Abstract
Objective
To investigate the impact of timing of implant placement and loading on implant survival and biological outcomes of multiple‐unit implant‐supported fixed dental prosthesis (FDPs).
Material and Methods
A literature search was performed by three independent reviewers for studies reporting on ≥10 patients with FPDs supported by ≥two implants over ≥3 years of follow‐up. Data were analyzed on implant survival and biological complications as primary outcomes and biological events, including changes in peri‐implant marginal bone level (MBL), probing depth, soft‐tissue level, and health condition as secondary outcomes.
Results
7002 titles were identified, 360 full‐texts were screened, and 14 studies were included. These comprised 6 randomized controlled studies (RCTs), 5 cohort studies, and 3 case series with identifiable implant placement and loading protocols in five of 09 possible combinations. All groups but one (IPIL) showed implant survival rates >90%. A meta‐analysis based on 3 RCTs found no differences in survival rate between DPIL and DPDL (p = .227).
Conclusions
High survival rates for all studied implant placement and loading combinations were shown for FPDs over ≥3 years of follow‐up. When a delayed implant placement protocol is applied, immediate or delayed loading demonstrated similar survival rates. The heterogeneity of the data did not allow to draw any further conclusions on the occurrence of biological complications related to timing of implant placement/loading.
Keywords: biological outcomes, bone level change, dental implant, implant loading protocols, implant placement protocols, success rates, survival rate
1. INTRODUCTION
Fixed dental prostheses supported by implants have become a well‐documented and reliable treatment option. Excellent survival rates of both the multiple‐unit prostheses and their supporting implants have been reported notably for conventional metal‐ceramic restorations (Sailer et al., 2018). Advances on the prosthetic materials, along with the development of different implant surfaces, digital planning tools and surgical techniques have contributed to the current success rates of implant‐supported restorations (Buser et al., 2017).
All the contemporary treatment and fabrication concepts have aimed to minimize treatment durations and patient visits while maintaining optimal clinical and patient‐related outcomes (Scheyer et al., 2017). This quest for greater efficiency also has resulted in a diversification of implant placement and loading protocols. Contemporary options include immediate, early, or placement, as well as immediate, early, or conventional loading (Gallucci et al., 2018). It is reasonable to assume that these expedited procedures and fewer patient visits involved in immediate or early placement or loading will reduce the cost of treatment, and possibly increase efficiency (Scheyer et al., 2017).
Numerous reviews have been published to classify these protocols and define their indications (Gallucci et al., 2009, 2014, 2018; Schrott et al., 2014). While both immediate/early placement and immediate/early loading can yield excellent results, they are subject to biological limitations and a need for careful patient selection and site assessment (Gallucci et al., 2018). Immediate or early placement requires a fair amount of residual bone for good primary stability of the implant (Benic et al., 2014; Gallucci et al., 2018).
Good primary stability is also crucial for immediate loading of implants. While surface modifications and advanced designs have improved the outcomes of all placement and loading protocols (Benic et al., 2014; Chu et al., 2020; Gallucci et al., 2018), immediate placement right after tooth extraction has repeatedly been shown not to prevent physiological remodeling of the alveolar bone (Sanz et al., 2017; Vignoletti et al., 2009). Thus, special care should be taken by clinicians in order to prevent biological and esthetic complications due to the natural ridge resorption and bone remodeling that will occur independently of implant placement (Araújo et al., 2005; Buser et al., 2009; Buser et al., 2013. These processes are accompanied by volume changes of the peri‐implant soft tissue, with loss of mucosa seen more often after immediate than early placement (Lee et al., 2020). Nonetheless, mucogingival tissue findings are contradictory. While they demonstrate that biotype (in addition to residual bone volume) is another major modifier of biological outcomes after immediate/early placement or loading (Lee et al., 2020; Prati et al., 2020; Sanz‐Martín et al., 2019), some authors have found significant mucosal recession around immediately placed and loaded implants (Blanco et al., 2019; Kolerman et al., 2016) whereas others have not (Chan et al., 2019; Östman et al., 2020; Parvini et al., 2020; Pohl et al., 2020; Yan et al., 2016).
Thus, it cannot be excluded that immediately inserted implants may be at higher risk of developing biological complications such as peri‐implant disease (Parvini et al., 2020).
The influence of soft‐tissue biotypes on the incidence of peri‐implant inflammation has been demonstrated in animal and clinical studies, suggesting the need for grafting procedures simultaneously to immediate implant placement (Chappuis et al., 2017; Perussolo et al., 2018).
Reversible inflammation affecting the soft tissue around the implant (mucositis) is a highly frequent condition that can progress to progressive bone loss (peri‐implantitis) and eventually implant loss. (Lee et al., 2017). Local and systemic conditions, such as poor oral hygiene, smoking, and diabetes, are already known risk factors for peri‐implant diseases, and the influence of recently developed implant materials and surfaces has been studied (De Bruyn et al., 2017; Dreyer et al., 2018; Peixoto & Almas, 2016). However, the role of recently developed surgical techniques including placement and loading shorter time protocols and their combinations in the index of these biological complications and implant survival is little known.
Further discussed are flapless approaches, a particularly efficient method often utilized for immediate procedures, offer advantages but are limited by local anatomy, ongoing infections and surgical skills (Barone et al., 2016).
With efficiency (shorter treatment durations, fewer patient visits, better affordability) being more desirable than ever in times of a pandemic crisis and global financial constraints, there is a need for evidence‐based insights into the biological indications and limitations of immediate/early placement and loading of implants, enabling clinicians to make appropriately efficient treatment decisions in carefully selected patients.
In this context, the present systematic review investigated the question of whether different timing protocols of implant placement and implant loading affect the biological outcomes and implant survival related to implant‐supported fixed partial dentures (FPDs) in partially edentulous patients.
2. MATERIAL AND METHODS
Ethics approval was not required for this systematic review and was registered in PROSPERO (IRD42020179528) and conducted in accordance with PRISMA (Liberati et al., 2009), PRISMA extension for abstracts (Beller et al., 2013), IOM (Institute of Medicine) standards (Institute of Medicine Committee on Standards for Developing Trustworthy Clinical Practice, 2011), and the Cochrane Handbook for Systematic Reviews of Interventions (Higgins & Green, 2017).
2.1. Focusing the question for review
The PICOS (population, intervention, comparison, outcome, and studies) principle was applied to focus the question posed for this review. As population, the focus was on partially edentulous patients treated by implant‐supported fixed partial dentures (FPDs). As intervention, the focus was on immediate or early placement or loading as compared to delayed placement or delayed loading. Outcome parameters included, as primary measures, implant survival and biological complications (e.g., peri‐implantitis, peri‐implant mucositis, and apical peri‐implantitis) and as secondary measures, the radiographic parameter of marginal bone levels (MBL) and the clinical parameters of soft‐tissue recession, bleeding on probing (BOP), probing depths (PD), preservation or loss of width of keratinized tissue (KT), and plaque index (PI) were analyzed (Vetter & Mascha, 2017). The study designs that were eligible for inclusion were prospective and retrospective comparative and non‐comparative clinical trials.
The focused question was as follows: Does immediate or early implant placement and loading influence the biological complication rate and implant survival in partially edentulous patients when compared with conventional protocols?
2.2. Protocols of implant placement and loading
Timing possibilities for implant placement and loading were defined as proposed by Gallucci et al., 2018; Siebers et al., 2010):
IPIL: immediate placement + immediate restoration/loading
IPEL: immediate placement + early loading
IPDL: immediate placement + delayed loading
EPIL: early placement + immediate restoration/loading
EPEL: early placement + early loading
EPDL: early placement + delayed loading
DPIL: delayed placement + immediate restoration/loading
DPEL: delayed placement + early loading
DPDL: delayed placement + delayed loading
Previous reports (Chen & Buser, 2009; Chen et al., 2004; Gallucci et al., 2018; Hämmerle et al., 2004; Siebers et al., 2010) provided the blueprint for the definition of protocols to be reviewed.
Immediate implant placement (IP): The implant is placed at the same day of tooth extraction.
Early implant placement (EP): The implant is placed between 1 and 4 months after tooth extraction.
Delayed implant placement (DP): Implant is placed >6 months after tooth extraction.
Immediate loading (IL): The prosthesis is connected to the implant within 1 week following implant placement.
Early loading (EL): Loading is performed between 1 week and 2 months after implant placement.
Delayed loading (DL): Prosthesis is connected to the implant later than 2 months after implant placement.
Definition of periodontal and peri‐implant diseases, conditions, health and complications were based on the proposed classification by the 2017 World Workshop, co‐sponsored by the American Academy of Periodontology and the European Federation of Periodontology (Araujo & Lindhe, 2018; Caton et al., 2018; Heitz‐Mayfield & Salvi, 2018; Schwarz et al., 2018).
2.3. Search strategy
The search strategy was developed in close collaboration with a research methodologist (University of Malmö, Sweden) and a “reference and education services librarian” (Medical University of Graz, Austria). The databases which were searched included PubMed/Medline, Embase, and Cochrane CENTER (Central Register of Controlled Trials) databases. Publications in English language were thus identified up to April 29, 2020. Whenever possible, controlled MeSH terms were included in the keyword combinations used for these database searches. The electronic search was complemented by an additional hand search that included the reference lists of all included publications and, in addition, systematic reviews on related topics.
For a detailed overview of search terms used in Embase and Cochrane, the reader is referred to Appendix S1. The basic terms used in PubMed, Embase, and Cochrane were as follows:
(dental implant) AND (immediate OR early OR late OR delayed OR conventional OR post‐extraction OR post‐extractive)
Filters: English, humans, year from 2000 up to April 29, 2020.
A reference management tool (EndNote X9.3.3; Clarivate Analytics, London, UK) was used for first entry of all references and elimination of double entries. Screening at the title, abstract, and full‐text levels was accomplished using a web‐based application for systematic reviews (rayyan.qcri.org) (Ouzzani et al., 2016).
2.4. Inclusion and exclusion criteria
Any multiple publications on the same populations were handled by considering only the results of the latest study (the one reporting the longest follow‐up) without making recourse to any of the preceding studies unless to retrieve truly additional information.
Studies meeting the criteria below were included:
Randomized and non‐randomized controlled trials
cohort and case–control studies
Prospective or retrospective case series
FPDs supported by ≥2 implants in partially edentulous patients
Root‐form or cylindrical implants supporting the FPDs
≥ 10 patients in each study arm and ≥3 years of follow‐up
Adequate reporting of implant placement protocols with timing
Adequate reporting of implant loading protocols with timing
Endosteal diameter of implant shoulder: 3−6 mm
Reporting of one or more biological outcomes
Studies meeting the criteria below were excluded:
Preclinical in vitro, experimental, or animal studies
Full‐arch dentures or removable superstructures
Implants placed in previously irradiated bone or in alveolar clefts
Medication compromising bone metabolism
Studies not based on clinical examinations (e.g., questionnaire surveys)
Studies published in languages other than English
Restorations other than permanently screw‐retained or cemented FPDs
Studies with non‐eligible designs
Inability to distinguish between placement/loading protocols
Inability to rule out single‐unit or full‐arch restorations
2.5. Screening and contacting
The retrieved reference publications were independently screened by three reviewers (LLA, JP, and GNA), including a first screening at the title/abstract level (LLA and JP) followed by a second run of full‐text screening conducted in duplicate (LLA, JP, and GNA). Any disagreements were settled either by discussion between the three reviewers or by obtaining a fourth and fifth opinion (IS and MP). The default approach was to include or exclude studies based on these full‐text screens, although this decision was deferred for studies regarded as potentially relevant. In these cases, the authors were emailed and asked to provide additional data. Likewise, authors of potentially relevant and already included studies were emailed as needed to resolve issues and fill in missing bits of information for the ensuing data extraction (see below). All this extra information was analyzed, and the data integrated for the final datasets.
2.6. Data extraction
As per the Cochrane recommendations, standardized pre‐piloted forms were designed for data extraction from all included papers. Three reviewers (LLA, JP, and GNA) extracted in duplicate a defined set of study characteristics (design, setting, funding, country, patient number, and mean age) and additional data pertinent to the PICO question.
Primary outcome measures
Implant survival rate (%)
Biological complication rate (peri‐implant mucositis and peri‐implantitis) (number of events)
Secondary outcome measures
Marginal bone levels (MBL) (in mm)
Bleeding on probing (BOP); modified Bleeding Index; Gingival Index; Sulcus Bleeding Index; Bleeding Index
Soft‐tissue recession (in mm)
Width of keratinized tissue (KT) (in mm)
Plaque index (PI)
Probing depths (PD) (in mm)
Miscellaneous information
Systemic condition of patients
Prescription of antibiotics
Time of implant placement after tooth loss or removal
Time of implant loading (functional or nonfunctional)
Mean follow‐up period
Implant numbers and locations
Implant diameters, lengths, surface characteristics
Implant materials, types and brands
Use and design of surgical access flaps
Use of bone grafting (material, technique)
Healing protocol (submerged, transmucosal)
Type and occlusal design of interim prosthesis
Design of the definitive FPD
Implant survival rate(s)
Prosthetic complications
2.7. Bias assessments and synthesis
Risk‐of‐bias assessments were conducted to rate the risk of bias in each individual study, using appropriate tools for each study designs. The Cochrane RoB 2.0 tool was applied to RCTs [Sterne et al., 2019], the Newcastle‐Ottawa scale to cohort studies (Wells et al., 2000), and the Joanna Briggs Institute's Critical Appraisal Checklist to case series (The Joanna Briggs Institute, 2017). It was planned to assess reporting biases by applying Egger's and Begg's tests to the main outcomes, to interpret tests for funnel plot asymmetry with visual inspection, and to perform post hoc sensitivity analyses by excluding studies one by one from the global estimation. To judge the strength of clinical recommendations derived from studies, their overall qualities of evidence were assessed based on the GRADE approach (Guyatt et al., 2011).
2.8. Statistical analysis
Cohen's kappa was used to determine inter‐rater (i.e., between the three reviewers) agreement and descriptive statistics to elucidate survival and biological complication rates and clinical outcomes. For each protocol, a mean cumulative survival rate was planned to be calculated and weighted by follow‐up durations and implant numbers. Thus, a weighted mean survival rate for each protocol was obtained by applying this formula:
where X is the reported survival rate, t the follow‐up period, and n the number of implants reported in each study (study 1 to study k).
As the implant placement is bound to be affected by patient and treatment‐related characteristics, a random‐effects model was a priori deemed appropriate to calculate the average distribution of true effects, based on clinical and statistical reasoning (Papageorgiou 2014), and an inverse variance estimator with the DerSimonian‐Laird estimator for tau2 was chosen (Langan et al., 2019).
Absolute and relative between‐trial heterogeneity was assessed using the t 2 and I 2 indices, respectively. The latter (I 2) index was defined as percentage variation in the global estimate due to heterogeneity, with I 2 scores of 25%, 50%, or 75% indicating low, moderate, or high heterogeneity, respectively. Forest plots were created to illustrate the effects in a meta‐analysis. SPSS Statistics (v. 26, IBM, Armonk, NY, USA) and R (v. 1.3; R Project for Statistical Computing, Vienna, Austria) software was used for all statistical operations. Differences were considered significant at p ≤ .05.
The potential of publication bias of this review was assessed by the funnel plot and an additional statistical test; the Egger’s test was performed (Figure 3).
FIGURE 3.
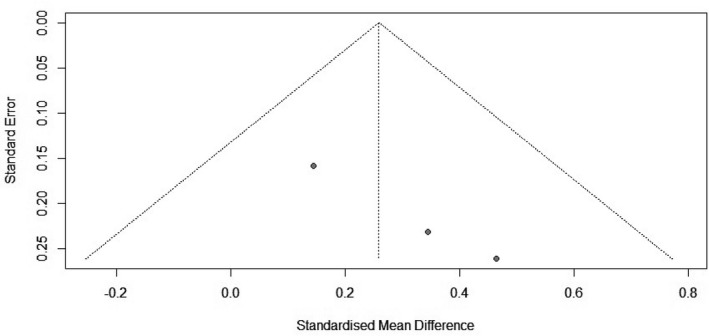
Funnel plot describing the publication bias assessment
3. RESULTS
3.1. Selected studies and their characteristics
The applied search strategy, returned a total of 7002 titles, after the identification and exclusion of 1593 duplicated hits (Figure 1). Screening at the title/abstract level left 360 articles for full‐text screening to assess their eligibility. Inter‐rater agreement (kappa score) was 0.63 for the title/abstract and 0.96 for the full‐text screens. A total of 153 studies were categorized as potentially relevant, eight of which could be included upon contacting their authors (Daher et al., 2019; Göthberg et al., 2018; Oxby et al., 2015; Payer et al., 2010; Si et al., 2016; Siebers et al., 2010; Simons et al., 2015; Vogl et al., 2019). Fourteen studies were finally included: Six RCTs, five cohort (four observational cohort and one case–control) studies, and three case series (two prospective and one retrospective).
FIGURE 1.
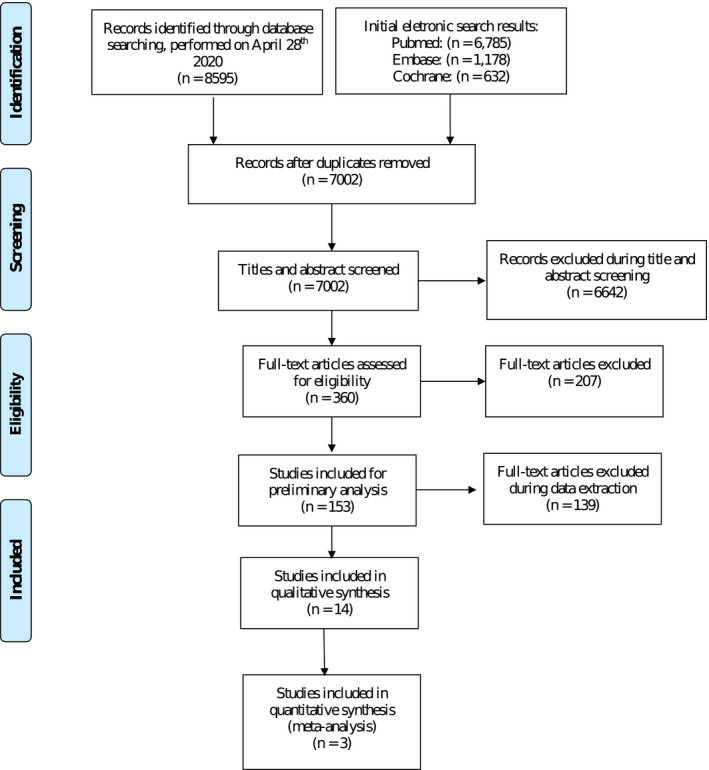
PRISMA flow diagram illustrating the search strategy
Each of these 14 studies was carefully selected based on parameters reported. In each assessment for eligibility, care was taken to identify well‐defined information on the placement and loading protocols used. Table 1 gives an overview of excluded studies and reasons for their exclusion. For additional information on the reasons for exclusion during full‐text screening, the reader is referred to Appendix S2. Table 2 lists the 14 included studies and their 21 cohort groups enabling us to analyze combined protocols of implant placement and loading.
TABLE 1.
Reasons for exclusion during data extraction
| Main reason for exclusion | N | Studies |
|---|---|---|
| Insufficient data for screening assessment | 67 |
Agliardi et al. (2014) Al Amri et al. (2017) Alasqah et al. (2018) Arlin et al. (2007) Bilhan et al. (2010) Bornstein et al. (2007) Bornstein et al. (2005) Bruschi et al. (2017) Cassetta et al. (2016) Cesaretti et al. (2015) Cochran et al. (2009) Crespi et al. (2010) Degidi et al. (2009a) Degidi et al. (2009b) Ferrini et al. (2018) Glauser et al. (2013) Glibert et al. (2016) Gomez‐Roman et al. (2001) Han et al. (2017) Harel et al. (2013) Jungner et al. (2014) Jungner et al. (2012) Kim et al. (2017) Kokovic et al. (2014) Maddalone et al. (2018) Malchiodi et al. (2011) Montero et al. (2012) Muelas‐Jiménez et al. (2015) Mura (2018) Nicolau et al. (2019) Nicolau et al. (2013) Peñarrocha‐Diago et al. (2012) Pettersson and Sennerby (2013) Polizzi et al. (2000) Polizzi et al. (2013) Pozzi et al. (2012) Pozzi et al. (2015) Rammelsberg et al. (2016) Rocci et al. (2012) Roccuzzo et al. (2018) Rocha et al. (2016) Rossi et al. (2017) Sato et al. (2014) Schliephake et al. (2012) Şener‐Yamaner et al. (2017) Sullivan et al. (2005) Sullivan et al. (2001) Tallarico and Meloni (2017) Testori et al. (2017) Valerón and Valerón (2007) Villa (2018) Wagenberg and Froum (2014) Zembić et al. (2010) Madani et al. (2018) Jung et al. (2016) Cochran et al. (2011a) Cochran et al. (2011b) Esposito et al. (2018) Bressan et al. (2017) Felice et al. (2019) Gastaldi et al. (2017) Maló and de Araújo Nobre (2016) Nedir et al. (2017) Prosper et al. (2010) Queridinha et al. (2016) Testori et al. (2001) Zuffetti et al. (2016) |
| Mean follow‐up less than 3 years | 3 |
Schwartz‐Arad et al. (2007) Cordaro et al. (2010) Degidi et al. (2008) |
| Less than 10 patients at 3 years | 1 | Ding and Wang (2017) |
| Absence of FPDs supported by ≥2 implants | 7 |
Prati et al. (2016) Kolinsky et al. (2013) Merli et al. (2020) Romeo et al. (2012) Salina et al. (2019) Crespi et al. (2016) Bruschi et al. (2014) |
| Insufficient data do separate different placement protocols | 4 |
Ferrini et al. (2018) Glauser et al. (2016) Glauser et al. (2006) Degidi et al. (2018) |
| Insufficient data to separate single/full mouth from multiple units | 44 |
Pozzi et al. (2014) Anitua et al. (2016) Botticelli et al. (2018) Cresp, et al. (2017) Crespi et al. (2016) Crespi et al. (2010a) Crespi et al. (2010b) Crespi et al. (2014) Degidi et al. (2012) Degidi et al. (2003) Galindo‐Moreno et al. (2014) Liu et al. (2019) Malchiodi et al. (2010) Maló et al. (2011) Maló et al. (2015) Maló et al. (2000) Malo et al. (2007) Maló et al. (2016) Malo et al. (2014) Martinez‐Rodriguez et al. (2018) Mengel et al. (2005) Merli et al. (2020) Mura et al. (2012) Nedir et al. (2004) Öskan et al. (2011) Paredes et al. (2018) Pozzi et al. (2014) Rocci et al. (2003a) Rodrigo et al. (2012) Soydan et al. (2013) Telleman et al. (2017) Wilson et al. (2013) Bettach et al. (2018) Cannizzaro et al. (2008) Crespi et al. (2016) Francetti et al. (2014) Mendonca et al. (2017) Wallkamm et al. (2015) Felice et al. (2018) Felice et al. (2015) Göthberg et al. (2016) Balmer et al. (2018) Temmerman et al. (2019) Mertens et al. (2011) |
| Time of implant placement/loading not reported | 13 |
Al Amri et al. (2017) ArReaje et al. (2019) Baelum et al. (2004) Blus et al. (2010) Bornstein et al. (2003) Bornstein et al. (2010) Cannizzaro et al. (2013) Chiapasco et al. (2020) Ibanes et al. (2003) Östman et al. (2018) Degidi et al. (2010) Harel et al. (2013) Rocci et al. (2003b) |
TABLE 2.
Overview on study, patient and implant characteristics of included studies
| Study | Study design | Setting/Country | Total number of patients | Drop‐outs |
Presence of smokers Yes/no (n) |
Patients with history of periodontitis included (n) | Reported timing of implant placement | Reported timing of restoration/loading | Type of implant placement and loading protocol | Number of implants | Implant material | Implant brand/Manufacturer |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| An et al. (2019) | Case series (prospective) | University/South Korea | 33 | 0 | NR | NR | NR | Day of implant placement | DPIL | 68 | Titanium | NR |
| Daher et al. (2019) | RCT (split‐mouth) | University/Lebanon | 24 | 2 | Yes (13) | Yes (NR) | >9 months | Immediately after implant placement | DPIL | 80 | Titanium | NobelActive/Nobel Biocare |
| >9 months | 3.5 months after implant placement | DPDL | 80 | Titanium | ||||||||
| Degidi et al. (2011) | Observational cohort (prospective) | Private practice/Italy | 24 | 3 | NR | NR | NR | Immediately after implant placement | DPIL | 48 | Titanium | ANKYLOS/Dentsply |
| Fung et al. (2011) | RCT (split‐mouth) | University/USA | 10 | 0 | Yes (2) | NR | ≥4 months | Within 24 h after implant placement | DPIL | 42 | Titanium | Brånemark System Mk IV TiUnite/Nobel Biocare |
| Göthberg et al. (2018) | RCT | University/Sweden | 50 | 0 | NR | Yes (NR) | >3 months post‐extraction | Within 48 h after implant placement | DPIL | 78 | Titanium | Brånemark System TiUnite/Nobel Biocare |
| >3 months post‐extraction | 3–4 months after implant placement | DPDL | 72 | Titanium | ||||||||
| Oxby et al. (2015) | Observational Cohort (prospective) | Private practice/Sweden | 39 | 4 | NR | NR | ≥3 months post‐extraction | Within 60 days after implant placement | DPEL | 107 | Titanium | Astra Tech/Dentsply |
| Immediately post‐extraction | Within 60 days after implant placement | IPEL | 67 | Titanium | ||||||||
| Payer et al. (2010) | Case Series (prospective) | University/Austria | 24 | 0 | NR | NR | 6 months post‐extraction | Immediately/within 1 week after implant placement | DPIL | 40 | Titanium | Xive/Dentsply |
| Romanos et al. (2016) | RCT (split‐mouth) | University/Germany | 24 | 4 | NR | NR | NR | Within 24 h after implant placement | DPIL | 36 | Titanium | ANKYLOS/Dentsply |
| NR | 3 months after healing | DPDL | 36 | Titanium | ||||||||
| Si et al. (2016) | Case Series (retrospective) | University/China | 10 | 0 | Yes (24) | Yes (41) | >3 months after tooth extraction | 3–4 months after healing | DPDL | 21 | Titanium | Straumann AG |
| Siebers et al. (2010) | Observational cohort (prospective) | Private practice/Germany | 45 | NR | Yes (15) | Yes (45) | Immediately after tooth extraction | Within 48 h after implant placement | IPIL | 20 | Titanium | Camlog; 3i; Lifecore |
| Healed sites | Within 48 h after implant placement | DPIL | 33 | Titanium | ||||||||
| Healed sites | 6 months after implant placement | DPDL | 46 | Titanium | ||||||||
| Spies et al. (2015) | Observational cohort (prospective) | University/Germany | 13 | 0 | NR | >3 months after tooth extraction | Immediately after implant placement | DPIL | 26 | Zirconia | Metoxit/Ziraldent | |
| Simons et al. (2015) | Case–control (retrospective)l | University/Belgium | 70 | NR | Yes (29) | Yes (267) | Healed sites | 3–6 months after implant placement | DPDL | 151 | Titanium | Branemark MK III/Nobel Biocare |
| Van Nimwegen et al. (2015) | RCT | University/Netherlands | 40 | 5 | NR | NR | ≥3 months post‐extraction | ≥3 months after implant placement | DPDL | 70 | Titanium | Nobel Perfect Groovy/Nobel Biocare |
| Vogl et al. (2019) | RCT | University/Austria | 20 | 0 | NR | NR | Healed sites | Immediately after implant placement | DPIL | 19 | Titanium | Xive/Dentsply |
| Healed sites | Immediately after implant placement | DPIL | 32 | Titanium |
Abbreviation: NR, not reported.
All 14 studies included information on implant survival and on one or more biological outcomes, but the biological outcomes reported across studies did differ. Since we would only consider MBL changes from prosthetic loading to follow‐up whereas some studies only reported MBL values measured at the time of implant placement, these latter values were not evaluable. Details on peri‐implant inflammation were reported based on clinical indices (Gingival Index, Sulcus Bleeding Index, Bleeding on Probing (BOP), modified Bleeding Index, Bleeding Index) so heterogeneous as to preclude a comparison across cohort groups. Group‐specific mean Plaque Index (PI) scores and Probing Depths (PD) were reported in few of the 14 studies, while mean soft‐tissue recession and mean width of keratinized tissue (KT) dimensions were reported in only one of them [Romanos et al., 2016].
Some studies indicated that implant placement had taken place >3 months (Göthberg et al., 2018; Oxby et al., 2015; Van Nimwegen et al., 2015), >4 months (Fung et al., 2011), or >3 to 6 months (Spies et al., 2015) after tooth extraction. Others were categorized as delayed placement based on statements that the implants had been inserted in healed (An et al., 2019; Degidi et al., 2011; Simons et al., 2015; Vogl et al., 2019) or edentulous (Romanos et al., 2016) ridge areas. As most placement and loading protocols were covered by few or no studies, only one direct comparison was performed (DPIL versus DPDL).
3.2. Within‐study risks of bias
Tables 3, 4, 5 summarizes the risk‐of‐bias assessments based on the Cochrane RoB 2.0 tool, Newcastle‐Ottawa scale, and Joanna Briggs Institute's Critical Appraisal Checklist.
TABLE 3.
Risk of bias assessments of RCTs based on the Cochrane RoB 2.0 tool
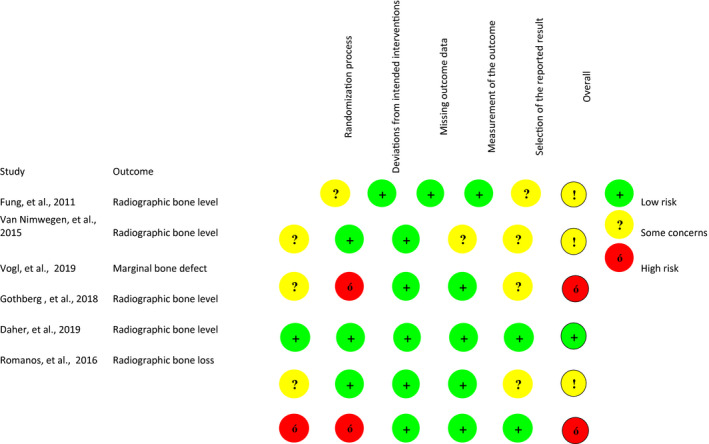
TABLE 4.
Risk of bias assessments of Cohort studies based on New Castle ‐ Ottawa Quality Assessment Scale
| Study | Representativeness of the exposed cohort | Selection of the non exposed cohort | Ascertainment of exposure | Outcome not present at the start of the study | Comparability of cases and controls | Assessment of outcome | Sufficient follow‐up time for outcomes to occur | Adequacy of follow‐up of cohorts | Total |
|---|---|---|---|---|---|---|---|---|---|
| Degidi et al. (2011), Oxby et al. (2015) | * | * | * | * | * | * | * | * | 8 |
| Oxby et al. (2015) | * | * | * | * | * | * | * | * | 8 |
| Siebers et al. (2010) | * | * | * | * | * | * | * | 7 | |
| Simons et al. (2015) | * | * | * | * | * | * | * | 7 | |
| Spies et al. (2015) | * | * | * | * | * | * | * | * | 8 |
Thresholds for converting the Newcastle‐Ottawa scales to AHRQ standards (good, fair, and poor):
Good quality: 3 or 4 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain.
Fair quality: 2 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain.
Poor quality: 0 or 1 star in selection domain OR 0 stars in comparability domain OR 0 or 1 stars in outcome/exposure domain.
TABLE 5.
Risk of bias assessments of Case Series based on Joanna Briggs Institute's Critical Appraisal Checklist
| Study | Were there clear criteria for inclusion in the case series? | Was the condition measured in a standard, reliable way for all participants included in the case series? | Were valid methods used for identification of the condition for all participants included in the case series? | Did the case series have consecutive inclusion of participants? | Did the case series have complete inclusion of participants? | Was there clear reporting of the demographics of the participants in the study? | Was there clear reporting of clinical information of the participants? | Were the outcomes of follow‐up results of cases clearly reported? | Was there clear reporting of the presenting site(s)/clinic (s) demographic information? | Was statistical analysis appropriate? | Overall appraisal |
|---|---|---|---|---|---|---|---|---|---|---|---|
| An et al. (2019) | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes* | Yes | Included |
| Payer et al. (2010) | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Included |
| Si et al. (2016) | Yes | Yes | Yes | Yes* | Unclear | Yes | Yes | Yes | Yes | Yes | Included |
All cohort and case series were rated with low risk of bias. Regarding RCT studies, 3 of them (Daher et al., 2019; Fung et al., 2011; Van Nimwegen et al., 2015) were evaluated as having some concerns in terms of risk bias. Two were rated with high risk of bias (Romanos et al., 2016; Vogl et al., 2019), and only one was rated with low risk of bias (Göthberg et al., 2018).
3.3. Within‐study results
Table 6 lists the data extracted from the included studies. None of these reported on IPDL, EPIL, EPDL, or EPDL combinations of placement and loading. Given the unspecific wording by which many authors refer to the timing of implant placement, any studies reporting on implants placed >3 months after tooth extraction without giving a time range (e.g., between 3 and 6 months) were considered delayed placement. Thus, eleven cohort groups were available for DPIL (delayed placement + immediate restoration/loading), seven for DPDL (delayed placement + delayed loading), one for DPEL (delayed placement +early loading), one for IPIL (immediate placement + immediate restoration/loading), and one for type IPEL (immediate placement + early loading).
TABLE 6.
Biological outcomes according to the implant placement and loading protocols (NA = not applicable; NR = not reported)
| Study | Placement and loading protocol | Type of loading | Mean ± SD follow‐up (months) | No. implants placed | No. implants available at follow‐up | Implant survival rate | Mean ± SD MBL changes at follow‐up (mm) | Mean ± SD on peri‐implant inflammation (different indexes) | Mean ± SD soft‐tissue recession at follow‐up (mm) | Mean ± SD width KT at follow‐up (mm) | Mean ± SD PI at follow‐up | Mean (SD) PD at follow‐up (mm) | No. of reported biological complications | Rate of biological complications (%) (except implant failure) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| An et al. (2019) | DPIL | Non‐functional | 36 | 68 | 68 | 100% | 0.42 ± 0.39 | 0.65 ± 0.81 (Gingival Index) | NR | NR | 0.35 ± 0.64 | 2.68 (1.00) | 0 | 0% |
| Daher et al. (2019) | DPIL | Functional | 36 | 80 | 69 | 95.5% | 0.78 ± 0.72 | NR | NR | NR | NR | NR | 0 | 0% |
| DPDL | NA | 36 | 80 | 71 | 96.3% | 0.91 ± 1.05 | NR | NR | NR | NR | NR | 2 implants with peri‐implantitis | 2.8% | |
| Degidi et al. (2011) | DPIL | Non‐functional | 36 | 48 | 48 | 100% | 0.57 ± 0.52 | NR | NR | NR | NR | NR | 0 | 0% |
| Fung et al. (2011) | DPIL | Functional | 36 | 42 | 40 | 95.2% | 0.26 ± 0.44 | 0.25 ± 0.30 (Sulcus Bleeding Index) | NR | NR | NR | 2.82 (0.75) | 0 | 0% |
| Göthberg et al. (2018) | DPIL | Functional | 60 | 78 | 62 | 94.9% | NR | NR | NR | NR | NR | 3.15 (0.87) | 3 implants with peri‐implantitis | 4.8% |
| DPDL | NA | 60 | 72 | 64 | 97.2% | NR | NR | NR | NR | NR | 3.18 (0.94) |
2 implants with peri‐implantitis 2 implants with fistula |
6.3% | |
| Oxby et al. (2015) | DPEL | NA | 55 | 107 | 107 | 100% | 0.28 ± 0.88 | NR | NR | NR | NR | NR | 1 implant with soft‐tissue recession | 0.9% |
| IPEL | NA | 55 | 67 | 67 | 100% | 0.34 ± 1.48 | NR | NR | NR | NR | NR | 1 implant with soft‐tissue recession | 1.5% | |
| Payer et al. (2010) | DPIL | Non‐functional | 96 | 40 | 18 | 95% | 0.88 ± 1.15 | NR | NR | NR | NR | NR | 1 implant with peri‐implantitis | 5.6% |
| Romanos et al. (2016) | DPIL | Functional | 145.7 ± 10.7 | 36 | 30 | 100% | 0.57 ± 1.06 | 0.07 ± 0.25 (Sulcus Bleeding Index) | 0.30 ± 0.84 | 1.73 ± 1.36 mm | 0.56 ± 0.94 | 2.53 (0.63) | 0 | 0% |
| DPDL | NA | 145.7 ± 10.7 | 36 | 30 | 100% | 1.12 ± 1.30 | 0.00 ± 0.00 (Sulcus Bleeding Index) | 0.20 ±0.48 | 2.00 ± 1.23 mm | 0.43 ± 0.63 | 2.6 (0.50) | 0 | 0% | |
| Si et al. (2016) | DPDL | NA | 66 | 21 | 19 | 90.5% | NR | NR | NR | NR | NR | NR | 2 implants with peri‐implantitis | 10.5% |
| Siebers et al. (2010) | DPIL | Both | 45.1 ± 7.2 | 33 | 32 | 97% | 2.15 ± 0.81 | 1.59 ± 1.39 (from 0 to 6) | NR | NR | NR | NR | NR | ‐ |
| DPDL | NA | 55.7 ± 16.2 | 46 | 46 | 100% | 2.46 ± 0.96 | 2.91 ± 2.11 (from 0 to 6) | NR | NR | NR | NR | NR | ‐ | |
| IPIL | Both | 47.64 ± 6.48 | 20 | 17 | 90% | 1.57 ± 0.91 | 1.76 ± 1.79 (from 0 to 6) | NR | NR | NR | NR | NR | ‐ | |
| Simons et al. (2015) | DPDL | NA | 48 | 151 | 151 | 98.3% | 0.5 ± 0.68 | NR | NR | NR | NR | NR | NR | ‐ |
| Spies et al. (2015) | DPIL | Non‐functional | 60 | 26 | 26 | 100% | 1.14 ± NR | 1.1 ± NA (modified Bleeding Index) | NR | NR | 0.72 ± NR | NR | 0 | 0 |
| Van Nimwegen et al. (2015) | DPDL | NA | 60 | 70 | 58 | 97.1% | NR | 40 ± NR (Bleeding Index) | NR | NR | NR | 3.33 (1.73) | 2 implants with peri‐implantitis | 3.4% |
| Vogl et al. (2019) | DPIL | Functional | 36 | 19 | 17 | 100% | 0.37 ± 0.46 | NR | NR | NR | 1.6 ± 0.7 | NR | 0 | 0% |
| DPIL | Non‐functional | 36 | 32 | 30 | 97% | 0.39 ± 0.47 | NR | NR | NR | 1.6 ± 0.7 | NR | 0 | 0% |
3.3.1. IPIL (immediate placement + immediate restoration/loading)
Only one prospective cohort study was available on this combination of protocols [Siebers et al., 2010]. It gave a mean follow‐up of 47.64 ± 6.48 months, two of these 20.
Implants failed (implant survival rate: 90%). Even though immediate placement and immediate restoration/loading tended to produce a lower survival in this specific study, the MBL changes appeared favorable compared to delayed placement protocols.
3.3.2. IPEL (immediate placement + early loading)
One prospective cohort study was available (Oxby et al., 2015). Based on a mean follow‐up of 55 months, none of the 67 implants in this category failed (survival rate: 100%) and merely one biological complication (soft‐tissue recession) was reported.
3.3.3. DPIL (delayed placement + immediate loading)
Data on this combination of protocols were available from five randomized controlled trials, three prospective cohort studies, and two prospective case series, including 11 cohort groups with data on implant outcomes. Overall, 14 of 502 implants in this category failed. Based on a mean follow‐up of 60.1 ± 37.8 months, a weighted cumulative survival of 97.2% was obtained. Data for 378 implants revealed a mean MBL change of 0.71 ± 0.66 mm and data for 361 implants a 2.6% rate of biological complications. Probing depths were reported in four studies (An et al., 2019; Fung et al., 2011; Göthberg et al., 2018; Romanos et al., 2016) resulting in a calculated mean of 2.83 ± 0.92 mm. A sub‐analysis on the type of loading revealed an approximately similar MBL change for functional (0.65 mm) versus nonfunctional (0.62 mm) loading.
3.3.4. DPEL—(delayed placement + early loading)
There was only one prospective cohort study [Oxby et al., 2015]. Based on a mean follow‐up of 55 months, none of the 107 implants in this category failed (survival rate: 100%) and merely one biological complication (soft‐tissue recession) was reported.
3.3.5. DPDL—(delayed placement + delayed loading)
Data on this combination of protocols were available from three randomized controlled trials, one prospective cohort study, one retrospective cohort study, and one retrospective case series. Overall, 14 of 476 implants in this category failed. Based on a mean follow‐up of 74.2 ± 43.4 months, the weighted cumulative survival was 98.1%. Data for 217 implants yielded a mean MBL change of 1.68 ± 0.97 mm and data for 242 implants a 3.7% cumulative rate of biological complications. From 3 studies, in a mean probing depth of 3.12 ± 1.08 mm was calculated (Göthberg et al., 2018; Romanos et al., 2016; Van Nimwegen et al., 2015).
3.4. Results of meta‐analysis
The reported results of analysis were based on data extracted directly from included studies but also on additional raw data provided by some of the authors (Daher et al., 2019; Göthberg et al., 2018; Oxby et al., 2015; Payer et al., 2010; Siebers et al., 2010; Simons et al., 2015; Vogl et al., 2019). Due to heterogeneity, mostly related to study designs and variable radiographic and clinical measures, only three RCTs comparing the same types of implant placement and implant loading protocols (DPIL vs. DPDL) were available for a quantitative synthesis (Daher et al., 2019; Göthberg et al., 2018; Romanos et al., 2016). The meta‐analysis revealed an overall effect size of 1.57 [95% CI: 0.19; 13.1], so that no significant difference in terms of survival rate (p = .227) emerged between the type DPIL (74 patients/188 implants) and DPDL (182 implants/72 patients) combinations of placement and loading (Figure 2). Between‐trial heterogeneity was minimal in absolute (t 2: 0.0022) and relative (I 2: 0) terms (p = .77).
FIGURE 2.
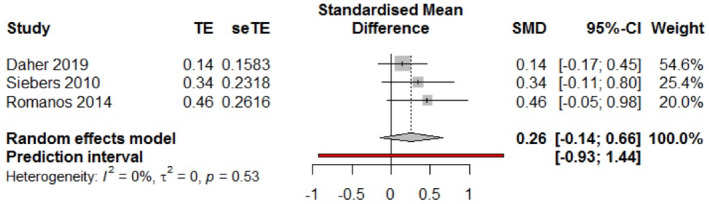
Forest plot with individual effects and heterogeneity measures
Regarding the publication bias assessment, Egger’s test does not indicate the presence of funnel plot asymmetry (Figure 3). However, since the meta‐analysis contains three studies (k = 3) the Egger's test may lack the statistical power to detect bias (i.e., k < 10).
3.5. Certainties of evidence
Table 7 illustrates the overall quality of meta‐evidence. The following outcome was assessed across the various combinations of implant placement and loading protocols: BOP, pocket depths, MBL changes, peri‐implantitis, peri‐implant mucositis, and implant survival rates. A GRADE summary‐of‐evidence compilation is provided (Table 7) for each of the four comparisons that could be made between any two of the evaluable placement‐plus‐loading combinations (DPIL vs. DPDL, IPIL vs. DPDL, DPEL vs. DPDL, and IPEL vs. DPDL). Both direct and indirect study comparisons and all (available) biological outcomes have been entered. A low certainty was identified for one comparison (DPIL vs. DPDL) and one outcome (BOP) based on one RCT exhibiting a high risk of bias (Romanos et al., 2016). Other than that, the certainty of evidence was rated as very low in all comparisons for all outcome parameters. In relation to the reference combination of protocols (type DPDL), all alternative combinations seem to improve biological outcomes and survival rates.
TABLE 7.
GRADE I‐IV summary‐of‐evidence compilation for each of the four comparisons that could be made between any two placement and loading combinations (DPIL vs. DPDL, IPIL vs. DPDL, DPEL vs. DPDL, IPEL vs. DPDL)
| Summary of findings: GRADE I | |||||
|---|---|---|---|---|---|
| Delayed placement and immediate loading (DPIL) compared to delayed placement and delayed loading (DPDL) for implant treatment in partially edentulous individuals | |||||
|
Patient or population: implant treatment in partially edentulous individuals (analysis at implant level) Setting: University/private clinic Intervention: delayed placement and immediate loading (DPIL) Comparison: delayed placement and delayed loading (DPDL) | |||||
| Outcomes | Anticipated absolute effects | No. of implants (contributing arm/studies) | Certainty of the evidence (GRADE) | Comments | |
| Weighted effect with delayed placement and delayed loading (DPDL) | Weighted effect with delayed placement and immediate loading (DPIL) | ||||
| Rx bone loss around the implant platform assessed with: Radiographic image d | The mean rx bone loss around the implant platform was 1.68 mm ± 0.97 | The mean rx bone loss around the implant platform was 0.71 mm ± 0.66 | 676 implants (4 RCTs, 6 observational studies) |
⨁◯◯◯ VERY LOW a |
Immediate loading after delayed placement seems to reduce potential bone loss after loading. Follow‐up period varied from 3 years up to 15 years |
| Bleeding on probing assessed with: Sulcus Bleeding Index c | The mean SBI was 0.066 (±0.253) | The mean SBI was 0.00 (±0.00) | 60 implants (1 RCT) |
⨁⨁◯◯ LOW b |
Immediate loading after delayed placement does not seems to affect the Sulcus Bleeding Index. Follow‐up was 15 years |
| Peri‐implant probing depth | The mean peri‐implant pocket depth was 3.12 mm ± 1.08 | The mean peri‐implant pocket depth was 2.83 mm ± 0.92 | 352 (4 RCTs, 1 observational studies) |
⨁◯◯◯ VERY LOW a |
Peri‐implant pocket depth does not exhibit substantial difference between immediate and delayed loading after delayed implant placement |
| Peri‐implantitis prevalence assessed with: Radiographic and clinical examination d | The mean percentage of implants with peri‐implantitis was 3.5% | The mean percentage of implants with peri‐implantitis was 0.9% | 535 (4 RCTs, 4 observational studies) |
⨁◯◯◯ VERY LOW a |
Evidence is scarce on peri‐implantitis and low rates were reported in the included studies. This could be in part due to poor reporting of the study of the clinical examination. Follow‐up period varied from 3 years up to 15 years |
| Mucositis | No muscositis was reported in all studies with data on peri‐implantitis | 535 (4 RCTs, 4 observational studies) |
⨁◯◯◯ VERY LOW a |
Evidence is scarce on mucositis. No cases were reported in the included studies but this could be in part due to poor reporting of the study of the clinical examination. Follow‐up period varied from 3 years up to 15 years | |
| Survival rate assessed with: Radiographic and clinical examination d | The mean survival rate was 98.1.2% | The mean survival rate was 97.2% % | 879 implants (6 RCTs, 7 observational studies) |
⨁◯◯◯ VERY LOW a |
Both delayed and immediate loading after delayed placement after delayed implant placement present high survival rates. Follow‐up period varied from 3 years up to 15 years |
| GRADE Working Group grades of evidence: High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
| Summary of findings: GRADE II | |||||
|---|---|---|---|---|---|
| Immediate placement and immediate loading (IPIL) compared to delayed placement and delayed loading (DPDL) for implant treatment in partially edentulous individuals | |||||
|
Patient or population: implant treatment in partially edentulous individuals Setting: University/private clinic Intervention: immediate placement and immediate loading Comparison: delayed placement and delayed loading | |||||
| Outcomes | Anticipated absolute effects | No. of implants (contributing arm/studies) | Certainty of the evidence (GRADE) | Comments | |
| Weighted effect with delayed placement and delayed loading (DPDL) | Weighted effect with immediate placement and immediate loading (IPIL) | ||||
| Rx bone loss around the implant platform assessed with: Radiographic image f | The mean rx bone loss around the implant platform was 1.68 mm ± 0.97 | The mean rx bone loss around the implant platform 1.57 mm ± 0.91 | 318 (2 RCTs, 3 observational studies) |
⨁◯◯◯ VERY LOW b |
Implants placed with immediate implant placement and immediate loading may exhibit comparable mean bone loss after loading. Follow‐up period varied from 3 years up to 15 years |
| Bleeding on probing assessed with: 0 to 6 scale (unknown reference) | Mean bleeding was 2.91 ± 2.11 | Mean bleeding was 1.76 ± 1.79 | 53 (1 observational study) |
⨁◯◯◯ VERY LOW b |
Implants placed with immediate implant placement and immediate loading may exhibit decreased bleeding on probing. Follow‐up period varied from 3 years up to 15 years |
| Peri‐implant probing depth | No comparison was possible | ‐ | ‐ | ‐ | |
| Peri‐implantitis prevalence | No comparison was possible | ‐ | ‐ | ‐ | |
| Mucositis | No comparison was possible | ‐ | ‐ | ‐ | |
| Survival rate assessed with: Radiographic and clinical examination f | Survival rate was 98.1% | Survival rate was 75% | 318 (2 RCTs, 3 observational studies) |
⨁◯◯◯ VERY LOW e |
Implants placed using both delayed placement with delayed loading may present higher survival rates compared to immediate placement with immediate loading. Follow‐up period varied from 3 years up to 15 years |
| GRADE Working Group grades of evidence: High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
| Summary of findings: GRADE III | ||||||
|---|---|---|---|---|---|---|
| Delayed placement and early loading (DPEL) compared to delayed placement and delayed loading (DPDL) for implant treatment in partially edentulous individuals | ||||||
|
Patient or population: implant treatment in partially edentulous individuals Setting: University/private clinic Intervention: delayed placement and early loading Comparison: delayed placement and delayed loading | ||||||
| Outcomes | Anticipated absolute effects | No. of participants (contributing arm/studies) | Certainty of the evidence (GRADE) | Comments | ||
| Weighted effect with delayed placement and delayed loading (DPDL) | Weighted effect with delayed placement and early loading (DPEL) | |||||
| Rx bone loss around the implant platform assessed with: Radiographic image f | The mean rx bone loss around the implant platform was 1.68 mm ± 0.97 | The mean rx bone loss around the implant platform was 0.28 ± 0.88 | 298 + 107 (2 RCTs, 3 observational studies) |
⨁◯◯◯ VERY LOW b |
Implants placed with delayed implant placement and early loading may exhibit decreased mean bone loss after loading. Follow‐up period varied from 3 years up to 15 years | |
| Bleeding on probing | No comparison was possible | ‐ | ‐ | ‐ | ||
| Peri‐implant probing depth | No comparison was possible | ‐ | ‐ | ‐ | ||
| Peri‐implantitis prevalence | No comparison was possible | ‐ | ‐ | ‐ | ||
| Mucositis | No comparison was possible | ‐ | ‐ | ‐ | ||
| Survival rate assessed with: Radiographic and clinical examination f | Survival rate was 98.1% | Survival rate was 100% | 439 + 107 (3 RCTs, 3 observational studies) |
⨁◯◯◯ VERY LOW e |
Implants placed using both delayed placement with delayed loading and delayed placement with early loading seem to present high survival rates. Follow‐up period varied from 3 years up to 15 years | |
| GRADE Working Group grades of evidence: High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
| Summary of findings: GRADE IV | |||||
|---|---|---|---|---|---|
| Immediate placement and early loading (IPEL) compared to delayed placement and delayed loading (DPDL) for implant treatment in partially edentulous individuals | |||||
|
Patient or population: implant treatment in partially edentulous individuals Setting: University/private clinic Intervention: immediate placement and early loading Comparison: delayed placement and delayed loading | |||||
| Outcomes | Anticipated absolute effects f (95% CI) | No. of participants (contributing arm/studies) | Certainty of the evidence (GRADE) | Comments | |
| Weighted effect with delayed placement and delayed loading (DPDL) | Weighted effect with immediate placement and early loading (IPEL) | ||||
| Rx bone loss around the implant platform assessed with: Radiographic image f | The mean rx bone loss around the implant platform was 1.68 mm ± 0.97 | The mean rx bone loss around the implant platform 0.99 ± 1.35 | 298+67 (2 RCTs, 3 observational study) |
⨁◯◯◯ VERY LOW b |
Implants placed with delayed implant placement and early loading may exhibit decreased mean bone loss after loading. Follow‐up period varied from 3 years up to 15 years |
| Bleeding on probing | No comparison was possible | ‐ | ‐ | ‐ | |
| Peri‐implant probing depth | No comparison was possible | ‐ | ‐ | ‐ | |
| Peri‐implantitis prevalence | No comparison was possible | ‐ | ‐ | ‐ | |
| Mucositis | No comparison was possible | ‐ | ‐ | ‐ | |
| Survival rate assessed with: Radiographic and clinical examination f | Survival rate was 98.1% | Survival rate was 100% | 439+67 (3 RCTs, 3 observational study) |
⨁◯◯◯ VERY LOW e |
Implants placed with both delayed placement with delayed loading and immediate placement with early loading present high survival rates. Follow‐up period varied from 3 years up to 15 years |
| GRADE Working Group grades of evidence: High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
All studies except for one RCT (Göthberg et al., 2018) showed from some concerns to high risk of bias; only 3 direct comparisons.
The study Romanos et al. (2014) was rated with high risk of bias.
Based on withing study comparisons.
Based on within and between study comparisons
All studies except for one RCT showed from some concerns to high risk of bias.
Based on between study comparisons.
4. DISCUSSION
It has been suggested as a fundamental principle in implant dentistry that the implant‐restoration complex should be considered as a single variable in assessing clinical outcomes (Garber & Belser, 1995) and consequently success of treatment. In the present review, this principle was adopted by evaluating all outcomes of placement and loading from the 14 studies in combination, as recently suggested (Gallucci et al., 2018). Five of the 9 categories are covered by the included studies: immediate placement combined with immediate or early loading (types IPIL and IPEL), and delayed placement combined with immediate, early, or delayed loading (types DPIL, DPEL, and DPDL). Three to 15 years after surgery, all groups showed implant survival rates >90%, except one observational study representing type IPIL (Siebers et al., 2010).
Heterogeneity in study designs, inconsistencies in outcome reporting, and a lack of comparative studies, reflected by the low level of evidence in the GRADE table, allowed to include only three RCT’s in one quantitative synthesis (Daher et al., 2019; Göthberg et al., 2018; Romanos et al., 2016). The meta‐analysis revealed no significant difference in terms of survival rate (p = .227) emerged between the type DPIL (74 patients/188 implants) and DPDL (182 implants/72 patients) combinations of placement and loading.
The only biological outcome measure that could be extracted from pooled data was mean MBL. However, their heterogeneity and quality did not allow to draw any conclusions on the effect of different timing of placement and loading protocols on peri‐implant marginal bone changes.
Biological complications were poorly reported in the studies here reviewed. Low rates of 2.6% or 3.7% emerged in two groups of delayed placement time combined with either immediate loading (type DPIL) or delayed loading (type DPDL). Our extraction of data on biological outcomes and complications was based on definitions of peri‐implant disease (Heitz‐Mayfield & Salvi, 2018; Schwarz et al., 2018) and health (Araujo & Lindhe, 2018) adopted by the 2017 World Workshop on the Classification of Periodontal and Peri‐implant Diseases and Conditions, co‐sponsored by the American Academy of Periodontology and the European Federation of Periodontology (Caton et al., 2018). Unfortunately, many studies do not clearly define peri‐implant diseases or do not consider clinical parameters in their definition, which can lead to inaccuracy and biased results. Thus, in this systematic review, only survival rates and mean bone level could be quantitatively assessed.
The results of this review are consistent with a previous finding of overall treatment outcomes being similar for immediately placed and loaded implants as in control groups of delayed placement and/or delayed loading (Parvini et al., 2020). In addition, a systematic review has reported survival rates >97% across all protocols of placement and loading (Gallucci et al., 2018), while another systematic review focusing on placement protocols did not find a significant difference between differently timed implant procedures (Bassir et al., 2018).
No solid conclusions arise on how smoking and histories of periodontitis relate to the biological outcomes of the various timing options. History of periodontitis has been postulated as a risk factor for peri‐implantitis (Schwarz et al., 2017), and there is some consensus on this despite some conflicting reports (Canullo et al., 2016; Dvorak et al., 2011; Marrone et al., 2013; Rokn et al., 2017; Schwarz et al., 2017). The majority of studies in the present review had specifically excluded patients with such histories or merely indicated that all included patients had been periodontally stable.
The integrity of the facial extraction socket wall has been regarded as a critical factor in deciding upon an implant placement protocol (Tonetti et al., 2019), and certainly, the anatomy of the extraction socket is a useful consideration regarding implant success and biological outcomes (Parvini et al., 2020). Most of the 14 studies dealt with healed sockets and yielded little information on bone grafting, which usually was performed simultaneously with the implant surgery, either in immediate or in delayed placement protocols (Oxby et al., 2015; Siebers et al., 2010). This suggests the presence of less‐than‐ideal socket anatomies even during immediate placement. Reference to post‐extraction socket anatomy was made in only one study, to the effect that grafting was performed when the buccal plate was “questionable” and preference given to submerged healing in the presence of a bone defect >3 mm (Siebers et al., 2010).
One strength of this systematic review is its broad literature base of over 7000 unique (i.e., deduplicated) publications which were returned by the search terms and carefully screened by the reviewers. Its methodology based on the Cochrane textbook is also a significant strength as well. Limitations arise from its inclusion of study designs that might weaken conclusions, as non‐RCT studies generally increase the risk of incurring biases in systematic reviews (Hoy et al., 2012). As shown in the GRADE listings (Table 7), certainty of evidence was very low for all outcomes across all combinations of protocols. One exception, with a low certainty of evidence based on one RCT (Romanos, et al.), was bleeding on probing compared between immediate and delayed loading in conjunction with delayed placement (type DPIL versus DPDL).
Another limiting factor was the small sample size (low number of included studies) the small number of implants included, and that only three studies were available for meta‐analysis. Thus, large parts of the conclusions from this systematic review are based on pooled data, which needs to mentioned as a limiting factor.
Yet this scarcity does reflect the current level of evidence on how different protocols of implant placement and loading may affect the risk of biological complications related to implant‐supported FPDs. Given this inadequate base of evidence to shed light on these issues, this systematic review cannot possibly yield any robust conclusions.
The need for well‐designed and adequately powered RCTs specifically reporting and evaluating biological outcomes of different implant placement as well as loading protocols is warranted.
5. CONCLUSION
Within its limitations, this review showed high rates of survival of all the studied implant placement and loading combinations for FPDs over ≥3 years of follow‐up. The small number of studies (n = 14), allowing data synthesis from only 3 trials, revealed no differences in terms of survival rates of implants immediately or delayed loaded after delayed placement. In addition, the analysis of pooled data did not reveal differences in survival rates nor marginal bone levels when DPDL and DPIM were compared.
The heterogeneity and quality of the data did not allow to draw any further conclusions on the occurrence of biological complications related to timing of implant placement/loading. Most comparisons across studies were precluded by major inconsistencies in outcome reporting, such as lack of definition of the peri‐implant diseases and scarcity of reported biological outcomes for each placement and loading combination. This suggests that the currently available evidence on the PICO question which was investigated is scarce and highlights the need for well‐designed and adequately powered RCTs comparing biological outcomes of different implant placement and loading protocols in the long term.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Louise Leite Aiquel: Data curation (equal); formal analysis; methodology (equal); project administration (equal); software (lead); writing‐original draft (equal); writing‐review & editing. Joao Pitta: Data curation (equal); Formal analysis (equal); methodology (equal); software (equal); writing‐original draft (equal); writing‐review & editing (equal). Georgios N. Antonoglou: Data curation (equal); formal analysis (equal); methodology (equal); validation (equal); writing‐original draft (equal); writing‐review & editing (equal). Irene Mischak: Data curation (lead); validation (equal). Irena Sailer: Conceptualization (equal); supervision (equal); writing‐original draft. Michael Payer: Conceptualization (equal); project administration (equal); supervision (equal); writing‐review & editing (equal).
ACKNOWLEDGEMENTS
This systematic review was performed in the context of the EAO Consensus Conference 2021. The reviewers would like to thank the EAO for the trust and the assignment for the conduction of this review and in addition all contacted authors that provided detailed information on their studies, that helped to complete the data set analyzed in the present work. Namely we received additional information and/or data from Dr. Susanne Vogl, Dr. Gert Oxby, Prof. Dr. Benedikt Spies, Dr. Zeina Majzoub, Dr. Paolo Capparé, Dr. Roberto Crespi, Dr. Derk Siebers, Prof. Dr. Georgios Romanos, Dr. Willem‐Frederik Simons, Prof. Dr. Andy Temmerman, Dr. Misi Si, Prof. Vibaeke Baelum, Dr. Zeev Ormianer, Prof. David Cochrane, Prof. Ralf Kohal, Prof. Alessandro Pozzi, Dr. Marco Esposito, Prof. Luca Cordaro, and Dr. Tom Wilson. The Authors acknowledge and are grateful for the support and contributions from all the above. The conduction of this systematic was further supported by the Division of Fixed Prosthodontics and Biomaterials University Clinics for Dental Medicine, University of Geneva, Switzerland (Chair: Prof. Dr. Irena Sailer) and the Department of Oral Surgery and Orthodontics, University Clinic of Dental Medicine and Oral Health, Medical University of Graz, Austria (Chair: Univ. Prof. DDr. Norbert Jakse). The authors would further like to thank Dr. Aron Naimi‐Akbar, DDS PhD research methodologist (University of Malmö, Sweden) for his expertise in developing the overall search strategy and Mag. Gregor Steinrisser reference and education services librarian (Medical University of Graz, Austria) for his help with electronic literature databases used in the present review and Mag. Wielfried Preinfalk for his support editing the manuscript.
Aiquel, L. L. , Pitta, J. , Antonoglou, G. N. , Mischak, I. , Sailer, I. , & Payer, M. (2021). Does the timing of implant placement and loading influence biological outcomes of implant‐supported multiple‐unit fixed dental prosthesis—A systematic review with meta‐analyses. Clinical Oral Implants Research, 32, 5–27. 10.1111/clr.13860
Louise Leite Aiquel and Joao Pitta contributed equally to this manuscript.
Funding information
Departmental funding was used exclusively.
DATA AVAILABILITY STATEMENT
Data available on request from the authors. The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- An, X. , Lee, C. , Fang, Y. , & Choi, B. H. (2019). Immediate nonfunctional loading of implants placed simultaneously using computer‐guided flapless maxillary crestal sinus augmentation with bone morphogenetic protein‐2/collagen matrix. Clinical Implant Dentistry and Related Research, 21, 1054–1061. 10.1111/cid.12831. [DOI] [PubMed] [Google Scholar]
- Araujo, M. G. , & Lindhe, J. (2018). Peri‐implant health. Journal of Periodontology, 89(Suppl 1), 249–256. 10.1002/JPER.16-0424. [DOI] [PubMed] [Google Scholar]
- Araújo, M. G. , Sukekava, F. , Wennström, J. L. , & Lindhe, J. (2005). Ridge alterations following implant placement in fresh extraction sockets: An experimental study in the dog. Journal of Clinical Periodontology, 32, 645–652. 10.1111/j.1600-051X.2005.00726.x. [DOI] [PubMed] [Google Scholar]
- Barone, A. , Toti, P. , Marconcini, S. , Derchi, G. , Saverio, M. , & Covani, U. (2016). Esthetic outcome of implants placed in fresh extraction sockets by clinicians with or without experience: A medium‐term retrospective evaluation. The International Journal of Oral & Maxillofacial Implants, 31, 1397–1406. 10.11607/jomi.4646. [DOI] [PubMed] [Google Scholar]
- Bassir, S. H. , Alhareky, M. , Wangsrimongkol, B. , Jia, Y. , & Karimbux, N. (2018). Systematic review and meta‐analysis of hard tissue outcomes of alveolar ridge preservation. The International Journal of Oral & Maxillofacial Implants, 33, 979–994. 10.11607/jomi.6399. [DOI] [PubMed] [Google Scholar]
- Beller, E. M. , Glasziou, P. P. , Altman, D. G. , Hopewell, S. , Bastian, H. , Chalmers, I. , Gøtzsche, P. C. , Lasserson, T. , & Tovey, D. , & PRISMA for Abstracts Group (2013). PRISMA for Abstracts: Reporting systematic reviews in journal and conference abstracts. PLoS Med, 10, e1001419. 10.1371/journal.pmed.1001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benic, G. I. , Mir‐Mari, J. , & Hämmerle, C. H. (2014). Loading protocols for single‐implant crowns: A systematic review and meta‐analysis. The International Journal of Oral & Maxillofacial Implants, 29(Suppl), 222–238. 10.11607/jomi.2014suppl.g4.1. [DOI] [PubMed] [Google Scholar]
- Blanco, J. , Carral, C. , Argibay, O. , & Liñares, A. (2019). Implant placement in fresh extraction sockets. Periodontology 2000, 79(1), 151–167. 10.1111/prd.12253. [DOI] [PubMed] [Google Scholar]
- Buser, D. , Chappuis, V. , Belser, U. C. , & Chen, S. (2017). Implant placement post extraction in esthetic single tooth sites: when immediate, when early, when late? Periodontology 2000, 73(1), 84–102. [DOI] [PubMed] [Google Scholar]
- Buser, D. , Chappuis, V. , Kuchler, U. , Bornstein, M. M. , Wittneben, J. G. , Buser, R. , Cavusoglu, Y. , & Belser, U. C. (2013). Long‐term stability of early implant placement with contour augmentation. Journal of Dental Research, 92(12 Suppl), 176–182. 10.1177/0022034513504949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser, D. , Halbritter, S. , Hart, C. , Bornstein, M. M. , Grütter, L. , Chappuis, V. , & Belser, U. C. (2009). Early implant placement with simultaneous guided bone regeneration following single‐tooth extraction in the esthetic zone: 12‐month results of a prospective study with 20 consecutive patients. Journal of Periodontology, 80, 152–162. 10.1902/jop.2009.080360. [DOI] [PubMed] [Google Scholar]
- Canullo, L. , Peñarrocha‐Oltra, D. , Covani, U. , Botticelli, D. , Serino, G. , & Penarrocha, M. (2016). Clinical and microbiological findings in patients with peri‐implantitis: A cross‐sectional study. Clinical Oral Implants Research, 27, 376–382. 10.1111/clr.12557. [DOI] [PubMed] [Google Scholar]
- Caton, J. G. , Armitage, G. , Berglundh, T. , Chapple, I. , Jepsen, S. , Kornman, K. S. , Mealey, B. L. , Papapanou, P. N. , Sanz, M. , & Tonetti, M. S. (2018). A new classification scheme for periodontal and peri‐implant diseases and conditions ‐ Introduction and key changes from the 1999 classification. Journal of Periodontology, 89(Suppl 1), 1–8. 10.1002/JPER.18-0157. [DOI] [PubMed] [Google Scholar]
- Chan, H.‐L. , George, F. , Wang, I.‐C. , Suárez López del Amo, F. , Kinney, J. , & Wang, H.‐L. (2019). A randomized controlled trial to compare aesthetic outcomes of immediately placed implants with and without immediate provisionalization. Journal of Clinical Periodontology, 46, 1061–1069. 10.1111/jcpe.13171. [DOI] [PubMed] [Google Scholar]
- Chappuis, V. , Araújo, M. G. , & Buser, D. (2017). Clinical relevance of dimensional bone and soft tissue alterations post‐extraction in esthetic sites. Periodontology 2000, 73(1), 73–83. 10.1111/prd.12167. PMID: 28000281. [DOI] [PubMed] [Google Scholar]
- Chen, S. T. , & Buser, D. (2009). Clinical and esthetic outcomes of implants placed in postextraction sites. The International Journal of Oral & Maxillofacial Implants, 24(Suppl), 186–217. [PubMed] [Google Scholar]
- Chen, S. T. , Wilson, T. G. Jr , & Hämmerle, C. H. (2004). Immediate or early placement of implants following tooth extraction: Review of biologic basis, clinical procedures, and outcomes. The International Journal of Oral & Maxillofacial Implants, 19(Suppl), 12–25. [PubMed] [Google Scholar]
- Chu, S. J. , Saito, H. , Östman, P. O. , Levin, B. P. , Reynolds, M. A. , & Tarnow, D. P. (2020). Immediate tooth replacement therapy in postextraction sockets: A comparative prospective study on the effect of variable platform‐switched subcrestal angle correction implants. The International Journal of Periodontics & Restorative Dentistry, 40, 509–517. 10.11607/prd.4440. [DOI] [PubMed] [Google Scholar]
- Daher, F. I. , Abi‐Aad, H. L. , Dimassi, H. I. , Cordioli, G. , & Majzoub, Z. (2019). Immediate versus conventional loading of variable‐thread tapered implants supporting three‐ to four‐unit fixed partial dentures in the posterior maxilla: 3‐year results of a split‐mouth randomised controlled trial. International Journal of Oral Implantology (Berlin, Germany), 12, 449–466. [PubMed] [Google Scholar]
- De Bruyn, H. , Christiaens, V. , Doornewaard, R. , Jacobsson, M. , Cosyn, J. , Jacquet, W. , & Vervaeke, S. (2017). Implant surface roughness and patient factors on long‐term peri‐implant bone loss. Periodontology 2000, 73(1), 218–227. 10.1111/prd.12177. [DOI] [PubMed] [Google Scholar]
- Degidi, M. , Nardi, D. , & Piattelli, A. (2011). One abutment at one time: Non‐removal of an immediate abutment and its effect on bone healing around subcrestal tapered implants. Clinical Oral Implants Research, 22, 1303–1307. 10.1111/j.1600-0501.2010.02111.x. [DOI] [PubMed] [Google Scholar]
- Dreyer, H. , Grischke, J. , Tiede, C. , Eberhard, J. , Schweitzer, A. , Toikkanen, S. E. , Glöckner, S. , Krause, G. , & Stiesch, M. (2018). Epidemiology and risk factors of peri‐implantitis: A systematic review. Journal of Periodontal Research, 53(5), 657–681. 10.1111/jre.12562. [DOI] [PubMed] [Google Scholar]
- Dvorak, G. , Arnhart, C. , Heuberer, S. , Huber, C. D. , Watzek, G. , & Gruber, R. (2011). Peri‐implantitis and late implant failures in postmenopausal women: A cross‐sectional study. Journal of Clinical Periodontology, 38, 950–955. 10.1111/j.1600-051X.2011.01772.x. [DOI] [PubMed] [Google Scholar]
- Esposito, M. , Grufferty, B. , Papavasiliou, G. , Dominiak, M. , Trullenque‐Eriksson, A. , & Heinemann, F. (2018). Immediate loading of occluding definitive partial fixed prostheses vs non‐occluding provisional restorations ‐ 3‐year post‐loading results from a pragmatic multicentre randomised controlled trial. European Journal of Oral Implantology, 11, 309–320. [PubMed] [Google Scholar]
- Fung, K. , Marzola, R. , Scotti, R. , Tadinada, A. , & Schincaglia, G. P. (2011). A 36‐month randomized controlled split‐mouth trial comparing immediately loaded titanium oxide‐anodized and machined implants supporting fixed partial dentures in the posterior mandible. The International Journal of Oral & Maxillofacial Implants, 26, 631–638. [PubMed] [Google Scholar]
- Gallucci, G. O. , Benic, G. I. , Eckert, S. E. , Papaspyridakos, P. , Schimmel, M. , Schrott, A. , & Weber, H. P. (2014). Consensus statements and clinical recommendations for implant loading protocols. The International Journal of Oral & Maxillofacial Implants, 29(Suppl), 287–290. 10.11607/jomi.2013.g4. [DOI] [PubMed] [Google Scholar]
- Gallucci, G. O. , Hamilton, A. , Zhou, W. , Buser, D. , & Chen, S. (2018). Implant placement and loading protocols in partially edentulous patients: A systematic review. Clinical Oral Implants Research, 29(Suppl 16), 106–134. 10.1111/clr.13276. [DOI] [PubMed] [Google Scholar]
- Gallucci, G. O. , Morton, D. , & Weber, H. P. (2009). Loading protocols for dental implants in edentulous patients. The International Journal of Oral & Maxillofacial Implants, 24(Suppl), 132–146. [PubMed] [Google Scholar]
- Garber, D. A. , & Belser, U. C. (1995). Restoration‐driven implant placement with restoration‐generated site development. Compendium of Continuing Education in Dentistry (Jamesburg, NJ), 16, 796–804. [PubMed] [Google Scholar]
- Göthberg, C. , Gröndahl, K. , Omar, O. , Thomsen, P. , & Slotte, C. (2018). Bone and soft tissue outcomes, risk factors, and complications of implant‐supported prostheses: 5‐Years RCT with different abutment types and loading protocols. Clinical Implant Dentistry and Related Research, 20, 313–321. 10.1111/cid.12587. [DOI] [PubMed] [Google Scholar]
- Guyatt, G. H. , Oxman, A. D. , Schünemann, H. J. , Tugwell, P. , & Knottnerus, A. (2011). GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology, 64, 380–382. 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Hämmerle, C. H. , Chen, S. T. , & Wilson, T. G. Jr (2004). Consensus statements and recommended clinical procedures regarding the placement of implants in extraction sockets. The International Journal of Oral & Maxillofacial Implants, 19(Suppl), 26–28. [PubMed] [Google Scholar]
- Heitz‐Mayfield, L. , & Salvi, G. E. (2018). Peri‐implant mucositis. Journal of Periodontology, 89(Suppl 1), 257–266. 10.1002/JPER.16-0488. [DOI] [PubMed] [Google Scholar]
- Higgins, J. P. T. , & Green, S. (2017). In Higgins J. P. T., & Green S. (Eds.), Cochrane handbook for systematic reviews of interventions version 5.2. The Cochrane Collaboration. [Google Scholar]
- Hoy, D. , Brooks, P. , Woolf, A. , Blyth, F. , March, L. , Bain, C. , Baker, P. , Smith, E. , & Buchbinder, R. (2012). Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. Journal of Clinical Epidemiology, 65, 934–939. 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine Committee on Standards for Developing Trustworthy Clinical Practice, G. (2011). Chapter 4: Current best practices and proposed standards for development of trustworthy CPGs: Part 1, Getting started. In Graham R., Mancher M., Miller Wolman D., Greenfield S., & Steinberg E. (Eds.), Clinical practice guidelines we can trust. National Academies Press (US). [PubMed] [Google Scholar]
- Kolerman, R. , Nissan, J. , Mijiritsky, E. , Hamoudi, N. , Mangano, C. , & Tal, H. (2016). Esthetic assessment of immediately restored implants combined with GBR and free connective tissue graft. Clinical Oral Implants Research, 27, 1414–1422. 10.1111/clr.12755. [DOI] [PubMed] [Google Scholar]
- Langan, D. , Higgins, J. P. , Jackson, D. , Bowden, J. , Veroniki, A. A. , Kontopantelis, E. , Viechtbauer, W. , & Simmonds, M. (2019). A comparison of heterogeneity variance estimators in simulated random‐effects meta‐analyses. Research Synthesis Methods, 10(1), 83–98. [DOI] [PubMed] [Google Scholar]
- Lee, C. T. , Huang, Y. W. , Zhu, L. , & Weltman, R. (2017). Prevalences of peri‐implantitis and peri‐implant mucositis: Systematic review and meta‐analysis. Journal of Dentistry, 62, 1–12. 10.1016/j.jdent.2017.04.011 [DOI] [PubMed] [Google Scholar]
- Lee, C. T. , Sanz‐Miralles, E. , Zhu, L. , Glick, J. , Heath, A. , & Stoupel, J. (2020). Predicting bone and soft tissue alterations of immediate implant sites in the esthetic zone using clinical parameters. Clinical Implant Dentistry and Related Research, 22, 325–332. 10.1111/cid.12910. [DOI] [PubMed] [Google Scholar]
- Liberati, A. , Altman, D. G. , Tetzlaff, J. , Mulrow, C. , Gøtzsche, P. C. , Ioannidis, J. P. , Clarke, M. , Devereaux, P. J. , Kleijnen, J. , & Moher, D. (2009). The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med, 6, e1000100. 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrone, A. , Lasserre, J. , Bercy, P. , & Brecx, M. C. (2013). Prevalence and risk factors for peri‐implant disease in Belgian adults. Clinical Oral Implants Research, 24, 934–940. 10.1111/j.1600-0501.2012.02476.x. [DOI] [PubMed] [Google Scholar]
- Östman, P. O. , Chu, S. J. , Drago, C. , Saito, H. , & Nevins, M. (2020). Clinical outcomes of maxillary anterior postextraction socket implants with immediate provisional restorations using a novel macro‐hybrid implant design: An 18‐ to 24‐Month Single‐Cohort Prospective Study. The International Journal of Periodontics & Restorative Dentistry, 40, 355–363. 10.11607/prd.4467. [DOI] [PubMed] [Google Scholar]
- Ouzzani, M. , Hammady, H. , Fedorowicz, Z. , & Elmagarmid, A. (2016). Rayyan — A web and mobile app for systematic reviews. Systematic Reviews, 5(1), 1–10. 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxby, G. , Oxby, F. , Oxby, J. , Saltvik, T. , & Nilsson, P. (2015). Early loading of fluoridated implants placed in fresh extraction sockets and healed bone: A 3‐ to 5‐year clinical and radiographic follow‐up study of 39 consecutive patients. Clinical Implant Dentistry and Related Research, 17, 898–907. 10.1111/cid.12210. [DOI] [PubMed] [Google Scholar]
- Parvini, P. , Obreja, K. , Becker, K. , Galarraga, M. E. , Schwarz, F. , & Ramanauskaite, A. (2020). The prevalence of peri‐implant disease following immediate implant placement and loading: A cross‐sectional analysis after 2 to 10 years. International Journal of Implant Dentistry, 6(1), 1–10. 10.1186/s40729-020-00259-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payer, M. , Heschl, A. , Wimmer, G. , Wegscheider, W. , Kirmeier, R. , & Lorenzoni, M. (2010). Immediate provisional restoration of screw‐type implants in the posterior mandible: Results after 5 years of clinical function. Clinical Oral Implants Research, 21, 815–821. 10.1111/j.1600-0501.2010.01919.x. [DOI] [PubMed] [Google Scholar]
- Peixoto, C. D. , & Almas, K. (2016). The implant surface characteristics and peri‐implantitis. An evidence‐based update. Odonto‐stomatologie Tropicale, 39(153), 23–35. [PubMed] [Google Scholar]
- Perussolo, J. , Souza, A. B. , Matarazzo, F. , Oliveira, R. P. , & Araújo, M. G. (2018). Influence of the keratinized mucosa on the stability of peri‐implant tissues and brushing discomfort: A 4‐year follow‐up study. Clinical Oral Implants Research, 29(12), 1177–1185. 10.1111/clr.13381. Epub 2018 Nov 15 PMID: 30346630. [DOI] [PubMed] [Google Scholar]
- Pohl, V. , Fürhauser, L. , Haas, R. , & Pohl, S. (2020). Gingival recession behavior with immediate implant placement in the anterior maxilla with buccal dehiscence without additional augmentation‐a pilot study. Clinical Oral Investigations, 24, 1455–1464. 10.1007/s00784-019-03176-5. [DOI] [PubMed] [Google Scholar]
- Prati, C. , Zamparini, F. , Canullo, L. , Pirani, C. , Botticelli, D. , & Gandolfi, M. G. (2020). Factors affecting soft and hard tissues around two‐piece transmucosal implants: A 3‐year prospective cohort study. The International Journal of Oral & Maxillofacial Implants, 35, 1022–1036. 10.11607/jomi.7778. [DOI] [PubMed] [Google Scholar]
- Rokn, A. , Aslroosta, H. , Akbari, S. , Najafi, H. , Zayeri, F. , & Hashemi, K. (2017). Prevalence of peri‐implantitis in patients not participating in well‐designed supportive periodontal treatments: A cross‐sectional study. Clinical Oral Implants Research, 28, 314–319. 10.1111/clr.12800. [DOI] [PubMed] [Google Scholar]
- Romanos, G. E. , Aydin, E. , Locher, K. , & Nentwig, G. H. (2016). Immediate vs. delayed loading in the posterior mandible: A split‐mouth study with up to 15 years of follow‐up. Clinical Oral Implants Research, 27, 74–79. 10.1111/clr.12542. [DOI] [PubMed] [Google Scholar]
- Sailer, I. , Strasding, M. , Valente, N. A. , Zwahlen, M. , Liu, S. , & Pjetursson, B. E. (2018). A systematic review of the survival and complication rates of zirconia‐ceramic and metal‐ceramic multiple‐unit fixed dental prostheses. Clinical Oral Implants Research, 29(Suppl 16), 184–198. 10.1111/clr.13277. [DOI] [PubMed] [Google Scholar]
- Sanz, M. , Lindhe, J. , Alcaraz, J. , Sanz‐Sanchez, I. , & Cecchinato, D. (2017). The effect of placing a bone replacement graft in the gap at immediately placed implants: A randomized clinical trial. Clinical Oral Implants Research, 28, 902–910. 10.1111/clr.12896. [DOI] [PubMed] [Google Scholar]
- Sanz‐Martín, I. , Encalada, C. , Sanz‐Sánchez, I. , Aracil, J. , & Sanz, M. (2019). Soft tissue augmentation at immediate implants using a novel xenogeneic collagen matrix in conjunction with immediate provisional restorations: A prospective case series. Clinical Implant Dentistry and Related Research, 21, 145–153. 10.1111/cid.12696. [DOI] [PubMed] [Google Scholar]
- Scheyer, E. T. , Richardson, C. , Mandelaris, G. , Pickering, S. , Nevins, M. , Pope, B. , Janakievski, J. , Toback, G. , & Heard, R. H. (2017). Retrospective study to determine patient satisfaction of immediately placed and provisionalized implants in the esthetic zone from a us private‐practice research network. Compendium of Continuing Education in Dentistry (Jamesburg, N.J: 1995), 38(2), 9–12. [PubMed] [Google Scholar]
- Schrott, A. , Riggi‐Heiniger, M. , Maruo, K. , & Gallucci, G. O. (2014). Implant loading protocols for partially edentulous patients with extended edentulous sites – A systematic review and meta‐analysis. International Journal of Oral Maxillofacial Implants, 29(Suppl), 239–255. 10.11607/jomi.2014suppl.g4.2. PMID: 24660201. [DOI] [PubMed] [Google Scholar]
- Schwarz, F. , Becker, K. , Sahm, N. , Horstkemper, T. , Rousi, K. , & Becker, J. (2017). The prevalence of peri‐implant diseases for two‐piece implants with an internal tube‐in‐tube connection: A cross‐sectional analysis of 512 implants. Clinical Oral Implants Research, 28, 24–28. 10.1111/clr.12609. [DOI] [PubMed] [Google Scholar]
- Schwarz, F. , Derks, J. , Monje, A. , & Wang, H. L. (2018). Peri‐implantitis. Journal of Clinical Periodontology, 45(Suppl 20), 246–266. 10.1111/jcpe.12954. [DOI] [PubMed] [Google Scholar]
- Si, M. S. , Shou, Y. W. , Shi, Y. T. , Yang, G. L. , Wang, H. M. , & He, F. M. (2016). Long‐term outcomes of osteotome sinus floor elevation without bone grafts: A clinical retrospective study of 4–9 years. Clinical Oral Implants Research, 27, 1392–1400. 10.1111/clr.12752. [DOI] [PubMed] [Google Scholar]
- Siebers, D. , Gehrke, P. , & Schliephake, H. (2010). Delayed function of dental implants: A 1‐ to 7‐year follow‐up study of 222 implants. The International Journal of Oral & Maxillofacial Implants, 25, 1195–1202. [PubMed] [Google Scholar]
- Simons, W. F. , De Smit, M. , Duyck, J. , Coucke, W. , & Quirynen, M. (2015). The proportion of cancellous bone as predictive factor for early marginal bone loss around implants in the posterior part of the mandible. Clinical Oral Implants Research, 26, 1051–1059. 10.1111/clr.12398. [DOI] [PubMed] [Google Scholar]
- Spies, B. C. , Balmer, M. , Patzelt, S. B. , Vach, K. , & Kohal, R. J. (2015). Clinical and patient‐reported outcomes of a zirconia oral implant: Three‐year results of a prospective cohort investigation. Journal of Dental Research, 94, 1385–1391. 10.1177/0022034515598962. [DOI] [PubMed] [Google Scholar]
- Sterne, J. A. C. , Savović, J. , Page, M. J. , Elbers, R. G. , Blencowe, N. S. , Boutron, I. , Cates, C. J. , Cheng, H.‐Y. , Corbett, M. S. , Eldridge, S. M. , Emberson, J. R. , Hernán, M. A. , Hopewell, S. , Hróbjartsson, A. , Junqueira, D. R. , Jüni, P. , Kirkham, J. J. , Lasserson, T. , Li, T. , … Higgins, J. P. T. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ (Clinical Research ed.), 366, l4898. 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- The Joanna Briggs Institute (2017). The Joanna Briggs Institute critical appraisal tools for use in JBI systematic reviews. Checklist for qualitative research. The Joanna Briggs Institute. [Google Scholar]
- Tonetti, M. S. , Jung, R. E. , Avila‐Ortiz, G. , Blanco, J. , Cosyn, J. , Fickl, S. , Figuero, E. , Goldstein, M. , Graziani, F. , Madianos, P. , Molina, A. , Nart, J. , Salvi, G. E. , Sanz‐Martin, I. , Thoma, D. , Van Assche, N. , & Vignoletti, F. (2019). Management of the extraction socket and timing of implant placement: Consensus report and clinical recommendations of group 3 of the XV European Workshop in Periodontology. Journal of Clinical Periodontology, 46(Suppl 21), 183–194. 10.1111/jcpe.13131. [DOI] [PubMed] [Google Scholar]
- Van Nimwegen, W. G. , Raghoebar, G. M. , Stellingsma, K. , Tymstra, N. , Vissink, A. , & Meijer, H. J. (2015). Treatment outcome of two adjacent implant‐supported restorations with different implant platform designs in the esthetic region: A five‐year randomized clinical trial. The International Journal of Prosthodontics, 28, 490–498. 10.11607/ijp.4199. [DOI] [PubMed] [Google Scholar]
- Vetter, T. R. , & Mascha, E. J. (2017). Defining the primary outcomes and justifying secondary outcomes of a study: Usually, the fewer, the better. Anesthesia and Analgesia, 125(2), 678–681. 10.1213/ANE.0000000000002224. [DOI] [PubMed] [Google Scholar]
- Vignoletti, F. , de Sanctis, M. , Berglundh, T. , Abrahamsson, I. , & Sanz, M. (2009). Early healing of implants placed into fresh extraction sockets: An experimental study in the beagle dog. III: Soft tissue findings. Journal of Clinical Periodontology, 36, 1059–1066. 10.1111/j.1600-051X.2009.01489.x. [DOI] [PubMed] [Google Scholar]
- Vogl, S. , Stopper, M. , Hof, M. , Theisen, K. , Wegscheider, W. A. , & Lorenzoni, M. (2019). Immediate occlusal vs nonocclusal loading of implants: A randomized prospective clinical pilot study and patient centered outcome after 36 months. Clinical Implant Dentistry and Related Research, 21, 766–774. 10.1111/cid.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells, A. , Shea, B. , O'Connell, D. , Peterson, J. , Welch, V. , Losos, M. , & Tugwell, P. (2000). The Newcastle‐Ottawa Scale (NOS) for assessing the quality of non‐randomised studies in meta‐analyses. Ottawa (Canada): Ottawa Hospital Research Institute. [Google Scholar]
- Yan, Q. , Xiao, L. Q. , Su, M. Y. , Mei, Y. , & Shi, B. (2016). Soft and hard tissue changes following immediate placement or immediate restoration of single‐tooth implants in the esthetic zone: A systematic review and meta‐analysis. The International Journal of Oral & Maxillofacial Implants, 31, 1327–1340. 10.11607/jomi.4668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors. The data that support the findings of this study are available from the corresponding author upon reasonable request.


