Abstract
COVID‐19 manifests as a mild disease in most people but can progress to severe disease in nearly 20% of individuals. Disease progression is likely driven by a cytokine storm, either directly stimulated by SARS‐CoV‐2 or by increased systemic inflammation in which the gut might play an integral role. SARS‐CoV‐2 replication in the gut may cause increased intestinal permeability, alterations to the fecal microbiome, and increased inflammatory cytokines. Each effect may lead to increased systemic inflammation and the transport of cytokines and inflammatory antigens from the gut to the lung. Few interventions are being studied to treat people with mild disease and prevent the cytokine storm. Serumderived bovine immunoglobulin/protein isolate (SBI) may prevent progression by (1) binding and neutralizing inflammatory antigens, (2) decreasing gut permeability, (3) interfering with ACE2 binding by viral proteins, and (4) improving the fecal microbiome. SBI is therefore a promising intervention to prevent disease progression in COVID‐19 patients.
Keywords: intestinal permeability, SARS‐CoV‐2, serum‐derived Bovine Immunoglobulin/protein isolate
Coronavirus disease (COVID‐19) due to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) emerged in late 2019 and has led to a world‐wide pandemic with devastating morbidity and mortality. Patients enter a state of hyper‐inflammation, leading to a cytokine storm, actute respiratory distress syndrome (ARDS), cardiac and gastrointestinal symptoms and postacute COVID‐19 syndrome (Jin et al., 2020; Lee et al., 2020; Nalbandian et al., 2021). While SARS‐CoV‐2 may directly stimulate the inflammatory response, other factors may also contribute. Thus, a systems view of the relationship between gastrointestinal health, systemic inflammation, and lung pathology could offer insight into disease progression and potential for new interventions.
Gastrointestinal (GI)‐associated symptoms including diarrhea, anorexia, and abdominal discomfort are among the initial symptoms in many people with COVID‐19 (Ahlawat et al., 2020). Indeed, the gut may be integral in driving inflammation and the cytokine storm (Figure 1). First, while the primary route of infection for SARS‐CoV‐2 is the binding of ACE2 on lung alveolar type 2 cells, ACE2 is also expressed on gut enterocytes (Gu et al., 2020). The SARS‐CoV‐2 viral nucleocapsid protein has been visualized in the cytoplasm of duodenal and rectal glandular epithelial cells, (Xiao et al., 2020) and infected enterocytes can produce SARS‐CoV‐2 (Lamers et al., 2020). Second, gut inflammation may exacerbate the cytokine storm through transport of cytokines and inflammatory antigens to the lung. The concept of different types of mucosa being interconnected through a common mucosal immune system is supported by findings of reduced systemic inflammation by oral plasma proteins (Maijo et al., 2012a, 2012b) and induction of Th17‐mediated autoimmune lung pathology by segmented filamentous bacteria in the gut (Bradley et al., 2017). Recruitment of Th17 cells to the lungs in response to a gut bacteria challenge is noteworthy as Th17 cell counts are high in COVID‐19 patients (De Biasi et al., 2020) and are associated with driving the cytokine storm (Fajgenbaum & June, 2020). A self‐perpetuating cycle of increasing gut inflammation, initiated by infiltration of plasma cells and lymphocytes, leading to increased intestinal permeability, results in higher cytokine production and translocation of bacteria/bacterial products that contribute to systemic inflammation through the common mucosal immune system, culminating in a cytokine storm. Patients with moderate to severe COVID‐19 show increased levels of zonulin, intestinal fatty acid binding protein, soluble CD14 (sCD14) (Utay et al., 2021), and lipopolysaccharide (LPS) binding protein (LBP), markers of increased gut permeability and LPS‐induced immune system activation (Giron et al., 2020; Hoel et al., 2021). And direct measures of bacterial and fungal translocation, LPS and (1–3)‐β‐D‐glucan, are high in serum or plasma samples from some COVID‐19 patients (Hoel et al., 2021; Sirivongrangson et al., 2020). Furthermore, sCD14 (indicating increased LPS‐induced monocyte activation), intestinal fatty acid binding protein (indicating intestinal permeability), and LBP increase with increasing COVID‐19 severity (Utay et al., 2021). In fact, similar associations of increased intestinal permeability have been associated with other pulmonary diseases such as chronic obstructive pulmonary disease exacerbations (Sprooten et al., 2018), and lipopolysaccharide has been shown to potentiate infections in porcine respiratory virus models (Van Reeth et al., 2002).
FIGURE 1.
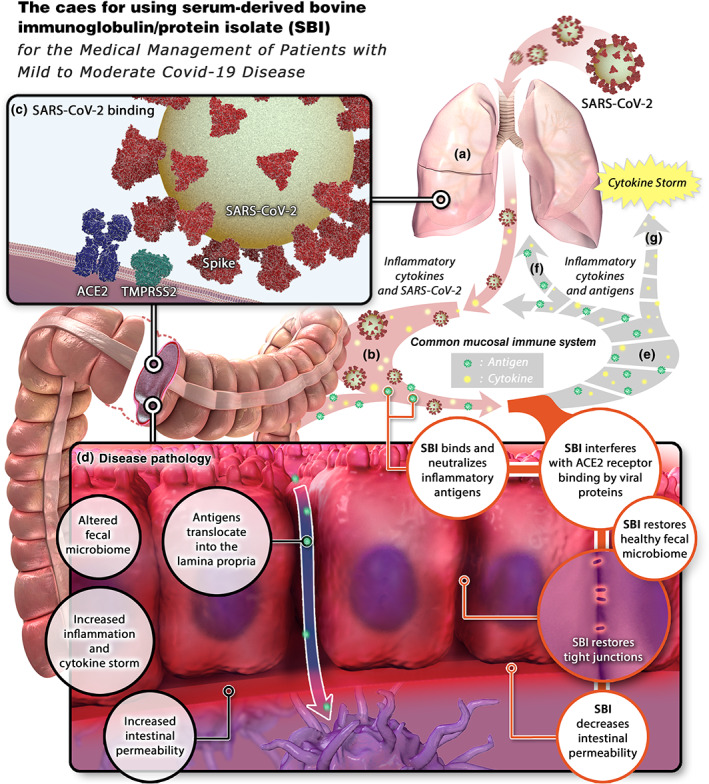
The case for using serum‐derived bovine immunoglobulin (SBI)/protein isolate. (a) SARS‐CoV‐2 enters the lungs and stimulates local inflammation. (b) Inflammatory cytokines and SARS‐CoV‐2 shuttle to the gastrointestinal (GI) tract via the common mucosal immune system. (c) SARS‐CoV‐2 binds to ACE2 on enterocytes, allowing entry and productive infection. (d) the presence of SARS‐CoV‐2 in the GI tract induces changes in the intestinal microbiome, increased intestinal inflammation and cytokine production, increased intestinal permeability, and translocation of microbial antigens into the lamina propria. (e) Antigens and cytokines are released from the GI tract (f) shuttle to the lungs via the common mucosal immune system, resulting in pulmonary inflammation and (g) into the systemic circulation, inducing a cytokine storm. SBI would interfere with SARS‐CoV‐2 binding to ACE2, restore a healthy microbiome, neutralize inflammatory antigens, restore tight junctions, and decrease intestinal permeability, ultimately preventing cytokine storm
Finally, alterations to the fecal microbiome can contribute to disease pathology and clinical outcomes. Increased abundance of Clostridium hathewayi, Clostridium ramosum, and Coprobacillus and lower levels of Faecalibacterium prausnitzii have been observed in patients with more severe COVID‐19 (Zuo et al., 2020). The fecal microbial communities found in COVID‐19 patients form three distinct clusters indicative of healthy gut genera, pathogenic genera or severe dysbiosis (Xu et al., 2021). These clusters shift from low to high diversity (eg. Pathogenic genera to healthy) as the days from symptom onset increase. Similar clustering and synchronous shifts toward higher diversity were observed for lung microbiomes. The richness of the two microbiome communities was negatively correlated with serum LPS levels (Xu et al., 2021). A separate study showed similar changes of gut microbiomes toward dysbiosis in patients with COVID‐19 compared to healthy controls. Commensal bacteria with immunomodulatory potential were depleted and stayed low up to 30 days after a negative PCR result. Dysbiosis correlated with both disease severity and elevation of cytokines and markers of inflammation such as IL‐10, TNF‐α, and C‐reactive protein (CRP) (Yeoh et al., 2021). The nature of microbiomes and disease is generally understood to be bidirectional. The gut microbiome also can prime innate immune responses against invading pathogens in the lungs (Moretó et al., 2020; Steed et al., 2017) and induces the production of circulating inflammatory mediators (Ahlawat et al., 2020). Immune cells can subsequently traffic between the gut and lung via the lymphatic system or systemic circulation (He et al., 2020). A healthy gut microbiome protects against other pulmonary bacterial and viral infections (Dang & Marsland, 2019).
Efforts to improve gut health and the microbiome may therefore attenuate this cytokine storm, improve pulmonary defenses against pathogens, strengthen the immunologic control of SARS‐CoV‐2 and thereby improve clinical outcomes in COVID‐19 patients. One intervention might be oral serum‐derived bovine immunoglobulin/protein isolate (SBI). SBI is a protein product (>90% protein) manufactured by fractionating edible grade bovine plasma. These fractionation processes enrich the target proteins: IgG (~50%), bovine serum albumin (~10%), transferrin (~6%), IgA and IgM (~5%). SBI is enriched in broadly reactive IgG against gut pathogens/antigens prone to translocation in bovine species. For instance, high titers against endotoxin, rotovirus, and E. coli have been measured. SBI is only partially impacted during initial digestive processes (Losonsky et al., 1985; Shaw et al., 2016), thus enabling SBI to improve GI function and morphology (Henderson et al., 2015; Utay et al., 2019; Wilson et al., 2013).
Animal and human clinical studies have shown that oral immunoglobulins improve mucosal immunity, both respiratory/pulmonary and gastrointestinal; they decrease systemic inflammation, reduce the severity of pulmonary inflammation and viral infections and lower the viral burden (Table 1). SBI reduces coronavirus disease severity in calves (Arthington et al., 2002), and humans and bovines display surface protein homology and may have common ancestry. SBI also alleviates symptoms in patients with irritable bowel syndrome (Petschow et al., 2014) and lowers systemic IL‐6 levels in individuals with HIV enteropathy (Utay et al., 2019).
TABLE 1.
Effects of plasma proteins on disease states in animal models and humans
| Author and year | Disease model | Outcome |
|---|---|---|
| Arthington JD, 2002 (Arthington et al., 2002) | Bovine calves subjected to coronavirus |
|
| Diaz I, 2010 (Diaz et al., 2010) | Piglets subjected to porcine reproductive and respiratory syndrome (PRRS) virus challenge |
|
| Duffy M, 2018 (Duffy et al., 2018) | Piglets subjected to acute porcine epidemic diarrhea virus (PEDV) |
|
| Maijo M, 2012 (Maijo et al., 2012a) | Mice challenged with intranasal lipopolysaccharide |
|
| Maijo M, 2012 (Maijo et al., 2012b) | Mice challenged with intranasal lipopolysaccharide |
|
| Petschow BW, 2014 (Petschow et al., 2014) | Individuals with diarrhea‐predominant irritable bowel syndrome |
|
| Utay NS, 2019 (Utay et al., 2019) | Individuals with HIV and chronic diarrhea |
|
| Asmuth DM, 2013 (Asmuth et al., 2013a) | Individuals with HIV and chronic diarrhea |
|
In vitro, the low gastric pH environment does not diminish the capacity of IgG to bind antigens (Jasion & Burnett, 2015) and in vivo orally ingested IgG remains bioactive in the small intestines (Roos et al., 1995). Proteolytic digestion of IgG does not eliminate binding and antigen‐neutralizing capacity when Fab monomers and dimers are left intact (Jasion & Burnett, 2015). Five grams SBI is recommended to be taken orally, twice daily, dissolved in 4 oz of water or mixed with a soft food like yogurt and consumed immediately. Following this guidance, bovine IgG is not absorbed and is either broken down or detected in fecal samples of healthy patients which demonstrates the ability of SBI to survive the GI tract (Shaw et al., 2016). SBI is well tolerated in both pediatric and adult populations with infrequent reports of abdominal discomfort, bloating or constipation and no significant or major side effects (Bégin et al., 2008) (Utay et al., 2019).
Here, we hypothesize that SBI might improve outcomes in COVID‐19 patients by reducing inflammation in the gut which in turn could decrease systemic and respiratory inflammation, thereby interrupting the positive feedback loop of inflammation in the gut‐lung axis that might contribute to a cytokine storm. Several mechanisms underpinning a reduction in gut inflammation are possible: (1) binding and neutralizing inflammatory antigens, (2) decreasing gut permeability, (3) interfering with ACE2 binding by viral proteins, and (4) improving the microbiota. First, by neutralizing microbial and viral antigens in the lumen, SBI could prevent stimulation of an excessive immune response, both in the intestine and systemically, as well as the migration of inflammatory antigens or pro‐inflammatory cytokines to the lungs (Figure 1, Utay et al., 2019). Second, SBI could restore gut integrity and thereby further decrease the translocation of microbial products into the common mucosa, leading to less inflammation in the gut and the lungs (Asmuth et al., 2013a; 2013b).
Third, cell entry of SARS‐CoV‐2 is initiated by the viral spike protein receptor binding domain (RBD) binding to ACE2 on the surface of host cells (Yan et al., 2020). Each of the Ig‐subtypes (IgA, IgM, and IgG) have been isolated and purified from convalescent plasma and shown to bind RBD and neutralize SARS‐CoV‐2 pseudovirus (Klingler et al., 2020). People who recovered from SARS‐CoV infections (76% homology to SARS‐CoV‐2) but had not been exposed to SARS‐CoV‐2 displayed antibodies that cross‐reacted with the SARS‐CoV‐2 spike protein (Zhu et al., 2020). The spike protein from these two viruses have 76% homology; a similar level of homology exists between bovine coronavirus and SARS‐CoV‐2 spike protein epitopes, 70–80% (Tilocca et al., 2020), which is likely due to the closely related evolution of group 2 coronaviruses (Vijgen et al., 2006). This indicates the potential for cross reactivity of antibodies from herds exposed to bovine coronavirus to antigens present in SARS‐CoV‐2. Therefore, plasma from herds of thousands of healthy cows that are likely immune to bovine coronavirus, due to its prevalence in the United States of America (Saif, 2008), may recognize SARS‐CoV‐2 proteins and neutralize the binding of SARS‐CoV‐2 spike to ACE2 on intestinal epithelial cells. Lastly, SBI could restore healthy gut microbiota and metabolome. Plasma proteins and SBI have been shown to change the microbiome in vitro and in vivo. Spray dried porcine plasma fed to C57BL/6 mice increased the abundance of Lactobacillus in stool samples (Moretó et al., 2020). A similar increase in Lactobacillus was observed in vitro when fecal microbiota from human subjects were grown on SBI (data on file at Entera Health). While patients experiencing HIV‐associated enteropathy show significant decreases in Ruminococcus after 8 weeks receiving 2 oral SBI, 2.5 g BID (Asmuth et al., 2013a), a Crohn's disease patient showed an increase in Shannon diversity and shift from overabundance of Bacteroides to levels consistent with their healthy parent (Casas & Hazan, 2020).
We therefore hypothesize that dietary supplementation with SBI might decrease systemic inflammation and prevent clinical deterioration in patients with COVID‐19. We anticipate that each of the potential mechanisms (Figure 1) will contribute independently over sustained administration for weeks to mitigating SARS‐CoV‐2 pathology and COVID‐19 disease progression, thus distinguishing it from a one‐time infusion of convalescent plasma that is based solely upon the neutralizing antibody mechanism of action. The different mechanisms, dosage, mode of administration, and duration of SBI treatment offers an advantage compared to convalescent plasma treatments in patients with mild COVID‐19 which have proven a disappointing intervention. SBI intervention would most likely be beneficial early in the infection by healing the gut and preventing the production and secretion of pro‐inflammatory cytokines, thereby preventing the cytokine storm.
In sum, recent data have suggested that intestinal infection by SARS‐CoV‐2 (Xiao et al., 2020), microbiome alterations (Zuo et al., 2020), and the ensuing intestinal damage (Ahlawat et al., 2020) may contribute to the systemic inflammatory response observed in patients with severe COVID‐19 (Effenberger et al., 2020). SBI represents a novel therapeutic approach to target intestinal pathology and thereby attenuate this response and improve clinical outcomes in COVID‐19 patients. Because of the extensive socioeconomic consequences of COVID‐19, reports of post‐COVID‐19 syndrome (Nicola et al., 2020) and the persistence of symptoms (Townsend et al., 2020), the medical management of mild COVID‐19 is of comparable public health importance as severe COVID‐19. SBI could serve as a cost‐effective option for international patients to prevent worsening of mild to severe COVID‐19. A randomized, open‐label, single‐center, pilot clinical study evaluating the impact of SBI on restoring health in people with COVID‐19 is currently ongoing in Barcelona, Spain, NCT04682041.
CONFLICTS OF INTEREST
Moises Contreras, Christopher D Warner, and Christopher J Detzel are employed by Entera Health, LLC, the manufacturer of serum‐derived bovine immunoglobulin/protein isolate (SBI). Netanya S Utay is co‐principal investigator of the SBI study in Barcelona, Spain.
ACKNOWLEDGMENTS
The authors wish to thank all the front‐line workers caring for patients with COVID‐19. The authors also want to acknowledge Doug Walp, MS, Axon Animation LLC, for the Medical Illustration, and Dr. Robert Güerri for his knowledge and insight.
Utay, N. S. , Asmuth, D. M. , Gharakhanian, S. , Contreras, M. , Warner, C. D. , & Detzel, C. J. (2021). Potential use of serum‐derived bovine immunoglobulin/protein isolate for the management of COVID‐19. Drug Development Research, 82(7), 873–879. 10.1002/ddr.21841
DATA AVAILABILITY STATEMENT
Data available upon request from authors.
REFERENCES
- Ahlawat, S. , Asha, & Sharma, K. K. (2020). Immunological co‐ordination between gut and lungs in SARS‐CoV‐2 infection. Virus Research, 286, 198103. 10.1016/j.virusres.2020.198103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthington, J. D. , Jaynes, C. A. , Tyler, H. D. , Kapil, S. , & Quigley, J. D., 3rd. (2002). The use of bovine serum protein as an oral support therapy following coronavirus challenge in calves. Journal of Dairy Science, 85(5), 1249–1254. 10.3168/jds.S0022-0302(02)74189-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmuth, D. M. , Ma, Z. M. , Albanese, A. , Sandler, N. G. , Devaraj, S. , Knight, T. H. , Flynn, N. M. , Yotter, T. , Garcia, J. C. , Tsuchida, E. , Wu, T. T. , Douek, D. C. , & Miller, C. J. (2013a). Oral serum‐derived bovine immunoglobulin improves duodenal immune reconstitution and absorption function in patients with HIV enteropathy. AIDS, 27(14), 2207–2217. 10.1097/QAD.0b013e328362e54c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmuth, D. M. , Ma, Z.‐M. , Albanese, A. , Sandler, N. G. , Devaraj, S. , Knight, T. H. , Flynn, N. M. , Yotter, T. , Garcia, J.‐C. , Tsuchida, E. , Wu, T.‐T. , Douek, D. C. , Miller, C. J. , (2013b). Changes in stool microbiota, bacterial translocation, and mucosal immunity after oral serum‐derived bovine immunoglobulin administration. Paper presented at the Conference on Retroviruses and Opportunistic Infections.
- Bégin, F. , Santizo, M. C. , Peerson, J. M. , Torún, B. , & Brown, K. H. (2008). Effects of bovine serum concentrate, with or without supplemental micronutrients, on the growth, morbidity, and micronutrient status of young children in a low‐income, peri‐urban Guatemalan community. European Journal of Clinical Nutrition, 62(1), 39–50. 10.1038/sj.ejcn.1602682 [DOI] [PubMed] [Google Scholar]
- Bradley, C. P. , Teng, F. , Felix, K. M. , Sano, T. , Naskar, D. , Block, K. E. , Huang, H. , Knox, K. S. , Littman, D. R. , & Wu, H.‐J. J. (2017). Segmented filamentous bacteria provoke lung autoimmunity by inducing gut‐lung Axis Th17 cells expressing dual TCRs. Cell Host & Microbe, 22(5), 697–704. 10.1016/j.chom.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas, D. A. , & Hazan, S. (2020). S2259 microbiome diversity in a patient treated with serum‐derived bovine immunoglobulin/protein isolate. Official Journal of the American College of Gastroenterology, 115, S1196. 10.14309/01.ajg.0000711084.27985.da [DOI] [Google Scholar]
- Dang, A. T. , & Marsland, B. J. (2019). Microbes, metabolites, and the gut‐lung axis. Mucosal Immunology, 12(4), 843–850. 10.1038/s41385-019-0160-6 [DOI] [PubMed] [Google Scholar]
- De Biasi, S. , Meschiari, M. , Gibellini, L. , Bellinazzi, C. , Borella, R. , Fidanza, L. , Gozzi, L. , Iannone, A. , Lo Tartaro, D. , Mattioli, M. , Paolini, A. , Menozzi, M. , Milic, J , Franceschi, G. , Fantini, R. , Tonelli, R. , Sita, M. , Sarti, M. , Trenti, T. , & Cossarizza, A. (2020). Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID‐19 pneumonia. Nature Communications, 11(1), 3434. 10.1038/s41467-020-17292-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz, I. , Lorca, C. , Galindo, I. , Campbell, J. , Barranco, I. , Kuzemtseva, L. , Rodriguez‐Gomez, I., Crenshaw, J., Russell, L., Polo, J., & Pujols, J. (2010). Potential positive effect of commercial spray‐dried porcine plasma on pigs challenged with PRRS virus. Paper presented at the Proc. 21st Intl. Pig Veterinary Society, Vancouver, Canada.
- Duffy, M. , Chen, Q. , Zhang, J. , Halibur, P. , & Opriessnig, T. (2018). Impact of dietary spray‐dried bovine plasma addition on pigs infected with porcine epidemic diarrhea virus. Translational Animal Science, 2(4), 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effenberger, M. , Grabherr, F. , Mayr, L. , Schwaerzler, J. , Nairz, M. , Seifert, M. , Hilbe, R. , Seiwald, S. , Scholl‐Buergi, S. , Fritsche, G. , Bellmann‐Weiler, R. , Weiss, G. , Müller, T. , Adolph, T. E. , & Tilg, H. (2020). Faecal calprotectin indicates intestinal inflammation in COVID‐19. Gut, 69(8), 1543–1544. 10.1136/gutjnl-2020-321388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajgenbaum, D. C. , & June, C. H. (2020). Cytokine storm. New England Journal of Medicine, 383(23), 2255–2273. 10.1056/NEJMra2026131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giron, L. B. , Dweep, H. , Yin, X. , Wang, H. , Damra, M. , Goldman, A. R. , Gorman, N., Palmer, C.S., Tang, H.Y., Shikh, M.W., Forsyth, C.B., Balk, R.A., Ziberstein, N.F., Liu, Q., Kossenkov, A., Keshavarzian, A., Landay, A., & Abdel‐Mohsen, M. (2020). Severe COVID‐19 Is fueled by disrupted gut barrier integrity. doi: 10.1101/2020.11.13.20231209 [DOI]
- Gu, J. , Han, B. , & Wang, J. (2020). COVID‐19: Gastrointestinal manifestations and potential fecal‐oral transmission. Gastroenterology, 158, 1518–1519. 10.1053/j.gastro.2020.02.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, L. H. , Ren, L. F. , Li, J. F. , Wu, Y. N. , Li, X. , & Zhang, L. (2020). Intestinal flora as a potential strategy to fight SARS‐CoV‐2 infection. Frontiers in Microbiology, 11, 1388. 10.3389/fmicb.2020.01388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, A. L. , Brand, M. W. , Darling, R. J. , Maas, K. J. , Detzel, C. J. , Hostetter, J. , Wannemuehler, M. J. , & Weaver, E. M. (2015). Attenuation of colitis by serum‐derived bovine immunoglobulin/protein isolate in a defined microbiota mouse model. Digestive Diseases and Sciences, 60(11), 3293–3303. 10.1007/s10620-015-3726-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoel, H. , Heggelund, L. , Reikvam, D. H. , Stiksrud, B. , Ueland, T. , Michelsen, A. E. , Otterdal, K. , Muller, K. E. , Lind, A. , Muller, F. , Dudman, S. , Aukrust, P. , Dyhol‐Riise, A. M. , Holter, J. C. , & Troseid, M. (2021). Elevated markers of gut leakage and inflammasome activation in COVID‐19 patients with cardiac involvement. Journal of Internal Medicine, 289(4), 523–531. 10.1111/joim.13178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasion, V. S. , & Burnett, B. P. (2015). Survival and digestibility of orally‐administered immunoglobulin preparations containing IgG through the gastrointestinal tract in humans. Nutritional Journal, 14, 22. 10.1186/s12937-015-0010-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, Y. , Yang, H. , Ji, W. , Wu, W. , Chen, S. , Zhang, W. , & Duan, G. (2020). Virology, epidemiology, pathogenesis, and control of COVID‐19. Viruses, 12(4), 1–17. 10.3390/v12040372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingler, J. , Weiss, S. , Itri, V. , Liu, X. , Oguntuyo, K. Y. , Stevens, C. , Ikegame, S. , Hung, C.‐T. , Enyindah‐Asonye, G. , Amanat, F. , Baine, I. , Arinsburg, S. , Bandres, J. C. , Milunka Kojic, E. , Stoever, J. , Jurczyszak, D. , Bermudez‐Gonzalez, M. , Nádas, A. , & Hioe, C. E. (2020). Role of IgM and IgA antibodies in the neutralization of SARS‐CoV‐2. 10.1101/2020.08.18.20177303 [DOI]
- Lamers, M. M. , Beumer, J. , van der Vaart, J. , Knoops, K. , Puschhof, J. , Breugem, T. I. , Ravelli, R. B. G. , Paul van Schayck, J. , Mykytyn, A. Z. , Duimel, H. Q. , van Donselaar, E. , Riesebosch, S. , Kuijpers, H. J. H. , Schipper, D. , van de Wetering, W. J. , de Graaf, M. , Koopmans, M. , Cuppen, E. , Peters, P. J. , … Clevers, H. (2020). SARS‐CoV‐2 productively infects human gut enterocytes. Science, 369, 50–54. 10.1126/science.abc1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. , Channappanavar, R. , & Kanneganti, T. D. (2020). Coronaviruses: Innate immunity, inflammasome activation, inflammatory cell death, and cytokines. Trends in Immunology, 41, 1083–1099. 10.1016/j.it.2020.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonsky, G. A. , Johnson, J. P. , Winkelstein, J. A. , & Yolken, R. H. (1985). Oral administration of human serum immunoglobulin in immunodeficient patients with viral gastroenteritis. A pharmacokinetic and functional analysis. The Journal of Clinical Investigation, 76(6), 2362–2367. 10.1172/JCI112248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maijo, M. , Miro, L. , Polo, J. , Campbell, J. , Russell, L. , Crenshaw, J. , Weaver, E. , Moreto, M. , & Perez‐Bosque, A. (2012a). Dietary plasma proteins attenuate the innate immunity response in a mouse model of acute lung injury. The British Journal of Nutrition, 107(6), 867–875. 10.1017/S0007114511003655 [DOI] [PubMed] [Google Scholar]
- Maijo, M. , Miro, L. , Polo, J. , Campbell, J. , Russell, L. , Crenshaw, J. , Weaver, E. , Moreto, M. , & Perez‐Bosque, A. (2012b). Dietary plasma proteins modulate the adaptive immune response in mice with acute lung inflammation. The Journal of Nutrition, 142(2), 264–270. 10.3945/jn.111.149070 [DOI] [PubMed] [Google Scholar]
- Moretó, M. , Miró, L. , Amat, C. , Polo, J. , Manichanh, C. , & Pérez‐Bosque, A. (2020). Dietary supplementation with spray‐dried porcine plasma has prebiotic effects on gut microbiota in mice. Scientific Reports, 10(1), 2926. 10.1038/s41598-020-59756-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian, A. , Sehgal, K. , Gupta, A. , Madhavan, M. V. , McGroder, C. , Stevens, J. S. , Cook, J. R. , Nordvig, A. S. , Shalev, D. , Sehrawat, T. S. , Ahluwalia, N. , Bikdeli, B. , Dietz, D. , der Nigoghossian, C. , Liyanage‐Don, N. , Rosner, G. F. , Bernstein, E. J. , Mohan, S. , Beckley, A. A. , … Wan, E. Y. (2021). Post‐acute COVID‐19 syndrome. Nature Medicine, 27(4), 601–615. 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola, M. , Alsafi, Z. , Sohrabi, C. , Kerwan, A. , Al‐Jabir, A. , Iosifidis, C. , Agha, M. , & Agha, R. (2020). The socio‐economic implications of the coronavirus pandemic (COVID‐19): A review. International Journal of Surgery, 78, 185–193. 10.1016/j.ijsu.2020.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petschow, B. W. , Blikslager, A. T. , Weaver, E. M. , Campbell, J. M. , Polo, J. , Shaw, A. L. , Burnett, B. P. , Klein, G. L. , & Rhoads, J. M. (2014). Bovine immunoglobulin protein isolates for the nutritional management of enteropathy. World Journal of Gastroenterology, 20(33), 11713–11726. 10.3748/wjg.v20.i33.11713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos, N. , Mahé, S. , Benamouzig, R. , Sick, H. , Rautureau, J. , & Tomé, D. (1995). 15N‐labeled immunoglobulins from bovine colostrum are partially resistant to digestion in human intestine. The Journal of Nutrition, 125(5), 1238–1244. 10.1093/jn/125.5.1238 [DOI] [PubMed] [Google Scholar]
- Saif, L. J. (2008). Coronaviruses of domestic livestock and poultry: Interspecies transmission, pathogenesis, and immunity nidoviruses. American Society of Microbiology. [Google Scholar]
- Shaw, A. L. , Mathews, D. W. , Hinkle, J. E. , Petschow, B. W. , Weaver, E. M. , Detzel, C. J. , Klein, G. L. , & Bradshaw, T. P. (2016). Absorption and safety of serum‐derived bovine immunoglobulin/protein isolate in healthy adults. Clinical and Experimental Gastroenterology, 9, 365–375. 10.2147/ceg.s120118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirivongrangson, P. , Kulvichit, W. , Payungporn, S. , Pisitkun, T. , Chindamporn, A. , Peerapornratana, S. , Pisitkun, P. , Chitcharoen, S. , Sawaswong, V. , Worasilchai, N. , Kampunya, S. , Putcharoen, O. , Thawitsri, T. , Leelayuwatanakul, N. , Kongpolprom, N. , Phoophiboon, V. , Sriprasart, T. , Samransamruajkit, R. , Tungsanga, S. , … Srisawat, N. (2020). Endotoxemia and circulating bacteriome in severe COVID‐19 patients. Intensive Care Medicine Experimental, 8(1), 72. 10.1186/s40635-020-00362-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprooten, R. T. M. , Lenaerts, K. , Braeken, D. C. W. , Grimbergen, I. , Rutten, E. P. , Wouters, E. F. M. , & Rohde, G. G. U. (2018). Increased small intestinal permeability during severe acute exacerbations of COPD. Respiration, 95(5), 334–342. 10.1159/000485935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steed, A. L. , Christophi, G. P. , Kaiko, G. E. , Sun, L. , Goodwin, V. M. , Jain, U. , Esaulova, E. , Artyomov, M. N. , Morales, D. J. , Holtzman, M. J. , Boon, A. C. M. , Lenschow, D. J. , & Stappenbeck, T. S. (2017). The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science, 357(6350), 498–502. 10.1126/science.aam5336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilocca, B. , Soggiu, A. , Musella, V. , Britti, D. , Sanguinetti, M. , Urbani, A. , & Roncada, P. (2020). Molecular basis of COVID‐19 relationships in different species: A one health perspective. Microbes and Infection, 22(4–5), 218–220. 10.1016/j.micinf.2020.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend, L. , Dyer, A. H. , Jones, K. , Dunne, J. , Mooney, A. , Gaffney, F. , O'Connor, L. , Leavy, D. , O'Brien, K. , Dowds, J. , Sugrue, J. A. , Hopkins, D. , Martin‐Loeches, I. , Ni Cheallaigh, C. , Nadarajan, P. , McLaughlin, A. M. , Bourke, N. M. , Bergin, C. , O'Farrelly, C. , … Conlon, N. (2020). Persistent fatigue following SARS‐CoV‐2 infection is common and independent of severity of initial infection. PLoS One, 15(11), e0240784. 10.1371/journal.pone.0240784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utay, N. S. , Shah, J. , Cantos, A. , Purpura, L. , & Yin, M. T. (2021). Intestinal permeability and microbial translocation are increased in severe COVID‐19 [CROI Abstract 212]. Abstracts from the virual 2021 Conference on Retroviruses and Opportunistic Infections. Topics in Antiviral Medicine, 29(1). [Google Scholar]
- Utay, N. S. , Somasunderam, A. , Hinkle, J. E. , Petschow, B. W. , Detzel, C. J. , Somsouk, M. , Fichtenbaum, C. J. , Weaver, E. , Shaw, A. L. , & Asmuth, D. M. (2019). Serum bovine immunoglobulins improve inflammation and gut barrier function in persons with HIV and enteropathy on suppressive ART. Pathogens and Immunity, 4(1), 124–146. 10.20411/pai.v4i1.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reeth, K. , Van Gucht, S. , & Pensaert, M. (2002). In vivo studies on cytokine involvement during acute viral respiratory disease of swine: Troublesome but rewarding. Veterinary Immunology and Immunopathology, 87(3–4), 161–168. 10.1016/s0165-2427(02)00047-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijgen, L. , Keyaerts, E. , Lemey, P. , Maes, P. , Van Reeth, K. , Nauwynck, H. , Pensaert, M. , & Van Ranst, M. (2006). Evolutionary history of the closely related group 2 coronaviruses: Porcine Hemagglutinating encephalomyelitis virus, bovine coronavirus, and human coronavirus OC43. Journal of Virology, 80(14), 7270–7274. 10.1128/jvi.02675-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, D. , Evans, M. , Weaver, E. , Shaw, A. L. , & Klein, G. L. (2013). Evaluation of serum‐derived bovine immunoglobulin protein isolate in subjects with diarrhea‐predominant irritable bowel syndrome. Clinical Medicine Insights Gastroenterol, 6, 49–60. 10.4137/CGast.S13200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, F. , Tang, M. , Zheng, X. , Liu, Y. , Li, X. , & Shan, H. (2020). Evidence for gastrointestinal infection of SARS‐CoV‐2. Gastroenterology, 158, 1831–1833. 10.1053/j.gastro.2020.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, R. , Lu, R. , Zhang, T. , Wu, Q. , Cai, W. , Han, X. , Wan, Z. , Jin, X. , Zhang, Z. , & Zhang, C. (2021). Temporal association between human upper respiratory and gut bacterial microbiomes during the course of COVID‐19 in adults. Communications Biology, 4(1), 240. 10.1038/s42003-021-01796-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, R. , Zhang, Y. , Li, Y. , Xia, L. , Guo, Y. , & Zhou, Q. (2020). Structural basis for the recognition of SARS‐CoV‐2 by full‐length human ACE2. Science, 367(6485), 1444–1448. 10.1126/science.abb2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh, Y. K. , Zuo, T. , Lui, G. C.‐Y. , Zhang, F. , Liu, Q. , Li, A. Y. , Chung, A. C. K. , Cheung, C. P. , Tso, E. Y. K. , Fung, K. S. C. , Chan, V. , Ling, L. , Joynt, G. , Hui, D. S. C. , Chow, K. M. , Ng, S. S. S. , Li, T. C. M. , Ng, R. W. Y. , Yip, T. C. F. , … Ng, S. C. (2021). Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID‐19. Gut, 70(4), 698–706. 10.1136/gutjnl-2020-323020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y. , Yu, D. , Han, Y. , Yan, H. , Chong, H. , Ren, L. , Wang, J. , Li, T. , & He, Y. (2020). Cross‐reactive neutralization of SARS‐CoV‐2 by serum antibodies from recovered SARS patients and immunized animals. Science Advances, 6(45), eabc9999. 10.1126/sciadv.abc9999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo, T. , Zhang, F. , Lui, G. C. Y. , Yeoh, Y. K. , Li, A. Y. L. , Zhan, H. , Wan, Y. , Chung, A. C. K. , Cheung, C. P. , Chen, N. , Lai, C. K. C. , Chen, Z. , Tso, E. Y. K. , Fung, K. S. C. , Chan, V. , Ling, L. , Joynt, G. , Hui, D. S. C. , Chan, F. K. L. , … Ng, S. C. (2020). Alterations in gut microbiota of patients with COVID‐19 during time of hospitalization. Gastroenterology, 159(3), 944–955. 10.1053/j.gastro.2020.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available upon request from authors.


