Summary
‘Monitoring of immune responses following mogamulizumab‐containing treatment in patients with adult T‐cell leukaemia–lymphoma (ATL)’ (MIMOGA) is a multicentre prospective clinical study (UMIN000008696). In the MIMOGA study, we found that a lower percentage of CD2−CD19+ B cells in peripheral blood mononuclear cells (PBMC) was a significant unfavourable prognostic factor for overall survival (OS). Accordingly, we then analysed the immunoglobulin G (IgG) heavy‐chain repertoire in PBMC by high‐throughput sequencing. Of the 101 patients enrolled in the MIMOGA study, for 81 a sufficient amount of PBMC RNA was available for repertoire sequencing analysis. Peripheral IgG B cells in patients with ATL had a restricted repertoire relative to those in healthy individuals. There was a significant positive correlation between the Shannon–Weaver diversity index (SWDI) for the IgG repertoire and proportions of B cells in the PBMC of the patients. Multivariate analysis identified two variables significantly affecting OS: a higher serum soluble interleukin‐2 receptor level, and a lower SWDI for the IgG repertoire [hazard ratio, 2·124; 95% confidence interval, 1·114–4·049; n = 44]. The present study documents the importance of humoral immune responses in patients receiving mogamulizumab‐containing treatment. Further investigation of strategies to enhance humoral immune responses in patients with ATL is warranted.
Keywords: adult T‐cell leukaemia–lymphoma, mogamulizumab, immunoglobulin G B‐cell diversity
Introduction
Adult T‐cell leukaemia–lymphoma (ATL) is a peripheral T‐cell neoplasm caused by human T‐cell lymphotropic virus type 1 (HTLV‐1). It has a grave prognosis, partially due to patients´ severely immunocompromised state. 1 , 2 , 3 , 4 Because CC chemokine receptor 4 (CCR4) is expressed on tumour cells from most patients with ATL, 5 , 6 a therapeutic anti‐CCR4 monoclonal antibody, mogamulizumab, has been developed to specifically target this molecule. 7 , 8 , 9 Although mogamulizumab treatment offers a clinical benefit to patients with ATL, some patients are initially refractory to the antibody, or acquire resistance on treatment. 10 , 11 , 12 , 13 , 14 , 15 , 16 In addition, many patients still suffer from lethal opportunistic infections. 3 , 4 , 17 In this context, we planned and conducted the ‘Monitoring of immune responses following mogamulizumab‐containing treatment in patients with ATL’ (MIMOGA), a multicentre prospective observational study (UMIN000008696), in order to establish the most effective and safe treatment strategy using mogamulizumab in patients with ATL. 18 Consequently, we have found that having a lower percentage of CD2−CD19+ B cells within the lymphocyte population in peripheral blood mononuclear cells (PBMC) were an independent and significant unfavourable prognostic factor for overall survival (OS) in patients with ATL. 18 This finding highlighted the potential importance of humoral immune responses during treatment for ATL, and prompted us to investigate the B‐cell immune system in detail by analysing the B‐cell repertoire in patients with ATL. In general, among human mature B cells, antigen‐experienced memory B cells are generated from antigen‐inexperienced naïve B cells in germinal centre reactions. The former cells possess several key characteristics distinguishing them from the latter which express immunoglobulin (Ig)‐M and/or IgD. 19 , 20 First, many memory B cells have undergone class switch recombination from IgM or IgD to IgG, IgA, or IgE. 21 Second, the vast majority harbour a mutated immunoglobulin variable gene by which they have gained higher affinity to the corresponding antigens. 22 Finally, they can survive longer compared to naïve B cells, and proliferate and/or differentiate faster and more efficiently than naïve B cells upon antigen stimulation. 23 , 24 Collectively, memory B cells play a critical role in the adaptive humoral immune system and for protecting the host from pathogenic antigens. Although human memory B cells consist of several subsets, in the present study we focused on IgG memory B cells. 25 , 26 One important reason for this is that secreted IgG is the most predominant isotype found in the human body, and another reason is that IgG is not expressed by naïve B cells; thus we can analyse memory B cells clearly separate from naïve B cells. Collectively, in the present study, we analysed the IgG heavy‐chain repertoire of PBMC in patients with ATL and explored its clinical and immunological significance, especially focussing on the degree of diversity.
Patients and methods
Patients and study design
The present study was affiliated with the MIMOGA study. For patients enrolled in this trial, three additional inclusion criteria were used: (i) those patients who provided written informed consent for genomic analysis at enrolment in the MIMOGA study, according to the principles of the Declaration of Helsinki, (ii) those patients in whom both the quality and quantity of total RNA extracted from their PBMC before mogamulizumab treatment reached the level required for IgG heavy‐chain repertoire analysis, and (iii) those patients in whom more than 200 in‐frame productive IgG sequence reads were obtained from their RNA. This third criterion was intended to minimize the possibility of including data with sequencing errors or insufficient RNA. Of the 101 patients who received mogamulizumab‐containing treatment in the MIMOGA study, 81 were eligible according to these criteria and they were all enrolled in the present substudy (Fig 1). The clinical information and immunological data of these patients, recorded in the MIMOGA study, were also available to the present study. 18
Fig 1.
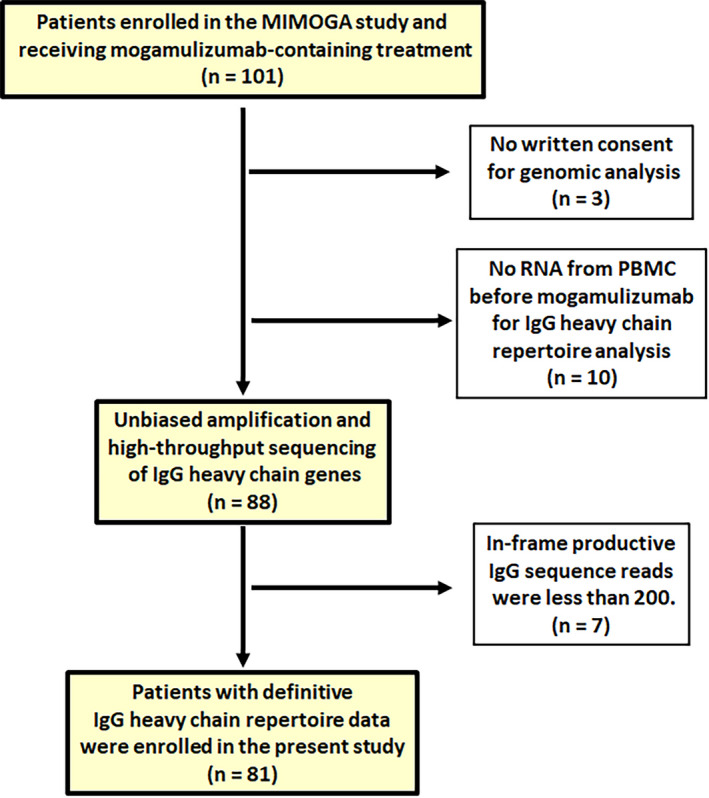
Reporting Recommendations for Tumour Marker Prognostic Studies (REMARK) diagram. The diagram details how the patients in the present study were selected from those in the MIMOGA study. IgG, immunoglobulin G; MIMOGA, monitoring of immune responses following mogamulizumab‐containing treatment in patients with adult T‐cell leukaemia–lymphoma; PBMC, peripheral blood mononuclear cells. [Colour figure can be viewed at wileyonlinelibrary.com]
Unbiased amplification and high‐throughput sequencing of IgG heavy‐chain genes
Data analyses
Each sequence read was analysed by bioinformatics software created by Repertoire Genesis Incorporation, Osaka Laboratory (Ibaraki, Japan), and the use of IgG heavy‐chain variable (IGHV) segments, diversity (IGHD), joining (IGHJ), and constant (IGHC) region segments, and CDR3 sequences were determined as previously reported. 27 , 28 Briefly, the identification of V, D, J, and C regions was determined by the sequence with the highest identity to reference sequence datasets available from the international ImMunoGeneTics (IMGT) database (http://www.imgt.org). Unproductive reads (out‐of‐frame reads) were excluded and only the productive reads (in‐frame reads) were used in further analyses. The identical V, D, J, and deduced amino acid sequences of CDR3 were defined as a unique sequence read. The unique reads with in‐frame CDR3 nucleotide sequences were used for calculation of diversity indices for the IgG heavy‐chain repertoire. The details are available in Data S1.
Repertoire diversity
The Shannon–Weaver diversity index (SWDI), 29 , 30 in which large numbers denote higher diversity, was calculated using R (R‐3·4.3) (http://www.R‐project.org) and its package vegan. The data analyses including SWDI were performed by Repertoire Genesis Incorporation.
Statistical analysis
Survival estimates were calculated using the Kaplan–Meier method. OS was measured from the day of the first dose of mogamulizumab to death resulting from any cause. The survival estimate was calculated with all transplanted patients (n = 10) censoring at the day of allogeneic haematopoietic stem cell transplantation, in the same manner as in a previous study. 17 Correlations between two variables were assessed using Spearman rank correlation coefficients (R s). Differences between the two groups were examined with a Mann–Whitney U‐test or Fisher´s exact test. Clinically meaningful cut‐off values of the SWDI for the IgG heavy‐chain repertoire in PBMC of patients with ATL have not been determined thus far. Hence, we attempted to divide such patients into two groups according to this parameter. The cut‐off values of SWDI for the IgG heavy‐chain repertoire were tested at six different points between the 30th and 70th percentiles (30th, 38th, 46th, 54th, 62nd, and 70th percentiles). Subsequently, univariate analysis for survival was performed using a Cox proportional hazards regression model according to the SWDI for the IgG heavy‐chain repertoire in PBMC at each of the six cut‐off points. In the present study, the cut‐off point yielding the minimum P value was selected as the most meaningful cut‐off value. 31 The cut‐off value of the percentage of CD2−CD19+ B cells within lymphocytes in PBMC was set at 0·15% according to our previous study. 18 Univariate and multivariate analyses using Cox proportional hazards regression models was applied to evaluate variables potentially affecting OS. In this study, P < 0·05 (two‐sided) was considered significant. The details are available in Data S1.
Results
Patients’ characteristics according to SWDI for the IgG heavy‐chain repertoire in PBMC
The SWDI for the IgG heavy‐chain repertoire in PBMC of 81 patients enrolled in the present study was 4·78, 5·25, and 1·02–10·13 (mean, median, and range), whereas these values were 8·46, 8·50, and 7·10–9·61 in healthy controls previously analysed in the same manner. 27 Thus, the IgG repertoire of peripheral B cells from patients with ATL was significantly less diverse than in healthy individuals (P < 0·001). A three‐dimensional graphical representation of the IgG heavy‐chain repertoire of an ATL patient before mogamulizumab‐containing treatment with the highest SWDI (SWDI = 10·13) in the approximately 30th (5·73), 50th (5·25), and 70th (3·53) percentiles in descending order, as well in as the lowest (1·02), is shown in Figs 2A–E, respectively. In addition, pie charts of the IgG heavy‐chain repertoire for all 81 patients are shown in Figure S1. In the present study, the cut‐off value of SWDI for the IgG heavy‐chain repertoire in PBMC was set at 5·38 (Table SI). The clinical characteristics according to the SWDI for the IgG heavy‐chain repertoire of such patients are summarized in Table I. The median ages were 69 (range, 41–86) and 68 years (range, 41–83) in patients with a lower and higher SWDI, respectively (not significantly different). There were also no significant differences between patients with a lower or higher SWDI regarding previous systemic chemotherapy (yes or no), sex (female or male), Eastern Cooperative Oncology Group performance status [ECOG PS; 0 (n = 23), 1 (n = 35), or 2 (n = 14), 3 (n = 7), 4 (n = 2)], serum soluble interleukin‐2 receptor (sIL‐2R; ≤20 000 or >20 000 U/ml), serum adjusted calcium (Ca; ≤11·0 or >11·0 mg/dl), or serum albumin (Alb; <3·5 or ≥3·5 g/dl). There were also no significant differences between patients with a lower or higher SWDI for clinical subtype (chronic [n = 11], smouldering [n = 2], or acute [n = 52], or lymphoma [n = 16]; Table I). In this context, there were also no significant differences in the SWDI for the IgG repertoire between patients with acute (4·84 [mean], 5·31 [median]) and lymphoma (4·56, 4·34) subtypes (P = 0·613). The HTLV‐1 provirus load (PVL) in PBMC tended to be higher in patients with a lower SWDI compared to those with a higher SWDI (P = 0·090; Table I).
Fig 2.
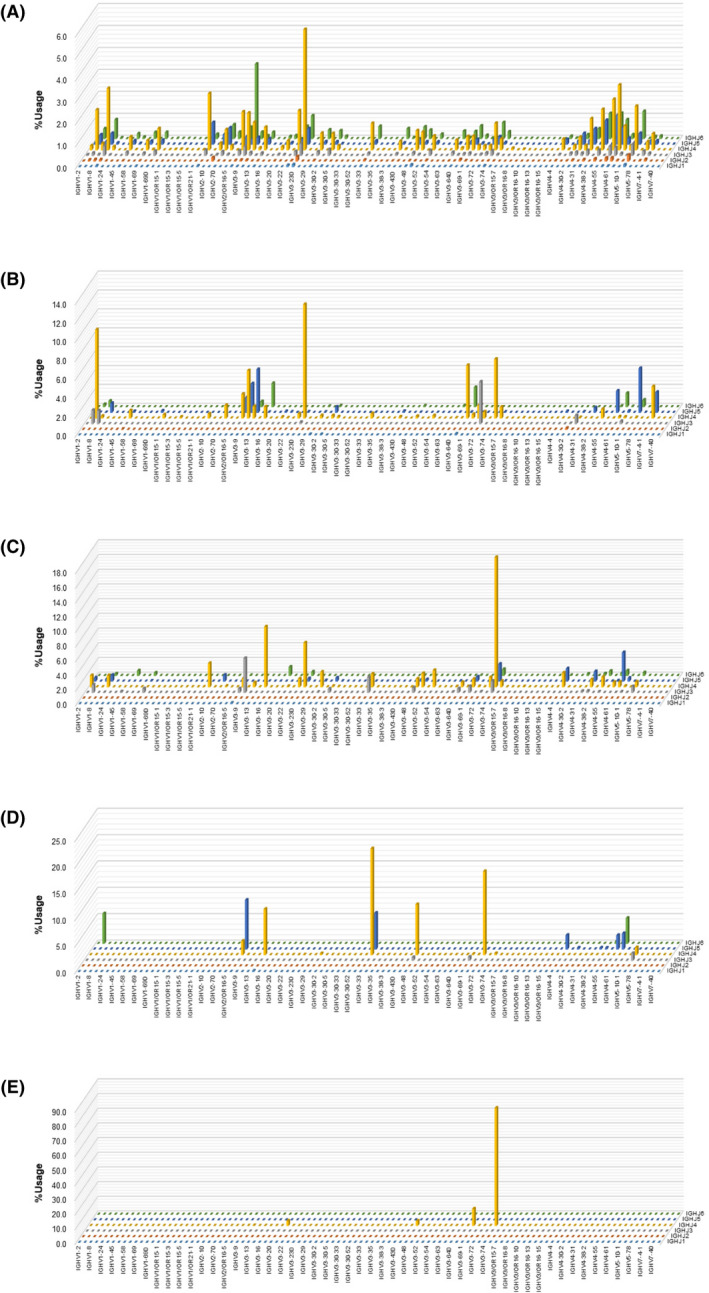
Three‐dimensional graphical representation of the immunoglobulin G (IgG) heavy‐chain repertoire in peripheral blood mononuclear cells (PBMC). The x‐axis (width) and y‐axis (depth) indicate V and J genes, respectively. The z‐axis (height) represents frequencies of V–J combinations. The total of the frequencies is 100%. The IgG heavy‐chain repertoire in PBMC in adult T‐cell leukaemia–lymphoma (ATL) patients with a Shannon–Weaver diversity index (SWDI) that is in the highest (SWDI = 10·13; A), approximately 30th (5·73; B), approximately 50th (5·25; C), and approximately 70th (3·53; D) percentiles, in descending order, and that in the lowest percentile (1·02; E) are shown. [Colour figure can be viewed at wileyonlinelibrary.com]
Table I.
Clinical characteristics of ATL patients according to SWDI for the IgG heavy‐chain repertoire in PBMC.
| Characteristics | SWDI for IgG heavy‐chain repertoire in PBMC | P value | |
|---|---|---|---|
| <5·38 | >5·38 | ||
| Number (%) | 44 (54) | 37 (46) | |
| Age (year) | 0·958 | ||
| Mean | 68 | 69 | |
| Median | 69 | 68 | |
| Range | 41–86 | 41–83 | |
| Previous systemic chemotherapy | 0·579 | ||
| Yes | 10 (23) | 6 (16) | |
| No | 34 (77) | 31 (84) | |
| Sex | 0·252 | ||
| Female | 30 (68) | 20 (54) | |
| Male | 14 (32) | 17 (46) | |
| Clinical subtype | 1·000 | ||
| Chronic, smouldering | 7 (16) | 6 (16) | |
| Acute, lymphoma | 37 (84) | 31 (84) | |
| ECOG PS | 0·323 | ||
| 0, 1 | 29 (66) | 29 (78) | |
| 2, 3, 4 | 15 (34) | 8 (22) | |
| Serum sIL‐2R (U/ml) | 0·278 | ||
| <20 000 | 37 (84) | 27 (73) | |
| >20 000 | 7 (16) | 10 (27) | |
| Serum Ca (mg/dl)*,† | 0·655 | ||
| <11·0 | 41 (95) | 33 (92) | |
| >11·0 | 2 (5) | 3 (8) | |
| Serum albumin (g/dl)* | 0·643 | ||
| >3·5 | 26 (79) | 24 (67) | |
| <3·5 | 17 (21) | 12 (33) | |
| HTLV‐1 PVL (copies/1 000 PBMC) | 0·090 | ||
| Mean | 571·6 | 379·2 | |
| Median | 458·6 | 219·9 | |
| Range | 0·8–3 093·7 | 0·5–1 919·5 | |
Alb, albumin; ATL, adult T‐cell leukaemia–lymphoma; Ca, calcium; ECOG, Eastern Cooperative Oncology Group; IgG, immunoglobulin G; PBMC, peripheral blood mononuclear cells; PS, performance status; sIL‐2R, soluble interleukin‐2 receptor; SWDI, Shannon–Weaver diversity index.
The data of two patients were unknown.
When serum Alb level was less than 4·0 g/dl, serum Ca was adjusted by the concentration of serum Alb as follows: adjusted Ca level (mg/dl) = measured Ca level (mg/dl) + [4‐albumin level (g/dl)]. HTLV‐1, human T cell lymphotropic virus type 1; PVL, provirus load.
Immunological characteristics according to SWDI for the IgG heavy‐chain repertoire in PBMC of patients with ATL are summarized in Table II. The percentage of CD2−CD19+ B cells was significantly higher in patients with a higher SWDI (P = 0·022). The percentage of CD11c+ monocytes within the monocyte population in PBMC was also significantly higher in patients with a higher SWDI compared to those with a lower SWDI (P = 0·029), but there were no differences in the percentages of CD3+CD8+ T cells or CD16+CD56+ natural killer (NK) cells between patients with a higher or lower SWDI (Table II). There were also no significant differences between percentages of CD4+, CD4+FOXP3lowCD45RA+, CD4+FOXP3highCD45RA−, or CD4+FOXP3lowCD45RA− T cells in patients with a higher or lower SWDI. Finally, there were also no differences for FOXP3lowCD45RA+, FOXP3highCD45RA−, or FOXP3lowCD45RA− T cells within CD4+ lymphocytes in patients with a higher or lower SWDI (Table II).
Table II.
Immunological characteristics of ATL patients according to SWDI for the IgG heavy‐chain repertoire in PBMC.
| Characteristics | SWDI for IgG heavy‐chain repertoire in PBMC | P value | |
|---|---|---|---|
| <5·38 | >5·38 | ||
| Number (%) | 44 (54) | 37 (46) | |
| CD2−CD19+ cells (%)* | 0·022 | ||
| Mean | 1·23 | 4·20 | |
| Median | 0·49 | 1·25 | |
| Range | 0·00–8·17 | 0·03–32·91 | |
| CD3+CD8+ cells (%)* | 0·857 | ||
| Mean | 14·70 | 12·78 | |
| Median | 8·94 | 9·65 | |
| Range | 0·10–71·73 | 0·60–52·33 | |
| CD16+CD56+ cells (%)* | 0·197 | ||
| Mean | 7·24 | 10·52 | |
| Median | 3·83 | 6·64 | |
| Range | 0·07–31·57 | 0·18–39·42 | |
| CD11c monocytes (%)† | 0·029 | ||
| Mean | 50·91 | 65·42 | |
| Median | 51·62 | 78·60 | |
| Range | 0·50–97·18 | 0·34–95·76 | |
| CD4+ cells (%)* | 0·308 | ||
| Mean | 64·90 | 59·55 | |
| Median | 68·86 | 62·67 | |
| Range | 11·16–98·14 | 14·60–97·86 | |
| CD4+FOXP3lowCD45RA+ cells (%)* | 0·271 | ||
| Mean | 0·21 | 0·34 | |
| Median | 0·07 | 0·16 | |
| Range | 0·00–1·27 | 0·00–4·71 | |
| FOXP3lowCD45RA+ cells (%)‡ | 0·135 | ||
| Mean | 0·34 | 0·62 | |
| Median | 0·14 | 0·26 | |
| Range | 0·00–1·50 | 0·00–7·64 | |
| CD4+FOXP3highCD45RA− cells (%)* | 0·712 | ||
| Mean | 19·08 | 15·41 | |
| Median | 2·16 | 2·66 | |
| Range | 0·00–87·26 | 0·00–83·09 | |
| FOXP3highCD45RA− cells (%)‡ | 0·649 | ||
| Mean | 24·63 | 21·11 | |
| Median | 4·84 | 6·17 | |
| Range | 0·00–90·91 | 0·00–88·80 | |
| CD4+FOXP3lowCD45RA− cells (%)* | 0·726 | ||
| Mean | 16·75 | 11·06 | |
| Median | 3·19 | 4·01 | |
| Range | 0·21–88·41 | 0·17–61·12 | |
| FOXP3lowCD45RA− cells (%)‡ | 0·528 | ||
| Mean | 21·22 | 16·66 | |
| Median | 6·30 | 7·69 | |
| Range | 0·39–98·04 | 0·26–87·58 | |
ATL, adult T‐cell leukaemia–lymphoma; IgG, immunoglobulin G; PBMC, peripheral blood mononuclear cells; SWDI, Shannon–Weaver diversity index.
The percentage among whole lymphocytes in PBMC.
The percentage among whole monocytes in PBMC.
The percentage among CD4+ lymphocytes in PBMC.
Correlations between the SWDI for the IgG heavy‐chain repertoire in PBMC and clinical or immunological parameters of ATL patients before starting mogamulizumab
No significant correlation was noted between the SWDI for the IgG heavy‐chain repertoire in PBMC and the HTLV‐1 PVL (R s = −0·124, P = 0·269), or serum sIL‐2R levels (R s = 0·057, P = 0·611). However, a weak but significant positive correlation between the SWDI for the IgG repertoire and percentages of CD2−CD19+ B cells was seen (R s = 0·291, P = 0·008). No correlations were seen between the SWDI and percentages of CD3+CD8+ T (R s = −0·078, P = 0·489), or CD16+CD56+ NK (R s = 0·075, P = 0·508) cells within the lymphocyte population, or CD11c+ monocytes within the monocyte population (R s = 0·182, P = 0·105). There were also no significant correlations between the SWDI for the IgG repertoire and percentages of CD4+ (R s = −0·125, P = 0·267), CD4+FOXP3lowCD45RA+ (R s = 0·058, P = 0·608), CD4+FOXP3highCD45RA− (R s = 0·043, P = 0·705), and CD4+FOXP3lowCD45RA− (R s = 0·061, P = 0·587) T cells. Finally, there were also no significant correlations between the SWDI and FOXP3lowCD45RA+ (R s = 0·070, P = 0·534), FOXP3highCD45RA− (R s = 0·045, P = 0·689), and FOXP3lowCD45RA− (R s = 0·084, P = 0·455) T cells within the CD4+ lymphocyte subset.
Overall survival of patients according to the SWDI for the IgG heavy‐chain repertoire in PBMC
In the entire patient cohort of the present study, the median OS was 16·0 months [95% confidence interval (CI), 9·9–22·1, Fig 3A)]. The SWDI for the IgG heavy‐chain repertoire tended to be associated with OS [lower compared with higher; hazard ratio (HR), 1·608; 95% CI, 0·876–2·951; Fig 3B]. However, the SWDI for the IgG heavy‐chain repertoire together with the percentage of CD2−CD19+ B cells within lymphocytes was significantly associated with OS [a lower SWDI and a lower percentage of CD2−CD19+ B cells within lymphocytes (≤0·15%) compared with the other; HR, 2·892; 95% CI, 1·347–6·206; Fig 3C].
Fig 3.
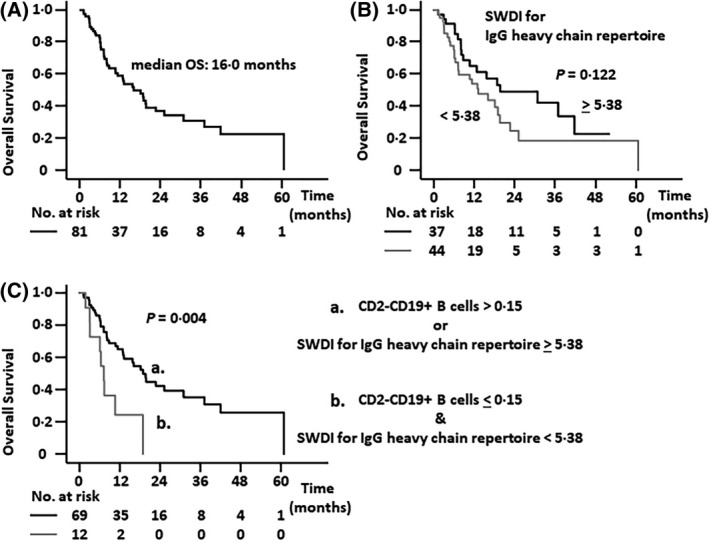
Overall survival (OS) of adult T‐cell leukaemia–lymphoma (ATL) patients. (A) OS of all patients with ATL enrolled in the study. (B) OS of patients with a lower Shannon–Weaver diversity index (SWDI) for the immunoglobulin G (IgG) heavy‐chain repertoire (<5·38) tended to be worse than for patients with a higher SWDI (≥5·38; median OS 13·2 vs. 19·7 months; P = 0·122). (C) OS of patients with both a lower SWDI and a lower percentage of CD2−CD19+ B cells within lymphocytes (≤0·15%) was significantly worse than other patients (median OS, 7·2 vs. 18·8 months; P = 0·004).
Univariate and multivariate analyses for OS including the SWDI for the IgG heavy‐chain repertoire in PBMC
Univariate analyses demonstrated that sex (male vs female; HR, 1·017; 95% CI, 0·552–1·876), and age (>70 vs ≤70 years; HR, 1·109; 95% CI, 0·603–2·039) were not associated with OS. Univariate analyses also indicated that clinical subtype (acute or lymphoma versus chronic or smouldering; HR, 2·997; 95% CI, 1·065–8·437), ECOG PS (2, 3 or 4, relative to 0 or 1; HR, 1·994; 95% CI, 1·066–3·729), and serum sIL‐2R level (>20 000 vs ≤20 000 U/ml; HR, 5·352; 95% CI, 2·649–10·812) were significantly associated with OS.
Subsequently, multivariate analysis of OS in the 81 patients was performed using the following six variables: sex, age, clinical subtype, ECOG PS, sIL‐2R, and the SWDI for the IgG heavy‐chain repertoire in PBMC. Of these, two variables significantly affected OS: a higher serum sIL‐2R (HR, 5·036; 95% CI, 2·377–10·669), and a lower SWDI for the IgG repertoire (HR, 2·124; 95% CI, 1·114–4·049; Table III). Furthermore, combining these six variables with the percentage of CD2−CD19+ B cells indicated that two variables significantly affected OS, namely, a higher serum sIL‐2R (HR, 4·907; 95% CI, 2·337–10·303), and a lower SWDI together with a lower percentage of CD2−CD19+ cells (HR, 2·909; 95% CI, 1·322–6·398; Table IV).
Table III.
Multivariate analysis including SWDI for the IgG heavy‐chain repertoire in PBMC for OS‡ in patients with ATL.
| Variables | Number | Hazard ratio | (95% CI) | P value |
|---|---|---|---|---|
| Sex | ||||
| Female | 50 | 1·000 | Reference | |
| Male | 31 | 1·175 | (0·612–2·255) | 0·628 |
| Age, years | ||||
| ≤70 | 52 | 1·000 | Reference | |
| >70 | 29 | 1·154 | (0·605–2·200) | 0·663 |
| Clinical subtype | ||||
| Chronic, smouldering | 13 | 1·000 | Reference | |
| Acute, lymphoma | 68 | 2·425 | (0·801–7·343) | 0·117 |
| ECOG PS | ||||
| 0, 1 | 58 | 1·000 | Reference | |
| 2, 3, 4 | 23 | 1·231 | (0·624–2·428) | 0·548 |
| sIL‐2R (U/ml) | ||||
| <20 000 | 64 | 1·000 | Reference | |
| >20 000 | 17 | 5·036 | (2·377–10·669) | <0·001 |
| SWDI for IgG heavy‐chain repertoire in PBMC | ||||
| >5·38 | 37 | 1·000 | Reference | |
| <5·38 | 44 | 2·124 | (1·114–4·049) | 0·022 |
ATL, adult T‐cell leukaemia–lymphoma; IgG, immunoglobulin G; SWDI, Shannon–Weaver diversity index; PBMC, peripheral blood mononuclear cells; CI, confidence interval.
OS, overall survival. The patients were censored at the day of allogeneic haematopoietic stem cell transplantation.
Table IV.
Multivariate analysis including SWDI for the IgG heavy‐chain repertoire in PBMC and the percentage of CD2−CD19+ B cells for OS in patients with ATL.
| Variables | Number | Hazard ratio | (95% CI) | P value |
|---|---|---|---|---|
| Sex | ||||
| Female | 50 | 1·000 | Reference | |
| Male | 31 | 0·971 | (0·521–1·809) | 0·927 |
| Age, years | ||||
| ≤70 | 52 | 1·000 | Reference | |
| >70 | 29 | 1·121 | (0·599–2·099) | 0·721 |
| Clinical subtype | ||||
| Chronic, smouldering | 13 | 1·000 | Reference | |
| Acute, lymphoma | 68 | 1·943 | (0·647–5·838) | 0·236 |
| ECOG PS | ||||
| 0, 1 | 58 | 1·000 | Reference | |
| 2, 3, 4 | 23 | 1·234 | (0·638–2·389) | 0·532 |
| sIL‐2R (U/ml) | ||||
| <20 000 | 64 | 1·000 | Reference | |
| >20 000 | 17 | 4·907 | (2·337–10·303) | <0·001 |
| SWDI for IgG heavy‐chain repertoire and CD2−CD19+ cells (%)† | ||||
| >5·38 or >0·15 | 69 | 1·000 | Reference | |
| <5·38 & <0·15 | 12 | 2·909 | (1·322–6·398) | 0·008 |
ATL, adult T‐cell leukaemia–lymphoma; CI, confidence interval; IgG, immunoglobulin G; PBMC, peripheral blood mononuclear cells; SWDI, Shannon–Weaver diversity index.
OS, overall survival. The patients were censored at the day of allogeneic haematopoietic stem cell transplantation.
The percentage among whole lymphocytes in PBMC.
Discussion
Here, we have analysed the IgG heavy‐chain repertoire in PBMC of patients who were prospectively enrolled in the MIMOGA study, and received mogamulizumab‐containing treatment. We explored the clinical and immunological significance of IgG B‐cell diversity in patients with ATL. The present finding, that peripheral IgG B cells in these patients were significantly less diverse than those in healthy individuals, is consistent with the fact that patients with ATL are severely immunocompromised. 3 , 4 In this context, it has been generally accepted that cellular immune responses are attenuated in patients with ATL, partially because the tumour cells from a subset of patients actually function as regulatory T (Treg) cells. 17 , 32 , 33 Furthermore, the present finding of a shrunken IgG B‐cell pool indicates that there is only a limited variety of memory B cells poised to respond to the corresponding pathogen antigens, which would lead to attenuated humoral immune responses.
The present study did not identify the definitive clinical factors correlating with higher or lower IgG B‐cell diversity in these ATL patients. Such clinical factors included serum sIL‐2R levels, which reflect the ATL tumour burden. 34 In this context, no significant correlation between the SWDI for the IgG heavy‐chain repertoire and serum sIL‐2R level was observed, indicating that ATL disease status seems not to be directly associated with the degree of IgG B‐cell diversity in PBMC. In comparison, the HTLV‐1 PVL in PBMC tended to be higher in patients with a lower SWDI for the IgG repertoire relative to those with a higher SWDI, although this difference did not achieve statistical significance. This may suggest that the HTLV‐1‐infected cells inhibited the generation and diversification of IgG B cells.
Regarding immunological parameters, the proportion of CD2−CD19+ B cells was significantly higher in patients with a higher SWDI for the IgG repertoire in PBMC, and a significant positive correlation between these two parameters was also observed. These findings possibly indicate that the more B cells, including IgG B cells, are present in the body, the more they can diversify. The proportion of CD11c+ monocytes within the monocyte population also tended to be higher in patients with a higher SWDI, although a significant correlation between these two parameters was not observed. The precise relationship between CD11c+ monocytes and IgG B‐cell diversity has not yet been elucidated, and thus further investigation regarding this matter is warranted. With respect to CD4+ T cells, including cells with an effector Treg phenotype, 35 , 36 there was no association with IgG B‐cell diversity. We have previously reported that the IgM heavy‐chain repertoire in PBMC became less diverse at the time of occurrence of skin‐related adverse events related to mogamulizumab treatment, 37 which results in the depletion of cells with an effector Treg phenotype. 38 , 39 The relationship between Treg phenotype and B‐cell diversity is not fully clarified as yet, thus also warranting further investigation.
The present study documented that the lower IgG B‐cell diversity in PBMC, especially together with lower percentages of CD2−CD19+ B cells, was an independent unfavourable prognostic factor in patients with ATL receiving mogamulizumab‐containing treatment. This may be because only a small set of memory B cells is poised to respond to the corresponding pathogen antigens in patients with a lower IgG B‐cell diversity. When the patients’ B‐cell counts are also low, this limited variety of memory B cells inevitably carries more severe implications. Accordingly, patients with a lower IgG B‐cell diversity would be expected to show weaker humoral immune responses, contributing to a poorer prognosis. From this point of view, appropriate intravenous polyclonal immunoglobulin therapy may be effective for treating opportunistic infections in patients with ATL receiving mogamulizumab‐containing treatment.
Although the present investigation offers significant observations regarding IgG B‐cell diversity for clinical outcomes in ATL patients undergoing mogamulizumab‐containing treatment, several limitations must be recognized. First, the study included both previously untreated patients and those treated with systemic chemotherapy. Second, some patients received mogamulizumab monotherapy, whereas others received different combination therapies. These variables might both affect the conclusions of the present study. Finally, the relationship and difference in IgG B‐cell diversity in different organs, such as lymphoid tissues or bone marrow, and not only in PBMC as studied here, also requires analysis in humans.
In conclusion, the present study demonstrated that peripheral IgG B cells in patients with ATL were less diverse than in healthy individuals. In addition, the study demonstrated that the lower IgG B‐cell diversity in PBMC, especially together with a lower percentage of CD2−CD19+ B cells, was an independent unfavourable prognostic factor. The present observations highlight the importance of humoral immune responses for the clinical outcomes of ATL patients receiving mogamulizumab‐containing treatment. Further investigation of strategies to enhance humoral immune responses, including the diversification of memory B cells in patients with ATL, is highly warranted.
Funding information
This work was supported by a grant‐in‐aid for scientific research (C) (21K08374 to KN), and by grants‐in‐aid from the Japan Agency for Medical Research and Development (No. 20ae0101074h0001 and 21ae0101074h0001 to RU, and No. 20cm0106301h0005 and 21cm0106301h0005 to TI).
Author contribution
Conception and design: KN, RU, TI. Acquisition and analysis of data: KN, SK, NN, IC, MY, YI, MH, HS, JM, EO, TJ, MO, AI, KY, HT, TaK, ToK, YS, KI, SI, TM, AU, TI. Data interpretation: KN, RU, TI. Manuscript writing and final approval of manuscript: all authors.
Conflicts of interest
KN has received consultancy fees, research funding and honoraria from Kyowa Kirin, research funding from Chugai Pharmaceutical, and honoraria from Celgene, Eisai, Meiji Seika Pharma, Janssen Pharmaceutical and Bristol Myers Squibb. SK has received consultancy fees, research funding and honoraria from Chugai Pharmaceutical, research funding and honoraria from Kyowa Kirin, Daiichi Sankyo, Takeda Pharmaceutical, Janssen Pharmaceutical and honoraria from Otsuka Pharmaceutical and Eisai. NN has received consultancy fees from JIMRO and honoraria from Novartis, Takeda Pharmaceutical, Chugai Pharmaceutical, Celgene, Otsuka Pharmaceutical, Nippon Shinyaku, Kyowa Kirin and Asahi Kasei Pharma. IC has no conflicts of interest to disclose. MY has received honoraria from Novartis International, Takeda Pharmaceutical, Sanofi, Otsuka and Chugai Pharmaceutical. YI has received honoraria from Kyowa Kirin, Celgene, Eisai, Bristol Myers Squibb and Sanofi. MH has received research funding from Chugai Pharmaceutical and honoraria from Nippon Shinyaku and Symbio Pharma., KY has received honoraria from Abbvie, Amgen, Celgene, Daiichi Sankyo, Eli Lilly Japan, Janssen Pharmaceutical, Kaken Pharmaceutical, Kyowa Kirin, Maruho, Minophagen Pharmaceutical, Novartis, Sanofi, Taiho Pharmaceutical, Torii Pharmaceutical, UCB Japan, Eisai, Sun Pharma Japan. HT has received honoraria from Ono Pharmaceutical, Chugai Pharmaceutical, Eisai, Novartis International and patients, and royalties from Mesoblast. YS has received research funding and honoraria from Kyowa Kirin and honoraria from Celgene and Bristol Myers Squibb., KI has received research funding and honoraria from Kyowa Kirin. SI has received research funding and honoraria from Takeda Pharmaceutical, Ono Pharmaceutical, Janssen Pharmaceutical, Sanofi, Celgene, and research funding from Chugai Pharmaceutical, Bristol Myers Squibb, Abbvie and Daiichi Sankyo. TM is an employee of Repertoire Genesis Incorporation. AU has received honoraria from Novartis International, Kyowa Kirin, Daiichi Sankyo, Bristol Myers Squibb, Celgene, Pfizer, Minophagen Pharmaceutical, Janssen Pharmaceutical, Chugai Pharmaceutical, HUYA Japan, JIMRO, Meiji Seika Pharma and Otsuka Medical Devices. RU has received research funding from Kyowa Kirin, Chugai Pharmaceutical and Ono Pharmaceutical. HS, JM, EO, TJ, MO, AI, TaK, ToK, and TI have no conflicts of interest to disclose.
Supporting information
Data S1. Supplementary Methods.
Fig S1. Pie charts of the immunoglobulin G (IgG) heavy‐chain repertoire in peripheral blood mononuclear cells (PBMC). Pie charts showing the IgG heavy‐chain repertoires in PBMC in 81 adult T‐cell leukaemia–lymphoma (ATL) patients. The unique reads with in‐frame CDR3 nucleotide sequences whose frequencies are 0·1% or higher are shown by individual colours, and those that are less than 0·1% are shown in grayscale. The pie charts are presented in ascending order of Shannon–Weaver diversity index (SWDI) for the IgG heavy‐chain repertoire, from upper left to lower right. The SWDIs are indicated to the upper left of the pie charts in the left, middle, and right columns. Pie charts labelled A, B, C, D, E at their upper right correspond to A, B, C, D, E, respectively, in the three‐dimensional graphical representation in Fig. 2. The upper 44 cases are those of the lower SWDI group (<5·38), and the lower 37 cases are included in the higher SWDI group (>5·38).
Table SI. Univariate Cox proportional hazard analysis for overall survival (OS) according to the Shannon–Weaver diversity index (SWDI) for the immunoglobulin G (IgG) heavy‐chain repertoire in peripheral blood mononuclear cells (PBMC).
Acknowledgements
We thank all nurses and clinical research coordinators who were involved in this study, for their patient care and schedule management. We also thank the Japan Institute of Statistical Technology (Tokyo, Japan) for their critical review of the statistical analyses, and for providing a certificate attesting the validity of the statistical methods used for the data analyses in the present manuscript. We are grateful to Mr. Hiroshi Iwata (SRL Medisearch Inc., Tokyo, Japan) for his support in scheduling samples from patients, and for sample preservation. We are also grateful to Dr. Satoshi Shinohara (Repertoire Genesis Incorporation) for his fruitful discussion with us. We also thank Professor Hiroyoshi Nishikawa (Nagoya University Graduate School of Medicine) for his helpful advice.
References
- 1. Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T‐cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481–92. [PubMed] [Google Scholar]
- 2. Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T‐cell leukemia‐lymphoma. A report from the Lymphoma Study Group (1984–1987). Br J Haematol. 1991;79:428–37. [DOI] [PubMed] [Google Scholar]
- 3. Ishitsuka K, Tamura K. Human T‐cell leukaemia virus type I and adult T‐cell leukaemia‐lymphoma. Lancet Oncol. 2014;15:e517–e526. [DOI] [PubMed] [Google Scholar]
- 4. Matsuoka M, Jeang KT. Human T‐cell leukaemia virus type 1 (HTLV‐1) infectivity and cellular transformation. Nat Rev Cancer. 2007;7:270–80. [DOI] [PubMed] [Google Scholar]
- 5. Yoshie O, Fujisawa R, Nakayama T, Harasawa H, Tago H, Izawa D, et al. Frequent expression of CCR4 in adult T‐cell leukemia and human T‐cell leukemia virus type 1‐transformed T cells. Blood. 2002;99:1505–11. [DOI] [PubMed] [Google Scholar]
- 6. Ishida T, Utsunomiya A, Iida S, Inagaki H, Takatsuka Y, Kusumoto S, et al. Clinical significance of CCR4 expression in adult T‐cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin Cancer Res. 2003;9:3625–34. [PubMed] [Google Scholar]
- 7. Ishida T, Iida S, Akatsuka Y, Ishii T, Miyazaki M, Komatsu H, et al. The CC chemokine receptor 4 as a novel specific molecular target for immunotherapy in adult T‐Cell leukemia/lymphoma. Clin Cancer Res. 2004;10:7529–39. [DOI] [PubMed] [Google Scholar]
- 8. Ito A, Ishida T, Utsunomiya A, Sato F, Mori F, Yano H, et al. Defucosylated anti‐CCR4 monoclonal antibody exerts potent ADCC against primary ATLL cells mediated by autologous human immune cells in NOD/Shi‐scid, IL‐2R gamma(null) mice in vivo. J Immunol. 2009;183:4782–91. [DOI] [PubMed] [Google Scholar]
- 9. Ishii T, Ishida T, Utsunomiya A, Inagaki A, Yano H, Komatsu H, et al. Defucosylated humanized anti‐CCR4 monoclonal antibody KW‐0761 as a novel immunotherapeutic agent for adult T‐cell leukemia/lymphoma. Clin Cancer Res. 2010;16:1520–31. [DOI] [PubMed] [Google Scholar]
- 10. Ishida T, Joh T, Uike N, Yamamoto K, Utsunomiya A, Yoshida S, et al. Defucosylated anti‐CCR4 monoclonal antibody (KW‐0761) for relapsed adult T‐cell leukemia‐lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30:837–42. [DOI] [PubMed] [Google Scholar]
- 11. Ishida T, Jo T, Takemoto S, Suzushima H, Uozumi K, Yamamoto K, et al. Dose‐intensified chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T‐cell leukaemia‐lymphoma: a randomized phase II study. Br J Haematol. 2015;169:672–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ishida T, Utsunomiya A, Jo T, Yamamoto K, Kato K, Yoshida S, et al. Mogamulizumab for relapsed adult T‐cell leukemia‐lymphoma: Updated follow‐up analysis of phase I and II studies. Cancer Sci. 2017;108:2022–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ishida T, Jo T, Takemoto S, Suzushima H, Suehiro Y, Choi I, et al. Follow‐up of a randomised phase II study of chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T‐cell leukaemia‐lymphoma: impact on allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2019;184:479–83. [DOI] [PubMed] [Google Scholar]
- 14. Sakamoto Y, Ishida T, Masaki A, Murase T, Yonekura K, Tashiro Y, et al. CCR4 mutations associated with superior outcome of adult T‐cell leukemia/lymphoma under mogamulizumab treatment. Blood. 2018;132:758–61. [DOI] [PubMed] [Google Scholar]
- 15. Sakamoto Y, Ishida T, Masaki A, Takeshita M, Iwasaki H, Yonekura K, et al. Clinical significance of CD28 gene‐related activating alterations in adult T‐cell leukaemia/lymphoma. Br J Haematol. 2021;192:281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sakamoto Y, Ishida T, Masaki A, Murase T, Takeshita M, Muto R, et al. Clinical significance of TP53 mutations in adult T‐cell leukemia/lymphoma. Br J Haematol. 2021; 10.1111/bjh.17749. [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ishida T, Ueda R. Immunopathogenesis of lymphoma: focus on CCR4. Cancer Sci. 2011;102:44–50. [DOI] [PubMed] [Google Scholar]
- 18. Yonekura K, Kusumoto S, Choi I, Nakano N, Ito A, Suehiro Y, et al. Mogamulizumab for adult T‐cell leukemia‐lymphoma: a multicenter prospective observational study. Blood Adv. 2020;4:5133–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–8. [DOI] [PubMed] [Google Scholar]
- 20. Klein U, Dalla‐Favera R. Germinal centres: role in B‐cell physiology and malignancy. Nat Rev Immunol. 2008;8:22–33. [DOI] [PubMed] [Google Scholar]
- 21. Kataoka T, Miyata T, Honjo T. Repetitive sequences in class switch recombination regions of immunoglobulin heavy chain genes. Cell. 1981;23:357–68. [DOI] [PubMed] [Google Scholar]
- 22. Klein U, Rajewsky K, Küppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–202. [DOI] [PubMed] [Google Scholar]
- 24. Good KL, Avery DT, Tangye SG. Resting human memory B cells are intrinsically programmed for enhanced survival and responsiveness to diverse stimuli compared to naive B cells. J Immunol. 2009;182:890–901. [DOI] [PubMed] [Google Scholar]
- 25. Tarlinton D. B‐cell memory: are subsets necessary? Nat Rev Immunol. 2006;6:785–90. [DOI] [PubMed] [Google Scholar]
- 26. Seifert M, Küppers R. Human memory B cells. Leukemia. 2016;30:2283–92. [DOI] [PubMed] [Google Scholar]
- 27. Kitaura K, Yamashita H, Ayabe H, Shini T, Matsutani T, Suzuki R. Different somatic hypermutation levels among antibody subclasses disclosed by a new next‐generation sequencing‐based antibody repertoire analysis. Front Immunol. 2017;8:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ichinohe T, Miyama T, Kawase T, Honjo Y, Kitaura K, Sato H, et al. Next‐generation immune repertoire sequencing as a clue to elucidate the landscape of immune modulation by host‐gut microbiome interactions. Front Immunol. 2018;9:668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Calderbank Robert, Sloane Neil J. A. Claude Shannon (1916–2001). Nature. 2001;410(6830):768. 10.1038/35071223 [DOI] [PubMed] [Google Scholar]
- 30. Shekhar TR, Kiran BR, Puttaiah ET, Shivaraj Y, Mahadevan KM. Phytoplankton as index of water quality with reference to industrial pollution. J Environ Biol. 2008;29:233–6. [PubMed] [Google Scholar]
- 31. Mazumdar M, Glassman JR. Categorizing a prognostic variable: review of methods, code for easy implementation and applications to decision‐making about cancer treatments. Stat Med. 2000;19:113–32. [DOI] [PubMed] [Google Scholar]
- 32. Kohno T, Yamada Y, Akamatsu N, Kamihira S, Imaizumi Y, Tomonaga M, et al. Possible origin of adult T‐cell leukemia/lymphoma cells from human T lymphotropic virus type‐1‐infected regulatory T cells. Cancer Sci. 2005;96:527–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yano H, Ishida T, Inagaki A, Ishii T, Kusumoto S, Komatsu H, et al. Regulatory T‐cell function of adult T‐cell leukemia/lymphoma cells. Int J Cancer. 2007;120:2052–7. [DOI] [PubMed] [Google Scholar]
- 34. Motoi T, Uchiyama T, Uchino H, Ueda R, Araki K. Serum soluble interleukin‐2 receptor levels in patients with adult T‐cell leukemia and human T‐cell leukemia/lymphoma virus type‐I seropositive healthy carriers. Jpn J Cancer Res. 1988;79:593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. [DOI] [PubMed] [Google Scholar]
- 36. Suzuki S, Ishida T, Yoshikawa K, Ueda R. Current status of immunotherapy. Jpn J Clin Oncol. 2016;46:191–203. [DOI] [PubMed] [Google Scholar]
- 37. Suzuki Y, Saito M, Ishii T, Urakawa I, Matsumoto A, Masaki A, et al. Mogamulizumab treatment elicits autoantibodies attacking the skin in patients with adult T‐Cell leukemia‐lymphoma. Clin Cancer Res. 2019;25:4388–99. [DOI] [PubMed] [Google Scholar]
- 38. Saito M, Ishii T, Urakawa I, Matsumoto A, Masaki A, Ito A, et al. Robust CD8+ T‐cell proliferation and diversification after mogamulizumab in patients with adult T‐cell leukemia‐lymphoma. Blood Adv. 2020;4:2180–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I, et al. Anti‐CCR4 mAb selectively depletes effector‐type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci U S A. 2013;110:17945–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplementary Methods.
Fig S1. Pie charts of the immunoglobulin G (IgG) heavy‐chain repertoire in peripheral blood mononuclear cells (PBMC). Pie charts showing the IgG heavy‐chain repertoires in PBMC in 81 adult T‐cell leukaemia–lymphoma (ATL) patients. The unique reads with in‐frame CDR3 nucleotide sequences whose frequencies are 0·1% or higher are shown by individual colours, and those that are less than 0·1% are shown in grayscale. The pie charts are presented in ascending order of Shannon–Weaver diversity index (SWDI) for the IgG heavy‐chain repertoire, from upper left to lower right. The SWDIs are indicated to the upper left of the pie charts in the left, middle, and right columns. Pie charts labelled A, B, C, D, E at their upper right correspond to A, B, C, D, E, respectively, in the three‐dimensional graphical representation in Fig. 2. The upper 44 cases are those of the lower SWDI group (<5·38), and the lower 37 cases are included in the higher SWDI group (>5·38).
Table SI. Univariate Cox proportional hazard analysis for overall survival (OS) according to the Shannon–Weaver diversity index (SWDI) for the immunoglobulin G (IgG) heavy‐chain repertoire in peripheral blood mononuclear cells (PBMC).


