Abstract
Background
Patients with short‐bowel syndrome and intestinal failure (SBS‐IF) require parenteral support (PS) and may need long‐term home‐care support. This survey assessed the impact of care provision on adult caregivers of adult patients receiving PS for SBS‐IF.
Methods
An online, cross‐sectional survey of caregivers of adults with a self‐reported physician diagnosis of SBS‐IF was conducted in France, Germany, Italy, the UK, and USA. Impact on caregivers was evaluated using the 18‐item Caregiver Strain Index (CSI), the Work Productivity and Activity Impairment Questionnaire: Specific Health Problem (WPAI:SHP), and self‐reporting impact questionnaires.
Results
Caregivers (N = 121; aged 51 ± 13.7 years; 59% women) provided assistance for a mean of 9.9 ± 12.53 years; 77% were providing care 7 days per week. Patients (51 ± 16.4 years; 56% women) of caregivers were typically family members: spouse/partner (61%), adult son/daughter (19%), or parent (10%). Caregivers reported experiencing some strain (CSI score 4 ± 3.4). Among 71 of 73 employed caregivers, the WPAI:SHP assessment showed that caregivers missed 7% ± 12.7% of work hours in the preceding week and were present but not productive at work 37% ± 23.1% of the time; 28% of caregivers reported a reduced number of working hours because of caregiving. Many caregivers reported limitations in recreational activities (53%), and ≥30% had difficulty spending time with family and friends. Caregivers (87%) also reported worrying about the patient's health.
Conclusions
Caregivers of adult patients with SBS‐IF experience negative daily personal impacts and loss of productivity arising from their caregiving responsibilities.
Keywords: caregiver, CSI, impact, intestinal failure, parenteral support, short‐bowel syndrome, WPAI:SHP
CLINICAL RELEVANCY STATEMENT
Long‐term parenteral support (PS) in patients with short‐bowel syndrome and intestinal failure (SBS‐IF) is thought to place a substantial burden upon caregivers, owing to the chronic, ongoing nature of the condition and the risk presented to patients by both PS and the condition of intestinal failure. As with caregivers of patients with other long‐term illnesses, caregivers of patients with SBS‐IF often have to modify their work schedules, reduce the number of hours at work, experience presenteeism while at work, and reduce their activities with friends and other family members. In light of this study's findings, treating physicians and other healthcare professionals should consider exploring the burden of care with patients and their caregivers and help them seek additional support as necessary.
INTRODUCTION
Patients with short‐bowel syndrome and intestinal failure (SBS‐IF) require parenteral support (PS) and often need long‐term home‐care support. PS encompasses the use of parenteral nutrition and/or intravenous fluids to maintain health. 1 , 2 Although PS is life‐saving, the risk of life‐threatening complications and the time necessary to deliver this support are likely to impact both patients and caregivers.
SBS‐IF is a rare and potentially life‐threatening condition in which intestinal failure occurs because of a reduction in the intestine's absorptive capacity. 1 , 3 In adults, SBS is anatomically defined by a remaining, continuous small‐bowel length of <200 cm and may develop as the result of surgical resections due to conditions such as mesenteric ischemia, Crohn's disease, radiation enteritis, or surgical complications. 3 In the USA, the prevalence of SBS‐IF is approximately two per million people. 4 In Europe, a considerable disparity exists between countries, with prevalence estimated to be between 0.4 and 50 per million people. 2 , 5
Owing to the reduction in the intestine's absorptive capacity, patients with SBS‐IF cannot absorb nutrients, fluids, electrolytes, and minerals adequately. 3 Although patients with SBS‐IF may have variations in terms of disease etiology, pathophysiology, and nutrition needs, they are, by definition, reliant on PS. 6 , 7 PS is vital for stabilizing the nutrition and hydration requirements of patients with SBS‐IF; however, PS is costly, time‐consuming, and invasive. Furthermore, PS is associated with numerous adverse events, including bacterial infections, osteoporosis, catheter‐related complications, blood clots, kidney disease, gall bladder disease, and liver problems, leading to frequent hospital admissions. 8 , 9 , 10 , 11
Caregivers of patients with chronic illnesses face psychosocial burdens and health‐related impairments in quality of life (QoL). Consistent with caregivers of patients with other chronic health conditions, caregivers of patients with SBS‐IF report impacts such as decreased social activities, disrupted relationships, loss of employment, and depression. 10 , 12 However, owing to the low prevalence of SBS‐IF, the burden that caregivers of patients with SBS‐IF face has not been particularly well characterized, with previous studies either not focusing on caregivers of patients specifically with SBS 12 or including only relatively small numbers of caregivers of patients with SBS. 13 Furthermore, studies have been limited in their geographic scope. 12 , 13 To address this gap, a multinational, online, cross‐sectional survey of adult caregivers of adult patients with SBS‐IF was conducted. This study's objective was to characterize further the burden experienced by adult caregivers of adult patients with SBS‐IF, using a sufficient and geographically diverse sample.
MATERIALS AND METHODS
An online, noninterventional, cross‐sectional survey of adult caregivers of adult patients with SBS‐IF was conducted in France, Germany, Italy, the UK, and the USA.
Recruitment
Caregivers were recruited via patient advocacy organizations (PAOs), healthcare providers (HCPs), online patient panels, and social media outreach.
Inclusion criteria
Caregivers were aged ≥18 years, and each was the primary caregiver who provided daily care for an adult patient (aged ≥18 years) with a self‐reported physician diagnosis of SBS‐IF.
Survey platform and consent
The web‐survey was hosted on a webserver secured using a “Secure Sockets Layer” protocol. Potential participants were provided with links to the survey via email. In order to proceed with the survey, participants had to be eligible for the study and had to provide consent.
Instruments
A number of instruments were used to assess the impact of being a caregiver for a patient with SBS‐IF.
Caregiver Strain Index
The 18‐item Caregiver Strain Index (CSI) is an expanded version of the original CSI 14 that includes five additional items around the positive aspect of caring compared with the original 13‐item CSI. 15 Caregivers were asked to respond “yes/no” to 18 statements (13 of which reflected strain relating to caregiving [such as "it is confining" or "it is a financial strain"] and five statements that are more positive [such as "I am happy to care for her/him"]) reflecting how they viewed their role as a caregiver in the previous 7 days. Scale scores range from −5 to 13, with higher values indicating higher levels of strain. Responses to the 13 strain‐related statements were scored 0 (no) or +1 (yes), with a "yes" response indicating greater strain. Responses to the five more positive statements were scored 0 (no) or −1 (yes), with a "yes" response indicating lower strain.
Work Productivity and Activity Impairment Questionnaire: Specific Health Problem
Work Productivity and Activity Impairment Questionnaire: Specific Health Problem (WPAI:SHP) 16 is a six‐item instrument assessing work and activity impairment due to a specific health problem, during the past 7 days. In this study, caregivers responded to each question about the effect that caring for a patient with SBS‐IF had on their ability to work and perform regular activities. The instrument elicits four scores: absenteeism, presenteeism, productivity loss, and regular activity productivity loss.
Absenteeism was measured as a percentage equal to: [hours missed from work due to caregiving/(hours missed due to caregiving + hours actually worked)] × 100. Presenteeism was defined as the degree to which caregiving affected productivity while at work, measured as follows: [(a number on a scale from 0 to 10)/10] × 100.
Productivity loss was measured as a percentage equal to: [absenteeism + (percentage of time worked × presenteeism)] × 100. Regular activity productivity loss was defined as the degree to which caregiving affected productivity while doing regular daily activities, measured as follows: [(a number on a scale from 0 to 10)/10] × 100. WPAI:SHP scores are percentages, with higher values indicating greater percentage impairment and lower work productivity.
Selected items from the Patient‐Reported Outcomes Measurement Information System–Short Forms
The Patient‐Reported Outcomes Measurement Information System–Short Forms (PROMIS‐SF) questionnaires are self‐administered validated short‐form instruments for different disease symptoms or impact areas. 17 , 18 Each short form consists of four questions with five response options; responses are summed to produce a total score for each short‐form instrument. For the purposes of comparison, these scores are then standardized using t‐scores; a score of 50 represents the general population, with a standard deviation (SD) of 10 points.
Three PROMIS‐SF questionnaires were utilized in this survey, relating to fatigue, sleep disturbance, and the ability to participate in social roles and activities. Each question was awarded 1–5 points according to the response option selected, from “not at all” (1 point) to "very much" (5 points). Thus, a range of 1–5 points per question provided an achievable range of 4 (minimum interference) to 20 points (maximum interference). Higher scores indicate greater impacts.
In the PROMIS‐SF Fatigue 4a, 19 the response options range from "not at all" to "very much." The questions address feelings of fatigue (question 1), having trouble starting things because of fatigue (question 2), feeling run‐down (question 3), and feeling fatigued (question 4).
In the PROMIS‐SF Sleep Disturbance 4a, 20 there are two sets of response options, ranging from "very poor" to "very good" or "not at all" to "very much." Questions address sleep quality (question 1), whether sleep was refreshing (question 2), whether there was a problem with sleep (question 3), and whether there was difficulty falling asleep (question 4).
In the PROMIS‐SF Ability to Participate in Social Roles and Activities 4a, 21 the response options range from "never" to "always." The questions address trouble doing regular leisure activities with others (question 1), trouble doing family activities (question 2), trouble doing usual work (question 3), and trouble doing activities with friends (question 4).
Survey items assessing other impacts of caregiving
Additional items addressed by the survey related to impacts of caregiving on employment, productivity, daily activities, relationships, and emotions. Caregivers were also asked about the clinical characteristics of the patient they cared for, amount of support provided, and types of support given.
Translation of instruments and survey items
The WPAI:SHP and CSI were translated from US English into French, German, and Italian by ICON Language Services. Individual survey questions were forward‐ and back‐translated into target languages and assessed for potential ambiguity.
Ethical compliance
Ethical approval was obtained from Salus Institutional Review Board and an institutional review board accredited by the Association for the Accreditation of Human Research Protection Programs. All data were collected in a manner consistent with the principles that have their origin in the Declaration of Helsinki.
Statistical analysis
Data were summarized with descriptive statistics (mean ± SD, median [interquartile range], or percentage).
RESULTS
Patient characteristics and recruitment
Caregivers (N = 121; aged 51 ± 13.7 years; 59% women [Table 1]) were recruited primarily from PAOs (62%) and HCPs (30%). Recruitment was via HCPs in Germany (36 of 36 caregivers) and via PAOs (75 of 85) and other sources (10 of 85) in the other countries. Patients (50 ± 16.4 years; 56% women) of caregivers were primarily the spouse/partner (61.2%), adult son/daughter (19.0%), or parent (9.9%) (Table 1).
TABLE 1.
Characteristics of caregivers of patients with short‐bowel syndrome and intestinal failure
| Characteristic | Overall (N = 121) |
|---|---|
| Age, mean (SD), years | 51.25 (13.70) |
| Gender, n (%) | |
| Male | 50 (41.3) |
| Female | 71 (58.7) |
| Country, n (%) | |
| France | 3 (2.5) |
| Germany | 36 (29.8) |
| Italy | 29 (24.0) |
| UK | 26 (21.5) |
| USA | 27 (22.3) |
| Employment status, n (%) | |
| Employed | 71 (58.7) |
| Unemployed | 10 (8.3) |
| Other | 40 (33.1) |
| Length of caregiving, mean (SD), years | 9.88 (12.53) |
| Relation of patient to caregiver, n (%) | |
| Spouse/partner | 74 (61.2) |
| Child | 23 (19.0) |
| Parent | 12 (9.9) |
| Another family member | 8 (6.6) |
| Friend | 2 (1.7) |
| Mother‐in‐law or father‐in‐law | 1 (0.8) |
| Acquaintance or neighbor | 1 (0.8) |
| Household income, n (%) | |
| <$50,000 or equivalent | 108 (59.7) |
| $50,000–$99,999 or equivalent | 26 (14.4) |
| ≥$100,000 or equivalent | 12 (6.6) |
| I prefer not to answer | 35 (19.3) |
Abbreviation: SD, standard deviation.
Length of care, amount of care, and types of assistance provided
Caregivers provided assistance for a mean of 9.9 ± 12.5 years (Table 1); 77% of caregivers reported providing care 7 days per week (Figure 1A). The majority (57.0%) of caregivers spent up to 3 h providing care each day (Figure 1B). Assistance provided commonly by caregivers included assistance with household‐related tasks (eg, household chores, shopping, and cooking), personal support (eg, spending time together and providing emotional support), and PS‐related activities (eg, connecting PS or changing the port needle) as well as providing transportation to doctors’ appointments (Figure 2).
FIGURE 1.
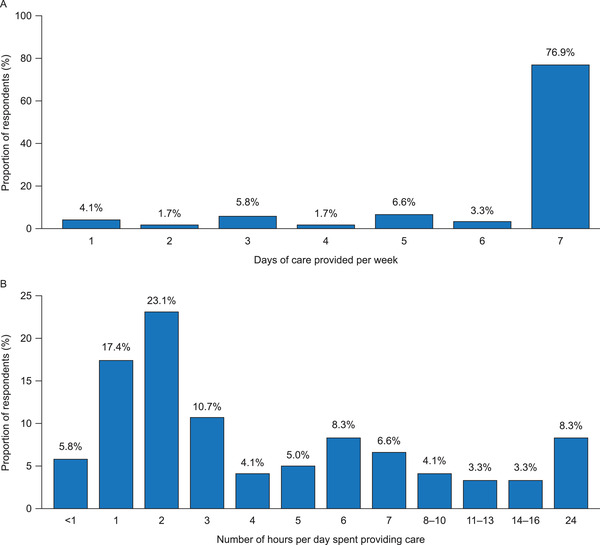
Amount of care given, self‐reported by caregivers of patients with short‐bowel syndrome and intestinal failure (N = 121). (A) Average number of days of care provided per week. (B) Average number of hours of care provided per day (free response)
FIGURE 2.
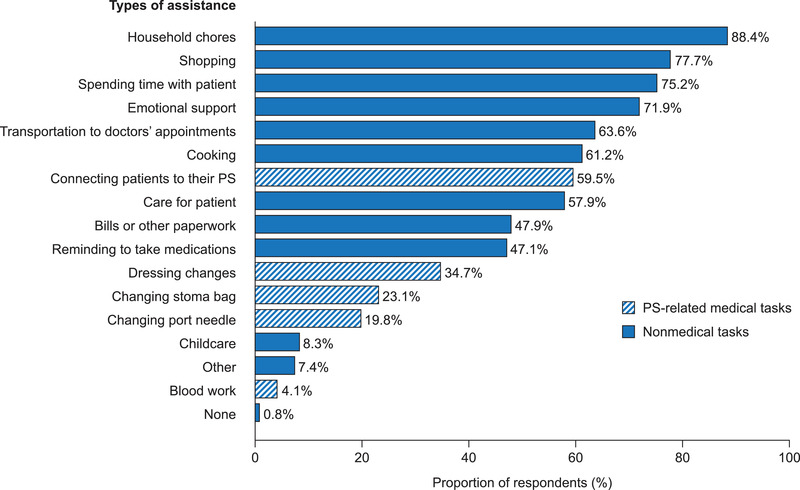
Types of assistance provided to patients with short‐bowel syndrome and intestinal failure by their caregivers (N = 121). PS, parenteral support
Caregiver burden and measures of fatigue
Caregivers experienced a mean reported CSI score of 4.0 (±3.4) (Table 2). Mean CSI scores were about 1 point lower (ie, better) for caregivers with patients receiving <9 L of PS per week than for caregivers with patients receiving at least 9 L of PS per week (see Table 2).
TABLE 2.
CSI and PROMIS‐SF Fatigue, Sleep Disturbance, and Social Roles and Activities in caregivers of patients with short‐bowel syndrome and intestinal failure: Overall scores and stratified by weekly volume of parenteral support
| Instrument | Overall (N = 121) | <9 L/week (n = 39) | 9–<18 L/week (n = 47) | ≥18 L/week (n = 35) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |
| CSI a | 4.01 (3.39) | 4.00 (2.00–7.00) | 3.36 (3.05) | 3.00 (2.00–5.00) | 4.30 (3.59) | 5.00 (2.00–7.00) | 4.34 (3.49) | 5.00 (1.00–7.00) |
| PROMIS‐SF Fatigue 4a b | 10.07 (3.99) | 9.00 (7.00–12.00) | 9.44 (3.55) | 9.00 (7.00–11.00) | 11.28 (4.07) | 11.00 (8.00–15.00) | 9.14 (4.04) | 8.00 (7.00–12.00) |
| PROMIS‐SF Sleep Disturbance 4a b | 13.40 (1.83) | 14.00 (12.00–15.00) | 13.77 (1.88) | 14.00 (12.00–15.00) | 13.34 (1.97) | 13.00 (12.00–15.00) | 13.09 (1.54) | 13.00 (12.00–14.00) |
| PROMIS‐SF Social Roles and Activities 4a b | 14.24 (3.70) | 14.00 (12.00–16.00) | 14.03 (4.06) | 14.00 (12.00–16.00) | 13.83 (3.28) | 13.00 (12.00–16.00) | 15.03 (3.81) | 16.00 (12.00–18.00) |
Abbreviations: CSI, Caregiver Strain Index; IQR, interquartile range; PROMIS‐SF, Patient‐Reported Outcomes Measurement Information System–Short Form; SD, standard deviation.
Range of scores possible for the 18‐item CSI instrument is −5 to 13.
Range of scores possible for the PROMIS‐SF Fatigue 4a, Sleep Disturbance 4a, and Social Roles and Activities 4a is 4 (minimum interference) to 20 points (maximum interference).
Mean scores for PROMIS‐SF questionnaires showed that caregivers experienced levels of fatigue, sleep disturbance, and social impacts similar to those of the general population (Table 2). For PROMIS‐SF Fatigue 4a, caregivers reported a mean score of 10.1 (±4.0), with a t‐score of approximately 53.1, which is close to the general population mean of 50. Mean fatigue scores were consistent across PS volumes (Table 2). For PROMIS‐SF Sleep Disturbance 4a, caregivers reported a mean score of 13.4 (±1.8), with a t‐score of approximately 56.1, a level of sleep disturbance similar to that of the general population. Mean sleep disturbance scores were consistent across PS volumes (Table 2). For PROMIS‐SF Social Roles and Activities 4a, caregivers reported a mean score of 14.2 (±3.7) (Table 2), with a t‐score of approximately 47.8, similar to that of the general population. Mean scores were reasonably consistent across PS volumes (Table 2).
Employment and work productivity
The most common life impact reported by caregivers was not being able to work full‐time (19.8%), with 14.9% reporting that their caregiving had limited their career progression (Figure 3A). Although a majority of caregivers reported being currently employed (58.7%), 21.5% of survey participants reported that they were unemployed either completely (9.1%) or partially (12.4%), owing to their caregiving responsibilities (Figure 3B). Among caregivers, 34 (28.1%) reported the most common impact on work productivity was a reduced number of working hours, and 9.1% reported lowering their career expectations (Figure 4A).
FIGURE 3.
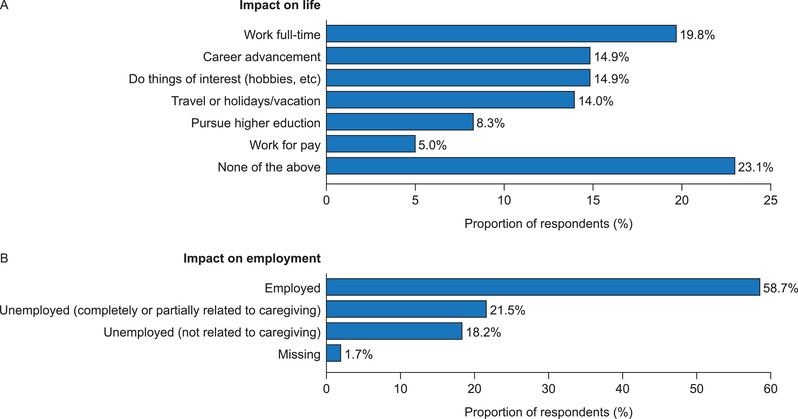
Impact of caregiving on (A) the life of caregivers (caregiving responsibilities have had an impact on plans or ability to do these activities) and (B) the employment of caregivers (employment status, or, if unemployed, whether this is related to caregiving roles) (N = 121)
FIGURE 4.
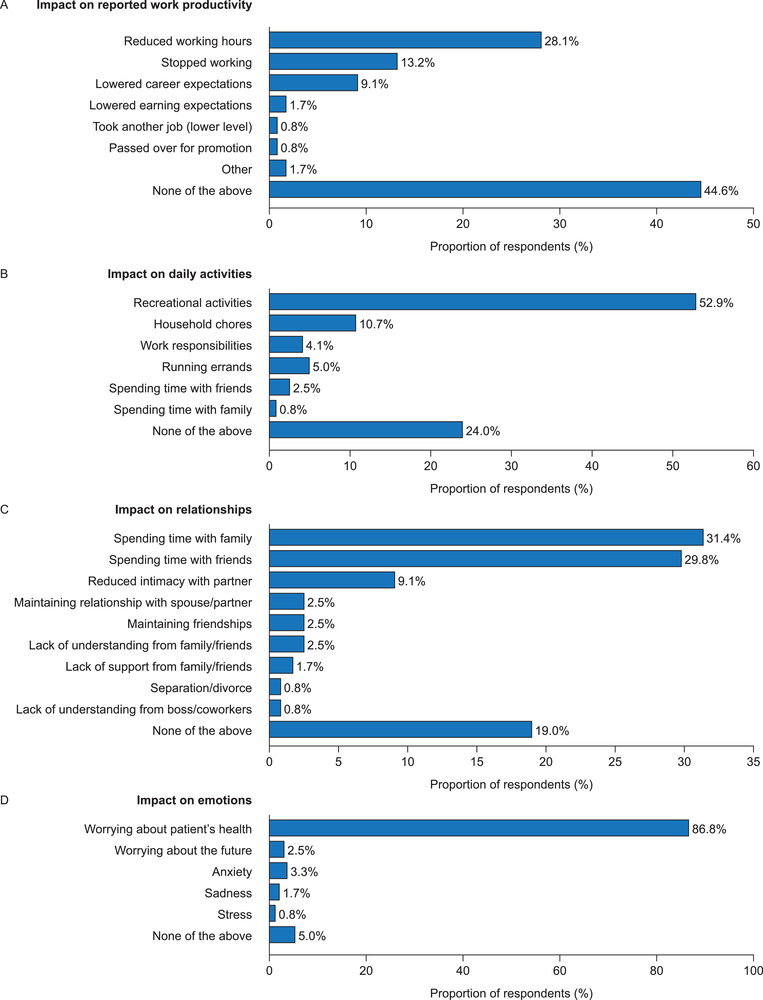
The impact of caregiving on (A) reported work productivity (including work for pay or volunteer work), (B) daily activities (caregiving responsibilities have had an impact on the listed daily activities), (C) relationships (have experienced any of the listed difficulties in relationships with others because of caregiving responsibilities), and (D) emotions (have experienced any of the listed emotional impacts from caregiving responsibilities) (N = 121)
The WPAI:SHP assessment showed that employed caregivers missed 7.2% ± 12.7% of working hours in the preceding week and were present but not productive at work 36.6% ± 23.1% of the time (Table 3). Overall, work productivity loss for the prior week was a mean of 40.0% ± 25.1% (Table 3). Caregivers reported being impaired in their activities for a mean of 35.1% ± 23.8% of their time in the prior week (Table 3).
TABLE 3.
Work Productivity and Activity Impairment Questionnaire: Specific Health Problem in caregivers of patients with short‐bowel syndrome and intestinal failure
| Assessment | Mean (SD) | Median (IQR) |
|---|---|---|
| Absenteeism a (N = 71) | 7.18 (12.74) | 0.00 (0.00–11.11) |
| Presenteeism b (N = 71) | 36.62 (23.11) | 30.00 (20.00–50.00) |
| Work productivity loss c (N = 71) | 40.03 (25.13) | 31.37 (20.00–60.00) |
| Activity impairment d (N = 121) | 35.12 (23.77) | 40.00 (20.00–50.00) |
Abbreviations: IQR, interquartile range; SD, standard deviation.
The percentage of hours of work reported as missed in the previous week.
The percentage of reported time present at work but not productive in the previous week.
The estimated percentage of productivity lost during the previous week.
The percentage of time that caregivers reported being impaired in their activities in the previous week.
Other impacts of caregiving
In total, 121 caregivers reported impacts across various areas owing to their responsibilities as caregivers of a patient with SBS‐IF. In the context of daily activities, 64 (53%) respondents reported having been impacted in their ability to participate in recreational activities (Figure 4B). In the context of relationships, 38 (31%) and 36 (30%) of caregivers reported that they had experienced difficulty spending time with family and friends, respectively (Figure 4C). When asked about the impact of caregiving on emotions, most caregivers (105 [87%]) reported worrying about the health of the patient (Figure 4D).
DISCUSSION
Although there has been an interest in the impact of PS on caregivers for nearly 30 years, 22 there have been very few studies that have looked specifically at the impact of PS on caregivers of patients with SBS‐IF. 13 , 23 The studies that have been conducted typically used low sample sizes and were limited to one country. The use of an online survey format enabled a robust sample size for a study of caregivers of patients with a rare disease and a diverse geographic scope.
This study showed that although caregivers show fatigue, sleep disturbance, and satisfaction with social roles and activities that are similar to those of the general population, they experience some strain, with markedly reduced productivity. Furthermore, caregiving responsibilities may limit time available for regular (daily) recreational activities, and many caregivers report having experienced difficulties spending time with family and friends.
The CSI scores reported in Table 2 are in line with another recent report of caregivers of patients with SBS‐IF. 13 The lower mean overall score reported here (4 vs 6), however, may be reflective of the use of the 18‐item version (score range: −5 to 13) that incorporates five strain‐reducing questionnaire items vs the 13‐item version (score range: 0–13) used in the Beurskens‐Meijerink study. 13
It is helpful to place results in the context of caregiving for other chronic illnesses, particularly ones that involve lengthy procedures. An obvious consideration would be home dialysis in end‐stage renal disease, which requires patients to undergo a multihour, life‐sustaining procedure at home multiple times a week. The burden experienced by caregivers of patients receiving dialysis has been explored in the literature 24 , 25 ; however, it does not appear to have been assessed using the measures reported in this study, making direct comparisons difficult. In terms of caregiving assistance given, the prominence of household chores and personal support is consistent with findings in caregivers of patients receiving renal dialysis and caregivers of older adults. 25 , 26 The lower reported frequency of providing assistance with PS‐related, medical‐related tasks, such as connecting the patients to their PS, compared with assistance with nonmedical tasks is worth noting; however, direct comparisons with medical‐related tasks in other chronic conditions are difficult to make. It may be that additional nursing support was available for patients to help with PS‐related medical tasks or that many patients are carrying out PS‐related medical tasks themselves; however, data relating to additional nursing care and patient self‐care were not collected as part of this study.
Patients receiving a higher volume of PS have been shown to report a lower QoL. 27 Interestingly, the CSI data stratified by PS volume do not appear to show any impact of PS volume on caregivers’ strain. This may be because the CSI is not sensitive enough to detect a difference, or it may be that any amount of PS is sufficient to cause a strain on caregivers (owing to the rigorous steps that must be taken to limit infection, oversupplementing, or other life‐threatening complications). For the PROMIS‐SF items included in the survey, caregivers had scores that were broadly similar to those of the general population, with no clear effect of patient PS volume on fatigue, sleep disturbance, or social roles.
This study is one of the first times that the WPAI:SHP has been applied to caregivers of patients with SBS‐IF. 23 Absenteeism was low in this study, with a median score of 0. However, rates of reported presenteeism are substantial and comparable to or higher than in similar studies involving primary caregivers of adult patients with conditions such as dementia and status epilepticus. 28 , 29 The low levels of absenteeism may reflect the recall period (7 days); however, longer recall periods are not necessarily associated with capturing a greater proportion of absences. 30 Absenteeism may also be influenced by levels of social support available, which may vary by country. 30 Alternatively, caregivers may have adjusted their work life around their caring responsibilities, mitigating absences from work. Nevertheless, the level of reported presenteeism and impact on career advancement suggest important impacts on work life, which may have lifelong effects on the caregiver and their family. 31 Interestingly, the WPAI:SHP scores reported by caregivers of patients with SBS‐IF are almost directly comparable to the patient scores reported by employed patients with myeloproliferative neoplasms (a constellation of chronic myeloproliferative disorders in which patients experience increased risk of mortality and symptoms such as fatigue, sleep disturbance, and concentration problems). 32 , 33 This suggests that providing care to a patient with SBS‐IF has similar impacts on productivity as experiencing a chronic disease.
Patient‐reported outcome (PRO) instruments that have been adapted to assess caregiver burden, such as the CSI, WPAI:SHP, and PROMIS‐SF subitems, may not be widely available to HCPs in clinical practice. Furthermore, standards for interpreting results are limited or not established. However, PRO instruments in general may be most helpful to individual caregivers as a means of prompting conversations around caregiver burden. In the absence of specific PRO instruments tailored for use in clinical practice, inquiries by HCPs about caregiver welfare are to be encouraged and may lead to productive conversations with the potential for improved outcomes for caregiver and patient alike. 34 During and after initiation of PS, conversations with caregivers about the potential challenges that PS will present to them and to their patient may be invaluable in promoting QoL for caregiver and patient, owing to the established relationship between expectation and QoL. 35
Limitations of study
As with any survey, responses were self‐reported, with no third‐party confirmation, which could lead to recall bias. Although the study's cross‐sectional nature may miss fluctuations in caregiving (based upon patient needs) that might be picked up in a longitudinal study, cross‐sectional surveys present slices of life experiences 36 that are likely to approximate to a longitudinal experience. There were few options for caregivers to provide free responses to survey questions. Consequently, it is possible that granularity was lost from these responses. Owing to the route of recruitment (referrals from PAOs, HCPs, online patient panels, and social media outreach), there may have been an element of selection bias; those caregivers who self‐selected to participate may have had more positive or more negative experiences with caregiving to share than caregivers who may have not been willing to participate. Finally, there are very few similar studies in this area to draw from; additional studies will strengthen confidence in this study's findings.
CONCLUSIONS
Informal caregivers to patients with SBS‐IF are predominantly family members (spouses/partners, adult children, or parents of adult children). Types of assistance caregivers provide include helping with household chores, shopping, emotional support, and connecting the patient to their PS. Most caregivers devote up to 3 h each day to caregiving activities, which impacts their employment, specifically their ability to work full‐time; leads to reductions in work productivity; and hinders career advancement. Whereas they do not experience physical impairments as a result of their caregiving role, caregivers experience emotional strain and negative daily impacts, including reduced time for leisure activities or hobbies, reduced time with family and friends, and worries about the patient's health.
Based on these findings, physicians and other HCPs should be encouraged to have open discussions with caregivers and patients around the burden and impact of caregiving, including ways to alleviate these strains (eg, by seeking additional professional support). HCPs are encouraged to collate country‐specific resources for caregivers, to facilitate discussions around coping with the burden of care. One example of a resource specifically for caregivers of patients receiving PS is the Oley Foundation website; 37 links to additional country‐specific patient groups are hosted by the website for the International Alliance of Patient Organisations for Chronic Intestinal Failure and Home Artificial Nutrition (PACIFHAN). 38 It should be noted that professional care available to support caregivers may be limited by the financial circumstances of the caregiver and/or constraints of country‐specific social care systems. 39 , 40 These data provide insight to payers and associated decision makers about providing and funding/reimbursing additional caregiving support to patients with SBS‐IF. Further research may be needed to support measures that could alleviate the burden placed currently on these unpaid, informal caregivers. 40
CONFLICT OF INTERESTS
Palle B. Jeppesen has has received consultancy fees from Albumedix A/S; ArTara Therapeutics; Baxter; Coloplast; Ferring Pharmaceuticals; Fresenius Kabi; GLyPharma Therapeutic; Naia Pharmaceuticals; Novo Nordisk Foundation; Shire, a Takeda company; Therachon; VectivBio AG; and Zealand Pharma. Kristina Chen, at the time of the study, was an employee of Shire Human Genetic Therapies, Inc, a Takeda company. Ryan Murphy and Saeid Shahraz are employees of ICON plc, contracted by Takeda to conduct the survey. Bridgett Goodwin is an employee of Takeda Development Center Americas, Inc, and a stockholder of Takeda Pharmaceutical Company Limited.
FUNDING INFORMATION
This study was funded by Shire Human Genetic Therapies, Inc, a Takeda company (Cambridge, MA, USA), and was conducted by ICON plc. Editorial support was funded by Shire International GmbH, a Takeda company (Zurich, Switzerland).
AUTHOR CONTRIBUTIONS
All authors contributed to the conception and design of the research and to the analysis and interpretation of the data. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
ACKNOWLEDGMENTS
This study was funded by Shire Human Genetic Therapies, Inc, a Takeda company (Cambridge, MA, USA), and was conducted by ICON plc. Editorial support, funded by Shire International GmbH, a Takeda company (Zurich, Switzerland) was provided by Richard Pye, PhD, of Oxford PharmaGenesis (Oxford, UK).
Jeppesen PB, Chen K, Murphy R, Shahraz S, Goodwin B. Impact on caregivers of adult patients receiving parenteral support for short‐bowel syndrome with intestinal failure: A multinational, cross‐sectional survey. Journal of Parenteral and Enteral Nutrition. 2022;46:00 905‐914 10.1002/jpen.2248.
Present address
Kristina Chen, PharmD, MS, Pfizer, Cambridge, Massachusetts, USA.
REFERENCES
- 1. Pironi L, Arends J, Bozzetti F, et al. ESPEN guidelines on chronic intestinal failure in adults. Clin Nutr. 2016;35(2):247‐307. [DOI] [PubMed] [Google Scholar]
- 2. Jeppesen PB. Spectrum of short bowel syndrome in adults: intestinal insufficiency to intestinal failure. JPEN J Parenter Enteral Nutr. 2014;38(1 Suppl):8S‐13S. [DOI] [PubMed] [Google Scholar]
- 3. Pironi L, Arends J, Baxter J, et al; Home Artificial Nutrition & Chronic Intestinal Failure; Acute Intestinal Failure Special Interest Groups of ESPEN. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin Nutr. 2015;34(2):171‐180. [DOI] [PubMed] [Google Scholar]
- 4. Buchman AL. Etiology and initial management of short bowel syndrome. Gastroenterology. 2006;130(2 Suppl 1):S5‐S15. [DOI] [PubMed] [Google Scholar]
- 5. Kelly DG, Tappenden KA, Winkler MF. Short bowel syndrome: highlights of patient management, quality of life, and survival. JPEN J Parenter Enteral Nutr. 2014;38(4):427‐437. [DOI] [PubMed] [Google Scholar]
- 6. Pironi L. Definitions of intestinal failure and the short bowel syndrome. Best Pract Res Clin Gastroenterol. 2016;30(2):173‐185. [DOI] [PubMed] [Google Scholar]
- 7. Nightingale J, Woodward JM; Small Bowel and Nutrition Committee of the British Society of Gastroenterology. Guidelines for management of patients with a short bowel. Gut. 2006;55 Suppl 4(Suppl 4):iv1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hofstetter S, Stern L, Willet J. Key issues in addressing the clinical and humanistic burden of short bowel syndrome in the US. Curr Med Res Opin. 2013;29(5):495‐504. [DOI] [PubMed] [Google Scholar]
- 9. Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, SJ O'Keefe. Randomised placebo‐controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60(7):902‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Winkler MF, Smith CE. Clinical, social, and economic impacts of home parenteral nutrition dependence in short bowel syndrome. JPEN J Parenter Enteral Nutr. 2014;38(1 Suppl):32S‐37S. [DOI] [PubMed] [Google Scholar]
- 11. Fuglsang KA, Brandt CF, Scheike T, Jeppesen PB. Hospitalizations in patients with nonmalignant short‐bowel syndrome receiving home parenteral support. Nutr Clin Pract. 2020;35(5):894‐902. [DOI] [PubMed] [Google Scholar]
- 12. Smith CE. Quality of life in long‐term total parenteral nutrition patients and their family caregivers. JPEN J Parenter Enteral Nutr. 1993;17(6):501‐506. [DOI] [PubMed] [Google Scholar]
- 13. Beurskens‐Meijerink J, Huisman‐de Waal G, Wanten G. Evaluation of quality of life and caregiver burden in home parenteral nutrition patients: a cross sectional study. Clin Nutr ESPEN. 2020;37:50‐57. [DOI] [PubMed] [Google Scholar]
- 14. Robinson BC. Validation of a Caregiver Strain Index. J Gerontol. 1983;38(3):344‐348. [DOI] [PubMed] [Google Scholar]
- 15. Al‐Janabi H, Frew E, Brouwer W, Rappange D, Van Exel J. The inclusion of positive aspects of caring in the Caregiver Strain Index: tests of feasibility and validity. Int J Nurs Stud. 2010;47(8):984‐993. [DOI] [PubMed] [Google Scholar]
- 16. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353‐365. [DOI] [PubMed] [Google Scholar]
- 17. Cella D, Yount S, Rothrock N, et al; PROMIS Cooperative Group. The Patient‐Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 Suppl 1):S3‐S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cella D, Gershon R, Lai JS, Choi S. The future of outcomes measurement: item banking, tailored short‐forms, and computerized adaptive assessment. Qual Life Res. 2007;16(Suppl 1):133‐141. [DOI] [PubMed] [Google Scholar]
- 19. Lai J‐S, Cella D, Choi S, et al. How item banks and their application can influence measurement practice in rehabilitation medicine: a PROMIS fatigue item bank example. Arch Phys Med Rehabil. 2011;92(10):S20‐S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buysse DJ, Yu L, Moul DE, et al. Development and validation of patient‐reported outcome measures for sleep disturbance and sleep‐related impairments. Sleep. 2010;33(6):781‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hahn EA, DeWalt DA, Bode RK, et al; PROMIS Cooperative Group. New English and Spanish social health measures will facilitate evaluating health determinants. Health Psychol. 2014;33(5):490‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith CE, Moushey L, Ross JA, Gieffer C. Responsibilities and reactions of family caregivers of patients dependent on total parenteral nutrition at home. Public Health Nurs. 1993;10(2):122‐128. [DOI] [PubMed] [Google Scholar]
- 23. Ballinger R, Macey J, Lloyd A, Chen K. Pam13 impact on carers of adult patients receiving parenteral support for short bowel syndrome‐associated intestinal failure. Value in Health. 2019;22(3):S417. [Google Scholar]
- 24. Gilbertson EL, Krishnasamy R, Foote C, Kennard AL, Jardine MJ, Gray NA. Burden of care and quality of life among caregivers for adults receiving maintenance dialysis: a systematic review. Am J Kidney Dis. 2019;73(3):332‐343. [DOI] [PubMed] [Google Scholar]
- 25. Hoang VL, Green T, Bonner A. Informal caregivers' experiences of caring for people receiving dialysis: a mixed‐methods systematic review. J Ren Care. 2018;44(2):82‐95. [DOI] [PubMed] [Google Scholar]
- 26. Wolff JL, Spillman BC, Freedman VA, Kasper JD. A national profile of family and unpaid caregivers who assist older adults with health care activities. JAMA Intern Med. 2016;176(3):372‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nordsten CB, Molsted S, Bangsgaard L, et al. High parenteral support volume is associated with reduced quality of life determined by the Short‐Bowel Syndrome Quality of Life Scale in nonmalignant intestinal failure patients. JPEN J Parenter Enteral Nutr. 2021;45(5):926‐932. [DOI] [PubMed] [Google Scholar]
- 28. Igarashi A, Fukuda A, Teng L, Ma FF, Dorey J, Onishi Y. Family caregiving in dementia and its impact on quality of life and economic burden in Japan‐web based survey. J Mark Access Health Policy. 2020;8(1):1720068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. King‐Stephens D, Wheless J, Krogh C, et al. Burden of disease in patients with a history of status epilepticus and their caregivers. Epilepsy Behav. 2020;112:107374. [DOI] [PubMed] [Google Scholar]
- 30. Zhang W, Bansback N, Boonen A, Young A, Singh A, Anis AH. Validity of the Work Productivity and Activity Impairment Questionnaire–General Health version in patients with rheumatoid arthritis. Arthritis Res Ther. 2010;12(5):R177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Piamjariyakul U, Yadrich DM, Ross VM, Smith CE, Clements F, Williams AR. Complex home care: part 2‐ family annual income, insurance premium, and out‐of‐pocket expenses. Nurs Econ. 2010;28(5):323‐329. [PMC free article] [PubMed] [Google Scholar]
- 32. Yu J, Parasuraman S, Paranagama D, et al. Impact of myeloproliferative neoplasms on patients' employment status and work productivity in the United States: results from the living with MPNs survey. BMC Cancer. 2018;18(1):420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bittencourt RI, Vassallo J, Chauffaille Mde L, et al. Philadelphia‐negative chronic myeloproliferative neoplasms. Rev Bras Hematol Hemoter. 2012;34(2):140‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith CE, Piamjariyakul U, Yadrich DM, Ross VM, Gajewski B, Williams AR. Complex home care: part III–economic impact on family caregiver quality of life and patients' clinical outcomes. Nurs Econ. 2010;28(6):393‐399. 414. [PMC free article] [PubMed] [Google Scholar]
- 35. Carr AJ, Gibson B, Robinson PG. Measuring quality of life: is quality of life determined by expectations or experience? BMJ. 2001;322(7296):1240‐1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Munnangi S, Boktor SW. Epidemiology of study design. In: StatPearls. StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 37. The Oley Foundation Caregivers/Parents. . Accessed June 25, 2021. https://oley.org/page/CaregiverParents
- 38.International Alliance of Patient Organisations for Chronic Intestinal Failure and Home Artificial Nutrition. Accessed June 25, 2021. http://pacifhan.org/
- 39. Piamjariyakul U, Ross VM, Yadrich DM, Williams AR, Howard L, Smith CE. Complex home care: part I–Utilization and costs to families for health care services each year. Nurs Econ. 2010;28(4):255‐263. [PMC free article] [PubMed] [Google Scholar]
- 40. Belza C, Wales PW. The Unsung Heroes: health‐related quality of life of caregivers and patients in pediatric intestinal failure. J Pediatr Gastroenterol Nutr. Accepted manuscript. Published online June 1, 2021. doi:10.1097/MPG.0000000000003193. 2021;73(3):291. [DOI] [PubMed] [Google Scholar]


