Summary
Being rooted in place, plants are faced with the challenge of responding to unfavourable local conditions. One such condition, heat stress, contributes massively to crop losses globally. Heatwaves are predicted to increase, and it is of vital importance to generate crops that are tolerant to not only heat stress but also to several other abiotic stresses (e.g. drought stress, salinity stress) to ensure that global food security is protected. A better understanding of the molecular mechanisms that underlie the temperature stress response in pollen will be a significant step towards developing effective breeding strategies for high and stable production in crop plants. While most studies have focused on the vegetative phase of plant growth to understand heat stress tolerance, it is the reproductive phase that requires more attention as it is more sensitive to elevated temperatures. Every phase of reproductive development is affected by environmental challenges, including pollen and ovule development, pollen tube growth, male–female cross‐talk, fertilization, and embryo development. In this review we summarize how pollen is affected by heat stress and the molecular mechanisms employed during the stress period, as revealed by classical and ‐omics experiments.
Keywords: heat stress (HS), heat stress response (HSR), multiomics, pollen development, thermotolerance
Introduction
Due to global warming, heatwaves are predicted to increase in many regions across the globe, posing a massive threat to agricultural security. Elevated temperatures, whether transient or constant, have an adverse impact on crop yields. Such conditions bring about changes in plant morphology, physiology, and biochemistry, which in turn negatively impact plant growth and development (Begcy & Dresselhaus, 2018). Extreme heatwaves are not solely responsible for these adverse impacts, as it has been shown that even mild temperature fluctuations have an impact. For example, it was found that for every 1°C increase in growing‐season minimum temperature, rice grain yields declined by 10% (Peng et al., 2004). Similarly, for every 1°C increase in temperature above 21°C in the day and 16°C at night, wheat (Triticum aestivum) yields declined by 5% (Tashiro & Wardlaw, 1989), while every day spent above 30°C resulted in a 1% decline in maize yields (Lobell et al., 2011).
Increased temperatures can often have a detrimental effect on plant sexual reproduction, which can lead to a reduction in the fertility of many species. Sexual reproduction in angiosperms comprises three phases: gametophyte development, the progamic phase, and embryo and seed development (from zygote to seed). The male gametophyte (pollen) plays a key role in plant reproduction and crop productivity, through the formation and delivery of male sperm cells to the female gametophyte for double fertilization (Carrizo García et al., 2017). Heat stress (HS) affects male and female gametophytes differently. Male gametophytes are more sensitive to HS throughout their development (Zinn et al., 2010; Hedhly, 2011); it affects pollen quantity and morphology, the architecture of cell walls and, importantly, pollen metabolism (Hedhly, 2011). However, sensitivity to HS varies between species when the high temperature modes are applied (Parrotta et al., 2016).
The heat stress response (HSR) of sporophytic tissues has been the subject of a plethora of studies; however, these research findings are not relevant to pollen, as it has evolved a distinct and rather complex HSR compared to that of sporophytes (Bokszczanin et al., 2013). This alone justifies the need for pollen‐specific HSR studies (Mesihovic et al., 2016). Due to advances in high‐throughput profiling methods and technologies related to pollen isolation and separation, our understanding of the HSR during the course of pollen development has dramatically improved. In this review we summarize what is known about the effects of HS on pollen development and the pollen HSR during various stages of development, using studies that utilized classical and multiomics approaches.
Cytological alteration during pollen development and pollen tube growth under heat stress
Plants experience heat stress when temperatures exceed a certain threshold (i.e. 5–10°C above their optimal growth temperatures; Kranner et al., 2010), with their response depending on the duration and intensity of the stress (Larkindale et al., 2005). The primary response, however, involves massive transcriptional and translational changes. Pollen development, specifically, represents a very narrow developmental window. It is a complex process that requires the coordinated activity of different gametophytic and sporophytic cell types and tissues (Hafidh et al., 2016), making it particularly sensitive to environmental challenges. The findings of a study using crosses of tomato (Solanum lycopersicum) and Brassica napus plants, with male and female reproductive organs independently subjected to various heat stresses, suggested that when high temperature stress is applied separately to male and female gametes before pollination, pollen represents the weakest link (Zinn et al., 2010). Hence, when HS is applied during this developmental window, it can potentially disturb reproductive development, leading to pollen abortion, which hampers the fertilization process.
Effects of temperature stress on pollen development and pollen tube growth
Within anthers, diploid pollen mother cells (microsporocytes) undergo meiosis to give rise to four haploid microspores held together in the tetrad by a thick callose wall. The callose wall later gets degraded by the enzyme callase (secreted by the tapetum), releasing the individual microspores. From here, microspores increase in size and vacuolize, and their nuclei migrate to the periphery. The polarized microspores then undergo a highly asymmetric mitotic division (pollen mitosis I, PMI) (Hafidh et al., 2016) to form a large vegetative cell and a small generative cell. The microspore is thus the pluripotent initial of the male germline that establishes cells with two different fates (Berger & Twell, 2011). The germ cell undergoes one more round of mitotic division (pollen mitosis II, PMII), to produce the two sperm cells required for double fertilization. Mature pollen is shed from the anthers in either a bicellular or tricellular form, depending on whether PMII occurs before pollen maturation or after pollen germination.
Successful and coordinated pollination and fertilization require the synchronous development of microspores within an anther. This process is controlled at several check‐points, and when it fails (e.g. due to stress), developmental asynchrony promotes physiological and metabolic differences among microspores (Fig. 1) (Giorno et al., 2013). It subsequently increases their competition, in terms of resources during development, water for rehydration on the stigma, and pollen tube growth. In most plants, the onset of meiosis and microspore development towards PMI seem to be the processes that are most sensitive to environmental stress conditions (De Storme & Geelen, 2014; Muller & Rieu, 2016; Rieu et al., 2017; Begcy et al., 2019). Indeed, the earliest heat‐induced defects in Arabidopsis male gametophyte development occur during meiosis, through an increased frequency of crossing over and homologous recombination (Boyko et al., 2005; Francis et al., 2007). Moreover, exposure of Arabidopsis and rose (Rosa spp.) plants to mild HS (e.g. 48 h at 36°C) results in meiotically restituted dyads and triads that contain unreduced, diploid male gametes instead of the standard haploid ones (Pecrix et al., 2011; De Storme & Geelen, 2020). In addition to affecting meiosis, HS also affects cytoskeletal dynamics and spindle orientation in Arabidopsis and tobacco (Nicotiana tabacum; De Storme & Geelen, 2013; Parrotta et al., 2016). In the context of changes to the secondary metabolome, HS brings about an increase in flavonoid abundance in polarized microspores in tomato (Paupière et al., 2017a). Flavonoids play an important role in reactive oxygen species (ROS) detoxification (Rice‐Evans et al., 1996). Moreover, the abundance of conjugated polyamines is 37% lower in late pollen developmental stages compared with polarized microspores following HS (Paupière et al., 2017a). Polyamines increase the activity of antioxidant enzymes, which play a role in ROS detoxification (Chen et al., 2018).
Fig. 1.
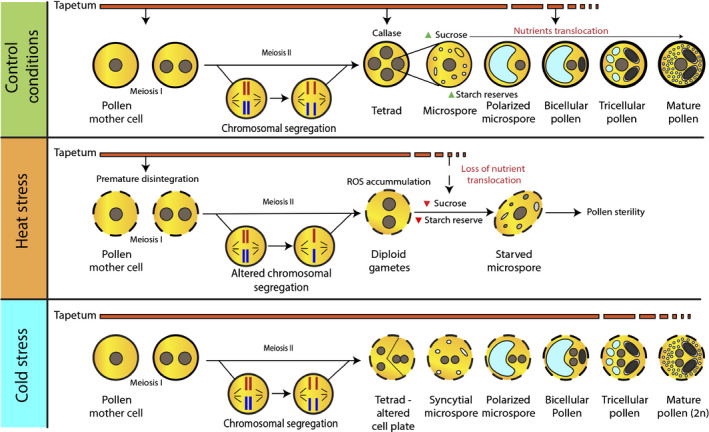
Schematic overview of cytological alterations imposed by heat and cold stress during male gametophyte development. Under heat stress conditions, the tapetum starts to degrade prematurely, which affects the nutritional supply to the developing pollen and leads to pollen sterility. Furthermore, the concentration of the soluble carbohydrate and starch reserves decreases in the developing anthers, followed by an increase in the reactive oxygen species (ROS) accumulation. Under cold stress, the tapetum does not undergo early degradation but rather shows abnormal expansion at the microspore stage and persists until pollen maturity. Cold treatment has a restitutive effect on the male meiosis; it significantly alters cell plate expansion and cell wall formation during meiotic division. Furthermore, cold‐stressed pollen mother cells produce microspores harbouring multiple haploid nuclei. Before pollen mitosis I (PMI), these nuclei fuse and develop into diploid or polyploid pollen.
Heat stress also affects the cell layers that surround microsporocytes. In common bean, (Phaseolus vulgaris) linear, looped, wavy or circular endoplasmic reticulum (ER) structure has been observed in tapetal cells following HS, instead of the customarily stacked rough ER observed under nonstressed conditions (Suzuki et al., 2001). Aberrations in tapetal development caused by HS have been reported in barley (Hordeum vulgare), cowpea (Vigna unguiculata), wheat and stiff brome (Brachypodium distachyon); (Saini et al., 1984; Ahmed et al., 1992; Abiko et al., 2005; Oshino et al., 2007; Sakata et al., 2010; Harsant et al., 2013). Heat stress also brings about premature degeneration of the tapetum, a layer of nutritive cells responsible for the nutrition of developing pollen grains. The tapetum is very rich in mitochondria compared to vegetative tissues (Lee & Warmke, 1979; Selinski & Scheibe, 2014). Under HS, the vast number of mitochondria most likely contribute to a dramatic rise in ROS generation as by‐product of aerobic metabolism (Mittler, 2017), and when vast amounts accumulate due to stress, they cause oxidative damage and cell death (Sharma et al., 2012). Reactive oxygen species signaling plays an important role in developmental programmed cell death (PCD) of the tapetum in dicot species such as Arabidopsis, tomato and tobacco, and in monocot species such as rice (Oryza sativa; Hu et al., 2011; Yu et al., 2017). However, the failure or premature PCD of the tapetum brings about male sterility (Kurusu & Kuchitsu, 2017). Indeed, during HS, ROS accumulates in anthers, causing an imbalance between ROS levels and ROS quenching enzymes, which induces premature PCD and degradation of the tapetal cell layer (Fig. 1) (Zhao et al., 2018).
However, during HS, ROS also participate in signaling cascades that produce detoxification enzymes (e.g. ascorbate peroxidase and catalase) that function to lower the amount of ROS in the cell, thereby forming a regulatory loop mechanism (Chaturvedi et al., 2013; Guan et al., 2013; Qu et al., 2013). This has been demonstrated in the pollen of wheat (Kumar et al., 2014) and Sorghum bicolor (Djanaguiraman et al., 2014), where an increase in ROS levels was accompanied by an increase in the abundance of detoxification enzymes. Finally, ROS accumulation has been shown to induce the expression of HEAT SHOCK TRANSCRIPTION FACTOR A1 (HsfA1), which stimulates HS‐responsive gene expression (Yoshida et al., 2011; Guan et al., 2013). Under cold stress conditions, however, the tapetum does not show early abortion, but rather shows abnormal expansion at the microspore stage (Oda et al., 2010) and persistence until pollen maturity stage (Fig. 1), a phenomenon observed in wheat, barley (Hordeum vulgare), stiff brome and rice (Oda et al., 2010; De Storme & Geelen, 2014; Muller & Rieu, 2016).
The functional, progamic phase commences once pollen reaches the stigma. Following rehydration on the stigma, the pollen grain germinates and produces a pollen tube that penetrates the pistil. Pollen tubes are highly specialized structures that deliver male gametes to the embryo sac for double fertilization (Hafidh et al., 2016). Pollen tube growth is an energetically demanding process that is dependent on the sufficient building and utilization of reserves, fast cell wall synthesis and adequate cell–cell communication. Mitochondrial decay under high temperatures causes defects in supporting these processes, as observed in rice pollen (Khatun & Flowers, 1995) and cultured tomato pollen tubes (Karapanos et al., 2009). At the ultrastructural level, HS induces changes in the isoform content and distribution of cytoskeletal subunits in tobacco pollen tubes, affecting the accumulation of secretory vesicles and the distribution of cellulose and callose synthases, enzymes involved in cell wall synthesis (Parrotta et al., 2016). Other effects of HS on the progamic phase include a decrease in ovule viability, altered stigma and style position, and a loss of stigma receptivity, which leads to impaired fertilization (Foolad & Sharma, 2005; Kumar et al., 2013; Gupta et al., 2015).
Stress sensing – setting in motion the heat stress response (HSR)
In order to respond to stress, plants first need to perceive it. Plant cells can sense stress at several interfaces using specific sensors. These include phospholipid membranes (due to their fluidity and permeability), Ca2+ flux, protein stability, the unfolded protein response (UPR) in the ER and cytoplasm, chromatin status and histone modifications, enzymatic reactions, and mRNA structure and stability. When stimulated, these pathways trigger signal transduction cascades that bring about the HSR, which functions to restore cellular homeostasis (Mittler et al., 2012; Zhu, 2016).
The accumulation of misfolded or unfolded proteins in the ER at elevated temperatures activates the UPR, which is conserved amongst eukaryotic organisms (Fig. 2). In order to set the UPR in motion, plants use UPR sensors to monitor the protein folding status in the ER (Iwata & Koizumi, 2005; Liu et al., 2007; Liu & Howell, 2010). The UPR pathway was shown to be active in both vegetative and reproductive development, defence, bacterial and viral immunity (Bao & Howell, 2017). In vegetative tissues, there are two arms of the UPR pathway. First, ER membrane‐localized RNA splicing factor INOSITOL REQUIRING ENZYME 1 (IRE1), harbouring both protein kinase and RNase domains in its cytoplasmic C‐terminal portion, is involved in the unconventional splicing of bZIP60 pre‐mRNA (Fig. 2). It results in the expression of functional transcription factor (TF) via the elimination of its transmembrane domain (Deng et al., 2011). The second UPR arm involves bZIP17 and bZIP28, a pair of ER membrane‐anchored TFs. They are typically retained in the ER membrane by their association with the luminal BiP protein. Following HS, they are released and relocated to the Golgi apparatus. There, bZIP17 and bZIP28 are cleaved by S1P and S2P proteases and, upon release, are transported to the nucleus where they activate stress‐responsive gene expression (Liu et al., 2007; Liu & Howell, 2010). When looking at plant reproductive development specifically, only the first arm of the UPR pathway has been shown to help to protect male gametophyte development from HS, even though the expression of genes active in both UPR arms were detected during pollen development (Fragkostefanakis et al., 2016a). Tissue profiling of Arabidopsis early and late flowers revealed distinct heat stress responses in vegetative and generative tissues, with genes participating in the UPR being enriched among heat‐upregulated reproductive tissue‐specific genes (S. S. Zhang et al., 2017). Moreover, in Arabidopsis, both the ire1a/ire1b double mutant and the bZIP60 single mutant showed male sterility at higher temperatures (Deng et al., 2016). Interestingly, two pollen‐expressed cytoplasm‐localized bZIP TFs, bZIP18 and bZIP52, were recently shown in Arabidopsis seedlings to accumulate in nuclei following HS. However, their re‐localization was triggered by dephosphorylation, probably at the serine residues within their conserved HXRXXS motifs (Wiese et al., 2021).
Fig. 2.
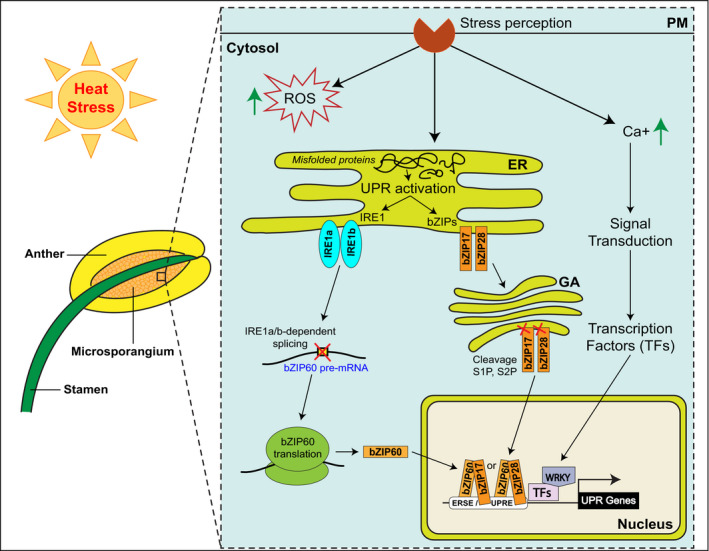
Heat stress sensing and response mechanism during male gametophyte development. Elevated temperature stress is perceived by the pollen vegetative cell, which triggers Ca2+ flux, ROS accumulation in the cytosol and activation of the unfolded protein response (UPR) in the endoplasmic reticulum (ER). The UPR pathway has two arms: (a) the ER membrane‐localized RNA splicing factor IRE1 is involved in the unconventional splicing of bZIP60 pre‐mRNA, resulting in the expression of functional transcription factor; (b) the pair of ER membrane‐anchored TFs, bZIP17 and bZIP28, is released and relocated to the Golgi apparatus, cleaved by S1P and S2P proteases, and transported to the nucleus. In the nucleus, bZIP17/60 and bZIP28/60 dimers activate stress‐responsive gene expression on the ERSE/UPRE. Abbreviations: bZIP, basic leucine zipper TF; Ca2+, calcium cation; ER, endoplasmic reticulum; ERSE, ER stress‐response element; GA, Golgi apparatus; IRE1, inositol requiring enzyme 1α; PM, plasma membrane; ROS, reactive oxygen species; S1P, site‐1 protease; S2P, site‐2 protease; TFs, transcription factors; UPR, unfolded protein response; UPRE, unfolded protein response element.
The accumulation of unfolded proteins in the cytoplasm elicits the UPR via heat shock protein (HSP)–heat shock factor (HSF) complexes (Bokszczanin et al., 2013). HsfA1 acts as a master regulator of the HS activation network in vegetative tissues (Mishra et al., 2002; Yoshida et al., 2011). Knockdown and multiple knockout mutants of HsfA1 genes in Arabidopsis and tomato vegetative tissues resulted in the reduced induction of many HS‐responsive genes, which concurrently produced HS‐sensitive phenotypes (Mishra et al., 2002; Yoshida et al., 2011). Upon HS, HsfA1 directly activates the second important player, DEHYDRATION‐RESPONSIVE ELEMENT BINDING PROTEIN 2A (DREB2A) and several other TFs, including HsfA2 (Yoshida et al., 2011). DREB2A integrates heat‐ and drought‐stress responses by activating the respective sets of genes (Ohama et al., 2017).
In tomato pollen, HsfA2 regulates a subset of HS‐induced genes (including several HSPs) and acts as an essential co‐activator of HsfA1a during the HSR (Giorno et al., 2010; Fragkostefanakis et al., 2016b). Heat shock proteins play a key role in mitigating the effects of HS on plant metabolism, acting predominantly as molecular chaperones and protecting proteins from the harmful effects of stress (e.g. conformation changes, aggregation), thereby maintaining protein homeostasis during the stress period (Vierling, 1991; Kotak et al., 2007; Mishra & Grover, 2015). HsfA2 suppression reduced pollen viability and germination when HS was applied during the meiosis and microspore formation stages, but had no effect later on. This highlights the fact that HsfA2 is an important player in the priming process that sustains pollen thermotolerance during microsporogenesis (Fragkostefanakis et al., 2016b). The AtREN1 (RESTRICTED TO NUCLEOLUS1) protein, a close homologue of HsfA5, also contributes to pollen thermotolerance. This protein is explicitly targeted to the nucleolus and is likely to be involved in ribosomal RNA biogenesis or other nucleolar functions. Atren1/‐ plants are defective in the HSR and produce a notably higher proportion of aberrant pollen grains (Reňák et al., 2014).
The ‐omics approach to unravelling unknown aspects of the HSR
To counter the negative effects brought on by HS, plants activate a HSR based on the initial stress, bringing about a hierarchical re‐programming of the transcriptome, proteome and metabolome (Mittler et al., 2012). With the advancement of isolation techniques over the years, researchers are now able to study multiple ‐omics in reproductive cells (e.g. pollen grains, sperm cells, egg cells, pollen tubes, Fig. 3) (Holmes‐Davis et al., 2005; Dai et al., 2006; Sheoran et al., 2007; Borges et al., 2008; Sheoran et al., 2009; Fíla et al., 2012; Chaturvedi et al., 2013; Obermeyer et al., 2013; Ischebeck et al., 2014; Chaturvedi et al., 2015; Fíla et al., 2016; Chaturvedi et al., 2016; Julca et al., 2020). In Fig. 3, we have assembled a summary of some of the crucial advances made in deciphering pollen development, under control and stress conditions, using ‐omics technologies. Similarly, Table 1 lists the stress response mechanisms elucidated for the male gametophytes of different species.
Fig. 3.
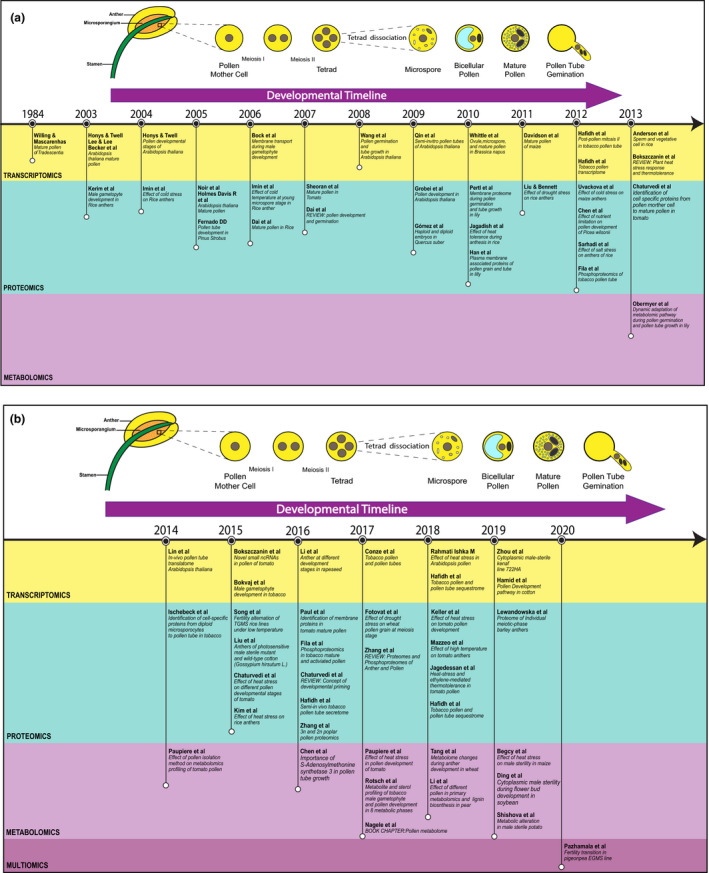
Timeline charting some of the important advances in male gametophyte (pollen) ‘‐omics’ studies based on developmental stages, cell types, techniques, and species. (a) Years 1984–2013; (b) Years 2014–2020.
Table 1.
Overview of the omics and classical studies published to understand stress response mechanism of male gametophyte in different plant species.
| Order | Plant species | Temperature (°C)/duration of exposure (h/d) | Effect on pollen | References |
|---|---|---|---|---|
| Poales | Brachypodium distachyon | 32°C | Decline in pollen viability, retention of pollen in anthers and pollen germination | Harsant et al. (2013) |
| 36°C | Abortion of microspores by the uninucleate stage, aberrations in tapetal development and degeneration | Harsant et al. (2013) | ||
| Hordeum vulgare | 30°C | Aberrations in tapetal development and degeneration | Abiko et al. (2005) | |
| Oryza sativa | 33°C | Reduced fertility and seed set | Ziska et al. (1996) | |
| Triticum aestivum | > 5°C above ambient temperature | Reduction in pollen production and viability | Stone & Nicolas (1995) | |
| 30°C/3 d | Tapetum degeneration | Saini et al. (1984) | ||
| Zea mays | 38°C | Affected pollen–stigma interactions | Mitchell & Petolino (1988) | |
| 35/25°C | Irregular tetrads | Begcy et al. (2019) | ||
| Vitales | Vitis vinifera | > 35°C | Alternative splicing | Jiang et al. (2017) |
| Fabales | Cicer arietinum | 35/20°C | Reduced pollen germination and tube growth | Devasirvatham et al. (2012) |
| 45/35°C | Decreases the concentration of soluble sugars in the anther walls of developing and mature pollen grains | Ismail & Hall (1999) | ||
| Phaseolus vulgaris | 32/27°C/1–5 d | Decreases the concentration of soluble sugars in the anther walls of developing and mature pollen grains | Suzuki et al. (2001) | |
| Pisum sativum | 35°C/4–7 d | Reduced pollen viability and the proportion of ovules that received a pollen tube | Jiang et al. (2019) | |
| Vigna unguiculata | 33/20°C | Aberrations in tapetal development and degeneration | Ahmed et al. (1992) | |
| Rosales | Pyrus bretschneideri | Low temperature of 4°C | Inhibits pollen tube growth | Gao et al. (2014) |
| Rosa sp. | 36°C/48 h | Alterations in male meiotic chromosome behaviour resulting in meiotically restituted dyads and triads | Pecrix et al. (2011) | |
| Brassicales | Arabidopsis thaliana | 30–32°C | Alterations in cross‐over distribution and induction of male meiotic restitution | De Storme & Geelen (2020) |
| Malvales | Gossypium hirsutum | > 30°C | Pollen sterility and abortion | Ismail & Hall (1999) |
| Malpighiales | Populus tremula | 38°C | Large spherical grains and pollen abortion | Wang et al. (2017) |
| Caryophyllales | Fagopyrum esculentum | 30°C | Ovules more sensitive compared to pollen grains | Płażek et al. (2019) |
| Solanales | Nicotiana tabacum | Heat | Altered the structure of cytoskeletal network of pollen tubes | Parrotta et al. (2016) |
| Solanum lycopersicum | 43–45°C/2 h | Reduced viability | Muller & Rieu (2016) | |
| 50°C/2 h | Decrease in germination rate | Firon et al. (2012), Jegadeesan et al. (2018) |
Heat stress response – re‐programming of the transcriptome
Transcriptome profiling provides a global snapshot of all RNA species (mRNA, tRNA, sRNA and microRNA) present in a sample at any given time point, which cannot be studied at the genomic level (Weckwerth et al., 2020). The Affymetrix ATH1 GeneChip has been the most widely used microarray for Arabidopsis cell type profiling of male and female reproductive lineages (Schmidt et al., 2012). Apart from microarrays, Next Generation Sequencing (NGS)‐based RNA sequencing (RNA‐seq) is now routinely used for transcriptional profiling. It has a broader dynamic range and higher sensitivity, and it offers whole‐genome coverage leading to the identification of unknown transcripts and novel splice variants (Schmidt et al., 2012; Loraine et al., 2013; Julca et al., 2020). The major advantage of RNA‐seq is the applicability to nonmodel species afforded by the continuous development of computational methods for data integration from various studies using different platforms and methods. Comparative transcriptomic analysis has revealed 5,365 genes that are differentially expressed in the heat‐stressed switchgrass (Panicum virgatum) cv. Alamo (Li et al., 2013). Moreover, HS has been shown to alter approximately 15% of the Arabidopsis pollen transcriptome when compared to control conditions (Rahmati Ishka et al., 2018). The earlier developmental stages were more sensitive to temperature fluctuations than the later stages (Raja et al., 2019), illustrating the limited capacity of the earlier stages in inducing a proper HSR.
Although the majority of HS‐responsive genes are regulated at the transcriptional level, transcriptomic studies also revealed the stress‐related regulation of gene expression at post‐transcriptional levels, namely mRNA processing and translation. A genome‐wide study in tomato pollen revealed alternative splicing (AS) as a new regulatory level for genes with a constitutive expression pattern (Keller et al., 2017). Alternative splicing is also implicated in the HSR in the vegetative tissues of grape (Vitis vinifera) (Jiang et al., 2017) and cabbage (Brassica oleracea), where genes upregulated by HS showed an increase in AS events (Lee et al., 2018). These genes included HSFs and HSPs, with the authors Lee et al. (2018) suggesting a role for AS in HS adaptation. The relationship between transcriptional and translational regulation under diverse and combined stress conditions (drought, heat) across different tissues was assessed by comparing transcriptomic and translatomic data (Matsuura et al., 2010; Li et al., 2018; Poidevin et al., 2020). For example, in vitro germinated Arabidopsis pollen responded to severe stress conditions by upregulating heat shock genes in a similar manner to vegetative tissues. Ribosome profiling combined with RNA‐seq revealed high correlation between transcriptional and translational responses to high temperatures, while specific regulations at the translational level were also observed (Poidevin et al., 2020). Post‐transcriptional regulation and ribosome re‐arrangement during the HSR involves the redistribution of mRNA and RNA‐binding proteins between actively translating ribosomes and cytoplasmic mRNA granules. In plants, they comprise stress granules (SGs) and processing bodies (PBs), where mRNA is sequestered and/or processed during HS (Weber et al., 2008; Chantarachot & Bailey‐Serres, 2017; Kosmacz et al., 2019). Such HS‐dependent mRNA rearrangement has been observed in pollen, as exemplified by the re‐distribution and accumulation of pollen‐expressed RNA‐binding protein ALBA4 in cytoplasmic granules during long‐term HS (Fig. 4). These cytoplasmic granules probably represent SGs, as suggested by the co‐localization of ALBA4 with PABP3 in pollen, but direct evidence is lacking (Náprstková et al., 2021). The suggested presence of SGs in pollen upon HS (Billey et al., 2020; Náprstková et al., 2021) would distinguish them from proposed general mRNA‐storage compartments, processing bodies (Scarpin et al., 2017) and nontranslating monosomes (Hafidh et al., 2018; Urquidi Camacho et al., 2020).
Fig. 4.
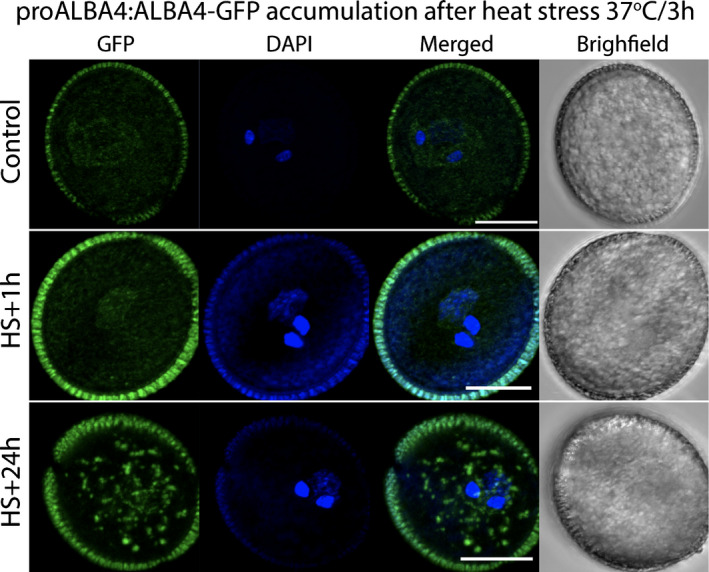
Effect of heat stress on the redistribution of ALBA‐family RNA‐binding protein ALBA4 (At1g76010) in pollen and its accumulation in large cytoplasmic granules 24 h after the heat stress (Náprstková et al., 2021). Arabidopsis plants cultivated at 22°C were heat‐stressed at 37°C for 3 h and then transferred back to their growth temperature. Mature pollen was collected 1 h and 24 h after the end of HS treatment and observed by bright field and fluorescent microscopy under control conditions (top row) and 1 h (middle row) and 24 h (bottom row) after the heat shock treatment (37°C for 3 h). Bars, 10 μm.
Several studies employing chromatin immunoprecipitation demonstrated the involvement of epigenetic processes in the pollen HSR (Chen et al., 2016). For example, HS affects DNA methylation and the expression of methyltransferase genes (Solis et al., 2012), and the methylation of genes encoding HSPs (Migicovsky et al., 2014; McCue et al., 2015), and influences chromatin conformation (Lang‐Mladek et al., 2010; Pecinka & Mittelsten Scheid, 2012). The importance of small noncoding RNAs (sncRNAs) in the regulation of pollen development at both transcriptional and epigenetic levels has been well documented (Slotkin et al., 2009; Calarco et al., 2012). A study performed by Bokszczanin et al. (2015) identified a complex set of tomato pollen sncRNAs (miRNAs, tRNAs, and snoRNAs) which were affected by HS in a stage‐specific manner. Interestingly, gene ontology enrichment analysis revealed that most target genes of all expressed miRNAs were significantly enriched in protein binding, transcription, and serine/threonine kinase activity. An outstanding result of this study demonstrated that tRNAs responded to HS not uniformly, but rather in an amino‐acid dependent manner, with the most dramatic response observed at later developmental stages (Bokszczanin et al., 2015).
Heat stress response – re‐programming of the proteome/restoring protein homeostasis
The cellular proteome does not fully reflect the transcriptome, especially in systems with high levels of translational regulation, such as the male gametophyte. Therefore, it is necessary to complement transcriptomics with translatomic and proteomic data to get a more realistic insight (Fíla et al., 2017). Compared to the transcriptomic approach, proteomic analyses require larger amounts of starting material, which represents a major limitation of using this technique for the younger developing stages of pollen (e.g. pollen mother cells, tetrads, microspores). For example, 40 µg of proteins isolated from different developmental stages of tomato pollen is required for a standard proteomic study (Chaturvedi et al., 2013; Chaturvedi et al., 2015). The availability of proteomic data demonstrated a low level of correlation between transcript and protein levels in the tomato pollen HSR (Jegadeesan et al., 2018; Keller & Simm, 2018). Since the transcriptome and translatome correlated well during Arabidopsis pollen HSR (Poidevin et al., 2020), post‐translational levels also seem to be involved. Interestingly, HS treatment of tomato pollen showed not only the uncoupling of the pollen HSR at transcriptional and post‐transcriptional levels, but also the differential upregulation of an unusually high proportion of pollen‐specific transcripts and proteins (Jegadeesan et al., 2018; Keller & Simm, 2018). These qualitative and quantitative differences, which only partly reflected the generally higher representation of specific genes in the male gametophyte (Honys & Twell, 2004; Rutley & Twell, 2015), should be attributed also to the sensitivity of the methods employed. Thus, in order to understand the pollen HSR in more detail, the integration of transcriptomic and proteomic datasets is essential.
As mentioned previously, HSFs regulate the expression of a diverse group of HSPs by recognizing heat stress elements (HSE) in their promoters, repetitive patterns of palindromic binding motifs (5′‐AGAAnnTTCT‐3′) upstream of the TATA box (Scharf et al., 2012). The majority of the transcriptome fraction induced by HS, however, encodes a diverse set of genes, not only HSPs but also genes encoding HSFs and metabolic enzymes, such as INOSITOL‐3‐PHOSPHATE SYNTHASE2 (IPS2) and GALACTINOL SYNTHASE1 (GOLS1) (Liu et al., 2011).
Several eukaryotic translation initiation factors (eIFs) are affected by stress treatment, and a few of them have even been shown to affect the stress response. Heterologous overexpression of several eIFs subunits in plants improved their tolerances to specific abiotic stresses. For example, transgenic Arabidopsis plants expressing Rosa chinensis RceIF5A, showed improved leaf thermotolerance and an increased resistance towards oxidative and osmotic stresses (Xu et al., 2011). Conversely, the Arabidopsis hot3 mutant, with impaired eIF5B1 expression, was unable to acquire tolerance to elevated temperatures (Hong & Vierling, 2000) and showed delayed recovery of translation apparatus following heat stress, accompanied by reduced translation efficiency of a subset of stress‐protective proteins (L. Zhang et al., 2017). The comparison of weak hot3‐1 and severe hot3‐2 alleles suggested the mechanism, whereby disrupting specific eIF5B interactions on the ribosome can affect translation, directly or indirectly (L. Zhang et al., 2017). Selective regulation of translation under heat stress conforms to the selective regulation of amino‐acid‐specific tRNAs under heat stress in tomato (Bokszczanin et al., 2015).
Heat stress response – restoring metabolite homeostasis
The plant metabolome is highly complex, as it emerges from both primary and secondary metabolism. It is estimated that there are c. 200 000 different metabolites present in plants (Sheth & Thaker, 2014). Unfortunately, the major limitation in high throughput metabolome profiling is the lack of a unified method that allows comprehensive measurements in terms of detection, quality, quantity and spatio‐temporal resolution. This is primarily because each metabolite differs in terms of concentration and chemical and analytical properties (Ghatak et al., 2018). A major analytical challenge as it relates to pollen is the removal of the pollenkitt and the other hydrophobic compounds on the pollen coat, which hinder the detection and identification of various metabolites (Obermeyer et al., 2013).
Heat stress has been shown to bring about metabolic imbalance (Kaplan et al., 2004). In this respect, the accumulation of reactive oxygen species (ROS) is a reliable marker of stress (Fig. 2, see above). An increase in ROS levels in stressed pollen correlates with a 60% reduction in the Arabidopsis and tomato pollen germination rate (Luria et al., 2019). With regards to the stress response, several studies have indicated a strong interaction between sugar‐ and abscisic acid (ABA)‐mediated signaling pathways (Gibson, 2004). Abscisic acid was shown to repress the expression of anther cell wall‐associated invertase (responsible for sucrose cleavage) in wheat, which resulted in a perturbation of sugar metabolism in developing spores (Ji et al., 2011). The application of exogenous auxin can enhance heat tolerance in rice reproductive organs (Zhang et al., 2018) and reduce the occurrence of male sterility. A similar phenomenon was observed in pigeonpea (Cajanus cajan), where the exogenous application of auxin could induce the expression of auxin transport proteins in pollen, which are required for cell wall development and nutritive supply under unfavourable conditions (Pazhamala et al., 2020). In this respect, temperature‐dependent decreases in auxin levels hamper normal cell wall development and contribute to male sterility in pigeonpea (Pazhamala et al., 2020). Other hormones have also been implicated in the stress response. For example, it has been proposed that the regulatory interaction between jasmonic acid and carbohydrate metabolism controls water transport into the anther, perhaps via induction of the AtSUC1 gene (Ishiguro et al., 2001).
Pollen development and germination comprise multiple steps of metabolic regulation, leading to significant metabolome dynamics (Nägele et al., 2017). Comprehensive profiling of metabolites under stress conditions and putting the obtained results in perspective with integrated transcriptomic and proteomic datasets, will be another step forward in producing stress‐tolerant crops.
Thermotolerance mechanisms – pollen perspectives
The adverse effects brought on by HS can be circumvented to some extent when plants undergo a ‘pre‐conditioning treatment’. Basal thermotolerance refers to a plant’s competence in withstanding nonlethal heat stress (e.g. acute heat stress; 36–45°C applied for 1–3 h) (Chaturvedi et al., 2015; Fragkostefanakis et al., 2015; Mesihovic et al., 2016). When a mild stress treatment is followed by a short recovery phase (i.e. pre‐conditioning), acquired thermotolerance is induced, which enables plants to withstand usually lethal heat stress. This phenomenon can be attributed to the ability of plant cells to store proteins which can enhance their tolerance to high temperatures that might otherwise be lethal (Larkindale & Vierling, 2008). For example, tomato Micro‐tom plants showed a dramatic decrease in their germination rate when subjected to 50°C temperature conditions for 2 h (Firon et al., 2012; Jegadeesan et al., 2018). However, a pre‐conditioning treatment (32°C for 1 h) followed by a recovery phase (25°C for 1 h), resulted in enhanced tolerance to 50°C for 2 h compared to plants that had not been pre‐conditioned (Firon et al., 2012). Such pre‐conditioning during the reproductive phase is not specific to pollen – it also affects seed development. A comparison between a gradual temperature increase and an acute 40°C stress treatment showed that the gradual acclimation enhanced seed thermotolerance (Stone & Nicolas, 1995). It not only confirmed the ability of reproductive tissues to acquire thermotolerance by applying a gradual and incrementally increasing HS treatment, but also highlighted the importance of using natural stress conditions in thermotolerance screens. Further studies on tomato have demonstrated that hormonal pre‐treatment is also beneficial for pollen fitness in stress conditions. Pre‐treating tomato plants with ethylene (ethephon) before HS caused a significant increase in pollen quality following a HS treatment (Jegadeesan et al., 2018). The induced pollen proteome of ethephon‐treated plants showed that there is an abundance of proteins that are implicated in the maintenance of the cellular redox state, which likely minimizes the effect of HS (Jegadeesan et al., 2018).
Pollen thermotolerance is an economically increasingly important trait for breeding; hence, it is necessary to develop suitable protocols which will allow high throughput evaluation of pollen quality in changing environmental conditions, together with the establishment of an appropriate pre‐treatment methodology that is applicable to crops growing in endangered areas.
Understanding natural variation in pollen thermotolerance
Exploring natural variation may offer insight into the genetic background of heat stress tolerance and can maintain diversity, which is favourable for breeding (Grandillo et al., 2007; Weckwerth et al., 2020). This also applies to heat tolerance of reproductive tissues, but so far there is minimal screening for variation in heat sensitivity in plant species with a reproductive system that is considered to be thermotolerant. There seem to be at least two significant reasons for this. Firstly, germplasm screening is limited to fruit sets. Fruit set is a very complex trait which is always accompanied by sub traits. For example, it has been shown that decreases in number of tomato fruits under long‐term mildly elevated temperatures correlated with lower pollen viability. (Pressman et al., 2002; Pressman et al., 2006). Therefore, investigating the individual traits separately can provide a better understanding of the genetic basis of reproductive thermotolerance under heat stress. Secondly, the choice of germplasm used for screening also plays an important role. It directly indicates the dimension of the genetic base used for screening; for example, several studies only use germplasm that consists mostly of cultivable tomato genotypes for thermotolerance screening (Dane et al., 1991; Grilli et al., 2007). Driedonks et al. (2018) investigated thermotolerance in reproductive tissues of 64 accessions across 13 wild species and seven tomato cultivars. These included a subset that showed satisfying reproductive thermotolerance under control conditions and following exposure to long‐term mild heat (LTMH). In this study, the LA1630 genotype showed the best performance in terms of pollen viability under LTMH, which demonstrates that the screening of wild germplasm can also enrich our knowledge of reproductive thermotolerance. In addition, the authors concluded that pollen viability and the quantity of pollen grains produced per flower are two main variables which can be used in further analysis, and pollen viability is an adaptive variable which depends on local conditions. However, it has also been proposed that further QTL analysis could be used to identify phenotypic traits under LTMH stress (Driedonks et al., 2018). Reproductive success depends on multiple traits; to identify these, and to significantly improve tolerance of the effects of heat stress on reproduction, more studies are needed at the genome‐wide level. The identified traits can be further transferred or used in marker‐assisted breeding for the development of resilient crops through genetic modification methods.
Concluding remarks and considerations for going forward
With regards to abiotic stress, temperature fluctuations are seldom an isolated event. Heat stress often coincides with drought stress and higher light intensities (Raja et al., 2019). Thus, how pollen responds to multiple co‐occurring stresses may differ greatly compared to its response to heat stress alone. This has been demonstrated for tobacco. In an attempt to simulate realistic environmental conditions, Rizhsky et al. (2002) subjected tobacco plants to either a single stress – heat or drought – or to both simultaneously. Using transcriptomics, the authors were able to show that plants responded differently when both stresses were applied at once, since the genes upregulated in the latter were not affected when a single stress was applied. Hence, experiments need to be designed that mimic real‐life conditions to yield information that breeders can use to generate crops tolerant to these scenarios. The ‐omics tools can be of great use in this regard, since they have already proved themselves fundamental in unravelling the mechanisms male gametophytes employ in response to heat stress. However, the majority of articles published on heat stress and gametophyte development have been based on transcriptomics and proteomics, with contributions from metabolomics and other more specialized ‐omics (e.g. lipidomics) still lagging (Paupière et al., 2017b; Mazzeo et al., 2018). Finally, the large quantities of data generated from the different ‐omics experiments need to be integrated, to provide plant breeders with targeted information on genes or alleles they can use to improve germplasm and to develop tolerant lines. In this review, we provided an overview of the stress response in the male gametophyte and highlighted pollen thermotolerance mechanisms. Furthermore, we elaborated on reproductive organ defects, regulation of gene expression, and the maintenance of protein and metabolite homeostasis under stress conditions, while summarizing the three central ‐omics approaches that deepen our understanding of the stress response mechanism.
Author contributions
PC and DH conceived the study; PC, AJW, AG, LZD, WW and DH drafted the manuscript; PC, AG, and DH designed the figures; and LZD prepared the data table. All authors have revised and approved the final version of the manuscript.
Acknowledgements
We thank the Austrian Science Fund (FWF, Der Wissenschaftsfonds), grant agreement no. W1257, for the financial support to Arindam Ghatak. We also thank the Czech Science Foundation (17‐23183S, 18‐02448S) and Ministry of Education, Youth and Sports of the Czech Republic (LTAIN19030, LTC20028). The work was supported by the European Regional Development Fund (CZ.02.1.01/0.0/0.0/16_019/0000738) and Prague Structural Funds (CZ.2.16/3.1.00/21519). We thank Alena Náprstková for providing ALBA4 images (Fig. 4) for this review. The authors declare no conflicting financial interest.
Contributor Information
Wolfram Weckwerth, Email: wolfram.weckwerth@univie.ac.at.
David Honys, Email: david@ueb.cas.cz.
References
- Abiko M, Akibayashi K, Sakata T, Kimura M, Kihara M, Itoh K, Asamizu E, Sato S, Takahashi H, Higashitani A. 2005. High‐temperature induction of male sterility during barley (Hordeum vulgare L.) anther development is mediated by transcriptional inhibition. Sexual Plant Reproduction 18: 91–100. [Google Scholar]
- Ahmed FE, Hall AE, DeMason DA. 1992. Heat injury during floral development in cowpea (Vigna unguiculata, Fabaceae). American Journal of Botany 79: 784–791. [Google Scholar]
- Anderson SN, Johnson CS, Jones DS, Conrad LJ, Gou XP, Russell SD, Sundaresan V. 2013. Transcriptomes of isolated Oryza sativa gametes characterized by deep sequencing: evidence for distinct sex‐dependent chromatin and epigenetic states before fertilization. The Plant Journal 76: 729–741. [DOI] [PubMed] [Google Scholar]
- Bao Y, Howell SH. 2017. The unfolded protein response supports plant development and defense as well as responses to abiotic stress. Frontiers in Plant Science 8: 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JD, Boavida LC, Carneiro J, Haury M, Feijo JA. 2003. Transcriptional profiling of Arabidopsis tissues reveals the unique characteristics of the pollen transcriptome. Plant Physiology 133: 713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begcy K, Dresselhaus T. 2018. Epigenetic responses to abiotic stresses during reproductive development in cereals. Plant Reproduction 31: 343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begcy K, Nosenko T, Zhou LZ, Fragner L, Weckwerth W, Dresselhaus T. 2019. Male sterility in maize after transient heat stress during the tetrad stage of pollen development. Plant Physiology 181: 683–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger F, Twell D. 2011. Germline specification and function in plants. Annual Review of Plant Biology 62: 461–484. [DOI] [PubMed] [Google Scholar]
- Billey E, Hafidh S, Cruz‐Gallardo I, Litholdo CG, Jean V, Carpentier M‐C, Picart C, Kulichova K, Honys D, Deragon J‐M et al. 2020. LARP6C regulates selective mRNA translation to promote pollen tube guidance in Arabidopsis thaliana . bioRxiv: doi: 10.1101/2020.11.27.401307. [DOI] [Google Scholar]
- Bock KW, Honys D, Ward JM, Padmanaban S, Nawrocki EP, Hirschi KD, Twell D, Sze H. 2006. Integrating membrane transport with male gametophyte development and function through transcriptomics. Plant Physiology 140: 1151–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokszczanin KL, Krezdorn N, Fragkostefanakis S, Müller S, Rycak L, Chen Y, Hoffmeier K, Kreutz J, Paupière MJ, Chaturvedi P et al. 2015. Identification of novel small ncRNAs in pollen of tomato. BMC Genomics 16: 714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokszczanin KL, Fragkostefanakis S; Consortium SPINS‐I . 2013. Perspectives on deciphering mechanisms underlying plant heat stress response and thermotolerance. Frontiers in Plant Science 4: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokvaj P, Hafidh S, Honys D. 2015. Transcriptome profiling of male gametophyte development in Nicotiana tabacum . Genom Data 3: 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges F, Gomes G, Gardner R, Moreno N, McCormick S, Feijo JA, Becker JD. 2008. Comparative transcriptomics of Arabidopsis sperm cells. Plant Physiology 148: 1168–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyko A, Filkowski J, Kovalchuk I. 2005. Homologous recombination in plants is temperature and day‐length dependent. Mutation Research–Fundamental and Molecular Mechanisms of Mutagenesis 572: 73–83. [DOI] [PubMed] [Google Scholar]
- Calarco J, Borges F, Donoghue M, Van Ex F, Jullien P, Lopes T, Gardner R, Berger F, Feijó JA, Becker JD et al. 2012. Reprogramming of DNA methylation in pollen guides epigenetic inheritance via small RNA. Cell 151: 194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrizo García C, Nepi M, Pacini E. 2017. It is a matter of timing: asynchrony during pollen development and its consequences on pollen performance in angiosperms – a review. Protoplasma 254: 57–73. [DOI] [PubMed] [Google Scholar]
- Chantarachot T, Bailey‐Serres J. 2017. Polysomes, stress granules and processing bodies: a dynamic triumvirate controlling cytoplasmic mRNA fate and function. Plant Physiology 176: 254–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi P, Doerfler H, Jegadeesan S, Ghatak A, Pressman E, Castillejo MA, Wienkoop S, Egelhofer V, Firon N, Weckwerth W. 2015. Heat‐treatment‐responsive proteins in different developmental stages of tomato pollen detected by targeted mass accuracy precursor alignment (tMAPA). Journal of Proteome Research 14: 4463–4471. [DOI] [PubMed] [Google Scholar]
- Chaturvedi P, Ghatak A, Weckwerth W. 2016. Pollen proteomics: from stress physiology to developmental priming. Plant Reproduction 29: 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi P, Ischebeck T, Egelhofer V, Lichtscheidl I, Weckwerth W. 2013. Cell‐specific analysis of the tomato pollen proteome from pollen mother cell to mature pollen provides evidence for developmental priming. Journal of Proteome Research 12: 4892–4903. [DOI] [PubMed] [Google Scholar]
- Chen D, Shao Q, Yin L, Younis A, Zheng B. 2018. Polyamine function in plants: metabolism, regulation on development, and roles in abiotic stress responses. Frontiers Plant Science 9: 1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Liu P, Hoehenwarter W, Lin J. 2012. Proteomic and phosphoproteomic analysis of Picea wilsonii pollen development under nutrient limitation. Journal of Proteome Research 11: 4180–4190. [DOI] [PubMed] [Google Scholar]
- Chen Y, Muller F, Rieu I, Winter P. 2016. Epigenetic events in plant male germ cell heat stress responses. Plant Reproduction 29: 21–29. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zou T, McCormick S. 2016. S‐adenosylmethionine synthetase 3 is important for pollen tube growth. Plant Physiology 172: 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conze LL, Berlin S, Le Bail A, Kost B. 2017. Transcriptome profiling of tobacco (Nicotiana tabacum) pollen and pollen tubes. BMC Genomics 18: 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S, Li L, Chen T, Chong K, Xue Y, Wang T. 2006. Proteomic analyses of Oryza sativa mature pollen reveal novel proteins associated with pollen germination and tube growth. Proteomics 6: 2504–2529. [DOI] [PubMed] [Google Scholar]
- Dai S, Wang T, Yan X, Chen S. 2007. Proteomics of pollen development and germination. Journal of Proteome Research 6: 4556–4563. [DOI] [PubMed] [Google Scholar]
- Dane F, Hunter AG, Cahambliss OL. 1991. Fruit set, pollen fertility, and combining ability of selected tomato genotypes under high‐temperature field conditions. Journal of the American Society for Horticultural Science 116: 906–910. [Google Scholar]
- Davidson RM, Hansey CN, Gowda M, Childs KL, Lin H, Vaillancourt B, Sekhon RS, de Leon N, Kaeppler SM, Jiang N et al. 2011. Utility of RNA sequencing for analysis of maize reproductive transcriptomes. Plant Genome 4: 191–203. [Google Scholar]
- De Storme N, Geelen D. 2013. Cytokinesis in plant male meiosis. Plant Signaling & Behavior 8: e23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Storme N, Geelen D. 2014. The impact of environmental stress on male reproductive development in plants: biological processes and molecular mechanisms. Plant, Cell & Environment 37: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Storme N, Geelen D. 2020. High temperatures alter cross‐over distribution and induce male meiotic restitution in Arabidopsis thaliana . Communications Biology 3: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Humbert S, Liu J, Srivastava R, Rothstein S, Howell SH. 2011. Heat induces the splicing by IRE1 of a mRNA encoding a transcription factor involved in the unfolded protein response in Arabidopsis. Proceedings of the National Academy of Sciences, USA 108: 7247–7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Srivastava R, Quilichini TD, Dong H, Bao Y, Horner HT, Howell SH. 2016. IRE1, a component of the unfolded protein response signaling pathway, protects pollen development in Arabidopsis from heat stress. The Plant Journal 88: 193–204. [DOI] [PubMed] [Google Scholar]
- Devasirvatham V, Gaur PM, Mallikarjuna N, Tokachichu RN, Trethowan RM, Tan DKY. 2012. Effect of high temperature on the reproductive development of chickpea genotypes under controlled environments. Functional Plant Biology 39: 1009–1018. [DOI] [PubMed] [Google Scholar]
- Ding XL, Wang X, Li Q, Yu LF, Song QJ, Gai JY, Yang SP. 2019. Metabolomics studies on cytoplasmic male sterility during flower bud development in soybean. International Journal of Molecular Sciences 20: 2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djanaguiraman M, Vara Prasad PV, Murugan M, Perumal R, Reddy UK. 2014. Physiological differences among sorghum (Sorghum bicolor L. Moench) genotypes under high temperature stress. Environmental and Experimental Botany 100: 43–54. [Google Scholar]
- Driedonks N, Wolters‐Arts M, Huber H, de Boer G‐J, Vriezen W, Mariani C, Rieu I. 2018. Exploring the natural variation for reproductive thermotolerance in wild tomato species. Euphytica 214: 67. [Google Scholar]
- Fernando DD. 2005. Characterization of pollen tube development in Pinus strobus (Eastern white pine) through proteomic analysis of differentially expressed proteins. Proteomics 5: 4917–4926. [DOI] [PubMed] [Google Scholar]
- Fíla J, Matros A, Radau S, Zahedi RP, Čapková V, Mock H‐P, Honys D. 2012. Revealing phosphoproteins playing role in tobacco pollen activated in vitro . Proteomics 12: 3229–3250. [DOI] [PubMed] [Google Scholar]
- Fíla J, Radau S, Matros A, Hartmann A, Scholz U, Feciková J, Mock HP, Čapková V, Zahedi RP, Honys D. 2016. Phosphoproteomics profiling of tobacco mature pollen and pollen activated in vitro . Molecular & Cellular Proteomics 15: 1338–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fíla J, Záveská Drábková L, Gibalová A, Honys D. 2017. When simple meets complex: pollen and the ‐omics. In: Obermeyer G, Feijó J, eds. Pollen tip growth . Cham, Switzerland: Springer, 247–292. [Google Scholar]
- Firon N, Pressman E, Meir S, Khoury R, Altahan L. 2012. Ethylene is involved in maintaining tomato (Solanum lycopersicum) pollen quality under heat‐stress conditions. AoB Plants 2012: pls024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foolad MR, Sharma A. 2005. Molecular markers as selection tools in tomato breeding. Acta Horticulturae 695: 225–240. [Google Scholar]
- Fotovat R, Alikhani M, Valizadeh M, Mirzaei M, Salekdeh GH. 2017. A proteomics approach to discover drought tolerance proteins in wheat pollen grain at meiosis stage. Protein and Peptide Letters 24: 26–36. [DOI] [PubMed] [Google Scholar]
- Fragkostefanakis S, Mesihovic A, Hu Y, Schleiff E. 2016a. Unfolded protein response in pollen development and heat stress tolerance. Plant Reproduction 29: 81–91. [DOI] [PubMed] [Google Scholar]
- Fragkostefanakis S, Mesihovic A, Simm S, Paupière MJ, Hu Y, Paul P, Mishra SK, Tschiersch B, Theres K, Bovy A et al. 2016b. HsfA2 controls the activity of developmentally and stress‐regulated heat stress protection mechanisms in tomato male reproductive tissues. Plant Physiology 170: 2461–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragkostefanakis S, Roth S, Schleiff E, Scharf KD. 2015. Prospects of engineering thermotolerance in crops through modulation of heat stress transcription factor and heat shock protein networks. Plant, Cell & Environment 38: 1881–1895. [DOI] [PubMed] [Google Scholar]
- Francis KE, Lam SY, Harrison BD, Bey AL, Berchowitz LE, Copenhaver GP. 2007. Pollen tetrad‐based visual assay for meiotic recombination in Arabidopsis. Proceedings of the National Academy of Sciences, USA 104: 3913–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y‐B, Wang C‐L, Wu J‐Y, Zhou H‐S, Jiang X‐T, Wu J, Zhang S‐L. 2014. Low temperature inhibits pollen tube growth by disruption of both tip‐localized reactive oxygen species and endocytosis in Pyrus bretschneideri Rehd. Plant Physiology and Biochemistry 74: 255–262. [DOI] [PubMed] [Google Scholar]
- Ghatak A, Chaturvedi P, Weckwerth W. 2018. Metabolomics in plant stress physiology. Plant Genetics and Molecular Biology 164: 187–236. [DOI] [PubMed] [Google Scholar]
- Gibson SI. 2004. Sugar and phytohormone response pathways: navigating a signalling network. Journal of Experimental Botany 55: 253–264. [DOI] [PubMed] [Google Scholar]
- Giorno F, Wolters‐Arts M, Grillo S, Scharf K‐D, Vriezen WH, Mariani C. 2010. Developmental and heat stress‐regulated expression of HsfA2 and small heat shock proteins in tomato anthers. Journal of Experimental Botany 61: 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorno F, Wolters‐Arts M, Mariani C, Rieu I. 2013. Ensuring reproduction at high temperatures: the heat stress response during anther and pollen development. Plants 2: 489–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez A, Lopez JA, Pintos B, Camafeita E, Bueno MA. 2009. Proteomic analysis from haploid and diploid embryos of Quercus suber L. identifies qualitative and quantitative differential expression patterns. Proteomics 9: 4355–4367. [DOI] [PubMed] [Google Scholar]
- Grandillo S, Chetelat R, Knapp S, Spooner D, Peralta I, Cammareri M, Perez O, Termolino P, Tripodi P, Chiusano ML. 2007. Solanum sect. Lycopersicon. In: Kole C, ed. Wild crop relatives: genomic and breeding resources. Heidelberg, Germany; Dordrecht, the Netherlands; London, UK; New York, USA: Springer, 129–215. [Google Scholar]
- Grilli GVG, Braz LT, Lemos EGM. 2007. QTL identification for tolerance to fruit set in tomato by fAFLP markers. Crop Breeding and Applied Biotechnology 7: 234–241. [Google Scholar]
- Grobei MA, Qeli E, Brunner E, Rehrauer H, Zhang RX, Roschitzki B, Basler K, Ahrens CH, Grossniklaus U. 2009. Deterministic protein inference for shotgun proteomics data provides new insights into Arabidopsis pollen development and function. Genome Research 19: 1786–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Q, Lu X, Zeng H, Zhang Y, Zhu J. 2013. Heat stress induction of miR398 triggers a regulatory loop that is critical for thermotolerance in Arabidopsis. The Plant Journal 74: 840–851. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Rai KN, Singh P, Ameta VL, Gupta SK, Jayalekha AK, Mahala RS, Pareek S, Swami ML, Verma YS. 2015. Seed set variability under high temperatures during flowering period in pearl millet (Pennisetum glaucum L. (R.) Br.). Field Crops Research 171: 41–53. [Google Scholar]
- Hafidh S, Breznenova K, Honys D. 2012a. De novo post‐pollen mitosis II tobacco pollen tube transcriptome. Plant Signaling & Behavior 7: 918–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafidh S, Breznenova K, Ruzicka P, Fecikova J, Capkova V, Honys D. 2012b. Comprehensive analysis of tobacco pollen transcriptome unveils common pathways in polar cell expansion and underlying heterochronic shift during spermatogenesis. BMC Plant Biology 12: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafidh S, Fila J, Honys D. 2016. Male gametophyte development and function in angiosperms: a general concept. Plant Reproduction 29: 31–51. [DOI] [PubMed] [Google Scholar]
- Hafidh S, Potěšil D, Müller K, Fíla J, Michailidis C, Herrmannová A, Feciková J, Ischebeck T, Valášek LS, Zdráhal Z et al. 2018. Dynamics of the pollen sequestrome defined by subcellular coupled omics. Plant Physiology 178: 258–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid R, Marashi H, Tomar RS, Shafaroudi SM, Sabara PH. 2019. Transcriptome analysis identified aberrant gene expression in pollen developmental pathways leading to CGMS in cotton (Gossypium hirsutum L.). PLoS ONE 14: e0218381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Chen SX, Dai SJ, Yang N, Wang T. 2010. Isobaric tags for relative and absolute quantification‐based comparative proteomics reveals the features of plasma membrane‐associated proteomes of pollen grains and pollen tubes from Lilium davidii . Journal of Integrative Plant Biology 52: 1043–1058. [DOI] [PubMed] [Google Scholar]
- Harsant J, Pavlovic L, Chiu G, Sultmanis S, Sage TL. 2013. High temperature stress and its effect on pollen development and morphological components of harvest index in the C3 model grass Brachypodium distachyon . Journal of Experimental Botany 64: 2971–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedhly A. 2011. Sensitivity of flowering plant gametophytes to temperature fluctuations. Environmental & Experimental Botany 74: 9–16. [Google Scholar]
- Holmes‐Davis R, Tanaka CK, Vensel WH, Hurkman WJ, McCormick S. 2005. Proteome mapping of mature pollen of Arabidopsis thaliana . Proteomics 5: 4864–4884. [DOI] [PubMed] [Google Scholar]
- Hong SW, Vierling E. 2000. Mutants of Arabidopsis thaliana defective in the acquisition of tolerance to high temperature stress. Proceedings of the National Academy of Sciences, USA 97: 4392–4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honys D, Twell D. 2003. Comparative analysis of the Arabidopsis pollen transcriptome. Plant Physiology 132: 640–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honys D, Twell D. 2004. Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biology 5: R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Liang W, Yin C, Cui X, Zong J, Wang X, Hu J, Zhang D. 2011. Rice MADS3 regulates ROS homeostasis during late anther development. Plant Cell 23: 515–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imin N, Kerim T, Rolfe BG, Weinman JJ. 2004. Effect of early cold stress on the maturation of rice anthers. Proteomics 4: 1873–1882. [DOI] [PubMed] [Google Scholar]
- Imin N, Kerim T, Weinman JJ, Rolfe BG. 2006. Low temperature treatment at the young microspore stage induces protein changes in rice anthers. Molecular & Cellular Proteomics 5: 274–292. [DOI] [PubMed] [Google Scholar]
- Ischebeck T, Valledor L, Lyon D, Gingl S, Nagler M, Meijon M, Egelhofer V, Weckwerth W. 2014. Comprehensive cell‐specific protein analysis in early and late pollen development from diploid microsporocytes to pollen tube growth. Molecular & Cellular Proteomics 13: 295–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro S, Kawai‐Oda A, Ueda J, Nishida I, Okada K. 2001. The DEFECTIVE IN ANTHER DEHISCENCE1 gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 13: 2191–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail AM, Hall AE. 1999. Reproductive‐stage heat tolerance, leaf membrane thermostability and plant morphology in cowpea. Crop Science 39: 1762–1768. [Google Scholar]
- Iwata Y, Koizumi N. 2005. An Arabidopsis transcription factor, AtbZIP60, regulates the endoplasmic reticulum stress response in a manner unique to plants. Proceedings of the National Academy of Sciences, USA 102: 5280–5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagadish SV, Muthurajan R, Oane R, Wheeler TR, Heuer S, Bennett J, Craufurd PQ. 2010. Physiological and proteomic approaches to address heat tolerance during anthesis in rice (Oryza sativa L.). Journal of Experimental Botany 61: 143–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegadeesan S, Chaturvedi P, Ghatak A, Pressman E, Meir S, Faigenboim A, Rutley N, Beery A, Harel A, Weckwerth W et al. 2018. Proteomics of heat‐stress and ethylene‐mediated thermotolerance mechanisms in tomato pollen grains. Frontiers in Plant Science 9: 1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X, Dong B, Shiran B, Talbot MJ, Edlington JE, Hughes T, White RG, Gubler F, Dolferus R. 2011. Control of abscisic acid catabolism and abscisic acid homeostasis is important for reproductive stage stress tolerance in cereals. Plant Physiology 156: 647–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Liu X, Liu C, Liu G, Li S, Wang L. 2017. Integrating omics and alternative splicing reveals insights into grape response to high temperature. Plant Physiology 173: 1502–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Lahlali R, Karunakaran C, Warkentin TD, Davis AR, Bueckert RA. 2019. Pollen, ovules, and pollination in pea: success, failure, and resilience in heat. Plant, Cell & Environment 42: 354–372. [DOI] [PubMed] [Google Scholar]
- Julca I, Ferrari C, Flores‐Tornero M, Proost S, Lindner A‐C, Hackenberg D, Steinbachová L, Michaelidis C, Pereira SG, Misra CS et al. 2020. Comparative transcriptomic analysis reveals conserved transcriptional programs underpinning organogenesis and reproduction in land plants. bioRxiv. doi: 10.1101/2020.10.29.361501. [DOI] [PubMed] [Google Scholar]
- Kaplan F, Kopka J, Haskell DW, Zhao W, Schiller KC, Gatzke N, Sung DY, Guy CL. 2004. Exploring the temperature‐stress metabolome of Arabidopsis. Plant Physiology 136: 4159–4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karapanos IC, Akoumianakis KA, Olympios CM, Passam HC. 2009. The effect of substrate, ADP and uncoupler on the respiration of tomato pollen during incubation in vitro at moderately high temperature. Sexual Plant Reproduction 22: 133–140. [DOI] [PubMed] [Google Scholar]
- Keller M, Hu Y, Mesihovic A, Fragkostefanakis S, Schleiff E, Simm S. 2017. Alternative splicing in tomato pollen in response to heat stress. DNA Research 24: 205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Simm S. 2018. The coupling of transcriptome and proteome adaptation during development and heat stress response of tomato pollen. BMC Genomics 19: 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerim T, Imin N, Weinman JJ, Rolfe BG. 2003. Proteome analysis of male gametophyte development in rice anthers. Proteomics 3: 738–751. [DOI] [PubMed] [Google Scholar]
- Khatun S, Flowers TJ. 1995. The estimation of pollen viability in rice. Journal of Experimental Botany 46: 151–154. [Google Scholar]
- Kim M, Kim H, Lee W, Lee Y, Kwon SW, Lee J. 2015. Quantitative shotgun proteomics analysis of rice anther proteins after exposure to high temperature. International Journal of Genomics 2015: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmacz M, Gorka M, Schmidt S, Luzarowski M, Moreno JC, Szlachetko J, Leniak E, Sokolowska EM, Sofroni K, Schnittger A et al. 2019. Protein and metabolite composition of Arabidopsis stress granules. New Phytologist 222: 1420–1433. [DOI] [PubMed] [Google Scholar]
- Kotak S, Larkindale J, Lee U, von Koskull‐Doring P, Vierling E, Scharf KD. 2007. Complexity of the heat stress response in plants. Current Opinion in Plant Biology 10: 310–316. [DOI] [PubMed] [Google Scholar]
- Kranner I, Minibayeva FV, Beckett RP, Seal CE. 2010. What is stress? Concepts, definitions and applications in seed science. New Phytologist 188: 655–673. [DOI] [PubMed] [Google Scholar]
- Kumar RR, Goswami S, Gadpayle KA, Singh K, Sharma SK, Singh GP, Pathak H, Rai RD. 2014. Ascorbic acid at pre‐anthesis modulate the thermotolerance level of wheat (Triticum aestivum) pollen under heat stress. Journal of Plant Biochemistry and Biotechnology 23: 293–306. [Google Scholar]
- Kumar S, Thakur P, Kaushal N, Malik JA, Gaur P, Nayyar H. 2013. Effect of varying high temperatures during reproductive growth on reproductive function, oxidative stress and seed yield in chickpea genotypes differing in heat sensitivity. Archives of Agronomy and Soil Science 59: 823–843. [Google Scholar]
- Kurusu T, Kuchitsu K. 2017. Autophagy, programmed cell death and reactive oxygen species in sexual reproduction in plants. Journal of Plant Research 130: 491–499. [DOI] [PubMed] [Google Scholar]
- Lang‐Mladek C, Popova O, Kiok K, Berlinger M, Rakic B, Aufsatz W, Jonak C, Hauser MT, Luschnig C. 2010. Transgenerational inheritance and resetting of stress‐induced loss of epigenetic gene silencing in Arabidopsis. Molecular Plant 3: 594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Hall JD, Knight MR, Vierling E. 2005. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiology 138: 882–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Vierling E. 2008. Core genome responses involved in acclimation to high temperature. Plant Physiology 146: 748–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Jung WY, Park HJ, Lee A, Kwon SY, Kim HS, Cho HS. 2018. Genome‐wide analysis of alternative splicing in an inbred cabbage (Brassica oleracea L.) line 'HO' in response to heat stress. Current Genomics 19: 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Lee DH. 2003. Use of serial analysis of gene expression technology to reveal changes in gene expression in Arabidopsis pollen undergoing cold stress. Plant Physiology 132: 517–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S‐LJ, Warmke HE. 1979. Organelle size and number in fertile and T‐cytoplasmic male‐sterile corn. American Journal of Botany 66: 141–148. [Google Scholar]
- Lewandowska D, Zhang R, Colas I, Uzrek N, Waugh R. 2019. Application of a sensitive and reproducible label‐free proteomic approach to explore the proteome of individual meiotic‐phase barley anthers. Frontiers in Plant Science 10: 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SM, Su XQ, Abdullah M, Sun YM, Li GH, Cheng X, Lin Y, Cai YP, Jin Q. 2018. Effects of different pollens on primary metabolism and lignin biosynthesis in pear. International Journal of Molecular Sciences 19: 2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y‐F, Wang Y, Tang Y, Gobal Kakani V, Mahalingam R. 2013. Transcriptome analysis of heat stress response in switchgrass (Panicum virgatum L.). BMC Plant Biology 13: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y‐F, Zheng Y, Vemireddy LR, Panda SK, Jose S, Ranjan A, Panda P, Govindan G, Cui J, Wei K et al. 2018. Comparative transcriptome and translatome analysis in contrasting rice genotypes reveals differential mRNA translation in salt‐tolerant Pokkali under salt stress. BMC Genomics 19: 935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZJ, Zhang PP, Lv JY, Cheng YF, Cui JM, Zhao HX, Hu SW. 2016. Global dynamic transcriptome programming of rapeseed (Brassica napus L.) anther at different development stages. PLoS ONE 11: e0154039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Chen PW, Chuang MH, Juntawong P, Bailey‐Serres J, Jauh GY. 2014. Profiling of translatomes of in vivo‐grown pollen tubes reveals genes with roles in micropylar guidance during pollination in Arabidopsis. Plant Cell 26: 602–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HC, Liao HT, Charng YY. 2011. The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant, Cell & Environment 34: 738–751. [DOI] [PubMed] [Google Scholar]
- Liu J, Pang C, Wei H, Song M, Meng Y, Ma J, Fan S, Yu S. 2015. iTRAQ‐facilitated proteomic profiling of anthers from a photosensitive male sterile mutant and wild‐type cotton (Gossypium hirsutum L.). Journal of Proteomics 126: 68–81. [DOI] [PubMed] [Google Scholar]
- Liu JX, Bennett J. 2011. Reversible and irreversible drought‐induced changes in the anther proteome of rice (Oryza sativa L.) genotypes IR64 and Moroberekan. Molecular Plant 4: 59–69. [DOI] [PubMed] [Google Scholar]
- Liu JX, Howell SH. 2010. bZIP28 and NF‐Y transcription factors are activated by ER stress and assemble into a transcriptional complex to regulate stress response genes in Arabidopsis. Plant Cell 22: 782–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JX, Srivastava R, Che P, Howell SH. 2007. An endoplasmic reticulum stress response in Arabidopsis is mediated by proteolytic processing and nuclear relocation of a membrane‐associated transcription factor, bZIP28. Plant Cell 19: 4111–4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobell DB, Bänziger M, Magorokosho C, Vivek B. 2011. Nonlinear heat effects on African maize as evidenced by historical yield trials. Nature Climate Change 1: 42–45. [Google Scholar]
- Loraine AE, McCormick S, Estrada A, Patel K, Qin P. 2013. RNA‐Seq of Arabidopsis pollen uncovers novel transcription and alternative splicing. Plant Physiology 162: 1092–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria G, Rutley N, Lazar I, Harper JF, Miller G. 2019. Direct analysis of pollen fitness by flow cytometry: implications for pollen response to stress. The Plant Journal 98: 942–952. [DOI] [PubMed] [Google Scholar]
- Matsuura H, Ishibashi Y, Shinmyo A, Kanaya S, Kato K. 2010. Genome‐wide analyses of early translational responses to elevated temperature and high salinity in Arabidopsis thaliana . Plant and Cell Physiology 51: 448–462. [DOI] [PubMed] [Google Scholar]
- Mazzeo MF, Cacace G, Iovieno P, Massarelli I, Grillo S, Siciliano RA. 2018. Response mechanisms induced by exposure to high temperature in anthers from thermo‐tolerant and thermo‐sensitive tomato plants: a proteomic perspective. PLoS ONE 13: e0201027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue AD, Panda K, Nuthikattu S, Choudury SG, Thomas EN, Slotkin RK. 2015. ARGONAUTE 6 bridges transposable element mRNA‐derived siRNAs to the establishment of DNA methylation. EMBO Journal 34: 20–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesihovic A, Iannacone R, Firon N, Fragkostefanakis S. 2016. Heat stress regimes for the investigation of pollen thermotolerance in crop plants. Plant Reproduction 29: 93–105. [DOI] [PubMed] [Google Scholar]
- Migicovsky Z, Yao Y, Kovalchuk I. 2014. Transgenerational phenotypic and epigenetic changes in response to heat stress in Arabidopsis thaliana . Plant Signal Behaviour 9: e27971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra RC, Grover A. 2015. ClpB/Hsp100 proteins and heat stress tolerance in plants. Critical Reviews in Biotechnology 36: 862–874. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Tripp J, Winkelhaus S, Tschiersch B, Theres K, Nover L, Scharf KD. 2002. In the complex family of heat stress transcription factors, HsfA1 has a unique role as master regulator of thermotolerance in tomato. Genes & Development 16: 1555–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JC, Petolino JF. 1988. Heat stress effects on isolated reproductive organs of maize. Journal of Plant Physiology 133: 625–628. [Google Scholar]
- Mittler R. 2017. ROS are good. Trends Plant Science 22: 11–19. [DOI] [PubMed] [Google Scholar]
- Mittler R, Finka A, Goloubinoff P. 2012. How do plants feel the heat? Trends in Biochemical Sciences 37: 118–125. [DOI] [PubMed] [Google Scholar]
- Muller F, Rieu I. 2016. Acclimation to high temperature during pollen development. Plant Reproduction 29: 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nägele T, Fragner L, Chaturvedi P, Ghatak A, Weckwerth W. 2017. Pollen metabolome dynamics: biochemistry, regulation and analysis. In: Obermeyer G, Feijó J, eds. Pollen tip growth. Cham, Switzerland: Springer, 319–336. [Google Scholar]
- Náprstková A, Malínská K, Záveská Drábková L, Billey E, Náprstková D, Sýkorová E, Bousquet‐Antonelli C, Honys D. 2021. Characterization of ALBA family expression and localization in Arabidopsis thaliana generative organs. International Journal of Molecular Sciences 22: 1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noir S, Brautigam A, Colby T, Schmidt J, Panstruga R. 2005. A reference map of the Arabidopsis thaliana mature pollen proteome. Biochemical and Biophysical Research Communications 337: 1257–1266. [DOI] [PubMed] [Google Scholar]
- Obermeyer G, Fragner L, Lang V, Weckwerth W. 2013. Dynamic adaption of metabolic pathways during germination and growth of lily pollen tubes after inhibition of the electron transport chain. Plant Physiology 162: 1822–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda S, Kaneko F, Yano K, Fujioka T, Masuko H, Park J‐I, Kikuchi S, Hamada K, Endo M, Nagano K et al. 2010. Morphological and gene expression analysis under cool temperature conditions in rice anther development. Genes & Genetic Systems 85: 107–120. [DOI] [PubMed] [Google Scholar]
- Ohama N, Sato H, Shinozaki K, Yamaguchi‐Shinozaki K. 2017. Transcriptional regulatory network of plant heat stress response. Trends in Plant Science 22: 53–65. [DOI] [PubMed] [Google Scholar]
- Oshino T, Abiko M, Saito R, Ichiishi E, Endo M, Kawagishi‐Kobayashi M, Higashitani A. 2007. Premature progression of anther early developmental programs accompanied by comprehensive alterations in transcription during high‐temperature injury in barley plants. Molecular Genetics and Genomics 278: 31–42. [DOI] [PubMed] [Google Scholar]
- Parrotta L, Faleri C, Cresti M, Cai G. 2016. Heat stress affects the cytoskeleton and the delivery of sucrose synthase in tobacco pollen tubes. Planta 243: 43–63. [DOI] [PubMed] [Google Scholar]
- Paul P, Chaturvedi P, Selymesi M, Ghatak A, Mesihovic A, Scharf KD, Weckwerth W, Simm S, Schleiff E. 2016. The membrane proteome of male gametophyte in Solanum lycopersicum . Journal of Proteomics 131: 48–60. [DOI] [PubMed] [Google Scholar]
- Paupière MJ, van Haperen P, Rieu I, Visser RGF, Tikunov YM, Bovy AG. 2017b. Screening for pollen tolerance to high temperatures in tomato. Euphytica 213: 130. [Google Scholar]
- Paupiere MJ, van Heusden AW, Bovy AG. 2014. The metabolic basis of pollen thermo‐tolerance: perspectives for breeding. Metabolites 4: 889–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paupière MJ, Muller F, Li HJ, Rieu I, Tikunov YM, Visser RGF, Bovy AG. 2017a. Untargeted metabolomic analysis of tomato pollen development and heat stress response. Plant Reproduction 30: 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazhamala LT, Chaturvedi P, Bajaj P, Srikanth S, Ghatak A, Chitikineni A, Bellaire A, Hingane A, Kumar CVS, Saxena Kb et al. 2020. Multiomics approach unravels fertility transition in a pigeonpea line for a two‐line hybrid system. Plant Genome 13: e20028. [DOI] [PubMed] [Google Scholar]
- Pecinka A, Mittelsten SO. 2012. Stress‐induced chromatin changes: a critical view on their heritability. Plant and Cell Physiology 53: 801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecrix Y, Rallo G, Folzer H, Cigna M, Gudin S, Le Bris M. 2011. Polyploidization mechanisms: temperature environment can induce diploid gamete formation in Rosa sp. Journal of Experimental Botany 62: 3587–3597. [DOI] [PubMed] [Google Scholar]
- Peng S, Huang J, Sheehy JE, Laza RC, Visperas RM, Zhong X, Centeno GS, Khush GS, Cassman KG. 2004. Rice yields decline with higher night temperature from global warming. Proceedings of the National Academy of Sciences, USA 101: 9971–9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertl H, Pockl M, Blaschke C, Obermeyer G. 2010. Osmoregulation in lilium pollen grains occurs via modulation of the plasma membrane H+ ATPase activity by 14‐3‐3 proteins. Plant Physiology 154: 1921–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Płażek A, Słomka A, Kopeć P, Dziurka M, Hornyák M, Sychta K, Pastuszak J, Dubert F. 2019. Effects of high temperature on embryological development and hormone profile in flowers and leaves of common buckwheat (Fagopyrum esculentum Moench). International Journal of Molecular Sciences 20: 1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poidevin L, Forment J, Unal D, Ferrando A. 2020. Transcriptome and translatome changes in germinated pollen under heat stress uncover roles of transporter genes involved in pollen tube growth. Plant, Cell & Environment. doi: 10.1111/pce.13972. [DOI] [PubMed] [Google Scholar]
- Pressman E, Harel D, Zamski E, Shaked R, Althan L, Rosenfeld K, Firon N. 2006. The effect of high temperatures on the expression and activity of sucrose‐cleaving enzymes during tomato (Lycopersicon esculentum) anther development. Journal of Horticultural Science and Biotechnology 81: 341–348. [Google Scholar]
- Pressman E, Peet MM, Pharr DM. 2002. The effect of heat stress on tomato pollen characteristics is associated with changes in carbohydrate concentration in the developing anthers. Annals of Botany 90: 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Leydon AR, Manziello A, Pandey R, Mount D, Denic S, Vasic B, Johnson MA, Palanivelu R. 2009. Penetration of the stigma and style elicits a novel transcriptome in pollen tubes, pointing to genes critical for growth in a pistil. PLoS Genetics 5: e1000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu AL, Ding YF, Jiang Q, Zhu C. 2013. Molecular mechanisms of the plant heat stress response. Biochemical and Biophysical Research Communications 432: 203–207. [DOI] [PubMed] [Google Scholar]
- Rahmati Ishka M, Brown E, Weigand C, Tillett RL, Schlauch KA, Miller G, Harper JF. 2018. A comparison of heat‐stress transcriptome changes between wild‐type Arabidopsis pollen and a heat‐sensitive mutant harboring a knockout of cyclic nucleotide‐gated cation channel 16 (cngc16). BMC Genomics 19: 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja MM, Vijayalakshmi G, Naik ML, Basha PO, Sergeant K, Hausman JF, Khan PSSV. 2019. Pollen development and function under heat stress: from effects to responses. Acta Physiologiae Plantarum 41: 47. [Google Scholar]
- Reňák D, Gibalová A, Šolcová K, Honys D. 2014. A new link between stress response and nucleolar function during pollen development in Arabidopsis mediated by AtREN1 protein. Plant, Cell & Environment 37: 670–683. [DOI] [PubMed] [Google Scholar]
- Rice‐Evans CA, Miller NJ, Paganga G. 1996. Structure‐antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biology and Medicine 20: 933–956. [DOI] [PubMed] [Google Scholar]
- Rieu I, Twell D, Firon N. 2017. Pollen development at high temperature: from acclimation to collapse. Plant Physiology 173: 1967–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizhsky L, Liang H, Mittler R. 2002. The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiology 130: 1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotsch AH, Kopka J, Feussner I, Ischebeck T. 2017. Central metabolite and sterol profiling divides tobacco male gametophyte development and pollen tube growth into eight metabolic phases. The Plant Journal 92: 129–146. [DOI] [PubMed] [Google Scholar]
- Rutley N, Twell D. 2015. A decade of pollen transcriptomics. Plant Reproduction 28: 73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini HS, Sedgley M, Aspinall D. 1984. Developmental anatomy in wheat of male‐sterility induced by heat‐stress, water deficit or abscisic‐acid. Australian Journal of Plant Physiology 11: 243–253. [Google Scholar]
- Sakata T, Oshino T, Miura S, Tomabechi M, Tsunaga Y, Higashitani N, Miyazawa Y, Takahashi H, Watanabe M, Higashitani A. 2010. Auxins reverse plant male sterility caused by high temperatures. Proceedings of the National Academy of Sciences, USA 107: 8569–8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarhadi E, Bazargani MM, Sajise AG, Abdolahi S, Vispo NA, Arceta M, Nejad GM, Singh RK, Salekdeh GH. 2012. Proteomic analysis of rice anthers under salt stress. Plant Physiology and Biochemistry 58: 280–287. [DOI] [PubMed] [Google Scholar]
- Scarpin MR, Sigaut L, Temprana SG, Boccaccio GL, Pietrasanta LI, Muschietti JP. 2017. Two Arabidopsis late pollen transcripts are detected in cytoplasmic granules. Plant Direct 1: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf KD, Berberich T, Ebersberger I, Nover L. 2012. The plant heat stress transcription factor (Hsf) family: structure, function and evolution. Biochimica et Biophysica Acta 1819: 104–119. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Schmid MW, Grossniklaus U. 2012. Analysis of plant germline development by high‐throughput RNA profiling: technical advances and new insights. The Plant Journal 70: 18–29. [DOI] [PubMed] [Google Scholar]
- Selinski J, Scheibe R. 2014. Pollen tube growth: where does the energy come from? Plant Signaling & Behavior 9: e977200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Jha AB, Dubey RS, Pessarakli M. 2012. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany 2012: 1–26. [Google Scholar]
- Sheoran IS, Pedersen EJ, Ross ARS, Sawhney VK. 2009. Dynamics of protein expression during pollen germination in canola (Brassica napus). Planta 230: 779–793. [DOI] [PubMed] [Google Scholar]
- Sheoran IS, Ross ARS, Olson DJH, Sawhney VK. 2007. Proteomic analysis of tomato (Lycopersicon esculentum) pollen. Journal of Experimental Botany 58: 3525–3535. [DOI] [PubMed] [Google Scholar]
- Sheth BP, Thaker VS. 2014. Plant systems biology: insights, advances and challenges. Planta 240: 33–54. [DOI] [PubMed] [Google Scholar]
- Shishova M, Puzanskiy R, Gavrilova O, Kurbanniazov S, Demchenko K, Yemelyanov V, Pendinen G, Shavarda A, Gavrilenko T. 2019. Metabolic alterations in male‐sterile potato as compared to male‐fertile. Metabolites 9: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin RK, Vaughn M, Borges F, Tanurdzic M, Becker JD, Feijo JA, Martienssen RA. 2009. Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell 136: 461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis MT, Rodriguez‐Serrano M, Meijon M, Canal MJ, Cifuentes A, Risueno MC, Testillano PS. 2012. DNA methylation dynamics and MET1a‐like gene expression changes during stress‐induced pollen reprogramming to embryogenesis. Journal of Experimental Botany 63: 6431–6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Liu Z, Tong J, Xiao L, Ma H, Zhang H. 2015. Comparative proteomics analysis reveals the mechanism of fertility alternation of thermosensitive genic male sterile rice lines under low temperature inducement. Proteomics 15: 1884–1905. [DOI] [PubMed] [Google Scholar]
- Stone PJ, Nicolas ME. 1995. A survey of the effects of high‐temperature during grain filling on yield and quality of 75 wheat cultivars. Australian Journal of Agricultural Research 46: 475–492. [Google Scholar]
- Suzuki K, Tsukaguchi T, Takeda H, Egawa Y. 2001. Decrease of pollen stainability of green bean at high temperatures and relationship to heat tolerance. Journal of the American Society for Horticultural Science 126: 571–574. [Google Scholar]
- Tang HL, Song YL, Guo JL, Wang JW, Zhang LL, Niu N, Ma SC, Zhang GS, Zhao HY. 2018. Physiological and metabolome changes during anther development in wheat (Triticum aestivum L.). Plant Physiology and Biochemistry 132: 18–32. [DOI] [PubMed] [Google Scholar]
- Tashiro T, Wardlaw IF. 1989. A comparison of the effect of high temperature on grain development in wheat and rice. Annals of Botany 64: 59–65. [Google Scholar]
- Urquidi Camacho RA, Lokdarshi A, von Arnim AG. 2020. Translational gene regulation in plants: a green new deal. WIREs RNA 11: e1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvackova L, Takac T, Boehm N, Obert B, Samaj J. 2012. Proteomic and biochemical analysis of maize anthers after cold pretreatment and induction of androgenesis reveals an important role of anti‐oxidative enzymes. Journal of Proteomics 75: 1886–1894. [DOI] [PubMed] [Google Scholar]
- Valero Galvan J, Valledor L, Navarro Cerrillo RM, Gil Pelegrin E, Jorrin‐Novo JV. 2011. Studies of variability in Holm oak (Quercus ilex subsp. ballota [Desf.] Samp.) through acorn protein profile analysis. Journal of Proteomics 74: 1244–1255. [DOI] [PubMed] [Google Scholar]
- Vierling E. 1991. The roles of heat shock proteins in plants. Annual Review of Plant Biology 42: 579–620. [Google Scholar]
- Wang J, Li D, Shang F, Kang X. 2017. High temperature‐induced production of unreduced pollen and its cytological effects in Populus . Scientific Reports 7: 5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang WZ, Song LF, Zou JJ, Su Z, Wu WH. 2008. Transcriptome analyses show changes in gene expression to accompany pollen germination and tube growth in Arabidopsis. Plant Physiology 148: 1201–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C, Nover L, Fauth M. 2008. Plant stress granules and mRNA processing bodies are distinct from heat stress granules. The Plant Journal 56: 517–530. [DOI] [PubMed] [Google Scholar]
- Weckwerth W, Ghatak A, Bellaire A, Chaturvedi P, Varshney RK. 2020. PANOMICS meets germplasm. Plant Biotechnology Journal 18: 1507–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle CA, Malik MR, Li R, Krochko JE. 2010. Comparative transcript analyses of the ovule, microspore, and mature pollen in Brassica napus . Plant Molecular Biology 72: 279–299. [DOI] [PubMed] [Google Scholar]
- Wiese AJ, Steinbachová L, Timofejeva L, Čermák V, Klodová B, Ganji RS, Limones‐Mendez M, Bokvaj P, Hafidh S, Potěšil D et al. 2021. Arabidopsis bZIP18 and bZIP52 accumulate in nuclei following heat stress where they regulate the expression of a similar set of genes. International Journal of Molecular Sciences 22: 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing RP, Mascarenhas JP. 1984. Analysis of the complexity and diversity of messenger‐RNAS from pollen and shoots of tradescantia. Plant Physiology 75: 865–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhang B, Jiang C, Ming F. 2011. RceIF5A, encoding an eukaryotic translation initiation factor 5A in Rosa chinensis, can enhance thermotolerance, oxidative and osmotic stress resistance of Arabidopsis thaliana . Plant Molecular Biology 75: 167–178. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Ohama N, Nakajima J, Kidokoro S, Mizoi J, Nakashima K, Maruyama K, Kim J‐M, Seki M, Todaka D et al. 2011. Arabidopsis HsfA1 transcription factors function as the main positive regulators in heat shock‐responsive gene expression. Molecular Genetics and Genomics 286: 321–332. [DOI] [PubMed] [Google Scholar]
- Yu SX, Feng QN, Xie HT, Li S, Zhang Y. 2017. Reactive oxygen species mediate tapetal programmed cell death in tobacco and tomato. BMC Plant Biology 17: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Li G, Chen T, Feng B, Fu W, Yan J, Islam MR, Jin Q, Tao L, Fu G. 2018. Heat stress induces spikelet sterility in rice at anthesis through inhibition of pollen tube elongation interfering with auxin homeostasis in pollinated pistils. Rice 11: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Liu X, Gaikwad K, Kou X, Wang F, Tian X, Xin M, Ni Z, Sun Q, Peng H et al. 2017. Mutations in eIF5B confer thermosensitive and pleiotropic phenotypes via translation defects in Arabidopsis thaliana . Plant Cell 29: 1952–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SS, Yang H, Ding L, Song ZT, Ma H, Chang F, Liu JX. 2017. Tissue‐specific transcriptomics reveals an important role of the unfolded protein response in maintaining fertility upon heat stress in Arabidopsis. Plant Cell 29: 1007–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XL, Zhang J, Guo YH, Sun P, Jia HX, Fan W, Lu MZ, Hu JJ. 2016. Comparative proteomic analysis of mature pollen in triploid and diploid Populus deltoides . International Journal of Molecular Sciences 17: 1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Hu M, Feng X, Gong A, Cheng L, Yuan H. 2017. Proteomes and phosphoproteomes of anther and pollen: availability and progress. Proteomics 17: 1600458. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Zhou L, Liu J, Cao Z, Du X, Huang F, Pan G, Cheng F. 2018. Involvement of CAT in the detoxification of HT‐induced ROS burst in rice anther and its relation to pollen fertility. Plant Cell Reports 37: 741–757. [DOI] [PubMed] [Google Scholar]
- Zhou B, Liu Y, Chen Z, Liu D, Wang Y, Zheng J, Liao X, Zhou AR. 2019. Comparative transcriptome analysis reveals the cause for accumulation of reactive oxygen species during pollen abortion in cytoplasmic male‐sterile Kenaf line 722HA. International Journal of Molecular Sciences 20: 5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK. 2016. Abiotic stress signaling and responses in plants. Cell 167: 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn KE, Tunc‐Ozdemir M, Harper JF. 2010. Temperature stress and plant sexual reproduction: uncovering the weakest links. Journal of Experimental Botany 61: 1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziska LH, Manalo PA, Ordonez RA. 1996. Intraspecific variation in the response of rice (Oryza sativa L.) to increased CO2 and temperature: growth and yield response of 17 cultivars. Journal of Experimental Botany 47: 1353–1359. [Google Scholar]


