as the first‐line treatment for pain due to knee and hip OA and are also the most commonly used analgesics for CLBP in Japan. 9 , 10 NSAIDs are also the first‐line therapy for mild to moderate or chronic musculoskeletal pain in the elderly. 11 , 12 However, NSAID use, particularly long‐term, is associated with adverse effects including gastrointestinal (GI) bleeding, deteriorating renal function, and cardiovascular complications. 13 , 14 , 15 Despite the known risk of adverse events, high proportions of elderly patients consulting general practitioners are prescribed NSAIDs regularly, and about one third of these patients used NSAIDs for more than 1 month. 16 In the elderly, polypharmacy including NSAIDs increases the potential for drug interaction and, thus, puts the elderly at a greater risk of adverse outcomes. 17 , 18 In a previous study, we used a commercial claims database—JMDC—and reported increased risk for GI, renal, and AMI events in patients with OA and CLBP using NSAIDs; however, as JMDC includes patients who are working, there were no patients >75 years and the data for patients >65 years were limited. 19 , 20 , 21 In these studies, comorbidities were associated with increasing risk for GI, renal, and AMI events, and the elderly presumably have a high prevalence of comorbidities. 3 As age is a known risk factor for these events, in the current study, we assessed the risk for GI, renal, and AMI events associated with the use of NSAIDs in the elderly (≥65 years) compared with younger (<65 years) patients with OA/CLBP by using the Medical Data Vision (MDV) database. We also quantified the effect of age on those events and estimated the risk by model analysis in the super‐elderly.
METHODS
Ethics
As the Japanese Ethical Guidelines for “Medical and Health Research Involving Human Subjects” do not apply to studies that use anonymized secondary data, review of an institutional review board/research ethics committee (IRB/REC) was not required for this study.
Study design and patient selection
This retrospective, noninterventional database analysis used anonymized claims data from the MDV database for patients with OA and CLBP who were prescribed NSAIDs in clinical practice in Japan. The MDV database is a nationwide hospital‐based claims database, covering patients treated as inpatients or outpatients and participating in the Diagnostic Procedure Combination/Per‐Diem payment system. The database covers patients of all age‐groups, making it appropriate for use for the study purpose. However, the MDV database does not cover data outside of the hospital and cannot follow up patients when they move to other medical institutes. All patient data were encrypted before entry. Diseases were coded according to the Japanese Claims Codes and the coding scheme of the World Health Organization International Classification of Diseases, 10th revision (ICD‐10).
For this study, eligible patients were ≥18 years at the index date, had an initial ICD‐10 diagnosis of OA (ICD‐10 codes M16 and M17) or CLBP (at least 2 ICD‐10 low back pain diagnoses [M40, M41, M43, M45‐M48, and M50‐M54] at an interval of ≥1 month within the previous 3 months), and had visited health‐care facilities (as recorded in the administrative database) between January 1, 2009, and December 31, 2018. Patients with malignancy (ICD10 codes: C00‐C97, D00‐D09) detected after the initial diagnosis of OA or CLBP were excluded. Also patients diagnosed with CLBP but having other diagnoses such as neck pain, radiculopathy and myelopathy, infection, vascular disease, acute low back pain, and limb symptoms (detailed ICD codes are described in the previous JMDC publication) were excluded. 19 , 20 , 21 Patients taking ≥2 prescriptions of the same or different analgesics at ≥1‐month interval after the initial diagnosis of OA or CLBP and at 6 months of the baseline period with no prescriptions for analgesics were included. The index date was defined as the date of the first prescription of an analgesic after the initial diagnosis of OA or CLBP. As this study involved anonymized structured data, no informed consent was sought from patients. The study is reported in compliance with the REporting of studies Conducted using Observational Routinely‐collected health Data (RECORD) statement. 21
Exposure
The exposure period was defined as a combination of series of NSAID treatments plus 3 months that ended on the day the first event occurred. A series of NSAID treatments (single or multiple treatment periods) within the same class of pain drugs prescribed had up to a 3‐month gap from the end date of previous prescription until the initial date of new prescription.
Study outcomes
The key outcome was the incidence of GI, renal, and AMI events in patients who were prescribed NSAIDs in the MDV database. GI events were defined in terms of bleeding and perforation. Renal events included acute/chronic tubulointerstitial nephritis, NSAID nephropathy, drug‐induced renal disease, acute kidney injury, chronic kidney disease (CKD), and end‐stage renal failure. Progression of CKD was defined as initiation of dialysis after treatment with NSAIDs in patients with renal disease not requiring dialysis at baseline. AMI events were defined as the occurrence of acute myocardial infarction (AMI) at any time during exposure. Detailed description of ICD10 codes for these events is provided in the previous articles. 19 , 20 , 21
Statistical analysis
All patients who met the eligibility criteria were included in the analysis set. Continuous variables were summarized using descriptive statistics or frequencies, with percentages for dichotomous and polychotomous variables of categorical data. The crude incidence (per 10,000 person‐years) of events and its 95% CI were calculated. The incidence rate was calculated as the number of patients who experienced an event divided by the total exposure period. The overdispersed Poisson regression model using the SAS GLIMMIX procedure with the log link function and the logarithm of the exposure period as an offset was used to identify factors associated with the risk of developing these events. Covariates included sex, baseline comorbidities, and NSAID treatment variables, namely, treatment duration, consistent use of NSAIDs (supply days of ≥70% of the total treatment duration), and means of administration. We compared the incidence of adverse events in elderly patients (≥65 years) with the younger patients (<65 years). We estimated that the effect of increasing age on the risk for developing these events using the Poisson regression model. In the model analysis, increasingly detailed age categories with 10‐year intervals (lowest category including 18–<20 years and 20–<30 years to avoid small sample size) were selected to evaluate gradual effects of aging, rather than evaluating only two large age categories of ≥65 years and <65 years; meanwhile, the relative risk in the super‐elderly was also estimated as their population is substantial in Japan. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). An alpha level of 0.05 was considered statistically significant.
RESULTS
Patient disposition
Between January 2009 and December 2018, a total of 1,819,836 patients were diagnosed with OA and/or CLBP in the MDV database, of which 1,209,106 were prescribed one or more analgesics at least twice (Figure 1). After excluding patients meeting the aforementioned exclusion criteria, 288,715 patients were included in the analysis. Patient demographics, clinical characteristics, and NSAID use are described in Table 1. The mean (SD) age of the patients was 67.7 (15.0) years at the index date. Of the total patients included in the analysis, a majority (68.5%) were ≥65 years (hereafter, the elderly patients).
FIGURE 1.
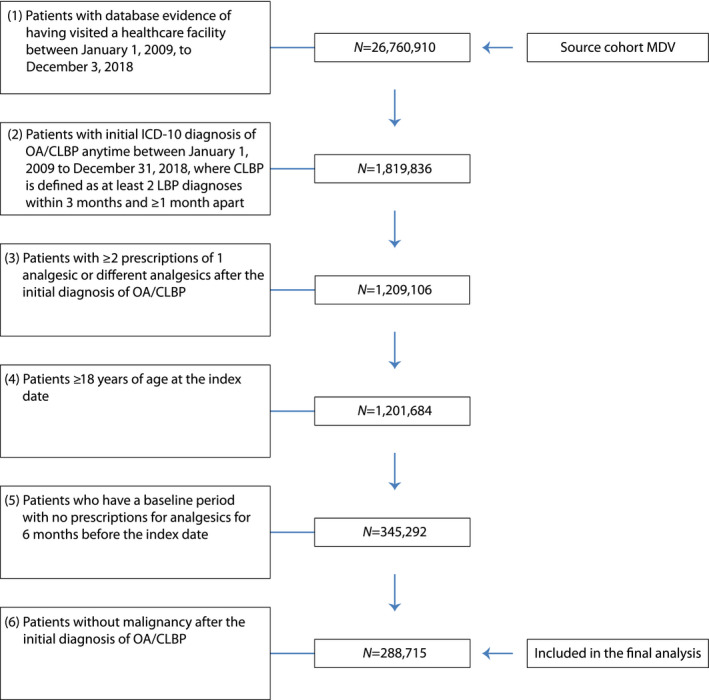
Patient flowchart. CLBP, chronic low back pain; MDV, Medical Data Vision; OA, osteoarthritis; ICD‐10, International Classification of Disease‐10
TABLE 1.
Patient demographic and clinical characteristics
| Characteristic | All patients (N = 288,715) | Patients <65 years (n = 96,940) | Patients ≥65 years (n = 191,775) |
|---|---|---|---|
| Age at index date (years), mean (SD) | 67.7 (15.0) | 50.5 (11.2) | 76.3 (7.1) |
| Age categories (years), n (%) | |||
| 18 to <30 | 5677 (2.0) | 5677 (5.9) | NA |
| 30 to <40 | 12,182 (4.2) | 12,182 (12.6) | NA |
| 40 to <50 | 20,689 (7.2) | 20,689 (21.3) | NA |
| 50 to <60 | 31,574 (10.9) | 31,574 (32.6) | NA |
| 60 to <70 | 65,653 (22.7) | 26,818 (27.7) | 38,835 (20.3) |
| 70 to <80 | 89,860 (31.1) | 0 (0.0) | 89,860 (46.9) |
| ≥80 | 63,080 (21.8) | 0 (0.0) | 63,080 (32.9) |
| Sex, male, n (%) | 118,976 (41.2) | 40,645 (41.9) | 78,331 (40.8) |
| Follow‐up duration (days), median (IQR) | 736.0 (329.0, 1295.0) | 775.0 (341.0, 1364.0) | 715.0 (323.0, 1262.0) |
| Follow‐up duration on treatment (days), median (IQR) | 445.0 (161.0, 960.0) | 458.0 (161.0, 1008.0) | 439.0 (161.0, 939.0) |
| Diagnosis, n (%) | |||
| OA | 68,556 (23.7) | 19,171 (19.8) | 49,385 (25.6) |
| CLBP | 174,637 (60.5) | 65,568 (67.6) | 107,069 (55.8) |
| OA and CLBP | 45,522 (15.8) | 10,201 (10.5) | 35321 (18.4) |
| Affected joints in OA patients, n (%) | |||
| Coxarthrosis | 19,255 (6.7) | 6,232 (6.4) | 13,023 (6.8) |
| Gonarthrosis | 94,823 (32.8) | 23,140 (23.9) | 71,683 (37.4) |
| Comorbidities, n (%) | |||
| Gastrointestinal disease | 47,034 (16.3) | 13,485 (13.9) | 33,549 (17.5) |
| Renal disease | 12,828 (4.4) | 2701 (2.8) | 10,127 (5.3) |
| Cardiovascular disease (excluding hypertension) | 101,320 (35.1) | 19,556 (20.2) | 81,764 (42.6) |
| Hypertension | 102,769 (35.6) | 22,068 (22.8) | 80,701 (42.1) |
| Diabetes mellitus | 66,343 (23.0) | 16,480 (17.0) | 49,863 (26.0) |
| Health‐care facility setup | |||
| Public (government) | 114,750 (39.7) | 40,303 (41.6) | 74,447 (38.8) |
| Public (local) | 50,135 (17.4) | 15,670 (16.2) | 34,465 (18.0) |
| University | 13,745 (4.8) | 5847 (6.0) | 7898 (4.1) |
| Private | 110,085 (38.1) | 35,120 (36.2) | 74,965 (39.1) |
Abbreviations: CLBP, chronic low back pain; IQR, interquartile range; OA, osteoarthritis.
The prevalence of concurrent occurrence of OA and CLBP was greater in the elderly patients than in the younger patients (18.4% vs. 10.5%). Hypertension (35.6%) was the most common comorbid condition, followed by other cardiovascular diseases (35.1%), diabetes (23%), GI diseases (16.3%), and renal diseases (4.4%). The prevalence of cardiovascular comorbidities, hypertension, and diabetes was greater in the elderly patients than in the younger patients. Patient characteristics were similar between the OA and CLBP subgroups (Table S1).
NSAID treatment
NSAIDs were used as first‐line analgesics in 232,183 (80.4%) patients (Figure 2). Overall, 43.4% of patients used oral NSAIDs and 45.4% used NSAID patches (Figure 2). Analysis of first‐line NSAID use by age‐group showed that elderly patients used patch NSAIDs more frequently than oral NSAIDs; patch and other transdermal/suppository NSAIDs were used by 50.7% and 57.8% in the elderly and younger patient groups, respectively. Oral NSAIDs were used by 38.7% and 52.8% of patients in the elderly and younger patient groups, respectively (Figure 2). A combination of oral and topical (patches, other transdermal, and suppository) NSAIDs was used by 51.4% of patients (Table S2). A total of 65.6% of the elderly patients and 73.9% of the younger patients, respectively, used NSAIDs for up to 1 year. Elderly patients used NSAIDs more consistently than the younger patients (42.7% vs. 32.1%). Among non‐NSAID analgesics prescribed as first‐line treatment, acetaminophen (12.2%) was the most frequent, followed by α2δ ligand (9.0%) and hyaluronate injection (5.2%) (Figure 2). Patterns of NSAID treatment remained similar between patients with OA and those with CLBP (Table S2).
FIGURE 2.
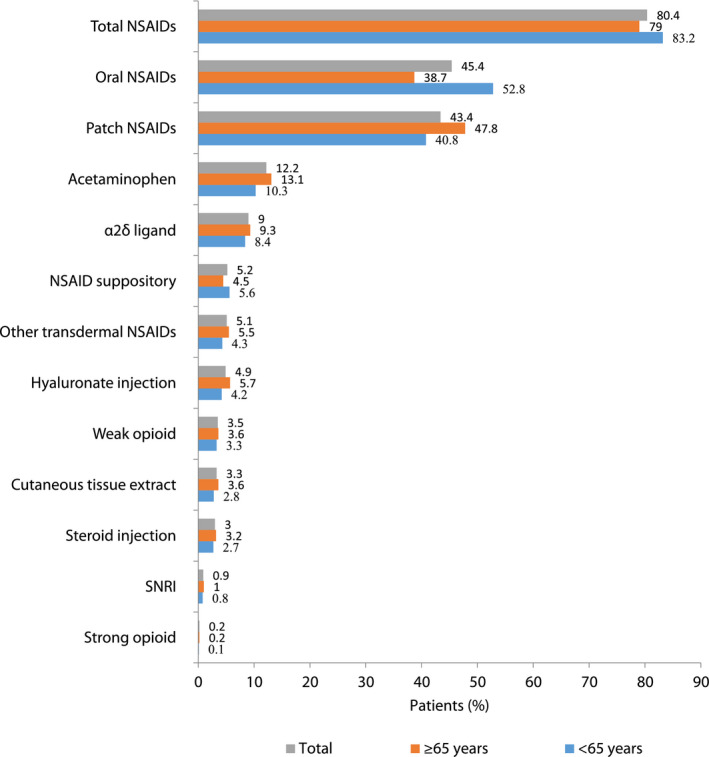
Prescription patterns of NSAIDs and other analgesics as first‐line pain medicines for entire population (N = 288,715) and for subgroup of patients <65 years (n = 96,940) and ≥65 years old (n = 191,775). NSAID, nonsteroidal anti‐inflammatory drug; SNRI, serotonin–norepinephrine reuptake inhibitors
Incidence rates of GI, renal, and AMI events
The incidence rate (95% CI) of GI, renal, and AMI events was higher in the elderly than in the younger patients—GI: 29.68 (27.67–31.68) vs. 16.61 (14.60–18.63), renal: 124.77 (120.56–128.99) vs. 39.88 (36.72–43.03) and AMI: 27.41 (25.48–29.35) vs. 10.90 (9.27–12.53), respectively (Table 2). In the model analysis, the risk of developing all 3 events significantly increased with age, which was significant for age greater than 60 years for renal and AMI events, and for age >80 years for GI events after adjusting for covariates, namely, gender, comorbidities, and variables of NSAID use (Table 3). In the super‐elderly, the risk ratio for the development of GI events was 2.19 (95% CI: 1.19–4.00); renal events, 10.29 (95% CI: 5.52–19.19); and AMI events, 6.56 (95% CI: 2.10–20.52) compared with patients aged 18–30 years. Men were found to be at a higher risk of developing these events than women. The presence of cardiovascular comorbidities and diabetes mellitus at baseline was found to increase the risk of developing GI, renal, and AMI events. In the model analysis, compared with the use of oral NSAIDs, the use of NSAID patches was associated with a similar risk of developing GI and AMI events and even a higher risk of renal events. The use of other transdermal NSAIDs (cream, gel, liquid, lotion, and ointments) were found to increase the risk for all 3 events. The incidence of GI, renal, and AMI events remained similar in patients with OA and those with CLBP (Table S3).
TABLE 2.
Incidence of gastrointestinal, renal, and acute myocardial infarction events
| Event | All patients | Patients <65 years | Patients ≥65 years | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Patients with an event (n) | Incidence rate per 10,000 person‐years (95% CI) | N a | Patients with an event (n) | Incidence rate per 10,000 person‐years (95% CI) | N a | Patients with an event (n) | Incidence rate per 10,000 person‐years (95% CI) | |
| Gastrointestinal | 231,079 | 1103 | 25.01 (23.53–26.48) | 80,410 | 262 | 16.61 (14.60–18.63) | 150,669 | 841 | 29.68 (27.67–31.68) |
| Renal | 223,155 | 3982 | 93.94 (91.02–96.85) | 78,803 | 614 | 39.88 (36.72–43.03) | 144,352 | 3,368 | 124.77 (120.56–128.99) |
| Acute myocardial infarction | 230,252 | 945 | 21.49 (20.12–22.86) | 80,343 | 172 | 10.90 (9.27–12.53) | 149,909 | 773 | 27.41 (25.48–29.35) |
Patients who had an event of interest at baseline were excluded.
TABLE 3.
Multivariate generalized linear model analysis with covariates for predicting event rates
| Gastrointestinal (n a = 1103) | Renal (n a = 3982) | Acute myocardial infarction (n a = 945) | ||||
|---|---|---|---|---|---|---|
| Risk ratio (95% CI) | p value | Risk ratio (95% CI) | p value | Risk ratio (95% CI) | p value | |
| Age (years) vs reference: 18 to <30 years | ||||||
| 30 to <40 | 0.77 (0.37–1.59) | 0.4858 | 1.23 (0.60–2.50) | 0.5683 | 0.25 (0.04–1.53) | 0.1342 |
| 40 to <50 | 1.17 (0.62–2.22) | 0.6312 | 1.86 (0.97–3.57) | 0.0627 | 1.77 (0.54–5.85) | 0.3469 |
| 50 to <60 | 0.92 (0.49–1.73) | 0.8006 | 2.96 (1.57–5.58) | 0.0008 | 2.89 (0.91–9.19) | 0.0714 |
| 60 to <70 | 1.12 (0.61–2.07) | 0.7064 | 3.47 (1.86–6.49) | <0.0001 | 3.67 (1.17–11.49) | 0.0256 |
| 70 to <80 | 1.37 (0.75–2.51) | 0.3043 | 5.62 (3.02–10.49) | <0.0001 | 4.66 (1.49–14.56) | 0.0081 |
| >80 | 2.19 (1.19–4.00) | 0.0113 | 10.29 (5.52–19.19) | <0.0001 | 6.56 (2.10–20.52) | 0.0012 |
| Sex, male | 1.20 (1.06–1.35) | 0.0035 | 1.70 (1.60–1.81) | <0.0001 | 2.19 (1.92–2.50) | <0.0001 |
| Baseline GI comorbidities | 1.56 (1.28–1.90) | <0.0001 | 1.08 (0.96–1.21) | 0.2278 | 1.08 (0.84–1.38) | 0.5492 |
| Baseline renal comorbidities | 2.59 (1.97–3.41) | <0.0001 | NA b | NA b | 1.54 (1.09–2.18) | 0.0155 |
| Baseline cardiovascular comorbidities | 1.63 (1.39–1.92) | <0.0001 | 1.62 (1.49–1.76) | <0.0001 | 1.58 (1.33–1.88) | <0.0001 |
| Baseline hypertension | 1.03 (0.85–1.24) | 0.7832 | 1.37 (1.25–1.51) | <0.0001 | 1.13 (0.92–1.39) | 0.2410 |
| Baseline diabetes mellitus | 1.48 (1.23–1.79) | <0.0001 | 1.61 (1.46–1.78) | <0.0001 | 1.45 (1.18–1.78) | 0.0004 |
| Duration of treatment vs 0 to <1 year | ||||||
| 1 to ≤3 months | 1.07 (0.80–1.43) | 0.6599 | NA c | NA | NA c | NA |
| >3 to ≤6 months | 0.95 (0.71–1.27) | 0.7137 | NA c | NA | NA c | NA |
| >6 to ≤12 months | 1.06 (0.80–1.41) | 0.6785 | NA c | NA | NA c | NA |
| >1 to ≤3 years | 0.74 (0.56–0.98) | 0.0361 | 0.91 (0.84–0.97) | 0.0073 | 0.75 (0.64–0.87) | 0.0001 |
| >3 to ≤5 years | 0.62 (0.45–0.85) | 0.0032 | 0.78 (0.70–0.87) | <0.0001 | 0.64 (0.51–0.80) | <0.0001 |
| >5 years | 0.55 (0.37–0.81) | 0.0029 | 0.69 (0.59–0.81) | <0.0001 | 0.50 (0.36–0.71) | <0.0001 |
| Consistent use of NSAIDs (supply days of ≥70%) | 1.55 (1.34–1.78) | <0.0001 | 1.35 (1.25–1.45) | <0.0001 | 1.56 (1.35–1.81) | <0.0001 |
| Method of administration vs oral | ||||||
| Patch NSAIDs | 1.10 (0.83–1.44) | 0.5118 | 1.48 (1.29–1.69) | <0.0001 | 1.30 (0.98–1.73) | 0.0658 |
| Other transdermal NSAIDs | 1.63 (1.28–2.07) | <0.0001 | 1.36 (1.20–1.54) | <0.0001 | 1.38 (1.06–1.79) | 0.0149 |
| Combination of oral and patch NSAIDs | 0.78 (0.68–0.90) | 0.0007 | 0.77 (0.71–0.83) | <0.0001 | 0.91 (0.78–1.06) | 0.2273 |
Abbreviations: GI, gastrointestinal; NSAIDs, nonsteroidal anti‐inflammatory drugs.
Patients with renal disease at baseline were excluded because these are the same as the disease for the renal event.
Patients who had disease of the event at baseline were excluded.
Short‐term intervals for renal and acute myocardial infarction events were not evaluated because these events are considered long‐term risk.
DISCUSSION
Our study attempted to assess the risk of NSAID‐associated GI, renal, and AMI events in elderly patients compared with younger patients with OA and/or CLBP and to quantify the effects of age on these events by using the claims data of a nationwide hospital‐based claims database—MDV. As expected, NSAIDs were the most commonly prescribed analgesics in patients with OA and/or CLBP in both the elderly and younger patients. Notably, the elderly patients used patch NSAIDs more frequently than oral NSAIDs compared with the younger patients. Incidences of all 3 events were higher in the elderly than in younger patients. The risk ratio for GI, renal, and AMI events increased by 2‐fold, 10‐fold, and 7‐fold, respectively, in patients >80 years than in patients aged 18–30 years. Using NSAID patches exhibited similar risk of developing GI and AMI events compared with using oral NSAIDs.
Multimorbidity complicates pain management in older adults. 22 Cardiovascular comorbidities are commonly reported in the elderly as well as in patients with OA and CLBP, further increasing the risk of developing cardiovascular events associated with NSAID use. 23 , 24 , 25 Accordingly, the Osteoarthritis Research Society International (OARSI) guidelines do not recommend the use of NSAIDs in patients with cardiovascular comorbidities. 10 However, regardless of this recommendation, patients with cardiovascular comorbidities continue to use NSAIDs frequently. 26 Several studies have shown an increase in the risk for developing cardiovascular events in patients using NSAIDs. 14 , 27 Furthermore, as hypertension and heart failure are often the common comorbid conditions in elderly patients with chronic pain, renin angiotensin system inhibitors and diuretics are frequently co‐prescribed with NSAIDs, which can potentially impede renal blood flow and worsen renal ischemia. 28 , 29 As many as 60% of the elderly patients were reportedly given medications for hypertension and/or congestive heart failure along with NSAIDs in Norway. 30 Expectedly, NSAID use in combination with diuretics was found to be a significant predictor of acute kidney injury in hospitalized elderly patients. 31 Moreover, our results validate the findings of all previous studies that showed an association between NSAIDs and adverse clinical events; however, these studies did not clearly indicate their trend in the elderly. 32 , 33 , 34 , 35
Aging itself is a known risk factor for increased GI bleeding. 36 The use of NSAIDs was reportedly found to increase the risk of GI bleeding by 4‐fold in elderly patients. 33 With increasing age, the severity and frequency of NSAID‐associated GI bleeding and ulceration were also found to increase. 32 , 33 , 34 , 35 Aging is also associated with worsening of cardiovascular functions, leading to increased risk for cardiovascular disease in older adults. 23 It is known to produce structural changes in the kidney, which reduces the kidney reserve and increases the risk for acute kidney injury in older individuals. 37 Our study quantified the additive effects of aging on the risk for developing AMI, GI, and renal events in elderly patients.
Several studies have identified the risk factors affecting the incidence of NSAID‐associated adverse events. Some of these risk factors include older age, prior history or presence of GI comorbidities, use of anticoagulants or corticosteroids, higher dose or multiple NSAIDs, cardiovascular and cerebrovascular disease, diabetes mellitus, and longer use of NSAIDs. 38 , 39 , 40 Diabetes and AMI comorbidities were associated with increased risks of all 3 events in our study. Male patients had higher risk for developing all 3 events; this was apparent for the AMI events in our study. However, previous studies have shown inconsistent results with respect to associating gender as a risk factor for these events.
In our study, elderly patients with OA and CLBP more frequently used patch NSAIDs than oral NSAIDs probably because NSAID patches tend to be used consistently over long term due to their better safety profile. Professional societies including the American Geriatric Society recommend caution when using NSAIDs and the use of the lowest effective dose for the shortest duration during episodic flares. 41 , 42 Common GI, renal, and cardiovascular side effects should be routinely monitored when NSAIDs are prescribed, particularly in elderly patients. This is important even in patients using patch NSAIDs as there is no difference in the risk for developing adverse events between patch and oral formulations of NSAIDs. 19 , 20 , 21 Repeated use or high doses of topical NSAIDs may increase systemic exposure of NSAIDs to levels similar to that of oral NSAIDs. Therefore, switching from oral to patch NSAIDs in high‐risk patients is not expected to offer any additional advantage in lowering the risk of developing these events. Moreover, as shown in an online survey in Japan, 37% of patients used patch NSAIDs along with oral NSAIDs owing to dissatisfaction with patches alone. 43 Therefore, when pain is not relieved by NSAIDs, switching to other treatments should be considered. 44
One of the limitations of this study is that the MDV only covers hospitals that provide acute in‐hospital care, and data from other medical institutions such as prescriptions given by general practitioners are not included in the database. Therefore, our study results cannot be generalized to the overall Japanese population. The MDV data do not capture any medical records other than the hospital records, and the record was censored if the patient changed the hospital; therefore, adverse events that developed while being treated in other hospitals or clinics could not be captured, leading to possible underestimation of their incidence rate. Estimation of the effect of NSAID variables, such as treatment duration, on the risk of events of interest was not possible. The increase in risk for developing adverse events when over‐the‐counter NSAIDs are used may not be estimated here. In addition, we assumed the first‐line analgesic by selecting patients with a 6‐month baseline period of no prescriptions for OA or CLBP; however, as MDV does not cover data of other hospitals, this remains an assumption. Furthermore, as this was an observational and retrospective study performed using a secondary data source, it may have potential errors in coding or record keeping.
CONCLUSION
NSAIDs are commonly used analgesics in patients with OA and/or CLBP, and their use continued in the elderly, despite the augmented risk of developing GI, renal, and AMI events with increasing age, especially in patients with multimorbidities. The elderly used topical NSAIDs more than oral NSAIDs, although the risk of events was similar in patients using patch or oral NSAIDs. The increase in risk for renal and AMI events was remarkable in the super‐elderly (ie, ≥80 years old). Therefore, to minimize NSAID‐associated adverse events, NSAIDs should be prescribed for the shortest duration possible at the lowest effective dose and with careful surveillance for GI, renal, and cardiovascular events, especially in elderly patients.
CONFLICT OF INTEREST
Kanae Togo, Nozomi Ebata, and Naohiro Yonemoto are employees of Pfizer Japan Inc., and shareholders of Pfizer Inc. Lucy Abraham is an employee and shareholder of Pfizer Ltd.
AUTHOR CONTRIBUTIONS
All authors contributed to the concept and design of the study, analysis and interpretation of data, drafting/revising the manuscript for important intellectual content, and approval of the final version to be published. KT and NE were also involved in the acquisition of data. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Supporting information
Tables S1–S3
ACKNOWLEDGMENTS
The authors thank Dr. Shogo Kikuchi, Dr. Takayuki Katsuno, and Dr. Koichi Fujii for their advice in planning this study. Editorial and/or medical writing support was provided by MedPro Clinical Research (Haruyoshi Ogata) and CBCC Global Research (Leena Patel and Sonali Dalwadi), and the study was funded by Pfizer Japan Inc. Data analytics support for this study was provided by the Institute of Japanese Union of Scientists & Engineers (Kazuhiko Hase and Yasushi Shimoda), who were paid contractors of Pfizer Japan Inc.
Togo K, Ebata N, Yonemoto N, Abraham L. Safety risk associated with use of nonsteroidal anti‐inflammatory drugs in Japanese elderly compared with younger patients with osteoarthritis and/or chronic low back pain: A retrospective database study. Pain Pract.2022;22:200–209. 10.1111/papr.13079
Funding information
The study was sponsored by Pfizer Japan Inc.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are not publicly available as all the rights for database ownership are reserved with Medical Data Vision. However, Pfizer Inc. have a contract with Medical Data Vision to use this database and publish the results.
REFERENCES
- 1. Briggs AM, Cross MJ, Hoy DG, Sànchez‐Riera L, Blyth FM, Woolf AD, et al. Musculoskeletal health conditions represent a global threat to healthy aging: a report for the 2015 World Health Organization world report on ageing and health. Gerontologist. 2016;56:S243–55. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Ageing and health. https://www.who.int/news‐room/fact‐sheets/detail/ageing‐and‐health. Accessed March 30, 2021.
- 3. Nojiri S, Itoh H, Kasai T, Fujibayashi K, Saito T, Hiratsuka Y, et al. Comorbidity status in hospitalized elderly in Japan: analysis from national database of health insurance claims and specific health checkups. Sci Rep. 2019;9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akazawa M, Mimura W, Togo K, Ebata N, Harada N, Murano H, et al. Patterns of drug treatment in patients with osteoarthritis and chronic low back pain in Japan: a retrospective database study. J Pain Res. 2019;12:1631–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ministry of Health Law Handbook of health and welfare statistics 2019 contents. https://www.mhlw.go.jp/english/database/db‐hh/1‐1.html. Accessed March 2, 2021.
- 6. Yoshimura N. Epidemiology of osteoarthritis in Japan: the ROAD study. Clin Calcium. 2011;21:821–5. [PubMed] [Google Scholar]
- 7. Iizuka Y, Iizuka H, Mieda T, Tsunoda D, Sasaki T, Tajika T, et al. Prevalence of chronic nonspecific low back pain and its associated factors among middle‐aged and elderly people: an analysis based on data from a musculoskeletal examination in Japan. Asian Spine J. 2017;11:989–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wong AY, Karppinen J, Samartzis D. Low back pain in older adults: risk factors, management options and future directions. Scoliosis Spinal Disord. 2017;12:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. The Japanese Orthopaedic Association . Clinical Practice Guideline on the Management of Low Back Pain, 2nd edn. Tokyo: Nankodo Co., Ltd; 2019. [Google Scholar]
- 10. Bannuru RR, Osani M, Vaysbrot E, Arden NK, Bennell K, Bierma‐Zeinstra SMA, et al. OARSI guidelines for the non‐surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578–89. [DOI] [PubMed] [Google Scholar]
- 11. AGS Panel on Persistent Pain in Older Persons . The management of persistent pain in older persons. J Am Geriatr Soc. 2002;50:S205–24. [DOI] [PubMed] [Google Scholar]
- 12. Ushida T. Burdensome problems of chronic musculoskeletal pain and future prospects. J Orthop Sci. 2015;20:958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gooch K, Culleton BF, Manns BJ, Zhang J, Alfonso H, Tonelli M, et al. NSAID use and progression of chronic kidney disease. Am J Med. 2007;120:280.e281–280.e287. [DOI] [PubMed] [Google Scholar]
- 14. Bally M, Dendukuri N, Rich B, Nadeau L, Helin‐Salmivaara A, Garbe E, et al. Risk of acute myocardial infarction with NSAIDs in real world use: Bayesian meta‐analysis of individual patient data. BMJ. 2017;357:j1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, et al. Vascular and upper gastrointestinal effects of non‐steroidal anti‐inflammatory drugs: meta‐analyses of individual participant data from randomised trials. Lancet. 2013;382:769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pilotto A, Franceschi M, Leandro G, Di Mario F. NSAID and aspirin use by the elderly in general practice. Drugs Aging. 2003;20:701–10. [DOI] [PubMed] [Google Scholar]
- 17. Al‐Azayzih A, Al‐Azzam SI, Alzoubi KH, Jarab AS, Kharaba Z, Al‐Rifai RH, et al. Nonsteroidal anti‐inflammatory drugs utilization patterns and risk of adverse events due to drug‐drug interactions among elderly patients: a study from Jordan. Saudi Pharm J. 2020;28:504–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abdu N, Mosazghi A, Teweldemedhin S, Asfaha L, Teshale M, Kibreab M, et al. Non‐Steroidal Anti‐Inflammatory Drugs (NSAIDs): usage and co‐prescription with other potentially interacting drugs in elderly: a cross‐sectional study. PLoS One. 2020;15:e0238868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kikuchi S, Togo K, Ebata N, Fujii K, Yonemoto N, Abraham L, et al. A retrospective database study of gastrointestinal events and medical costs associated with nonsteroidal anti‐Inflammatory drugs in Japanese patients of working age with osteoarthritis and chronic low back pain. Pain Med. 2021;22:1029–38. [DOI] [PubMed] [Google Scholar]
- 20. Katsuno T, Togo K, Ebata N, Fujii K, Yonemoto N, Abraham L, et al. Burden of renal events associated with nonsteroidal anti‐inflammatory drugs in patients with osteoarthritis and chronic low back pain: a retrospective database study. Pain Ther. 2021;10:443–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kikuchi S, Togo K, Ebata N, Fujii K, Yonemot N, Abraham L, et al. Database analysis on the relationships between nonsteroidal anti‐inflammatory drug treatment variables and incidence of acute myocardial infarction in Japanese patients with osteoarthritis and chronic low back pain. Adv Ther. 2021;38:1601–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yarnall AJ, Sayer AA, Clegg A, Rockwood K, Parker S, Hindle JV. New horizons in multimorbidity in older adults. Age Ageing. 2017;46:882–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rodgers JL, Jones J, Bolleddu SI, Vanthenapalli S, Rodgers LE, Shah K, et al. Cardiovascular risks associated with gender and aging. J Cardiovasc Dev Dis. 2019;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramanathan S, Hibbert P, Wiles L, Maher CG, Runciman W. What is the association between the presence of comorbidities and the appropriateness of care for low back pain? A population‐based medical record review study. BMC Musculoskelet Disord. 2018;19:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Swain S, Sarmanova A, Coupland C, Doherty M, Zhang W. Comorbidities in osteoarthritis: a systematic review and meta‐analysis of observational studies. Arthritis Care Res. 2020;72:991–1000. [DOI] [PubMed] [Google Scholar]
- 26. McCarberg BH. NSAIDs in the older patient: balancing benefits and harms. Pain Med. 2013;14:S43–4. [DOI] [PubMed] [Google Scholar]
- 27. Fanelli A, Ghisi D, Aprile PL, Lapi F. Cardiovascular and cerebrovascular risk with nonsteroidal anti‐inflammatory drugs and cyclooxygenase 2 inhibitors: latest evidence and clinical implications. Ther Adv Drug Saf. 2017;8:173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lapi F, Azoulay L, Yin H, Nessim SJ, Suissa S. Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non‐steroidal anti‐inflammatory drugs and risk of acute kidney injury: nested case‐control study. BMJ. 2013;346:e8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adhiyaman V, Asghar M, Oke A, White AD, Shah IU. Nephrotoxicity in the elderly due to co‐prescription of angiotensin converting enzyme inhibitors and nonsteroidal anti‐inflammatory drugs. J R Soc Med. 2001;94:512–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vandraas KF, Spigset O, Mahic M, Slørdal L. Non‐steroidal anti‐inflammatory drugs: use and co‐treatment with potentially interacting medications in the elderly. Eur J Clin Pharmacol. 2010;66:823–9. [DOI] [PubMed] [Google Scholar]
- 31. Kate RJ, Perez RM, Mazumdar D, Pasupathy KS, Nilakantan V. Prediction and detection models for acute kidney injury in hospitalized older adults. BMC Med Inform Decis Mak. 2016;16:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fries JF, Williams CA, Bloch DA, Michel BA. Nonsteroidal anti‐inflammatory drug‐associated gastropathy: incidence and risk factor models. Am J Med. 1991;91:213–22. [DOI] [PubMed] [Google Scholar]
- 33. Hernández‐Díaz S, Rodríguez LAG. Association between nonsteroidal anti‐inflammatory drugs and upper gastrointestinal tract bleeding/perforation: an overview of epidemiologic studies published in the 1990s. Arch Inter Med. 2000;160:2093–9. [DOI] [PubMed] [Google Scholar]
- 34. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non‐steroidal anti‐inflammatory drug use in the elderly. Aging Dis. 2018;9:143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abraham NS, Noseworthy PA, Inselman J, Herrin J, Yao X, Sangaralingham LR, et al. Risk of gastrointestinal bleeding increases with combinations of antithrombotic agents and patient age. Clin Gastroenterol Hepatol. 2020;18:337–46.e319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abdulla A, Adams N, Bone M, Elliott AM, Gaffin J, Jones D, et al. Guidance on the management of pain in older people. Age Ageing. 2013;42:i1–57. [DOI] [PubMed] [Google Scholar]
- 37. Denic A, Glassock RJ, Rule AD. Structural and functional changes with the aging kidney. Adv Chronic Kidney Dis. 2016;23:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sostres C, Gargallo CJ, Lanas A. Nonsteroidal anti‐inflammatory drugs and upper and lower gastrointestinal mucosal damage. Arthritis Res Ther. 2013;15:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Laine L, Curtis S, Cryer B, Kaur A, Cannon C. Risk factors for NSAID‐associated upper GI clinical events in a long‐term prospective study of 34 701 arthritis patients. Aliment Pharmacol Ther. 2010;32:1240–8. [DOI] [PubMed] [Google Scholar]
- 40. Chi T‐Y, Zhu H‐M, Zhang M. Risk factors associated with nonsteroidal anti‐inflammatory drugs (NSAIDs)‐induced gastrointestinal bleeding resulting on people over 60 years old in Beijing. Medicine. 2018;97:e0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meara AS, Simon LS. Advice from professional societies: Appropriate use of NSAIDs. Pain Med. 2013;14:S3–S10. [DOI] [PubMed] [Google Scholar]
- 42. Fixen DR. 2019 AGS beers criteria for older adults. Pharmacy Today. 2019;25:42–54. [Google Scholar]
- 43. Takeda O, Chiba D, Ishibashi Y, Tsuda E. Patient–physician differences in desired characteristics of NSAID plasters: an online survey. Pain Res Manag. 2017;2017:5787854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42:1235–481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available as all the rights for database ownership are reserved with Medical Data Vision. However, Pfizer Inc. have a contract with Medical Data Vision to use this database and publish the results.


