Abstract
Infants admitted to neonatal intensive care units are repeatedly stimulated by painful events, especially if intubated. Preterm infants are known to have greater pain perception than full term infants due to immaturity of descending inhibitory circuits and poor noxious inhibitory modulation. Newborns exposed to repetitive painful stimuli are at high risk of impairments in brain development and cognition. Chronic pain is induced and supported by proinflammatory cytokines, free radicals, and reactive oxygen species creating a self‐ sustaining vicious circle. Melatonin is a neurohormone secreted by the pineal gland with antioxidant and anti‐inflammatory functions. This review describes the in‐depth beneficial effects of melatonin for pain control in ventilated preterm newborns. As yet, a minimal amount of literature has been undertaken to consider all its promising bioactivities. The rationale behind the use of melatonin for pain control has also been taken into account in this review. Besides, this review addresses safety concerns and dosages. The potential benefits of melatonin have been assessed against neurological disorders, respiratory distress, microbial infections, and as analgesic adjuvant during ventilation. Additionally, a possible approach for the use of melatonin in ventilated newborns will be discussed.
Keywords: mechanical ventilation, melatonin, newborn, pain
INTRODUCTION
The International Association for the Study of Pain defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.” Because subjective reporting of pain is a fundamental prerequisite of this definition, infants would be excluded, but it is now recognized that the inability to communicate verbally does not negate the possibility that an individual is experiencing pain and needs appropriate pain‐relieving treatment. 1 Pain perception and stress response may be greater in premature than in full term infants. Indeed, nociceptive pathways are fully functional by the 24th week of gestation, whereas dorsal horn synaptic connectivity and descending inhibitory circuits are still immature, causing poor input sensory localization and discrimination and poor noxious inhibitory modulation. 2 , 3 , 4 , 5
Preterm infants are also likely to experience more pain because their stays in the neonatal intensive care unit (NICU) are longer than those of less premature or full‐term infants. Invasive mechanical ventilation is a main painful stress that exposes patients to other complications, such as ventilator‐induced lung injury, pneumonia, and chronic lung disease. Moreover, research proved that pain experienced by preterm infants may alter brain development with long‐term effects on the infant’s behavior and neurological outcome. 6
Repeated painful stimuli during early brain development can alter the brain microstructure and topographical organization of afferent thalamo‐cortical projections, resulting in the development of the atypical somatosensory cortex. 7
Despite that, a large prospective study conducted by France in 2008 found that specific pharmacological or non‐pharmacological analgesia was administered in only about 50% of infants undergoing painful procedures. 8
Melatonin is a neurohormone secreted by the pineal gland with antioxidant and anti‐inflammatory functions. 9 , 10 Moreover, melatonin shows analgesic properties, with a promising role to control pain during mechanical ventilation in infants admitted to the NICU. 11
The purpose of this article is to highlight the potential role of melatonin as an analgesic adjuvant drug to control pain in ventilated preterm infants, illustrating the most relevant evidences in this field.
PAIN, OXIDATIVE STRESS, AND BRAIN DEVELOPMENT IN PRETERM NEWBORNS
Painful stimuli activate both ascending signaling pathways and descending inhibitory feedback mechanism.
Hyperalgesia is the result of continuous peripheral afferent stimulation, which leads to the production of free radicals, the release of glutamate, and subsequent spinal sensitization. 12 Generally, a dynamic balance between reactive oxygen species (ROS) generation and elimination is maintained by antioxidant enzymes, such as superoxide dismutase, catalase, and glutathione peroxidase. However, painful stimuli cause an increase in free radicals that cannot be properly neutralized by the antioxidant system in preterm newborns. 13
Preterm infants have a poor antioxidant system. Indeed, fetal levels of antioxidant enzymes increase progressively, with an exponential increase during the last 4–6 weeks of gestation. 13
The increased energy requirement, following pain, causes a greater breakdown of adenosine triphosphate (ATP) into adenosine diphosphate and adenosine monophosphate (AMP), whereas adenosine is converted into hypoxanthine, xanthine, and acid uric, generating ROS. In turn, ROS promotes central sensitization of dorsal horn cells and activate spinal glial cells (SGCs) through several mechanisms involving the N‐methyl‐D‐aspartate (NMDA) receptor, inhibition of gamma‐aminobutyric acid transmission, and the activation of the transient potential of the receptor superfamily. 14 , 15 , 16 NMDA receptors involved in the pain stimuli transmission are more active during early life, inducing a great release of glutamate. 17 Glutamate, in turn, may trigger both oxidative stress and inflammatory reactions. 18 , 19 ROS induces also cyclooxygenase enzymes and prostaglandins production, amplifying the perception of pain. 20
Finally, another mechanism that may increase ROS is inflammation and cytokine production. Cytokines are produced by peripheral nerve afferents, dorsal root ganglia, and SGCs, making a communication network between immune and neuronal cells. 21 A recent review analyzing the role of cytokines during pain states showed that initially circulating cytokines induce nociceptors to stimulate second order neurons in the spinal cord, and subsequently cytokines released by SGCs contribute to the cascade of inflammatory signals that lead to persistent pain. 22
Increased oxidative stress biomarkers have been demonstrated in plasma of preterm and term infants undergoing painful procedures. 13 , 23 , 24
A schematic diagram about cytokines and ROS involvement during pain transmission is shown in Figure 1.
FIGURE 1.
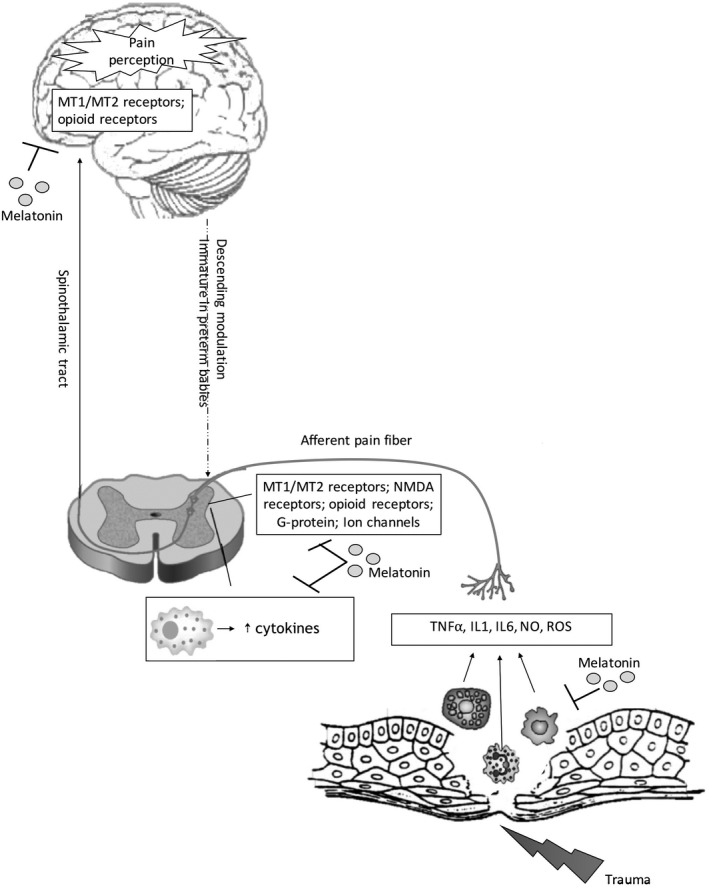
Schematic diagram of the role of cytokines and ROS during pain states. Melatonin acts via its MT1/MT2 receptors in the spinal cord and in brain and by its interaction with other receptors such as opioid and NMDA. Abbreviations NO, nitric oxide; ROS, reactive oxygen species, IL, interleukin); TNF, tumor necrosis factor; MT1/MT2, melatonin receptors; NMDA, N‐methyl‐D‐aspartate
On the clinical side, early exposure of premature infants to painful stimuli may cause brain cells dysmaturation, impairing neurological and sensorimotor development. 25 , 26 , 27 Brain dysmaturation, expressed as white matter injuries and gray matter lesions, is the most important predictor of neurodevelopmental disorders. The exposure to procedural pain of prematurely born infants is associated with reduced width of the frontal and parietal loves of the brain, reduced functional connectivity in the temporal lobes, and smaller volumes of subcortical brain structures, including amygdala, thalamus, and basal ganglia. 28 , 29 , 30 Finally, Tortora et al. also demonstrated a relationship between alterations in thalamus and insular cortex connectivity and exposure to early postnatal painful events of premature infants. 7 Reduced brain volumes in very preterm infants have been associated with poor functional outcomes in childhood, such as impaired intelligence quotient, speech and attention, and poor behavioral outcomes. 31 , 32
These results are supported by animal studies that have shown similar impairments in brain development and cognition in rat models with neonatal pain. Rats exposed to repetitive stimulation of the needle prick during the first weeks of life presented memory impairments, reduced locomotor activity, anxiety, depression, and reduced social behavior. 33 , 34 Likewise, preterm infants exposed to neonatal pain have a higher rate of internalizing behaviors, such as withdrawal, depression, and / or anxiety, than full‐term infants later in life. 35 , 36 These negative behaviors can be evident as early as 18 months of age and persist throughout childhood and adulthood. 37 , 38
ANALGESIC EFFECTS OF MELATONIN
Melatonin (N‐acetyl‐5‐methoxytryptamine) is a neurohormone secreted by the pineal gland with several important functions, including regulation of the circadian rhythms, modulation of season changes, and antioxidant and anti‐inflammatory effects. 39 , 40 , 41 , 42 Released melatonin has a half‐life of 20–30 min and is metabolized primarily in the liver. The endogenous indoleamine melatonin in the fetus has a maternal origin, and, after birth, the full‐term newborns have an irregular melatonin secretion for 3–5 months, leading to a transient melatonin deficiency in neonatal period and in the first months of life. Prematurity delays the maturation of the neurological network that controls melatonin secretion, leading to poor secretion for an even longer period. 43 Therefore, exogenous melatonin administration appears to be a promising strategy in the treatment of neonatal morbidities in which free radicals or inflammatory mediators have a leading role. 44 Several studies on the effect of melatonin in nociceptive modulation have reported its analgesic capacity for various types of pain. 45 , 46 , 47 The physiological mechanism of the analgesic effect of melatonin is not yet clear, however, it seems to act on two levels: both directly on the Gi‐coupled melatonin receptors, MT1 and MT2, distributed in the pain control regions of the central nervous system such as lamina I‐V and X of the spinal cord, thalamus, hypothalamus, spinal trigeminal tract, and trigeminal nucleus leads, and indirectly through its inflammatory and antioxidants effects. 48 , 49 Activation of melatonin receptors leads to reduction of cyclic AMP levels, activation of K+ channels and inhibition of Ca2+ channels, resulting in inhibition of the membrane potential of neurons. 50 Furthermore, melatonin interacts with other receptors, including opioidergic, benzodiazepinergic, muscarinic, nicotinic, serotonergic, and adrenergic receptors, and also promotes the release of β‐endorphin from the pituitary gland. 49 , 51 As for the anti‐inflammatory and antioxidant effects, melatonin appears to decrease hyperalgesia by reducing inflammation and tissue damage. In fact, it inhibits the production of nitric oxide (NO), proinflammatory cytokines by lymphocytes, and macrophages, on the other hand it neutralizes ROS by acting directly as a free radical scavenger and stimulating antioxidant enzymes, such as glutathione peroxidase, glutathione reductase, and superoxide dismutase 10 , 52 , 53
The analgesic activity of melatonin during endotracheal intubation and mechanical ventilation was evaluated in 60 premature infants of 32 weeks of gestation or less. Two groups, each of 30 newborns, treated with 10 mg/kg of intravenous melatonin + fentanyl or fentanyl alone were compared. 54 Neonatal Infant Pain Scale (NIPS) score resulted similar in the two groups before, during, and after 5 min from intubation procedure. After intubation, pain response was assessed at 12, 24, 48, and 72 h of invasive ventilation using the Premature Infant Pain Profile (PIPP) scale. The pain scale was significantly lower in the group of patients treated with intravenous melatonin 10 mg/kg and fentanyl compared to infants managed with standard medication. The use of melatonin as an adjunct analgesic therapy during procedural pain was suggested. There might be a role for melatonin in premedication for nonemergency intubation in the neonate, especially if preterm.
Melatonin can be administered intravenously and orally. The most frequent method of administration is oral. The pharmacokinetic profile of oral pharmacological doses of melatonin in preterm neonates was recently reported by Carloni et al. 55 using three different doses of melatonin: 0.1, 0.5, and 5 mg. The results showed that a single intragastric administration of these drug doses to preterm infants resulted in high peak plasma concentrations, higher time of maximum plasma concentration (Tmax) values than in adults, and a plasma half‐life of melatonin ranging from 7.98 to 10.94 h. 55 The high peak plasma concentrations and the long half‐life indicate that, in the neonatal clinical setting, it is possible to obtain and maintain high serum concentrations using a single administration of 0.5 mg melatonin repeated every 12/24 h. Long‐term studies have never shown major side effects after oral administration of melatonin in children. 52
MELATONIN ADMINISTRATION DURING MECHANICAL VENTILATION
Tracheal intubation is a stressful, painful, and dangerous procedure both for changes in vital parameters and for possible trauma to the airways. 56 In addition to the intubation procedure, mechanical ventilation is also a stressful and painful event that promotes the production of proinflammatory cytokines with long‐term effects on the newborn’s brain and behavioral development. 57
The premedication with sedatives, analgesics, and muscle relaxants has long been a consolidated practice in adults and children, but the role of continuous analgesia or sedation in preterm infants is controversial. However, to date, premedication prior to endotracheal intubation is recommended in neonates, because studies on both premature and full‐term infants showed that it makes it easier for the intubation procedure and limits pain, stress, and worsening of vital signs. 58
Latest guidelines of the Italian Society of Neonatology on the management of pain control in the newborn suggested premedication of intubation with fast‐acting opioids (fentanil or remifentanil) associated or not with benzodiazepines (commonly midazolam), in order to facilitate the procedure and reduce pain and stress. 59 When rapid recovery of spontaneous respiratory activity is required, it is preferable to use drugs with a short half‐life (remifentanil among opioids) or with a low degree of respiratory depression (propofol). 60 , 61
Although several studies in children and adolescents have shown that melatonin may have benefits as an analgesic therapy for procedural pain, data on its efficacy in premature infants are still lacking. 43 , 61
Over the last year, the role of melatonin in intubated and mechanically ventilated patients has also been investigated in adults with coronavirus disease 2019 (COVID‐19). Ramlall et al. 62 demonstrated that melatonin exposure is associated with a positive outcome both in terms of duration of mechanical ventilation and attenuation of lung inflammation.
The reduction in mechanical ventilation time could be explained by the fact that melatonin not only has analgesic and sedative power without any respiratory depressant effect, but its antioxidant effect also protects against ventilator‐associated lung damage. 63 The treatment of melatonin for ventilated preterm infants might reduce pain‐related stress and it may protect the lungs from oxidative stress and inflammation.
The protective role of melatonin in ventilated infants has been demonstrated. 11 , 54 In 2005, the pro‐inflammatory cytokines were measured before and after treatment with melatonin in 110 preterm newborns with respiratory distress syndrome of III or IV degrees that needed invasive mechanical ventilation. 11 In this randomized study, the authors divided the patients into two groups (melatonin and placebo) and demonstrated that if infants received melatonin they had lower pro‐inflammatory cytokines in the tracheobronchial aspirate and better clinical outcomes in terms of chronic lung disease development. 11 In 2012, Gitto et al. 54 evaluated also plasma concentration of IL‐6, IL‐8, IL‐10, and IL‐12 before and after 24, 72 h, and 7 days of mechanical ventilation. Comparing preterm newborns treated with and without melatonin, serum levels of pro‐inflammatory cytokines were similar before endotracheal intubation, whereas it was significantly lower at subsequent times in patients treated with melatonin.
Although available data on the use of melatonin in infants support safe and effective use, further well‐designed studies are needed before clear consensus and guidelines can be developed.
CONCLUSIONS
Preterm infants requiring intensive care are exposed to painful stimuli during hospitalization, such as invasive mechanical ventilation. Because pain can be harmful because it causes hemodynamic instability, low oxygenation, and impaired brain development, neonatal pain prevention and management is imperative. To date it is well known that pro‐inflammatory cytokines play a key role in the induction and maintenance of pain.
The analgesic power of melatonin seems to be linked both to its direct action on melatonergic, opioidergic, benzodiazepinergic, muscarinic, nicotinic, serotonergic, and adrenergic receptors, and indirectly to its inflammatory and antioxidant effects. Furthermore, the antioxidant effect of melatonin appears to decrease lung inflammation, shortening the duration of ventilation, and improving the outcome of lung disease.
In summary, given the various positive effects in controlling chronic pain and reducing oxidative stress, melatonin may be a neuroprotective agent with analgesic properties and without any respiratory depressants or other significant side effects. These characteristics make melatonin a safe and promising analgesic substance suitable for the treatment of preterm newborns with lung diseases undergoing mechanical ventilation. Further studies are warranted to investigate the analgesic and sedative effects of melatonin in preterm infants with lung disease undergoing invasive mechanical ventilation.
CONFLICTS OF INTEREST
No conflicts of interest exist for this article.
ACKNOWLEDGEMENT
Open Access Funding provided by Universita degli Studi di Parma within the CRUI‐CARE Agreement. [Correction added on 24 May 2022, after first online publication: CRUI funding statement has been added.]
List of abbreviations
| NICU | neonatal intensive care unit |
| ATP | adenosine triphosphate |
| AMP | adenosine monophosphate |
| ROS | reactive oxygen species |
| SGCs | spinal glial cells |
| NMDA | N‐methyl‐D‐aspartate |
| NO | nitric oxide |
Cannavò L, Perrone S, Marseglia L, Viola V, Di Rosa G, Gitto E. Potential benefits of melatonin to control pain in ventilated preterm newborns: An updated review. Pain Pract.2022;22:248–254. 10.1111/papr.13069
REFERENCES
- 1. Troels SJ, Gebhart GF. New pain terminology: a work in progress. Pain. 2008;140:399–400. [DOI] [PubMed] [Google Scholar]
- 2. Lee SJ, Ralston HJ, Drey EA, Partridge JC, Rosen MA. Fetal pain: a systematic multidisciplinary review of the evidence. JAMA. 2005;294:947–54. [DOI] [PubMed] [Google Scholar]
- 3. Slater R, Cantarella A, Gallella S, Worley A, Boyd S, Meek J, et al. Cortical pain responses in human infants. J Neurosci. 2006;26:3662–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fitzgerald M, Beggs S. The neurobiology of pain: developmental aspects. Neuroscientist. 2001;7:246–257. [DOI] [PubMed] [Google Scholar]
- 5. Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6:507–20. [DOI] [PubMed] [Google Scholar]
- 6. Grunau R. Early pain in preterm infants: a model of long‐term effects. Clin Perinatol. 2002;29:373–94. [DOI] [PubMed] [Google Scholar]
- 7. Tortora D, Severino M, Di Biase C, Malova M, Parodi A, Minghetti D, et al. Early pain exposure influences functional brain connectivity in very preterm neonates. Front Neurosci. 2019;13:899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carbajal R, Rousset A, Danan C, Coquery S, Nolent P, Ducrocq S, et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA. 2008;300:60–70. [DOI] [PubMed] [Google Scholar]
- 9. Gordon N. The therapeutics of melatonin: a paediatric perspective. Brain Dev. 2000;22:213–7. [DOI] [PubMed] [Google Scholar]
- 10. D’Angelo G, Cannavò L, Reiter RJ, Melatonin GE. Melatonin administration from 2000 to 2020 to human newborns with hypoxic‐ischemic encephalopathy. Am J Perinatol. Online ahead of print. 2020. 10.1055/s-0040-1719151 [DOI] [PubMed] [Google Scholar]
- 11. Gitto E, Reiter RJ, Sabatino G, Buonocore G, Romeo C, Gitto P, et al. Correlation among cytokines, bronchopulmonary dysplasia and modality of ventilation in preterm newborns: improvement with melatonin treatment. J Pineal Res. 2005;39:287–93. [DOI] [PubMed] [Google Scholar]
- 12. Ilari S, Dagostino C, Malafoglia V, Lauro F, Giancotti LA, Spila A, et al. Protective effect of antioxidants in nitric oxide/COX‐2 interaction during inflammatory pain: the role of nitration. Antioxidants. 2020;9:1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Slater L, Asmerom Y, Boskovic DS, Bahjri K, Plank MS, Angeles KR, et al. Procedural pain and oxidative stress in premature neonates. J Pain. 2012;13(5):90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herzberg D, Strobel P, Chihuailaf R, Ramirez‐Reveco A, Müller H, Werner M, et al. Spinal reactive oxygen species and oxidative damage mediate chronic pain in lame dairy cows. Animals (Basel). 2019;9:693. 10.3390/ani9090693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carrasco C, Naziroǧlu M, Rodríguez AB, Pariente JA. Neuropathic pain: delving into the oxidative origin and the possible implication of transient receptor potential channels. Front Physiol. 2018;14:95. 10.3389/fphys.2018.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shahid M, Subhan F, Islam NU, Ahmad N, Farooq U, Abbas S, et al. The antioxidant N‐(2‐mercaptopropionyl)‐glycine (tiopronin) attenuates expression of neuropathic allodynia and hyperalgesia. Naunyn Schmiedebergs Arch Pharmacol. 2021;394:603–17. [DOI] [PubMed] [Google Scholar]
- 17. Vinall J, Grunau RE. Impact of repeated procedural pain‐related stress in infants born very preterm. Pediatr Res. 2014;75:584–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xie SS, Fan WG, Liu Q, Li JZ, Zheng MM, He HW, et al. Involvement of nNOS in the antinociceptive activity of melatonin in inflammatory pain at the level of sensory neurons. Eur Rev Med Pharmacol Sci. 2020;24:7399–411. [DOI] [PubMed] [Google Scholar]
- 19. Sugiyama T, Shinoda M, Watase T, Honda K, Ito R, Kaji K, et al. Nitric oxide signaling contributes to ectopic orofacial neuropathic pain. J Dent Res. 2013;92:1113–7. [DOI] [PubMed] [Google Scholar]
- 20. Salvemini D, Kim SF, Mollace V. Reciprocal regulation of the nitric oxide and cyclooxygenase pathway in pathophysiology: relevance and clinical implications. Am J Physiol Regul Integr Comp Physiol. 2013;304:R473–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol. 2009;194:417–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gonçalves Dos Santos G, Delay L, Yaksh TL, Corr M. Neuraxial cytokines in pain states. Front Immunol. 2020;10:3061. 10.3389/fimmu.2019.03061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bellieni CV, Iantorno L, Perrone S, Rodriguez A, Longini M, Capitani S, et al. Even routine painful procedures can be harmful for the newborn. Pain. 2009;147:128–31. [DOI] [PubMed] [Google Scholar]
- 24. Perrone S, Bellieni CV, Negro S, Longini M, Santacroce A, Tataranno ML, et al. Oxidative stress as a physiological pain response in full‐term newborns. Oxid Med Cell Longev. 2017;2017:3759287. 10.1155/2017/3759287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burnett AC, Cheong JLY, Doyle LW. Biological and social influences on the neurodevelopmental outcomes of preterm infants. Clin Perinatol. 2018;45:485–500. [DOI] [PubMed] [Google Scholar]
- 26. McPherson C, Miller SP, El‐Dib M, Massaro AN, Inder TE. The influence of pain, agitation, and their management on the immature brain. Pediatr Res. 2020;88:168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brummelte S, Grunau RE, Chau V, Poskitt KJ, Brant R, Vinall J, et al. Procedural pain and brain development in premature newborns. Ann Neurol. 2012;71:385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Synnes A, Luu TM, Moddemann D, Church P, Lee D, Vincer M, et al. Determinants of developmental outcomes in a very preterm Canadian cohort. Arch Dis Child Fetal Neonatal Ed. 2017;102:F235–4. [DOI] [PubMed] [Google Scholar]
- 29. Duerden EG, Grunau RE, Guo T, Foong J, Pearson A, Au‐Young S, et al. Early procedural pain is associated with regionally‐specific alterations in thalamic development in preterm neonates. J Neurosci. 2018;38:878–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chau CMY, Ranger M, Bichin M, Park MTM, Amaral RSC, Chakravarty M, et al. Hippocampus, amygdala, and thalamus volumes in very preterm children at 8 years: neonatal pain and genetic variation. Front Behav Neurosci. 2019;13:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ranger M, Zwicker JG, Chau CM, Park MT, Chakravarthy MM, Poskitt K, et al. Neonatal pain and infection relate to smaller cerebellum in very preterm children at school age. J Pediatr. 2015;167:292–8. [DOI] [PubMed] [Google Scholar]
- 32. Linsell L, Johnson S, Wolke D, O’Reilly H, Morris JK, Kurinczuk JJ, et al. Cognitive trajectories from infancy to early adulthood following birth before 26 weeks of gestation: a prospective, population‐based cohort study. Arch Dis Child. 2018;103:363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anand KJS, Coskun V, Thrivikraman KV, Nemeroff CB, Plotksy PM. Long‐term behavioral effects of repetitive pain in neonatal rat pups. Physiol Behav. 1999;66:627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burke NN, Finn DP, McGuire BE, Roche M. Psychological stress in early life as a predisposing factor for the development of chronic pain: clinical and preclinical evidence and neurobiological mechanisms. J Neurosci Res. 2017;95:1257–70. [DOI] [PubMed] [Google Scholar]
- 35. Talge NM, Holzman C, Wang J, Lucia V, Gardiner J, Breslau N. Late‐preterm birth and its association with cognitive and socioemotional outcomes at 6 years of age. Pediatrics. 2010;126:1124–31. [DOI] [PubMed] [Google Scholar]
- 36. Schmidt LA, Miskovic V, Boyle M, Saigal S. Frontal electroencephalogram asymmetry, salivary cortisol, and internalizing behavior problems in young adults who were born at extremely low birth weight. Child Dev. 2010;81:183–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vinall J, Miller SP, Synnes AR, Grunau RE. Parent behaviors moderate the relationship between neonatal pain and internalizing behaviors at 18 months corrected age in children born very prematurely. Pain. 2013;154:1831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Loe IM, Lee ES, Luna B, Feldman HM. Behavior problems of 9–16 year old preterm children: biological, sociodemographic, and intellectual contributions. Early Hum Dev. 2011;87:247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brzezinski A. Melatonin in humans. N Engl J Med. 1997;336:186–95. [DOI] [PubMed] [Google Scholar]
- 40. D’Angelo G, Chimenz R, Reiter RJ, Gitto E. Use of melatonin in oxidative stress related neonatal diseases. Antioxidants (Basel). 2020;9:477. 10.3390/antiox9060477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. D’Angelo G, Marseglia L, Reiter RJ, Buonocore G, Gitto E. Melatonin and neonatal sepsis: a promising antioxidant adjuvant agent. Am J Perinatol. 2017;34:1382–8. [DOI] [PubMed] [Google Scholar]
- 42. Marseglia L, D’Angelo G, Manti S, Reiter RJ, Gitto E. Potential utility of melatonin in preeclampsia, intrauterine fetal growth retardation, and perinatal asphyxia. Reprod Sci. 2016;23:970–7. [DOI] [PubMed] [Google Scholar]
- 43. Biran V, Decobert F, Bednarek N, Boizeau P, Benoist JF, Claustrat B, et al. Melatonin levels in preterm and term infants and their mothers. Int J Mol Sci. 2019;20:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Perrone S, Negro S, Tataranno ML, Buonocore G. Oxidative stress and antioxidant strategies in newborns. J Matern Fetal Neonatal Med. 2010;23:63–5. [DOI] [PubMed] [Google Scholar]
- 45. Srinivasan V, Pandi‐Perumal SR, Spence DW, Moscovitch A, Trakht I, Brown GM, et al. Potential use of melatonergic drugs in analgesia: mechanisms of action. Brain Res Bull. 2010;81:362–71. [DOI] [PubMed] [Google Scholar]
- 46. Zhu C, Xu Y, Duan Y, Li W, Zhang L, Huang Y, et al. Exogenous melatonin in the treatment of pain: a systematic review and meta‐analysis. Oncotarget. 2017;8:100582–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oh SN, Myung SK, Jho HJ. Analgesic efficacy of melatonin: a meta‐analysis of randomized, double‐blind, placebo‐controlled trials. J Clin Med. 2020;9:1553. 10.3390/jcm9051553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zahn PK, Lansmann T, Berger E, Speckmann EJ, Musshoff U. Gene expression and functional characterization of melatonin receptors in the spinal cord of the rat: implications for pain modulation. J Pineal Res. 2003;35:24–31. [DOI] [PubMed] [Google Scholar]
- 49. Ambriz‐Tututi M, Rocha‐Gonzalez HI, Cruz SL, Granados‐Soto V. Melatonin: a hormone that modulates pain. Life Sci. 2009;84:489–98. [DOI] [PubMed] [Google Scholar]
- 50. Ayar A, Martin DJ, Ozcan M, Kelestimur H. Melatonin inhibits high voltage activated calcium currents in cultured rat dorsal root ganglion neurones. Neurosci. 2001;313:73–7. [DOI] [PubMed] [Google Scholar]
- 51. Xie S, Fan W, He H, Huang F. Role of melatonin in the regulation of pain. J Pain Res. 2020;13:331–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Marseglia L, D’Angelo G, Manti S, Aversa S, Arrigo T, Reiter RJ, et al. Analgesic, anxiolytic and anaesthetic effects of melatonin: new potential uses in pediatrics. Int J Mol Sci. 2015;16:1209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen WW, Zhang X, Huang WJ. Pain control by melatonin: physiological and pharmacological effects. Exp Ther Med. 2016;12:1963–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gitto E, Aversa S, Salpietro CD, Barberi I, Arrigo T, Trimarchi G, et al. Pain in neonatal intensive care: role of melatonin as an analgesic antioxidant. J Pineal Res. 2012;52:291–5. [DOI] [PubMed] [Google Scholar]
- 55. Carloni S, Proietti F, Rocchi M, Longini M, Marseglia L, D’Angelo G, et al. Melatonin pharmacokinetics following oral administration in preterm neonates. Molecules. 2017;22(12):2115. 10.3390/molecules22122115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Caldwell CD, Watterberg KL. Effect of premedication regimen on infant pain and stress response to endotracheal intubation. J Perinatol. 2015;35:415–8. [DOI] [PubMed] [Google Scholar]
- 57. Cannavò L, Rulli I, Falsaperla R, Corsello G, Gitto E. Ventilation, oxidative stress and risk of brain injury in preterm newborn. Ital J Pediatr. 2020;46:100. 10.1186/s13052-020-00852-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ancora G, Lago P, Garetti E, Merazzi D, Savant LP, Bellieni CV, et al. Evidence‐based clinical guidelines on analgesia and sedation in newborn infants undergoing assisted ventilation and endotracheal intubation. Acta Paediatr. 2019;108:208–17. [DOI] [PubMed] [Google Scholar]
- 59. Gitto E, Marseglia L, D’Angelo G, Manti S, Crisafi C, Montalto AS, et al. Melatonin versus midazolam premedication in children undergoing surgery: a pilot study. J Paediatr Child Health. 2016;52:291–5. [DOI] [PubMed] [Google Scholar]
- 60. Badiee Z, Vakiliamini M, Mohammadizadeh M. Remifentanil for endotracheal intubation in premature infants: a randomized controlled trial. J Res Pharm Pract. 2013;2:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Procaccini D, Lobner K, Azamfirei R, Kudchadkar SR. Melatonin for anaesthetic indications in paediatric patients: a systematic review. Anaesthesia. 2021;76:837–49. 10.1111/anae.15249 [DOI] [PubMed] [Google Scholar]
- 62. Ramlall V, Zucker J, Tatonetti N. Melatonin is significantly associated with survival of intubated COVID‐19 patients. medRxiv [Preprint]. 2020. 10.1101/2020.10.15.20213546 [DOI] [Google Scholar]
- 63. Wu GC, Peng CK, Liao WI, Pao HP, Huang K, Chu SJ. Melatonin receptor agonist protects against acute lung injury induced by ventilator through up‐regulation of IL‐ 10 production. Respir Res. 2020;21:65. 10.1186/s12931-020-1325-2 [DOI] [PMC free article] [PubMed] [Google Scholar]


