Abstract
Introduction
We tested our hypothesis that atrial entrainment pacing (EP) of a) the common‐type (com‐) fast‐slow (F/S‐) atypical atrioventricular nodal reentrant tachycardia (AVNRT) using a typical slow pathway (SP), or b) the superior‐type (sup‐) F/S‐AVNRT using a superior SP, both modify the retrograde conduction time across the SP immediately after termination of EP (retro‐SP‐time).
Methods
We measured the difference in the His‐atrial interval (HA difference) immediately after cessation of EP, performed at 2 ± 2 rates from the high right atrium (HA[1]‐HRA) versus from the proximal coronary sinus (HA[1]‐CS) in 17 patients with com‐F/S‐AVNRT and 11 patients with sup‐F/S‐AVNRT. We also measured the atrial‐His and HA intervals of the first and second cycles immediately after cessation of EP and during stable tachycardia.
Results
Unequal responses, defined as a ≥ 20‐ms HA difference at ≥1 EP rates, were observed in 16 patients (57%), including 7 with com‐ and 9 with sup‐F/S‐AVNRT. Irrespective of the EP rate, all unequal responses of com‐F/S‐AVNRT were due to a shorter HA[1]‐CS than HA[1]‐HRA, with a mean 34 ± 11 ms HA difference, whereas all unequal responses of sup‐F/S‐AVNRT were due to a longer HA[1]‐CS than HA[1]‐HRA, with a mean 49 ± 25 ms HA difference. The unequal responses resolved within two cycles after the cessation of EP.
Conclusions
We have identified a little‐known pacing site‐ and pacing rate‐dependent shortening of the retro‐SP‐time.
Keywords: atrioventricular nodal reentrant tachycardia, entrainment pacing, fast atrioventricular nodal pathway, programmed electrical stimulation, slow atrioventricular nodal pathway
1. INTRODUCTION
The electrophysiological response of reentrant tachycardia after cessation of entrainment pacing (EP) is an important clue to clarify the mechanisms of tachycardia as well as the conductive properties and anatomical location of the reentry circuit. This implies the entrainment rule by which the site of entrainment has no effect on the orthodromic conduction time across the reentry circuit immediately after cessation of pacing. This rule has been amply verified in macroreentrant arrhythmias, such as atrial flutter or ventricular tachycardia associated with structural heart disease, 1 , 2 in which case the conduction time across the circuit is technically measurable. It has not, however, been unequivocally verified in atypical atrioventricular (AV) nodal reentrant tachycardia (NRT). We have recently observed a unique electrophysiological response upon atrial induction of fast‐slow (F/S‐) AVNRT, characterized by a shortening of the retrograde conduction time across the slow pathway (SP), which represents a large fraction of the orthodromic conduction time. 3 Therefore, we tested our hypothesis that atrial EP of a) the common‐type (com‐) F/S‐AVNRT using a typical SP, or b) the superior‐type (sup‐) F/S‐AVNRT using a superior SP, 4 both modify the retrograde conduction time immediately after termination of EP (retro‐SP‐time).
2. METHODS
2.1. Study sample, electrophysiological study, and catheter ablation
We prospectively identified 17 patients with com‐F/S‐ and 11 with sup‐F/S‐AVNRT, who underwent electrophysiological studies and successful EP at ≥1 pacing rates from the high right atrium (HRA) and the proximal coronary sinus (prox‐CS), as described later, followed by the successful ablation of an SP. This study, which complied with the guidelines of the Declaration of Helsinki, was approved by the Institutional Review Board of Gunma University Hospital, and all patients were granted their written informed consent to participate.
Electrophysiologic study and catheter ablation were performed as described in detail in previous reports. 4 , 5 We excluded the diagnosis of AVNRT, AV reentrant tachycardia, and atrial tachycardia, using standard criteria (Table 1). 4 , 5
Table 1.
Diagnosis of atrioventricular nodal reentrant tachycardia (AVNRT)
| AVNRT | |||
|---|---|---|---|
|
All (n = 28) |
com‐F/S‐ (n = 17) |
sup‐F/S‐ (n = 11) |
|
| Criteria of exclusion of atrioventricular reentrant tachycardia | |||
|
7 (25) 25 (100) |
3 (18) 17 (100) |
4 (36) 11 (100) |
| Criteria of exclusion of atrial tachycardia | |||
|
|
16 (57) 5 (18) 13 (46) 19 (68) 1 (4) |
11 (65) 0 (0) 8 (42) 16 (94) 0 (0) |
5 (45) 5 (45) 5 (45) 3 (27) 1 (9) |
2.2. Study protocol of entrainment pacing and measurement of retro‐SP‐time
EP from the HRA (EP‐HRA) and from the prox‐CS (EP‐CS) was performed during episodes of tachycardia, keeping the cycle lengths (CL) stable and in rapid sequence to blunt the effects of variations in autonomic activity on AV nodal conduction. The CL of all episodes of tachycardia were stable before the initiation of EP. The first stimulus of EP was synchronized to the atrial electrogram during tachycardia, at an interval 10‐ms shorter than the tachycardia CL (TCL). The first EP was attempted at an S‐S interval 10–30 ms shorter than the TCL; if successful, the next attempts were performed at a gradually shorter S‐S interval in decrements of 10 ms, until failure of EP. EP was successful when (1) all atrial and subsequent His bundle electrograms were captured 1:1 via the fast pathway, while keeping the atrio‐His (AH) interval constant during EP‐HRA and EP‐CS at a fixed S‐S interval, for up to 10 consecutive paced cycles, and (2) F/S‐AVNRT was re‐induced and persisted after the cessation of EP. When EP was unsuccessful at baseline, because of termination of the tachycardia during or after pacing, another attempt was made during infusion of isoproterenol. When a > 20‐ms difference in stable TCL immediately following EP was observed between EP‐HRA and EP‐CS, suggesting changes in AV nodal conduction between both EP, the recordings were not retained for the analysis.
As detailed later, the difference in the retro‐SP‐time between EP‐HRA and EP‐CS corresponds to the difference in the His‐atrial (HA) interval measured immediately after cessation of EP (HA difference) between EP‐HRA and EP‐CS (HA[1]‐HRA and HA[1]‐CS, respectively). The HA difference measured after each EP attempt was classified as an unequal response when ≥20 ms, versus equal response when <20 ms. Moreover, to examine the dynamics of the unequal responses, we measured the AH and HA intervals of the first and second cycles after cessation of each EP, and during the tachycardia immediately after its CL had stabilized.
2.3. Statistical analysis
Continuous variables are expressed as means ± standard deviation (SD), and categorical variables as counts and percentages. Differences between two continuous variables were examined by paired or unpaired t‐test, while categorical variables were compared using Fisher's exact test. A p‐value <.05 was considered statistically significant.
3. RESULTS
Between 1 and 8 rates per patient (mean = 2 ± 2) of the EP protocol were successfully delivered among all patients studied (Table 2). Paired measurements made immediately before delivery of EP revealed a <10‐ms difference in the TCL, suggesting that the autonomic nervous tone was not significantly changed by EP, irrespective of the pacing site.
Table 2.
Patients and procedural characteristics
| AVNRT | |||||
|---|---|---|---|---|---|
| All | com‐F/S‐ | sup‐F/S‐ | p | ||
| Patients, n | 28 | 17 | 11 | ||
| Age, year | 61 ± 15 | 55 ± 16 | 70 ± 6 | .003 | |
| Men | 9 (32) | 4 (24) | 5 (45) | .41 | |
|
Anterograde AV nodal function, ms AH interval Longest overdrive pacing cycle length associated with Wenckebach type atrioventricular block Effective refractory period of the fast pathway Dual atrioventricular nodal pathway |
87 ± 21 392 ± 78 299 ± 70 19 (68) |
83 ± 18 364 ± 70 299 ± 65 10 (59) |
89 ± 26 434 ± 68 301 ± 75 9 (82) |
.51 .03 .94 .03 |
|
| Retrograde conduction via typ‐SP during ventricular pacing | 16 (57) | 14 (82) | 2 (18) | .001 | |
|
Attempts at successful entrainment pacing, n Patients with successful entrainment pacing attempt at a single rate Patients with successful entrainment pacing attempts at multiple rates |
2 ± 2 13 (46) 15 (54) |
2 ± 2 6 (35) 11 (65) |
2 ± 1 7 (64) 4 (36) |
.21 .25 .25 |
|
| Use of isoproterenol to promote successful entrainment pacing | 11 (44) | 8 (47) | 5 (33) | .93 | |
|
Tachycardia intervals, ms Cycle length AH HA |
385 ± 64 103 ± 35 285 ± 65 |
374 ± 69 92 ± 26 286 ± 71 |
403 ± 46 116 ± 41 287 ± 52 |
.20 .12 .96 |
|
Note: Values are means ± SD or percent (%) of observations.
Abbreviations: AH, atrial‐His interval; HA, His‐atrial interval; SP, slow pathway.
Equal responses at ≥1 rates of EP were observed in 19 patients (68%), including 16 patients presenting with com‐F/S‐AVNRT and three with sup‐F/S‐AVNRT (Figure 1). Unequal responses at ≥1 rates of EP were observed in 16 patients (57%), including seven patients with com‐ (Figure 2) and nine with sup‐F/S‐AVNRT (Figure 3). Among these 16 patients, seven (25% of the overall study sample), including six with com‐F/S‐ and 1 with sup‐F/S‐AVNRT presented with both unequal and equal responses. It is noteworthy that, irrespective of the EP rate, all unequal responses of com‐F/S‐AVNRT were due to a shorter HA[1]‐CS than HA[1]‐HRA, with a mean 34 ± 11 ms difference in HA, whereas all unequal responses of sup‐F/S‐AVNRT were due to a longer HA[1]‐CS than HA[1]‐HRA, with a mean 49 ± 25 ms difference in HA. Moreover, all ≥20‐ms HA differences (unequal responses) resolved within two cycles after the cessation of EP (Figure 4).
Figure 1.
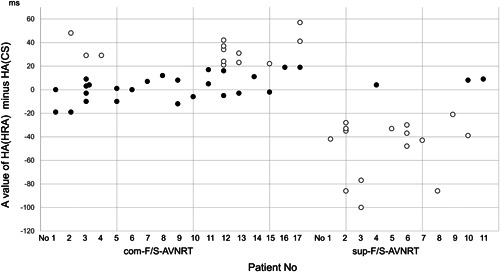
Scatter plot of the HA interval after EP from the HRA (HA‐HRA) minus the HA interval after EP from the proximal coronary sinus (HA‐CS) in all EP attempts of com‐ and sup‐F/S‐AVNRT in each patient. The filled and unfilled circles represent the equal and unequal responses, respectively. AVNRT, atrioventricular nodal reentrant tachycardia; CS, coronary sinus; EP, entrainment pacing; HA, His‐atrial interval; HRA, high right atrium
Figure 2.
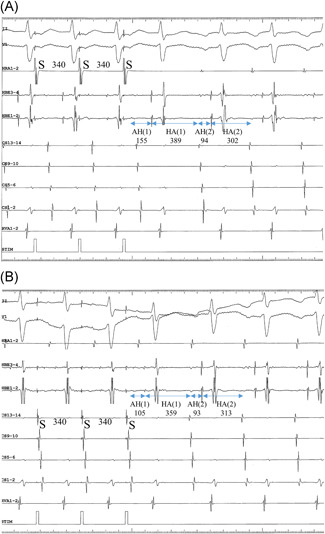
Intracardiac electrograms of com‐F/S‐AVNRT illustrating the unequal response in Patient no 8. (A) The 340‐ms pacing CL (S‐S) is identical during EP from the high right atrium (EP‐HRA) and (B) EP from the proximal coronary sinus (EP‐CS). The 359‐ms HA interval in the first cycle [HA(1)] following EP‐CS is considerably shorter than the 389‐ms HA(1) after EP‐HRA. In contrast, the HA interval in the seecond cycle [HA(2)] after EP‐HRA (313 ms) is nearly identical to the HA(2) after EP‐CS (302 ms). I, II and V1 = surface electrocardiogram. AVNRT, AVNRT, atrioventricular nodal reentrant tachycardia; HRA, high right atrium; HBE, His bundle electrogram; CS13‐14 to 1‐2 = proximal to distal CS recordings; RVA, right ventricular apex
Figure 3.
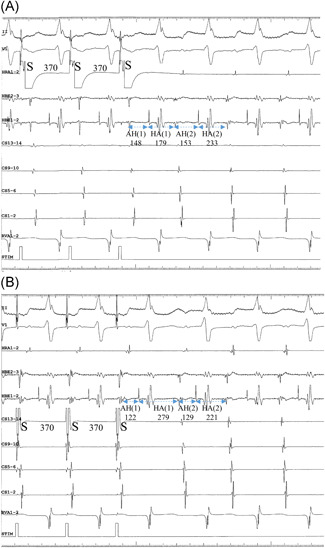
Intracardiac electrograms of sup‐F/S‐AVNRT illustrating the unequal response in Patient no 3. (A) The 370‐ms pacing CL (S‐S) is identical during EP from the high right atrium (EP‐HRA) and (B) EP from the proximal coronary sinus (EP‐CS). The HA interval in the first cycle [HA(1)] following EP‐HRA (179 ms) is considerably shorter than the HA(1) after EP‐CS (279 ms). However, the 233 ms HA interval in the second cycle [HA(2)] after EP‐HRA is nearly identical to the HA(2) after EP‐CS (221 ms). AVNRT, AVNRT, atrioventricular nodal reentrant tachycardia; HRA, high right atrium
Figure 4.
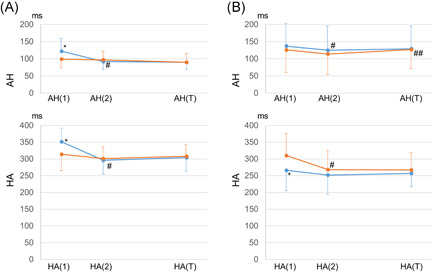
Change in the AH (upper graph) and HA (lower graph) intervals, after cessation of EP from the high right atrium (EP‐HRA; blue lines) and from the proximal coronary sinus (EP‐CS; orange lines), in patients presenting with the unequal response in com‐F/S‐AVNRT (A) and sup‐F/S‐AVNRT (B). *p < .05 versus AH or HA interval after EP‐CS; # p < .05 versus AH(1) or HA(1); ## p < .05 versus AH(2) or HA(2). AVNRT, AVNRT, atrioventricular nodal reentrant tachycardia; HRA, high right atrium
In both types of AVNRT, the atrial activation sequence during tachycardia remained unchanged after EP, and no relationship between rate of EP and equal or unequal response was observed.
4. DISCUSSION
In a high proportion of com‐ and sup‐F/S‐AVNRT, a change in the HA interval (unequal response) was observed immediately after EP‐HRA and after EP‐CS, depending on the rate of EP. Furthermore, the pacing site associated with a shorter HA interval was also related to the type of F/S‐AVNRT, that is, the prox‐CS site was associated with com‐F/S‐AVNRT and the HRA site with sup‐F/S‐AVNRT. Unequal response was limited to the first cycle after cessation of EP.
4.1. Putative mechanism of the unequal response
This observational study was not designed to clarify the mechanism responsible for the unequal response. Previous studies, however, might suggest some mechanisms, including an enhanced sympathetic tone, 11 impulse summation, 12 accelerating retrograde conduction via the AV nodal pathway, a shift in retrograde conduction over multiple SP, 13 a frequency‐dependent, orthodromic conduction delay via the AV nodal pathways, 14 or a re‐induction of the tachycardia after its termination by EP. However, these mechanisms hardly explain the existence of pacing site‐specific differences in HA intervals at certain EP rates, without change in the atrial activation sequence, and limited to the first cycle after cessation of EP. A disturbance in the tachycardia CL after EP may have occurred, due to the decremental conduction properties of the AV node. 15 However, the difference in the HA interval of the first cycle does not seem attributable to this simple property of the AV node, since a cycle of orthodromic propagation through the AV nodal pathways, up the first cycle after EP, corresponds to a cycle of EP, irrespective of the site of pacing.
Since AH and HA are arbitrary time intervals that reflect conduction across the lower common pathway up to the His bundle recording site, they do not accurately represent the anterograde or retrograde conduction times across the AV nodal pathways during F/S‐AVNRT. However, since the anterograde conduction time over the lower common pathway during EP is expected to remain constant, irrespective of the pacing site, the HA difference may reflect a difference in the retro‐SP‐time. A shorter AH interval after EP‐CS versus EP‐HRA is a well‐known pacing site‐dependent effect on the anterograde AV nodal conduction time 16 , 17 (Figure 4A), which is not associated with the subsequent retrograde conduction time over the SP reflected in the HA interval.
Atrial stimulation has an effect on subsequent retrograde conduction over the AV nodal pathway. The shortening by atrial pacing of the retrograde conduction time via the fast pathway has been observed in several experimental 18 , 19 and clinical 20 , 21 studies. Similar shortenings of non‐reentrant 22 or reentrant 3 retro‐SP‐times by atrial stimulation have been reported. These shortening effects of the retrograde conduction time over the AV nodal pathway after atrial stimulation can be reasonably explained by frequency‐dependent decremental properties of the AV nodal pathway, caused by its anterograde concealed penetration by atrial stimulation. 3 , 20 , 21 , 22
We hypothesized that the mechanism of the unequal response is based on the pacing site‐dependent effect on the retro‐SP‐time, limited to the first cycle after cessation of EP. We presumed initially that, in presence of anterograde conduction in the SP during EP, the antidromic wavefront penetrates the SP and collides with the orthodromic wavefront inside the pathway (Figure 5). Conduction of the orthodromic wavefront within the SP may be decrementally delayed due to an EP rate faster than that of the tachycardia, while the last antidromic wavefront inside the SP may facilitate conduction of the next orthodromic wavefront inside the SP, depending on its frequency‐dependent decremental conduction property (Figure 5). The net balance of these two effects would, therefore, determine the length of the HA interval of the first cycle after cessation of EP. Moreover, the depth of antidromic penetration of the SP varies, depending on the site of pacing relative to the AV nodal pathways, increasing during EP‐CS versus during EP‐HRA in com‐F/S‐AVNRT (left panels in Figure 5A) and during EP‐HRA versus during EP‐CS in sup‐F/S‐AVNRT (left panels in Figure 5B). On the other hand, the amount of decremental delay of the orthodromic wavefront inside the SP in response to EP may depend on the decremental properties of the pathway, or the depth of its orthodromic penetration, or both. From these assumptions, the retro‐SP‐time with deeper antidromic penetration of the SP, that is, EP‐HRA of sup‐F/S‐AVNRT and EP‐CS of com‐F/S‐AVNRT, may be shortened, to a greater extent than the decremental delay of the orthodromic wavefront inside the SP (right panels in Figure 5A,B), causing an unequal response. We further hypothesize that the EP rate is related to the depth of antidromic penetration of the SP by modulating the anterograde or retrograde conductivity of the SP during EP, explaining the pacing rate‐dependent variations in HA. Conversely, during EP with no or little antidromic penetration due to conduction block or collision with an orthodromic wavefront, or a decremental conduction delay within the SP canceling the facilitation of orthodromic conduction inside the pathway, this shortening effect does not vary significantly regardless of the EP site, resulting in an equal response. The equal versus unequal response is, therefore, determined by a complex interaction among several factors, including the orthodromic, decremental conduction delay inside the orthodromic SP, the facilitating effect of pre‐exciting segments of the SP by the antidromic wavefront, and a difference in antidromic penetration into the SP depending on the site and rate of EP.
Figure 5.
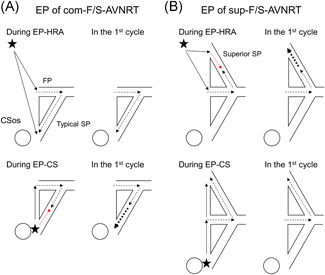
Schematic illustration of the putative mechanism of shortening of retrograde conduction over the slow pathway (SP) after atrial EP of com‐F/S‐AVNRT (A) and sup‐F/S‐AVNRT (B). The thin arrows show the directions of propagation in the atrium, and the black and red dotted arrows show the orthodromic and antidromic directions of propagation in the AV nodal pathways. The bold, dotted arrows illustrate the accelerated retrograde conduction over the atrial side of the typical or superior SP. (A) When antidromic penetration into the typical SP is deeper during EP from the proximal coronary sinus (EP‐CS) than from the high right atrium (EP‐HRA; left panel), the retrograde conduction time over the typical SP in the first cycle is shorter after EP‐CS than after EP‐HRA (right panel), causing an unequal response. (B) When antidromic penetration into the superior SP is deeper during EP‐HRA than during EP‐CS (left panel), the retrograde conduction time over the superior SP in the first cycle is shorter after EP‐CS than after EP‐HRA (right panel), causing an unequal response. See text for details. CSos, ostium of coronary sinus, FP, fast pathway. The asterisks indicate the EP site in the HRA or proximal CS. AVNRT, atrioventricular nodal reentrant tachycardia; CS, coronary sinus; EP, entrainment pacing; HRA, high right atrium
We observed no linear relationship between the rate of EP and HA, suggesting that the EP rate is not linearly correlated with the depth of antidromic penetration into the SP, perhaps because of a complex combination of retrograde and anterograde conductive properties of the SP during EP. Furthermore, a faster EP rate does not necessarily increase the antidromic penetration of the SP. The mechanism of the higher incidence of unequal response and the wider difference in HA interval observed in the sup‐F/S‐ versus the com‐F/S‐AVNRT remains unclear, though might be due to differences in the electrophysiological properties of the two SP, causing a greater antidromic penetration of the superior SP during EP.
Our proposed mechanism may not be strictly based on objective observations within the AV nodal reentry circuit and are, at least in part, speculative. However, the facilitation of conduction in AV nodal tissue due to collision of the orthodromic with the antidromic wavefront has been confirmed in both the anterograde and retrograde directions. 20 , 21 , 22
4.2. Clinical implications
Previous studies have suggested that the absence of VA linking eliminates the diagnosis of AVNRT. 7 , 23 However, our study suggests that, since the unequal response was observed at a specific rate of EP in patients with F/S‐AVNRT, an evaluation of the VA relationship is not an infallible means of diagnosing supraventricular tachycardia. When an unequal response is observed during a study of long RP tachycardia, differential atrial EP should be repeated at several different pacing rates to avoid an erroneous diagnosis of F/S‐AVNRT.
Moreover, our study offers new insights into the resetting of the F/S‐AVNRT cycle by atrial pacing. In previous studies, entrainment mapping or mapping of the return of single extrastimuli during AVNRT was used to analyze the electrophysiological or structural properties of AV nodal pathways. 20 , 24 , 25 These maneuvers are based on the understanding that the postpacing (PPI) or post extrastimulus interval through the critical pathway of the reentry circuit is equal to the TCL, since the conduction velocity of the wavefront depolarized by paced events is the same as that of the tachycardia. However, our study suggests that the PPI after atrial entrainment of F/S‐AVNRT can be modified by shortening of the retro‐SP‐time, and is not invariably indicative of the location of the EP site relative to the critical circuit. For example, in Figure 6, where the retro‐SP‐time that modified the PPI is evidenced by an HA shorter than the subsequent HA interval during tachycardia, immediately after the cessation of EP‐CS, EP may not have been delivered to the critical pathway despite the precisely similar PPI and TCL. The site of successful ablation was, in fact, located away from the pacing site. Thus, the analysis of F/S‐AVNRT on the basis of the PPI, mapping of the return cycle, or both, may erroneously locate the reentry circuit.
Figure 6.
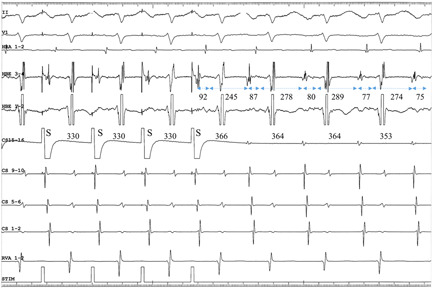
Intracardiac electrograms during EP from the proximal CS at an S‐S CL of 330 ms, in Patient no 13, presenting with com‐F/S‐AVNRT. Since the 245‐ms HA interval immediately following the cessation of EP is shorter than the 278‐ms subsequent HA interval, the 368‐ms PPI at the CS15‐16 pacing site is nearly identical to the atrial CL. The dashed, bidirectional arrows and numbers underneath indicate the AH or HA intervals in ms. The numbers on the CS15‐16 channel indicate the interpacing stimuli or interatrial intervals in ms. AVNRT, atrioventricular nodal reentrant tachycardia; CS, coronary sinus; EP, entrainment pacing; HA, His‐atrial interval; PPI, postpacing interval
Our study describes a new effect of shortening orthodromic SP conduction by its antidromic penetration in F/S‐AVNRT. This effect is distinct from simple orthodromic 20 , 24 , 25 or antidromic 26 resetting. The absence of the ability to record AV nodal signals makes it difficult to detect this phenomenon. Moreover, in the absence of strict criteria, measurements of individual interelectrogram intervals do not reliably confirm the presence of paradoxical resetting, which, in our study, was documented by differential pacing. Since a similar effect has not been observed in macroreentrant atrial flutter 27 or in scar‐related ventricular tachycardia, 28 this resetting may be characteristic of tachycardias originating from AV nodal tissue.
Finally, the presence of an upper common pathway just above the AV nodal reentry circuit can be excluded in some AVNRT associated with an unequal response elicited by pacing from two different sites. The existence of an upper common pathway remains controversial. 29 However, if it were present, this site selectivity would not be observed. Thus, the upper limb of these reentrant circuits must consist of atrial tissue surrounding the AV node. 24
5. CONCLUSIONS
We have identified a little‐known pacing site‐ and pacing rate‐dependent shortening of the retrograde conduction time over the SP immediately after EP.
Kaneko Y, Nakajima T, Tamura S, et al. Pacing site‐ and rate‐dependent shortening of retrograde conduction time over the slow pathway after atrial entrainment of fast‐slow atrioventricular nodal reentrant tachycardia. J Cardiovasc Electrophysiol. 2021;32:2979‐2986. 10.1111/jce.15242
Disclosures: None.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, [Y.K.], upon reasonable request.
REFERENCES
- 1. Olgin JE, Kalman JM, Fitzpatrick AP, Lesh MD. Role of right atrial endocardial structures as barriers to conduction during human type I atrial flutter. Activation and entrainment mapping guided by intracardiac echocardiography. Circulation. 1995;92:1839‐1848. [DOI] [PubMed] [Google Scholar]
- 2. Nitta T, Schuessler RB, Mitsuno M, et al. Return cycle mapping after entrainment of ventricular tachycardia. Circulation. 1998;31(97):1164‐1175. [DOI] [PubMed] [Google Scholar]
- 3. Tamura S, Nakajima T, Iizuka T, et al. Unique electrophysiological properties of fast‐slow atrioventricular nodal reentrant tachycardia characterized by a shortening of retrograde conduction time via a slow pathway manifested during atrial induction. J Cardiovasc Electrophysiol. 2020;31:1420‐1429. [DOI] [PubMed] [Google Scholar]
- 4. Kaneko Y, Naito S, Okishige K, et al. Atypical fast‐slow atrioventricular nodal reentrant tachycardia incorporating a “superior” slow pathway: a distinct supraventricular tachyarrhythmia. Circulation. 2016;133:114‐123. [DOI] [PubMed] [Google Scholar]
- 5. Kaneko Y, Nakajima T, Irie T, Iizuka T, Tamura S, Kurabayashi M. Atrial and ventricular activation sequence after ventricular induction/entrainment pacing during fast‐slow atrioventricular nodal reentrant tachycardia: new insight into the use of V‐A‐A‐V for the differential diagnosis of supraventricular tachycardia. Heart Rhythm. 2017;14:1615‐1622. [DOI] [PubMed] [Google Scholar]
- 6. Knight BP, Zivin A, Souza J, et al. A technique for the rapid diagnosis of atrial tachycardia in the electrophysiology laboratory. J Am Coll Cardiol. 1999;33:775‐781. [DOI] [PubMed] [Google Scholar]
- 7. Maruyama M, Kobayashi Y, Miyauchi Y, et al. The VA relationship after differential atrial overdrive pacing: a novel tool for the diagnosis of atrial tachycardia in the electrophysiologic laboratory. J Cardiovasc Electrophysiol. 2007;18:1127‐1133. [DOI] [PubMed] [Google Scholar]
- 8. Kaneko Y, Nakajima T, Tamura S, et al. Superior‐type fast‐slow Atrioventricular nodal reentrant tachycardia phenotype mimicking the slow‐fast type. Circ Arrhythm Electrophysiol. 2020;13:e008732. [DOI] [PubMed] [Google Scholar]
- 9. Dandamudi G, Mokabberi R, Assal C, et al. A novel approach to differentiating orthodromic reciprocating tachycardia from atrioventricular nodal reentrant tachycardia. Heart Rhythm. 2010;7:1326‐1329. [DOI] [PubMed] [Google Scholar]
- 10. AlMahameed ST, Buxton AE, Michaud GF. New criteria during right ventricular pacing to determine the mechanism of supraventricular tachycardia. Circ Arrhythm Electrophysiol. 2010;3:578‐584. [DOI] [PubMed] [Google Scholar]
- 11. Sadr‐Ameli MA, Shenasa M, Lacombe P, Faugère G, Nadeau R. Effect of autonomic nervous system modulation on retrograde atrioventricular nodal conduction in the human heart. Cardiovasc Res. 1987;21:45‐54. [DOI] [PubMed] [Google Scholar]
- 12. Antzelevitch C, Moe GK. Electrotonic inhibition and summation of impulse conduction in mammalian Purkinje fibers. Am J Physiol. 1983;245:H42‐H53. [DOI] [PubMed] [Google Scholar]
- 13. Hwang C, Martin DJ, Goodman JS, et al. Atypical atrioventricular node reciprocating tachycardia masquerading as tachycardia using a left‐sided accessory pathway. J Am Coll Cardiol. 1997;30:218‐225. [DOI] [PubMed] [Google Scholar]
- 14. Kinjo T, Sasaki S, Kimura M, et al. Long postpacing interval after entrainment of tachycardia including a slow conduction zone within the circuit. J Cardiovasc Electrophysiol. 2016;27:923‐929. [DOI] [PubMed] [Google Scholar]
- 15. Crawford TC, Mukerji S, Good E, et al. Utility of atrial and ventricular cycle length variability in determining the mechanism of paroxysmal supraventricular tachycardia. J Cardiovasc Electrophysiol. 2007;18:698‐703. [DOI] [PubMed] [Google Scholar]
- 16. Leon FAY, Denes P, Wu D, Pietras RJ, Rosen KM. Effects of atrial pacing site on atrial and atrioventricular nodal function. Br Heart J. 1975;37:576‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aranda J, Castellanos A, Moleiro F, Befeler B. Effects of the pacing site on A‐H conduction and refractoriness in patients with short P‐R intervals. Circulation. 1976;53:33‐39. [DOI] [PubMed] [Google Scholar]
- 18. Moore EN, Spear JF. Experimental studies on the facilitation of AV conduction by ectopic beats in dogs and rabbits. Circ Res. 1971;29:29‐39. [DOI] [PubMed] [Google Scholar]
- 19. Moe GK, Childers RW, Merideth J. An appraisal of “supernormal” A‐V conduction. Circulation. 1968;38:5‐28. [DOI] [PubMed] [Google Scholar]
- 20. Shenasa M, Denker S, Mahmud R, Lehmann M, Gilbert CJ, Akhtar M. Atrioventricular nodal conduction and refractoriness after intranodal collision from antegrade and retrograde impulses. Circulation. 1983;67:651‐660. [DOI] [PubMed] [Google Scholar]
- 21. Mahmud R, Lehmann M, Denker S, Gilbert CJ, Akhtar M. Atrioventricular sequential pacing: differential effect on retrograde conduction related to level of impulse collision. Circulation. 1983;68:23‐32. [DOI] [PubMed] [Google Scholar]
- 22. Kaneko Y, Nakajima T, Irie T, Kurabayashi M. Shortening of retrograde conduction time over slow pathway after atrial stimulation. Heart Rhythm. 2015;12:1097‐1099. [DOI] [PubMed] [Google Scholar]
- 23. Sarkozy A, Richter S, Chierchia GB, et al. A novel pacing manoeuvre to diagnose atrial tachycardia. Europace. 2008;10:459‐466. [DOI] [PubMed] [Google Scholar]
- 24. Yamabe H, Shimasaki Y, Honda O, Kimura Y, Hokamura Y. Demonstration of the exact anatomic tachycardia circuit in the fast‐slow form of atrioventricular nodal reentrant tachycardia. Circulation. 2001;104:1268‐1273. [DOI] [PubMed] [Google Scholar]
- 25. Nawata H, Yamamoto N, Hirao K, et al. Heterogeneity of anterograde fast‐pathway and retrograde slow‐pathway conduction patterns in patients with the fast‐slow form of atrioventricular nodal reentrant tachycardia: electrophysiologic and electrocardiographic considerations. J Am Coll Cardiol. 1998;32:1731‐1740. [DOI] [PubMed] [Google Scholar]
- 26. Kay GN, Epstein AE, Plumb VJ. Resetting of ventricular tachycardia by single extrastimuli: relation to slow conduction within the reentrant circuit. Circulation. 1990;81:1507‐1519. [DOI] [PubMed] [Google Scholar]
- 27. Morton JB, Sanders P, Deen V, Vohra JK, Kalman JM. Sensitivity and specificity of concealed entrainment for the identification of a critical isthmus in the atrium: relationship to rate, anatomic location and antidromic penetration. J Am Coll Cardiol. 2002;39:896‐906. [DOI] [PubMed] [Google Scholar]
- 28. Cabo C, Deruyter B, Coromilas J, Wit AL. Mechanisms for absence of inverse relationship between coupling intervals of premature impulses initiating reentrant ventricular tachycardia and intervals between premature and first tachycardia impulses. Circulation. 1997;96:3136‐3147. [DOI] [PubMed] [Google Scholar]
- 29. Valderrábano M. Atypical atrioventricular nodal reentry with eccentric atrial activation. Is the right target on the left? Heart Rhythm. 2007;4:433‐434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [Y.K.], upon reasonable request.


