Abstract
Background & Aims
The relationship between the frequency of drinking and fatty liver in the general population is still poorly understood. This study analysed data from a large cohort who underwent health checkups in Japan between 2008 and 2019 to investigate the prevalence and incidence of fatty liver by alcohol consumption and risk factors for fatty liver.
Methods
The prevalence of fatty liver diagnosed with ultrasonography was calculated in 75,670 residents. The incidence of fatty liver in 31,062 residents who underwent ultrasonography at least twice during the period without fatty liver at the first time was calculated using the person‐year method. Multivariate logistic analysis was performed to investigate risk factors associated with the prevalence and incidence of fatty liver.
Results
The prevalence of fatty liver was 27.6% (95% confidence interval [CI], 27.2‐27.9) in non‐drinkers, 28.5% (27.5‐29.5) in moderate‐drinkers and 28.0% (26.0‐29.9) in heavy‐drinkers. The incidence of fatty liver was 3,084/100,000 person‐years (2,997‐3,172/100,000) in non‐drinkers, 3,754/100,000 person‐years (3,481‐4,042/100,000) in moderate‐drinkers and 3,861/100,000 person‐years (3,295‐4,497/100,000) in heavy‐drinkers. The prevalence and incidence of fatty liver were not associated with drinking status. Obesity was the most important independent risk factor (prevalence: adjusted odds ratio [AOR], 6.3; 95% CI, 6.0‐6.5; incidence: AOR, 2.4; 95% CI, 2.3‐2.6).
Conclusions
Drinking status does not affect the prevalence or incidence of fatty liver in Japanese residents undergoing health checkups. From a public health perspective, measures for obesity control must be prioritised to reduce the burden of disease of fatty liver in Japan.
Keywords: drinking status, fatty liver, incidence, Japan, NAFLD, prevalence
Abbreviations
- AFLD
alcoholic fatty liver disease
- AOR
adjusted odds ratio
- BMI
body mass index
- CI
confidence interval
- HCV
hepatitis C virus
- MAFLD
metabolic dysfunction‐associated fatty liver disease, HBV, hepatitis B virus
- NAFLD
non‐alcoholic fatty liver disease
- OR
odds ratio
- SD
standard deviation
1. INTRODUCTION
Non‐alcoholic fatty liver disease (NAFLD) is a global health problem that is becoming more common with changes in dietary habits and increasing prevalence of obesity. The prevalence of NAFLD varies across countries or regions: it has been reported to be 27.37% in Asia, 31.79% in the Middle East, 24.13% in North America, 30.45% in South America, 23.71% in Europe and 13.48% in Africa. 1
To diagnose NAFLD, alcoholic fatty liver disease (AFLD) needs to be ruled out. Japanese guidelines define the upper limit of alcohol consumption for diagnosis of NAFLD as 30 g/day of pure ethanol in males and 20 g/day of pure ethanol in females. 2 However, some argue that the distinction between NAFLD and AFLD has little clinical significance. 3 , 4 Recently, defining fatty liver disease as metabolic dysfunction‐associated fatty liver disease (MAFLD), regardless of alcohol consumption or other concomitant liver diseases, has been proposed. 5 However, the prevalence and incidence of fatty liver and risk factors for fatty liver by alcohol consumption in the general population remain unclear. This study aimed to investigate these factors through the analysis of data from a large cohort who underwent health checkups in Japan.
2. MATERIALS AND METHODS
This study was designed as a retrospective observational study. It included residents who underwent health checkups in Hiroshima or Iwate Prefecture, Japan. This cohort consisted of all residents who underwent a health checkup provided by the Foundation for Community Health and Medicine Promotion in Hiroshima Prefecture between April 2013 and July 2018 (5 years) or the Iwate Prefectural Preventive Medicine Association between April 2008 and March 2019 (11 years). Among total 3,817,770 (Hiroshima: 172,819, Iwate: 3,644,951) number of health checkup data, 856,296 were available after excluding duplicates (Figure 1). Analyses were performed using anonymised data collected from a questionnaire completed at the health checkup, laboratory testing results and abdominal ultrasonography results. Ultrasonography is not a mandatory item for health checkups but an optional test for those who wish. Fatty liver was diagnosed if any of the following four ultrasonic findings were present: bright liver, hepato‐renal contrast, deep attenuation and vascular blurring. 6 A clinical laboratory technician made the primary diagnosis, and a radiologist reviewed the images to make the final diagnosis.
FIGURE 1.
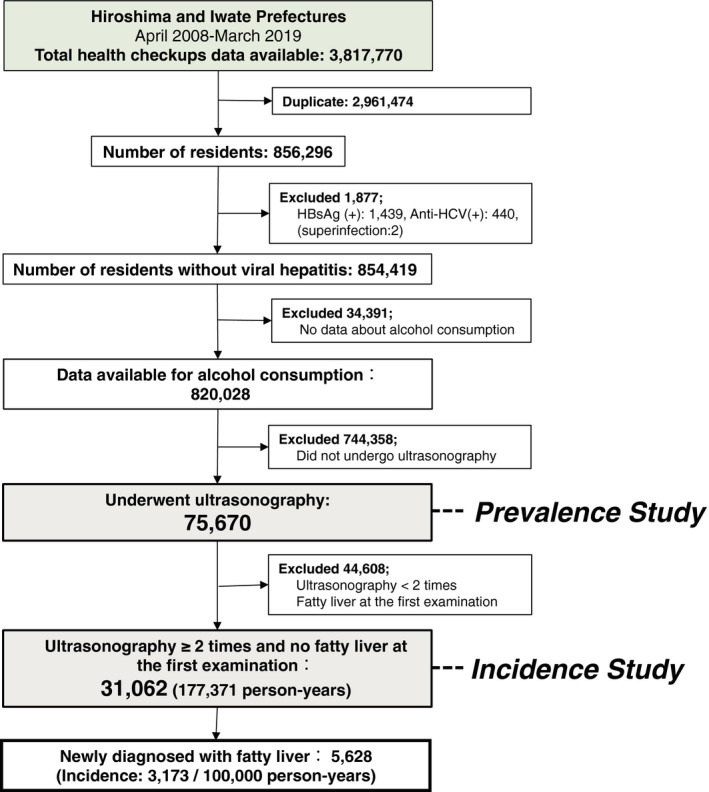
Flow of the study. Flowchart of selecting study subjects from the health checkup database in Hiroshima (April 2013‐July 2018) and Iwate (April 2008‐March 2019) prefectures, Japan
2.1. Alcohol consumption
Alcohol consumption was evaluated in 820,028 residents without hepatitis B virus (HBV) or hepatitis C virus (HCV) infection who responded to a questionnaire about alcohol use at their first health checkup during the study period (Figure 1). Of them, the number of subjects ≥70 years old was smaller than the other age groups, but all the other age groups were almost the same number (Figure 2A). HBV and HCV infection status were defined based on the results of screening tests which were carried out for applicants at health checkups during the study period. The questionnaire for alcohol intake was based on the standard form prescribed by the Ministry of Health, Labor and Welfare in Japan for the Specific Health Check and Guidance program (Tokutei‐Kenshin) provided by all insurers to all adults over 40 years old in Japan. Subjects were asked to indicate their drinking frequency (daily, sometimes and rarely) and amount of alcohol consumed in units of glasses of sake. A glass (180 mL) of this traditional Japanese alcohol beverage contains 20 g of pure ethanol. For other alcohol types, an equivalence in glasses of sake was used (500 mL for beer, 60 mL for whisky, 240 mL for wine and 110 mL for shochu, another traditional Japanese beverage). The results of the alcohol consumption questionnaire were used to classify the respondents into three categories: non‐drinkers, moderate drinkers and heavy drinkers. A non‐drinker was defined as a male with alcohol consumption (pure ethanol equivalent) of <30 g/day or a female who consumed <20 g/day. A moderate drinker was defined as a male who consumed 30‐60 g/day or a female who consumed 20‐40 g/day. A heavy drinker was defined as a male who consumed ≥60 g/day or a female who consumed ≥40 g/day. The distribution of alcohol consumption was evaluated by sex and age group.
FIGURE 2.
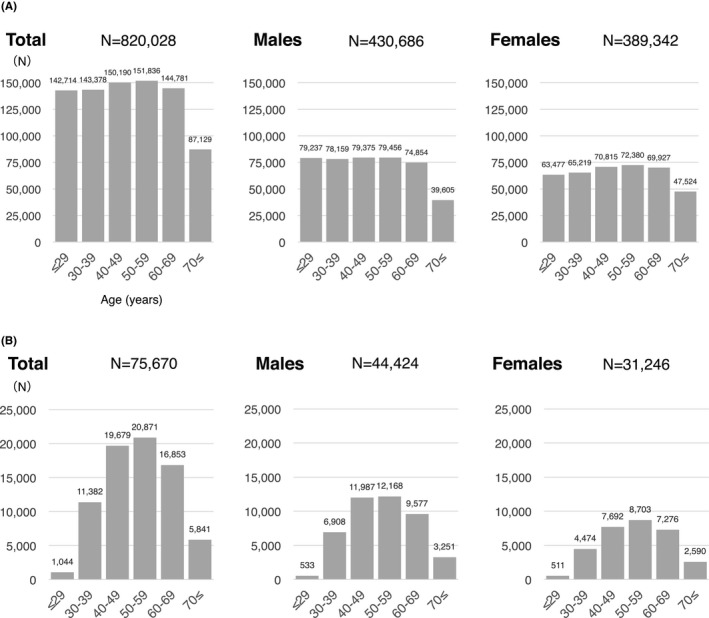
Age distribution of (A) all subjects who underwent health checkups with or without ultrasonography and answered for alcohol consumption, B) subjects who underwent ultrasonography at health checkups and answered for alcohol consumption. The largest and smallest age groups for those who underwent ultrasonography were 50‐59 years and 20‐29 years for both males and females. Age is the age at the time of the survey
2.2. Prevalence of fatty liver
Among the 820,028 study participants, the prevalence of fatty liver was investigated in 75,670 residents (44,424 males and 31,246 females) who underwent abdominal ultrasonography at least once during the study period (Figure 1). The largest age group for males and females was 50‐59 years (Figure 2B). For this study, fatty liver was defined as fatty liver detected by abdominal ultrasonography. The prevalence of fatty liver based on the results of the first abdominal ultrasonography examination during the study period was calculated by alcohol consumption, sex, and age group. Fatty liver in non‐drinkers who did not test positive for HBV or HCV was defined as NAFLD.
2.3. Incidence of fatty liver
The incidence of fatty liver was calculated using longitudinal data. Among the 75,670 residents mentioned above, 31,062 who underwent ultrasonography at least twice during the study period and were not diagnosed with fatty liver based on the first ultrasonography examination were included in the analysis (Figure 1). The incidence of fatty liver was calculated by sex, age, and alcohol consumption using the person‐year method.
2.4. Statistical analysis
Chi‐square test with post hoc multiple comparisons by Bonferroni correction were used to compare groups. Multiple logistic regression analysis was performed to determine risk factors associated with the prevalence or incidence of fatty liver using age group, sex, alcohol consumption, presence or absence of diabetes, and body mass index (BMI). Statistical analyses were conducted using JMP14.2.0 software (SAS Institute Inc, Cary, NC, USA); p values of less than 0.05 were regarded as statistically significant.
2.5. Ethics considerations
This was a retrospective observational study using existing data without any interventions. The existing data were converted into an anonymous database to protect privacy. This study received ethics approval for the use of data with an opt‐out methodology based on the low risk of harm to the participants. The ethics committees for epidemiological research at Hiroshima University, Japan waived the need for further informed consent and approved the study (approval number, E‐1082). All study activities were performed in accordance with relevant guidelines and regulations.
3. RESULTS
3.1. Alcohol consumption
Figure 3 and Supplementary Table S1 show alcohol consumption among study participants in Hiroshima and Iwate by sex and age group. The percentage of residents who drank alcohol every day was highest in males aged 50‐59 years (50.9%) and in females aged 40‐49 years (15.2%). The percentage of residents who drank three glasses or more per day was highest among males aged 40‐49 years (3.7%). The distribution of alcohol consumption by sex and age group was similar in Hiroshima and Iwate despite being geographically far from east to west (Supplementary Table S1).
FIGURE 3.
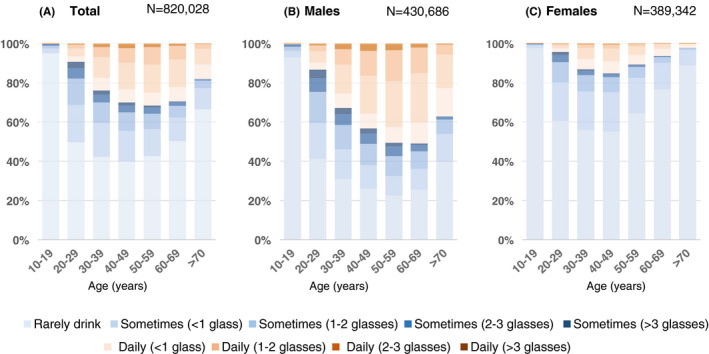
Frequency of alcohol consumption in Japan. This figure shows the frequency of alcohol consumption in Japan (N = 820,028) by sex and age group. Each colour represents the amount of alcohol consumption ranging from rarely drinking (light blue) to more than three glasses per day (dark brown). Age is the age at the time of the survey
3.2. Characteristics of study participants who underwent ultrasonography stratified by alcohol consumption
After excluding duplicates, there were 856,296 residents who underwent health checkups. After excluding 1,877 residents who tested positive for HBV or HCV and 34,391 residents with no data about alcohol consumption from the questionnaire, there were 820,028 potential study participants. Among them, 75,670 (9.2%) underwent ultrasonography at a health checkup (Figure 1). Of these 75,670 residents, 65,194 (89.2%) were non‐drinkers, 8,424 (8.9%) were moderate drinkers and 2,052 (1.9%) were heavy drinkers. Table 1 shows the characteristics of study participants by alcohol consumption. The percentage of males in non‐drinkers was lower than those in moderate or heavy drinkers (56.2%, 75.9% and 72.8% respectively). The percentage of obese individuals (BMI ≥25 kg/m2) was 28.6% in non‐drinkers, 31.4% in moderate drinkers, and 27.6% in heavy drinkers. The percentage of residents with diabetes was 6.3% in non‐drinkers, 6.6% in moderate drinkers, and 5.0% in heavy drinkers. In study participants with the fatty liver (N = 20,944), 59.9% of non‐drinkers (N = 17,969), 62.6% of moderate drinkers (N = 2,401) and 57.5% of heavy drinkers (N = 574) were obese and 12.8%, 13.7% and 10.6% had diabetes respectively.
TABLE 1.
Characteristics of non‐drinkers, moderate drinkers and heavy drinkers
| Total (N = 75,670) | Fatty liver (N = 20,944) | |||||
|---|---|---|---|---|---|---|
| Non‐drinkers | Moderate drinkers | Heavy drinkers | Non‐drinkers (NAFLD) | Moderate drinkers | Heavy drinkers (AFLD) | |
| N (%) | 65,194 (89.2%) | 8,424 (8.9%) | 2,052 (1.9%) | 17,969 (85.8%) | 2,401 (11.5%) | 574 (2.7%) |
| Male (%) | 56.2% | 75.9% | 72.8% | 70.9% | 90.1% | 82.1% |
| Age: years (mean ±SD) | 52.6 ± 12.2 | 50.2 ± 10.3 | 48.8 ± 9.8 | 52.9 ± 11.0 | 51.1 ± 9.7 | 50.0 ± 9.7 |
| BMI (mean ±SD) | 23.4 ± 3.5 | 23.7 ± 3.5 | 23.3 ± 3.4 | 26.2 ± 3.5 | 26.3 ± 3.6 | 25.7 ± 3.6 |
| BMI ≥25.0 kg/m2 | 28.6% | 31.4% | 27.6% | 59.9% | 62.6% | 57.5% |
| Diabetes | 6.3% | 6.6% | 5.0% | 12.8% | 13.7% | 10.6% |
| Fatty liver | 27.6% | 28.5% | 28.0% | |||
AFLD, alcoholic fatty liver disease; BMI, body mass index; NAFLD, non‐alcoholic fatty liver disease; SD, standard deviation.
Non‐drinker: Male with alcohol consumption (pure ethanol equivalent) of <30 g/day, female with alcohol consumption of <20 g/day.
Moderate drinkers: Male with alcohol consumption of 30‐60 g/day, female with alcohol consumption of 20‐40 g/day.
Heavy drinker: Male with alcohol consumption of ≥60 g/day, female with alcohol consumption of ≥40 g/day.
3.3. Prevalence of fatty liver
Fatty liver was detected in 27.7% (20,994/75,670, 95% confidence interval [CI]; 27.4‐28.1) of residents who underwent ultrasonography at a health checkup. Non‐drinkers made up 85.8% of residents with fatty liver. Therefore, 23.7% (N = 17,969) of the 75,670 residents were considered to have NAFLD. The prevalence of fatty liver was 27.6% (17,969/65,194, 95% CI; 27.2‐27.9) in non‐drinkers, 28.5% (2,401/8,424, 95% CI; 27.5‐29.5) in moderate drinkers and 28.0% (574/2,052, 95% CI; 26.0‐29.9) in heavy drinkers. There was no significant difference in the prevalence of fatty liver in the three groups (P =.1844). In males, fatty liver was observed in 34.8% (N = 12,740/36,624, 95% CI; 34.3‐35.3) of non‐drinkers, 34.3% (N = 2,162/6,307, 95% CI; 33.1‐33.5) of moderate drinkers and 31.5% (N = 471/1,493, 95% CI; 29.2‐33.9) of heavy drinkers (Figure 4). In females, fatty liver was observed in 18.3% (N = 5,239/28,570, 95% CI; 17.9‐18.8) of non‐drinkers, 11.3% (N = 240/2,117, 95% CI; 10.0‐12.7) of moderate drinkers and 18.4% (N = 103/559, 95% CI; 15.2‐21.6) of heavy drinkers (Figure 4). Males had a higher prevalence of NAFLD (34.8%) than females (18.3%) (P <.0001). Among males, the prevalence of fatty liver was highest in the 40‐49 age group (39.7%), whereas in females, the prevalence was highest in the 60‐69 age group (25.0%) (Figure 4, Supplementary Table S2).
FIGURE 4.
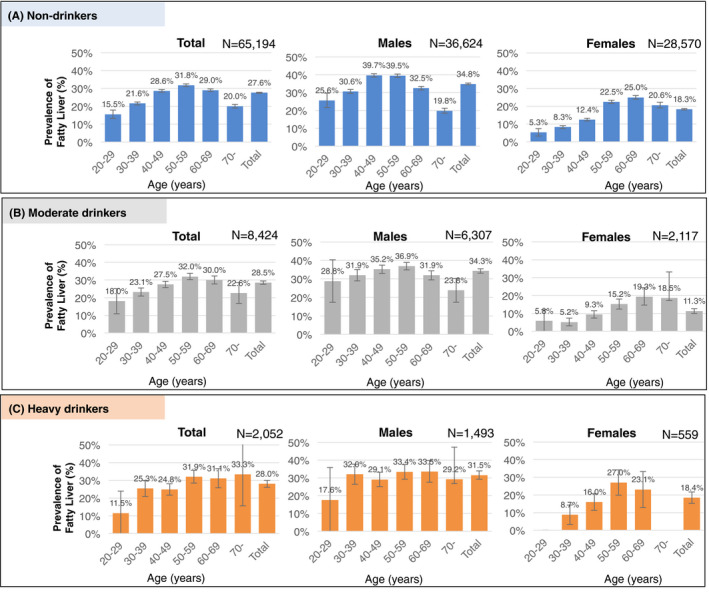
Age and sex‐specific prevalence of fatty liver stratified by alcohol consumption among residents who underwent ultrasonography at health checkups in Japan. This figure shows the prevalence of fatty liver stratified by alcohol consumption by sex and age group in Japan in A) Non‐drinkers (pure ethanol consumption <30 g/day for males or <20 g/day for females), B) Moderate drinkers (30‐60 g/day for males or 20‐40 g/day for females), and C) Heavy drinkers (≥60 g/day for males or a ≥ 40 g/day for females). Age is the age at the time of the survey
3.4. Risk factors associated with the prevalence of fatty liver
Table 2 shows the results of the univariate and multivariate analyses of risk factors associated with the prevalence of fatty liver. Significant risk factors in the multivariate analysis were age of 40‐59 years (adjusted odds ratio [AOR], 1.5; 95% confidence interval [CI], 1.4‐1.6; P <.0001), age ≥60 years (AOR, 1.2; 95% CI 1.1‐1.2; P <.0001), male sex (AOR, 2.0; 95% CI, 1.9‐2.0; P <.0001), moderate drinking (AOR, 0.8; 95% CI, 0.8‐0.9; P <.0001), diabetes (AOR, 2.5; 95% CI, 2.3‐2.7; P <.0001), BMI ≥25 kg/m2 (AOR, 6.3; 95% CI, 6.5‐7.0; P <.0001) and BMI <18.5 kg/m2 (AOR, 0.1; 95% CI, 0.0‐0.1; P <.0001). There were no significant differences in the prevalence of fatty liver between non‐drinkers and heavy drinkers (P =.1614).
TABLE 2.
Logistic regression analysis on factors affecting fatty liver prevalence among 75,670 participants in Japan
| Factor | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| Prevalence | OR (95% CI) | p | AOR (95% CI) | p | |||
|
Fatty liver + N (%) |
Fatty liver – N (%) |
||||||
| Age, years | <40 | 2,653 (21.4) | 9,773 (78.7) | 1 | 1 | ||
| 40‐59 | 12,203 (30.1) | 28,347 (69.9) | 1.6 (1.5‐1.7) | <.0001 | 1.5 (1.4‐1.6) | <.0001 | |
| ≥60 | 6,088 (26.8) | 16,606 (73.2) | 1.4 (1.3‐1.4) | <.0001 | 1.2 (1.1‐1.2) | <.0001 | |
| Sex | Male | 15,373 (34.6) | 29,051 (65.4) | 2.4 (2.4‐2.5) | <.0001 | 2.0 (1.9‐2.0) | <.0001 |
| Female | 5,571 (17.8) | 25,675 (82.2) | 1 | 1 | |||
| Alcohol consumption | Heavy drinker | 574 (28.0) | 1,478 (72.0) | 1.0 (0.9‐1.1) | 1 | 0.9 (0.8‐1.0) | .1614 |
| Moderate drinker | 2,401 (28.5) | 6,023 (71.5) | 1.0 (0.9‐1.0) | .1394 | 0.8 (0.8‐0.9) | <.0001 | |
| Non‐drinker | 17,696 (27.6) | 47,225 (72.4) | 1 | 1 | |||
| Diabetes | Yes | 2,693 (56.1) | 2,107 (43.9) | 3.7 (3.5‐3.9) | <.0001 | 2.5 (2.3‐2.7) | <.0001 |
| No | 18,251 (25.8) | 52,619 (74.3) | 1 | 1 | |||
| BMI, kg/m2 | <18.5 | 48 (1.2) | 4,144 (98.9) | 0.1 (0.0‐0.1) | <.0001 | 0.1 (0.1‐0.1) | <.0001 |
| 18.5‐24.9 | 8.303 (16.7) | 41,341 (83.3) | 1 | 1 | |||
| ≥25.0 | 12,593 (57.7) | 9,240 (42.3) | 6.8 (6.5‐7.0) | <.0001 | 6.3 (6.0‐6.5) | <.0001 | |
AOR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; OR, odds ratio.
Univariate analysis: χ2 test.
Multivariate analysis: Logistic regression analysis; R2 = 0.1849; Model P <.0001.
3.5. Incidence of fatty liver
The incidence of fatty liver in 31,062 residents who underwent ultrasonography at least twice between 2008 and 2019 and were not diagnosed with fatty liver at the first ultrasonography examination was evaluated using the person‐year method. The mean observation period was 6.0 years (standard deviation, 3.0; range, 1‐11 years). The total observation period was 177,371 person‐years. During the target period, 5,628 residents had newly diagnosed fatty liver. Therefore, the incidence of fatty liver was estimated to be 3,173/100,000 person‐years (95% CI, 3,091‐3,257). By alcohol consumption, incidence was estimated to be 3,084/100,000 person‐years (95% CI, 2,997‐3,172) in non‐drinkers (N = 26,809), 3,754/100,000 person‐years (95% CI, 3,481‐4,042) in moderate drinkers (N = 3,466) and 3,861/100,000 person‐years (95% CI: 3,295‐4,497) in heavy drinkers (N = 787). The incidence of fatty liver by sex, age and alcohol consumption is shown in Figure 5 and Supplementary Table S3. There was a high incidence of NAFLD in males aged 40‐59 years and females aged 60‐69 years.
FIGURE 5.
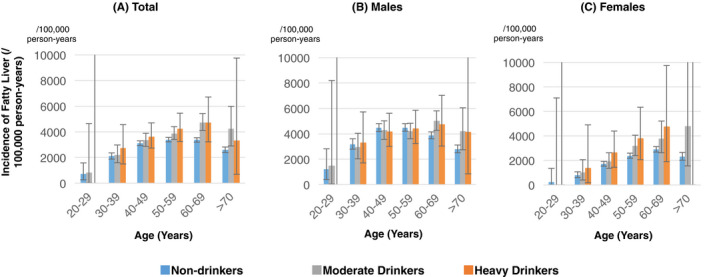
Age and sex‐specific incidence of fatty liver stratified by alcohol consumption among residents who underwent ultrasonography at health checkups in Japan. The incidence of fatty liver stratified by alcohol consumption was compared by age group in males and females among residents who underwent ultrasonography at health checkups in Japan. Blue bars represent non‐drinkers, grey bars moderate drinkers and orang bars heavy drinkers. Non‐drinkers: pure ethanol consumption <30 g/day for males or <20 g/day for females; Moderate drinkers: 30‐60 g/day for males or 20‐40 g/day for females; Heavy drinkers: ≥60 g/day for males or a ≥ 40 g/day for females. Age is the age at the time of the survey
3.6. Risk factors associated with the incidence of fatty liver
Table 3 shows the results of the univariate and multivariate analyses of risk factors associated with the incidence of fatty liver. Significant risk factors in the multivariate analysis include age of 40‐59 years (AOR, 1.3; 95% CI, 1.2‐1.4; P <.0001), male sex (AOR, 1.5; 95% CI, 1.4‐1.6; P <.0001), diabetes (AOR, 1.5; 95% CI, 1.3‐1.7; P <.0001), BMI ≥25 kg/m2 (AOR, 2.4; 95% CI, 2.3‐2.6; P <.0001) and BMI <18.5 kg/m2 (AOR, 0.2; 95% CI, 0.2‐0.3; P <.0001). There were no significant associations between alcohol consumption and fatty liver incidence.
TABLE 3.
Logistic regression analysis on factors affecting fatty liver incidence
| Factor | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| Incidence | OR (95% CI) | P | AOR (95% CI) | P | |||
|
Fatty liver + N (%) |
Fatty liver – N (%) |
||||||
| Age, years | <40 | 751 (15.3) | 4,155 (84.7) | 1 | 1 | ||
| 40‐59 | 3,322 (19.5) | 13,695 (80.5) | 1.3 (1.2‐1.5) | <.0001 | 1.3 (1.2‐1.4) | <.0001 | |
| ≥60 | 1,555 (17.0) | 7,584 (83.0) | 1.1 (1.0‐1.2) | .0184 | 1.0 (0.9‐1.1) | .7657 | |
| Sex | Male | 3,739 (21.6) | 13,550 (78.4) | 1.7 (1.6‐1.8) | <.0001 | 1.5 (1.4‐1.6) | <.0001 |
| Female | 1,889 (13.7) | 11,884 (86.3) | 1 | 1 | |||
| Alcohol consumption | Heavy drinker | 178 (22.6) | 609 (77.4) | 1.3 (1.1‐1.6) | .0010 | 1.2 (1.0‐1.4) | .0877 |
| Moderate drinker | 680 (19.6) | 2,786 (80.4) | 1.2 (1.0‐1.2) | .0168 | 1.0 (0.9‐1.1) | .7235 | |
| Non‐drinker | 4,770 (17.8) | 22,039 (82.2) | 1 | 1 | |||
| Diabetes | Yes | 340 (28.4) | 856 (71.6) | 1.8 (1.6‐2.1) | <.0001 | 1.5 (1.3‐1.7) | <.0001 |
| No | 5,288 (17.7) | 24,578 (82.3) | 1 | 1 | |||
| BMI, kg/m2 | <18.5 | 77 (3.5) | 2,104 (96.5) | 0.2 (0.2‐0.2) | <.0001 | 0.2 (0.2‐0.3) | <.0001 |
| 18.5‐24.9 | 3,825 (16.2) | 19,788 (83.8) | 1 | 1 | |||
| ≥25.0 | 1,726 (32.8) | 3,542 (67.2) | 2.5 (2.4‐2.7) | <.0001 | 2.4 (2.3‐2.6) | <.0001 | |
AOR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; OR, odds ratio.
Univariate analysis: χ2 test.
Multivariate analysis: Logistic regression analysis; R2 = 0.0490; Model P <.0001.
4. DISCUSSION
In this study, the prevalence and incidence of fatty liver by alcohol consumption were estimated based on the analysis of a general population who received ultrasonography at health checkups in Japan. The strength of this study is the large cohort size, so that heavy drinkers, whose proportion is small at 1.9% of the total, could be evaluated.
According to the literature, fatty liver develops in about 90% of individuals who drink more than 60 g/day of alcohol. 7 However, in this study, the prevalence of fatty liver in heavy drinkers was 28.0%. This discrepancy is thought to be because of the difference between hospital‐based data and health checkups data.
In this study, subjects were recruited among those who attended health checkups, and the amount of alcohol drinking was self‐reported. Therefore, there is a possibility of selection bias and under‐reporting of alcohol consumption. Indeed, according to the 2019 National Health and Nutrition Survey, 8 the percentage of heavy drinkers was accounted for 6.7%. However, it is unlikely that the amount of alcohol consumed was over‐reported, so those classified as heavy drinkers in this study are most probably to be individuals with high levels of alcohol consumption. Data on heavy drinkers in the general population in Japan are scarce. Thus, the large number of heavy drinkers included in this study is of particular interest and allows reliable interpretation of the findings for this group.
The prevalence of NAFLD in this study is consistent with previously reported results. 9 , 10 , 11 Multivariate analysis showed no significant differences in the prevalence of fatty liver among non‐drinkers and heavy drinkers and that obesity is the most important independent risk factor for the prevalence of fatty liver. Diabetes, male sex, age 40 years and older, were also independent and significant risk factors for the prevalence of fatty liver. Takahashi et al also reported that alcohol intake was not associated with fatty liver prevalence in males, but heavy alcohol intake was a significant risk of fatty liver in females in Japan. 11 However, the small number of females with heavy drinking in their study (N = 78) suggests caution in interpreting these results.
As for the incidence of NAFLD, there are only a few reports. 10 , 12 Although a meta‐analysis reported that the incidence of NAFLD in Asia is 52.34 per 1,000 persons (95% CI, 28.31‐96.77), 1 there have been no large‐scale retrospective cohort studies in the general population. This study is the first report to reveal the incidence of fatty liver by alcohol drinking status in Japanese cohort undergoing health checkups. In the results of the risk factor analysis, like the prevalence results, the incidence of fatty liver was not associated with alcohol consumption but associated with obesity, diabetes, male sex and age 40‐59 years independently. Further studies are needed to investigate the cure rate for fatty liver in a general population because fatty liver is a reversible condition.
The definition of NAFLD was developed based on the fact that some non‐drinkers present with histopathological findings that are very similar to those observed in patients with AFLD. 13 , 14 NAFLD diagnosis needs to exclude AFLD because of the apparent different pathogenetic mechanisms between alcoholic factor and non‐alcoholic factors (obesity and metabolic factors) in fatty liver. However, it has recently been reported that both diseases have common causes, such as hypernutrition and mitochondrial dysfunction, 15 , 16 , 17 and increased endotoxin burden because of increased intestinal permeability. 18 , 19 , 20 In addition, it is not uncommon for obesity and drinking factors to coexist in patients with fatty liver. In the present study, 57.5% of heavy drinkers with fatty liver were obese. There is a discussion on the necessity of distinguishing between these diseases. 5 Furthermore, it is unclear whether NAFLD and AFLD can be distinguished based on the amount of alcohol consumed because people tend to underreport their drinking, and lifelong drinking is not taken into consideration when defining these diseases. Also, the non‐drinker category does not only include residents who do not consume any alcohol; it also includes males who consume <30 g/day and females who consume <20 g/day. Therefore, in some patients diagnosed with NAFLD, alcohol consumption cannot be completely ruled out and might have contributed to fatty liver.
Nevertheless, studies on the prognosis of alcoholic and non‐alcoholic fatty liver should be continued. Recently, a large population‐based study reported synergy between alcohol and metabolic/obesity risk when fibrosis is considered. 21 Another study reported that the risk of liver cancer in patients with AFLD is higher than in those with NAFLD, 22 although no consensus has been reached yet.
According to a modelling study based on epidemiological data, 23 the total number of NAFLD patients in Japan in 2016, estimated at 22.7 million, will not change in 2030, but the proportion of advanced liver fibrosis is expected to increase because of the ageing of the population. To reduce the burden of fatty liver disease, measures for obesity control such as assistance in changing lifestyle habits are the most important.
4.1. Limitations
In this study, the prevalence of fatty liver was estimated in residents who underwent ultrasonography at health checkups. However, less than 10% of residents who underwent health checkups underwent ultrasonography. According to the results of the age distribution, young people in their 30s and younger were less probably to undergo ultrasonography. Since those previously diagnosed with fatty liver tend to undergo ultrasonography, selection bias might have led to an overestimation of the prevalence of fatty liver. Further studies are needed to investigate the prevalence of fatty liver in the general population of randomly selected residents.
5. CONCLUSION
This study was conducted to investigate the epidemiology of fatty liver in a general population based on a large dataset from health checkup programmes in Japan. In the results, drinking status did not affect the prevalence or incidence of fatty liver. From a public health perspective, measures for obesity must be prioritised to reduce the burden of disease of fatty liver in Japan.
CONFLICT OF INTEREST
The authors declare no conflicts of interest that pertain to this work.
AUTHORS CONTRIBUTIONS
Study concept and design: JT. Acquisition of data: TH, TS, MK and JT. Data management: AK, TH, TS, and MK. Data analysis: AS, AK, TA, MK, and JT. Statistical analysis: AS, TA and JT. Interpretation of data: AS, and JT. Manuscript development: AS, SO, BE, KK, AR, HA and JT. Study supervision: JT. All authors reviewed and approved the final version of the manuscript.
Supporting information
Table S1‐S3
ACKNOWLEDGEMENTS
This research was partly funded by a grant from the Japanese Ministry of Health, Labor, and Welfare (19HC1001), AMED (20fk0210040h0003) and JSPS KAKENHI Grant‐in‐Aid for Scientific Research B (19H03886).
Sugiyama A, Kurisu A, Ouoba S, et al. Relationship between drinking frequency and fatty liver prevalence or incidence in Japanese undergoing health checkup in 2008‐2019. Liver Int. 2021;41:2914–2923. 10.1111/liv.15055
Funding information
This research was partly funded by a grant from the Japanese Ministry of Health, Labour, and Welfare (19HC1001), AMED (20fk0210040h0003) and JSPS KAKENHI Grant‐in‐Aid for Scientific Research B (19H03886).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease‐Meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73‐84. [DOI] [PubMed] [Google Scholar]
- 2. Evidence‐based Clinical Practice Guidelines for Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis . The Japanese Society of Gastroenterology/The Japan Society of Hepatology. Nankodo; 2020. [Google Scholar]
- 3. Sanyal AJ, American GA. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123(5):1705‐1725. [DOI] [PubMed] [Google Scholar]
- 4. Volzke H. Multicausality in fatty liver disease: is there a rationale to distinguish between alcoholic and non‐alcoholic origin? World J Gastroenterol. 2012;18(27):3492‐3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction‐associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202‐209. [DOI] [PubMed] [Google Scholar]
- 6. Hamaguchi M, Kojima T, Itoh Y, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol. 2007;102(12):2708‐2715. [DOI] [PubMed] [Google Scholar]
- 7. O'Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51(1):307‐328. [DOI] [PubMed] [Google Scholar]
- 8. Ministry of Health Labor and Welfare Japan . National Health and Nutrition Survey. 2019. https://www.mhlw.go.jp/content/000711008.pdf
- 9. Eguchi Y, Hyogo H, Ono M, et al. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol. 2012;47(5):586‐595. [DOI] [PubMed] [Google Scholar]
- 10. Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143(10):722‐728. [DOI] [PubMed] [Google Scholar]
- 11. Takahashi H, Ono M, Hyogo H, et al. Biphasic effect of alcohol intake on the development of fatty liver disease. J Gastroenterol. 2015;50(11):1114‐1123. [DOI] [PubMed] [Google Scholar]
- 12. Suzuki A, Angulo P, Lymp J, et al. Chronological development of elevated aminotransferases in a nonalcoholic population. Hepatology. 2005;41(1):64‐71. [DOI] [PubMed] [Google Scholar]
- 13. Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55(7):434‐438. [PubMed] [Google Scholar]
- 14. Schaffner F, Thaler H. Nonalcoholic fatty liver disease. Prog Liver Dis. 1986;8:283‐298. [PubMed] [Google Scholar]
- 15. Arteel GE. Oxidants and antioxidants in alcohol‐induced liver disease. Gastroenterology. 2003;124(3):778‐790. [DOI] [PubMed] [Google Scholar]
- 16. Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122(6):1649‐1657. [DOI] [PubMed] [Google Scholar]
- 17. Sakaguchi S, Takahashi S, Sasaki T, Kumagai T, Nagata K. Progression of alcoholic and non‐alcoholic steatohepatitis: common metabolic aspects of innate immune system and oxidative stress. Drug Metab Pharmacokinet. 2011;26(1):30‐46. [DOI] [PubMed] [Google Scholar]
- 18. Diehl AM. Nonalcoholic fatty liver disease: implications for alcoholic liver disease pathogenesis. Alcohol: Clin Exp Res. 2001;25(Supplement):8S‐14S. [DOI] [PubMed] [Google Scholar]
- 19. Forgione A, Miele L, Cefalo C, Gasbarrini G, Grieco A. Alcoholic and nonalcoholic forms of fatty liver disease. Minerva Gastroenterol Dietol. 2007;53(1):83‐100. [PubMed] [Google Scholar]
- 20. Sabaté J‐M, Jouët P, Harnois F, et al. High prevalence of small intestinal bacterial overgrowth in patients with morbid obesity: a contributor to severe hepatic steatosis. Obes Surg. 2008;18(4):371‐377. [DOI] [PubMed] [Google Scholar]
- 21. Abeysekera KWM, Fernandes GS, Hammerton G, et al. Prevalence of steatosis and fibrosis in young adults in the UK: a population‐based study. Lancet Gastroenterol Hepatol. 2020;5(3):295‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sørensen HT, Mellemkjær L, Jepsen P, et al. Risk of cancer in patients hospitalized with fatty liver: a Danish cohort study. J Clin Gastroenterol. 2003;36(4):356‐359. [DOI] [PubMed] [Google Scholar]
- 23. Estes C, Anstee QM, Arias‐Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69(4):896‐904. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S3
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


