Abstract
Introduction
Emicizumab is a humanised, bispecific monoclonal antibody mimicking the cofactor function of activated factor (F)VIII. It is indicated for routine prophylaxis of bleeding episodes in persons with haemophilia A (PwHA) with/without FVIII inhibitors.
Aim
To evaluate the development of anti‐emicizumab antibodies and their impact on pharmacokinetics (PK), pharmacodynamics (PD), efficacy and safety in PwHA.
Methods
Data from seven completed or ongoing phase 3 studies were pooled. The assessment of the immunogenicity profile of emicizumab included anti‐drug antibody (ADA) measurement and the association of ADAs with PK, PD, bleeding events, and adverse events.
Results
Of 668 PwHA evaluable for immunogenicity analysis, 34 (5.1%) developed ADAs after exposure to emicizumab. ADAs were transient in 14/34 PwHA (41.2%). ADAs were neutralising in vitro in 18/34 PwHA (52.9%) and associated with decreased emicizumab concentration in 4/668 evaluable PwHA (.6%); of those, one (.1%) discontinued emicizumab due to loss of efficacy. ADAs without decreased exposure did not impact emicizumab efficacy. The proportion of PwHA who had injection‐site reactions (ISRs) was higher in ADA‐positive PwHA (29.4% vs. 20.8%); however, the safety profile was similar between ADA‐positive and ADA‐negative PwHA, overall. No cases of anaphylaxis or hypersensitivity were reported in ADA‐positive participants.
Conclusion
The immunogenicity risk of emicizumab in phase 3 studies was low. ADAs, including in vitro neutralising ADAs, were not associated with a change in safety profile. Routine surveillance is, therefore, not warranted; however, in cases where a loss and/or waning of efficacy are observed, prompt evaluation by a healthcare provider should be sought.
Keywords: clinical study, emicizumab, monoclonal antibodies, pharmacodynamics, pharmacokinetics
1. INTRODUCTION
Persons with haemophilia A (PwHA) have frequent bleeds and develop long‐term complications such as haemophilic arthropathy if inadequately treated. 1 Historically, replacement therapy with factor (F)VIII was the standard treatment for PwHA without FVIII inhibitors, while treatment for those with inhibitors was limited to bypassing agents (BPAs) such as activated prothrombin complex concentrates (aPCC) or recombinant activated human FVII (rFVIIa). 2 FVIII prophylaxis has been proven to minimise bleeding events but, requires more than two intravenous (IV) infusions per week for maintaining protective trough levels (for standard half‐life FVIII products). 3 BPAs have suboptimal haemostatic effects and a high treatment burden associated with significant limitations (short half‐life, slow IV infusion rate). 4 , 5
Emicizumab is a subcutaneously administered recombinant, humanised, bispecific antibody, which mimics missing FVIIIa by bridging FIXa and FX to promote effective haemostasis in PwHA. 6 Emicizumab has a half‐life of approximately 4 weeks, 7 , 8 which allows prophylactic dosing once weekly (QW), 9 , 10 , 11 , 12 every 2 weeks (Q2W) 10 , 11 , 13 or every 4 weeks (Q4W) in PwHA with/without FVIII inhibitors. 13 , 14 Significant reductions in treated annualised bleed rates (ABRs) were confirmed with all three dosing regimens in phase 3 studies, regardless of FVIII inhibitor status, and the majority of participants (55.6%‐90%) receiving emicizumab in HAVEN 1‐5 reported zero treated bleeds. 9 , 10 , 11 , 14 , 15 A pooled, long‐term analysis of HAVEN 1‐4 showed that across a median (interquartile range [IQR]) efficacy period of 120.4 (89.0‐164.4) weeks, the model‐based ABR for treated bleeds (95% confidence interval [CI]) was 1.4 (1.1‐1.7) following emicizumab prophylaxis; in addition, at Weeks 121‐144, 82.4% of participants had zero treated bleeds. 16 Overall, emicizumab was well tolerated. The most common treatment‐related adverse event (AE) in the HAVEN 1‐4 studies was injection‐site reaction (ISR), which was reported in 26.8% of PwHA; the majority of ISRs were mild in intensity and none required treatment modification. 16 As previously reported, three thrombotic microangiopathies (TMA) and two thrombotic events (TE) were documented in the HAVEN 1 study in participants treated with cumulative aPCC doses exceeding 100 U/kg/24 h while receiving emicizumab prophylaxis. 9 This pharmacodynamic (PD) interaction is in part explained by the synergistic effect of aPCC, which contains the target antigens for emicizumab, FIX and FX, on thrombin generation. 17 , 18 Adherence to dosing guidance for BPA treatment during emicizumab prophylaxis, provided in the prescribing information, has resulted in no further reports of aPCC‐related TMA/TE events in clinical studies. 19 , 20 A small number of additional TE (not associated with aPCC) have been reported with emicizumab; these were associated with known comorbidities or pre‐existing risk factors. 12 , 21
As with all therapeutic proteins, there is potential for an immune response with subsequent development of anti‐drug antibodies (ADAs). ADAs can be associated with effects of various clinical consequences, varying from no impact at all to potentially serious adverse events (SAEs). Besides safety concerns such as hypersensitivity reactions, anaphylaxis, or injection‐site reactions (ISRs), the presence of ADAs may be associated with a reduction in therapeutic response and a loss of efficacy through inhibition of the drug activity and/or acceleration of the drug clearance. 22 , 23 , 24 , 25 Of note, ADAs directed against emicizumab will not affect a person's underlying haemophilia or FVIII inhibitor status, nor the ability to manage bleeding events with conventional therapies, including BPAs. 26 The current analysis aims to evaluate the immunogenicity of emicizumab in seven phase 3/3b clinical studies in PwHA, to report the incidence of ADAs against emicizumab and to assess their impact on pharmacokinetics (PK), PD, efficacy and safety.
2. MATERIALS AND METHODS
2.1. Clinical study design and sample collection
Study protocols were approved by the relevant institutional review board/independent ethics committee. All enrolled PwHA, or their legal representative, provided informed consent to participate. Seven clinical studies (HAVEN 1‐5, HOHOEMI and STASEY; see Table S1) were included in this analysis. The designs of these studies have been published previously. 9 , 10 , 11 , 12 , 13 , 14 , 15 The studies included paediatric and adult PwHA, with or without FVIII inhibitors. With the exception of the PK run‐in cohort in HAVEN 4, each PwHA received emicizumab prophylaxis starting with weekly loading doses of 3 mg/kg QW for 4 weeks, followed by maintenance doses of 1.5 mg/kg QW, 3 mg/kg Q2W or 6 mg/kg Q4W. Treatment doses could be up‐titrated if suboptimal bleed control was observed. These regimens have identical cumulative doses, and provided effective bleeding control over the entire dosing interval. 27
Endpoints were aligned across the phase 3 studies. Data from these seven phase 3/3b studies were subsequently pooled to provide an aggregated analysis of immunogenicity.
Blood samples for the detection of ADAs, for the determination of emicizumab concentration and for PD analyses were collected at baseline prior to dosing and at trough at regular intervals during treatment. 28 , 29 , 30 ADA samples were also collected at early withdrawal visit and 24 weeks after treatment cessation. For the PK run‐in cohort of HAVEN 4 (n = 7 participants), samples were collected prior to and after dosing at numerous time points during the first 8 weeks of study, and less frequently thereafter. 14
2.2. Anti‐emicizumab antibody and neutralising antibody assays
Plasma samples were assessed for the presence of anti‐emicizumab antibodies using a validated bridging enzyme‐linked immunosorbent assay (ELISA). ADA‐positive samples were further analysed for neutralising capacity (neutralising ADA [nADA]) using a modified FVIII chromogenic assay measuring emicizumab activity. Further details of the assays are provided in the Supplementary Material.
2.3. Pharmacokinetic and pharmacodynamic assessments
Active emicizumab plasma concentrations were determined by a dual‐binding competent ELISA. 8 , 31 The lower limit of quantitation was 100 ng/ml in human plasma. PD markers included FVIII‐like activity, thrombin generation (not measured in HAVEN 2 and STASEY studies) and activated partial thromboplastin time (aPTT); see Supplement for details.
2.4. Statistical analysis
Participant immunogenicity status was determined based on the harmonised definitions for therapeutic proteins (Table 1). 32 Participants were evaluable for immunogenicity analysis if they received at least one dose of emicizumab and had at least one ADA assessment following drug administration. Descriptive statistics were used to assess the incidence of ADA, nADA, and ADA with decreased exposure. The kinetics of ADAs were examined through time to first ADA detection, the transient or persistent nature of the ADAs and distribution of maximum post‐baseline titres.
TABLE 1.
Definition of ADA status
| Participant status | Definition | |
|---|---|---|
| ADA‐negative | ADA‐negative all samples | All baseline (pre‐treatment a ) and post‐dose samples were negative |
| Treatment‐unaffected ADAs | Pre‐treatment a sample was positive and post‐dose samples were either negative or positive with a <4‐fold increase in titre compared with baseline | |
| ADA‐positive | Treatment‐induced ADAs | Pre‐treatment a sample was negative and at least one post‐dose sample was positive |
| Treatment‐boosted ADAs | Pre‐treatment a sample was positive and at least one post‐dose sample was positive with a ≥4‐fold increase in titre compared with baseline | |
| Transient ADA | Treatment‐induced or boosted ADAs detected only at one post‐dose sample (with the exclusion of the last sampling time point) | |
| Persistent ADA | Treatment‐induced or boosted ADAs detected at two or more post‐dose samples or detected on last sampling time point | |
| Neutralising ADA | Treatment‐induced or boosted ADAs with in vitro neutralising capacity (i.e., based on a neutralising antibody assay) | |
| ADA with decreased exposure | ADA‐positive participant with decreased emicizumab concentrations |
Abbreviation: ADA, anti‐drug antibody.
Missing sample at baseline was considered negative.
To determine the potential impact of ADA on PK, time‐course of plasma emicizumab concentrations in ADA‐positive PwHA were visualised graphically. Decreased emicizumab concentrations were corroborated by time profiles of PD markers. Intra‐individual mean emicizumab trough concentrations at steady state were calculated (as the mean of all steady state trough concentrations in a given PwHA) and their distributions were compared among the ADA categories (negative vs. positive with or without in vitro neutralising capacity). The potential impact of ADA on emicizumab efficacy was evaluated by comparing the model‐based ABR (using a negative‐binomial regression model 14 ) or median ABR among the ADA categories (negative vs. positive with or without decreased exposure). Treated bleeds and all bleeds were calculated as previously described. 16 The potential impact of emicizumab ADAs on safety was assessed by comparing the incidences of AEs in ADA‐positive participants with those in ADA‐negative participants for the following categories: AE, drug‐related AE, ISR, SAE, drug‐related SAE and hypersensitivity/anaphylactic reactions; safety profiles were assessed throughout the entire study duration, that is, pre‐ and post‐ADA detection.
3. RESULTS
3.1. Participants
A total of 668 PwHA (98 children aged <12 years; 570 adolescents and adults aged ≥12 years) from the seven phase 3/3b studies were evaluable for immunogenicity analysis. Participants were all male and had a median (range) age of 28 (.3‐80) years (Table S2). The majority of participants (62.1%) had FVIII inhibitors at baseline. Overall, participants were exposed to emicizumab for a median (IQR) of 103.1 (82.4‐148.1) weeks.
3.2. ADA incidence
The proportions of PwHA with ADAs detected at baseline and during treatment are shown in Figure 1 and Table 2. Overall, 3.6% (n = 24/668) of participants had a positive test for ADAs at baseline, all with a low titre (≤20). A total of 34/668 participants developed ADAs across the seven phase 3/3b studies, resulting in an overall incidence of ADAs of 5.1%. ADA incidence was comparable across five of these studies (HAVEN 1‐4 and STASEY) and, overall, ranged from 0% (n = 0/13) in HOHOEMI to 12.5% (n = 8/64) in HAVEN 5. The majority of ADA‐positive PwHA had treatment‐induced ADAs, with only three PwHA having treatment‐boosted ADAs.
FIGURE 1.
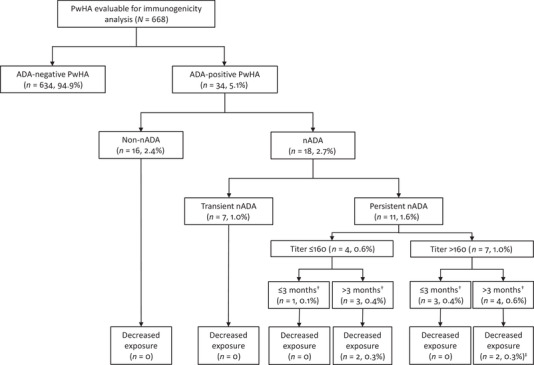
Summary of overall immunogenicity status. †Time of ADA persistence. ‡One participant withdrew from the study due to a loss of efficacy. Proportions of PwHA based on a total of 668 evaluable participants; percentages are rounded to the nearest decimal point. ADA, anti‐drug antibody; nADA, neutralising antibody; PwHA, person with haemophilia A
TABLE 2.
Overall immunogenicity status
| HAVEN 1 | HAVEN 2 | HAVEN 3 | HAVEN 4 | HAVEN 5 | HOHOEMI | STASEY | Total | |
|---|---|---|---|---|---|---|---|---|
| No. of PwHA evaluable for ADA | 111 | 88 | 151 | 48 | 64 | 13 | 193 | 668 |
| n (%) of PwHA with positive test for ADAs at baseline a | 2 (1.8) | 4 (4.5) | 4 (2.6) | 3 (6.3) | 4 (6.3) | 2 (15.4) | 5 (2.6) | 24 (3.6) |
| n (%) of ADA‐negative PwHA | 109 (98.2) | 82 (93.2) | 145 (96.0) | 46 (95.8) | 56 (87.5) | 13 (100) | 183 (94.8) | 634 (94.9) |
| Negative (all samples) | 107 (96.4) | 78 (88.6) | 142 (94.0) | 43 (89.6) | 52 (81.3) | 11 (84.6) | 180 (93.3) | 613 (91.8) |
| Negative (treatment unaffected) | 2 (1.8) | 4 (4.5) | 3 (2.0) | 3 (6.3) | 4 (6.3) | 2 (15.4) | 3 (1.6) | 21 (3.1) |
| n (%) of ADA‐positive PwHA | 2 (1.8) | 6 (6.8) | 6 (4.0) | 2 (4.2) | 8 (12.5) | 0 (0) | 10 (5.2) | 34 (5.1) |
| Positive (treatment induced) | 2 (1.8) | 6 (6.8) | 5 (3.3) | 2 (4.2) | 8 (12.5) | 0 (0) | 8 (4.2) | 31 (4.6) |
| Positive (treatment boosted) | 0 (0) | 0 (0) | 1 (.7) | 0 (0) | 0 (0) | 0 (0) | 2 (1.0) | 3 (.5) |
| n (%) of PwHA with neutralising ADAs | 2 (1.8) | 3 (3.4) | 4 (2.6) | 1 (2.1) | 3 (4.7) | 0 (0) | 5 (2.6) | 18 (2.7) |
| n (%) of PwHA with ADAs with decreased exposure b | 1 (.9) | 2 (2.3) | 0 (0) | 0 (0) | 1 (1.6) | 0 (0) | 0 (0) | 4 (.6) |
Abbreviations: ADA, anti‐drug antibody; PwHA, persons with haemophilia A.
Missing sample at baseline was considered negative.
All PwHA with ADAs with decreased exposure had neutralising ADAs.
The incidence of ADA development was similar across participant age (<12 vs. ≥12 years), emicizumab dosing regimen and FVIII inhibitor status (Table S3).
3.3. ADA kinetics and titre
A summary of duration from initiation of emicizumab treatment to first ADA detection is shown in Table 3. The vast majority of ADAs (88.2%, n = 30/34) were first detected within 6 months of treatment initiation with emicizumab. Most ADA‐positive PwHA (70.6%, n = 24/34) had maximum ADA titres ≤160. The highest measured titre (10,200) was observed in a PwHA who developed ADAs associated with decreased emicizumab exposure. Of the 34 ADA‐positive PwHA, 41.2% (n = 14/34) had transient ADAs (i.e., detected at a single time point). Among the remaining 20 ADA‐positive PwHA, 75% (n = 15/20) tested negative for ADAs at their last time point, after a median (IQR) duration of ADA positivity of 20 (12‐33) weeks.
TABLE 3.
Summary of time to first ADA detection during treatment with emicizumab
| Time to first treatment‐induced or treatment‐boosted ADA | ||||||
|---|---|---|---|---|---|---|
| PwHA (N = 668) | n | ≤1 month | >1 to ≤2 months | >2 to ≤3 months | >3 to ≤6 months | >6 months |
| ADA‐positive PwHA | 34 | 3 (8.8%) | 4 (11.8%) | 9 (26.5%) | 14 (41.2%) | 4 (11.8%) |
| nADA‐positive PwHA a | 18 | 2 (11.1%) | 2 (11.1%) | 7 (38.9%) | 6 (33.3%) | 1 (5.6%) |
Abbreviations: ADA, anti‐drug antibody; nADA, neutralising anti‐drug antibody; PwHA, persons with haemophilia A.
Including ADA‐positive PwHA with decreased exposure.
3.4. Neutralising ADAs
ADAs were neutralising in vitro in 18 participants (2.7%; Table 2 and Figure 1); of whom, seven had detectable nADAs at a single time point only, six had a positive signal at two consecutive time points, four had positive signals at several time points and, for one, nADAs were detected throughout the analysis period of approximately 35 weeks. For six participants, the signal was low and close to the detection cut‐off. Of these 18 participants, only four developed nADAs associated with decreased emicizumab exposure.
3.5. Impact of ADAs on PK and PD
In four PwHA (.6%), the presence of nADAs was associated with a decrease in emicizumab concentration (i.e., ADAs with decreased exposure; Figure 1). The decline in exposure was corroborated by a coinciding decrease in FVIII‐like activity and thrombin generation (when measured), and occasionally accompanied by prolonged aPTT (Figures S1–S4). In one of these participants, high‐titre ADAs were detected at 4 weeks after treatment initiation with emicizumab; emicizumab plasma concentrations became undetectable within the first 8 weeks of treatment and remained so with emicizumab 3 mg/kg QW (Figure S1). The participant eventually withdrew from the study due to a loss of efficacy, as previously described. 10 , 33 A second participant exhibited emicizumab plasma concentrations that consistently declined with time despite dose up‐titration; a final concentration of <4 μg/ml was measured when the participant withdrew from the study for personal preference at Week 64 (Figure S2). A third participant demonstrated decreased plasma concentrations that stabilised around 15 μg/ml before increasing at the last measurement, at which time this participant tested negative for ADAs (Figure S3). A fourth participant experienced transient decrease of exposure over approximately 20 weeks, reaching a minimum of 10 μg/ml before increasing, even in the presence of ADAs (Figure S4).
Excluding the four participants with ADAs with decreased exposure, the distribution of emicizumab trough concentrations at steady state (Ctrough,ss) in ADA‐positive PwHA generally overlapped with that in ADA‐negative PwHA, regardless of the positivity or negativity of nADAs (Figure 2). Across the dosing regimens, 17/30 (56.7%) ADA‐positive PwHA (excluding ADA‐positive PwHA with decreased exposure) had a Ctrough,ss within the first quartile (Figure 2).
FIGURE 2.
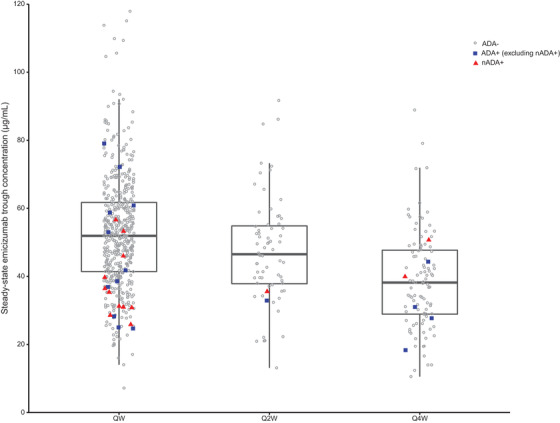
Distribution of intra‐individual mean steady‐state trough plasma emicizumab concentrations by ADA status and dosing regimen, excluding participants with ADAs with decreased exposure (n = 4). Box plots represent 25th and 75th percentiles, bold lines within the box represent median values and whiskers represent 1.5 interquartile range for all concentration points regardless of the ADA status. ADA, anti‐drug antibody; nADA, neutralising ADA; Q2W, once every 2 weeks; Q4W, once every 4 weeks; QW, once weekly
3.6. Impact of ADAs on efficacy
The ADA‐positive and ADA‐negative PwHA had a comparable median (IQR) efficacy period of 103 (78‐160) weeks and 103 (84‐150) weeks, respectively; the start of the efficacy period for each participant was the first day of emicizumab prophylaxis and the end of the efficacy period was the day of clinical cut‐off or treatment discontinuation, regardless of up‐titration. Emicizumab was highly effective in controlling bleeding events in PwHA with or without ADAs, regardless of nADA status (except in participants with ADAs with decreased emicizumab exposure), with similar model‐based ABRs (95% CI) for treated bleeds (ADA‐positive PwHA, .7 [.37‐1.33]; ADA‐negative PwHA, .9 [.79‐1.11]) or all bleeds (ADA‐positive PwHA, 1.2 [.75‐1.91]; ADA‐negative PwHA, 2.3 [1.99‐2.60]) observed. Additionally, similar proportions of participants with zero treated bleeds and zero all bleeds were observed, regardless of ADA or nADA status (Table 4 and Table S4).
TABLE 4.
ABR for PwHA treated with emicizumab by ADA status a
| ADA‐negative PwHA (N = 634) | ADA‐positive PwHA b (N = 30) | ADA‐positive PwHA with decreased exposure (N = 4) | |
|---|---|---|---|
|
Duration of efficacy period Median [IQR], weeks |
103 [84‐150] |
103 [78‐160] |
57 c [16; 65; 80; 50] |
|
Annualised rate of treated bleeding events Model‐based d [95% CI] |
.9 [.79‐1.11] |
.7 [.37‐1.33] |
14.1 c [23.0; 28.0; 0; 5.3] |
|
Proportion of participants with zero treated bleeds, n (%) [95% CI e ] |
374 (59.0) [55.1‐62.9] |
16 (53.3) [34.3‐71.7] |
1 (25.0) [.6‐80.6] |
|
Annualised rate of all bleeding events Model‐based d [95% CI] |
2.3 [1.99‐2.60] |
1.2 [.75‐1.91] |
15.2 c [23.0; 28.0; 0; 7.4] |
|
Proportion of participants with zero all bleeds n (%) [95% CI e ] |
224 (35.3) [31.6‐39.2] |
7 (23.3) [9.9‐42.3] |
1 (25.0) [.6‐80.6] |
Abbreviations: ABR, annualised bleed rate; ADA, anti‐drug antibody; CI, confidence interval; IQR, interquartile range; PwHA, persons with haemophilia A.
Data includes the up‐titration period.
Excluding participants A, B, C, and D with ADA with decreased exposure.
Median [individual data for participants A, B, C, and D, respectively] (Figures S1–S4).
Model‐based ABR were derived using a negative‐binomial regression model.
CIs were derived using the exact method.
One of the four participants with ADAs with decreased exposure withdrew from the study early due to loss of efficacy and so the duration of emicizumab treatment was short, at 16 weeks. This participant had an ABR of 23.0 for both treated and all bleeds. The other three participants with ADAs with decreased exposure had treatment durations of 50, 65, and 80 weeks, with ABRs for treated bleeds of 5.3, 28.0, and 0, respectively, and for all bleeds of 7.4, 28.0, and 0, respectively (Table 4).
3.7. Impact of ADAs on safety
The safety profile of ADA‐positive PwHA (including nADAs) did not appear to differ from that of ADA‐negative PwHA; the proportions of PwHA with AEs, drug‐related AEs, SAEs, and drug‐related SAEs were similar for ADA‐positive and ADA‐negative PwHA (Table 5). The proportion of PwHA who reported at least one ISR was slightly higher in ADA‐positive PwHA (29.4% [n = 10/34] vs. 20.8% [n = 132/634]); however, the majority of ADA‐positive PwHA reporting ISRs (n = 8/10) did so prior to ADA detection (i.e., on or before the day of last negative sample); of whom, two also reported ISRs after the detection of ADAs (Table S5). All ISRs were mild and did not require treatment. Furthermore, no cases of anaphylactic reactions or severe hypersensitivity reactions were reported, nor were any events indicative of potential immune complex deposition, in ADA‐positive PwHA.
TABLE 5.
Safety summary in ADA‐negative and ADA‐positive PwHA treated with emicizumab a
| ADA‐negative PwHA (N = 634) | ADA‐positive PwHA (N = 34) | |
|---|---|---|
|
Duration of exposure period Median (IQR), weeks |
103 (83‐148) | 100 (55‐159) |
| PwHA with at least one AE, n (%) | 575 (90.7) | 31 (91.2) |
| PwHA with at least one drug‐related AE, n (%) | 187 (29.5) | 11 (32.4) |
| PwHA with at least one SAE, n (%) | 122 (19.2) | 7 (20.6) |
| PwHA with at least one drug‐related SAE, n (%) | 6 (.9) | 1 (2.9) |
| PwHA with at least one ISR, n (%) | 132 (20.8) | 10 (29.4) |
| PwHA with at least one hypersensitivity, anaphylactic or anaphylactoid reaction, n (%) | 2 (.3) | 0 (0) |
Abbreviations: ADA, anti‐drug antibody; AE, adverse event; IQR, interquartile range; ISR, injection‐site reaction; PwHA, persons with haemophilia A; SAE, serious AE.
Data includes the up‐titration period.
The presence of nADAs in ADA‐positive PwHA did not affect the safety profile of emicizumab (Table S6).
4. DISCUSSION
We assessed the immunogenicity results in PwHA treated with emicizumab for a median duration of approximately 2 years across seven phase 3/3b studies. In this analysis, 34 of the 668 PwHA (5.1%) treated with emicizumab, at a maintenance dose of either 1.5 mg/kg QW, 3 mg/kg Q2W, or 6 mg/kg Q4W, developed binding antibodies against emicizumab. More than 40% of the PwHA had transient ADAs (detected on a single occasion only) and the majority of ADAs were of relatively low titre (≤160). The incidence of ADAs was low, as expected for humanised monoclonal antibodies, 34 and consistent with that reported in an earlier analysis. 35 It was generally consistent across studies, with no ADAs detected in HOHOEMI 13 (possibly due to the relatively small sample size) and a slightly higher incidence observed in HAVEN 5. 15 There were no clear clinical characteristics (e.g., history of hypersensitivity) predictive of ADA development, and the likelihood of developing ADAs was also independent of participant age, dosing regimen or FVIII inhibitor status.
Overall, 18 PwHA (2.7%) developed ADAs that were neutralising in an in vitro neutralising antibody assay; of these, only four participants developed nADAs associated with a decrease in emicizumab concentration, corroborated by a loss of PD effect. As nADAs bind to the active binding site of emicizumab, or in close proximity to it, they can neutralise its effect and can preclude its detection via the dual‐binding competent PK assay. This may result in an apparent decrease in emicizumab concentration, regardless of whether the ADAs really have capacity to promote the clearance of emicizumab in vivo. No effects on PK were observed in the other 14 nADA‐positive PwHA. This is most likely a consequence of the low titre and transient nature of their nADAs. Likewise, in these 14 PwHA, the presence of nADAs had no impact on the PD of emicizumab (data not shown). Of note, decreased emicizumab concentrations were also observed in some ADA‐negative PwHA. The cause remains unidentified, but these decreases may not be attributable to lack of compliance. It is unlikely that these were the consequence of undetected ADAs as the validated ELISA has been shown to be drug tolerant, for example, low‐titre ADAs were detected in PwHA in the presence of emicizumab concentrations >50 μg/ml, and emicizumab concentrations were relatively low (<10 μg/ml) in the majority of the samples in these PwHA.
Even ADAs with neutralising capacity in vitro did not impact the efficacy of emicizumab, if they had no effect on PK. Low ABRs for treated bleeds were observed in ADA‐negative (.9) and ADA‐positive PwHA (.7). In contrast, ADAs leading to decreased emicizumab exposure could decrease emicizumab efficacy in line with the known exposure‐response relationship. 27 The incidence of nADAs associated with decreased exposure was low (.6%); among the four PwHA with these ADAs, two participants withdrew from the study, one due to a loss of efficacy 10 , 33 and the other on personal preference. The third PwHA was still participating in the study at the time of clinical cut‐off date and the fourth PwHA completed the study (at which point this participant was ADA‐negative with restoration of anticipated emicizumab plasma concentrations and moved to commercial emicizumab).
In clinical studies of emicizumab, a loss of efficacy due to ADAs is an infrequent (≥1/1000 to <1/100) event. Overall, the incidence of clinically important ADAs (e.g., ADAs associated with decreased emicizumab exposure) is lower compared with FVIII inhibitor development in PwHA treated with FVIII in this analysis population (.6% vs. 25%‐30%, respectively), 36 highlighting the low immunogenicity observed. In the post‐marketing setting, where there is no commercialised assay for measuring ADAs, there are only a few documented cases reported to the Safety Database where clinical signs (e.g., breakthrough bleeding) alongside surrogates for loss of efficacy due to presumptive ADAs are present (e.g., lowered emicizumab concentration and/or prolonged aPTT). More than 10,000 PwHA have been treated with emicizumab to date (Data on file, F. Hoffmann‐La Roche, Ltd.).
No association was found between the presence of ADAs and the safety profile of emicizumab.
The limitations of HAVEN 1‐5, STASEY and HOHOEMI studies, which have been published previously, 9 , 10 , 11 , 12 , 13 , 14 , 15 also apply to this immunogenicity analysis. Conversely, the strengths of the data presented in this prospective analysis are attributed to the relatively large participant sample–a total of 668 participants across all seven studies were evaluable for immunogenicity analysis–and participants were followed for approximately 2 years.
In conclusion, emicizumab is associated with a low incidence of ADAs, a large proportion being transient in nature and/or of low titre. nADAs impacting PK occurred in .6% of PwHA and the efficacy of emicizumab was otherwise not impacted by ADAs. Regardless of their neutralising capacity in vitro, the presence of ADAs did not alter the safety profile of emicizumab.
Given the low incidence of ADAs, particularly those impacting PK and PD, detection of ADAs, including nADAs, has limited impact on clinical management, suggesting that routine laboratory surveillance is not warranted for the clinical use of emicizumab. A progressive loss of efficacy manifested by an increase in breakthrough bleeding events despite adherence to emicizumab dosing regimen, reduced emicizumab concentration, diminished chromogenic FVIII‐like activity using an assay with human factors or prolonged aPTT could be indicators of the presence of clinically important ADAs.
CONFLICTS OF INTEREST
Christophe Schmitt is an employee of F. Hoffmann‐La Roche Ltd, holds stock in F. Hoffmann‐La Roche Ltd and is a co‐inventor of a patent related to an anti‐FIXa/FX bispecific antibody. Thomas Emrich is an employee of Roche Diagnostics GmbH and holds stock in F. Hoffmann‐La Roche Ltd. Sammy Chebon is an employee of F. Hoffmann‐La Roche Ltd and holds stock in F. Hoffmann‐La Roche Ltd. Elena Fernandez is an employee of F. Hoffmann‐La Roche Ltd and holds stock in F. Hoffmann‐La Roche Ltd. Claire Petry is an employee of F. Hoffmann‐La Roche Ltd and holds stock in F. Hoffmann‐La Roche Ltd. Koichiro Yoneyama is an employee of Chugai Pharmaceutical Co., Ltd and an inventor/co‐inventor of patents related to anti‐FIXa/FX bispecific antibodies. Anna Kiialainen is an employee of F. Hoffmann‐La Roche Ltd and holds stock in F. Hoffmann‐La Roche Ltd. Monet Howard is an employee of Hoffmann‐La Roche Ltd and holds stock in F. Hoffmann‐La Roche Ltd. Markus Niggli is an employee of F. Hoffmann‐La Roche Ltd. Ido Paz‐Priel is an employee of Genentech, Inc. and holds stock in F. Hoffmann‐La Roche Ltd. Tiffany Chang was an employee of Genentech, Inc. at the time of the study and holds stock in F. Hoffmann‐La Roche Ltd.
AUTHOR CONTRIBUTIONS
Christophe Schmitt wrote the manuscript; Christophe Schmitt, Koichiro Yoneyama, Thomas Emrich contributed to/designed the research; Christophe Schmitt, Thomas Emrich, Sammy Chebon, Elena Fernandez, Claire Petry, Koichiro Yoneyama, Anna Kiialainen, Monet Howard, Markus Niggli, Ido Paz‐Priel, and Tiffany Chang performed the research; Christophe Schmitt, Markus Niggli, Elena Fernandez analysed the data. All authors reviewed and approved the final version submitted.
Supporting information
Supporting material
ACKNOWLEDGEMENTS
The authors thank all investigators, study participants and all members of the clinical study teams. Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Adele Blair, PhD, of Ashfield MedComms, an Ashfield Health company, and funded by F. Hoffmann‐La Roche Ltd. This research was sponsored by F. Hoffmann‐La Roche Ltd and Chugai Pharmaceutical Co., Ltd.
Schmitt C, Emrich T, Chebon S, et al. Low immunogenicity of emicizumab in persons with haemophilia A. Haemophilia. 2021;27:984–992. 10.1111/hae.14398
DATA AVAILABILITY STATEMENT
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
REFERENCES
- 1. Mannucci PM, Tuddenham EG. The hemophilias–from royal genes to gene therapy. N Engl J Med. 2001;344(23):1773‐1779. [DOI] [PubMed] [Google Scholar]
- 2. Witmer C, Young G. Factor VIII inhibitors in hemophilia A: rationale and latest evidence. Ther Adv Hematol. 2013;4(1):59‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Srivastava A, Elena S, Dougall A, et al. WFH guidelines for the management of hemophilia. Haemophilia. 2020;26(Suppl 6):1‐158. [DOI] [PubMed] [Google Scholar]
- 4. Lambert T, Benson G, Dolan G, et al. Practical aspects of extended half‐life products for the treatment of haemophilia. Ther Adv Hematol. 2018;9(9):295‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ljung R, Gretenkort Andersson N. The current status of prophylactic replacement therapy in children and adults with haemophilia. Br J Haematol. 2015;169(6):777‐786. [DOI] [PubMed] [Google Scholar]
- 6. Kitazawa T, Igawa T, Sampei Z, et al. A bispecific antibody to factors IXa and X restores factor VIII hemostatic activity in a hemophilia A model. Nat Med. 2012;18(10):1570‐1574. [DOI] [PubMed] [Google Scholar]
- 7. Yoneyama K, Schmitt C, Kotani N, et al. A pharmacometric approach to substitute for a conventional dose‐finding study in rare diseases: example of phase III dose selection for emicizumab in hemophilia A. Clin Pharmacokinet. 2018;57(9):1123‐1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Retout S, Schmitt C, Petry C, Mercier F, Frey N. Population pharmacokinetic analysis and exploratory exposure‐bleeding rate relationship of emicizumab in adult and pediatric persons with hemophilia A. Clin Pharmacokinet. 2020;59(12):1611‐1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377(9):809‐818. [DOI] [PubMed] [Google Scholar]
- 10. Young G, Liesner R, Chang T, et al. A multicenter, open‐label, phase 3 study of emicizumab prophylaxis in children with hemophilia A with inhibitors. Blood. 2019;134(24):2127‐2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mahlangu J, Oldenburg J, Paz‐Priel I, et al. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. 2018;379(9):811‐822. [DOI] [PubMed] [Google Scholar]
- 12. Jimenez‐Yuste V, Klamroth R, Castaman G, et al. Second interim analysis results from the STASEY trial: a single‐arm, multicenter, open‐label, phase III clinical trial to evaluate the safety and tolerability of emicizumab prophylaxis in people with hemophilia A (PwHA) with FVIII inhibitors. Res Prac Thromb Haemostas. 2020;4(Suppl1). [Google Scholar]
- 13. Shima M, Nogami K, Nagami S, et al. A multicentre, open‐label study of emicizumab given every 2 or 4 weeks in children with severe haemophilia A without inhibitors. Haemophilia. 2019;25(6):979‐987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pipe SW, Shima M, Lehle M, et al. Efficacy, safety, and pharmacokinetics of emicizumab prophylaxis given every 4 weeks in people with haemophilia A (HAVEN 4): a multicentre, open‐label, non‐randomised phase 3 study. Lancet Haematol. 2019;6(6):E295‐E305. [DOI] [PubMed] [Google Scholar]
- 15. Wang S, Zhou X, Wang X, et al. A randomized, multicenter, open‐label, phase III clinical trial to evaluate the efficacy, safety and pharmacokinetics of prophylactic emicizumab versus no prophylaxis in persons with hemophilia A in the Asia‐Pacific region (HAVEN 5). Res Prac Thromb Haemost. 2020;4(Suppl 1). [Google Scholar]
- 16. Callaghan M, Negrier C, Paz‐Priel I, et al. Long‐term outcomes with emicizumab prophylaxis for hemophilia A with or without FVIII inhibitors from the HAVEN 1–4 studies. Blood. 2021;137(16):2231‐2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hartmann R, Feenstra T, Valentino L, Dockal M, Scheiflinger F. In vitro studies show synergistic effects of a procoagulant bispecific antibody and bypassing agents. J Thromb Haemost. 2018;16:1580‐1591. [DOI] [PubMed] [Google Scholar]
- 18. Schultz NH, Glosli H, Biornsen S, Holme PA. The effect of bypassing agents in combination with emicizumab‐treatment. Res Prac Thromb Haemost. 2020;4(Suppl 1). [Google Scholar]
- 19. Collins PW, Liesner R, Makris M, et al. Treatment of bleeding episodes in haemophilia A complicated by a factor VIII inhibitor in patients receiving emicizumab. Interim guidance from UKHCDO inhibitor working party and executive committee. Haemophilia. 2018;24(3):344‐347. [DOI] [PubMed] [Google Scholar]
- 20. Callaghan M, Negrier C, Young G, et al. Use of bypassing agents prior to and post bypassing agent dosing guidance during emicizumab prophylaxis: analyses from the HAVEN 1 study. Blood. 2017;130:3668. [Google Scholar]
- 21. Lee L, Moreno K, Kuebler P, et al. Summary of thrombotic events or thrombotic microangiopathy events in persons taking emicizumab. EAHAD. 2020:P131. [Google Scholar]
- 22. Chirmule N, Jawa V, Meibohm B. Immunogenicity to therapeutic proteins: impact on PK/PD and efficacy. AAPS J. 2012;14:296‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mok CC, van der Kleij D, Wolbink GJ. Drug levels, anti‐drug antibodies, and clinical efficacy of the anti‐TNF alpha biologics in rheumatic diseases. Clin Rheumatol. 2013;32:1429‐1435. [DOI] [PubMed] [Google Scholar]
- 24. Saint‐Remy JM, Lacroix‐Desmazes S, Oldenberg J. Inhibitors in haemophilia: pathophysiology. Haemophilia. 2004;10:146‐151. [DOI] [PubMed] [Google Scholar]
- 25. Vermeire S, Gils A, Accossato P, Lula S, Marren A. Immunogenicity of biologics in inflammatory bowel disease. Ther Adv Gastroenterol. 2018;11:1756283X17750355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. National Hemophilia Foundation for all bleeding disorders . MASAC Document 258‐Recommendation on the Use and Management of Emicizumab‐kxwh (Hemlibra®) for Hemophilia A with and without Inhibitors. https://www.hemophilia.org/healthcare-professionals/guidelines-on-care/masac-documents/masac-document-258-recommendation-on-the-use-and-management-of-emicizumab-kxwh-hemlibrar-for-hemophilia-a-with-and-without-inhibitors. Accessed April 2021. [Google Scholar]
- 27. Jonsson F, Schmitt C, Petry C, Mercier F, Frey N, Retout S. Exposure‐bleeding count modeling of emicizumab for the prophylaxis of bleeding in persons with hemophilia A with /without inhibitors against factor VIII. Clin Pharmacokinet. 2021;60(7):931‐941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmitt C, Adamkewicz JI, Xu J, et al. Pharmacokinetics and pharmacodynamics of emicizumab in persons with hemophilia A with factor VIII inhibitors: HAVEN 1 study. Thromb Haemost. 2021;121(3):351‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kiialainen A, Schmitt C, Adamkewicz JI, et al. Pharmacokinetics and biomarkers in persons with haemophilia A (PwHA) receiving emicizumab every 2 or 4 weeks. EAHAD. 2019:P021. [Google Scholar]
- 30. Kiialainen A, Schmitt C, Oldenberg J, et al. Pharmacokinetics and biomarkers in persons with haemophilia A (PwHA) without FVIII inhibitors receiving emicizumab once weekly in the phase 3 HAVEN 3 study. EAHAD. 2019:P022. [Google Scholar]
- 31. Heinrich J, Staack RF, Stubenrauch K‐G, Papadimitriou A. Proposal for a harmonized descriptive analyte nomenclature for quantitative large‐molecule bioanalysis. Bioanalysis. 2015;7(24):3057‐3062. [DOI] [PubMed] [Google Scholar]
- 32. Shankar G, Cocea SAL, Devanarayan V, et al. Assessment and reporting of the clinical immunogenicity of therapeutic proteins and peptides‐harmonized terminology and tactical recommendations. AAPS J. 2014;16(4):658‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Valsecchi C, Gobbi M, Beeg M, et al. Characterisation of the neutralizing anti‐emicizumab antibody in a patient with haemophilia A and inhibitor. J Thromb Haemost. 2021;19(3):711‐718. [DOI] [PubMed] [Google Scholar]
- 34. van Brummelen EM, Ros W, Wolbink G, Beijnen JH, Schellens JH. Antidrug antibody formation in oncology: clinical relevance and challenges. Oncologist. 2016;21:1260‐1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Paz‐Priel I, Chang T, Asikanius E, et al. Immunogenicity of emicizumab in people with hemophilia A (PwHA): results from the HAVEN 1–4 studies. Blood. 2018;132(Suppl 1):633. [Google Scholar]
- 36. Franchini M, Mannucci PM. Hemophilia A in the third millennium. Blood Rev. 2013;27(4):179‐184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting material
Data Availability Statement
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).


