Abstract
Monocytes play a critical role in inflammation and immune response, their activity being sex‐dependent. However, the basis of sex differences is not well understood. Therefore, we investigated the lipopolysaccharide (LPS) effects on tumor necrosis factor‐α (TNF‐α) release, autophagy, and chemotaxis in freshly isolated monocytes from healthy young men and women. In basal conditions, male and female monocytes had similar TNF‐α release, chemotaxis, and estrogen receptors (ER‐α) and ER‐β expression, while the LC3II/I ratio was significantly higher in males. LPS treatment induced qualitative and quantitative sex differences. It reduced autophagy and increased TNF‐α release only in male monocytes, while, chemotaxis was significantly influenced only in female cells. Moreover, it reduced the expression of ER‐α only in female cells, while ER‐β expression was reduced in both sexes, but more markedly in female cells. Finally, the interplay between LPS treatment and 17‐β‐estradiol (E2) was present only in female cells. Globally, these findings expand the concept that sex plays a role in regulating monocytes' functions, being sex differences cell‐ and parameter‐specific.
Keywords: 17‐β‐estradiol, chemotaxis, estrogen receptors, human monocytes, LPS, sex differences
Lipopolysaccharide (LPS) treatment reduced autophagy and increased tumor necrosis factor‐α (TNF‐α) release only in male monocytes, chemotaxis and ER‐α expression were affected by LPS only in female cells An interplay between LPS treatment and 17‐β‐estradiol (E2) was present only in female cells.
1. INTRODUCTION
Sex can affect the risk factors, incidence, prevalence, diagnosis, prognosis, and treatments of diseases, changing both genetic, hormonal, and environmental effects. Knowledge of the sex impact will contribute to providing more appropriate prevention and care (Khramtsova et al., 2019; Legato, 2017). One of the important examples of sex impact involves inflammatory and immune response (Lotter & Altfeld, 2019), as also shown by the recent pandemic Covid‐19 (Takahashi et al., 2020). Among inflammatory cells, monocytes and macrophages play a crucial role in innate immune response, having a strong role in the battle against viral and bacterial infections (Meidaninikjeh et al., 2021) through the production of cytokines such as tumor necrosis factor‐α (TNF‐α) (Aldrich & Sevick‐Muraca, 2013; Campesi et al., 2013, 2017; Franconi et al., 2017; Meidaninikjeh et al., 2021).
Interestingly, their responses strongly depend on sex (Campesi et al., 2017; Franconi et al., 2017; Ruggieri et al., 2014). For example, lipopolysaccharide (LPS)‐induced TNF‐α release is higher in human monocyte‐derived macrophages in nonsmoker women in comparison with no‐smoker men (Campesi et al., 2013). Estrogens contribute to the sex differences of immune responses (Bhatia et al., 2014). In fact, in human monocyte‐derived macrophages, an interplay between estrogen receptors (ER) and LPS was reported. In both sexes, LPS upregulates the expression and the activity of ER‐α, the effect being more marked in male cells. By contrast, ER‐β is downregulated by LPS only in female cells (Campesi et al., 2017). This component of Gram‐negative bacteria binds the TLR4 receptor (Lu et al., 2008) being able, for example, to affect monocyte chemotaxis (Teh et al., 2019). More recently, it was shown that LPS is also able to modulate autophagy in human monocyte‐derived dendritic cells by increasing some proteins including microtubule‐associated protein 1A/1B‐light chain 3B (LC3B) (Monaci et al., 2020). In LPS‐treated macrophages and monocytes, the inhibition of autophagy is associated with increased secretion of several cytokines (Lee et al., 2016; Saitoh et al., 2008). Although autophagy is a fundamental process for monocytes' life (Zhang et al., 2012), to the best of our knowledge, no data are available regarding sex differences in monocytes' autophagy. Autophagy appears sexually dimorphic in several cells such as human umbilical vein endothelial cells (HUVEC), in human and rat vascular smooth muscle cells and neurons (Addis et al., 2014; Campesi et al., 2016; Du et al., 2009; Straface et al., 2009; Wang et al., 2015).
Only one study, to our knowledge, investigates the influence of sex on chemotaxis in human monocytes. It shows that LPS is more effective in inducing chemotaxis in female than in male cells (Ruggieri et al., 2014). Previously, it was shown that 17‐β‐estradiol (E2; 10−9–10−6 M) inhibits it in a monocyte‐like cell line 1 (Okada et al., 2010). E2 (10− 12−10− 4 M) also inhibits the chemotaxis induced by monocyte‐chemoattractant protein 1 in human monocytes whereas tamoxifen and clomiphene, two ER antagonists, restore it (Yamada et al., 1996).
On this basis, we checked if LPS treatment had a sex‐specific effect on monocytes functions (TNF‐α release, autophagy, and chemotaxis) and if physiological doses of E2 (10–9 M and 10–10 M) were able to modify LPS influence on chemotaxis of monocytes freshly isolated from healthy young men and women.
2. METHODS
2.1. Population
Fourteen healthy adult men and 13 healthy adult women aged between 18 and 37 years were enrolled. All women were fertile and premenopausal, with regular menstrual cycles (28–30 days) without reported precocious and/or surgically induced menopause, and were free from hormonal contraceptives use for at least 3 months to ensure an adequate wash‐out period, and were all analyzed during the follicular phase of their menstrual cycles. This study was approved by the Independent Ethical Committee of Azienda Ospedaliero Universitaria (AOU Cagliari; prot. PG/2019/6280). Written informed consent was obtained for each participant and all procedures were conducted following the Declaration of Helsinki.
2.2. Monocytes isolation
Monocytes of individual subjects were isolated from 15 ml of blood, withdrawn from healthy men and women according to Campesi et al. (2012). Purified monocytes were obtained by adhesion; non‐adherent cells (mainly lymphocytes) were removed by gentle washes with phosphate buffer after 2 h of culture in a 5% CO2 incubator at 37°C in RPMI‐1640 medium containing 20% fetal bovine serum, 2 mM glutamine, 10 mM HEPES, and antibiotics/antimycotics. The method used for monocyte culture and purification is a well‐recognized and widely and currently used method, as evidenced by numerous recently published papers (Antonelli et al., 2021; Chaudhary et al., 2021; Filipek et al., 2020; Hummitzsch et al., 2020; Lappalainen et al., 2021; Marchini et al., 2020; Nogieć et al., 2020).
2.3. TNF‐α release quantification
Male and female monocytes (4 × 104/cm2) were incubated in a 96‐well plate for 24 h in the absence or presence of 100 ng/ml LPS (Sigma‐Aldrich). The supernatants were then collected and stored at −80°C until analysis of the TNF‐α release using a commercial ELISA kit (human TNF‐α/TNFSF1A DuoSet ELISA kit; R&D Systems) following the manufacturer's instructions. All samples were assayed in duplicate.
2.4. Chemotaxis assay
For chemotaxis assay, monocytes (8.0 × 104 cells/well) were suspended in RPMI‐1640 medium only or suspended in RPMI‐1640 medium +100 mg/ml LPS. The LPS dose was selected as previously described (Monguió‐Tortajada et al., 2018; Sinistro et al., 2007; Tucureanu et al., 2018; Watanabe et al., 2002). Monocytes were placed into the upper chamber of a 24‐well modified Boyden Chamber (8.0 µm cell culture inserts; BD Bioscience).
Cells inserts, with porous membranes, were placed over a bottom chamber containing RPMI‐1640 medium only, or E2 (10–9 M, 10–10 M), or LPS (100 ng/ml) as chemoattractant agents. After 5 h of incubation at 37°C, the cells that had migrated to the lower side of the filter were stained with DAPI (0.2 mg/ml; Sigma‐Aldrich), and 5 unit fields per filter were counted using a fluorescence microscope (Motic AE31). The percentage of migrated monocytes was calculated using the ImageJ software (NIH), counting nuclei at each condition in comparison with the baseline. Each sample was examined in duplicate.
2.5. Western blot analysis
Monocytes were cultured for 24 h RPMI‐1640 medium in the absence or presence of 100 ng/ml LPS and then were lyzed (Cell Lysis Buffer). The protein concentration was quantified using the BCA protein assay kit (Thermo Fisher Scientific). For the western blot analysis, 15 µg of solubilized proteins were electrophoretically resolved by 4%–15% SDS‐PAGE (100 V, 2 h, 24°C) and then transferred to a PVDF membrane (250 mA, 65 min, 4°C) using a mini‐PROTEAN tetra cell system (Bio‐Rad). The membranes were blocked in 5% (w/v) skim‐milk (Sigma‐Aldrich) in 150 mM Tris buffer (Sigma‐Aldrich) and 20 mM Tris‐HCl, pH 7.2 (Sigma‐Aldrich) at 24°C for 1 h. Then membranes were incubated overnight at 4°C with the following antibodies: rabbit ER‐α, ER‐β (1:500; Thermo Fisher Scientific), or LC3 (LC3‐I and LC3‐II) (1:1000; MBL), a reliable and worldwide used method for monitoring autophagy (Klionsky et al., 2021). The LC3II/I ratio allows, in fact, to understand the state of the process as an increase in the ratio indicates increased autophagy and therefore greater phagolysosome formation (Klionsky et al., 2021).
The rabbit polyclonal antibody anti‐α‐actin (1:1000; Sigma‐Aldrich) was used as a loading control to normalize protein levels; no difference in α‐actin levels was reported between sexes as demonstrated by western blot images (2,594,221.4 ± 2,335,420.7053 optical density [OD] for females, and 170,801.64 ± 2,604,547.38 OD for males; N = 5 for each sex; p = .48). After washing, the blots were incubated for 1 h with anti‐rabbit IgG horseradish peroxidase (HRP)‐conjugated secondary antibodies (Cell Signaling Technology) (1:2000). Antibody binding was detected using a chemiluminescence reaction (Cell Signalling Technology) with the Bio‐Rad Chemi Doc instrument (Berkeley). Band volume analysis was performed using the Image Lab 4.0 software (Bio‐Rad Laboratories).
2.6. Statistical analysis
Data were reported as the mean ± SD. Statistical analysis was performed by t test to compare population characteristics and by two‐way analysis of variance (two‐way ANOVA) followed by the pairwise multiple comparison procedures to analyze the effect of sex and treatment with LPS or E2 using Sigma‐Stat 3.1 software (Systat Software). The distribution of samples was assessed by the Kolmogorov–Smirnov and Shapiro tests. A p ≤ 0.05 was considered statistically significant.
3. RESULTS
3.1. Basal characteristics of blood donors
Thirteen healthy women and 14 men were enrolled. They were well matched for age (women 26.6 ± 5.8 years, men 26.4 ± 4.3 years, p = 0.45). As expected, men were taller (women 1.63 ± 0.06 m, men 1.77 ± 0.07 m, p < 0.001), weighed significantly more (women 53.7 ± 9.1 kg, men 77.8 ± 14.9 kg, p < 0.001) and had a higher body mass index (women 20.2 ± 2.9 kg/m2, men 24.8 ± 3.9 kg/m2, p < 0.001).
3.2. LPS effects on TNF‐α release and autophagy
In basal conditions, the cytokine release was similar in both sexes (Figure 1a). The LPS incubation increased the release of TNF‐α of about 6,000% and 20,000% in female and male monocytes, respectively. Thus, the LPS effect is much higher in male monocytes than in female ones (Figure 1a).
Figure 1.
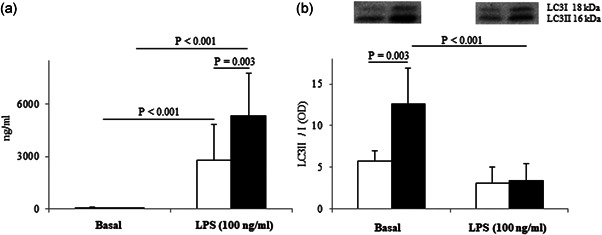
(a) Effect of LPS on TNF‐α release; (b) effect of LPS on LC3II/I ratio. Data are expressed as the mean of experiments ± SD of at least 10 independent samples for each sex for TNF‐α (performed in duplicate) and five independent samples for each sex for LC3II/I ratio. Connectors represent the statistical differences. White bars, female monocytes; black bars, male monocytes. LPS, lipopolysaccharide; TNF‐α, tumor necrosis factor‐α
In basal conditions, male monocytes were more autophagic than female ones: the LC3II/I ratio was 2.2‐fold significantly higher in male monocytes than in female ones (Figure 1b). Only in male cells, exposure to LPS significantly downregulated at 3.7‐fold the LC3II/I ratio. The reduction observed in female monocytes did not reach statistical significance (Figure 1b).
3.3. LPS effects on monocytes chemotaxis
In basal conditions, male and female monocytes did not present any significant difference in the number of migrated cells (4973 ± 2491 cells for females and 5286 ± 2267 for males, n = 12 for each sex; p = 0.69; Figure 2a). Treatment of monocytes with LPS (cells + LPS in the upper chamber, Figure 2b) significantly reduced chemotaxis (of about 33%) only in female cells, while no statistically significant effect was highlighted in male monocytes (Figure 3a).
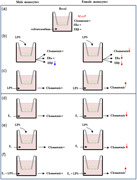
Figure 2.
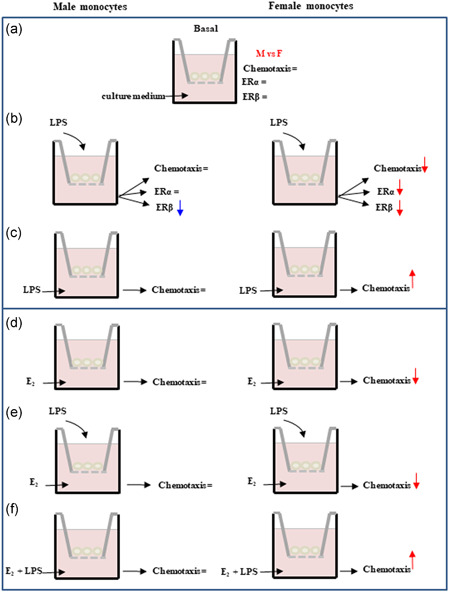
Schematic representation of the different experimental conditions used for chemotaxis assay and a summary of observed sex differences. LPS, lipopolysaccharide; M, males; F, females. Rows represent an increase or a decrease. The simbol '=' indicate a lack of effect.
Figure 3.
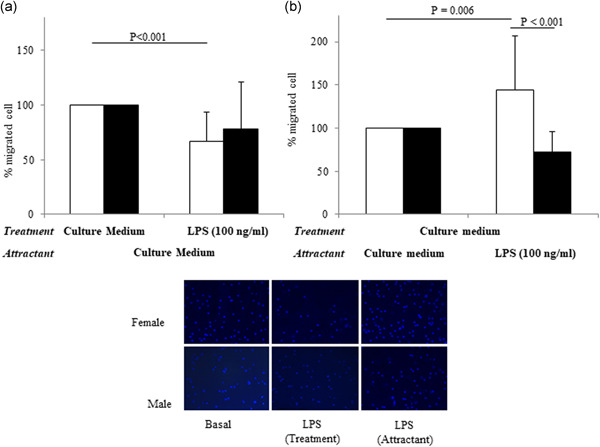
Effect of LPS treatment (a) and the effect of LPS used as a chemoattractant (b) on female and male monocytes chemotaxis, and representative photographs of chemotaxis assay at 5 h, taken at ×20 magnification (bottom). Data are expressed as the mean ± SD of at least 10 independent samples for each sex. Connectors represent the statistical differences. White bars, female monocytes; black bars, male monocytes
When LPS was used as a chemoattract agent (in the bottom of migration chamber, Figure 2c), a significantly higher percentage of female monocytes migrated when compared to basal conditions (Figure 3b). In male monocytes, the LPS exposure induced a non statistically significant decrease in chemotaxis versus basal conditions and to LPS‐treated female cells.
3.4. ER‐α and ER‐β expression in basal conditions and after LPS stimulation
Basal expression of ER‐α and ER‐β was similar in female and male monocytes (Figure 4a). LPS stimulation significantly downregulated ER‐β expression in female and male monocytes, the effect was more marked in female cells (Figure 4b). However, LPS significantly decreased ER‐α expression only in female cells.
Figure 4.
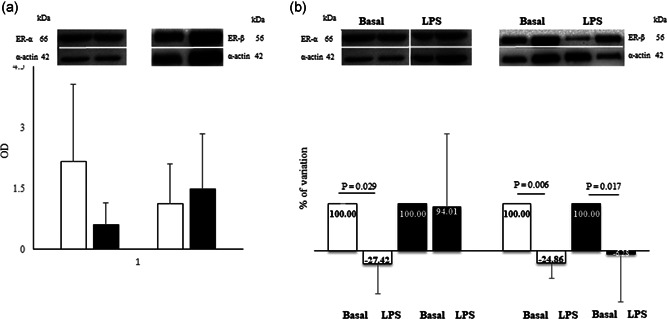
(a) Western blot (up) and densitometric analysis (bottom) for ER‐α and ER‐β expression; (b) percentage of variation of ER‐α and ER‐β expression after 100 ng/ml LPS. The value of the percentages is indicated within the histograms. Data are expressed as the mean ± SD of at least five independent samples for each sex. Connectors represent the statistical difference. Black bars, male monocytes; white bars, female monocytes. The white dividing line between the bands has been used for the purpose of presentation as there is splicing between the basal and LPS lanes of ER‐α and α‐actin bands The bands relative to the basal expression are identical in panels (a) and (b) and have been adapted to the images for representative purposes
3.5. E2 effects on chemotaxis
In basal female cells, both concentrations of E2, added in the bottom of the migration chamber (Figure 2d), significantly decreased the chemotaxis by about 30% and 35%, respectively (Figure 5a).
Figure 5.
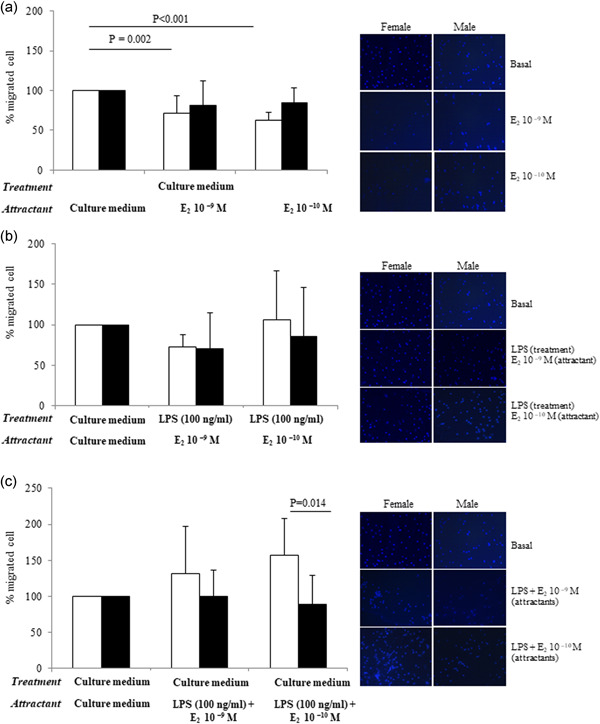
(a) Effect of E2 on chemotaxis and representative photographs at 5 h, taken at ×20 magnification. (b) Effect of LPS treatment (upper chamber) and E2 used as chemoattractant on chemotaxis and representative photographs (right) at 5 h, taken at ×20 magnification. (c) Effect of LPS and E2 used both as chemoattractant on chemotaxis and representative photographs at 5 h, taken at ×20 magnification. Data are expressed as the mean ± SD of at least 10 independent samples for each sex. Connectors represent the statistical differences. Black bars, male monocytes; white bars, female monocytes
To understand the interplay between LPS and E2, the chemotaxis assay was performed treating cells with LPS and using E2 as the attracting agent (Figure 2e). In this experimental condition, the inhibitory effect of E2 on female cell migration disappeared (Figure 5b). Finally, the chemotaxis assay was carried out using both LPS and E2 as chemoattractant agents at the same time (Figure 2f): the attractant effect of LPS prevailed over the inhibitory effect E2, only in females and at the dose of 10–10 M (Figure 5c). No effects were observed in male monocytes, in none of the tested conditions (Figure 5b,c).
4. DISCUSSION
In humans, the immune response is sexual divergent, with men exhibiting larger infection rates than women for a variety of microorganisms, and women having a higher prevalence of autoimmune diseases compared to men (Angum et al., 2020; Chakravarty et al., 2020; Ngo et al., 2014; Úbeda & Jansen, 2016). However, the mechanisms of sex differences are not clearly understood. The sexual hormones elicit direct effects on the function of immune cells (Shepherd et al., 2021), including monocytes (Franconi et al., 2017). Here, male and female monocytes express both ER‐α and ER‐β receptors without statistically significant differences in basal conditions. No consensus exists in the literature on this point. Pelekanou et al. (2016) found that human monocytes express only ER‐α 36 kDa independently from sex and without significant modifications of the expression related to the menstrual phase. Others reported that monocytes express low levels of both ER‐α and ER‐β messenger RNA, ER‐α being prevalent in male monocytes more than in female ones (Murphy et al., 2009; Phiel et al., 2005). Villablanca et al. (2010) reported that ER‐β is the major receptor in monocytes. The discrepant results can be attributed to differences in the monocyte isolation procedures, the purity of the preparations, the specificity of the antibodies used for the detection of ER.
In basal conditions, the only sex difference among tested parameters regards autophagy, which prevails in male monocytes, while no sex differences were observed in the basal release of TNF‐α. This suggests that sex differences in monocytes are parameter specific as occurs in other cells such as HUVEC, in vascular smooth muscle cells, neurons, macrophages (Addis et al., 2014; Campesi et al., 2013, 2015, 2016, 2017; Du et al., 2009). This also indicates that sex differences are also cell‐specific: in basal conditions, male macrophages derived from monocytes release significantly more TNF‐α in comparison with female ones (Campesi et al., 2013).
Interestingly, LPS stimulation increases sex differences. LPS‐induced TNF‐α release is much higher in men as also reported in other experimental conditions (Asai et al., 2001; Beenakker et al., 2020; Bouman et al., 2004), but not in all (Campesi et al., 2013). The higher releases of TNF‐α in men after LPS stimulation may contribute to the higher mortality reported in men with septic shock (Lefèvre et al., 2012) and in COVID‐19‐related deaths (Williamson et al., 2020). Furthermore, LPS reduces a fundamental process: autophagy for monocytes life of unknown sex (Zhang et al., 2012). Here, autophagy is reduced only in males, who also release much more TNF‐α than female ones. The interplay between TNF‐α release and inhibition of autophagy has already been described (Lee et al., 2016; Saitoh et al., 2008). However, the previous findings did not report the sex influence on this relationship, here, instead, the interplay is present only when male cells are considered and to the best of our knowledge, no data are available regarding sex differences in monocytes autophagy.
LPS also affects the expression of ER in a sex‐specific way. In particular, it reduces the expression of ER‐α only in female cells, while ER‐β expression is reduced in both sexes although the reduction prevails in females. However, in other experimental models (human macrophages derived from monocytes), LPS upregulates the expression of ER‐α in both sexes, the effect being bigger in male cells and downregulates ER‐β only in females (Campesi et al., 2017). Globally, the impact of sex on ER expression confirms that sex influence is cell‐specific.
Besides this, LPS affects monocytes chemotaxis only in female cells and the effect depends on experimental conditions: LPS significantly reduces or promotes chemotaxis when used as a treatment or as a chemoattractant, respectively (Figure 2). Previously, it was showed that treatment with LPS is more effective in inducing migration in female than in male cells (Ruggieri et al., 2014). This last study involved an older population in comparison to our cohort and did not include the hormonal status of women in inclusion and exclusion criteria. Moreover, chemotaxis has been performed in different experimental conditions (such as LPS dose and incubation time).
Some of the sex‐dependent activities of LPS could be ascribed to the fact that LPS binds the Toll‐like receptors TLR4 (Lu et al., 2008), which are more expressed in male human monocytes and neutrophils (Aomatsu et al., 2013; Bannister et al., 2013). In this regard, it is relevant to recall that in human leukocytes, 1217 autosomal, 54 X‐linked genes have sex‐specific responses to LPS, as well as 71 autosomal and one X‐linked sex‐specific expression quantitative trait loci (Stein et al., 2021), suggesting that LPS activity may occur through multiple pathways.
Importantly, when the monocytes' chemotaxis is considered, a complex interplay between estrogens and LPS in female cells emerges. In detail, LPS treatment decreases chemotaxis (33%) and reduces ER‐α and ER‐β expression, whereas E2 inhibits chemotaxis reducing it by about 30% and 35% (for 10–9 M and 10–10 M, respectively) as also observed in female rat vascular smooth muscle cells (Pellegrini et al., 2014). However, when LPS and E2 are simultaneously used as chemoattractants chemotaxis increase measured in the absence of estrogen is preserved. In male monocytes, LPS does not modify chemotaxis but induces a small but significant decrease of ER‐β, and E2 does not affect chemotaxis in any experimental condition used. Globally, in female cells, the chemotaxis seems to be modulated by estrogens probably through ER‐α. This is in line with results obtained in macrophages derived from monocytes (Campesi et al., 2017).
Here, we expand the concept that sex plays a crucial role in regulating the activity of monocytes. However, the major novelty is the LPS effect, which amplifies sex differences qualitatively and quantitatively, including the interplay between E2 and LPS observed in the female cell chemotaxis.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ACKNOWLEDGMENTS
We thank Dr. Antonella Mattana for providing antibodies for ERs detection. This study was partially funded by a grant of INAIL “Bando BRIC 2016.” Open access funding provided by Universita degli Studi di Sassari within the CRUI‐CARE Agreement.
Campesi, I. , Montella, A. , & Franconi, F. (2022). Human monocytes respond to lipopolysaccharide (LPS) stimulation in a sex‐dependent manner. J Cell Physiol, 237, 580–588. 10.1002/jcp.30503
DATA AVAILABILITY STATEMENT
All data have been presented in the figures. Other related information is available upon reasonable request to the corresponding author
REFERENCES
- Addis, R. , Campesi, I. , Fois, M. , Capobianco, G. , Dessole, S. , Fenu, G. , Montella, A. , Cattaneo, M. G. , Vicentini, L. M. , & Franconi, F. (2014). Human umbilical endothelial cells (HUVECs) have a sex: Characterisation of the phenotype of male and female cells. Biology of Sex Differences, 5(1), 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich, M. B. , & Sevick‐Muraca, E. M. (2013). Cytokines are systemic effectors of lymphatic function in acute inflammation. Cytokine, 64(1), 362–369. 10.1016/j.cyto.2013.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angum, F. , Khan, T. , Kaler, J. , Siddiqui, L. , & Hussain, A. (2020). The prevalence of autoimmune disorders in women: A narrative review. Cureus, 12(5), 8094. 10.7759/cureus.8094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli, A. , Scarpa, E. S. , & Magnani, M. (2021). Human red blood cells modulate cytokine expression in monocytes/macrophages under anoxic conditions. Frontiers in Physiology, 12, 632682. 10.3389/fphys.2021.632682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aomatsu, M. , Kato, T. , Kasahara, E. , & Kitagawa, S. (2013). Gender difference in tumor necrosis factor‐α production in human neutrophils stimulated by lipopolysaccharide and interferon‐γ. Biochemical and Biophysical Research Communications, 441(1), 220–225. 10.1016/j.bbrc.2013.10.042 [DOI] [PubMed] [Google Scholar]
- Asai, K. , Hiki, N. , Mimura, Y. , Ogawa, T. , Unou, K. , & Kaminishi, M. (2001). Gender differences in cytokine secretion by human peripheral blood mononuclear cells: Role of estrogen in modulating LPS‐induced cytokine secretion in an ex vivo septic model. Shock, 16(5), 340–343. 10.1097/00024382-200116050-00003 [DOI] [PubMed] [Google Scholar]
- Bannister, E. G. , Smith, C. , Visvanathan, K. , Thompson, A. , & Hardikar, W. (2013). TLR2 and TLR4 in healthy children: Age and gender differences. Journal of Paediatrics and Child Health, 49(12), 1082–1083. 10.1111/jpc.12437 [DOI] [PubMed] [Google Scholar]
- Beenakker, K. G. M. , Westendorp, R. G. J. , De Craen, A. J. M. , Chen, S. , Raz, Y. , Ballieux, B. E. P. B. , Nelissen, R. G. H. H. , Later, A. F. L. , Huizinga, T. W. , Slagboom, P. E. , Boomsma, D. I. , & Maier, A. B. (2020). Men have a stronger monocyte‐derived cytokine production response upon stimulation with the Gram‐negative stimulus lipopolysaccharide than women: A pooled analysis including 15 study populations. Journal of Innate Immunity, 12(2), 142–153. 10.1159/000499840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia, A. , Sekhon, H. K. , & Kaur, G. (2014). Sex hormones and immune dimorphism. The Scientific World Journal, 2014, 159150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouman, A. , Schipper, M. , Heineman, M. J. , & Faas, M. M. (2004). Gender difference in the non‐specific and specific immune response in humans. American Journal of Reproductive Immunology, 52(1), 19–26. 10.1111/j.1600-0897.2004.00177.x [DOI] [PubMed] [Google Scholar]
- Campesi, I. , Capobianco, G. , Dessole, S. , Occhioni, S. , Montella, A. , & Franconi, F. (2015). Estrogenic compounds have divergent effects on human endothelial progenitor cell migration according to sex of the donor. Journal of Vascular Research, 52(4), 273–278. 10.1159/000443403 [DOI] [PubMed] [Google Scholar]
- Campesi, I. , Carru, C. , Zinellu, A. , Occhioni, S. , Sanna, M. , Palermo, M. , Tonolo, G. , Mercuro, G. , & Franconi, F. (2013). Regular cigarette smoking influences the transsulfuration pathway, endothelial function, and inflammation biomarkers in a sex‐gender specific manner in healthy young humans. American Journal of Translational Research, 5(5), 497–509. [PMC free article] [PubMed] [Google Scholar]
- Campesi, I. , Marino, M. , Montella, A. , Pais, S. , & Franconi, F. (2017). Sex differences in estrogen receptor α and β levels and activation status in LPS‐stimulated human macrophages. Journal of Cellular Physiology, 232(2), 340–345. 10.1002/jcp.25425 [DOI] [PubMed] [Google Scholar]
- Campesi, I. , Occhioni, S. , Capobianco, G. , Montella, A. , Dessole, S. , & Franconi, F. (2016). Sex‐specific pharmacological modulation of autophagic process in human umbilical artery smooth muscle cells. Pharmacology Research, 113, 166–174. 10.1016/j.phrs.2016.08.014 [DOI] [PubMed] [Google Scholar]
- Campesi, I. , Sanna, M. , Zinellu, A. , Carru, C. , Rubattu, L. , Bulzomi, P. , Seghieri, G. , Tonolo, G. , Palermo, M. , Rosano, G. , Marino, M. , & Franconi, F. (2012). Oral contraceptives modify DNA methylation and monocyte‐derived macrophage function. Biology of Sex Differences, 3, 4. 10.1186/2042-6410-3-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campesi, I. , Straface, E. , Occhioni, S. , Montella, A. , & Franconi, F. (2013). Protein oxidation seems to be linked to constitutive autophagy: A sex study. Life Sciences, 93(4), 145–152. 10.1016/j.lfs.2013.06.001 [DOI] [PubMed] [Google Scholar]
- Chakravarty, D. , Nair, S. S. , Hammouda, N. , Ratnani, P. , Gharib, Y. , Wagaskar, V. , Mohamed, N. , Lundon, D. , Dovey, Z. , Kyprianou, N. , & Tewari, A. K. (2020). Sex differences in SARS‐CoV‐2 infection rates and the potential link to prostate cancer. Communications Biology, 3(1), 1–12. 10.1038/s42003-020-1088-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary, N. , Que Nguyen, T. N. , Maguire, A. , Wynne, C. , & Meade, A. D. (2021). Comparison of sample preparation methodologies towards optimisation of Raman spectroscopy for peripheral blood mononuclear cells. Analytical Methods, 13(8), 1019–1032. 10.1039/d0ay02040k [DOI] [PubMed] [Google Scholar]
- Du, L. , Hickey, R. W. , Bayir, H. , Watkins, S. C. , Tyurin, V. A. , Guo, F. , Kochanek, P. M. , Jenkins, L. W. , Ren, J. , Gibson, G. , Chu, C. T. , Kagan, V. E. , & Clark, R. S. B. (2009). Starving neurons show sex difference in autophagy. Journal of Biological Chemistry, 284(4), 2383–2396. 10.1074/jbc.M804396200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipek, A. , Mikołajczyk, T. P. , Guzik, T. J. , & Naruszewicz, M. (2020). Oleacein and foam cell formation in human monocyte‐derived macrophages: A potential strategy against early and advanced atherosclerotic lesions. Pharmaceuticals, 13(4), 10.3390/ph13040064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franconi, F. , Rosano, G. , Basili, S. , Montella, A. , & Campesi, I. (2017). Human cells involved in atherosclerosis have a sex. International Journal of Cardiology, 228, 983–1001. [DOI] [PubMed] [Google Scholar]
- Hummitzsch, L. , Berndt, R. , Kott, M. , Rusch, R. , Faendrich, F. , Gruenewald, M. , Steinfath, M. , Albrecht, M. , & Zitta, K. (2020). Hypoxia directed migration of human naïve monocytes is associated with an attenuation of cytokine release: Indications for a key role of CCL26. Journal of Translational Medicine, 18(1), 404. 10.1186/s12967-020-02567-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khramtsova, E. A. , Davis, L. K. , & Stranger, B. E. (2019). The role of sex in the genomics of human complex traits. Nature Reviews Genetics, 20, 173–190. 10.1038/s41576-018-0083-1 [DOI] [PubMed] [Google Scholar]
- Klionsky, D. J. , Abdel‐Aziz, A. K. , Abdelfatah, S. , Abdellatif, M. , Abdoli, A. , Abel, S. , Abeliovich, H. , Abildgaard, M. H. , Abudu, Y. P. , Acevedo‐Arozena, A. , Adamopoulos, I. E. , Adeli, K. , Adolph, T. E. , Adornetto, A. , Aflaki, E. , Agam, G. , Agarwal, A. , Aggarwal, B. B. , Agnello, M. , … Bartek, J. (2021). Guidelines for the use and interpretation of assays for monitoring autophagy (4th ed.), Autophagy, 17, 1–382. 10.1080/15548627.2020.1797280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappalainen, J. , Yeung, N. , Nguyen, S. D. , Jauhiainen, M. , Kovanen, P. T. , & Lee‐Rueckert, M. (2021). Cholesterol loading suppresses the atheroinflammatory gene polarization of human macrophages induced by colony stimulating factors. Scientific Reports, 11(1), 4923. 10.1038/s41598-021-84249-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. P. W. , Foote, A. , Fan, H. , Peral de Castro, C. , Lang, T. , Jones, S. A. , Gavrilescu, N. , Mills, K. H. G. , Leech, M. , Morand, E. F. , & Harris, J. (2016). Loss of autophagy enhances MIF/macrophage migration inhibitory factor release by macrophages. Autophagy, 12(6), 907–916. 10.1080/15548627.2016.1164358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre, N. , Corazza, F. , Duchateau, J. , Desir, J. , & Casimir, G. (2012). Sex differences in inflammatory cytokines and CD99 expression following in vitro lipopolysaccharide stimulation. Shock, 38(1), 37–42. 10.1097/SHK.0b013e3182571e46 [DOI] [PubMed] [Google Scholar]
- Legato, M. (2017). Principles of gender‐specific medicine (3rd ed). Academic Press. https://www.elsevier.com/books/principles-of-gender-specific-medicine/legato/978-0-12-803506-1 [Google Scholar]
- Lotter, H. , & Altfeld, M. (2019). Sex differences in immunity. Seminars in Immunopathology, 41, 133–135. 10.1007/s00281-018-00728-x [DOI] [PubMed] [Google Scholar]
- Lu, Y. C. , Yeh, W. C. , & Ohashi, P. S. (2008). LPS/TLR4 signal transduction pathway. Cytokine, 42(2), 145–151. 10.1016/j.cyto.2008.01.006 [DOI] [PubMed] [Google Scholar]
- Marchini, J. F. , Manica, A. , Crestani, P. , Dutzmann, J. , Folco, E. J. , Weber, H. , Libby, P. , & Croce, K. (2020). Oxidized low‐density lipoprotein induces macrophage production of prothrombotic microparticles. Journal of the American Heart Association, 9(15), 015878. 10.1161/JAHA.120.015878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meidaninikjeh, S. , Sabouni, N. , Marzouni, H. Z. , Bengar, S. , Khalili, A. , & Jafari, R. (2021). Monocytes and macrophages in COVID‐19: Friends and foes. Life Sciences, 269, 269. 10.1016/j.lfs.2020.119010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaci, S. , Aldinucci, C. , Rossi, D. , Giuntini, G. , Filippi, I. , Ulivieri, C. , Marotta, G. , Sozzani, S. , Carraro, F. , & Naldini, A. (2020). Hypoxia shapes autophagy in LPS‐activated dendritic cells. Frontiers in Immunology, 11, 11. 10.3389/fimmu.2020.573646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monguió‐Tortajada, M. , Franquesa, M. , Sarrias, M.‐R. , & Borràs, F. E. (2018). Low doses of LPS exacerbate the inflammatory response and trigger death on TLR3‐primed human monocytes. Cell Death & Disease, 9(5), 499. 10.1038/s41419-018-0520-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, A. J. , Guyre, P. M. , Wira, C. R. , & Pioli, P. A. (2009). Estradiol regulates expression of estrogen receptor ERα46 in human macrophages. PLoS One, 4(5), 5539. 10.1371/journal.pone.0005539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo, S. T. , Steyn, F. J. , & McCombe, P. A. (2014). Gender differences in autoimmune disease. Frontiers in Neuroendocrinology, 35(3), 347–369. 10.1016/j.yfrne.2014.04.004 [DOI] [PubMed] [Google Scholar]
- Nogieć, A. , Bzowska, M. , Demczuk, A. , Varol, C. , & Guzik, K. (2020). Phenotype and response to PAMPs of human monocyte‐derived foam cells obtained by long‐term culture in the presence of oxLDLs. Frontiers in Immunology, 11, 11. 10.3389/fimmu.2020.01592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada, K. , Inamori, M. , Imajyo, K. , Chiba, H. , Nonaka, T. , Shiba, T. , Sakaguchi, T. , Atsukawa, K. , Takahashi, H. , Hoshino, E. , & Nakajima, A. (2010). Gender differences of low‐dose aspirin‐associated gastroduodenal ulcer in Japanese patients. World Journal of Gastroenterology, 16(15), 1896–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelekanou, V. , Kampa, M. , Kiagiadaki, F. , Deli, A. , Theodoropoulos, P. , Agrogiannis, G. , Patsouris, E. , Tsapis, A. , Castanas, E. , & Notas, G. (2016). Estrogen anti‐inflammatory activity on human monocytes is mediated through cross‐talk between estrogen receptor ERα36 and GPR30/GPER1. Journal of Leukocyte Biology, 99(2), 333–347. 10.1189/jlb.3a0914-430rr [DOI] [PubMed] [Google Scholar]
- Pellegrini, M. , Bulzomi, P. , Lecis, M. , Leone, S. , Campesi, I. , Franconi, F. , & Marino, M. (2014). Endocrine disruptors differently influence estrogen receptor beta and androgen receptor in male and female rat VSMC. Journal of Cellular Physiology, 229(8), 1061–1068. 10.1002/jcp.24530 [DOI] [PubMed] [Google Scholar]
- Phiel, K. L. , Henderson, R. A. , Adelman, S. J. , & Elloso, M. M. (2005). Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunology Letters, 97(1), 107–113. 10.1016/j.imlet.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Ruggieri, A. , Gambardella, L. , Maselli, A. , Vona, R. , Anticoli, S. , Panusa, A. , Malorni, W. , & Matarrese, P. (2014). Statin‐induced impairment of monocyte migration is gender‐related. Journal of Cellular Physiology, 229(12), 1990–1998. 10.1002/jcp.24657 [DOI] [PubMed] [Google Scholar]
- Saitoh, T. , Fujita, N. , Jang, M. H. , Uematsu, S. , Yang, B. G. , Satoh, T. , Omori, H. , Noda, T. , Yamamoto, N. , Komatsu, M. , Tanaka, K. , Kawai, T. , Tsujimura, T. , Takeuchi, O. , Yoshimori, T. , & Akira, S. (2008). Loss of the autophagy protein Atg16L1 enhances endotoxin‐induced IL‐1β production. Nature, 456(7219), 264–268. 10.1038/nature07383 [DOI] [PubMed] [Google Scholar]
- Shepherd, R. , Cheung, A. S. , Pang, K. , Saffery, R. , Novakovic, B. , De Winther, M. , Agostinis, C. , & Bansal, K. (2021). Sexual dimorphism in innate immunity: The role of sex hormones and epigenetics. Frontiers in Immunology, 11, 1. 10.3389/fimmu.2020.604000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinistro, A. , Ciaprini, C. , Natoli, S. , Sussarello, E. , Carducci, F. C. , Almerighi, C. , Capozzi, M. , Bolacchi, F. , Rocchi, G. , & Bergamini, A. (2007). Lipopolysaccharide desensitizes monocytes‐macrophages to CD40 ligand stimulation. Immunology, 122(3), 362–370. 10.1111/j.1365-2567.2007.02648.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, M. M. , Conery, M. , Magnaye, K. M. , Clay, S. M. , Billstrand, C. , Nicolae, R. , Naughton, K. , Ober, C. , & Thompson, E. E. (2021). Sex‐specific differences in peripheral blood leukocyte transcriptional response to LPS are enriched for HLA region and X chromosome genes. Scientific Reports, 11(1), 1107. 10.1038/s41598-020-80145-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straface, E. , Vona, R. , Gambardella, L. , Ascione, B. , Marino, M. , Bulzomi, P. , Canu, S. , Coinu, R. , Rosano, G. , Malorni, W. , & Franconi, F. (2009). Cell sex determines anoikis resistance in vascular smooth muscle cells. FEBS Letters, 583(21), 3448–3454. 10.1016/j.febslet.2009.09.052 [DOI] [PubMed] [Google Scholar]
- Takahashi, T. , Ellingson, M. K. , Wong, P. , Israelow, B. , Lucas, C. , Klein, J. , Silva, J. , Mao, T. , Oh, J. E. , Tokuyama, M. , Lu, P. , Venkataraman, A. , Park, A. , Liu, F. , Meir, A. , Sun, J. , Wang, E. Y. , Casanovas‐Massana, A. , Wyllie, A. L. , … Iwasaki, A. (2020). Sex differences in immune responses that underlie COVID‐19 disease outcomes Overview of the study design. Nature, 588, 315–320. 10.1038/s41586-020-2700-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh, Y. C. , Ding, J. L. , Ng, L. G. , & Chong, S. Z. (2019). Capturing the fantastic voyage of monocytes through time and space. Frontiers in Immunology, 10, 834. 10.3389/fimmu.2019.00834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucureanu, M. M. , Rebleanu, D. , Constantinescu, C. A. , Deleanu, M. , Voicu, G. , Butoi, E. , Calin, M. , & Manduteanu, I. (2018). Lipopolysaccharide‐induced inflammation in monocytes/macrophages is blocked by liposomal delivery of Gi‐protein inhibitor. International Journal of Nanomedicine, 13, 63–76. 10.2147/IJN.S150918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villablanca, A. C. , Jayachandran, M. , & Banka, C. (2010). Atherosclerosis and sex hormones: Current concepts. Clinical Science (Lond), 119(12), 493–513. 10.1042/CS20100248 [DOI] [PubMed] [Google Scholar]
- Wang, L. F. , Yokoyama, K. K. , Chen, T. Y. , Hsiao, H. W. , Chiang, P. C. , Hsieh, Y. C. , Lo, S. , & Hsu, C. (2015). Male‐specific alleviation of iron‐induced striatal injury by inhibition of autophagy. PLOS One, 10(7), 0131224. 10.1371/journal.pone.0131224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, K. , Jose, P. J. , & Rankin, S. M. (2002). Eotaxin‐2 generation is differentially regulated by lipopolysaccharide and IL‐4 in monocytes and macrophages. Journal of Immunology, 168(4), 1911–1918. 10.4049/jimmunol.168.4.1911 [DOI] [PubMed] [Google Scholar]
- Williamson, E. J. , Walker, A. J. , Bhaskaran, K. , Bacon, S. , Bates, C. , Morton, C. E. , Curtis, H. J. , Mehrkar, A. , Evans, D. , Inglesby, P. , Cockburn, J. , McDonald, H. I. , MacKenna, B. , Tomlinson, L. , Douglas, I. J. , Rentsch, C. T. , Mathur, R. , Wong, A. , Grieve, R. , … Goldacre, B. (2020). Factors associated with COVID‐19‐related death using OpenSAFELY. Nature, 584(7821), 430–436. 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, K. , Hayashi, T. , Kuzuya, M. , Naito, M. , Asai, K. , & Iguchi, A. (1996). Physiological concentration of 17 beta‐estradiol inhibits chemotaxis of human monocytes in response to monocyte chemotactic protein 1. Artery, 22(1), 24–35. [PubMed] [Google Scholar]
- Zhang, Y. , Morgan, M. J. , Chen, K. , Choksi, S. , & Liu, Z. G. (2012). Induction of autophagy is essential for monocyte‐macrophage differentiation. Blood, 119(12), 2895–2905. 10.1182/blood-2011-08-372383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Úbeda, F. , & Jansen, V. A. A. (2016). The evolution of sex‐specific virulence in infectious diseases. Nature Communications, 7(1), 1–9. 10.1038/ncomms13849 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data have been presented in the figures. Other related information is available upon reasonable request to the corresponding author


