Abstract
Objectives
To report the long‐term follow‐up outcomes of masculinizing surgery in disorders/differences of sex development (DSD), including both physicians' and patients’ perspectives on appearance and functional outcome, including sexuality.
Patients and Methods
In total, 1040 adolescents (age ≥16 years) and adults with a DSD took part in this multicentre cross‐sectional clinical study in six European countries in 2014/2015. Of those, 150 living in other than the female gender had some kind of masculinizing surgery: hypospadias repair, orchidopexy, breast reduction and/or gonadectomy. The study protocol included medical data collection, an optional genital examination, and patient‐reported outcomes including satisfaction with appearance and current sexual functioning.
Results
Diagnoses included partial and mixed gonadal dysgenesis (45,XO/46,XY; n = 38), Klinefelter syndrome/46,XX males (n = 57), and various 46,XY DSDs (n = 42; e.g. partial androgen insensitivity syndrome, severe hypospadias) and 13 with other diagnoses. Of the participants, 84 underwent hypospadias surgery, 86 orchidopexy, 52 gonadectomy and 32 breast reduction (combinations possible). Physicians evaluated anatomical appearance at genital examination as poor in approximately 11% of patients. After hypospadias surgery, 38% of participants reported that they were (very) dissatisfied with anatomical appearance and 20% with function. The physician and patient evaluations were moderately correlated (r = 0.43).
Conclusion
The majority of participants were neutral to satisfied with the appearance and function in the long‐term after masculinizing surgery. Given the initial severe phenotype and a risk of unsatisfactory results after masculinizing surgery in DSD, treatment should be handled by experienced multidisciplinary teams in order to optimize the postoperative results.
Keywords: disorders of sex development, masculinizing surgery, urological outcome, sexual outcome, hypospadias, patient‐reported outcome, #Urology
Abbreviations
- CAH
congenital adrenal hyperplasia
- DSD
disorders/differences of sex development
- GD
gonadal dysgenesis
- KS
Klinefelter syndrome
- PAIS
partial androgen insensitivity syndrome
- PRO
patient‐reported outcome
Introduction
Disorders/differences of sex development (DSD) are defined as congenital conditions in which the development of chromosomal, gonadal and anatomical sex is atypical, according to the Chicago Consensus Meeting statement [1]. DSD includes sex chromosome conditions and conditions with a 46,XY or 46,XX karyotype. In the present paper, we use the acronym DSD, and refer to the specific condition as appropriate [2].
Management of DSD conditions is complex, and surgery was historically both the early, and only, available treatment for patients with DSD and genital variance/malformations. Hypospadias surgery and orchidopexy are the most common masculinizing procedures and are frequently performed. The prevalence of hypospadias in newborn boys is approximately one in 125 in Sweden [3], and the prevalence of undescended testis is 4–5% in term infants and up to 30% in premature infants [4]. Masculinizing surgery in DSD also includes surgery of penile chordee, phalloplasty and scrotoplasty, and may include removal of Müllerian duct remnants and breast reduction. The reconstructive part of masculinizing surgery targets good voiding function with a glandular meatus, a straight penis during erection, and satisfactory aesthetic appearance, with or without prepuce [5, 6].
Hypospadias surgery is generally recommended at 1–2 years of age [7]. There are numerous surgical techniques that have evolved during decades and that are related to grade of severity [8, 9, 10]. Long‐term follow‐up after hypospadias surgery reveals problems with voiding, curvature of the penis and concerns about penile size, even to the extent that phalloplasty is requested by the affected male [8, 11, 12, 13, 14, 15]. Other problems are anejaculation and lower fertility outcomes [16, 17]. Study results with regard to sexual activity and satisfaction with sex life vary [8, 17, 18].
Undescended testes often accompany severe hypospadias. Undescended testes are divided into palpable and nonpalpable testes [19]. Orchidopexy to place the testis into the scrotum is recommended to be performed at 1 year of age in order to reduce the risk of secondary damage of the testis and to bring it to a position where it is palpable for follow‐up examination [19]. In individuals with DSD, testis/gonadal dysgenesis is more common, and with an increased risk of tumours and dysfunction warranting orchidectomy [1].
In the present study we describe the masculinizing surgical treatments performed in a large pan‐European cohort of individuals with DSD, and compare physicians’ and participants’ evaluations in relation to different diagnoses and surgical procedures. Finally, we assess whether anatomical and functional outcomes, and body (part) satisfaction were associated with the surgical procedures performed. We expected significant variability in outcomes, largely based on underlying diagnosis and surgical procedures. Also, we expected some discrepancy between patient and physician evaluations given the different factors each group takes into consideration.
Participants and Methods
Study Design and Participants
The methods used in the multicentre cross‐sectional clinical evaluation study dsd‐LIFE, described in detail elsewhere [20], are summarized below. The dsd‐LIFE consortium consisted of 16 European partners from Germany, France, the Netherlands, Poland, Sweden and the UK. Recruitment of adolescents (≥16 years) and adults was performed from 3100 eligible persons with a DSD condition through patient record and support groups, as described in the classification system of the Chicago Consensus Conference [1], of whom 1040 took part in the study.
Dsd‐LIFE consisted of two study parts. The first part included a medical interview with an optional medical/gynaecological/urological examination and a retrospective chart review collecting data on appearance and age at diagnosis, past surgical treatments, type of surgery and past medical examinations. All tasks were carried out by trained researchers following standard operation procedures. The second part of the study consisted of a patient‐reported outcome (PRO) questionnaire. The PRO questionnaire was administered as a secured online version; if needed, a paper‐and‐pencil version was provided. The PRO questionnaire included sociodemographic data (including age, a standardized European education level based on the European Survey on International Standard Classification of Education, living conditions, and family status), self‐constructed questions related to received surgical treatments, and scores for satisfaction with anatomical and functional outcomes after surgery.
For the present analyses, all participants who had received masculinizing surgery were included. Masculinizing surgery included genital surgery (hypospadias surgery or phalloplasty in all participants), gonadal surgery in males (gonadectomy, orchidopexy or testicle implants), removal of Müllerian structures, or breast reduction in males/other genders. Patients identifying neither as female nor male were checked individually for masculinizing surgery in their history. Individuals were included if either the clinical report form or patient‐reported data indicated masculinizing surgery, as defined above, and the concordance and accuracy were reviewed manually. This resulted in a total of 150 study participants. We present outcome data for each diagnosis and compare those with a non‐hypospadias operated group of male participants, mainly with Klinefelter syndrome (KS).
Outcome Measurements
Genital examination included standardized evaluation of external genital appearance, surgical results and sensitivity tests (comparing to the inner thigh) using predefined answer categories. Genital satisfaction was assessed by answering the self‐constructed questions ‘Are you satisfied with the appearance of your genitals after surgery?’ and ‘Are you satisfied with how your genitals function after surgery?’ on a five‐point scale (very satisfied to very dissatisfied). Genital body image was measured via the genital items from the Body Image Scale [21, 22]: satisfaction with penis, scrotum and testes was scored on a five‐point scale ranging from very satisfied to very dissatisfied. With regard to sexual satisfaction, participants were surveyed on their satisfaction with penile length/shape/erectability, general arousability and orgasmic capacity (self‐constructed; five‐point scale from very dissatisfied to very satisfied). Also, participants were provided with a list of sexual dysfunctions, and were asked whether they had ever experienced any of the following (self‐constructed measure; yes/no): pain during/after intercourse; frequent urogenital infections; premature ejaculation; erectile dysfunction; or delayed orgasm. Lastly, participants rated their satisfaction with their sex life in general (self‐constructed; five‐point scale from very satisfied to very dissatisfied) and were asked whether or not they attributed their (dis)satisfaction to surgery (no/partially/yes). The impact of surgery on life was measured by responses to the question ‘Overall, how do you think that [surgical procedure] has affected your life?’ on a five‐point scale (very negative to very positive). With regard to attitudes towards surgery, participants were asked to what extent they agreed that they would have ‘been better off without surgery as a minor/adult', and what the preferred age for genital surgery in DSD would be (self‐constructed; infant/minor age, adolescent/adult or any time the individual could consent).
Statistical Analyses
For the background descriptive data and surgical characteristics, all participants with masculinizing surgery were included, regardless of surgery type, and the data were reported per diagnosis. For surgery‐specific outcomes, the cohort was divided into those with and without hypospadias surgery and orchidopexy/gonadectomy and described according to underlying diagnosis (for outcomes of hypospadias surgery) or by type of gonadal surgery. Background characteristics, surgical data, urological examination and patient‐reported data were displayed as frequencies and means as appropriate. Statistical comparisons were performed using ANOVA or chi‐squared tests. Post hoc testing was performed via Bonferroni analysis (for continuous variables) or standardized residuals (for nominal/ordinal variables). Overlap in hypospadias surgery, gonadectomy and orchidopexy surgery in an individual was displayed in a Venn diagram. The experienced effect of surgery on life was evaluated per surgical intervention. For the whole cohort and for those participants who underwent hypospadias surgery, the associations between examined penile length and satisfaction with penile length were calculated through bivariate correlations. Similarly, key (scale) physician‐ and patient‐reported outcomes were correlated with age at participation, Prader stage at diagnosis, age at first surgery, and total number of surgeries. The operated groups with and without ambiguous genitalia at diagnosis were compared in terms of penile length and glans sensitivity at follow‐up (using two‐sided t‐tests). Significance was set at P < 0.05 and all analyses were performed using SPSS statistics 26.0. (IBM Corp. Armonk, NY, USA)
Ethics
The research complied with the requirement of the Helsinki Declaration. We obtained ethical approval as appropriate to each country, e.g. Ethics Commission of the Charite Universitatsmedizin; reference number EA2/069/13. We obtained written informed consent from all participants, and both the participants and the parents of young people below the age of 18 years gave written informed consent.
Results
Of the participants in the whole dsd‐LIFE cohort (n = 1040) [20], 150 had undergone masculinizing surgery during their lifetime, of whom 57 had KS (including 47,XXY, 47,XXY/46,XY, and 46,XX males), 25 had severe (isolated) hypospadias, 17 had partial androgen insensitivity syndrome (PAIS), 38 had partial 46,XY or mixed 46,XY/45,X0 gonadal dysgenesis, and 13 had other diagnoses (e.g. ovotestes, steroid synthesis errors, congenital adrenal hyperplasia [CAH]). These participants' sociodemographic and medical characteristics are presented in Table 1. In total, 145 identified as males and the participants' ages ranged up to 72 years. A total of 56 were married or living with a partner, and eight reported having biological children. Ambiguous genitalia with Prader stage between 3 and 4 at diagnosis was reported in 79 (61%) of the participants who underwent surgery, but in 85% when excluding KS, indicating severe forms of hypospadias.
Table 1.
Sample characteristics.
|
All (N= 150) |
Klinefelter/XX males (N = 57) |
Hypospadias N = 25) |
PAIS (N = 17) |
Partial/mixed GD (N = 38) |
Other diagnoses (N = 13) |
Test statistics | |
|---|---|---|---|---|---|---|---|
| Gender, n (%) | |||||||
| Male | 145 (96.7) | 57 (100) | 24 (96.0) | 17 (100) | 38 (100) | 9 (69.2) | χ 2(8) = 48.3** |
| Female | 4 (2.7) † | – | – | – | – | 4 (30.8) | |
| Other ‡ | 1 (.7) † | – | 1 (4.0) | – | – | – | |
| Age, n (%) | |||||||
| 16–19 years | 38 (25.3) | 5 (8.8) | 11 (44.0) | 2 (11.8) | 18 (47.4) | 2 (15.4) | χ 2(16) = 70.2** |
| 20–24 years | 25 (16.7) | 4 (7.0) | 4 (16.0) | 8 (47.1) | 8 (21.1) | 1 (7.7) | |
| 25–44 years | 55 (36.7) | 20 (35.1) | 9 (36.0) | 7 (41.2) | 12 (31.6) | 7 (53.8) | |
| 45–64 years | 26 (17.3) | 23 (40.4) | 1 (4.0) | – | – | 2 (15.4) | |
| ≥ 65 years | 6 (4.0) | 5 (8.8) | – | – | – | 1 (7.7) | |
| Education level, n (%) | |||||||
| Low | 41 (28.7) | 19 (33.3) | 5 (20.0) | 2 (11.8) | 15 (39.5) | 2 (15.4) | n.s. |
| Middle | 63 (42.0) | 22 (38.6) | 9 (36.0) | 9 (52.0) | 17 (44.7) | 6 (46.2) | |
| High | 33 (22.0) | 12 (21.1) | 8 (32.0) | 5 (29.4) | 5 (13.2) | 3 (23.1) | |
| Other/n.a. | 11 (7.3) | 4 (7.0) | 3 (12.0) | 1 (5.9) | 1 (2.6) | 2 (15.4) | |
| Experienced income, n (%) | |||||||
| Living comfortably | 32 (21.3) | 12 (21.1) | 8 (32.0) | 5 (29.4) | 4 (10.5) | 3 (23.1) | χ 2(6) = 23.0* |
| Coping | 64 (42.7) | 31 (54.4) | 6 (24.0) | 6 (35.3) | 14 (36.8) | 7 (53.8) | |
| (Very) Difficult | 17 (11.3) | 9 (15.8) | 2 (8.0) | 1 (5.9) | 4 (10.5) | 1 (7.7) | |
| Other/n.a. | 37 (24.7) | 5 (8.8) | 9 (36.0) | 5 (29.4) | 16 (42.1) | 2 (15.4) | |
| Relationship status, n (%) | |||||||
| Single | 27 (19.7) | 10 (17.9) | 7 (33.3) | 1 (6.3) | 5 (13.5) | 4 (30.8) | χ 2(8) = 43.8** |
| Married/ Partner | 56 (37.0) | 37 (66.1) | 4 (19.0) | 5 (31.3) | 5 (13.5) | 5 (38.5) | |
| Other/with parents | 60 (43.3) | 9 (16.1) | 10 (47.6) | 10 (62.5) | 27 (73.0) | 4 (30.8) | |
| Biological children | |||||||
| No, n (%) | 135 (94.4) | 52 (94.5) | 21 (95.5) | 16 (100) | 33 (89.2) | 13 (100) | n.s. |
| Yes, n (%) | 8 (5.6) | 3 (5.5) | 1 (4.5) | – | 4 (10.8) | – | |
| Number, mean (range) | 1.88 (1–3) | 2.3 (2–3) | 1 (–) | 1.8 (1–2) | n.s. | ||
| Ambiguous genitalia § | |||||||
| No, n (%) | 50 (38.8) | 44 (93.6) | 1 (5.3) | 2 (13.3) | 1 (2.8) | 2 (16.7) | χ 2(4) = 94.8** |
| Yes, n (%) | 79 (61.2) | 3 (6.4) | 18 (94.7) | 13 (86.7) | 35 (97.2) | 10 (83.3) | |
| Prader stage, mean (SD) | 3.4 (0.91) | 3.5 (0.7) | 3.6 (0.5) | 3.3 (1.2) | 3.3 (0.8) | 3.2 (1.5) | n.s. |
GD, gonadal dysgenesis; n.a., not applicable; n.s., not significant; PAIS, partial androgen insensitivity syndrome. In the Klinefelter group were included 49x 47, XXY; 2x 47, XXY/46, XY; 1x 47, XXY/46, XX; 3x other/unknown; 2x XX males. GD were 13x mixed GD; 25x partial GD, among other diagnosis were 8x CAH, 2x XY ovotestes, 1x CAIS, 1x steroid synthesis error, 1x other.
Masculinizing surgery performed in the four women and one other was hypospadias surgery (n=3), orchidopexy and gonadectomy was performed in three participants with CAH, one with CAIS and one with hypospadias.
Gender identity = inter.
At diagnosis.
P ≤ 0.05; ** P ≤ 0.001.
Type of Surgery
Eighty‐four men had undergone surgery for hypospadias, orchidopexy was performed in 76 and gonadectomy was performed in 52, on one or both sides (Table 2). The main indication for gonadectomy was dysgenesis. There was a substantial overlap among the procedures undergone, as illustrated in Fig. 1. Removal of Müllerian ducts/uterus was performed in 31 participants, of whom 28 had gonadal dysgenesis (GD), and was performed mainly at a young age. Breast reduction was performed in 32 participants, mainly in those with KS and PAIS, at a mean (SD) age of 21.9 (9.6) years. Approximately one‐quarter of male participants with KS underwent surgery as adults, mostly through gonadal surgery and/or breast reduction. The mean number of surgeries per person was 5.3, but ranged from one to 60 procedures, with the highest numbers among men who underwent surgery for hypospadias. The large number of surgeries per participant was attributable both to the indication and to complications and revision procedures.
Table 2.
Surgical characteristics. †
|
All (N = 150) |
Klinefelter/XX males (n = 57) |
Hypospadias (N = 25) |
PAIS (n = 17) |
Partial/mixed GD (N = 38) |
Other diagnoses (N = 13) |
Test statistics | |
|---|---|---|---|---|---|---|---|
| Age at 1st surgery, mean (SD) | 6.2 (10.2) | 27.9 (16.6) | 2.4 (3.2) | 1.8 (1.5) | 4.1 (4.5) | 7.8 (10.8) | F(4,79) = 26.2*** |
| Hypospadias surgery, n (%) | 84 (58.7) | 3 (5.7) | 25 (100.0) | 15 (93.8) | 34 (94.4) | 7 (53.8) | χ 2(4) = 106.3*** |
| Orchidopexy, n (%) | 76 (52.4) | 24 (44.4) | 13 (54.2) | 14 (82.4) | 20 (52.6) | 5 (41.7) | n.s. |
| Gonadectomy, n (%) | 52 (35.4) | 11 (20.0) | 3 (12.0) | 1 (5.9) | 30 (78.9) | 7 (58.3) | χ 2(4) = 52.5*** |
| One‐sided | 25 (59.5) | 3 | 2 | 1 | 17 | 2 | |
| Two‐sided | 17 (40.5) | 2 | – | – | 12 | 3 | |
| Prepubertal gonadectomy, n (%) | 10 (35.7) | – | 1 (50.0) | – | 8 (57.1) | 1 (33.3) | χ 2(3) = 8.0* |
| Removal uterus/Mullerian ducts, n (%) | 31 (32.0) | – | – | 2 (13.3) | 28 (75.7) | 1 (16.7) | χ 2(4) = 53.9*** |
| Breast reduction, n (%) | 32 (21.3) | 15 (26.3) | 1 (4.0) | 11 (64.7) | 2 (5.3) | 3 (23.1) | χ 2(4) = 30.2*** |
| Total surgeries, mean (range) | 5.3 (1–60) | 2.8 (1–30) | 9.5 (2–60) | 4.7 (1–12) | 8.1 (1–26) | 5.4 (1–15) | F(4,85) = 2.8* |
| Gender reassignment, n (%) | 15 (10.0) | – | 2 (8.0) | 2 (11.8) | 7 (18.4) | 4 (30.8) | χ 2(8) = 21.8** |
| After puberty, n | 4 | – | – | – | 2 | 2 |
GD, gonadal dysgenesis; n.s., not significant; PAIS, partial androgen insensitivity syndrome.
The following surgeries were not assessed structurally: phalloplasty (n = 2) and testicle implantation (n = 4).
P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Fig. 1.
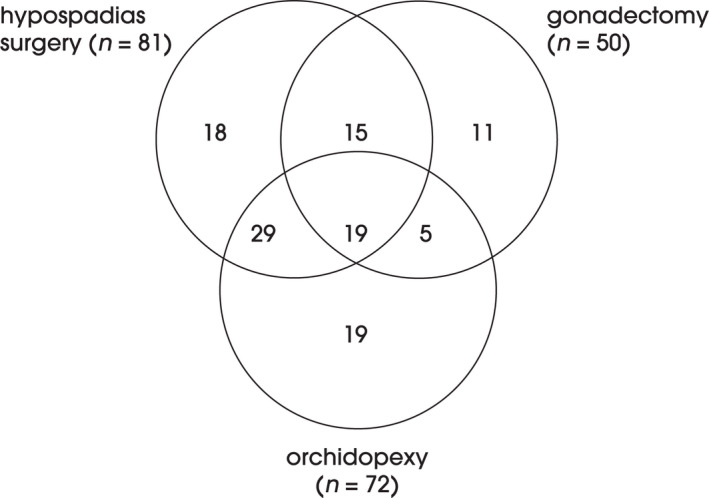
Frequencies of combinations of genital masculinizing surgeries. Missing variables, n = 11 and other than one of the three procedures displayed, n = 23.
Data on the type of surgical procedures performed in hypospadias surgery were available for 45 participants only: the most common were Duplay/Tubularized incised plate (TIP) (n = 23) and Duckett (n = 8). Revision surgery after hypospadias operations was performed in 60%, mainly as a result of urethral stenosis (through urethral dilatations or meatus disposition) and none as a result of lichen sclerosis et atrophicus. Urine dribbling and urogenital infections were quite commonly described (12/45 participants).
Altogether, 15 participants had their sex reassigned, of whom only four underwent this after puberty (all partial/mixed GD or other diagnosis, none in PAIS). Thirteen participants changed from female to male, of whom seven had GD, two had PAIS, one had ovotesticular DSD, one had hypospadias, one had CAH and one had an unknown androgen synthesis defect. One participant with CAH changed from male to female and, finally, one participant with hypospadias underwent sex reassignment but in an unknown direction. Two participants underwent phalloplasty, both twice; one had partial GD and one had 11beta‐hydroxylase deficiency.
Urological Examination
Altogether, urological examination was performed in 110 participants (73%), of whom 74 had undergone surgery for hypospadias (Table 3A). The general appearance after hypospadias surgery, as evaluated by the examiner, was poor in seven participants (11.3%), and most frequent in participants with PAIS (25.0%). No such outcome was reported for non‐surgery controls. It was very uncommon to observe the presence of a foreskin after surgery. Grouped by diagnosis, participants with PAIS had the shortest mean penile length (46 [SD 21] mm), and most often described having a bifid and hypoplastic scrotum. A normal positioned meatus was described in 74% of participants and remaining chordee in six (9.8%). Severe or moderate scars were judged to be present in 25% of participants. Three males who underwent surgery for hypospadias had no sensitivity on the glans, while four described an increased sensation or pain during examination, which was more than in the group who did not undergo surgery for hypospadias. No differences were found in glans sensitivity between the groups.
Table 3.
Hypospadias outcomes from genital examination and participant‐reported measures in males.
|
All operated n = 74 |
Hypospadias n = 25 |
PAIS n = 15 |
Partial/mixed GD n = 34 |
Non‐operated clinical controls † n = 50 |
Test statistics | |
|---|---|---|---|---|---|---|
| A) Genital examinations | ||||||
| Genital examination performed, n (%) | 64 (86.5) | 18 (72.0) | 13 (86.7) | 33 (97.1) ‡ | 35 (71.4) ‡ | χ 2(3) = 10.0* |
| Overall genital appearance, n (%) | ||||||
| Good | 30 (48.4) | 12 (66.7) | 1 (8.3) ‡ | 17 (53.1) | 19 (82.6) ‡ | χ 2(6) = 21.0** |
| Satisfactory | 25 (40.3) | 4 (22.2) | 8 (66.7) ‡ | 13 (40.6) | 4 (17.4) ‡ | |
| Poor | 7 (11.3) | 2 (11.1) | 3 (25.0) ‡ | 2 (6.3) | 0 (0) | |
| Foreskin present, n (%) | ||||||
| No | 50 (80.6) | 13 (76.5) | 8 (72.7) | 29 (87.9) ‡ | 5 (22.7) ‡ | χ 2(6) = 50.6*** |
| Incomplete | 8 (12.9) | 4 (23.5) ‡ | 1 (9.1) | 3 (9.1) ‡ | 0 (0) | |
| Hooded/complete | 3 (4.8) | 0 (0) ‡ | 2 (18.2) | 1 (3.0) | 17 (77.3) ‡ | |
| Stretched dorsal penile length (mm), mean (SD) | 63 (–) | 68 (31) | 46 (21) ‡ | 68 (15) | 89 (25) ‡ | F(3,81) = 10.6*** |
| Meatus position, n (%) | ||||||
| Normal | 46 (74.2) | 14 (77.8) | 8 (66.7) | 24 (75.0) | 27 (100) | χ 2(3) = 9.1* |
| Other § | 16 (25.8) | 4 (22.2) | 4 (33.3) | 8 (25.0) | 0 (0) ‡ | |
| Persistent chordee, n (%) | ||||||
| No | 55 (90.2) | 13 (76.5) | 12 (100) | 30 (93.8) | 22 (88.0) | n.s. |
| Yes | 6 (9.8) | 4 (23.5) | 0 (0) | 2 (6.3) | 3 (12.0) | |
| Scars, n (%) | ||||||
| No | 9 (15.3) | 5 (31.3) | 0 (0) ‡ | 4 (12.9) ‡ | 16 (76.2) ‡ | χ 2(9) = 33.8*** |
| Minor | 34 (57.6) | 6 (37.5) | 9 (75.0) ‡ | 19 (61.3) | 5 (23.8) ‡ | |
| Moderate | 15 (25.4) | 5 (31.3) | 3 (25.0) | 7 (22.6) | 0 (0) ‡ | |
| Severe | 1 (1.7) | 0 (0) | 0 (0) | 1 (3.2) | 0 (0) | |
| sensitivity glans, n (%) | ||||||
| No | 3 (5.4) | 2 (13.3) | 0 (0) | 1 (3.3) | 1 (4.0) | n.s. |
| Same as inner thigh | 19 (33.9) | 7 (46.7) | 3 (27.3) | 9 (30.0) | 11 (44.0) | |
| More than inner thigh | 30 (53.6) | 6 (40.0) | 7 (63.6) | 17 (56.7) | 13 (52.0) | |
| Much more/pain | 4 (7.1) | 0 (0) | 1 (9.1) | 3 (10.0) | 0 (0) | |
| B) Participant‐reported measures | ||||||
| Satisfaction postoperative genital appearance, n (%) | ||||||
| (Very) Satisfied | 17 (30.4) | 6 (30.0) | 1 (8.3) | 10 (41.7) | n.a. | n.s. |
| Neither satisfied nor dissatisfied | 18 (32.1) | 6 (30.0) | 3 (25.0) | 9 (37.5) | ||
| (Very) Dissatisfied | 21 (37.5) | 8 (40.0) | 8 (66.7) ‡ | 5 (20.8) ‡ | ||
| Satisfaction postoperative genital function, n (%) | ||||||
| (Very) Satisfied | 25 (44.6) | 9 (45.0) | 5 (41.7) | 11 (45.8) | n.a. | n.s. |
| Neither satisfied nor dissatisfied | 20 (35.7) | 6 (30.0) | 2 (16.7) | 12 (50.0) | ||
| (Very) Dissatisfied | 11 (19.6) | 5 (25.0) | 5 (41.7) ‡ | 1 (4.2) ‡ | ||
| Penile body image, n (%) | ||||||
| (Very) Satisfied | 16 (23.2) | 4 (18.2) | 1 (6.7) ‡ | 11 (34.4) | 25 (53.2) ‡ | χ 2(6) = 21.3** |
| Neither Satisfied nor Dissatisfied | 10 (14.5) | 5 (22.7) | 0 (0) | 5 (15.6) | 6 (12.8) | |
| (Very) Dissatisfied | 43 (62.3) | 13 (59.1) | 14 (93.3) ‡ | 16 (50.0) | 16 (34.0) ‡ | |
| Satisfaction penile length, n (%) | ||||||
| (Very) Satisfied | 9 (13.4) | 3 (15.0) | 0 (0) ‡ | 6 (18.8) | 24 (54.5) ‡ | χ 2(6) = 29.9*** |
| Neither satisfied nor dissatisfied | 15 (22.4) | 4 (20.0) | 1 (6.7) | 10 (31.3) ‡ | 5 (11.4) | |
| (Very) Dissatisfied | 43 (64.2) | 13 (65.0) | 14 (93.3) ‡ | 16 (50.0) | 15 (34.1) ‡ | |
| Satisfaction penile shape, n (%) | ||||||
| (Very) Satisfied | 19 (28.4) | 6 (30.0) | 2 (13.3) ‡ | 11 (34.4) | 27 (61.4) ‡ | χ 2(6) = 25.6*** |
| Neither satisfied nor dissatisfied | 20 (29.9) | 3 (15.0) | 3 (20.0) | 14 (43.8) ‡ | 8 (18.2) | |
| (Very) Dissatisfied | 28 (41.8) | 11 (55.0) ‡ | 10 (66.7) ‡ | 7 (21.9) | 9 (20.5) ‡ | |
| Satisfaction erectability, n (%) | ||||||
| (Very) Satisfied | 44 (66.7) | 14 (70.0) | 9 (60.0) | 21 (67.7) | 29 (67.4) | n.s. |
| Neither satisfied nor dissatisfied | 17 (25.8) | 5 (25.0) | 5 (33.3) | 7 (22.6) | 9 (20.9) | |
| (Very) Dissatisfied | 5 (7.6) | 1 (5.0) | 1 (6.7) | 3 (9.7) | 5 (11.6) | |
| Satisfaction orgasm ability, n (%) | ||||||
| (Very) Satisfied | 37 (68.5) | 10 (52.6) | 10 (76.9) | 17 (77.3) | 24 (64.9) | χ 2(6) = 15.9* |
| Neither satisfied nor dissatisfied | 14 (25.9) | 9 (47.4) ‡ | 1 (7.7) | 4 (18.2) | 5 (13.5) | |
| (Very) Dissatisfied | 3 (5.6) | 0 (0) | 2 (15.4) | 1 (4.5) | 8 (21.6) ‡ | |
| Sexual problems experienced, n (%) | ||||||
| Pain during intercourse | 4 (8.0) | 2 (11.1) | 1 (11.1) | 1 (4.3) | 9 (25.0) ‡ | n.s. |
| Frequent urogenital infections | 7 (10.1) | 2 (9.5) | 4 (26.7) ‡ | 1 (3.0) | 2 (4.3) | χ 2(3) = 9.3* |
| Premature ejaculation | 8 (14.5) | 0 (0) ‡ | 4 (28.6) | 4 (17.4) | 14 (36.8) ‡ | χ 2(3) = 9.9* |
| Erectile dysfunction | 12 (20.3) | 4 (21.1) | 3 (21.4) | 5 (19.2) | 15 (37.5) | n.s. |
| Delayed orgasm | 14 (20.0) | 7 (31.8) | 4 (26.7) | 3 (9.1) ‡ | 15 (31.9) | n.s. |
| Overall sex life evaluation, n (%) | ||||||
| (Very) Satisfied | 20 (28.2) | 5 (21.7) | 3 (20.0) | 12 (36.4) | 17 (35.4) | n.s. |
| Neither satisfied nor dissatisfied | 26 (36.6) | 8 (34.8) | 4 (26.7) | 14 (42.4) | 16 (33.3) | |
| (Very) Dissatisfied | 25 (35.2) | 10 (43.5) | 8 (53.3) | 7 (21.2) | 15 (31.3) | |
GD, gonadal dysgenesis; n.a., not applicable; n.s., not significant; PAIS, partial androgen insensitivity syndrome.
Clinical controls include participants with Klinefelter syndrome/XX males without hypospadias surgery.
Difference in post hoc testing.
15 glandular/coronal, 1 posterior.
P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Gonadal surgery, either gonadectomy, orchidopexy or both, was performed in 101 males (Tables 2 and 4). Of those, 82 participated in the genital evaluation. Most commonly, the scrotum was fused and symmetrical, but was hypoplastic in one‐third of participants. In two‐thirds of all participants, the gonads were in the scrotal position, and were impalpable in one‐third. Altogether, eight participants had undergone surgical treatment with testicle prosthesis (all had KS and partial GD).
Table 4.
Outcomes after gonadal surgery (Gonadectomy/orchidopexy) from genital examination and participant‐reported measures in males.
|
All operated (N = 101) |
Gonadectomy (N = 26) |
Gonadectomy and orchidopexy (N = 25) |
Orchidopexy n = 50 |
Test statistics | |||||
|---|---|---|---|---|---|---|---|---|---|
| Genital examinations | |||||||||
| Genital examination performed | 82 (82.0) | 19 (76.0) | 22 (88.0) | 41 (82.0) | n.s. | ||||
| Scrotal appearance | |||||||||
| Fused | 53 (73.6) | 13 (72.2) | 15 (71.4) | 25 (75.8) | n.s. | ||||
| Symmetrical | 53 (77.9) | 13 (81.3) | 13 (61.9) † | 27 (87.1) | n.s. | ||||
| Hypoplastic | 24 (36.4) | 6 (37.5) | 6 (28.6) | 12 (41.4) | n.s. | ||||
| Gonad localization (left/right) | |||||||||
| Scrotal | 48 (64.9) | 50 (67.6) | 8 (50.0) | 5 (31.3) † | 8 (38.1) † | 9 (42.9) † | 32 (86.5) † | 36 (97.3) † | Left: χ 2(4) = 17.7** |
| Ectopic | 3 (4.1) | 2 (2.7) | 0 (0) | 0 (0) | 2 (9.5) | 2 (9.5) † | 1 (2.7) | 0 (0) | Right: χ 2(4) = 34.5*** |
| Impalpable | 23 (31.1) | 22 (29.7) | 8 (50.0) | 11 (68.8) † | 11 (52.4) † | 10 (47.6) † | 4 (10.8) † | 1 (2.7) † | |
| Participant‐reported measures | |||||||||
| Scrotal body image | |||||||||
| (Very) Satisfied | 33 (37.9) | 4 (21.1) | 10 (45.5) | 19 (41.3) | χ 2(4) = 11.1* | ||||
| Neither satisfied nor dissatisfied | 20 (23.0) | 3 (15.8) | 2 (9.1) | 15 (32.6) † | |||||
| (Very) Dissatisfied | 34 (39.1) | 12 (63.2) † | 10 (45.5) | 12 (26.2) † | |||||
| Testicular body image | |||||||||
| (Very) Satisfied | 23 (27.4) | 3 (17.6) | 9 (40.9) | 11 (24.4) | n.s. | ||||
| Neither satisfied nor dissatisfied | 15 (17.9) | 1 (5.9) | 3 (13.6) | 11 (24.4) | |||||
| (Very) Dissatisfied | 46 (54.8) | 13 (76.5) | 10 (45.5) | 23 (51.1) | |||||
n.s., not significant.
Difference in post hoc testing.
P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Patient‐Reported Outcomes
The males who underwent surgery for hypospadias expressed the whole spectrum of satisfaction/ dissatisfaction with their genital appearance, but were generally more satisfied with function than with appearance (Table 3B). The highest dissatisfaction was generally reported by men with PAIS. The whole group were more often dissatisfied with penile body image, penile length and shape than those who did not undergo surgery, again with the poorest scores reported by males with PAIS and the best scores by the KS non‐operated control group. Body image of the scrotum ranged within the spectrum of satisfaction/dissatisfaction, with a higher dissatisfaction after gonadectomy. Testicular body image had more dissatisfaction scores.
Sexual problems were prevalent across the sample. Males who underwent surgery had generally no more problems with erectability or orgasm than reported by the non‐operated clinical controls. More frequent sexual problems included urogenital infections, described mostly in men with PAIS. The overall satisfaction with sex life was moderate to poor and showed no significant differences between diagnoses and operated versus non‐operated groups. No significant differences in PROs were observed between the participants with and without gender reassignment, possibly because reassignment was mainly performed when participants were young children.
Comparing Urological Examination with Patient‐Reported Outcomes
In the whole cohort, objective penile length was moderately positively correlated with satisfaction with the penis (r = 0.28, P = 0.008) and satisfaction with penile length (r = 0.39, P < 0.001). In participants with hypospadias surgery, penile evaluation by the examiner was moderately positively correlated with satisfaction as evaluated by the participant (r = 0.43, P < 0.001). Mean dissatisfaction with penile length was higher amongst participants with ambiguous genitalia at birth (4.5 [SD 0.6]) compared to those without (3.8 [SD 1.2]); however, no statistical significance was reached because of an insufficient sample size for the first group. No significant associations were observed between patient‐reported satisfaction with genital function and appearance, and Prader stage at surgery, age at surgery, and number of surgeries. Older age at participation was associated with more dissatisfaction at follow‐up regarding genital appearance (r = 0.31, P = 0.01) and function (r = 0.35, P = 0.003).
Satisfaction with Surgery and Timing of Surgery
In the whole cohort, a minority (strongly) agreed that they would have been better off without surgery as a minor (n = 11, 10.5%) or as an adult (n = 9, 9.8%). KS/46,XX male participants significantly more often preferred surgery in adolescence or adulthood (38%), whereas the PAIS and partial/mixed GD groups and hypospadias group preferred surgery during infancy (approximately 60–75%; χ 2[20] = 56.5; P < 0.001). Surgery at any age ‘only if the person decides’ was chosen less frequently (KS/46,XX males 6%, PAIS and partial/mixed GD 6–9%, hypospadias 14%).
The impact of individual surgical procedures on later life is shown in Fig. 2 and seems to show a slightly positive or indifferent point of view about the procedures. The procedures viewed most favourably were removal of the Müllerian ducts/uterus (mainly in GD) and breast reduction (mainly in KS and PAIS) as adults.
Fig. 2.
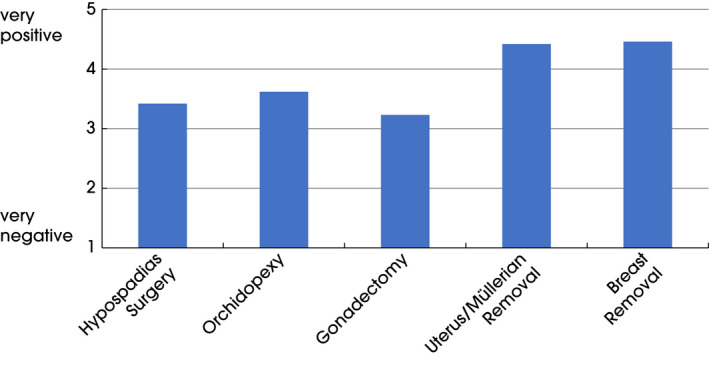
Experienced effects of surgical procedures on life. Scale: 1 = very negative to 5 = very positive. Hypospadias, n = 59 (SD 1.3); orchidopexy, n = 50 (SD 1.1); gonadectomy, n = 30 (SD 1.3); uterus removal, n = 12 (SD 0.5); breast removal, n = 28 (SD 1.04).
Discussion
This study reports results from a large European study on long‐term outcomes of hypospadias surgery, orchidopexy, gonadectomy and also Müllerian duct removal and breast reduction in individuals born with DSD. We focused on PROs with regard to appearance and function, and correlated those results with diagnoses, surgical procedures and findings during genital examinations. The satisfaction levels with genital appearance after surgery differed widely and were evaluated as higher by the examiner than the participant, while participant satisfaction with genital function was generally higher.
The different diagnoses included in the study were associated with different surgical procedures conducted. Masculinizing surgery was common in men with non‐KS DSD (83%, 93/112) and at an early age, while men with KS underwent surgery to a lesser extent (27%), and had predominantly testicular and breast surgery as adults. Gonadectomy was mainly performed in males with partial/mixed GD, probably because of the risk of malignancy [23], as well as in participants with KS after puberty. Müllerian duct remnants were seldom removed in men with PAIS, as compared with those with partial/mixed GD (75%). This approach might not be recommended nowadays due to the aim of preserving this tissue [1]. Gynaecomastia is a frequent finding in both males with KS and those with PAIS, and thus breast reduction was performed more frequently in these groups, and generally at the patient's own request [24, 25]. It is possible that this surgery is an option in more individuals with DSD identifying as males. Gender reassignment took place in 10% of the participants, mainly from female to male and in only four participants after puberty. The reported incidence of gender reassignment is still more prevalent than in the general population [26].
Hypospadias surgery is generally regarded as a reconstructive procedure as no or a minor amount of tissue is removed, and is mostly functionally motivated. This surgery was the most common procedure performed as part of masculinizing genital surgery in our cohort. Almost all who underwent this surgery for DSD had ambiguous genitalia at birth, indicating severe hypospadias, and underwent surgery at an early age. Hypospadias reconstruction was performed in 84 males, with revisions in 60%, which is what would be expected based on the literature [27]. In the present study, no definitive conclusion about complications and revision procedures after hypospadias surgeries and technical approaches can be drawn because of insufficient data, but our limited results are in concordance with the literature on frequency of redo procedures and with stenosis and fistulae being the main reason for revision [8, 9, 10].
Penile length was shorter (7 ± 2.7 cm) in the whole group and shortest in men with PAIS (4.6 ± 2.1 cm) and also correlated to satisfaction with appearance. This is in accordance with earlier studies [11, 14, 28]. A smaller penile size cannot be ‘corrected’ with surgery, apart from straightening procedures, degloving and chordee resection. Two‐stage procedures with mobilization of the urethral plate and lengthening of the corpora ventrally probably have a better potential to improve penile length [29, 30]. Nowadays, flap phalloplasty is an alternative, which is undergone by transgender men and patients with severe penile insufficiency/injury, but more comparative long‐term outcome studies are needed, especially in men with a micropenis.
One not so frequently described finding may be of interest, namely, the affected glans sensitivity after hypospadias surgery in seven of 74 participants; pain, in particular, was exclusively described in the group that underwent surgery. The finding that fewer than half of participants seemed to have normal sensitivity and one‐third claimed a sensitivity as compared to the inner thigh could be a reason for dissatisfaction of function and sexual dysfunction. This finding may be the result of a lack of foreskin after surgery because reduced sensitivity is also shown after circumcision in adults [31], but may also be caused by surgical procedures with dorsal plications for correcting chordee.
Dissatisfaction often remains high after hypospadias surgery, especially with regard to appearance and sexuality [32], and this was confirmed in the present study. Most patients have no insight into preoperative status as surgery was performed early in life, which also gives them no experience of being non‐operated. The aesthetic outcome related to scarring is a reason for dissatisfaction, although was not so obvious in the present study, with the exception of the examiners' view in males with PAIS. It has been shown previously that after a hypospadias surgery men are less satisfied with penile appearance compared to controls, even if the objectively assessed aesthetic results are good [12, 14, 33]. We were not able to correlate Prader stage at birth, or age at and number of surgeries with follow‐up satisfaction, possibly due to the sample heterogeneity. Older age at the time of participation in the study, however, was associated with more dissatisfaction with postoperative appearance and function, which may be attributed to improved surgical techniques and more affirmative healthcare practices. Compared with a 2011 review [12], the present study showed similar rates of dissatisfaction with postoperative appearance (37% vs 38%) and with function (29% vs 20%). However, satisfaction with appearance has also been shown to be very low in individuals with nonoperated hypospadias [34]. Because appearance is an important outcome, and the results are to a large extent unsatisfactory, especially in proximal hypospadias, such surgery should be performed only by experienced specialists [6, 14]. In our group with severe hypospadias, participants were slightly more satisfied with their penile function than with the aesthetics. This may imply that many have acceptable voiding and sexual function. Earlier studies reported that the quality of erection in men with distal hypospadias is comparable to that in non‐affected men [13], but may be impaired in patients with proximal hypospadias [33]. Our results indicate a fairly satisfied view on penile erectability, arousability and orgasmic capacity, however, approximately one‐quarter of patients experienced premature ejaculation and erectile dysfunction with delayed orgasm. These symptoms were reported most frequently in (control) participants with KS and delayed orgasm was most frequently reported in those with hypospadias and PAIS. These findings support follow‐up by a skilled urologist/andrologist after puberty, with possible referral to a sexologist and adequate hormone substitution.
The findings on gonadal surgery are complex to evaluate because of differences between the left and right gonad and cases in which both orchidopexy and later gonadectomy have been performed. One‐third of participants underwent gonadectomy on one or two sides and half had orchidopexy performed. In the majority, the scrotum looked fused and symmetrical but almost one‐third had impalpable gonads. The participants themselves provided various different ratings for scrotal and testicle body image, but were mostly dissatisfied after gonadectomy. Gonadectomy was primarily performed in individuals with GD because of the risk of germ cell neoplasia. The risk of neoplasia was previously reported to be 14% in XY DSD, 23% in partial GD and in 8% in mixed GD, none in PAIS or KS in our cohort [23]. It remains unknown whether more insight into the motivations behind performing (early) gonadectomy to prevent tumour development would positively influence scrotal/testicular body image at follow‐up.
Dissatisfaction with regard to penile body image in those who underwent surgery for hypospadias was much higher than in non‐operated clinical controls (with KS), and worst among those with PAIS. The scrotum was regarded as a little more satisfactory, but not after gonadectomy. No cross‐condition data with which to compare these results have been published before. However, in the present study, participants with PAIS also scored worse on multiple aspects of genital examination (e.g. shorter penile length, poorer overall appearance and more scarring), which may contribute to the poorer patient‐reported outcomes.
Patient‐reported outcomes with regard to sexuality indicate that many problems can occur at follow‐up after masculinizing surgery. Many participants were not satisfied with their sex life, although the (hypospadias) groups who did and did not undergo surgery scored alike. Possibly related to the low satisfaction is the published finding that sexual activity is generally lower in individuals with a DSD when compared with the general population [32], although sex life satisfaction was lower in individuals with DSD who underwent surgery compared to those who did not, possibly indicating more severe phenotypes. In the present study, the highest dissatisfaction with penile appearance and shape was reported among men with PAIS and they also experienced more urogenital infections and premature ejaculation. Generally, orgasm function and erection were regarded as satisfactory. Our results are in accordance with earlier studies reporting that appearance is generally not satisfactory, but function and sex life are appreciated to a higher degree [12, 13, 14, 16, 28, 33, 35]. This finding is probably attributable to the fact that genital appearance and function are only some of the factors contributing to a satisfactory sex life (e.g. partner support, coping skills and adequate sex hormones). Lastly, similar to the decreased sexual satisfaction, sexual problems, including erectile dysfunction and premature ejaculation were more common in our sample that underwent surgery (all surgeries) compared with normative values.
The question on timing of surgery is much debated and, generally, the participants were satisfied with early surgery for hypospadias and cryptorchidism. The procedures performed in adulthood were in general regarded as more satisfactory. There is a tendency to prefer the timing of surgery according to one’s individual experience. However, there was limited and likely biased data to support these hypotheses.
Lessons that we can learn from earlier studies and the present study are that surgical treatment is not satisfactory for all in the long term, and that treatment requires experienced multidisciplinary teams and a respect for surgical procedures, especially those that are irreversible [1]. It is also known that non‐operated adult men with hypospadias reported lower sexual health, with ventral curvature, difficulties with intercourse and voiding, and also lower penile perception scores, and these were worse in proximal hypospadias [36]. It is essential to focus on performing surgery that is medically necessary. This is further underlined by our finding that it was mostly the surgeries that participants chose themselves at an older age (e.g. breast reduction) that had the most positive impact on their lives.
The present study describes a large group of men, who underwent surgery for DSD with diverse diagnoses and ages, for whom we collected many different (patient‐reported) variables, which is a significant strength of the study. However, describing and evaluating the impact of masculinizing surgery has some limitations, and in the present study one of these was that the overall response rate was only 34%. Nevertheless, these participants contributed a large amount of data, given on a group level. The participating sample may have been biased towards more positive patients who were more inclined to participate in this clinical follow‐up study, and the reverse cannot be excluded either. Reconstructive surgeries in individuals with DSD are often complex, which is reflected in the mean of 5.3 surgeries per participant, thus there was an overlap of these procedures, with some surgeries being infrequently performed (e.g. phalloplasty), which makes generalizable interpretations on group levels more difficult. To improve future follow‐up evaluations we would recommend that surgeons standardize and document the clinical findings concerning phenotype severity as well as details of the surgical procedure.
In conclusion, the present data suggest that long‐term satisfaction with aesthetic and functional outcomes depends on both initial anatomy and the surgeries performed, and not necessarily on the specific diagnosis, although men from certain DSD groups tended to report poorer outcomes. We found moderate satisfaction with appearance after hypospadias surgery, and higher satisfaction with regard to function and sexuality, even though many participants experienced some degree of sexual problems. The individual’s and the observer’s perspectives on the anatomical and functional outcomes of masculinization surgery often do not correspond well. Clinicians should be attentive to these problems when following up on this group and collaborate with psychologists and sexologists when required. Our findings suggest some characteristics to consider since poorer average (patient‐reported) outcomes were found in older participants and those with a PAIS diagnosis, with (extensive) hypospadias surgery and/or gonadectomy.
Conflicts of Interest
None declared.
Acknowledgements
We are grateful to the individuals who participated in dsd‐LIFE and all of the study centres for their enthusiasm and dedication in contacting potential participants and collecting high‐quality data, and especially we would like to thank Tabea Schroder and Sofia Mouttalib. We also thank the support groups in the different countries that have supported the study. For an overview of all contributors we refer to our study protocol by Röhle et al. [20].
We publish this paper in memoriam and with the greatest thanks to PD Dr Birgit Kohler (Charite Universitatsmedizin, Berlin), the principal investigator of the European Consortium dsd‐LIFE, who died in March 2019. We honour Birgit Kohler's dedicated leadership, energy and enthusiasm for the dsd‐LIFE project and her promotion of collaboration of clinicians, patients and support groups aiming to improve clinical care in DSD. dsd‐LIFE was funded by the European Union Seventh Framework Programme no. 305373 (FP7/2007–2013). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Appendix 1. The dsd‐LIFE group
Birgit Kohler and Uta Neumann, Berlin; Peggy Cohen‐Kettenis, Baudewijntje Kreukels and Annelou de Vries, Amsterdam; Wiebke Arlt, Birmingham; Claudia Wiesemann, Gottingen; Jolanta Slowikowska‐Hilczer, Lodz; Ute Thyen and Marion Rapp, Lubeck; Aude Brac de la Perriere, Lyon; Charles Sultan and Francoise Paris, Montpellier; Nicole Reisch, Munich; Annette Richter‐Unruh, Munster and Bochum; Hedi Claahsen–van der Grinten, Nijmegen; Claire Bouvattier and Lise Duranteau, Paris; Anna Nordenström and Agneta Nordenskjöld, Stockholm; Catherine Pienkowski, Toulouse; Maria Szarras‐Czapnik, Warsaw.
T.C.G. and M.R. contributed equally to this work as first authors.
Trial registration: Trial registry name: Klinische europäische Studie zu Langzeitergebnissen chirurgischer und hormoneller und psychologische Behandlung von Patienten mit Störungen der Geschlechtsentwicklung (DSD). Registration identification number: DRKS00006072 (German Clinical Trials Register); URL: http://drks‐neu.uniklinik‐freiburg.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00006072.
Contributor Information
Tim C. van de Grift, Email: t.vandegrift@amsterdamumc.nl.
the dsd‐LIFE group:
Birgit Kohler, Uta Neumann, Peggy Cohen‐Kettenis, Baudewijntje Kreukels, Annelou de Vries, Wiebke Arlt, Claudia Wiesemann, Jolanta Slowikowska‐Hilczer, Ute Thyen, Marion Rapp, Aude Brac de la Perriere, Charles Sultan, Francoise Paris, Nicole Reisch, Annette Richter‐Unruh, Hedi Claahsen–van der Grinten, Claire Bouvattier, Lise Duranteau, Anna Nordenström, Agneta Nordenskjöld, Catherine Pienkowski, and Maria Szarras‐Czapnik
References
- 1. Hughes IA, Houk CP, Ahmed SF, Lee PA, LWPES Consensus Group, ESPE Consensus Group . Consensus statement on management of intersex disorders. Arch Dis Child 2006; 91: 554–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benneke E, Köhler B, Röhle R et al. Disorders or differences of sex development? Views of affected individuals on DSD terminology. J Sex Res 2020. 10.1080/00224499.2019.1703130 [DOI] [PubMed] [Google Scholar]
- 3. Nordenvall AS, Frisén L, Nordenström A, Lichtenstein P, Nordenskjöld A. Population based nationwide study of hypospadias in Sweden, 1973 to 2009: incidence and risk factors. J Urol 2014; 191: 783–9 [DOI] [PubMed] [Google Scholar]
- 4. Ghirri P, Ciulli C, Vuerich M et al. Incidence at birth and natural history of cryptorchidism: a study of 10,730 consecutive male infants. J Endocrinol Invest 2002; 25: 709–15 [DOI] [PubMed] [Google Scholar]
- 5. Snodgrass W. Tubularized, incised plate urethroplasty for distal hypospadias. J Urol 1994; 151: 464–5 [DOI] [PubMed] [Google Scholar]
- 6. Ruppen‐Greef NK, Weber DM, Gobet R, Landolt MA. What is a good looking penis? How women rate the penile appearance of men with surgically corrected hypospadias. J Sex Med 2015; 12: 1737–45 [DOI] [PubMed] [Google Scholar]
- 7. Springer A, Baskin LS. Timing of hypospadias repair in patients with disorders of sex development. Endocr Dev 2014; 27: 197–202 [DOI] [PubMed] [Google Scholar]
- 8. Sircili MHP, de Queiroz e Silva FA, Costa EMF et al. Long‐term surgical outcome of masculinising genitoplasty in large cohort of patients with disorders of sex development. J Urol 2010; 184: 1122–7 [DOI] [PubMed] [Google Scholar]
- 9. McNamara ER, Schaeffer AJ, Logvinenko T et al. Management of proximal hypospadias with 2‐stage repair: 20 year experience. J Urol 2015; 194: 1080–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spinoit AF, Poelaert F, van Praet C, Groen LA, van Laecke E, Hoebeke P. Grade of hypospadias is the only factor predicting for re‐intervention after primary hypospadias repair: A multivariate analysis from a cohort of 474 patients. J Pediatr Urol 2015; 11(70): e1–70.e6 [DOI] [PubMed] [Google Scholar]
- 11. Moriya K, Kakazaki H, Tanaka H et al. Long‐term patient reported outcome of urinary symptoms after hypospadias surgery: Norm related study in adolescents. J Urol 2007; 178(4S): 1659–62 [DOI] [PubMed] [Google Scholar]
- 12. Rynja SP, de Jong TPVM, Bosch JLHR, de Kort LMO. Functional, cosmetic and psychosexual results in adult men who underwent hypospadias correction in childhood. J Ped Urol 2011; 7: 504–15 [DOI] [PubMed] [Google Scholar]
- 13. Ruppen‐Greeff MK, Weber DM, Gobet R, Landolt MA. Health‐related quality of life in men with corrected hypospadias: an explorative study. J Pediatr Urol 2013; 9: 551–8 [DOI] [PubMed] [Google Scholar]
- 14. Örtqvist L, Fossum M, Andersson M et al. Long‐term follow up of men born with hypospadias: urological and cosmetic results. J Urol 2015; 193: 975–81 [DOI] [PubMed] [Google Scholar]
- 15. Andersson M, Sjostrom S, Doroszkiewicz M et al. Urological results and patient satisfaction in adolescents after surgery for proximal hypospadias in childhood. J Ped Urol 2020; 16: 660.e1–e8 [DOI] [PubMed] [Google Scholar]
- 16. Örtqvist L, Fossum M, Andersson M et al. Sexuality and fertility in men with hypospadias; improved outcome. Andrology 2017; 5: 286–93 [DOI] [PubMed] [Google Scholar]
- 17. Skarin Nordenvall A, Chen Q, Norrby C et al. Fertility in adult men born with hypospadias: a nationwide register‐based cohort study on birthrates, the use of assisted reproductive technologies and infertility. Andrology 2020; 8: 372–80 [DOI] [PubMed] [Google Scholar]
- 18. Andersson M, Sjostrom S, Wangqvist M, Ortqvist L, Nordenskjold A, Holmdahl G. Psychosocial Outcomes in adolescents following surgery for proximal hypospadias in childhood. J Urol 2018; 200: 1362–70 [DOI] [PubMed] [Google Scholar]
- 19. Radmayr C, Dogan HS, Hoebeke P et al. Management of undescended testes: European Association of Urology/European Society for Paediatric Urology Guidelines. J Pediatr Urol 2016; 12: 335–43 [DOI] [PubMed] [Google Scholar]
- 20. Röhle R, Gehrmann K, Szarras‐Czapnik M et al. Participation of adults with disorders/differences of sex development (DSD) in the clinical study dsd‐LIFE: design, methodology, recruitment, data quality and study population. BMC Endocr Disord 2017; 17: 52–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van de Grift TC, Cohen‐Kettenis PT, Elaut E et al. A network analysis of body satisfaction of people with gender dysphoria. Body Image 2016; 17: 184–90 [DOI] [PubMed] [Google Scholar]
- 22. Lindgren TW, Pauly IB. Body image scale for evaluating transsexuals. Arch Sex Behav 1975; 4: 639–56 [DOI] [PubMed] [Google Scholar]
- 23. Slowikowska‐Hilczer J, Szarras‐Czapnik M, Duranteau L et al. Risk of gonadal neoplasia in patients with disorders/differences of sex development. Cancer Epidemiol 2020; 69: 101800 [DOI] [PubMed] [Google Scholar]
- 24. Aksglaede L, Shakkebaek NE, Almstrup K, Juul A. Clinical and biological parameters in 166 boys, adolescents and adults with nonmosaic Klinefelter syndrome: a Copenhagen experience. Acta Paediatr 2011; 100: 793–806 [DOI] [PubMed] [Google Scholar]
- 25. Hughes IA, Davies JD, Bunch TI, Pasterski V, Mastroyannopoulou K, MacDougall J. Androgen insensitivity syndrome. Lancet 2012; 380: 1419–28 [DOI] [PubMed] [Google Scholar]
- 26. Kreukels BPC, Köhler B, Nordenström A et al. Gender dysphoria and gender change in disorders of sex development/intersex conditions: results from the dsd‐LIFE Study. J Sex Med 2018; 15: 777–85. [DOI] [PubMed] [Google Scholar]
- 27. Pippi Salle JL, Sayed S, Salle A et al. Proximal hypospadias: a persistent challenge. Single institution outcome analysis of three surgical techniques over a 10‐year period. J Ped Urol 2015; 12: 28.e1–e7 [DOI] [PubMed] [Google Scholar]
- 28. van de Zwan YG, Callens N, van Kuppenveld J et al. Long‐Term outcomes in males with disorders of sex development. J Urol 2013; 190: 1038–42. [DOI] [PubMed] [Google Scholar]
- 29. Callens N, Hoebeke P. Phalloplasty: a panacea for 46,XY disorder of sex development conditions with penile deficiency. Endocr Dev 2014; 27: 222–33. [DOI] [PubMed] [Google Scholar]
- 30. Snodgrass WT, Bush N. Staged tubularized autograft repair for primary proximal hypospadias with 30‐degree or greater ventral curvature. J Urol 2017; 198: 680–6 [DOI] [PubMed] [Google Scholar]
- 31. Bronselaer GA, Schober JM, Meyer‐Bahlburg HFL, T’Sjoen G, Vlietnick R, Hoebeke PB. Male circumcision decreases penile sensitivity as measured in a large cohort. BJU Int 2013; 111: 820–7 [DOI] [PubMed] [Google Scholar]
- 32. Kreukels BPC, Cohen‐Kettenis PT, Roehle R et al. Sexuality in adults with differences/ disorders of sex development (DSD): findings from the dsdLIFE Study. J Sex Marital Ther 2019; 45: 688–705 [DOI] [PubMed] [Google Scholar]
- 33. Even L, Bouali O, Moscovici J et al. Long‐term outcomes after hypospadias surgery: sexual reported outcomes and quality of life in adulthood. Prog Urol 2015; 25: 655–64 [DOI] [PubMed] [Google Scholar]
- 34. Keays MA, Starke N, Lee SC, Bernstein I, Snodgrass WT, Bush N. Patient reported outcome in preoperative and postoperative patients with hypospadias. J Urol 2015; 195: 1215–20 [DOI] [PubMed] [Google Scholar]
- 35. Aho MO, Tammela OK, Somppi EM, Tammela TL. Sexual and social life of men operated in childhood for hypospadias and phimosis. A comparative study. Eur Urol 2000; 37: 95–100 [DOI] [PubMed] [Google Scholar]
- 36. Schlomer B, Breyer B, Copp H, Baskin L, DiSandro M. Do adult men with untreated hypospadias have adverse outcomes? A pilot study using social media advertised survey. J Pediatr Urol 2014; 10: 672–9 [DOI] [PMC free article] [PubMed] [Google Scholar]


