Abstract
Background
Eosinophilic esophagitis (EoE) is a food allergen driven disease that is accompanied by interleukin (IL) 13 overexpression and esophageal barrier dysfunction allowing transepithelial food allergen permeation. Nutraceuticals, such as short‐chain fatty acids (SCFAs) that restore barrier function and increase immune fitness may be a promising tool in the management of EoE. Here, we investigated the effects of the SCFAs acetate, propionate, and butyrate on an IL‐13‐compromised human esophageal epithelial barrier, including the mechanisms involved.
Methods
An air‐liquid interface culture model of differentiated human EPC2‐hTERT (EPC2) was used to study whether SCFAs could restore barrier function after IL‐13‐induced impairment. Esophageal epithelial barrier function was monitored by transepithelial electrical resistance (TEER) and FITC‐dextran paracellular flux, and was further examined by qPCR and immunohistochemical analysis. G protein‐coupled receptor (GPR) GPR41, GPR43, GPR109a, or histone deacetylase (HDAC) (ant)agonists were used to assess mechanisms of action of SCFAs.
Results
IL‐13 stimulation decreased TEER and increased FITC flux, which was counteracted by butyrate and propionate, but not acetate treatment. Barrier proteins FLG and DSG1 mRNA expression was upregulated following butyrate and propionate treatment, whereas expression of eosinophil chemoattractant CCL26 and protease CAPN14 was downregulated. Similarly, butyrate and propionate restored FLG and DSG1 protein expression. Similar effects were observed with an HDAC antagonist but not with GPR agonists.
Conclusion
Nutraceuticals butyrate and propionate restore the barrier function of esophageal epithelial cells after an inflammatory insult and may be of therapeutic benefit in the management of EoE.
Keywords: barrier function, dietary intervention, eosinophilic esophagitis, interleukin 13, short‐chain fatty acids
The SCFAs butyrate and propionate counteracted the compromising effects of IL‐13 on barrier function of human esophageal epithelium cultured under ALI conditions. The increase in barrier function induced by these SCFAs was associated with restored expression of proinflammatory mediators and esophageal epithelial barrier proteins. An HDAC antagonist induced similar effects as butyrate and propionate, whereas GPR agonists did not.
Abbreviations: ALI, air‐liquid interface; CAPN14, calpain‐14; CCL26, (C‐C motif) ligand 26; DSG1, desmoglein‐1; FLG, filaggrin; GPR, G protein‐coupled receptor; HDAC, histone deacetylase; IL‐13, interleukin 13; SCFA, short‐chain fatty acid.
1. INTRODUCTION
The epithelial barrier of the esophagus forms the first line of chemical, physical, and immunologic defenses, and provides a protective wall against environmental factors including microbes and food allergens. 1 In eosinophilic esophagitis (EoE), a chronic food allergen‐mediated disease of the esophagus, the esophageal barrier is frequently disrupted, leading to exposure to food allergens in the esophageal mucosa and the subsequent induction of a local type 2 immune response. 2 , 3 Current treatment options for EoE consist of topical steroids and dietary restrictions 4 , 5 but are sometimes unpopular with patients. Thus, there is a demand for novel treatment protocols that restore esophageal barrier function and mitigate esophageal inflammation to reestablish esophageal immune fitness.
Recent studies have demonstrated a link between the type 2 cytokine interleukin (IL) 13 in esophageal epithelial proliferation and esophageal barrier dysfunction. 3 , 6 , 7 In fact, esophageal epithelial cells express each subunit of the IL‐13 receptor including IL‐4Rα, IL‐13Rα1, and IL‐13Rα2. 8 Transcriptomics studies have shown that IL‐13 is overexpressed during active EoE, but its major cellular source or sources remain to be elucidated. 8 Subsequently, IL‐13 disrupts the esophageal barrier, mediated in part by the loss of the epithelial barrier proteins desmoglein‐1 (DSG1) and filaggrin (FLG). 3 , 9 In addition, IL‐13 induces marked overexpression of eosinophil chemoattractant chemokine (C‐C motif) ligand 26 (CCL26, encoding eotaxin‐3) and protease calpain‐14 (CAPN14). 10 , 11 Notably, the EoE transcriptome can be partially reproduced in IL‐13‐treated immortalized esophageal epithelial cells cultured under air‐liquid interface (ALI) conditions, indicating that IL‐13‐induced gene expression in esophageal epithelial cells may make an important contribution to the EoE pathogenesis. 6
Short‐chain fatty acids (SCFAs) –in particular acetate, propionate, and butyrate– are produced by bacterial fermentation of dietary fiber in the gut, where they serve as an energy source for colonocytes, maintain intestinal homeostasis, and promote gut barrier function. 12 , 13 , 14 SCFAs are agonists of G protein‐coupled receptor (GPR) GPR41, GPR43, and GPR109a, inducing anti‐inflammatory pathways upon binding. 15 , 16 , 17 , 18 In addition, butyrate and propionate influence the activity of histone deacetylase (HDAC), a class of histone modification enzymes that regulates gene transcription and has the potential to influence biological processes. 19 , 20 , 21 , 22 Although mainly produced in the gut, SCFAs have also been shown to have immunomodulatory effects in other barrier organs such as the lungs and skin. 23 , 24 , 25 , 26 , 27 , 28 , 29
In this study, we use a model that resembles differentiated (ie, stratified squamous) human esophageal epithelium to investigate the potential barrier‐restorative effects of the SCFAs acetate, propionate, and butyrate on an IL‐13‐compromised barrier. In addition, we aimed to determine the underlying mechanisms of the observed functional effects.
2. MATERIALS AND METHODS
2.1. EPC2‐hTERT culture
The immortalized human esophageal epithelial cell line EPC2‐hTERT (EPC2) was given by Dr Anil Rustgi (University of Pennsylvania, Philadelphia, PA, USA). 30 , 31 , 32 EPC2 were cultured in low‐calcium (0.09 mM) keratinocyte serum‐free medium (KSFM; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with bovine pituitary extract (20 mg/ml), epidermal growth factor (1 ng/ml), penicillin (10,000 U/ml), and streptomycin (10,000 µg/ml).
2.2. Air‐liquid interface (ALI) culture system and SCFA treatment
The 3D ALI culture protocol was adapted from Kc et al. 6 A schematic representation of the experimental timeline is shown in Figure 1A. Briefly, EPC2 were grown to confluence on semipermeable membranes (0.4 µm; Corning Incorporated, Corning, NY, USA) in low‐calcium KSFM for 3 days. Initial differentiation of confluent monolayers was induced by switching to high‐calcium (1.8 mM) KSFM from culture day 3 to 7. Terminal epithelial differentiation and stratification were induced by removing the media from the apical chamber and exposing the cells to the ALI from culture day 7 to 14. Cells were exposed to IL‐13 (100 ng/ml; Prospec, Rehovot, Israel) in the basolateral chamber at the start of ALI culture.
FIGURE 1.
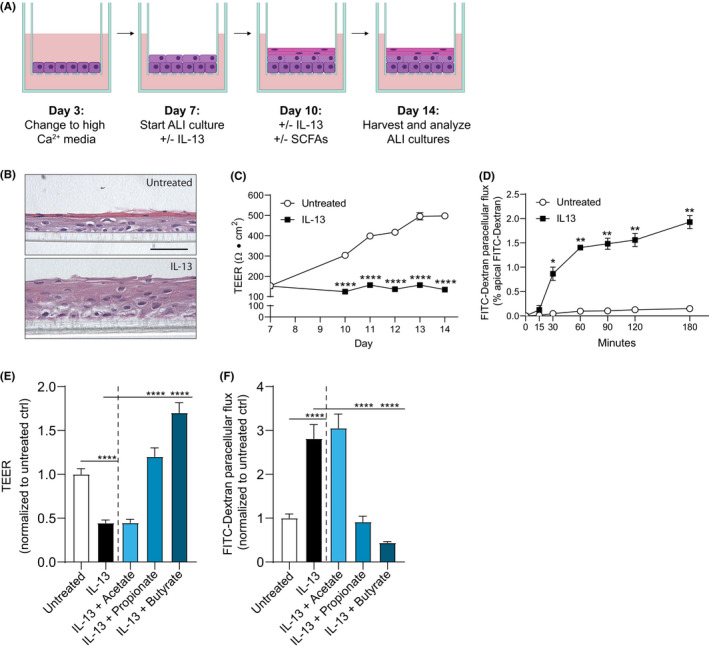
Butyrate and propionate restore IL‐13‐induced barrier dysfunction in EPC2 ALI cultures A, Schematic diagram of the ALI culture model. Culture day 1 to 7 allows initial differentiation, and culture day 7 to 14 (ALI) induces terminal differentiation and stratification of the EPC2. EPC2 are stimulated with IL‐13 (100 ng/ml) from day 7 to 14. EPC2 are treated with SCFAs acetate (10 mM), propionate (10 mM), or butyrate (5 mM) from day 10 to 14. B, Hematoxylin and eosin staining of EPC2 differentiated at the ALI in the absence (untreated) or presence of IL‐13 (100 ng/ml). Scale bar =50 µm. C, TEER development of EPC2 in the absence (untreated) or presence of IL‐13 (100 ng/ml) during differentiation under ALI conditions. D, Kinetic FITC flux analysis of EPC2 differentiated at the ALI in the absence (untreated) or presence of IL‐13 (100 ng/ml). E and F, Day 14 TEER (E) and FITC flux (180 min.) (F) of IL‐13‐stimulated EPC2 ALI cultures treated with acetate (10 mM), propionate (10 mM) or butyrate (5 mM). Images and data in panels B‐D are representative of twelve independent experiments (n=6 wells/group). Data in panels E and F are representative of two to eight independent experiments (n=4 wells/group). Data are presented as mean +SEM. Statistical significance was tested with one‐way ANOVA followed by Dunnett's multiple comparisons test: *p < .05, ** p < .01, ****p < .0001
Sodium acetate was purchased from BDH Laboratory Supplies (Poole, England, cat. no. 102364Q). Sodium propionate and sodium butyrate were purchased from Sigma‐Aldrich (Saint‐Louis, MO, USA, cat. no. P1880 (propionate) and 303410 (butyrate)). All SCFAs were used in preliminary work in a range of concentrations from 5 to 20 mM (acetate and propionate) and 2 to 10 mM (butyrate) (Figure S1). In final experiments, acetate (10 mM), propionate (10 mM), or butyrate (5 mM) was added to the basolateral chamber of IL‐13‐stimulated EPC2 ALI cultures from day 10 to 14. EPC2 ALI cultures were also treated with SCFAs in the absence of IL‐13 (Figure S2). Media plus IL‐13 and SCFAs were refreshed every other day. ALI cultures were then collected for total RNA isolation and immunohistochemistry.
2.3. Assessment of mechanisms of action of SCFAs
The following (ant)agonists were used to investigate the involvement of GPR41, GPR43, GPR109a, and HDAC in the barrier‐restorative effects of SCFAs: GPR41 agonist AR420626 (1 µM), GPR43 agonist 4‐CMTB (10 µM), GPR109A agonist niacin (10 mM), and HDAC antagonist trichostatin A (TSA, 2 µM). All (ant)agonists were dissolved in DMSO or 1 M NaOH according to the manufacturer's instructions and were purchased from Sigma‐Aldrich (cat. no. SML1339 (AR420626), SML0302 (4‐CMTB), N4126 (niacin), and T8552 (TSA)). All (ant)agonists were used in preliminary ALI experiments in a range of concentrations from 1 to 100 µM (AR420626), 0.1 to 50 µM (4‐CMTB), 1 to 20 mM (niacin), and 0.1 to 10 µM (TSA) (Figures S4 and S5). In final experiments, (ant)agonists were added to the basolateral chamber of the IL‐13‐stimulated EPC2 ALI cultures from day 10 to 14. Media plus IL‐13 and (ant)agonist were refreshed every other day. ALI cultures were then collected for total RNA isolation and immunohistochemistry.
2.4. Transepithelial electrical resistance (TEER), paracellular flux assays and LDH toxicity test
TEER was measured during ALI culture using a Millicell ERS‐2 Volt‐ohm meter (Merck Millipore, Burlington, MA, USA). High‐calcium KSFM was added to the apical chamber one hour before TEER measurement. Paracellular flux assays were performed one hour after TEER measurement on day 14. 4‐kDa fluorescein isothiocyanate (FITC)‐dextran (0.1 mg/µL; Sigma‐Aldrich) was added to the apical chamber, and fluorescein levels in the basolateral chamber were detected after 15, 30, 60, 90, 120, and 180 minutes using a GloMax Discover Microplate Reader (Promega, Madison, WI, USA) at Ex/Em =492/518. Cytotoxicity was measured in 50 µL supernatant collected at day 14 using the Cytotoxicity Detection Kit (LDH) (Roche, Basel, Switzerland) (Figure S7) per manufacturer's instructions.
2.5. Quantitative real‐time PCR
Total RNA was treated with DNase I (Qiagen, Hilden, Germany) and isolated from EPC2 ALI cultures using the RNeasy Mini Kit (QIAGEN) according to the manufacturer's instructions. cDNA was generated from 500 ng RNA using the iScript™ cDNA Synthesis Kit (BioRad, Hercules, CA, USA). qPCR was performed using SYBR Green (BioRad). All primers used for amplification were purchased from BioRad (Unique Assay ID: qHsaCID0017001 (CAPN14), qHsaCED0041923 (CCL26), qHsaCED0044569 (DSG1), and qHsaCED0036604 (FLG)). Results were normalized to ribosomal protein S13 (RPS13; Unique Assay ID:qHsaCID0038672) expression for each sample. mRNA expression levels were calculated using the following formula: fold change =2−ΔΔCt and were normalized to the untreated control.
2.6. Histology and Immunofluorescence
Formalin‐fixed, paraffin‐embedded EPC2 ALI cultures were cut into 5 µm sections and deparaffinized using xylene followed by graded ethanol washes. For histology, sections were stained in hematoxylin, rinsed in tap water and then stained in eosin, followed by dehydration in graded ethanol washes and xylene before mounting with Pertex (Histolab, Askim, Sweden) and xylene (1:1). For immunofluorescence, heat‐induced antigen retrieval in sodium citrate buffer (10 mM citric acid, pH 6.0) was used on deparaffinized sections, and endogenous peroxidase activity was quenched using 3% H2O2 in methanol. After rinsing in 0.2% Tween in PBS, sections were blocked in 3% BSA in PBS containing 5% normal goat serum (Dako, Jena, Germany) for 90 minutes, followed by overnight incubation at 4℃ with rabbit anti‐DSG1 (1 µg/ml; Abcam, Cambridge, UK, cat. no. ab209490) or rabbit anti‐FLG (1 µg/ml; Abcam, cat. no. ab234406). Sections were rinsed and incubated for 1 hour with goat anti‐rabbit AF594 (10 µg/ml; Invitrogen, Carlsbad, CA, USA, cat. no. A11072). Sections were mounted with ProLong™ Gold antifade reagent with DAPI (Invitrogen) for nuclei staining. Immunofluorescent images were acquired using the Keyence Fluorescence Microscope BZ‐9000, and immunofluorescence intensity was quantified using ImageJ software.
2.7. Nuclear extract preparation and HDAC activity
EPC2 were grown in 12 well culture plates (Costar) in low‐calcium KSFM until confluent, followed by stimulation with acetate (10 mM), propionate (10 mM), butyrate (5 mM), or TSA (2 µM) in high‐calcium KSFM. Cytoplasmic and nuclear extracts were isolated 48 hours after stimulation. Briefly, EPC2 were trypsinized, collected by centrifugation (1000 rpm, 4 min, 4℃) and washed twice in ice‐chilled PBS. EPC2 were resuspended in 100 µL ice‐chilled buffer 1 (Table S1) and incubated on a rotator for 10 minutes at 4℃. After vortexing, lysates were spun down (12,000 rpm; 1 min; 4℃) and the cytoplasmic protein fractions were collected and stored at −80℃. Nuclear pellets were washed twice with ice‐chilled PBS, disrupted with 40 µL Buffer 2 (Table S1) and incubated on ice for 30 minutes with regular vortexing followed by sonication for 3 x 10 seconds. The suspension was spun down (12,000 rpm; 15 min; 4℃) and the nuclear fractions were collected and stored at −80℃. Total protein content was quantified using the Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific). HDAC activity was measured in 2 ng nuclear extract using the colorimetric epigenase HDAC Activity/Inhibition Direct Assay Kit (EpiGentek, Farmingdale, NY, USA) according to the manufacturer's instructions.
3. RESULTS
3.1. Short‐chain fatty acids butyrate and propionate restored esophageal barrier resistance and permeability in IL‐13‐stimulated EPC2 ALI cultures
We used an ALI culture model that resembles human‐differentiated esophageal epithelium to investigate the potential barrier‐restorative effects of SCFAs as depicted schematically in Figure 1A. Following 7 days of differentiation at the ALI, EPC2 formed a stratified squamous epithelial layer indicating the development of differentiated esophageal epithelium (Figure 1B). Prolonged IL‐13 exposure resulted in marked morphologic changes including decreased epithelial differentiation and expansion of the epithelial layer (Figure 1B). Furthermore, IL‐13 induced a significant decrease in TEER from day 10 and onwards (Figure 1C), and a significant increase in FITC‐dextran paracellular flux (FITC flux) on day 14 (Figure 1D). Together, these results indicate that IL‐13 induces barrier dysfunction in EPC2 ALI cultures as reported previously. 3 , 6
To study the barrier‐restorative effects of SCFAs on IL‐13‐stimulated EPC2 ALI cultures, acetate, propionate, or butyrate were added to the basolateral chamber from day 10 to 14. At day 14, IL‐13 stimulation showed a 2.2‐fold decrease in TEER compared with untreated cultures. Propionate and butyrate counteracted the effect of IL‐13 on TEER as shown by a 2.7‐fold increase in propionate‐treated ALI cultures and 3.8‐fold increase in butyrate‐treated cultures compared with IL‐13‐stimulated EPC2 ALI cultures (Figure 1E). FITC flux assays confirm these findings. IL‐13‐stimulated EPC2 ALI cultures had a significantly increased FITC flux at day 14, which was counteracted by propionate and butyrate treatment (Figure 1F). In addition, SCFAs –in particular butyrate– restored IL‐13‐induced barrier dysfunction measured by TEER and FITC flux in a culture model of apical SCFA treatment, supporting our data on basolateral SCFA treatment (Figure S3). Together, these data show that butyrate and propionate, but not acetate, restore esophageal barrier resistance and permeability after IL‐13‐induced impairment.
3.2. Butyrate and propionate restored mRNA expression of key EoE genes
qPCR analysis was used to assess whether SCFAs changed mRNA expression of proinflammatory factor CCL26, protease CAPN14, and barrier proteins DSG1 and FLG. IL‐13 treatment significantly increased CCL26 and CAPN14 mRNA expression and significantly decreased DSG1 and FLG mRNA expression by day 14. This was counteracted by propionate and butyrate as they decreased the expression of CCL26 and CAPN14, while increasing the expression of FLG and DSG1 compared with IL‐13‐stimulated EPC2 ALI cultures (Figure 2A, B). These results correspond with the observed improved barrier function after butyrate and propionate treatment and further indicate that treatment with these SCFA have an anti‐inflammatory action.
FIGURE 2.
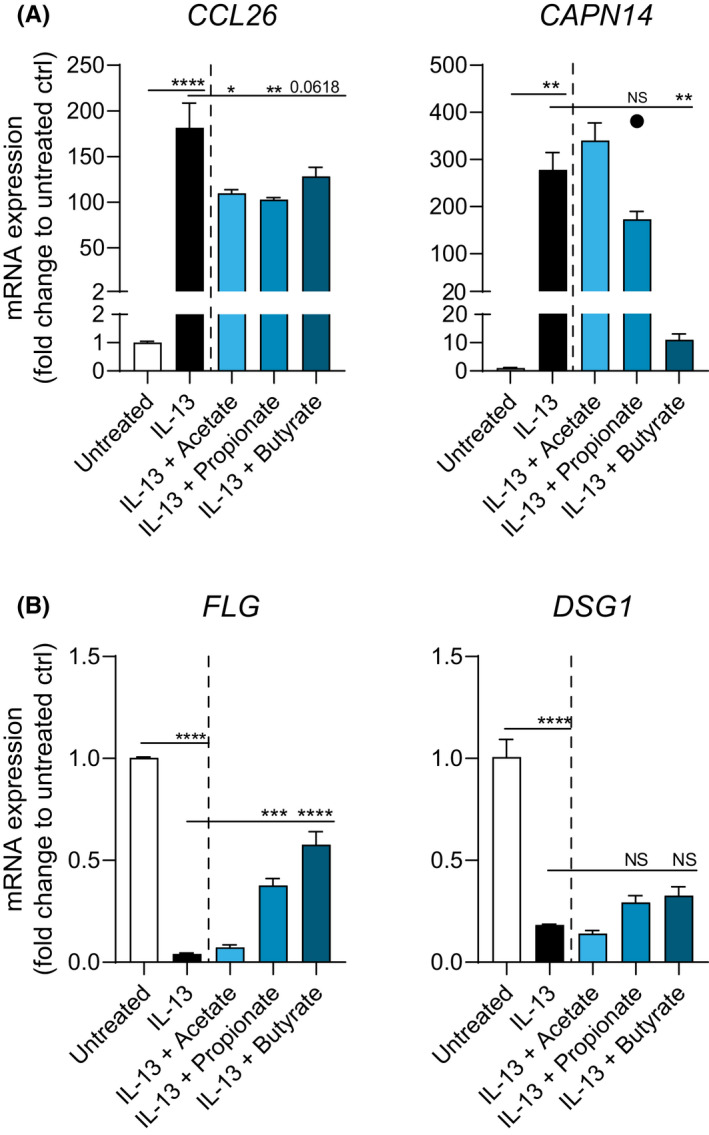
Butyrate and propionate restore mRNA expression of EPC2 ALI cultures. A, mRNA expression of proinflammatory factor CCL26 and protease CAPN14 in IL‐13‐stimulated EPC2 ALI cultures treated with acetate (10 mM), propionate (10 mM), or butyrate (5 mM). B, mRNA expression of esophageal barrier proteins FLG and DSG1 in IL‐13‐stimulated EPC2 ALI cultures treated with acetate (10 mM), propionate (10 mM), or butyrate (5 mM). Data are representative of two to six independent experiments (n=3 wells/group). Outlier is shown as a separate data point. Data are presented as mean +SEM. Statistical significance was tested with one‐way ANOVA followed by Dunnett's multiple comparisons test: *p<.05; **p<.01; ***p<.001; ****p<.0001; NS, not significant
3.3. Butyrate and propionate restored DSG1 and FLG protein expression
To test the effects of SCFA treatment on esophageal barrier protein expression, we examined day 14 DSG1 and FLG expression by immunofluorescent staining. DSG1 and FLG expression was decreased in IL‐13‐stimulated EPC2 ALI cultures compared with untreated ALI cultures. Consistent with the mRNA expression data, butyrate, and to a lesser extent propionate, restored the expression of DSG1 and FLG in IL‐13‐stimulated EPC2 ALI cultures (Figure 3A). Quantification of fluorescence intensity confirms the upregulation of DSG1 and FLG in IL‐13‐stimulated EPC2 ALI cultures after butyrate and propionate treatment (Figure 3B, C).
FIGURE 3.
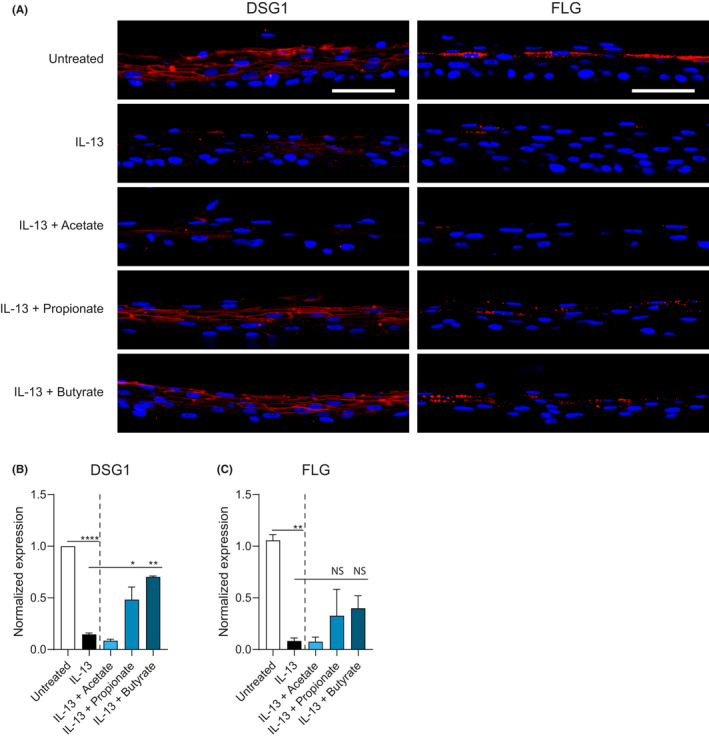
Butyrate and propionate upregulate DSG1 and FLG protein expression in ALI cultures of EPC2 treated with IL‐13. A, Immunofluorescent staining for barrier proteins DSG1 (left) and FLG (right) in red with a blue DAPI nuclear counterstain in IL‐13‐stimulated EPC2 ALI cultures treated with acetate (10 mM), propionate (10 mM), or butyrate (5 mM). Scale bar =50 µm. B, Quantification of DSG1 and FLG expression in IL‐13‐stimulated EPC2 ALI cultures treated with SCFA. Images in panel A are representative of three independent experiments performed in duplicate or triplicate and are taken at 40x magnification. Data in panel B are pooled from three independent experiments performed in duplicate or triplicate and are presented as mean +SEM. Statistical significance was tested with one‐way ANOVA followed by Dunnett's multiple comparisons test: *p<.05, **p<.01; NS, not significant
3.4. Barrier‐restorative effects of butyrate and propionate are independent of the free fatty acid receptors GPR41, GPR43, and GPR109a
Next, we investigated whether the barrier‐restorative effects of butyrate and propionate depend on signaling through the free fatty acid receptors GPR41, GPR43, and GPR109a. All three GPRs were found expressed in EPC2 on mRNA and protein level (data not shown). Direct stimulation of GPRs with specific agonists did not affect neither TEER (Figure 4A) nor FITC flux (Figure 4B). In line with these observations, expression of genes associated with EoE and altered by IL‐13 stimulation of EPC2 grown under ALI conditions was unaffected by GPR stimulation (Figure 4C).
FIGURE 4.
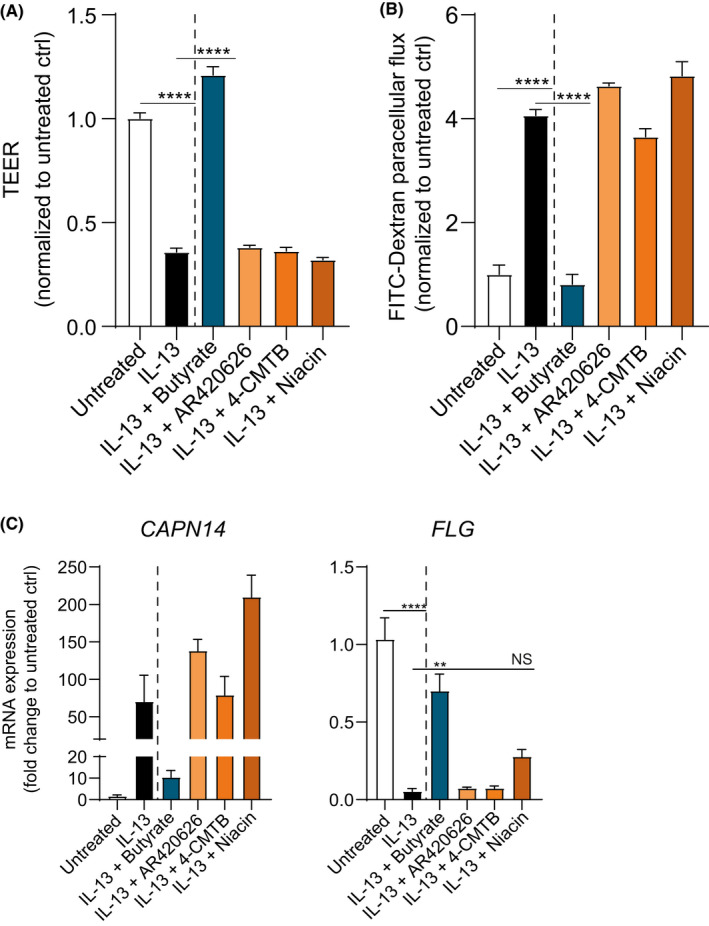
The effects of SCFAs are independent of GPR41, GPR43, and GPR109a stimulation. Day 14 TEER (A) and FITC flux (180 min.) (B) of IL‐13‐stimulated EPC2 ALI cultures treated with GPR agonists AR420626 (1 µM, GPR41), 4‐CMTB (10 µM, GPR43), or niacin (10 mM, GPR109a). C, CAPN14 and FLG mRNA expression in IL‐13‐stimulated EPC2 ALI cultures treated with GPR agonists. Data are representative of two independent experiments (n=3 wells/group) and are presented as mean +SEM. Statistical significance was tested with one‐way ANOVA followed by Dunnett's multiple comparisons test: **p<.01; ***p<.001; ****p<.0001; NS, not significant
To confirm the ability of these agonist to stimulate GPRs and reduce inflammation, human umbilical vein endothelial cells (HUVECs) were stimulated with lipopolysaccharide (LPS) following treatment with GPR agonists. Stimulation of GPRs with specific agonists decreased LPS‐induced IL‐6 and IL‐8 release in a concentration‐dependent manner (Figure S6), confirming the ability of the GPR agonists used in this study to stimulate GPR signaling. Together, these data indicate that the barrier‐restorative effects of butyrate and propionate measured by TEER, FITC flux, and mRNA and protein expression are most likely not mediated via stimulation of GPR41, GPR43, or GPR109a in EPC2.
3.5. HDACs may be involved in barrier‐restorative effects of butyrate and propionate
It has been demonstrated that SCFAs are also effective inhibitors of HDAC activity. 19 , 21 , 22 Since the effects of butyrate and propionate are independent of GPR signaling, we investigated whether the barrier‐restorative effects of SCFAs may be related to inhibition of HDAC. To study the functional effects of HDAC inhibition, TSA, a potent and specific inhibitor of HDAC activity was added to EPC2 ALI cultures. Despite the minimal effect on TEER (Figure 5A), TSA significantly decreased FITC flux in IL‐13‐stimulated EPC2 ALI cultures, although with a smaller impact than butyrate (Figure 5B). CAPN14 and FLG mRNA expression was not affected by TSA (Figure 5C). Furthermore, butyrate and propionate treatment led to attenuated HDAC activity in EPC2 (Figure 6). These data suggest that inhibition of HDAC activity can partly mimic the restorative effects on epithelial barrier function as observed by butyrate and propionate.
FIGURE 5.
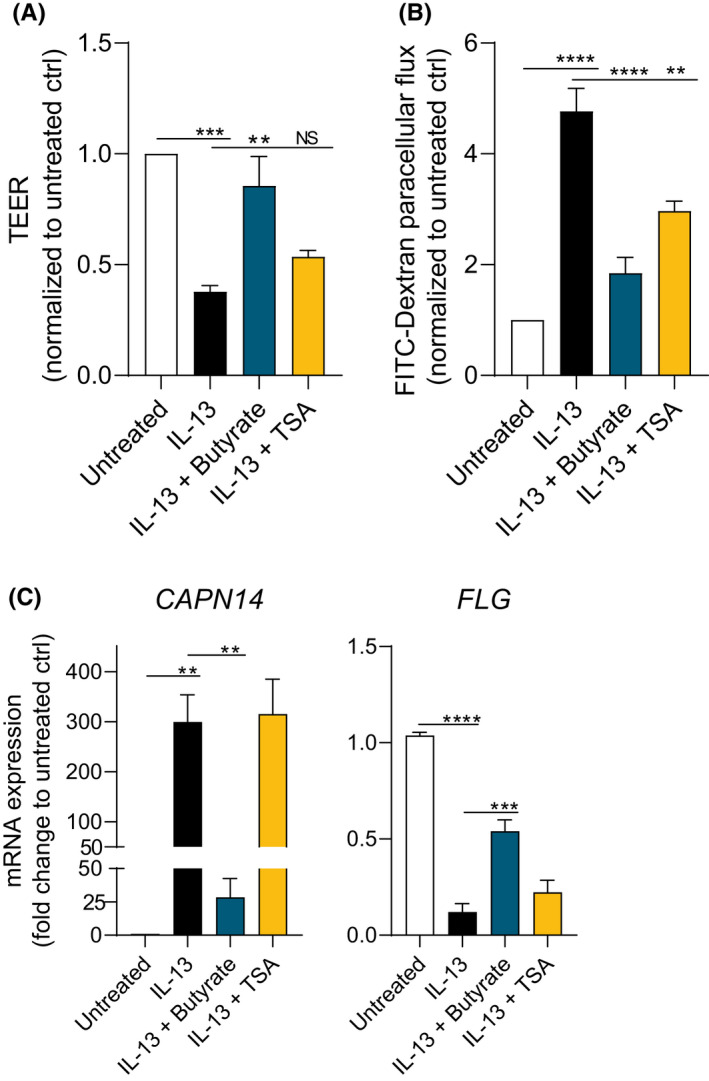
The HDAC inhibitor TSA partially mimics effects in EPC2 ALI cultures. Day 14 TEER (A) and FITC flux (180 min.) (B) of IL‐13‐stimulated EPC2 ALI cultures treated with TSA (2 µM). C, CAPN14 and FLG mRNA expression in IL‐13‐stimulated EPC2 ALI cultures treated with TSA (2 µM). Data are pooled from four independent experiments performed in duplicate, triplicate, or quadruplicate and are presented as mean +SEM. Statistical significance was tested with one‐way ANOVA followed by Dunnett's multiple comparisons test: **p<.01; ***p<.001; ****p<.0001; NS, not significant
FIGURE 6.
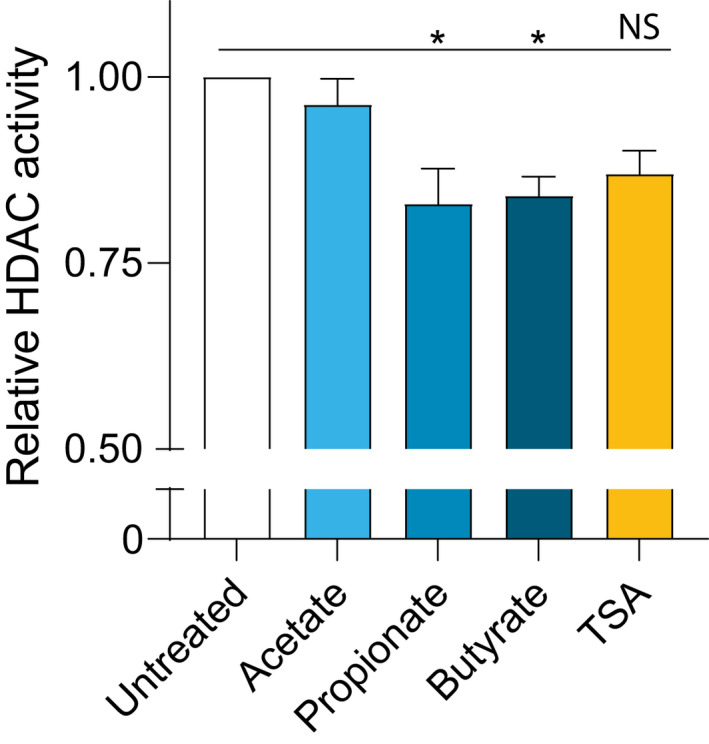
Butyrate and propionate decrease HDAC activity in EPC2. HDAC activity was measured in 2 ng nuclear proteins after treating confluent EPC2 for 48 hours with acetate (10 mM), propionate (10 mM), butyrate (5 mM), or TSA (2 µM) and was normalized to the untreated control. Data are pooled from three independent experiments performed in triplicate, and are presented as mean +SEM. Statistical significance was tested with one‐way ANOVA followed by Dunnett's multiple comparisons test: *p<.05; ****p<.0001
4. DISCUSSION
The data presented in this study demonstrate that the SCFAs butyrate and propionate, but not acetate, restore esophageal epithelial barrier function after IL‐13‐induced impairment using an ALI culture model resembling differentiated human esophageal epithelium. First, we demonstrate that butyrate and propionate restore epithelial barrier resistance and permeability, as assessed by TEER and FITC flux. Second, we show that butyrate and propionate restore mRNA expression of genes associated with inflammation in EoE, such as CCL26, and barrier function, such as CAPN14, DSG1, and FLG. Third, we show that butyrate and propionate increase DSG1 and FLG protein expression. Fourth, our studies suggest that the barrier‐restorative effects of butyrate and propionate are independent of GPR signaling, but may, in part, be dependent on inhibition of nuclear HDAC activity.
Although acetate is the most abundant SCFA in the gut and periphery, butyrate is the most potent immunomodulatory SCFA. 33 Indeed, we observed that butyrate has the highest potency to enhance esophageal barrier function after IL‐13‐induced impairment. Also propionate, but not acetate, significantly augmented barrier function despite with a lower activity than butyrate. Our data add to the growing body of literature linking SCFAs to immunomodulation and epithelial barrier function. Nonetheless, Wen et al. have reported a potential proinflammatory effect of SCFAs in Th2 cell‐associated responses, 34 indicating that immunomodulatory effects of SCFAs are cell type‐dependent. Our findings are consistent with effects of SCFAs on cytokine‐compromised monolayers of Caco‐2 and T84 human colorectal carcinoma cells and 16HBE human bronchial epithelial cells, where butyrate enhanced barrier function and tight junction protein expression at millimolar level. 35 , 36 , 37 , 38 We used relatively high SCFA concentrations compared with these studies, which could be attributed to characteristics of the stratified esophageal epithelial layer that may contribute to SCFA sensitivity.
Here, we focused on the response of CAPN14 protease and esophageal barrier proteins DSG1 and FLG expression to SCFA treatment because of their suggested role in esophageal barrier function. 3 , 9 , 11 , 39 Expression of the epithelium‐derived proinflammatory factor CCL26 was studied because of its strong correlation with disease severity. 10 The increase in TEER and decrease in FITC flux induced by butyrate and propionate were associated with a decrease in mRNA expression of CCL26 and CAPN14, and an increase in mRNA and protein expression of DSG1 and FLG. CAPN14 activity is specific for esophageal tissue and its overexpression results in loss of epithelial barrier function. 11 , 40 , 41 , 42 Furthermore, previous studies have shown that DSG1 and FLG are downregulated in inflamed esophageal mucosa of EoE patients, 3 , 6 , 9 but are restored after successful therapeutical treatment and are associated with improved mucosal integrity. 43 , 44 Whereas, DSG1 is specifically linked to EoE pathology, 3 IL‐13‐mediated downregulation of FLG has also been described in atopic dermatitis. 45 , 46 The role of other epithelial barrier proteins including claudins, occludin, involucrin, E‐cadherin, and keratins in maintaining esophageal epithelial integrity is less evident. 6 , 47 Interestingly, rather than changes in tight junction proteins, DSG1 and FLG dysregulation contributes to esophageal barrier dysfunction. 44 Current findings indicate that SCFAs can restore dysregulated expression of DSG1 and FLG leading to restoration of esophageal barrier function. In addition to IL‐13, transforming growth factor (TGF) β1 and IL‐9 have also been found to diminish esophageal barrier function of esophageal epithelial cells grown under ALI conditions. 39 , 48 Further studies characterizing the effects of SCFAs on TGF‐β1 and IL‐9‐induced barrier dysfunction will clarify the full impact of SCFA treatment on the compromised esophageal barrier.
We considered signaling via free fatty acid receptors GPR41, GPR43, and GPR109a as a potential mechanism for the barrier‐restorative effects of butyrate and propionate. AR420626, 4‐CMTB, and niacin, agonists for GPR41, GPR43, and GPR109a, were used to investigate whether the activation of these receptors could mimic the effects of SCFAs on EPC2. GPR agonists did not increase epithelial integrity as measured by TEER and FITC flux in IL‐13‐stimulated EPC2 ALI cultures contrasting the effects of butyrate and propionate. Also, CAPN14 and FLG mRNA expression was unaffected by GPR agonists, indicating that the barrier‐restorative effects of SCFAs are independent of GPR stimulation. Furthermore, as a positive control for GPR stimulation, we studied the effect of GPR agonists on LPS‐induced IL‐6 and IL‐8 production by HUVECs, since it has been shown that this is partially mediated via GPRs. 21 We observed a dose‐dependent decrease in LPS‐induced IL‐6 and IL‐8 production, indicating that the lack of a response in EPC2 ALI cultures is not caused by biologically inactive GPR agonists but by the inability of these GPR agonists to induce SCFA‐like effects. Our findings are in line with other studies demonstrating that SCFAs can exert their effects independent of free fatty acid receptors GPR41, GPR43, and GPR109a. 49 , 50 , 51
Alternatively, SCFAs can directly act as nuclear HDAC inhibitors. 19 , 21 , 22 Indeed, both butyrate and propionate attenuated HDAC activity in EPC2. To further investigate whether HDAC inhibition could potentially contribute to the barrier‐restorative effects of butyrate and propionate the pan‐HDAC inhibitor TSA was used. 21 , 49 , 50 TSA is structurally unrelated to butyrate and propionate but is 1000 times more potent in inhibiting HDAC than these SCFAs. 52 HDAC inhibition results in histone hyperacetylation, leading to changes in chromatin structure that facilitate access for transcription factors to the promotor region of certain genes which then induces gene transcription. However, despite the overall correlation between histone acetylation and transcriptional activity, active gene transcription rather relates to the transcriptional competence of the gene than the high levels of histone acetylation. 53 , 54 , 55 This could explain why the effects of TSA on barrier function in EPC2 ALI cultures measured by TEER and FITC flux were modest compared with those of butyrate and propionate. Thus, the ability of these SCFAs to directly inhibit HDAC activity may only be in part involved in their barrier‐restorative effects.
Nevertheless, our studies have some limitations. Exposure to air in the ALI culture is essential for terminal epithelial differentiation and stratification. EPC2 ALI cultures were therefore treated with SCFAs in the basolateral compartment, but similar high concentrations of SCFAs may be difficult to reach systemically. 56 However, our data on apical SCFA treatment suggest that SCFA exposure from the apical side of the epithelium also supports the restoration of the esophageal epithelial barrier. Interestingly, it has been shown that increased dietary fiber intake influences the esophageal microbiome, which might lead to increased local SCFA concentrations in the esophagus. 57 Furthermore, we used the immortalized human esophageal epithelial cell line EPC2‐hTERT to study the effects of SCFA treatment on an IL‐13‐compromised barrier. It may support our study to confirm our findings in primary human esophageal epithelial cells derived from EoE patients despite the marked transcriptional and morphologic overlap between IL‐13‐stimulated EPC2 ALI cultures and inflamed esophageal tissue. 3 , 6
The esophageal epithelial barrier during active EoE is impaired and selectively permeable to food allergens that can remain in the esophageal epithelium for up to 4 days. 58 The presence and subsequent recognition of food allergens in the esophageal mucosa generates a local type 2 immune response, 59 , 60 , 61 forming a pathogenic cycle to further exacerbate allergic inflammation. Butyrate and propionate may break this cycle by restoring barrier function and thus preventing the penetration of food allergens into the esophageal mucosa and subsequent inflammation.
The interest in dietary therapies for EoE has recently emerged as a result of the limitations associated with other therapies, and its effectiveness in achieving and maintaining clinical remission while avoiding the need for drugs. 62 A recent meta‐analysis has shown that empiric elimination diets have moderate response rates (71%), but require a large number of endoscopies, whereas the efficacy of skin allergy testing‐directed food elimination is questionable (45%). 63 Interestingly, complete dietary allergen avoidance using an elemental diet is highly effective in both children and adults (90.8%), 63 and restores esophageal mucosal integrity. 44 , 64 It would be interesting to investigate whether a dietary intervention with SCFA formulations could restore esophageal immune fitness and improve symptoms.
In conclusion, our findings demonstrate that butyrate and propionate restore esophageal barrier function after IL‐13‐induced impairment, and that this is at least in part mediated by their ability to directly inhibit HDAC activity. Deeper knowledge of the mechanisms underlying the beneficial effects of butyrate and propionate could lead to novel approaches to restore esophageal barrier function. Our data highlight a potential role for butyrate and propionate in the management of EoE.
CONFLICTS OF INTEREST
MTJVA, JG and BCAMVE are partly employed by Danone Nutricia Research and salary does not depend on the outcomes of this study. All other authors report no conflict of interest.
AUTHOR CONTRIBUTIONS
Study design: MTAK, FAR, and BCAMVE. Data collection: MTAK with assistance of BMH. Data analysis and interpretation: MTAK, FAR, and BCAMVE. Drafting of the manuscript: MTAK. Critical review of the manuscript: MLH, MTJVA, AJB, JG, FAR, and BCAMVE. All authors have read and agreed to the published version of the manuscript.
Supporting information
Figure S1‐S7
Table S1
Method S1
ACKNOWLEDGMENTS
This research is funded within the Partnership between NWO domain Applied and Engineered Sciences and Danone Nutricia Research and with additional financial support from Topsector Agri and Food, project number 16495 with the acronym LOIRE. We thank Dr. Anil Rustgi (University of Pennsylvania) for the EPC2‐hTERT cell line.
Kleuskens MTA, Haasnoot ML, Herpers BM, et al. Butyrate and propionate restore interleukin 13‐compromised esophageal epithelial barrier function. Allergy.2022;77:1510–1521. 10.1111/all.15069
REFERENCES
- 1. Celebi Sozener Z, Cevhertas L, Nadeau K, Akdis M, Akdis CA. Environmental factors in epithelial barrier dysfunction. J Allergy Clin Immunol 2020;145(6):1517‐1528. [DOI] [PubMed] [Google Scholar]
- 2. Furuta GT, Katzka DA. Eosinophilic Esophagitis. N Engl J Med 2015;373(17):1640‐1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sherrill JD, Kc K, Wu D, et al. Desmoglein‐1 regulates esophageal epithelial barrier function and immune responses in eosinophilic esophagitis. Mucosal Immunol 2014;7(3):718‐729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dellon ES, Gonsalves N, Hirano I, et al. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol 2013;108(5):679‐692. quiz 93. [DOI] [PubMed] [Google Scholar]
- 5. Rothenberg ME. Biology and treatment of eosinophilic esophagitis. Gastroenterology 2009;137(4):1238‐1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kc K, Rothenberg ME, Sherrill JD. In vitro model for studying esophageal epithelial differentiation and allergic inflammatory responses identifies keratin involvement in eosinophilic esophagitis. PLoS One 2015;10(6):e0127755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blanchard C, Stucke EM, Burwinkel K, et al. Coordinate interaction between IL‐13 and epithelial differentiation cluster genes in eosinophilic esophagitis. J Immunol 2010;184(7):4033‐4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blanchard C, Mingler MK, Vicario M, et al. IL‐13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J Allergy Clin Immunol 2007;120(6):1292‐1300. [DOI] [PubMed] [Google Scholar]
- 9. Wu L, Oshima T, Li M, et al. Filaggrin and tight junction proteins are crucial for IL‐13‐mediated esophageal barrier dysfunction. Am J Physiol Gastrointest Liver Physiol 2018;315(3):G341‐G350. [DOI] [PubMed] [Google Scholar]
- 10. Blanchard C, Wang N, Stringer KF, et al. Eotaxin‐3 and a uniquely conserved gene‐expression profile in eosinophilic esophagitis. J Clin Invest 2006;116(2):536‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis BP, Stucke EM, Khorki ME, et al. Eosinophilic esophagitis‐linked calpain 14 is an IL‐13‐induced protease that mediates esophageal epithelial barrier impairment. JCI Insight 2016;1(4):e86355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koh A, De Vadder F, Kovatcheva‐Datchary P, Backhed F. From Dietary Fiber to Host Physiology: Short‐Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016;165(6):1332‐1345. [DOI] [PubMed] [Google Scholar]
- 13. Donohoe DR, Garge N, Zhang X, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab 2011;13(5):517‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Correa‐Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MA. Regulation of immune cell function by short‐chain fatty acids. Clin Transl Immunology 2016;5(4):e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown AJ, Goldsworthy SM, Barnes AA, et al. The Orphan G protein‐coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 2003;278(13):11312‐11319. [DOI] [PubMed] [Google Scholar]
- 16. Le Poul E, Loison C, Struyf S, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem 2003;278(28):25481‐25489. [DOI] [PubMed] [Google Scholar]
- 17. Singh N, Gurav A, Sivaprakasam S, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014;40(1):128‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thangaraju M, Cresci GA, Liu K, et al. GPR109A is a G‐protein‐coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res 2009;69(7):2826‐2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr 2003;133(7 Suppl):2485S‐2493S. [DOI] [PubMed] [Google Scholar]
- 20. Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet 2009;10(1):32‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li M, van Esch B, Henricks PAJ, Folkerts G, Garssen J. The Anti‐inflammatory Effects of Short Chain Fatty Acids on Lipopolysaccharide‐ or Tumor Necrosis Factor alpha‐Stimulated Endothelial Cells via Activation of GPR41/43 and Inhibition of HDACs. Front Pharmacol 2018;9:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sanchez HN, Moroney JB, Gan H, et al. B cell‐intrinsic epigenetic modulation of antibody responses by dietary fiber‐derived short‐chain fatty acids. Nat Commun 2020;11(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smolinska S, Groeger D, O'Mahony L. Biology of the Microbiome 1: Interactions with the Host Immune Response. Gastroenterol Clin North Am 2017;46(1):19‐35. [DOI] [PubMed] [Google Scholar]
- 24. Cait A, Hughes MR, Antignano F, et al. Microbiome‐driven allergic lung inflammation is ameliorated by short‐chain fatty acids. Mucosal Immunol 2018;11(3):785‐795. [DOI] [PubMed] [Google Scholar]
- 25. Thio CL, Chi PY, Lai AC, Chang YJ. Regulation of type 2 innate lymphoid cell‐dependent airway hyperreactivity by butyrate. J Allergy Clin Immunol 2018;142(6):1867‐1883 e12. [DOI] [PubMed] [Google Scholar]
- 26. Thorburn AN, McKenzie CI, Shen S, et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun 2015;6:7320. [DOI] [PubMed] [Google Scholar]
- 27. Krejner A, Bruhs A, Mrowietz U, Wehkamp U, Schwarz T, Schwarz A. Decreased expression of G‐protein‐coupled receptors GPR43 and GPR109a in psoriatic skin can be restored by topical application of sodium butyrate. Arch Dermatol Res 2018;310(9):751‐758. [DOI] [PubMed] [Google Scholar]
- 28. Sanford JA, Zhang LJ, Williams MR, Gangoiti JA, Huang CM, Gallo RL. Inhibition of HDAC8 and HDAC9 by microbial short‐chain fatty acids breaks immune tolerance of the epidermis to TLR ligands. Sci Immunol 2016;1(4):eaah4609. 10.1126/sciimmunol.aah4609 [DOI] [PubMed] [Google Scholar]
- 29. Schwarz A, Bruhs A, Schwarz T. The Short‐Chain Fatty Acid Sodium Butyrate Functions as a Regulator of the Skin Immune System. J Invest Dermatol 2017;137(4):855‐864. [DOI] [PubMed] [Google Scholar]
- 30. Kalabis J, Wong GS, Vega ME, et al. Isolation and characterization of mouse and human esophageal epithelial cells in 3D organotypic culture. Nat Protoc 2012;7(2):235‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Okawa T, Michaylira CZ, Kalabis J, et al. The functional interplay between EGFR overexpression, hTERT activation, and p53 mutation in esophageal epithelial cells with activation of stromal fibroblasts induces tumor development, invasion, and differentiation. Genes Dev 2007;21(21):2788‐2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oyama K, Okawa T, Nakagawa H, et al. AKT induces senescence in primary esophageal epithelial cells but is permissive for differentiation as revealed in organotypic culture. Oncogene 2007;26(16):2353‐2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meijer K, de Vos P, Priebe MG. Butyrate and other short‐chain fatty acids as modulators of immunity: what relevance for health? Curr Opin Clin Nutr Metab Care 2010;13(6):715‐721. [DOI] [PubMed] [Google Scholar]
- 34. Wen T, Aronow BJ, Rochman Y, et al. Single‐cell RNA sequencing identifies inflammatory tissue T cells in eosinophilic esophagitis. J Clin Invest 2019;129(5):2014‐2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Feng Y, Wang Y, Wang P, Huang Y, Wang F. Short‐Chain Fatty Acids Manifest Stimulative and Protective Effects on Intestinal Barrier Function Through the Inhibition of NLRP3 Inflammasome and Autophagy. Cell Physiol Biochem 2018;49(1):190‐205. [DOI] [PubMed] [Google Scholar]
- 36. Valenzano MC, DiGuilio K, Mercado J, et al. Remodeling of Tight Junctions and Enhancement of Barrier Integrity of the CACO‐2 Intestinal Epithelial Cell Layer by Micronutrients. PLoS One 2015;10(7):e0133926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Suzuki T, Yoshida S, Hara H. Physiological concentrations of short‐chain fatty acids immediately suppress colonic epithelial permeability. Br J Nutr 2008;100(2):297‐305. [DOI] [PubMed] [Google Scholar]
- 38. Richards LB, Li M, Folkerts G, Henricks PAJ, Garssen J, van Esch B. Butyrate and Propionate Restore the Cytokine and House Dust Mite Compromised Barrier Function of Human Bronchial Airway Epithelial Cells. Int J Mol Sci 2020;22(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nguyen N, Fernando SD, Biette KA, et al. TGF‐beta1 alters esophageal epithelial barrier function by attenuation of claudin‐7 in eosinophilic esophagitis. Mucosal Immunol 2018;11(2):415‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Litosh VA, Rochman M, Rymer JK, Porollo A, Kottyan LC, Rothenberg ME. Calpain‐14 and its association with eosinophilic esophagitis. J Allergy Clin Immunol 2017;139(6):1762‐1771 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Martin LJ, He H, Collins MH, et al. Eosinophilic esophagitis (EoE) genetic susceptibility is mediated by synergistic interactions between EoE‐specific and general atopic disease loci. J Allergy Clin Immunol 2018;141(5):1690‐1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miller DE, Forney C, Rochman M, et al. Genetic, Inflammatory, and Epithelial Cell Differentiation Factors Control Expression of Human Calpain‐14. G3: Genes ‐ Genomes ‐ Genetics 2019;9(3):729‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van Rhijn BD, Verheij J, van den Bergh Weerman MA, et al. Histological Response to Fluticasone Propionate in Patients With Eosinophilic Esophagitis Is Associated With Improved Functional Esophageal Mucosal Integrity. Am J Gastroenterol 2015;110(9):1289‐1297. [DOI] [PubMed] [Google Scholar]
- 44. Warners MJ, Vlieg‐Boerstra BJ, Verheij J, et al. Esophageal and Small Intestinal Mucosal Integrity in Eosinophilic Esophagitis and Response to an Elemental Diet. Am J Gastroenterol 2017;112(7):1061‐1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Drislane C, Irvine AD. The role of filaggrin in atopic dermatitis and allergic disease. Ann Allergy Asthma Immunol 2020;124(1):36‐43. [DOI] [PubMed] [Google Scholar]
- 46. Howell MD, Kim BE, Gao P, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol 2009;124(3 Suppl 2):R7‐R12. [DOI] [PubMed] [Google Scholar]
- 47. Simon D, Page B, Vogel M, et al. Evidence of an abnormal epithelial barrier in active, untreated and corticosteroid‐treated eosinophilic esophagitis. Allergy 2018;73(1):239‐247. [DOI] [PubMed] [Google Scholar]
- 48. Doshi A, Khamishon R, Rawson R, et al. Interleukin 9 Alters Epithelial Barrier and E‐cadherin in Eosinophilic Esophagitis. J Pediatr Gastroenterol Nutr 2019;68(2):225‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Martin‐Gallausiaux C, Beguet‐Crespel F, Marinelli L, et al. Butyrate produced by gut commensal bacteria activates TGF‐beta1 expression through the transcription factor SP1 in human intestinal epithelial cells. Sci Rep 2018;8(1):9742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Folkerts J, Redegeld F, Folkerts G, et al. Butyrate inhibits human mast cell activation via epigenetic regulation of FcepsilonRI‐mediated signaling. Allergy 2020;75(8):1966‐1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schilderink R, Verseijden C, Seppen J, et al. The SCFA butyrate stimulates the epithelial production of retinoic acid via inhibition of epithelial HDAC. Am J Physiol Gastrointest Liver Physiol 2016;310(11):G1138‐G1146. [DOI] [PubMed] [Google Scholar]
- 52. Yoshida M, Kijima M, Akita M, Beppu T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem 1990;265(28):17174‐17179. [PubMed] [Google Scholar]
- 53. Andoh A, Shimada M, Araki Y, Fujiyama Y, Bamba T. Sodium butyrate enhances complement‐mediated cell injury via down‐regulation of decay‐accelerating factor expression in colonic cancer cells. Cancer Immunol Immunother 2002;50(12):663‐672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Arts J, Lansink M, Grimbergen J, Toet KH, Kooistra T. Stimulation of tissue‐type plasminogen activator gene expression by sodium butyrate and trichostatin A in human endothelial cells involves histone acetylation. Biochem J 1995;310(Pt 1):171‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pazin MJ, Kadonaga JT. What's up and down with histone deacetylation and transcription? Cell 1997;89(3):325‐328. [DOI] [PubMed] [Google Scholar]
- 56. Bloemen JG, Venema K, van de Poll MC, Olde Damink SW, Buurman WA, Dejong CH. Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clin Nutr 2009;28(6):657‐661. [DOI] [PubMed] [Google Scholar]
- 57. Nobel YR, Snider EJ, Compres G, et al. Increasing Dietary Fiber Intake Is Associated with a Distinct Esophageal Microbiome. Clin Transl Gastroenterol 2018;9(10):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ravi A, Marietta EV, Alexander JA, et al. Mucosal penetration and clearance of gluten and milk antigens in eosinophilic oesophagitis. Aliment Pharmacol Ther 2021;53(3):410‐417. [DOI] [PubMed] [Google Scholar]
- 59. Le‐Carlson M, Seki S, Abarbanel D, Quiros A, Cox K, Nadeau KC. Markers of antigen presentation and activation on eosinophils and T cells in the esophageal tissue of patients with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 2013;56(3):257‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mulder DJ, Pooni A, Mak N, Hurlbut DJ, Basta S, Justinich CJ. Antigen presentation and MHC class II expression by human esophageal epithelial cells: role in eosinophilic esophagitis. Am J Pathol 2011;178(2):744‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Philpott H, Lee SZ, Arrington A, McGee SJ, Dellon ES. Impact of food challenge on local oesophageal immunophenotype in eosinophilic oesophagitis. Clin Exp Allergy 2020;50(4):463‐470. [DOI] [PubMed] [Google Scholar]
- 62. Lucendo AJ. Meta‐Analysis‐Based Guidance for Dietary Management in Eosinophilic Esophagitis. Curr Gastroenterol Rep 2015;17(10):464. [DOI] [PubMed] [Google Scholar]
- 63. Arias A, Gonzalez‐Cervera J, Tenias JM, Lucendo AJ. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: a systematic review and meta‐analysis. Gastroenterology 2014;146(7):1639‐1648. [DOI] [PubMed] [Google Scholar]
- 64. Warners MJ, Vlieg‐Boerstra BJ, Verheij J, et al. Elemental diet decreases inflammation and improves symptoms in adult eosinophilic oesophagitis patients. Aliment Pharmacol Ther 2017;45(6):777‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1‐S7
Table S1
Method S1


