Abstract
BACKGROUND
Many groups of insects utilize substrate‐borne vibrations for intraspecific communication. This characteristic makes them a suitable model for exploring the use of vibrations as a tool for pest control as an alternative to the use of chemicals. Detailed knowledge of species communication is a prerequisite to select the best signals to use. This study explored the use of substrate‐borne vibrations for pest control of the brown marmorated stink bug (BMSB), Halyomorpha halys Stål (Heteroptera: Pentatomidae). For this purpose, we first identified the spectral and temporal characteristics that best elicit male responsiveness. Bioassays were conducted with artificial signals that mimicked the natural female calling signal. Second, we used the acquired knowledge to synthesize new signals endowed with different degrees of attractiveness in single‐ and two‐choice bioassays using a wooden custom‐made T stand.
RESULTS
The results from this study showed that males were attracted to female signals along a high range of amplitudes, especially starting from a threshold of 100 μm s−1, a high pulse repetition time (1 s) and frequency peak corresponding to the first harmonic (76 Hz). This resulted in an “optimal” signal for use to attract males, while the choice test in the T arena showed that this signal elicits searching behavior and attracts BMSB males towards a stimulation point.
CONCLUSION
We confirm the use of vibrational signals as a strong tool for behavioral manipulation of male BMSB and suggest its possible use in the development of field traps and further management of this pest. © 2021 The Authors. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: biotremology, Halyomorpha halys, vibrational communication, playbacks, insects, brown marmorated stink bug
Vibrotaxis experiments allowed us to define the signal parameters that best attract Halyomorpha halys to vibrotraps. In the near future, this strategy of behavioral manipulation could become a new solution for stinkbug pest management.
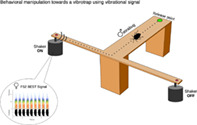
1. INTRODUCTION
Behavioral manipulation of a target species (with attraction to/repellence from a source of attractive/repelling signals) and mating disruption are two alternative methods for insect pest management in agriculture to minimize the risk that pesticides pose to human health and the environment. 1 , 2 Pest control through interference with cues used for inter‐ and intraspecific communication, theorized since the 1940s with the idea of releasing sexual pheromones in the field to catch moths, 3 is nowadays successfully applied worldwide to control several crop pests, particularly Lepidoptera. 4 , 5
More recently, a new technique of mating disruption based on the use of substrate‐borne vibrations in place of pheromones has been described and effectively tested in semi‐field and field trials. 6 , 7 , 8 In many insect species, vibrational signals (that is, emission, reception, and correct interpretation of signals) are essential to accomplish mating. 9 , 10 , 11 This is also true for many Hemiptera (that is, leafhoppers, planthoppers, stinkbugs), in which the mating process is composed of several behavioral steps, after the initial reciprocal (that is, male–female) identification, passing through mate location, courtship and eventually ending with copulation. As a general rule, the sender (more commonly the male) initially emits a call to elicit a response from the receiver (the potential mate), thus establishing a vibrational duet with him/her. 12 However, single emission of a call does not automatically elicit a behavioral response in the receiver, and it is fundamental that the emitted signal contains certain spectral/temporal features capable of positively motivating the receiver to respond and search for the sender. 13 Indeed, even small differences in the structure of a vibrational signal (that is, the secondary components of a song frequency pattern) could drastically affect the motivation of an individual to establish a duet with a potential partner. 10 , 11 , 14 A direct correlation between signal and emitter quality is well documented in animals 15 ; therefore, it is of interest to define the roles and values of spectral and temporal parameters affecting signal efficacy. 16
In this study, we focused on the brown marmorated stinkbug (BMSB) Halyomorpha halys (Hemiptera: Pentatomidae), an insect pest native to east Asia that recently became invasive in North America and Europe, causing severe economic damage to numerous crops. 17 , 18 , 19 In this species, long‐range mating communication is mediated by male‐emitted aggregation pheromones, 20 , 21 whereas vibrational signals mediate behavioral interactions at short distances. 7 In particular, males emit a low‐frequency signal (MS1), the behavioral meaning of which is not yet known, 22 whereas their interaction with females is associated with a pulsed signal (MS2) to which the females reply with two types of signals (FS1 and FS2). 23
Pest control for the species is currently based on commercial traps that use two‐component aggregation pheromone dispensers to attract BMSB to the vicinity. 21 , 24 However, this strategy does not always ensure that the animals enter the trap, but instead leads to aggregation of individuals in the surrounding area, once pheromones are efficient for medium range attraction. 25 , 26 Therefore, the use of attractive vibrational signals towards the inside of the trap is an alternative to cope with this problem. Indeed, a recent study 11 demonstrated that males of BMSB can be attracted to an artificial source point (that is, a mini‐shaker) by playback of FS2 both in plants and artificial arenas. These results suggest that FS2 could be used to capture males for monitoring or mass‐trapping purposes. Indeed, integrating pheromone and vibrational traps could increase the capture rate and would constitute an important innovation for the sector. 11
However, a single emission of a signal does not automatically elicit a behavioral response in insects. Vibration signals within a species have a range of spectral and temporal features that vary between individuals 7 , 23 and failing to send the correct signal could imply a miscommunication. Therefore, the aim of this study was to identify the exact spectral and temporal components of FS2 that best trigger searching behavior in BMSB male receivers. In this regard, we stimulated males with different types of FS2 playbacks and designed a new T stand arena for one‐ and two‐choice tests of vibrotaxis. Our ultimate goal was to synthesize the optimal attractive FS2 signal for BMSB field trapping.
2. MATERIAL AND METHODS
2.1. Insect rearing
Colonies of BMSB were initiated from adults and nymphs collected in the Province of Trento, Italy, during spring and summer 2018 and 2019. Insects were reared in transparent plastic mesh cages in climatic chambers (under 16:8 h light/dark photoperiod at 25 ± 1°C and 60 ± 5% relative humidity) in a greenhouse at the Institute Fondazione Edmund Mach (San Michele all'Adige, Italy), according to the protocol of a previous study. 23 All experiments were performed with sexually mature individuals (7 days after maturation molt).
2.2. Playback experiments
Data collection took place from March to August in 2018 and 2019. Experiment 1 was conducted in 2018 and experiment 2 in 2019. All trials were carried out in the Laboratory of Bioacoustics, Fondazione Edmund Mach, inside an acoustically insulated chamber (24 ± 1°C under artificial lighting conditions) on an anti‐vibration table. The signal, FS2 (taken from our signal library) was used as a template for all playbacks 11 and consisted of a series of approximately 0.5‐s long and regularly repeated pulses [pulse repetition time (PRT) of approximately 1.0 s] with a dominant frequency on the first harmonic, 80 Hz and a total duration of 11.5 s. We tested the male response to FS2 playbacks in three different settings: (i) a potted bean plant (Phaseolus vulgaris, 20 cm tall, with two well‐developed opposite leaves); (ii) a custom‐made cardstock arena (Figure 1) and (iii) a wooden custom‐made T stand (Figure 2a). The variability in the arenas was aimed at answering different questions: from more basic parameters following protocol of Mazzoni et al. 11 in experiment 1, to more complex ones, assessing the capacity of individuals to make choices between different directions at the same time in experiment 2 with a proposed new arena (T). Each arena had a “release point” (RP), where the individual was initially released, and a “stimulation point” (SP) correspondence to the tip of a mini‐shaker (model 4810; Bruel & Kjaer) that was the source of FS2 playbacks.
FIGURE 1.
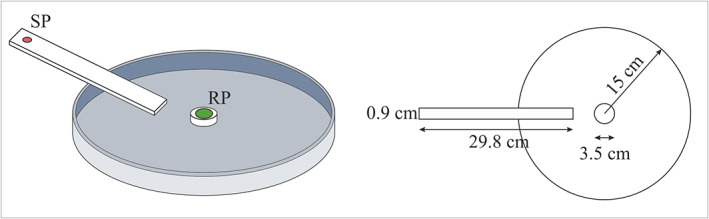
Cardstock arena used for Exp1c: dominance of the harmonics and Exp1d: pulse repetition time (PRT).
FIGURE 2.
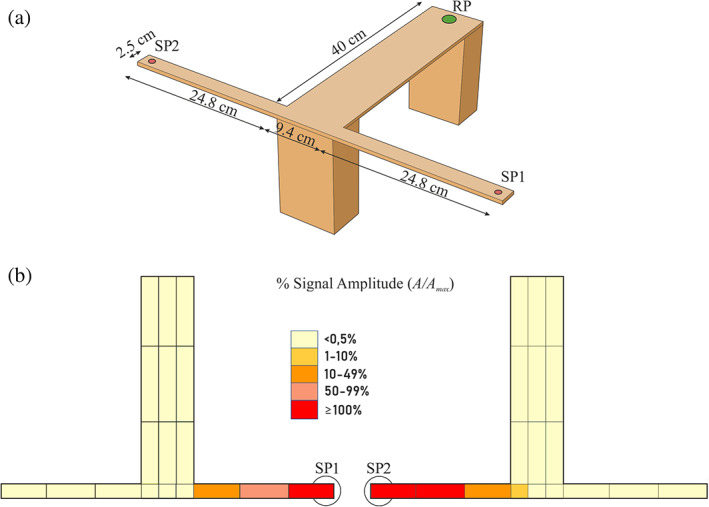
(a) Plywood T‐shaped arena with dimensions; the thickness of the lateral arms is 0.4 cm. The green circle shows the release point (RP), and the red circles identify the stimulation points (SP). (b) Results of the signal amplitude propagation by changing the source of the stimulus. Values are normalized to the maximum amplitude recorded on the arena.
Each trial began with a BMSB male placed at the RP under a Falcon vial cap (diameter: 3.2 mm). After 30 s (experiment 1) and 1 min (experiment 2), the playback was turned on and the cap was lifted, thus freeing the insect (see Table 1 for descriptions of the FS2 used for each test). Depending on the tests, the trials ended when either the given time ran out (details in experiments session) or the male left the plant/arena or reached the SP. Analysis for each test was primarily based on determining which versions of the female signal most triggered the searching behavior. Searching, for this species, is defined as alternation between walking and stopping during pulse emission when the male stretches his legs, presumably in a posture of “listening”. 23
TABLE 1.
Summary of tests and parameters used in each experiment
| Experiment | Test | Tested parameter | Type of arena | Sample size | Variations of FS2 |
|---|---|---|---|---|---|
| Exp 1 | a. Amplitude of the playback | Amplitude of the signal measured as substrate velocity (μm s−1) | Bean plant | 42 males |
FS2 (0–8000 μm s−1); Control – silence |
| b. Continuity of the playback | Continuity of the signal (with or without silent breaks) | Bean plant | 20 males |
FS2‐continuous – FS2 played in loop with no breaks; FS2‐discontinuous – 11.5 s FS2‐2 playback with breaks of 11 s of silence; Control – silence |
|
| c. Dominance of the harmonics | Dominant frequency (Hz) | Round arena | 20 males |
FS2‐76 – peak of frequency at the first harmonic (76 Hz); FS2‐even – first and second harmonics equally important; FS2‐152 – peak of frequency at the second harmonic (152 Hz); |
|
| d. Pulse repetition time | PRT (s) | Round arena | 50 males |
FS2‐fast – PRT at 1.0 s; FS2‐standard – PRT at 1.5 s |
|
| Exp 2 | a. One‐choice test | Optimal Exp1 features (a, b, c, d) | T arena | 59 males | FS2‐Best – FS2 continuous play, 76 Hz and 1 s PRT; |
| b. Simple two‐choice test | Dominant frequency (Hz) | T arena | 38 males |
FS2‐76 – peak of frequency at the first harmonic (76 Hz); FS2‐152 – peak of frequency at the second harmonic (152 Hz); |
|
| c. Complex two‐choice test | Dominant frequency (Hz) and PRT (s) | T arena | 80 males |
FS2‐Best – FS2 with 100–150 μm s−1, continuous play, 76 Hz and fast PRT; FS2‐Worst – FS2 with 100–150 μm s−1, continuous play, 152 Hz and standard PRT; FS2‐Sub1 – FS2 with 100–150 μm s−1, continuous play, 76 Hz and standard PRT; FS2‐Sub2 – FS2 with 100–150 μm s−1, continuous play, 152 Hz and fast PRT; |
2.3. Design and validation of the T‐shaped arena
The T‐shaped arena was built of plywood. The three‐dimensional (3D) scheme is shown in Figure 2a: the two arms of the T arena can oscillate at their free ends, while two thick pillars, one at the base and the other at the front, support the main stem. Stimulation points SP1 and SP2 were set on the free ends of the T arena (red circles in Figure 2a).
Before performing the experiments, we tested vibrational signal propagation using a laser vibrometer (Polytec PDV 100) associated with an acquisition device (LAN XI, Brüel & Kjaer) to verify the symmetry of the setup when the SP was switched from one arm to the other. This preliminary test was also performed to characterize the T arena by describing the vibrational landscape and the possible occurrence of amplitude gradients. For the recording, we used a sample rate of 8192 Hz. Spectral analysis was done by applying a fast Fourier transform with a Hanning window length of 400 lines, 8 Hz of frequency resolution and 66.7% overlap.
2.4. Bioassays
Two sets of experiments were performed (summarized in Table 1): the first one was composed of four different tests, each targeting a different parameter (either spectral or temporal) of FS2. The four tests were as follows: Exp1a—amplitude, measured as velocity of substrate vibration (μm s−1); Exp1b—continuity of emission (with or without interruption); Exp1c—dominance of the harmonics between the first and second harmonic of the signal; Exp1d—signal emission pattern, measured as PRT. After each test, the FS2 playback was adjusted by fixing the parameter that best triggered a positive behavioral response in the male. This means that Exp1b benefited from the experience gained after test Exp1a, and Exp1c, from information gathered from both Exp1a and Exp1b; finally Exp1d, benefited from the information gathered from all previous tests. The second set of experiments consisted of one‐ (Exp2a) and two‐choice tests (Exp2b and Exp2c). FS2 signals were designed to validate the information gathered in experiment 1, and establish which one, between dominant frequency and PRT, was more important to motivate males. In this way, optimal and suboptimal (that is, deprived of either the optimal frequency or temporal pattern) FS2 signals were designed and played back into the T‐arena. Each male was tested only once per test. Different treatments for the same test were randomized, alternating the side of emission to minimize any possible time/position effect as well as the position of them on each side of the T arena.
2.4.1. Experiment 1a: amplitude of the playback
The aim was to determine an optimal range for the signal amplitude among the tested values. RP was set on one bean leaf and SP on the other. Before each trial, the FS2 amplitude was measured, as substrate velocity (μm s−1) using a laser vibrometer at two points: 1 cm from RP and 1 cm from SP. Males were individually tested using a randomized FS2 velocity (0 to 8000 μm s−1, at the RP; see Table 1 for detail). As a control, we used males placed on the plant without any playback transmission (n = 10). Each male was left on the plant for a maximum time of 10 min. We counted the number of individuals that reached the source (that is, the mini‐shaker).
2.4.2. Exp1b: continuity of the playback
The aim of this test was to assess whether interruptions to FS2 affected: (i) males' motivation to express searching behavior; and (ii) the accuracy of locating the SP. Therefore, FS2 was played back in a continuous loop (without any interruption), or as a discontinuous loop, with a silent break of 11 s between consecutive signals. A third silent treatment was used as a control. The analysis considered both video and audio recordings. Videos were used to measure the number of “right” (towards the SP) and “wrong” (away from SP) choices made along the bean plant in the direction of the SP. The numbers of MS1 and MS2 signals emitted by the males were also counted. The maximum time for the experiment was 10 min.
2.4.3. Exp1c: dominance of the harmonics
Because the ultimate aim was to use the FS2 playback as an attractant in field traps, it was important to assess whether any alteration in the frequency pattern (that may be caused by the different substrates, that is, matters, size, shape, crossed by the FS2) could affect the male behavioral response. In particular, we created three different FS2 playbacks with variations in the first two harmonics (that is, 76 and 152 Hz). In FS2‐76, the first harmonic (76 Hz) was dominant; in FS2‐even the first two harmonics were of equal amplitude, which was achieved by amplifying the second one; and FS2‐152 was generated from FS2‐even by applying a 20 dB reduction in the first harmonic (Table 1; Figure 3a–c). The cardstock arena described above was used in this test. For each trial, all three signals were played in a random order to each insect; each playback was turned on for 1 min and with 30 s of silence in between. We counted the number of individuals that showed searching behavior, in correspondence with or immediately after each playback.
FIGURE 3.
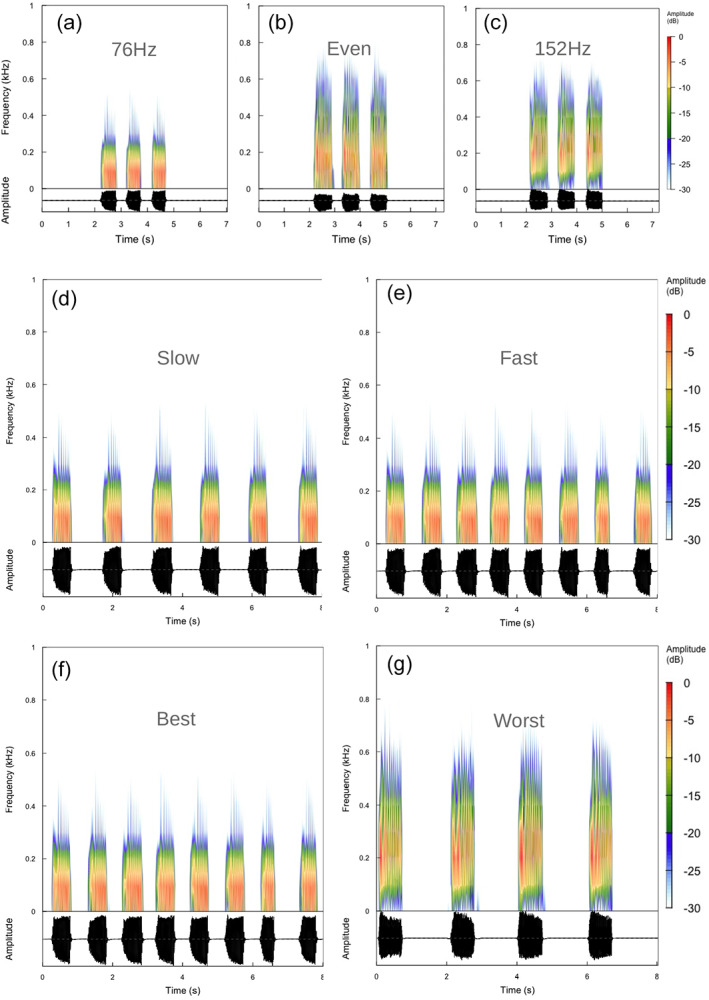
Spectrograms (upper) and oscillograms (lower) of FS2 signals used in the playback experiments. (a) FS2‐76, peak frequency at the first harmonic (76 Hz). (b) FS2‐even, first and second harmonics are equal. (c) FS2‐152, peak frequency at the second harmonic (152 Hz). (d) FS2‐standard, PRT at 1.5 s. (e) FS2‐fast, PRT at 1.0 s; standard. (f) FS2‐Best, FS2 with 100–150 μm s−1, continuous play, 76 Hz and 1 s PRT. (g) FS2‐Worst, FS2 with 100–150 μm s−1, continuous play, 152 Hz and 1.5 s PRT.
2.4.4. Exp1d: pulse repetition time
Exp1d tested the pulse emission rate, or PRT, of FS2 and how variation in this can affect the male behavioral response. The PRT can be defined as the time between the onset of two consecutive pulses. Two values of PRT, fast and standard, were chosen based on the natural range reported by Polajnar et al. 23 We called the signal with PRT around 1.0 s, which was also used as a model in the previous tests “FS2‐fast”, and we created a “FS‐standard” by adding a 0.5 s silent gap between each pulse (PRT of approximately 1.5 s), which corresponds to the average parameter found in nature (Table 1; Figure 3d,e). Each version of FS2 was randomly played to a male with a 30 s break in between each playback. The total time given to each male was 2 min. We counted the number of individuals that showed searching behavior, in correspondence with or immediately after each playback.
2.4.5. Experiment 2a: one‐choice test
The goal of this test was to measure the ability of BMSB to reach the vibrational target males when stimulated with FS2 using optimal parameters (FS2‐Best) (Figure 3f) derived from experiment 1 (see Table 1) in the absence of other stimuli. We used the wooden T arena (Figure 2a) and placed two mini‐shakers (one on and one off) at the end of each of the outstretched arms, one of which was muted and served as a control. Only one, chosen at random, was playing during each trial. RP was set at the base of the T, at the opposite end from the arms (SP) and was receiving the signal at around 10 μm s−1 to elicit searching behavior in males. The signal increased towards the SP with the shaker, reaching up to 2000 μm s−1 (Figure 2b). Each trial used one male and ran for up to 7 min with the playback on a loop. The analysis was based on whether or not the insect showed searching behavior, moved towards or away from the SP, and whether it touched the functioning mini‐shaker within the stipulated time.
2.4.6. Ex2b: simple two‐choice test
The purpose of this test was to assess whether males search towards a preferred FS2 signal when stimulated by two sources coming from distinct directions at the same time, and with different spectral characteristics (that is, the dominant frequency of the harmonics). We used the same setup as Exp2a, but this time both mini‐shakers were turned on during the trial. We used the preferred signal FS2‐76 that was compared with FS2‐152 (see in Results, Exp1c; Table 1; Figure 3a). Before each trial, the playback was switched between the two mini‐shakers.
2.4.7. Exp2c: complex two‐choice test
We combined within the same FS2 optimal and non‐optimal features (that is, frequency and PRT) to further test males in two‐choice tests. In a first set of trials, we compared two new FS2 versions in a two‐choice test: FS2‐Best (FS2‐fast + FS2‐76) versus FS2‐Worst (FS2‐standard + FS2‐152) (Table 1; Figure 3f,g); in a second set of trials, we compared two suboptimal FS2 versions: FS2‐sub1 (FS2‐fast + FS2‐152) and FS2‐sub2 (FS2‐standard + FS2‐76). The latter set of trials aimed to establish which feature, between frequency and PRT, is more relevant in determining male choice. The arena and protocols were the same as in Exp2b.
2.5. Data analysis
In Exp1a, we explored the effect of signal amplitude measured at the SP in terms of velocity (0‐8000 μm s−1) on the number of BMSB males reaching the mini‐shaker using a generalized linear model (GLM) with binomial distribution, with velocity (μm s−1) as an explanatory variable. To better understand the minimum threshold of the BMSB response to the signal, we also performed a polynomial regression with binomial distribution to the signal over a range of 0–400 μm s−1.
For Exp1b (continuity of the signal emission) we used a non‐parametric Kruskal–Wallis test comparing differences between treatments, continuous and discontinuous emission, and the silent control for the following parameters: (i) number of movements toward and away from the signal source; (ii) time spent by males in emitting MS1 and MS2; and (iii) number of individuals that “search” and that reached the “target”. When the test was significant, we applied a post hoc test (Dunn's test).
We explored the effect of signal frequency (76 Hz, even, 152 Hz) on insect responsiveness using a GLM with binomial distribution, and frequency as explanatory variable (Exp. 1c). The same analysis was performed to test the effect of signal speed (“fast” versus “standard”) on insect responsiveness with speed as the explanatory variable (Exp1d).
For the choice tests in experiment 2, we analyzed the effect of signal source positioning (one‐choice tests) and of the specific signal source (see Table 1, two‐choice tests) on the right or left arm of the T arena on the number of active individuals that reached the target using a William's corrected G likelihood‐ratio test.
We ran the models using packages “lme4” 27 and “MASS”. 28 We checked the models for residual distribution using the “car” package. 29 In the case of model significance, we additionally performed Tukey's test for pairwise comparisons using the “emmeans” package. 30 Other non‐parametric analyses were conducted with the package “PMCMR”. 31 Figures were built using “seewave”, “dyplr”, “tidyr” and “ggplot” packages. All analyses were performed in R v.3.5.3. 32 All data are available upon request.
3. RESULTS
3.1. Experiment 1
3.1.1. Exp1a: amplitude of the playback
As expected, no males reached the mini‐shaker in the silent control. Signal amplitude emitted by the shaker was a significant factor for the number of BMSB movements towards it (z = 2.373, P < 0.0176). According to the outcome of the GLM, there was a significant positive correlation between male responsiveness and signal amplitude, with the best response in the velocity range 100–200 μm s−1, according to polynomial regression (z = −3.259, P < 0.0011). Individuals were responsive even at the highest amplitudes tested (Figure 4a; Table TABLE 2).
FIGURE 4.
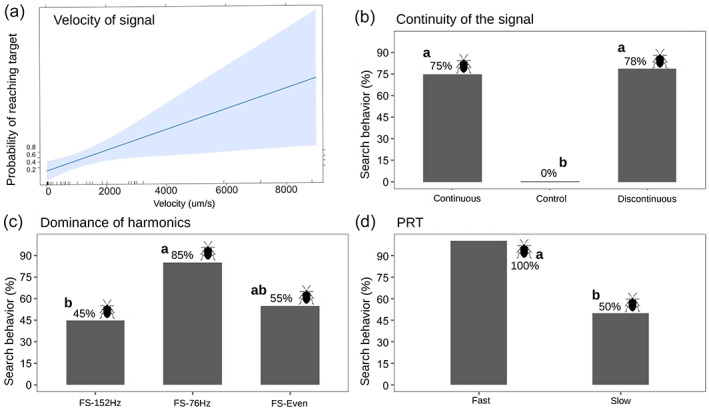
Experiment 1: individual behavior according to different signal parameters tested (a) GLM model of the response of individuals to the amplitude of playback (μm s−1) with confidence interval (blue). (b) Continuity of signal emission. (c) Dominance of harmonics. (d) Pulse repetition time of signal. Silhouette of BMSB next to the percentage represents treatments in which individuals moved towards the emission of a signal. Letters (a and b) represent significant differences between treatments for each parameter tested.
TABLE 2.
Summary of the results found in this study
| Test | Results |
|---|---|
| Exp1a. Intensity of the playback | Significant positive correlation between male responsiveness and signal intensity, with the best response between 100 and 200 μm s−1. |
| Exp1b. Continuity of the playback | FS‐continuous helps with decision‐making: males make fewer mistakes. |
| Exp1c. Dominance of the harmonics | Male searching is more elicited during FS2‐76. |
| Exp1d. Pulse repetition time | Males preferred FS2‐fast – FS2 with PRT 1.0 s. |
| Exp2a. One‐choice test | Males can easily find a source of a FS2 signal (FS2‐Best) on a T‐stand arena. |
| Exp2b. Simple two‐choice test | Males preferred FS2‐76 more than the altered one (FS2‐152). |
| Exp2c. Complex two‐choice test |
Males preferred FS2‐Best that FS2‐Worst. Males did not show a preference when exposed to FS2 with mixed good and bad features (FS2‐sub2 versus FS2‐sub3). |
3.1.2. Exp1b: continuity of the playback
Movements toward the signal source were significantly different between treatments (x2 = 17.7, P = 0.001), lower in the control compared with both the continuous (P = 0.002) and discontinuous (P = 0.0001) signals, with no differences between the latter (P = 0.423) (Figure 4b; Table TABLE 2). Similarly, movements away from the source were significantly different between treatments (x2 = 18.56, P = 0.001) for both the continuous (P = 0.004) and discontinuous signals (P = 0.0001) signals compared with the control (where no movements were observed), with no differences between the continuous and discontinuous signals (P = 0.423). Moreover, no differences were observed among signals for time spent by BMSB in emitting MS1 (x 2 = 0.38, P = 0.823), although MS2 was significantly longer in both continuous (x 2 = 20.501, P = 0.001) and discontinuous (x 2 = 22.95, P ≤ 0.001) signals compared with the control. Median MS2 time was far greater in response to the continuous signal (18%) than in response to the discontinuous signal (1.5%). In our experiment, signal continuity had no significant role in terms of number of males that performed “search” (P = 0.337) and reached the “target” (P = 0.408).
3.1.3. Exp1c: dominance of the harmonics
The best signal frequency in terms of insect responsiveness was FS2‐76 Hz which more often elicited searching behavior in males than FS2‐152 (z = −2.478, P = 0.0352). By contrast, we did not find any significant difference between FS2‐even and FS2‐76 (z = −1.553, P = 0.266) or 152 Hz (z = −1.110, P = 0.508) (Figure 4c; Table TABLE 2 and S1).
3.1.4. Exp1d: pulse repetition time
BMSB male responsiveness was significantly greater when stimulated with FS2‐fast signal compared with FS2‐standard (z = 2.458, P = 0.014) (Figure 4d; Tables TABLE 2 and S1).
3.2. Validation of the T‐shaped arena
Signal propagation tests revealed that most of the signal energy (over 99%) dissipated immediately after the signal left the free arm with the active mini‐shaker. Along this free arm, there was a clear amplitude gradient, although the highest value does not necessarily coincide with the SP. The T‐stem showed a rather homogenous pattern of amplitude values with amplitude never exceeding 1.0% of the SP; values were lowest at the center (Figure 2b) and increased towards the edges of the T.
3.3. Experiment 2
3.3.1. Exp2a: one‐choice test
In accordance with experiment 1, we chose the standard values of the FS2‐Best parameters to stimulate searching in BMSB males: 100 μm s−1 of amplitude (as the lower threshold of the best response) at 76 Hz peak frequency and 1.0 s PRT, transmitted continuously without any silent break. Twenty‐eight of 59 tested individuals were active and of these, 22 (78%) moved towards the active shaker (signal source); only two moved towards the end with no signal and four did not reach any SP (G test: G = 24.7, df = 2; P < 0.001) (Table TABLE 2; Figure 5a).
FIGURE 5.
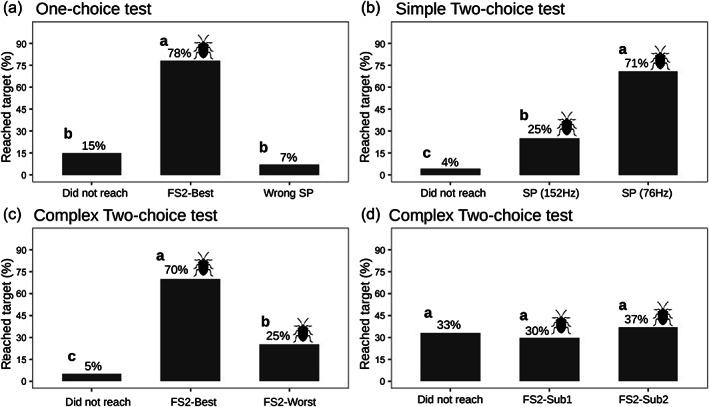
Experiment 2: percentage of individuals that arrived at the signal emission target in each of the experiments. Silhouette of BMSB next to the percentage represents treatments in which individuals moved towards the emission of a signal. Letters (a,b and c) represent significant differences between treatments for each parameter tested.
3.3.2. Exp2b: simple two‐choice test
For FS2‐76 versus FS2‐156, 24 of the 38 males tested were active, of these 17 (71%) reached the FS2‐76 shaker, significantly more than the single male that moved toward the FS2‐156 shaker and six that did not reach any SP (G‐test: G = 18.0; df = 2; P < 0.001) (Table TABLE 2; Figure 5b).
3.3.3. Exp2c: complex two‐choice test
For FS2‐Best versus FS2‐Worst, 20 of 32 males tested were active. Of those, 70% (14/20) preferred FS2‐Best (fast and low dominance frequency), significantly more (G test: G = 14.9, df = 2; P = 0.001) than those that reached the shaker emitting FS2‐Worst (standard and high dominance frequency) (2/20) and those that did not reach any shaker in the given time (4/20) (Table TABLE 2; Figure 5c).
For FS2‐Sub1 versus FS2‐Sub2, 27 of 48 males tested were active. We did not find a significant difference (G test: G = 0.75, df = 2; P = 0.33) between SP‐FS2‐Sub1 (ten males), FS2‐Sub2 (eight males) and the nine males that did not reach any of the shakers (Table TABLE 2; Figure 5d).
4. DISCUSSION
Our study shows that BMSB male responsiveness to playbacks improved proportionally with amplitude, especially with a minimum threshold of 100 μm s−1, a peak frequency associated with the first harmonic at 76 Hz and the fast PRT (1 s). Furthermore, continuous playback emission did not increase the number of individuals reaching the target, but helped animals to locate it with fewer mistakes. In terms of insect performance in the T‐arena, we found that males showed a significant orientation towards the female signal, which validates this method for future vibrotaxis studies.
In acoustic signaling systems, the amplitude of a signal can affect male mating success. Higher amplitude signals have greater broadcast distances, and females in many species prefer higher amplitude signals in playback tests. 33 , 34 , 35 For this reason, we first tested signal amplitude to establish a standard amplitude for subsequent trials. Signal amplitude strongly depends on the structure and architecture of the substrate through which the signal travels (that is, a plant). 36 Consequently, the active area of a species signal also depends on its amplitude, 12 thus defining the range of the signal activity (that is, efficacy to attract males) to determine the optimal interspace between attractive systems (for example, traps). The results of these experiments showed that at low levels of amplitude, few individuals showed searching behavior, but starting from a minimum threshold of 100 μm s−1, the behavioral response increased. We did not find a reduction in male responsiveness even at values 10–20 dB higher. Previous studies with Nezara viridula (Hemiptera: Pentatomidae) proved that individuals were responding to playbacks with a signal amplitude of up to 1000 μm s−1. 37 , 38 Here, we found that males were positively reacting to playback up to 8000 μm s−1. This is important for application purposes because it indicates that it will be possible to considerably increase the power of the trap vibration generator to amplify the active space without affecting the male response. 38 , 39 The next research step should be to further investigate this aspect to define the highest amplitudes with which males could be stimulated without suffering any repelling/saturation effects. However, in this work, we have not determined the actual basal threshold of sensitivity. In our trials, although few individuals showed searching behavior at values below 100 μm s−1, we believe that the signal should still be perceptible, just not enough to elicit a high searching behavior. Indeed, we know that the threshold curves calculated for neurons in response to vibratory signals in N. viridula indicate that values around 10 μm s−1 are the lowest threshold 40 and this value is suggested to be similar in BMSB (A. Ibrahim, unpublished data). We hypothesize that male motivation can vary according to the perceived distance from a potential mate. In fact, animals that perceive a mating call from a long enough distance could decide not to search in order to remain inconspicuous to rivals and predators while they are scanning their surroundings using sensors (chemical and mechanical) to gather more information. 41 , 42 , 43 Choice is based on an analysis of costs/benefits, when the cost of beginning to search outweighs the chance to find a mate. 44 Together with these risks, a distant signal also suggests accuracy reduction and thus a waste of energy. 41 , 45 In this way, we can assume that a threshold of approximately 100 μm s−1 could be an acceptable and reliable amplitude at which to start searching, as shown by the results of the polynomial regression. We must also consider that a natural female signal, measured from the same leaf as the female, was on average 460 μm s−1. 23 In this way, values of 100–400 μm s−1 would presumably indicate to the male the presence of a female in his close vicinity, whereas values at lower amplitude, even if perceived, would likely represent a more distant conspecific, and thus a more difficult and risky target to reach. This hypothesis needs to be validated with more experiments using different substrates.
The second factor we tested was the duration of the playback, in particular the use of continuous and discontinuous signals. Although we have not found any differences between continuous and discontinuous signals in our trials, males may have a better vibrational contact with the sender and thus better directional accuracy with a continuous emission. Thus, we decided to use the continuous signal for subsequent experiments. Our results also suggest that males are capable of obtaining directional information while walking and not only during pauses. In the case of N. viridula, directionality is given by comparison of the signal simultaneously received from the legs while standing at a fork point. 46 According to previous studies, 38 , 47 the sensitivity of the sensory system of a species to frequencies higher than 120 Hz enables males to detect higher harmonics. The ability to detect those changes may serve as an orientation cue for the searching male. Furthermore, many species, including leafhoppers and treehoppers, have the capacity to correct their direction after choosing the wrong course. 12 , 45 , 48 Regarding signal emission by males during this part of the test, individuals exposed to the FS2 playback also emit MS2, which confirms it as an interactive signal with a possible role in maintaining female motivation to emit FS2 until the animals find each other. 23
The third signal feature analyzed was peak frequency. Males were more attracted to FS2 with a fundamental and dominant frequency at 76 Hz, which also corresponds to the signal emitted in situ by this species. By contrast, males were significantly less motivated to search when exposed to the FS2‐152 (second harmonic), whereas an intermediate result was observed when exposed to FS2‐even (first‐second harmonics of identical amplitude). Unlike amplitude and continuity of emission, which are temporal (and thus quantitative) parameters, frequency is qualitative. Results from a previous study with the glassy winged sharpshooter, Homalodisca vitripennis, 10 showed that a shift in energy from the natural dominant harmonic to a different one, or simply the use of two dominant harmonics of identical amplitude, led to a significant reduction of male responsiveness. This observation is important when thinking about the use of mechanical devices to transmit the attraction signal from a constructed trap. In vibrational signals, transmission of seismic and bending waves is frequency dependent and higher frequencies tend to dissipate before lower ones. 34 However, construction of a trap must consider the materials, shapes and position of the site. Underestimating the importance of producing undesired harmonics could be the difference between a successful device and failure. For this reason, particular attention should be given to both the design of the signal generator (that is, the shaker) and the coupling effects between the device and the overall system. 49 , 50 , 51
Finally, the fourth feature that we tested was the PRT of FS2. We tested the fastest and slowest PRT among those registered within the natural range of the species, 23 and found a clear prevalence of male responsiveness when stimulated with the FS2‐Fast. In insects, the signal (that is, pulses, chirp) emission rate can be associated with different physiological parameters such as age, 52 size 53 and nutritional condition, 54 and could therefore be an important element by which to evaluate fitness. Most studies of signal preference and attractiveness focus mainly on the female's choice, with less literature about male choice and the role of the signal emission rate. Our research indicates that males can also show choosiness because they preferred a fast signal probably associated with a female of higher quality. However, we cannot exclude a reduction in recognition due to alteration of the signal.
In experiment 2, we found that males exposed to single‐ and double‐choice tests in the T arena showed significant orientation towards the female signal. During the single‐choice test, most of the males exposed to FS2‐Best reached the SP. Indeed, several behavioral studies have demonstrated the ability of insects to locate exactly the source of vibrations, 42 including Pentatomidae, for either prey location 55 or mate location. 56 Our previous paper 11 demonstrated that BMSB can also locate the source of an attractive signal but, in addition, we herein demonstrated that males can distinguish between two sources that are transmitting signals with different spectral/temporal features. The “simple” two‐choice test provided evidence that males can distinguish between two different types of FS2, when the qualitative difference is large enough. This means that males still reached the supposedly preferred FS2, composed of a low frequency and high repetition rate (FS2‐Best) both in “simple” (only one manipulated parameter) and “complex” two‐choice (two parameters) tests. However, when “good” and “bad” characters (FS‐Sub1 and FS‐Sub2) were mixed in additional signals, males did not show a preference and the choices were split between the two SPs. This result may indicate that there is no hierarchy of parameters and would suggest that frequency and PRT equally affect FS2 attractiveness to males.
This study also indicated the importance of a T‐stand arena as a powerful tool to study and select which parameters of the vibrational signal are most efficient in triggering insect behavior. Indeed, when we switched the SP from one arm of the T‐stand arena to the other male responses remained consistent. In the arena used, the signal enhances on the two edges towards the shaker (SP) and reduces drastically towards the center (RP), so even in two choice test, when emitting signals simultaneously, the orientation of males was towards the shaker. In a previous study with H. halys, 11 the authors mentioned that in general, males were able to locate the stimulation points on plants and in artificial arenas. The authors assumed that time delay was the cue males used, basing their orientation on either amplitude difference 7 or time delay. 57 Furthermore, plywood, the material used for the arena, permitted the emission of the chosen signal with quality, without losing the important parameters that trigger searching in males. Moreover, application of this setup can be extended to several other species, adapting its shape and size as needed. The number of potential species that can be tested with a T arena is large when considering that many insects communicate by means of vibrational signals and those that use mechanical channels. In particular, among these insects, 80% use vibrational signals alone or in combination with other mechanical signals, and 74% use vibrational signals alone. 9
In conclusion, because animals respond to combinations of signal values that they find attractive, 58 we identified some of the features that most elicit activation of searching behavior in BMSB males. To trap insects, it is essential to have a signal that not only mimics the natural FS2 emitted by females to sexually attract males, but also indicates the high fitness quality of the artificial calling female. FS2, like other vibrational signals, has multiple components 59 characterized by a range of temporal and spectral parameters. The range of variability depends on numerous factors that can be related to the female physiological status (that is, age, health) and to the environmental conditions (for example, temperature and relative humidity). During our experiments, all the values used were reported to be within the natural range of BMSB females 11 to ensure signal compatibility recognition within the thresholds of acceptability by the tested males. 58 In fact, in all the experiments, male exposure to our playbacks elicited the response (that is, searching behavior and or MS2 emission) at least in some individuals that showed a clear preference for certain characteristics. In this work, we created an “optimal” attractive signal, given by multiple components that interact with each other thus positively affecting the receiver's response. 60 , 61 Giving the strong directional orientation of males towards the FS2 source, which was clearly demonstrated in trials conducted on the T arena, we consider this study to be important for the development of new concept traps for BMSB, and furthermore in synergy with the pheromones already available for the commercial devices. Current pheromone traps guide insects towards the area surrounding the trap, often not succeeding in luring animals inside the trap. We suggest the combination of long‐distance pheromone attraction with short‐distance vibrations (guiding individuals inside the trap) as an interesting trap design for H. halys pest control. Further experiments will be realized in future to correlate the BMSB female physiology (that is, age, health, size, mating status ) and spatial location with signal variability and preferences.
Supporting information
TABLE S1. Estimated regression coefficients, standard errors, and confidence intervals for GLM of variable response to vibratory playback stimuli. The reference category in Dominance of harmonic experiment was 152 Hz and for the PRT experiment was Fast playback.
ACKNOWLEDGEMENTS
This work was partially funded by CBCEurope Ltd. (Milano, Italy). MT was supported by the project Agroecology in practice. DC was supported by European Union's Horizon 2020 and Marie Skłodowska‐Curie grant agreement No 835732 XYL‐SPIT. AB was supported by Fondazione CARITRO, Cassa di Risparmio di Trento e Rovereto, No. 2019.0216. We further acknowledge Marco Deromedi, Livia Zapponi, Jalal Fouani for their help.
AUTHORS CONTRIBUTIONS
Valerio Mazzoni, Michele Torriani, Damiano Moser, Alice Berardo, Lara Maistrello and Nicola M. Pugno conceived and designed the research. Valentina Caorsi, Karen E. Wells, Damiano Moser, Michele Torriani and Roberto Miselli conducted the experiments. Valentina Caorsi, Daniele Cornara, Valerio Mazzoni and Alice Berardo analysed the data. Valentina Caorsi and Valerio Mazzoni wrote the manuscript. Karen E. Wells revised the English language. All authors read and approved the manuscript.
Funding information: CBCEurope Ltd (Milan, Italy); Fondazione CARITRO, Cassa di Risparmio di Trento e Rovereto, Grant/Award Number: 2019.0216; European Union Horizon 2020; Marie Skłodowska‐Curie, Grant/Award Number: 835732 XYL‐SPIT
Contributor Information
Valentina Caorsi, Email: valenzc@gmail.com.
Daniele Cornara, Email: danielecornara@berkeley.edu.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Foster SP and Harris MO, Behavioral manipulation methods for insect pest‐management. Annu Rev Entomol 42:123–146 (1997). [DOI] [PubMed] [Google Scholar]
- 2. Baker BP, Green TA and Loker AJ, Biological control and integrated pest management in organic and conventional systems. Biol Control 140:104095 (2020). [Google Scholar]
- 3. Götz B, Über weitere Versuche zur Bekämpfung der Traubenwickler mit Hilfe des Sexualduftstoffes. Anz Schädlingskd 15:109–114 (1939). [Google Scholar]
- 4. Welter S, Pickel C, Millar J, Cave F, Van Steenwyk R and Dunley J, Pheromone mating disruption offers selective management options for key pests. Calif Agric 59:16–22 (2005). [Google Scholar]
- 5. Koul O, Cuperus GW and Elliott N, Areawide Pest Management: Theory and Implementation. CABI, Wallingford: (2008). [Google Scholar]
- 6. Eriksson A, Anfora G, Lucchi A, Lanzo F, Virant‐Doberlet M and Mazzoni V, Exploitation of insect vibrational signals reveals a new method of pest management. PLoS One 7:e32954 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Polajnar J, Eriksson A, Virant‐Doberlet M and Mazzoni V, Mating disruption of a grapevine pest using mechanical vibrations: from laboratory to the field. J Pest Sci 89:909–921 (2016). [Google Scholar]
- 8. Mazzoni V, Nieri R, Eriksson A, Virant‐Doberlet M, Polajnar J, Anfora G et al., Mating disruption by vibrational signals: state of the field and perspectives, in Biotremology: Studying Vibrational Behavior. Springer, Cham, Switzerland, pp. 331–354 (2019). [Google Scholar]
- 9. Cocroft RB and Rodriguez RL, The behavioral ecology of insect vibrational communication. Bioscience 55:323–334 (2005). [Google Scholar]
- 10. Mazzoni V, Gordon SD, Nieri R and Krugner R, Design of a candidate vibrational signal for mating disruption against the glassy‐winged sharpshooter, Homalodisca vitripennis . Pest Manag Sci 73:2328–2333 (2017). [DOI] [PubMed] [Google Scholar]
- 11. Mazzoni V, Polajnar J, Baldini M, Stacconi MVR, Anfora G, Guidetti R et al., Use of substrate‐borne vibrational signals to attract the brown marmorated stink bug, Halyomorpha halys . J Pest Sci 90:1219–1229 (2017). [Google Scholar]
- 12. Polajnar J, Eriksson A, Stacconi MVR, Lucchi A, Anfora G, Virant‐Doberlet M et al., The process of pair formation mediated by substrate‐borne vibrations in a small insect. Behav Processes 107:68–78 (2014). [DOI] [PubMed] [Google Scholar]
- 13. Johnstone RA, The evolution of animal signals. Behavioural Ecology: An Evolutionary Approach: 155178, ed. by, Krebs JR and Davies NB, 4th, Blackwell Science Ltd 155–178 (1997). [Google Scholar]
- 14. Mazzoni V, Polajnar J and Virant‐Doberlet M, Secondary spectral components of substrate‐borne vibrational signals affect male preference. Behav Processes 115:53–60 (2015). [DOI] [PubMed] [Google Scholar]
- 15. Seyfarth RM and Cheney DL, The origin of meaning in animal signals. Anim Behav 124:339–346 (2017). [Google Scholar]
- 16. Polajnar J, Eriksson A, Lucchi A, Anfora G, Virant‐Doberlet M and Mazzoni V, Manipulating behaviour with substrate‐borne vibrations ‐ potential for insect pest control. Pest Manag Sci 71:15–23 (2015). [DOI] [PubMed] [Google Scholar]
- 17. Leskey TC and Nielsen AL, Impact of the invasive brown marmorated stink bug in North America and Europe: history, biology, ecology, and management. Annu Rev Entomol 63:599–618 (2018). [DOI] [PubMed] [Google Scholar]
- 18. Maistrello L, Vaccari G, Caruso S, Costi E, Bortolini S, Macavei L et al., Monitoring of the invasive Halyomorpha halys, a new key pest of fruit orchards in northern Italy. J Pest Sci 90:1231–1244 (2017). [Google Scholar]
- 19. Maistrello L, Dioli P, Dutto M, Volani S, Pasquali S and Gilioli G, Tracking the spread of sneaking aliens by integrating crowdsourcing and spatial modeling: the Italian invasion of Halyomorpha halys . Bioscience 68:979–989 (2018). [Google Scholar]
- 20. Harris C, Abubeker S, Yu M, Leskey T and Zhang A, Semiochemical production and laboratory behavior response of the brown marmorated stink bug, Halyomorpha halys . PLoS One 10:e0140876 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khrimian A, Zhang A, Weber DC, Ho H‐Y, Aldrich JR, Vermillion KE et al., Discovery of the aggregation pheromone of the brown marmorated stink bug (Halyomorpha halys) through the creation of stereoisomeric libraries of 1‐bisabolen‐3‐ols. J Nat Prod 77:1708–1717 (2014). [DOI] [PubMed] [Google Scholar]
- 22. Bedoya CL, Brockerhoff EG, Hayes M, Leskey TC, Morrison WR III, Rice KB et al., Brown marmorated stink bug overwintering aggregations are not regulated through vibrational signals during autumn dispersal. R Soc Open Sci 7:201371 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Polajnar J, Maistrello L, Bertarella A and Mazzoni V, Vibrational communication of the brown marmorated stink bug (Halyomorpha halys). Physiol Entomol 41:249–259 (2016). [Google Scholar]
- 24. Weber DC, Leskey TC, Walsh GC and Khrimian A, Synergy of aggregation pheromone with methyl (E, E, Z)‐2, 4, 6‐decatrienoate in attraction of Halyomorpha halys (Hemiptera: Pentatomidae). J Econ Entomol 107:1061–1068 (2014). [DOI] [PubMed] [Google Scholar]
- 25. James DG, Heffer R and Amaike M, Field attraction of Biprorulus bibax Breddin (Hemiptera: Pentatomidae) to synthetic aggregation pheromone and (E)‐2‐hexenal, a pentatomid defense chemical. J Chem Ecol 22:1697–1708 (1996). [DOI] [PubMed] [Google Scholar]
- 26. Aldrich JR, Khrimian A, Chen X and Camp MJ, Semiochemically based monitoring of the invasion of the brown marmorated stink bug and unexpected attraction of the native green stink bug (Heteroptera: Pentatomidae) in Maryland. Fla Entomol 92:483–491 (2009). [Google Scholar]
- 27. Bates D, Mächler M, Bolker B and Walker S, Fitting linear mixed‐effects models using lme4. J Stat Softw 67:1–48 (2015). [Google Scholar]
- 28. Venables WN and Ripley BD, Random and mixed effects, in Modern Applied Statistics with S. Springer, New York, pp. 271–300 (2002). [Google Scholar]
- 29. Fox J and Weisberg S, Multivariate linear models in R, in An R Companion to Applied Regression. SAGE Publications, Los Angeles, CA, Thousand Oaks, CA (2011). [Google Scholar]
- 30. Lenth R, Singmann H, Love J and Buerkner PH, emmeans: estimated marginal means, aka least‐squares means. R package v. 1.3. 4. (2019).
- 31. Pohlert, T. . “The pairwise multiple comparison of mean ranks package (PMCMR).” (2016).
- 32. R Core Team , R: a language and environment for statistical computing. R. Foundation for Statistical Computing, Vienna: (2014). [Google Scholar]
- 33. Morris GK, Kerr GE and Fullard JH, Phonotactic preferences of female meadow katydids (Orthoptera: Tettigoniidae: Conocephalus nigropleurum). Can J Zool 56:1479–1487 (1978). [Google Scholar]
- 34. Ryan MJ and Keddy‐Hector A, Directional patterns of female mate choice and the role of sensory biases. Am Nat 139:S4–S35 (1992). [Google Scholar]
- 35. Gerhardt HC and Huber F, Acoustic Communication in Insects and Anurans: Common Problems and Diverse Solutions. University of Chicago Press, Chicago: (2002). [Google Scholar]
- 36. Michelsen A, Fink F, Gogala M and Traue D, Plants as transmission channels for insect vibrational songs. Behav Ecol Sociobiol 11:269–281 (1982). [Google Scholar]
- 37. Čokl A, Functional poperties of viboreceptors in the legs of Nezara viridula (L.)(Heteroptera, Pentatomidae). J Comp Physiol 150:261–269 (1983). [Google Scholar]
- 38. Zorović M, Prešern J and Čokl A, Morphology and physiology of vibratory interneurons in the thoracic ganglia of the southern green stinkbug Nezara viridula (L.). J Comp Neurol 508:365–381 (2008). [DOI] [PubMed] [Google Scholar]
- 39. Stritih N, Anatomy and physiology of a set of low‐frequency vibratory interneurons in a nonhearing ensiferan (Troglophilus neglectus, Rhaphidophoridae). J Comp Neurol 516:519–532 (2009). [DOI] [PubMed] [Google Scholar]
- 40. Prešern J, Polajnar J, De Groot M, Zorović M and Virant‐Doberlet M, On the spot: utilization of directional cues in vibrational communication of a stink bug. Sci Rep 8:1–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bell WJ, Searching behavior patterns in insects. Annu Rev Entomol 35:447–467 (1990). [Google Scholar]
- 42. Virant‐Doberlet M, Čokl A and Zorovic M, Use of substrate vibrations for orientation: from behaviour to physiology, in Insect Sounds and Communication. Physiology, Behaviour, Ecology and Evolution. Taylor and Francis, Boca Raton, FL, pp. 81–89 (2006). [Google Scholar]
- 43. Virant‐Doberlet M, Kuhelj A, Polajnar J and Šturm R, Predator‐prey interactions and eavesdropping in vibrational communication networks. Front Ecol Evol 7:203 (2019). [Google Scholar]
- 44. Jackson RR and Wilcox RS, Aggressive mimicry, prey‐specific predatory behaviour and predator‐recognition in the predator‐prey interactions of Portia fimbriata and Euryattus sp., jumping spiders from Queensland. Behav Ecol Sociobiol 26:111–119 (1990). [Google Scholar]
- 45. Gibson JS and Cocroft RB, Vibration‐guided mate searching in treehoppers: directional accuracy and sampling strategies in a complex sensory environment. J Exp Biol 221:jeb175083 (2018). [DOI] [PubMed] [Google Scholar]
- 46. Čokl A, Virant‐Doberlet M and McDowell A, Vibrational directionality in the southern green stink bug, Nezara viridula (L.), is mediated by female song. Anim Behav 58:1277–1283 (1999). [DOI] [PubMed] [Google Scholar]
- 47. Čokl A, Stink bug interaction with host plants during communication. J Insect Physiol 54:1113–1124 (2008). [DOI] [PubMed] [Google Scholar]
- 48. Legendre F, Marting PR and Cocroft RB, Competitive masking of vibrational signals during mate searching in a treehopper. Anim Behav 83:361–368 (2012). [Google Scholar]
- 49. Bertini L, Paolo N CS, Design and optimization of a compact high‐frequency electromagnetic Sh, Presented at 11th International Conference on Engineering Vibration, ICoEV 2015, 7–10 September 2015, Ljubljana, Slovenia, 7–10.
- 50. Hoffait S, Marin F, Simon D, Peeters B and Golinval J‐C, Measured‐based shaker model to virtually simulate vibration sine test. Case Stud Mech Syst Signal Process 4:1–7 (2016). [Google Scholar]
- 51. Calabrese L, Berardo A, De Rossi D, Gei M, Pugno NM and Fantoni G, A soft robot structure with limbless resonant, stick and slip locomotion. Smart Mater Struct 28:104005 (2019). [Google Scholar]
- 52. Jacot A, Scheuber H and Brinkhof MWG, The effect of age on a sexually selected acoustic display. Ethology 113:615–620 (2007). [Google Scholar]
- 53. Simmons LW and Zuk M, Variability in call structure and pairing success of male field crickets, Gryllus bimaculatus: the effects of age, size and parasite load. Anim Behav 44:1145–1152 (1992). [Google Scholar]
- 54. Scheuber H, Jacot A and Brinkhof MWG, The effect of past condition on a multicomponent sexual signal. Proc R Soc London Ser B Biol Sci 270:1779–1784 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pfannenstiel RS, Hunt RE and Yeargan KV, Orientation of a hemipteran predator to vibrations produced by feeding caterpillars. J Insect Behav 8:1–9 (1995). [Google Scholar]
- 56. Ota D and Čokl A, Mate location in the southern green stink bug, Nezara viridula (Heteroptera: Pentatomidae), mediated through substrate‐borne signals on ivy. J Insect Behav 4:441–447 (1991). [Google Scholar]
- 57. Hager FA and Kirchner WH, Directionality in insect vibration sensing: behavioral studies of vibrational orientation, in Biotremology: Studying Vibrational Behavior, ed. by Hill P, Lakes‐Harlan R, Mazzoni V, Narins P, Virant‐Doberlet M and Wessel A. Springer, Cham, pp. 235–255 (2019). [Google Scholar]
- 58. Mendelson TC and Shaw KL, The (mis) concept of species recognition. Trends Ecol Evol 27:421–427 (2012). [DOI] [PubMed] [Google Scholar]
- 59. Hebets EA and Papaj DR, Complex signal function: developing a framework of testable hypotheses. Behav Ecol Sociobiol 57:197–214 (2005). [Google Scholar]
- 60. Hasson O, Amplifiers and the handicap principle in sexual selection: a different emphasis. Proc R Soc London Ser B Biol Sci 235:383–406 (1989). [DOI] [PubMed] [Google Scholar]
- 61. Guilford T and Dawkins MS, Receiver psychology and the evolution of animal signals. Anim Behav 42:1–14 (1991). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1. Estimated regression coefficients, standard errors, and confidence intervals for GLM of variable response to vibratory playback stimuli. The reference category in Dominance of harmonic experiment was 152 Hz and for the PRT experiment was Fast playback.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


