Summary
Plasma cell leukaemia (PCL) is a rare and very aggressive plasma cell disorder. Preventing a dismal outcome of PCL requires early diagnosis with appropriate analytical tools. Therefore, the investigation of 33 patients with primary and secondary PCL was done when the quantity of circulating plasma cells (PCs) using flow cytometry (FC) and morphology assessment was evaluated. The phenotypic profile of the PCs was also analysed to determine if there is an association with clinical outcomes and to evaluate the prognostic value of analysed markers. Our results revealed that FC is an excellent method for identifying circulating PCs as a significantly higher number was identified by FC than by morphology (26·7% vs. 13·5%, P = 0·02). None of secondary PCL cases expressed CD19 or CD20. A low level of expression with similar positivity of CD27, CD28, CD81 and CD117 was found in both PCL groups. A decrease of CD44 expression was detected only in secondary PCL. Expression of CD56 was present in more than half of PCL cases as well as cytoplasmic nestin. A decreased level of platelets, Eastern Cooperative Oncology Group score of 2–3 and lack of CD20+ PC were associated with a higher risk of death. FC could be incorporated in PCL diagnostics not only to determine the number of circulating PCs, but also to assess their phenotype profile and this information should be useful in patients’ diagnosis and possible prognosis.
Keywords: plasma cell leukaemia, multiple myeloma, plasma cell, flow cytometry, prognosis, phenotype
Introduction
Plasma cell leukaemia (PCL) is a very rare and an aggressive plasma cell disorder (PCD). Unlike multiple myeloma (MM), where clonal plasma cells (PCs) accumulate mostly in bone marrow (BM), 1 PCL is primarily characterised by an increased number of clonal circulating plasma cells (cPCs) in peripheral blood (PB). PCL diagnostic criteria were published in 1974; they are based on the relative number of cPCs exceeding 20% of total leukocytes and/or the absolute number of cPCs exceeding 2 × 109/l in whole PB; 2 these criteria are still valid today. 3 , 4
Since PCL is a very rare entity, international data are not available in commonly used databases [e.g. GLOBOCAN (https://gco.iarc.fr/)]; thus, information is based only on isolated studies or case reports. 5 , 6 , 7 , 8 For example, the incidence of PCL in Europe, according to the HAEMACARE project, is 0·4 cases per million. 9 The incidence of PCL in the Czech Republic was reported at 0·57 cases per million between 2000–2015, and the ratio of PCL to MM was 3:100. 5
PCL can be divided in two groups: primary PCL (pPCL) and secondary PCL (sPCL). pPCL originates de novo, with no previous history of MM 10 and represents approximately 60% of all PCL cases. In recent years, a decreased incidence has been observed; currently, it is about 50% of all PCL cases. 7 , 11 Median age at diagnosis of pPCL patients is 55 years. pPCL is characterised by a higher tumour burden, typically accompanied by anaemia, hypercalcaemia, thrombocytopenia and renal failure, 4 , 7 , 10 but fewer osteolytic lesions than in MM. 7 sPCL, on the other hand, develops as a leukaemic transformation of MM with a median age at diagnosis of approximately 65 years. sPCL patients have a significantly shorter overall survival (OS) than pPCL patients. 7 , 12
Treatment of PCL should start immediately after diagnosis to eliminate the risk of early death. 13 As with MM patients, modern drugs (proteasome inhibitors, immunomodulatory drugs) increase the survival of PCL patients. New treatment strategies, including monoclonal antibodies or chimeric antigen receptor T cells, might also be a promising treatment in PCL. 14
Although current PCL diagnostic criteria are based on morphological assessment, flow cytometry (FC) is a more helpful method, allowing not only detection and enumeration of PCs, but also their phenotypic characterisation and clonality analysis. 15 , 16 FC is able to distinguish abnormal myelomatous PCs from normal plasmablasts/PCs. These are produced in the lymph nodes and circulate through PB until they reach the BM niche required for their survival and differentiation into long‐living PCs. 17 This is especially important in differential diagnostics of PCDs and reactive plasmacytosis. 18
Similarly to MM, PCs in PCL express CD38, CD138 and typically lack CD19 and CD45. 6 , 19 , 20 Despite the low incidence of PCL, some studies demonstrated differences in the PC phenotypic profile in comparison to MM. 21 , 22 There are very few studies describing the correlation between phenotypic profile and disease behavior, although different antigens were tested. 20 Nowadays, FC analyses are focused mostly on possibilities of the detection and number of cPCs. 23 Both pPCL and sPCL share very similar phenotypic profiles. However, as pPCL should be considered a specific entity, distinct from sPCL, which generally constitutes the leukaemic evolution of a pre‐existing, end‐stage relapsed/refractory MM, 24 there is an expectation that both PCL can differ in expression of specific markers. According to some studies, absence of CD56 is typical for both forms of PCL at the time of diagnosis and disease progression. This observation also suggests that sPCL could emerge preferentially from CD56−/weak MM, 6 , 19 but this is not consistent with other studies where expression of CD56 seems not to be uniformly negative. 20 , 25 However, only CD28, which correlates with progression and tumour burden and can be upregulated during disease evolution, is more frequently expressed in sPCL and, possibly, even discriminates sPCL from pPCL. 19 , 26
This study focused on flowcytometric cPC/PC assessment in PCLs, phenotypic analysis and clarifying differences between the phenotypic profile of pPCL and sPCL in patients diagnosed in one institution between 2008 and 2019. Association of the phenotypic profile with clinical outcomes of PCL patients and evaluation of the prognostic value of cPC immunophenotype was also performed.
Materials and methods
Clinical characteristics of patients
In total, 33 PCL patients (18 pPCL and 15 sPCL) were diagnosed at the Department of Internal Medicine ‐ Hematology and Oncology at the University Hospital Brno, Czech Republic, between 2008 and 2019. Patients were identified either by FC analysis alone using presence ≥20% of CD38+CD138+ cPCs or by a combination of FC and morphology assessment using ≥2 × 109/l cPCs as disease criteria.
Patients were included only after they signed the informed consent form approved by the ethics committee of the University Hospital Brno, Czech Republic (2017) in accordance with the current version of the Helsinki Declaration.
The median age of all PCL patients was 67 years (range 42–85) and 42% were male. In the pPCL group, median age was 66 years (range 42–80) and in sPCL group, median age was 68 years (range 52–85). Patient characteristics are summarised in Table I and Table SI.
Table I.
Baseline characteristics of plasma cell leukaemia (PCL) patients at the time of PCL diagnosis.
| Baseline characteristics at PCL diagnosis* | pPCL (N = 18) | sPCL (N = 15) | P‐value † |
|---|---|---|---|
| Sex | N = 18 | N = 15 | |
| Women | 11 (61·1) | 8 (53·3) | 0·733 |
| Men | 7 (38·9) | 7 (46·7) | |
| Age at PCL diagnosis | N = 18 | N = 15 | |
| ≤50 | 2 (11·1) | 0 (0·0) | 0·459 |
| 51–60 | 4 (22·2) | 4 (26·7) | |
| 61–70 | 5 (27·8) | 5 (33·3) | |
| 71–80 | 7 (38·9) | 4 (26·7) | |
| >80 | 0 (0·0) | 2 (13·3) | |
| >65 | 10 (55·) | 10 (66·7) | 0·722 |
| Median (min–max) | 66 (42–80) | 68 (52–85) | 0·436 |
| Follow‐up (months) | N = 18 | N = 15 | |
| Median (min–max) | 11·0 (0·2–103·0) | 0·9 (0·1–25·2) | 0·001 |
| % PC in peripheral blood by morphology | N = 18 | N = 12 | |
| Median (min–max) | 15·0 (0·5–57·0) | 12·0 (0·0–63·5) | 0·271 |
| % PC in bone marrow by morphology | N = 17 | N = 14 | |
| Median (min–max) | 51·4 (20·6–76·8) | 64·3 (30·0–95·0) | 0·152 |
| ECOG PS | N = 18 | N = 12 | |
| 0 | 2 (11·1) | 1 (8·3%) | 0·806 |
| 1 | 9 (50·0) | 4 (33·3) | |
| 2 | 5 (27·8) | 5 (41·7) | |
| 3 | 2 (11·1) | 2 (16·7) | |
| ISS | N = 17 | N = 12 | |
| Stage 1 | 0 (0·0) | 1 (8·3) | 0·081 |
| Stage 2 | 4 (23·5) | 6 (50·0) | |
| Stage 3 | 13 (76·5) | 5 (41·7) | |
| Durie‐Salmon stage | N = 18 | N = 10 | |
| I | 1 (5·6) | 1 (10·0) | 0·443 |
| II | 5 (27·8) | 1 (10·0) | |
| III | 12 (66·7) | 8 (80·0) | |
| Durie‐Salmon substage | N = 18 | N = 10 | |
| A | 10 (55·6) | 9 (90·0) | 0·098 |
| B | 8 (44·4) | 1 (10·0) |
PC, plasma cell; pPCL, primary plasma cell leukaemia; sPCL, secondary plasma cell leukaemia.
Data presented as N (%) in categorical variables and median (minimum–maximum) in continuous variables.
P‐value of Fisher’s exact test in categorical variables and Mann‐Whitney U test in continuous variables. ECOG, Eastern Cooperative Oncology Group; ISS, International staging system.
Flow cytometry
Paired samples of PB and BM from 33 PCL patients were analysed by polychromatic FC. Analysis was performed on whole PB and BM from each patient. All samples were processed within 24 h after aspiration. Eight‐color combinations of monoclonal antibodies (MoAbs) were used for PC identification (CD38+CD138+ leukocytes), and analyses of PC surface antigenic profile as follows: CD38‐PB [Exbio (Vestec Czech Republic); clone HIT2], CD138‐PerCP (Exbio; MI15), CD45‐PO (Exbio; HI30), CD19‐PC7 [Beckman Coulter (Brea, CA, USA); J3‐119], CD56‐FITC (Exbio; MEM‐188), CD56‐PE [Exbio; LT56 or Dako (Santa Clara, CA, USA); C5.9]; CD56‐APC (Exbio; LT56), CD81‐APC‐H7 [Becton Dickinson (Franklin Lakes, NJ, USA); JS‐81], CD27‐PE (Exbio; LT27), CD27‐APC‐AF750 (Beckman Coulter; 1A4CD27), CD28‐FITC (Exbio; CD28.2), CD117‐APC (Becton Dickinson or Exbio; 104D2), CD44‐APC‐eF780 [eBioscience (Thermo Fisher Scientific, Waltham, MA, USA); IM7], CD200‐PE (Exbio; OX‐104).
In the case of cytoplasmic analyses, surface staining was followed by fixation and permeabilization using IntraPrep reagent (Beckman Coulter). Polyclonal kappa light chain‐FITC (Beckman Coulter; and/or Dako), polyclonal lambda light chain‐PE (Beckman Coulter and/or Dako) were used for clonality assessment and/or nestin‐APC (R&D; clone 196908).
Analysis was performed according to European Myeloma Network recommendations. 18 The relative count of CD38+CD138+ PB cPCs and BM PCs, their phenotypic profile (% of expressed marker) and positivity were analysed. Cell population was considered positive if the expression of any given marker was higher than the 20% cut‐off. 27 Also, clonality verification of phenotypically abnormal and normal PCs was done in BM, according to their cytoplasmic expression of kappa/lambda light chains, to evaluate clonal PCs (a‐PCs). 28
Flow cytometry analyses were performed on BD FACSCanto II (BD Biosciences, San Jose, CA, USA) equipped with three lasers (violet 405 nm, blue 488 nm, red 633 nm) and with software BD FACS Diva 6.1.3 (BD Biosciences). Data re‐analysis was done using Infinicyt 1.6 software (Cytognos SL, Salamanca, Spain).
Statistical analysis
Data were described by absolute and relative frequencies for categorical variables and median (minimum–maximum) for quantitative variables. Differences between groups of patients with pPCL and sPCL were tested using the Fisher’s exact and Mann Whitney U tests. OS from time of PCL diagnosis was plotted using Kaplan–Meier methodology. The log‐rank test was used to estimate the statistical significance of the difference between the curves. The Cox proportional hazards model was performed to explore the univariate association of risk factors with OS. Cut‐off for the smallest P‐value of log‐rank test was selected. In regard to FC parameters, cut‐offs with a 1% increase and for the thrombocyte count a 10‐unit increase were tested. P < 0·05 was considered statistically significant (all tests two‐sided). Analysis was performed using the SPSS software, version 25.0 (IBM Corp., Armonk, NY, USA.) and R software, version 3.3.0 (www.r‐projec.org).
Results
Clinical characteristics
Clinical characteristics of pPCL and sPCL patients were compared but no statistically significant differences were found. The only statistically significant difference was an increased level of thrombocytes found at the time of pPCL diagnosis when compared with sPCL: median 129·5 × 109/l (range 37·6–411·0) vs. 49·1 × 109/l (range 26·5–186·0), P = 0·004 respectively (Table SI). Biochemically detected kappa light chain type prevailed in the group of patients with pPCL; however, lambda light chain was unexpectedly more frequent in the sPCL group and a statically significant difference between light chain type was detected (P = 0·043).
Results of PCL treatment are summarised in Tables SII–SIV. Previous therapy in sPCL patients is described in Table SV.
Survival of PCL patients
Median time to progression (TTP) of newly diagnosed MM to sPCL was 26·9 months (range 4·6–67·3). Median length of follow‐up from time of PCL diagnosis was 11 months (range 0·2–103·0) in the pPCL and 0·9 months (range 0·1–25·2) in the sPCL group, which was statistically significant (P = 0·001). Of the group of pPCL patients, five are still alive and undergoing treatment; 28 patients died.
Median OS was 17·6 months (range 6·7 – not reached) in pPCL and 0·9 months (range 0·4–4·0) in sPCL, (P < 0·001); Figure 1. Only two patients were alive 12 months after sPCL diagnosis.
Fig 1.
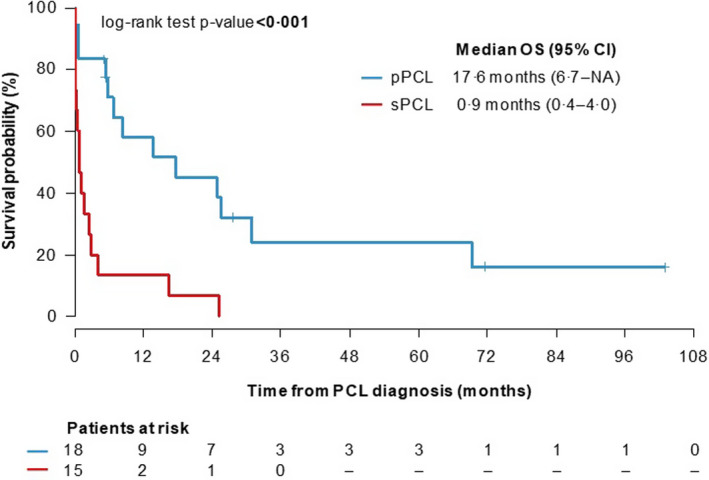
Overall survival (OS) of plasma cell leukaemia (PCL) patients from PCL diagnosis. [Colour figure can be viewed at wileyonlinelibrary.com]
Flow cytometric analysis
In this study, the quantification of PCs and phenotypic profile analyses of pPCL and sPCL were done in PB and BM. Although the number of analysed PCL patients was not sufficient for statistical analysis in some cases (expression of CD44, CD81, CD200 and nestin), there were some interesting findings, presented in Tables II and III.
Table II.
Relative expression (%) of analysed antigens on CD38+CD138+ PCs and their differences between pPCL and sPCL.
| Antigen | pPCL (N = 18) | sPCL (N = 15) | P * | ||
|---|---|---|---|---|---|
| N | Median % (min–max) | N | Median % (min–max) | ||
| CD19+ (PB) | N = 18 | 0·0 (0·0–94·1) | N = 14 | 0·0 (0·0–0·3) | 0·151 |
| CD19+ (BM) | N = 17 | 0·1 (0·0–92·6) | N = 13 | 0·0 (0·0–0·2) | 0·146 |
| CD56+ (PB) | N = 18 | 65·1 (0·0–99·9) | N = 14 | 46·7 (0·0–99·8) | 0·888 |
| CD56+ (BM) | N = 17 | 97·6 (0·0–99·9) | N = 13 | 93·7 (0·0–99·8) | 0·642 |
| CD20+ (PB) | N = 18 | 1·4 (0·0–94·4) | N = 14 | 0·1 (0·0–14·1) | 0·091 |
| CD20+ (BM) | N = 17 | 1·4 (0·0–92·8) | N = 12 | 0·1 (0·0–0·2) | 0·010 |
| CD27+ (PB) | N = 17 | 0·7 (0·0–50·6) | N = 14 | 0·4 (0·0–79·0) | 0·776 |
| CD27+ (BM) | N = 17 | 0·6 (0·0–81·4) | N = 12 | 0·3 (0·0–62·1) | 0·398 |
| CD28+ (PB) | N = 18 | 0·1 (0·0–99·6) | N = 13 | 0·4 (0·0–84·8) | 0·626 |
| CD28+ (BM) | N = 17 | 0·1 (0·0–99·9) | N = 10 | 0·9 (0·0–89·0) | 0·431 |
| CD117+ (PB) | N = 18 | 0·1 (0·0–96·6) | N = 13 | 0·2 (0·0–97·6) | 0·219 |
| CD117+ (BM) | N = 17 | 0·0 (0·0–95·0) | N = 10 | 0·1 (0·0–95·9) | 0·266 |
| CD44+ (PB) | N = 14 | 98·6 (2·3–100·0) | N = 8 | 99·9 (83·5–100·0) | 0·202 |
| CD44+ (BM) | N = 13 | 85·4 (13·4–100·0) | N = 5 | 98·2 (95·8–100·0) | 0·166 |
| CD81+ (PB) | N = 12 | 1·6 (0·2–99·5) | N = 9 | 0·7 (0·2–98·1) | 0·663 |
| CD81+ (BM) | N = 13 | 1·3 (0·2–99·1) | N = 7 | 3·3 (0·5–99·9) | 0·574 |
| CD200+ (PB) | N = 5 | 99·7 (0·0–99·9) | N = 5 | 51·5 (26·3–87·4) | 0·548 |
| CD200+ (BM) | N = 5 | 94·5 (0·1–99·9) | N = 2 | 48·2 (35·1–61·2) | 0·857 |
| nestin+ (PB) | N = 14 | 9·3 (0·0–99·1) | N = 8 | 32·0 (0·1–99·4) | 0·244 |
| nestin+ (BM) | N = 13 | 32.0 (0.0–99.1) | N = 6 | 32.9 (0.1–99.5) | 0.506 |
| a‐PC (BM) | N = 16 | 100.0 (99.8–100.0) | N = 12 | 100.0 (100–100.0) | 0.053 |
P‐value of Mann‐Whitney U test, P < 0·1 in bold. BM, bone marrow; PB, peripheral blood; PC, plasma cell; pPCL, primary plasma cell leukaemia; sPCL secondary plasma cell leukaemia; a‐PC, clonal PCs.
Table III.
Positivity of CD38+CD138+ PCs for analysed antigens in peripheral blood and bone marrow.
| Antigen | pPCL positivity % (N) | sPCL positivity % (N) |
|---|---|---|
| CD19+ (PB) | 11·1 (2/18) | 0 (0/14) |
| CD19+ (BM) | 11·8 (2/17) | 0 (0/13) |
| CD56+ (PB) | 61·1 (11/18) | 57·1 (8/14) |
| CD56+ (BM) | 64·7 (11/17) | 69·2 (9/13) |
| CD20+ (PB) | 22·2 (4/18) | 0 (0/14) |
| CD20+ (BM) | 23·5 (4/17) | 0 (0/12) |
| CD27+ (PB) | 17·6 (3/17) | 14·2 (2/14) |
| CD27+ (BM) | 23·5 (4/17) | 16·7 (2/12) |
| CD28+ (PB) | 22·2 (4/18) | 30·8 (4/13) |
| CD28+ (BM) | 23·5 (4/17) | 20 (2/10) |
| CD117+ (PB) | 11·1 (2/18) | 15·4 (2/13) |
| CD117+ (BM) | 11·8 (2/17) | 30 (3/10) |
| CD44+ (PB) | 92·9 (13/14) | 100 (8/8) |
| CD44+ (BM) | 92·3 (12/13) | 100 (5/5) |
| CD81+ (PB) | 25 (3/12) | 22·2 (2/9) |
| CD81+ (BM) | 23·1 (3/13) | 28·6 (2/7) |
| CD200+ (PB) | 80 (4/5) | 100 (5/5) |
| CD200+ (BM) | 80 (4/5) | 100 (2/2) |
| nestin+ (PB) | 42·9 (6/14) | 50 (4/8) |
| nestin+ (BM) | 53·8 (7/13) | 66·7 (4/6) |
Data are presented as % (N). BM, bone marrow; PB, peripheral blood; pPCL, primary plasma cell leukaemia; sPCL secondary plasma cell leukaemia.
According to FC, the median relative number of cPCs in PB was lower in pPCL than in sPCL patients [25·1% (range 17·2–79·0) vs. 30·2% (range 17·4–80·3)]. The number of PCs in BM was significantly lower in pPCL than in sPCL [39·2% (range 24·6–78·3) vs. 64·9% (range 20·3–81·6); P = 0·02]. However, the possibility of BM contamination with PB should be considered.
Further, a statistically significant difference in the relative number of PCs was found when results of FC analysis and PB smear assessment (Wright‐Giemsa‐stained samples) of analysed PCL patients were compared. All patients were analysed using both methods, except for three cases of sPCL that did not undergo morphological evaluation. The morphological determination of PB smears was performed on 200 cells and verified by at least two individuals (External peoples). FC analysis was performed with a sensitivity 10−5, and panels of combination of MoAb are listed in Table SVII. The median of cPCs as assessed by FC was 26·7% (range 17·2–80·3), whereas PB smear assessment revealed only 13·5% (range 0–63·5) of cPCs (P = 0·02). Surprisingly, two patients were morphologically classified as cPC negative because suspect cPCs were incorrectly classified as atypical lymphocytes, blasts or undifferentiated cells.
In pPCL, the phenotypic profile of PB cPCs correlated with BM PCs in all cases, although there were small differences, as mentioned below (Tables II and III). As medians of expression of some markers were generally low, a description of the phenotype using positivity terms was more useful in selected cases. There were two cases of CD19+ pPCL. In the first case, CD19 was expressed on the whole PC population of IgM positive pPCL case; in the second IgG positive case, all PCs were CD56+ and a some of them co‐expressed CD19 and CD27. Although 22·2% of pPCL cases were positive for CD20, the median of CD20 expression was very low in PB and BM. Interestingly, co‐expression of CD28 and CD56 with no expression of CD117 was observed in 75% (3/4) of CD20+ cases. Median of CD56 expression was decreased in pPCL PB when compared with BM, but positivity was the same in both PB and BM. Also, the median of cytoplasmic nestin expression was lower in PB than BM in pPCL, and the decrease under the positivity cut‐off was detected in PB of one of six BM positive cases. Positivity for CD81 was found in a quarter of pPCL patients with low median of expression in PB and BM. On the other hand, almost all pPCL expressed CD44 with a high median of expression in PB and BM. Also, most of the patients were CD200 positive in PB and BM with a high median of expression.
The phenotypic profile of sPCL was also similar in PB when compared to BM (Table II and III). No CD19 and CD20 positivity was detected in sPCL. All sPCL cases were positive for CD44 with a median of almost 100% in BM and with slightly lower median in PB. All sPCL cases were CD200 positive with a median expression of about 50%. The median CD56 expression was again lower in PB when compared to BM (46·7% vs. 93·7%). Although CD56 was decreased in three of nine BM positive cases, there was a change of positivity only in one case (shift from positive BM to negative PB). The change of positivity in PB was also found for CD117 in one of three BM positive cases (shift to negativity in PB), while the median of expression was not affected, thus the majority of sPCL patients was negative for CD117.
Differences in expression and positivity in pPCL versus sPCL are presented in Figs 2, 3, 4 and Figure S1.
Fig 2.
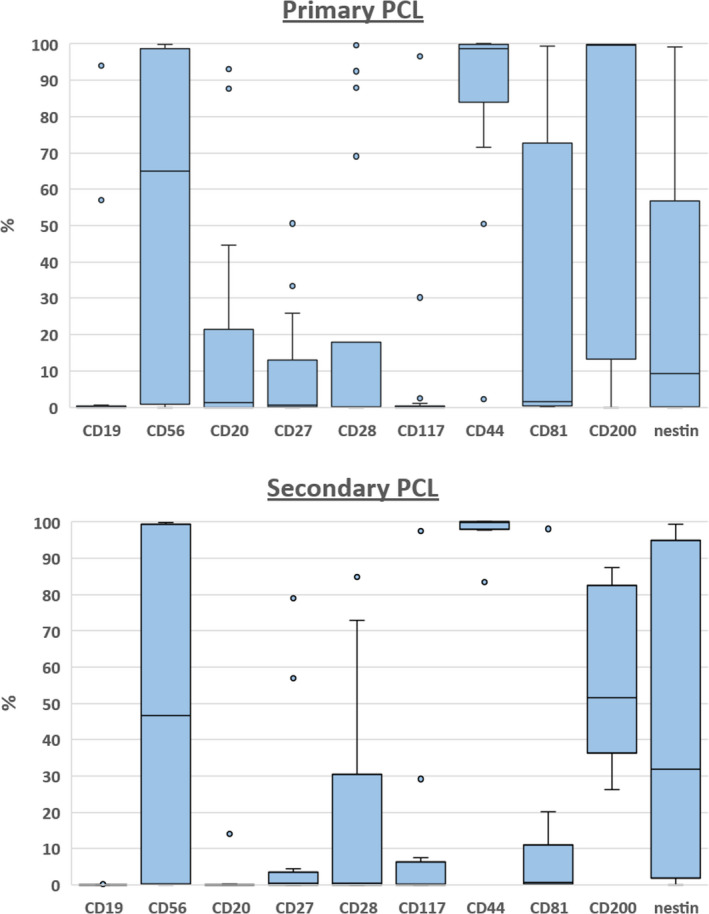
Relative expression (%) of analysed antigens on circulating plasma cells (cPCs) in peripheral blood of primary plasma cell leukaemia and secondary plasma cell leukaemia. [Colour figure can be viewed at wileyonlinelibrary.com]
Fig 3.
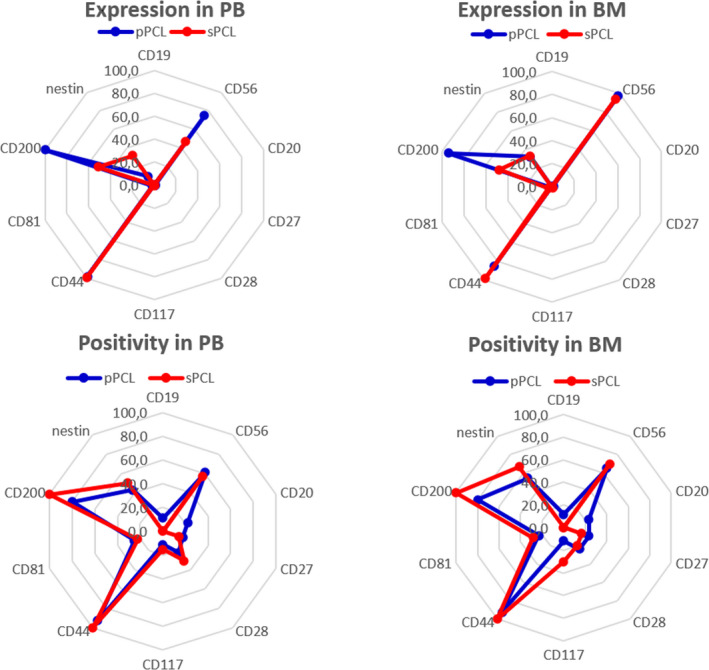
Differences in expression and positivity of analysed markers in peripheral blood (PB) and bone marrow (BM) of primary plasma cell leukaemia (pPCL) (blue) and secondary plasma cell leukaemia (sPCL) (red). [Colour figure can be viewed at wileyonlinelibrary.com]
Fig 4.
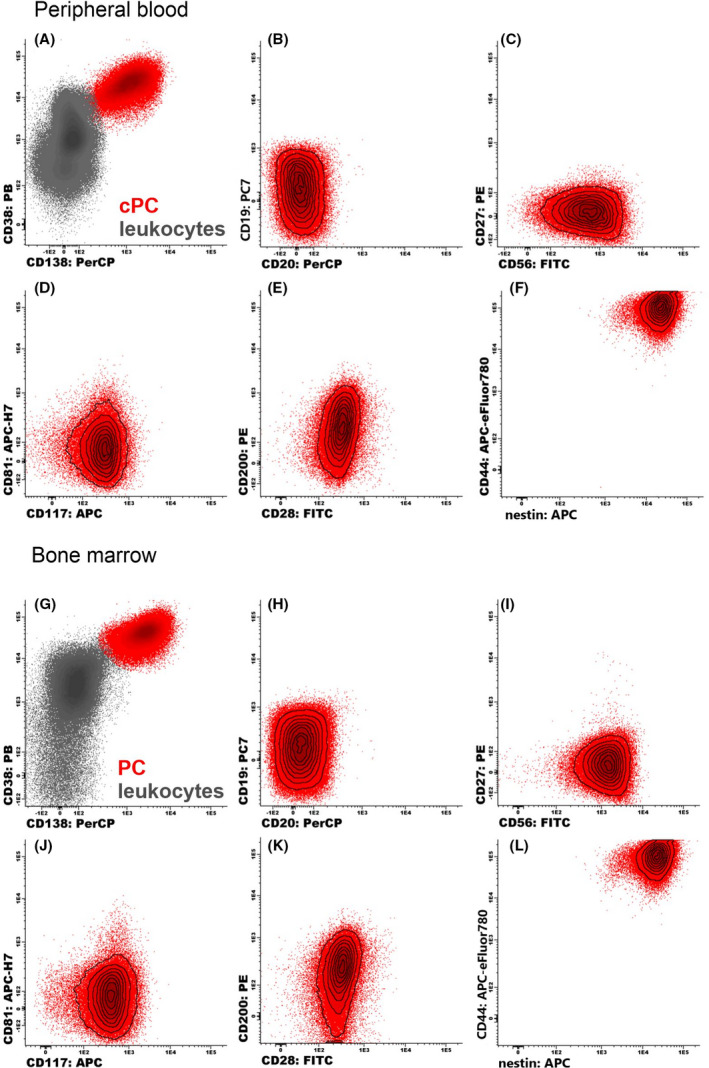
Flow cytometry identification of circulating plasma cells (cPCs) and plasma cells (PCs) (red dots; A and G) and their phenotypic analysis in (B‐F) peripheral blood and (H‐L) bone marrow of a single patient. [Colour figure can be viewed at wileyonlinelibrary.com]
Generally, the phenotypic profile of pPCL was very close to sPCL in PB and BM, although there were some alterations. Unlike pPCL, none of the sPCL cases expressed CD19 and CD20 (Tables II and III). A significantly different expression of CD20 was found in pPCL because of four positive cases, although medians of expression were low in both types of PCLs. Low level of expression and similar positivity of CD27, CD28, CD81 and CD117 were found in PB and BM of both types of PCL. Expression of CD56 was insignificantly decreased in PB when compared to BM, which was more obvious in PB of sPCL than pPCL, but expression in BM was similar. A lower median of CD200 expression was found in sPCL when compared to pPCL in PB as well as in BM, but CD200 was positive in the entire sPCL group. Although positivity of nestin was similar for both types of PCL in PB and BM, a lower median of expression was found in PB of pPCL, but not in BM. Only clonal a‐PCs were detected in the BM of both types of PCL.
Risk factors associated with survival
Risk factors significantly associated with OS of both PCLs are shown in Table SVI. Patients with a thrombocyte count below 70 × 109/l had a 3·9 times higher risk of death in comparison to patients with a higher thrombocyte count (Fig 5A). Moreover, the total lack of CD20 on PC was associated with a higher risk of death. Patients with ≤5% CD20+ PCs in PB had shorter OS when compared to cases with >5% CD20+ PCs (3·4 vs. 47·4 months; P = 0·044) as well as for patients with ≤4% CD20+ PCs in BM when compared to cases with >4% CD20+ PCs (2·9 vs. 47·4 months; P = 0·007) (Fig 5B,C). Nevertheless, high expression of CD20 on PCs was not connected to OS. Patients with Eastern Cooperative Oncology Group performance status (ECOG) 2–3 had a three times higher risk of death compared to patients with ECOG 0–1 (Fig 5D). Patients with M‐protein type other than IgG, IgA and light chain (LC) had only shorter OS (Fig 5E).
Fig 5.
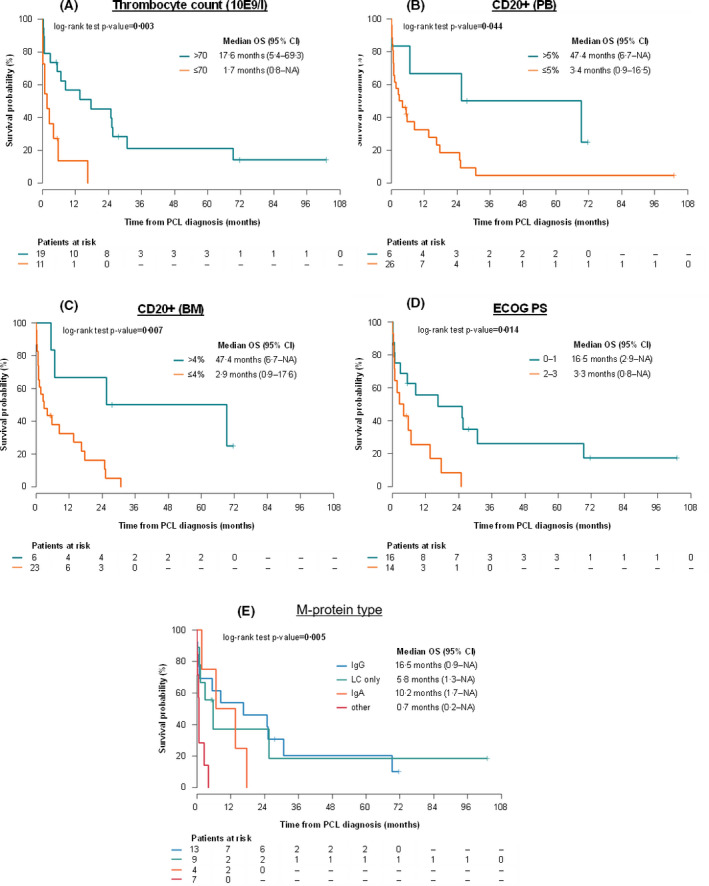
Overall survival from plasma cell leukaemia (PCL) diagnosis according to (A) thrombocyte count; (B, C) percentage of CD20+ PCs in peripheral blood PB and bone marrow (BM); (D) Eastern Cooperative Oncology Group ECOG; and (E) according to M‐protein type. [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
This investigation was focused on the comparison of cPCs and PCs phenotypic profile in PCL and its correlation with clinical characteristics. FC is a dynamically evolving method that is nowadays routinely used in analyses of monoclonal gammopathy (MG) cases. Despite the very rare presence of PCL, in the past 11 years, 33 PCL cases were diagnosed at one institution. Patients were identified either by FC analysis alone or by combination of FC and morphological assessment. Thus, although the value of cPC in some patients did not meet the given morphological criterion of the disease, the diagnosis of PCL was made by the physician, based on complete clinical information about the patient.
Unexpectedly, comparison of different approaches showed significant discrepancies in the number of cPCs between these methods. Routine morphology can identify a significantly lower number of cPCs in PB than sensitive and robust FC in whole PB. Given the unambiguous identification by FC, this finding is alarming as some patients would not be identified with PCL based only on morphology assessment. Unfortunately, two patients were even diagnosed as cPC negative as these suspect cells were incorrectly classified into the categories of atypical lymphocytes, blasts or undifferentiated cells.
Although FC is not a diagnostic method in MGs, mainly because of the underestimation of number of PCs in BM, it seems that there is a completely different situation in PB; FC is more accurate in cPC assessment than morphology. Similar results were reported by Evans et al., who recommended incorporation of multiparametric FC into the conventional morphological assessment of a PB smear to diagnose pPCL. 29 Of note, their study analysed the mononuclear fraction of PB, and therefore, some discrepancies in the number of cPCs could not be avoided in these manipulated samples. However, this favours FC as a complementary or even a primary diagnostic method in PCL that should be changed in diagnostic criteria. It is known that exact enumeration of cPCs by conventional cell count could be complicated by lower sensitivity; 30 on contrary, morphology assessment of PB smear by subjectivity. 31 Also, evaluation experience is needed as PCL with atypical and/or ambiguous morphology was documented as well. 32 FC, a progressively evolving method, allows clear identification of PCs and, thus, overcomes the limitations of morphology assessment mentioned above. Moreover, clonality verification of cPCs is possible only when FC is done. 29 As the number of cPCs can dramatically increase over a few days, the exact and timely quantification of cPCs by FC was proven to be a valuable method of eliminating the risk of missing PCL diagnosis at a specific moment. The question remains if the PCL cut‐off value should not be decreased from 20% to 5% cPCs as patients with >5% cPCs < 20% seem clinically very similar to PCL. 30
Because of the biological similarities of MM and PCL, the phenotypic profile of PCL was often compared to MM. Although the typical phenotypic profile of MM is known, 18 limited information about the PCL profile is available. There is no recent publication focused on the phenotype of PCLs, despite the fact that the phenotypic profile of cPCs has been analysed by a highly sensitive next generation flow (NGF) approach and applied to different MG cases. 23 , 33 Also, some of the analysed markers have not been sufficiently studied, especially their correlation with patients’ prognosis. There is also a possibility that different treatment regimens, which were applied in our sPCL group as well as many cycles of previously applied therapy, made it impossible to find an association of immunophenotypic profile with behaviour of PCL.
CD19 and CD56, are essential PC antigens, generally allowing discrimination between normal and abnormal PCs. 18 CD19+ PCs are usually normal and their verification by clonality assessment should be done in unclear cases; however, expression of CD19 on clonal PCs in MM results in a worse disease outcome. 27 None of the sPCL group expressed CD19 on PCs in this assessment, but two pPCL cases expressing CD19+ PC differed in production of monoclonal immunoglobulin (IgM vs. IgG), suggesting that data are not sufficient to draw any conclusion regarding prognosis of CD19+ cases.
Even though CD20 is not included in a group of essential markers in MGs, its expression on myelomatous cells is relevant. 16 In this assessment, CD20 was expressed only in pPCL with 22% of positive cases, similar to MM. However, higher expression of CD20 in 50% of pPCL (50% in pPCL vs. 17% in MM) has previously been published. 6 Interestingly, CD20+ cases in this study were almost (in 75% of cases) CD56+ and CD28+CD117−. It was previously published that the CD28+CD117− phenotype stratified MM patients into a high‐risk group, 27 but 50% of analysed CD20+CD56+CD28+CD117− patients reached very good partial response and are still alive. Further, six of 14 CD20− cases reached a minimal response and/or progression of disease. Thus, at least in this study, lack of CD20 on PCs was associated with higher risk of death. Of course, the prognostic value of phenotype depends on the treatment approach and usually is studied on a large uniformly treated cohort of patients, therefore, our results cannot simply be applied to any patient.
The neural cell adhesive molecule (NCAM, CD56) is expressed in 60–75% of MM patients; its absence is probably associated with shorter OS, but divergent information had been published. 34 , 35 In concordance with the fact that CD56 is a molecule involved in cell‐cell or cell‐matrix adhesion, the lack or weak expression of CD56 in MM should not only predict PC migration to PB, but also, possibly, their contribution to this process or even development of sPCL. 19 , 36 Although some studies showed that PCLs do not usually express CD56, neither in PB nor BM, 19 , 36 positivity of CD56 was detected in more than half of the pPCL and sPCL cases (61·1 and 57·1% of PB respectively). Similarly, a Polish study found CD56 positivity in 45% of pPCL and 75% of sPCL cases. 20 As our group published previously, expression of CD56 was also not changed in different MM cases (extramedullary vs. intramedullary relapses), so its loss probably should not be considered as a marker of extramedullary spread. 37 A larger decrease in expression of CD56 in PB than in BM is not easily explained. Rawstron et al. revealed the same composite phenotype in paired samples of PB and BM in MM, with significantly lower expression of CD56 and CD138 in PB. 38 The question of a decrease of CD56 expression in PB has not been solved yet, although one study reported that CD56 is often downregulated during cell migration and re‐expressed after cells reach a target tissue or organ. 39 Therefore, some MM cells seem to develop a similar expression regulation strategy. 40
Lack of CD27 expression is generally a sign of poorer survival in MM; association with aggressive clinical course was found in both MM and PCL. 36 , 41 Despite the low level of positivity of CD27 on PCs in this assessment, there was no association of CD27 with OS.
Positivity of CD28 reached 22·2% of pPCL and 30·8% of sPCL cases in PB, which is less when compared to newly diagnosed MM (36% cases). 27 On the other hand, Pellat‐Deceunynck et al. demonstrated CD28 expression in 92% of sPCL cases, suggesting that CD28 is able to discriminate sPCL from pPCL. 19 The same authors found CD28 expressed in the whole group of sPCL patients and 93% of extramedullary relapses, which is in concordance with the emergence of CD28+ myeloma cells with tumour expansion and treatment failure. 18 , 24 Although CD28 is mentioned as a negative prognostic marker in MM, this was not proved in this study, probably because of the low positivity level of this marker, which interacts with the BM microenvironment.
Positivity of CD81 in both PCLs was generally lower than in previous studies of MM, where positivity of this marker correlated with shorter progression free survival (PFS) and OS. 42 This is consistent with Paiva et al., who showed that CD81 represents a novel adverse prognostic marker in MM. 43 Confirmation of that fact was not possible in our study.
Similarly, CD117+ PCs were found only in a small number of both PCL patients, which is in contrast to a previous study of PCL 20 and MM. 27 Positivity for CD117 has been described to be associated with longer PFS and OS in MM, 27 , 35 , 42 but this information is not available for any PCL study. Therefore, the low number of identified CD117+ PCLs did not allow us to prove any advantage of CD117 positivity for these patients.
As for CD200, so far, several controversial studies have suggested that it is expressed in more than 70% of MM patients and seems to be mostly useful for monitoring MM for detection of clonal PCs. 44 Despite a low number of analysed PCLs, the heterogeneous expression of the immunosuppressive molecule CD200 in sPCL with a decreased median of expression than in pPCL, we found should correspond to more aggressive behaviour and worse clinical outcome of sPCL, similarly to MM. 45
Standard molecule CD44 analysed in this study showed high and homogenous expression in both PCLs, as also published by a Polish group. 20 Thus, it seems that this molecule has probably no impact in PCLs, although its negativity was detected in one pPCL case. Unfortunately, there is no information about significance of standard CD44 molecule loss in MM, as only CD44 variant isoforms were analysed. 46 On the other hand, overexpression of CD44 has been reported to be a putative biomarker of sensitivity to lenalidomide. 47 Further, extramedullary disease in MM expresses more CD44 than MM without extramedullary involvement; 37 thus, CD44 should be related to possible extramedullary involvement in MGs.
There is not much information about the relevance of cytoplasmic nestin (the neural stem cell protein) in MGs. Nestin was initially described as a marker of neural stem cells. It is also expressed in various types of malignancies, including MGs. 48 , 49 High nestin levels in MM patients were strongly associated with the presence of 1q21 gain and seemed to be a predictor of worse response to therapy; its higher expression was observed in disease progression. 49 , 50 Increased nestin expression was also found in MM with extramedullary involvement. 37 Nestin was described only on a limited number of PCLs with a low level of expression in pPCL. 50 Here we have presented the largest group of patients with PCL, to date, in which nestin has been analysed. This assessment showed similar positivity of nestin in both types of PCL, although a heterogenic pattern with a lower median of expression was mostly detected in the PB of pPCL patients. Despite this, analysis of more cases is needed to prove its significance for prognosis of PCLs, which remains unclear at this moment.
It seems that FC is an indispensable method for PCL diagnosis as well as for other PCDs. 15 , 16 , 31 , 33 Nowadays, standardisation of FC analyses by the Euroflow PCD protocol is available and recommended, not only for clinical studies. 51 As the second generation of the Euroflow PCD protocol contains a restricted number of markers, combination of backbone markers with other MoAbs in Euroflow settings is possible, to cover a wide phenotypic PC profile in specific cases, such as PCL. This can further elucidate its behaviour and the clinical impact of the PCL immunophenotypic profile. 33 , 52
Since sPCL evolves from existing MM, significantly longer median OS of pPCL than sPCL was expected. Other groups also found similar data of longer median OS in pPCL than sPCL. 7 , 8 , 12 However, two patients in our sPCL cohort were alive at 12 months after diagnosis suggesting that the introduction of new drugs (bortezomib, lenalidomide, thalidomide) and autologous stem cell transplant (ASCT) as these patients underwent ASCT three times: 1st before progression to sPCL, 2nd and 3rd after diagnosis of sPCL can prolong survival of patients. Similarly, Katodritou et al. published that a bortezomib‐based regimen prolonged OS of sPCL from 2 to 7 months. 12
The median age of pPCL at the time of diagnosis was 66 years, which is inconsistent with results of some studies, where pPCL patients were almost a decade younger. 7 , 53 , 54 Despite this, pPCL patients were younger than sPCL patients at diagnosis, which corresponds to the biology of PCL and is in concordance with previously published results. 6 , 7 , 8 , 20 , 54 , 55
Thrombocytopenia often accompanies both types of PCLs. Finding a more decreased thrombocytes count in sPCL was probably caused by the biology of the disease and as a consequence of previous treatment.
Interestingly, sPCL patients showed more frequent expression of the lambda light chain than pPCL. It is a unique result, not previously published, but it could also be randomly generated.
Finally, the unique role of FC in PCL diagnostics was identified. Thus, confirmation of all PCL samples should be done not by morphological assessment alone but both methods should be utilised. Differences in the phenotypic profile of both PCLs included only CD20; its lack is also connected to shorter survival. On the other hand, missing CD56 was rare. Expression of selected antigens should be further analysed on a large cohort of patients. Analysis of markers is important in the analysis of monoclonal gammopathies development as some antigens (CD44, nestin etc.) may contribute to identification of patients with later extramedullary involvement.
Conclusions
PCL represents a very rare and aggressive form of MG with an adverse prognosis. This investigation proved FC as a unique and sensitive diagnostic tool for accurate cPCs enumeration, even better than morphology assessment, which is crucial for preventing late diagnosis, especially in the case of PCL. Low level of expression and similar positivity of CD27, CD28, CD81, and CD117 were found in both PCLs. Decrease of CD200 expression was found only in sPCL cases and cytoplasmic nestin was expressed in more than 50% of both PCLs, both with an unknown impact on patient’s prognosis. However, the lack of CD20+ PC was associated with higher risk of death. We have also shown that CD56 expression is common in both PCL groups. Although the phenotypic profile of both PCL groups did not differ too much, comparison of PCL with other MGs may be important for detection of antigens associated with extramedullary spread, high risk of progression and shortened survival. But still, routine use of FC can be beneficial, not only for newly diagnosed MM patients, but also for early detection of leukaemic transformation of MM patients.
Funding
This investigation was supported by grant AZV NV18‐03‐00203.
Author contributions
Conceptualization, LR, RH, SS; methodology, RB, LR; investigation, RB, TJ, RK, PP, PV; data curation, MS, MA, IB, ZK, MP, LP; data analysis, LB, JJ; writing: original draft preparation, RB, LR, SS; writing: review and editing, RB, LR, TJ, SS. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
Table S1. Basic characteristics at PCL diagnosis – continuation.
Table S2. First line therapy in patients after PCL diagnosis.
Table S3. Following therapy of pPCL patients (N = 18).
Table S4. Following therapy in sPCL patients (N = 15).
Table S5. Previous therapy in any treatment line before PCL diagnosis.
Table S6. Clinical and flow cytometry parameters significantly associated with overall survival.
Table S7. Basic 8‐colour panel used in PCL patients*.
Fig S1. Visualization of positivity and negativity of cPC for a given antigen analysed in peripheral blood of different PCLs.
Acknowledgments
The authors wish to thank all the patients and their caregivers for participating in this investigation.
Data Availability Statement
Flowcytometric data are available on request from the corresponding author.
References
- 1. Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos M‐V, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–48. [DOI] [PubMed] [Google Scholar]
- 2. Kyle RA, Maldonado JE, Bayrd ED. Plasma cell leukemia. Report on 17 cases. Arch Intern Med. 1974;133(5):813–8. [DOI] [PubMed] [Google Scholar]
- 3. Noel P, Kyle RA. Plasma cell leukemia: an evaluation of response to therapy. Am J Med. 1987;83(6):1062–8. [DOI] [PubMed] [Google Scholar]
- 4. Fernández de Larrea C, Kyle RA, Durie BGM, Ludwig H, Usmani S, Vesole DH, et al. Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia. 2013;27(4):780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zapletalova M, Krejci D, Jarkovský J, Muzik J, Dusek L, Pour L. Epidemiology of plasma cell leukemia in the Czech Republic. Klin Onkol. 2019;32(1):47–51. [DOI] [PubMed] [Google Scholar]
- 6. García‐Sanz R, Orfão A, González M, Tabernero MD, Bladé J, Moro MJ, et al. Primary plasma cell leukemia: clinical, immunophenotypic, DNA ploidy, and cytogenetic characteristics. Blood. 1999;93(3):1032–7. [PubMed] [Google Scholar]
- 7. Tiedemann RE, Gonzalez‐Paz N, Kyle RA, Santana‐Davila R, Price‐Troska T, Van Wier SA, et al. Genetic aberrations and survival in plasma cell leukemia. Leukemia. 2008;22(5):1044–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jurczyszyn A, Castillo JJ, Avivi I, Czepiel J, Davila J, Vij R, et al. Secondary plasma cell leukemia: a multicenter retrospective study of 101 patients. Leuk Lymphoma. 2019;60(1):118–23. [DOI] [PubMed] [Google Scholar]
- 9. Sant M, Allemani C, Tereanu C, De Angelis R, Capocaccia R, Visser O, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. 2010;116(19):3724–34. [DOI] [PubMed] [Google Scholar]
- 10. Bladé J, Kyle RA. Nonsecretory myeloma, immunoglobulin D myeloma, and plasma cell leukemia. Hematol Oncol Clin North Am. 1999;13(6):1259–72. [DOI] [PubMed] [Google Scholar]
- 11. Mina R, D’Agostino M, Cerrato C, Gay F, Palumbo A. Plasma cell leukemia: update on biology and therapy. Leuk Lymphoma. 2017;58(7):1538–47. [DOI] [PubMed] [Google Scholar]
- 12. Katodritou E, Terpos E, Kelaidi C, Kotsopoulou M, Delimpasi S, Kyrtsonis M‐C, et al. Treatment with bortezomib‐based regimens improves overall response and predicts for survival in patients with primary or secondary plasma cell leukemia: analysis of the Greek myeloma study group. Am J Hematol. 2014;89(2):145–50. [DOI] [PubMed] [Google Scholar]
- 13. Jelinek T, Kryukov F, Rihova L, Hajek R. Plasma cell leukemia: from biology to treatment. Eur J Haematol. 2015;95(1):16–26. [DOI] [PubMed] [Google Scholar]
- 14. Musto P, Statuto T, Valvano L, Grieco V, Nozza F, Vona G, et al. An update on biology, diagnosis and treatment of primary plasma cell leukemia. Expert Rev Hematol. 2019;12(4):245–53. [DOI] [PubMed] [Google Scholar]
- 15. Paiva B, Almeida J, Pérez‐Andrés M, Mateo G, López A, Rasillo A, et al. Utility of flow cytometry immunophenotyping in multiple myeloma and other clonal plasma cell‐related disorders. Cytometry B Clin Cytom. 2010;78(4):239–52. [DOI] [PubMed] [Google Scholar]
- 16. Jelinek T, Bezdekova R, Zatopkova M, Burgos L, Simicek M, Sevcikova T, et al. Current applications of multiparameter flow cytometry in plasma cell disorders. Blood Cancer J. 2017;7(10):e617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caraux A, Klein B, Paiva B, Bret C, Schmitz A, Fuhler GM, et al. Circulating human B and plasma cells. Age‐associated changes in counts and detailed characterization of circulating normal CD138‐ and CD138+ plasma cells. Haematologica. 2010;95(6):1016–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rawstron AC, Orfao A, Beksac M, Bezdickova L, Brooimans RA, Bumbea H, et al. Report of the European Myeloma Network on multiparametric flow cytometry in multiple myeloma and related disorders. Haematologica. 2008;93(3):431–8. [DOI] [PubMed] [Google Scholar]
- 19. Pellat‐Deceunynck C, Barillé S, Jego G, Puthier D, Robillard N, Pineau D, et al. The absence of CD56 (NCAM) on malignant plasma cells is a hallmark of plasma cell leukemia and of a special subset of multiple myeloma. Leukemia. 1998;12(12):1977–82. [DOI] [PubMed] [Google Scholar]
- 20. Kraj M, Kopeć‐Szlęzak J, Pogłód R, Kruk B. Flow cytometric immunophenotypic characteristics of 36 cases of plasma cell leukemia. Leuk Res. 2011;35(2):169–76. [DOI] [PubMed] [Google Scholar]
- 21. Říhová L, Bezdekova R, Všianská P, Kučerová P, Suská R, Penka M, et al. Does characteristic phenotype for plasma cell leukaemia exist? Haematologica 2014; 99(s1):98, abstract n.335.
- 22. Kumar S. Multiple myeloma – current issues and controversies. Cancer Treat Rev. 2010;36:S3–11. [DOI] [PubMed] [Google Scholar]
- 23. Sanoja‐Flores L, Flores‐Montero J, Puig N, Contreras‐Sanfeliciano T, Pontes R, Corral‐Mateos A, et al. Blood monitoring of circulating tumor plasma cells by next generation flow in multiple myeloma after therapy. Blood. 2019;134(24):2218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gavriatopoulou M, Musto P, Caers JO, Merlini G, Kastritis E, van de Donk N, et al. European myeloma network recommendations on diagnosis and management of patients with rare plasma cell dyscrasias. Leukemia. 2018;32(9):1883–98. [DOI] [PubMed] [Google Scholar]
- 25. Kraj M, Sokołowska U, Kopeć‐Szlęzak J, Pogłód R, Kruk B, Woźniak J, et al. Clinicopathological correlates of plasma cell CD56 (NCAM) expression in multiple myeloma. Leuk Lymphoma. 2008;49(2):298–305. [DOI] [PubMed] [Google Scholar]
- 26. Robillard N, Jego G, Pellat‐Deceunynck C, Pineau D, Puthier D, Mellerin MP, et al. CD28, a marker associated with tumoral expansion in multiple myeloma. Clin Cancer Res. 1998;4(6):1521–6. [PubMed] [Google Scholar]
- 27. Mateo G, Montalbán MA, Vidriales M‐B, Lahuerta JJ, Mateos MV, Gutiérrez N, et al. Prognostic value of immunophenotyping in multiple myeloma: a study by the PETHEMA/GEM cooperative study groups on patients uniformly treated with high‐dose therapy. J Clin Oncol. 2008;26(16):2737–44. [DOI] [PubMed] [Google Scholar]
- 28. Rihova L, Raja KRM, Leite LAC, Vsianska P, Hajek R. Immunophenotyping in Multiple Myeloma and Others Monoclonal Gammopathies. 2013 [cited 2017 Jun 27]. Available from: http://www.intechopen.com/books/multiple‐myeloma‐a‐quick‐reflection‐on‐the‐fast‐progress/immunophenotyping‐in‐multiple‐myeloma‐and‐others‐monoclonal‐gammopathies.
- 29. Evans LA, Jevremovic D, Nandakumar B, Dispenzieri A, Buadi FK, Dingli D, et al. Utilizing multiparametric flow cytometry in the diagnosis of patients with primary plasma cell leukemia. Am J Hematol. 2020;95(6):637–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ravi P, Kumar SK, Roeker L, Gonsalves W, Buadi F, Lacy MQ, et al. Revised diagnostic criteria for plasma cell leukemia: results of a Mayo Clinic study with comparison of outcomes to multiple myeloma. Blood Cancer J. 2018;8(12):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gounari E, Tsavdaridou V, Koletsa T, Nikolaidou A, Kaiafa G, Papaioannou M, et al. Utility of hematology analyzer and flow cytometry in timely and correct detection of circulating plasma cells: report of three cases. Cytometry B Clin Cytom. 2016;90(6):531–7. [DOI] [PubMed] [Google Scholar]
- 32. Loureiro AD, Gonçalves MV, Ikoma MRV, Silva MRR, Colleoni GWB, de Chauffaille M, et al. Plasma cell leukemia with t(11;14)(q13;q32) simulating lymphoplasmacytic lymphoma ‐ a diagnostic challenge solved by flow cytometry. Rev Bras Hematol E Hemoter. 2017;39(1):66–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sanoja‐Flores L, Flores‐Montero J, Garcés JJ, Paiva B, Puig N, García‐Mateo A, et al. Next generation flow for minimally‐invasive blood characterization of MGUS and multiple myeloma at diagnosis based on circulating tumor plasma cells (CTPC). Blood Cancer J. 2018;8(12):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hundemer M, Klein U, Hose D, Raab M‐S, Cremer FW, Jauch A, et al. Lack of CD56 expression on myeloma cells is not a marker for poor prognosis in patients treated by high‐dose chemotherapy and is associated with translocation t(11;14). Bone Marrow Transplant. 2007;40(11):1033–7. [DOI] [PubMed] [Google Scholar]
- 35. Pan Y, Wang H, Tao Q, Zhang C, Yang D, Qin H, et al. Absence of both CD56 and CD117 expression on malignant plasma cells is related with a poor prognosis in patients with newly diagnosed multiple myeloma. Leuk Res. 2016;40:77–82. [DOI] [PubMed] [Google Scholar]
- 36. Morgan TK, Zhao S, Chang KL, Haddix TL, Domanay E, Cornbleet PJ, et al. Low CD27 expression in plasma cell dyscrasias correlates with high‐risk disease: an immunohistochemical analysis. Am J Clin Pathol. 2006;126(4):545–51. [DOI] [PubMed] [Google Scholar]
- 37. Rihova L, Vsianska P, Bezdekova R, Adam Z, Penka M, Jelinek T, et al. Identification of phenotype profile related to the extramedullary involvement in multiple myeloma relapse. Blood. 2016;128(22):5653. [Google Scholar]
- 38. Rawstron AC, Owen RG, Davies FE, Johnson RJ, Jones RA, Richards SJ, et al. Circulating plasma cells in multiple myeloma: characterization and correlation with disease stage. Br J Haematol. 1997;97(1):46–55. [DOI] [PubMed] [Google Scholar]
- 39. Edvardsen K, Chen W, Rucklidge G, Walsh FS, Obrink B, Bock E. Transmembrane neural cell‐adhesion molecule (NCAM), but not glycosyl‐phosphatidylinositol‐anchored NCAM, down‐regulates secretion of matrix metalloproteinases. Proc Natl Acad Sci USA. 1993;90(24):11463–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pellat‐Deceunynck C, Bataille R, Robillard N, Harousseau JL, Rapp MJ, Juge‐ Morineau N, et al. Expression of CD28 and CD40 in human myeloma cells: a comparative study with normal plasma cells. Blood. 1994;84(8):2597–603. [PubMed] [Google Scholar]
- 41. Moreau P, Robillard N, Jego G, Pellat C, Gouill SL, Thoumi S, et al. Lack of CD27 in myeloma delineates different presentation and outcome. Br J Haematol. 2006;132(2):168–70. [DOI] [PubMed] [Google Scholar]
- 42. Chen F, Hu Y, Wang X, Fu S, Liu Z, Zhang J. Expression of CD81 and CD117 in plasma cell myeloma and the relationship to prognosis. Cancer Med. 2018;7(12):5920–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Paiva B, Gutiérrez N‐C, Chen X, Vídriales M‐B, Montalbán M‐Á, Rosiñol L, et al. Clinical significance of CD81 expression by clonal plasma cells in high‐risk smoldering and symptomatic multiple myeloma patients. Leukemia. 2012;26(8):1862–9. [DOI] [PubMed] [Google Scholar]
- 44. Douds JJ, Long DJ, Kim AS, Li S. Diagnostic and prognostic significance of CD200 expression and its stability in plasma cell myeloma. J Clin Pathol. 2014;67(9):792–6. [DOI] [PubMed] [Google Scholar]
- 45. Alapat D, Coviello‐Malle J, Owens R, Qu P, Barlogie B, Shaughnessy JD, et al. Diagnostic usefulness and prognostic impact of CD200 expression in lymphoid malignancies and plasma cell myeloma. Am J Clin Pathol. 2012;137(1):93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van Driel M, Günthert U, Stauder R, Joling P, Lokhorst HM, Bloem AC. CD44 isoforms distinguish between bone marrow plasma cells from normal individuals and patients with multiple myeloma at different stages of disease. Leukemia. 1998;12(11):1821–8. [DOI] [PubMed] [Google Scholar]
- 47. Bjorklund CC, Baladandayuthapani V, Lin HY, Jones RJ, Kuiatse I, Wang H, et al. Evidence of a role for CD44 and cell adhesion in mediating resistance to lenalidomide in multiple myeloma: therapeutic implications. Leukemia. 2014;28(2):373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Svachova H, Pour L, Sana J, Kovarova L, Raja KRM, Hajek R. Stem cell marker nestin is expressed in plasma cells of multiple myeloma patients. Leuk Res. 2011;35(8):1008–13. [DOI] [PubMed] [Google Scholar]
- 49. Bernal A, Arranz L. Nestin‐expressing progenitor cells: function, identity and therapeutic implications. Cell Mol Life Sci. 2018;75(12):2177–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Svachova H, Kryukov F, Kryukova E, Sevcikova S, Nemec P, Greslikova H, et al. Nestin expression throughout multistep pathogenesis of multiple myeloma. Br J Haematol. 2014;164(5):701–9. [DOI] [PubMed] [Google Scholar]
- 51. van Dongen JJM, Lhermitte L, Böttcher S, Almeida J, van der Velden VHJ, Flores‐Montero J, et al. EuroFlow antibody panels for standardized n‐dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012;26(9):1908–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tarín F, López‐Castaño F, García‐Hernández C, Beneit P, Sarmiento H, Manresa P, et al. Multiparameter flow cytometry identification of neoplastic subclones: a new biomarker in monoclonal gammopathy of undetermined significance and multiple myeloma. Acta Haematol. 2019;141(1):1–6. [DOI] [PubMed] [Google Scholar]
- 53. Dimopoulos MA, Palumbo A, Delasalle KB, Alexanian R. Primary plasma cell leukaemia. Br J Haematol. 1994;88(4):754–9. [DOI] [PubMed] [Google Scholar]
- 54. Ganzel C, Rouvio O, Avivi I, Magen H, Jarchowsky O, Herzog K, et al. Primary plasma cell leukemia in the era of novel agents for myeloma ‐ a multicenter retrospective analysis of outcome. Leuk Res. 2018;68:9–14. [DOI] [PubMed] [Google Scholar]
- 55. Gonsalves WI, Rajkumar SV, Go RS, Dispenzieri A, Gupta V, Singh PP, et al. Trends in survival of patients with primary plasma cell leukemia: a population‐based analysis. Blood. 2014;124(6):907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Basic characteristics at PCL diagnosis – continuation.
Table S2. First line therapy in patients after PCL diagnosis.
Table S3. Following therapy of pPCL patients (N = 18).
Table S4. Following therapy in sPCL patients (N = 15).
Table S5. Previous therapy in any treatment line before PCL diagnosis.
Table S6. Clinical and flow cytometry parameters significantly associated with overall survival.
Table S7. Basic 8‐colour panel used in PCL patients*.
Fig S1. Visualization of positivity and negativity of cPC for a given antigen analysed in peripheral blood of different PCLs.
Data Availability Statement
Flowcytometric data are available on request from the corresponding author.


