Abstract
The number of patients with myelofibrosis (MF) undergoing an allogeneic hemopoietic stem cell transplantation (HSCT) is increasing: in the analysis of the European Group for Blood and Marrow Transplantation (EBMT) the number of MF has increased from 515 in 2014 to 748 in 2018 . This reflects the fact that HSCT is currently the only curative treatment, capable of inducing prolonged disease‐free survival. Nevertheless, several problems prevent more patients from undergoing an allogeneic HSCT: we will be discussing indications for HSCT, comorbidities, splenomegaly, older age and disease phase. Donor type and stem cell source are less of a problem. Several transplant platforms exist, including different strategies for graft versus host disease (GvHD) prophylaxis, Age tailored conditioning regimens need to be implemented, to allow older and fragile patients to undergo an allogeneic HSCT.
1. INTRODUCTION
Myelofibrosis (MF) is a Philadelphia chromosome‐negative myeloproliferative neoplasm that can be primary or secondary to polycythemia vera or essential thrombocythemia. Its clinical course may vary from an indolent disease evolving over decades, but generally progressive to a disorder with severe cytopenia, resulting in transfusion requirement and eventually evolving to an acute phase, resembling acute leukemia. Several prognostic scoring systems have been developed over the past years, to identify patients who are likely to progress, and are therefore at higher risk of morbidity and mortality. 1 , 2 , 3 , 4 Despite the approval of JAK inhibitors and various other exciting non‐transplant treatments in development, allogeneic hemopoietic stem cell transplantation (HSCT) remains at present the only curative therapy for patients with MF, and prognostic scores are used to select patients for the procedure, possibly including also molecular markers of the disease. 3 The number of MF patients undergoing an allogeneic HSCT annually is increasing. In a recent analysis European Group for Blood and Marrow Transplantation (EBMT) reported the number of MF has increased from 515 in 2014 to 748 in 2018. 5 This reflects the fact that HSCT has become safer with reduction in non‐relapse mortality (NRM) over the years, making the choice of an HSCT less morbid for the patient and the non‐transplant hematologist. However, several challenges remain, and prevent more patients from undergoing an allogeneic HSCT. Selecting a patient requires careful consideration of a multitude of factors before proceeding to HSCT. We will be discussing indications for HSCT, as well as impact of comorbidities, splenomegaly, older age, and disease phase. Donor type and stem cell source are currently less of a problem, although matching for HLA remains an important issue. Several transplant platforms exist, including different strategies for graft versus host disease (GvHD) prophylaxis. Age‐tailored conditioning regimens need to be implemented, to allow older and fragile patients to undergo an allogeneic HSCT.
2. ELIGIBILITY OF ALLOGENEIC STEM CELL TRANSPLANTATION
Prior to HSCT in myelofibrosis (MF) patients, a suitable donor is identified. This is followed by a thorough assessment to ensure a patient has sufficient organ function reserve to withstand physiological stress from the transplant process due to conditioning regimen, graft versus host disease (GVHD) prophylaxis, and prolonged cytopenia. In addition, a very important component is thorough psychosocial evaluation. Cardiac, pulmonary, hepatic, and renal function are assessed and generally, each institution has predefined criteria to proceed to HCT. Cardiac function is generally measured by echocardiogram or MUGA scan and EF of 50% is generally the cutoff. Special attention is needed to pulmonary artery systolic pressure (PASP). We recently reported pulmonary hypertension (PASP > 24 mm Hg) significantly associated with inferior OS 58.9% vs 88.8% P = 0.024) mainly due to increase NRM (21.6% vs. 7.1% p = 0.007). 6 Pulmonary function is assessed by complete pulmonary function studies with the adequate pulmonary function defined as DLCO and FEV1 > 50%. Renal function is generally assessed using estimated GFR or actual creatine clearance of > 60 ml/min. Hepatic function measurement to ensure bilirubin is equal to less than two times the upper limit of normal unless the patient has Gilbert's syndrome.
So, MF is a disease of the elderly with a median age at the time of diagnosis of 67 years. 7 There is generally no defined upper limit of age for HSCT. However, as organ function and comorbidities are more prevalent in the elderly, thus historically HSCT was offered to patients with age < 60 years when myeloablative conditioning (MAC) regimen was more frequently used. Over the last couple of decades use of reduced‐intensity conditioning (RIC) has improved access to patients to this life‐saving and curative procedure. Kroger et al. reported in patients with a median age of 67 years (65–74 years) outcome was comparable to other reports with 6 years estimated progression‐free survival and OS of 60% and 64%; one‐year NRM was 21%. 8 This study predominantly included fludarabine and busulfan (FB) RIC. Another study using melphalan based conditioning regimen in patients > 70, included 53 patients with any diagnosis including six MF patients: after 31 months follow up 2‐year OS, PFS, NRM was respectively 69%, 64%, and 17%. 9 Thus, generally, higher chronological age is not prohibitive of the decision of HSCT and rather physiological age with thorough organ function assessment is used to consider the eligibility of SCT.
In the US, the majority of myelofibrosis patients are Medicare beneficiaries (age 65 or older) as the median age at the time of diagnosis is 67 years. Currently, the Center of Medicare and Medicaid Services (CMS) has determined HSCT will be covered for its beneficiaries using the Coverage with Evidence Development (CED) paradigm. This requires beneficiaries with DIPSS‐plus intermediate‐2 or high risk and participating in an approved prospective clinical study which must address if HSCT improve outcomes compare to non‐transplant treatment options.
3. IMPACT OF SPLENOMEGALY
Splenomegaly is a hallmark of myelofibrosis. However, massive splenomegaly has been associated with poor HCT outcomes due to increased risk of poor graft function, and graft failure.10, 11 This may be due to early pooling of CD34+ cells in the spleen and homing defect in the bone marrow due to loss of vascular cell adhesion molecule 1 (VCAM‐1), a cell adhesion molecule. 12 A detailed analysis of the impact of spleen size and splenectomy was done in a recent study by EBMT that shed light on the impact of spleen and spleen size on the HCT outcome. 13 Patients with less bulky spleen had faster engraftment with 28 days cumulative incidence of ANC recovery was 87% in spleen < 5 cm compared to 81%‐82% in larger size group. It was noted that increasing spleen size at the time of HCT was significantly associated with worse OS. This finding was related to an excess of NRM noted in larger spleen size > 15 cm below LCM, however, there was no difference in a relapse in different spleen sizes. Also effect of spleen size on OS was no longer significant in MAC population in contrast in patients undergoing RIC where OS was reduced in larger spleen size compared to smaller spleen size. In a comparison of spleen > 15 cm with splenectomized patients, the 36 months OS was 59% in splenectomized compared to 49% in non‐splenectomized patients. There was although a mild increase in relapse among splenectomized patients. Thus, there may be some role of spleen for GVL.
4. CONDITIONING REGIMENTS
The role of the conditioning regimen is to reduce the neoplastic clone and provide immunosuppression to allow successful engraftment of donor stem cells. A regimen can be myeloablative (MAC), reduced intensity (RIC), or non‐myeloablative regimen (NMA). 14 Within each category, there is significant heterogeneity of intensity. There has been significant improvement in making myeloablative and non‐myeloablative regimens safer. Optimal conditioning regimens are not defined in patients with myelofibrosis. Direct comparison in prospective trials is not available to understand the exact impact of outcomes. However, several medium to large retrospective studies are available. For patients with lower performance status and age older than 60 years, generally, RIC conditioning is used. 15 A retrospective study compared fludarabine and busulfan (FB) with fludarabine and melphalan (FM), and showed FM to have better control of disease but more NRM mainly due to GVHD. In this group, lower GVHD may have been due to anti‐thymocyte globulin (ATG) in the FB group and hence improved NRM. To improve FM, the dose was lowered from traditional 140 to 100 mg in AML, which showed lower NRM which was seen in patients with lower PS. 16 In AML it was shown to be better than FB and FM 140. A recent study showed a 5‐year OS of 65% in 110 MF patients after a median follow‐up of 64 months. The median age was relatively higher, compared to other studies with a median age of 59 years. Below is the list of some of the retrospective transplant outcomes reports in reverse chronological order. It is to note that earlier studies used more MAC and had a younger age at the time of SCT, compared to more recent reports. In addition, the OS has improved and NRM reduced due to improvement in conditioning regimens, as targeted busulfan, and also supportive care during and after SCT Table 1.
TABLE 1.
Survival and non‐relapse mortality of selected studies
| Study | Years | N | Conditioning Regimen | Median Age | Median follow‐up | OS% (years) | NRM |
|---|---|---|---|---|---|---|---|
| Ali et al. | 2004–2017 | 110 | RIC Flu/Mel | 59 | 64 Months | 65%(5) | 17% |
| Kroger et al. | 2002–2007 | 103 | RIC Flu/Bu | 55 | 33 Months | 67% (5) | 16% |
| Chiusolo et al. | 2000−2019 | 120 | RIC and MAC | 56 | 22 Months | 62% (5) | 22% |
| Gupta et al. | 1997–2010 | 233 | RIC | 55 | 50 Months | 47% (5) | 24% |
| Robin et al. | 1997–2008 | 147 | RIC and MAC | 53 | 35 Months | 39% (4) | 39% |
| Lussana et al. | 1994–2010 | 250 | RIC and MAC | 56 | 13 Months | 55% (3) | 28% |
| Scott et al. | 1990–2009 | 170 | RIC and MAC | 51 | 71 Months | 57% (5) | 34% |
| Ballen et al. | 1989–2002 | 289 | RIC and MAC | 47 | 41–46 Months | 37%‐30% (5) | 35%‐50% |
| Patriarca et al. | 1986–2006 | 100 | RIC and MAC | 49 | 34 Months | 42% (3) | 43% |
5. PREDICTING OUTCOME
Outcome prediction models in the non‐transplant setting have been discussed extensively in earlier updates. 17 Most of these prognostic scoring systems have also been shown to predict outcomes in MF patients undergoing HSCT. Thus, DIPSS was studied and showed an increase in NRM (HR 3.4) and overall mortality (HR 4.11) in high‐risk disease when it was compared to low‐risk disease. After a median follow up of 5.9 years, the median survival for low and intermediate‐1 did not reach and was 7 years for intermediate‐2 and only 2.5 years for high‐risk patients respectively. 18 In addition, DIPSS‐plus classification which included in addition to DIPSS variables also cytogenetics, thrombocytopenia, and transfusion dependency predicted outcomes of myelofibrosis patients undergoing HCT. Low/int‐1 have better survival with 5‐year OS of 78% compared to 5‐year OS of high risk of only 35%. 19
More recently we reported the utility of MIPSS70 to predict HCT outcomes. Intermediate risk showed better OS (HT, 0.49; p = 0.039) when compared with high risk. Similarly, pre‐HCT MIPSS70+ v2.0 better OS for intermediate (HR0.29) compared to high risk, much lower OS when VHR were compared to HR (5.05). 20 A new risk scoring combing clinical‐molecular characteristics for MF patients undergoing HCT has been devised. It combines age >57, KPS <90, plt <150, WBC >25, HLA mismatched donor, ASXL1 mutation, and non‐CALR/MPL driving mutation. Using them it was divided into four risk groups, low (score 0–2), intermediate (score 3–4), high (score 5), and very high (score > 5). The 5‐year OS was 83%, 64%, 37%, and 22% respectively. 21
Transplant is therefore offered to eligible patients with the available donor for Intermediate, HR, and VHR population in various risk categories. Risk vs benefits of transplant is individually discussed with patients.
6. STEM CELL SOURCE AND STEM CELL DONORS
We and others have reported better outcomes of matched sibling donors in MF. 22 Outcomes of patients undergoing transplant from matched sibling donor (MSD) is better than the matched unrelated donor (MUD) and mismatched unrelated donor (mMUD). Gupta et al. reported in a CIBMTR study of 233 patients with PMF undergoing HCT, that in multivariate analysis 5‐year OS of MSD, MUD, and mMUD was 56%, 48%, and 34% respectively. 23 In another study we reported similar findings with 5‐year OS was 75%, 63%, and 29% in MSD, MUD, and mMUD respectively. 13 As haploidentical transplants are being commonly performed for various hematological malignant and non‐malignant conditions, it is also being evaluated for MF patients requiring transplant but who do not have suitable HLA‐matched donors. In a recent study, Kunte et al. reported estimates of 2 years OS, RFS, and NRM of 69%, 52%, and 21%. 24 Similar findings were reported by the EBMT group. 25 However, there was a higher incidence of graft failure. Thus, in summary, MSD is best option if available followed by MUD and mMUD with more research needed on alternative donors including haploidentical.
Most studies reporting HCT outcomes have employed predominantly PBSC as the stem cell source. There are no studies directly comparing the outcomes of BM vs PBSC.
7. GVHD PROPHYLAXIS
Graft versus host disease (GvHD) prophylaxis is a crucial component of the transplant platform. Two major strategies can be chosen: ex vivo manipulation of the graft, or in vivo treatment of the recipient. The former may be less ideal for patients with MF because it combines two predictors of slow engraftment and/or rejection: T cell depletion per se, 26 and marrow fibrosis. 27 The second option is in vivo treatment of the recipient, by administration of one or more immunosuppressive drugs, which results in a protective effect against both acute and chronic GvHD. The standard GvHD prophylaxis is a combination of a calcineurin inhibitor (CNI) ‐either, tacrolimus or cyclosporine‐ and methotrexate (CNI + MTX); in Europe, this is combined with anti‐thymocyte globulin (ATG) for patients undergoing an unrelated donor transplant. 28 A transient hyperbilirubinemia has been reported in MF patients receiving ATG, with no effect on non‐relapse mortality (NRM). 29 Other regimens for GvHD prophylaxis in patients with MF, have been the combination of ATG and post‐transplant cyclophosphamide (PTCY), 30 ruxolitinib and PTCY, 31 peritransplant ruxolitinib, 32 and tacrolimus‐sirolimus, with or without methotrexate (MTX). 33 In these studies, the risk of acute GvHD grade III‐IV ranges between 10% and 60%, and the risk of moderate/severe chronic GvHD, requiring treatment, between 15% and 60%. However, these figures per se are not instructive of a global outcome. The success of allogeneic HSCT depends on our ability to mitigate GvHD, without losing the GvL effect: this is why the transplant platform, including the conditioning regimen, the stem cell source, and GvHD prophylaxis need to be considered in the equation. Patients with MF are older, and appropriate protection against G HD should be a priority.
8. HEMATOLOGIC RECONSTITUTION AND GRAFT FAILURE
Patients with MF are at higher risk of slow hematologic recovery: in 1311 patients allografted 2000–2020 (unpublished), of whom 152 with myelofibrosis and 1159 with other hematologic malignancies, the median platelet count on day 30 after HSCT was 39 vs 102 × 109/L respectively (p < 0.001). The difference was evident also when selecting for patients over the age of 60 years, with a median platelet count of 30 × 109/L for MF patients and 71 × 109/L for other malignancies (p = 0.001). On day 90 after HSCT, in the same population, the proportion of patients with platelet counts less than 20 × 109/L, was 34% versus 17% respectively (p < 0.001). These data highlight the problem of patients with myelofibrosis: very slow recovery, as compared to other diagnoses, with a significant number of patients remaining transfusion‐dependent months after HSCT. The spleen volume has been reported to correlate with poor recovery: in a GITMO study on MF, 34 patients with splenomegaly before HSCT had significantly slower neutrophil and platelet recovery (HR, 0.51, p = 0.03 and HR, 0.41, p = 0.005). In the same study patients, splenectomized had significantly faster neutrophil and platelet recovery. The median time to neutrophil and platelet recovery, was 19 and 20 days for patients with splenomegaly, significantly longer when compared to the recovery of MF patients, who were either splenectomized or without spleen enlargement before HSCT (16 and 14 days, respectively; p < 0.001).34 Therefore, one needs to acknowledge that recovery is slow, and, for patients with a large spleen, splenectomy or splenic irradiation may be considered. Whether the use of TPO‐mimetics, such as eltrombopag, would accelerate hematologic recovery, needs to be proven in a prospective study.
Poor graft function (PGF), defined as transfusion dependence with complete donor chimerism, can be rescued either with a boost infusion of donor‐derived CD34 selected cells, 35 or with a prolonged course of a thrombopoietin mimetic drug 36 : trilineage hematologic recovery can be achieved in the majority of patients, but it requires several months to occur.
9. DISEASE MARKERS AND CHIMERISM POST HSCT
Driver mutations (JAK2, MPL, or CALR), can be used to assess minimal or measurable residual disease (MRD) after an allogeneic HSCT and may correlate with donor chimerism. The conditioning regimen appears to have a relevant role in determining the degree of donor chimerism, and thus the risk of relapse of myelofibrosis: in a randomized study, a busulfan‐fludarabine regimen (BU‐FLU) was compared to a thiotepa‐fludarabine regimen.34 The proportion of patients with full donor chimerism on day +100 was 24% for the BU‐FLU regimen and 68% for the THIO‐FLU regimen. The overall cumulative incidence of relapse was 36% (BU‐FLU) versus 24% (THIO‐FLU).34 Therefore, the combination of fludarabine with one alkylating agent results in a low degree of complete donor chimerism and a high rate of relapse. A recent study with the BU‐FLU regimen, confirms a high rate of mixed chimerism (43%) in patients with MF, associated with a high rate of relapse (40%). 37 On the contrary the use of two alkylating agents, either thiotepa and busulfan 38 , 39 or BU‐FLU melphalan, 40 are associated with a high degree of complete donor chimerism (over 90%) and a low risk of relapse (less than 20%). It is important to reduce the dose of the two alkylating agents in older or fragile patients: in our experience 39 we have reduced the dose of busulfan to one single day, still maintaining a good eradicating effect.
Thus, monitoring patients with donor chimerism and driver mutations is essential to assess the degree of engraftment and the risk of relapse: these two events, achievement of full donor chimerism and relapse, are strongly dependent on the type of conditioning regimen given to the patient; apparently, the combination of two alkylating agents appears to offer an advantage in terms of control of the underlying disease, and possibly also in terms of survival. 39 , 40
10. TREATMENT OF RELAPSE
Relapse of the original disease is a negative event for any patient, particularly for patients with acute leukemia, but also for patients with chronic disorders like myelofibrosis. In a recent study of the European Group for Blood and Marrow Transplantation group (EBMT), the risk of relapse has apparently decreased in the past two decades41 this may be due to greater use of ruxolitinib pre‐transplant, which has been shown to have a favorable effect when given pre‐transplant, especially in responders.42 We should probably distinguish molecular relapse, from hematologic relapse43: the former is seen in patients with the reappearance of a driver mutation, with or without a decline in donor chimerism, but an otherwise normal blood count and possibly a normal bone marrow biopsy. A patient with a hematologic relapse has positive molecular markers, combined with hematologic abnormalities (leuko‐thrombocytosis) or cytopenia, with or without circulating blasts, an enlarged spleen, and a marrow biopsy with fibrosis. Unfortunately, these hematologic signs of MF do not disappear 1 month after transplant, and splenomegaly or marrow fibrosis may persist for months or years. It is, therefore, necessary to combine molecular markers with hematologic signs, to assess whether the patient is experiencing a molecular or a hematologic relapse.
Figure 1 outlines the approach one can consider in patients with MF, experiencing either persistence or reappearance of a molecular marker. After an allogeneic HSCT, a patient usually shows disappearance of driver mutation and full donor chimerism (FDC) (far left): he then goes in follow‐up. However, some patients (center) may have a slow clearance of their driver mutations with or without mixed donor chimerism (MDC): if the patient is still on immunosuppressive therapy (IST), this should be discontinued. If this action results in a negative molecular marker and FDC, the patient will go in follow up. 28 If instead the patient is already off IST and is free of chronic GvHD, he should receive planned sequential donor lymphocyte infusions (DLI); the initial dose may depend on the degree of HLA matching. A conservative starting dose may be 1 × 105 CD3/kg for HAPLO mismatched and unrelated mismatched grafts, and 1 × 106 CD3/kg for HLA matched related and unrelated grafts. The dose should be escalated every 21–28 days, in the absence of GvHD. Donor chimerism should be assessed monthly. In case the patient returns to FDC and has a negative driver marker, he should go in follow‐up. If the patient instead does not convert to FDC, he may be considered for lymphodepletion (usually FLU‐CY) or lymphodepletion plus myeloablation (a dose of melphalan) followed again by DLI. 44 If all these approaches fail, and the patient has a hematologic relapse, a second transplant is the only possible curative approach. The advantage of a second transplant procedure should be considered together with the clinical conditions, and the age of the patient: we have recently had a relapse in a patient, 10 years after his first transplant for MF, now aged 75 years: splenectomy reduced his transfusion requirement, and improved his quality of life, without affecting the underlying disease.
FIGURE 1.
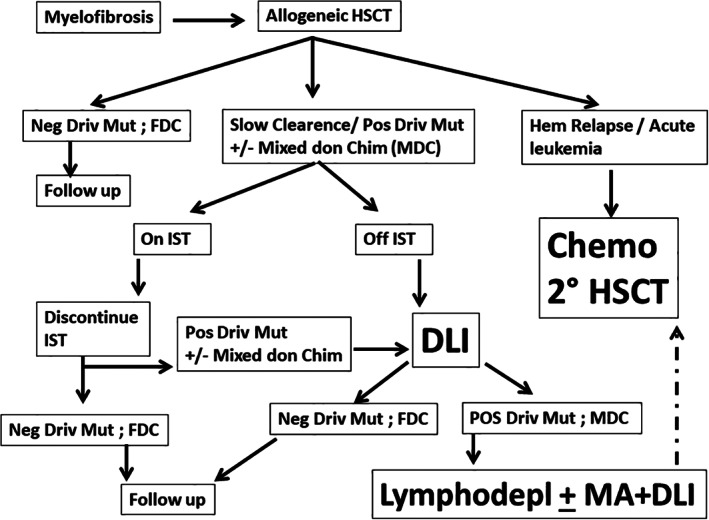
possible post‐transplant approach to monitor engraftment and relapse in patients with myelofibrosis after an allogeneic HSCT. FDC, full donor chimerism; HSCT, hemopoietic stem cell transplantation; IST, immunosuppressive therapy; lymphodepl, lymphodepletion; MA, myeloablation; MDC, mixed donor chimerism; Pos Driv Mut, positive driver mutations
11. CONCLUSIONS
Allogeneic transplantation for patients with MF remains a difficult task, due to several disease‐specific characteristics, such as a large spleen, an unfavorable marrow environment with fibrosis, a transfusion burden, often significant, and advanced age. However, at the same, it is also gratifying as it offers only curative option for these patients.
Indications for HSCT are currently based on prognostic scores, also including NGS‐based mutational analysis. Reduction of NRM has come with reduced intensity regimens, better supportive care and optimal selection of HLA matched unrelated donors.
However, NRM remains high in older patients (over the age of 60) and requires adaptation of conditioning regimens, and possible selection of HLA matched donors only.
ACKNOWLEDGMENTS
This work was supported by Associazione Italiana Ricerca sul Cancro (AIRC), Milan Italy. Open Access Funding provided by Universita Cattolica del Sacro Cuore within the CRUI‐CARE Agreement.
Ali H, Bacigalupo A. 2021 Update on allogeneic hematopoietic stem cell transplant for myelofibrosis: A review of current data and applications on risk stratification and management. Am J Hematol. 2021;96(11):1532-1538. doi: 10.1002/ajh.26349
REFERENCES
- 1. Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG‐MRT (international working Group for Myeloproliferative Neoplasms Research and Treatment). Blood. 2010;115(9):1703‐1708. doi: 10.1182/blood-2009-09-245837 [DOI] [PubMed] [Google Scholar]
- 2. Gangat N, Caramazza D, Vaidya R, et al. DIPSS plus: a refined dynamic international prognostic scoring system for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29(4):392‐397. doi: 10.1200/10.1200/JCO.2010.32.2446 [DOI] [PubMed] [Google Scholar]
- 3. Guglielmelli P, Lasho TL, Rotunno G, Mudireddy M, Mannarelli C. Nicolosi met al. MIPSS70: mutation‐enhanced international prognostic score system for transplantation‐age patients with primary Myelofibrosis. J Clin Oncol. 2018;36(4):310‐318. [DOI] [PubMed] [Google Scholar]
- 4. Tefferi A, Nicolosi M, Mudireddy M, et al. Driver mutations and prognosis in primary myelofibrosis: Mayo‐Careggi MPN alliance study of 1095 patients. Am J Hematol. 2018;93(3):348‐355. doi: 10.1002/10.1002/ajh.24978 [DOI] [PubMed] [Google Scholar]
- 5. Passweg J, Baldomero H, Chabannon C, et al. The EBMT activity survey on hematopoietic‐cell transplantation and cellular therapy 2018: CAR‐T's come into focusBone marrow. Transplantation. 2020;55:1604‐1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gupta R, Jamal F, Yang D, et al. Pulmonary hypertension is associated with increased nonrelapse mortality after allogeneic hematopoietic cell transplantation for myelofibrosis. Bone Marrow Transplant. 2020;55(5):877‐883. doi: 10.1038/s41409-019-0741-8 [DOI] [PubMed] [Google Scholar]
- 7. Mesa RA, Silverstein MN, Jacobsen SJ, Wollan PC, Tefferi A. Population‐based incidence and survival figures in essential thrombocythemia and agnogenic myeloid metaplasia: an Olmsted County study, 1976–1995. Am J Hematol. 1999;61(1):10‐15. [DOI] [PubMed] [Google Scholar]
- 8. Daghia G, Zabelina T, Zeck G, et al. Allogeneic stem cell transplantation for myelofibrosis patients aged ≥65 years. Eur J Haematol. 2019;103(4):370‐378. [DOI] [PubMed] [Google Scholar]
- 9. Al Malki MM, Nathwani N, Yang D, et al. Melphalan‐based reduced‐intensity conditioning is associated with favorable disease control and acceptable toxicities in patients older than 70 with hematologic malignancies undergoing allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2018;24(9):1828‐1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guardiola P, Anderson JE, Bandini G, et al. Allogeneic stem cell transplantation for agnogenic myeloid metaplasia: a European Group for Blood and Marrow Transplantation, Société Française de Greffe de Moelle, Gruppo Italiano per il Trapianto del Midollo Osseo, and Fred Hutchinson Cancer Research Center collaborative study. Blood. 1999;93(9):2831‐2838. [PubMed] [Google Scholar]
- 11. Li Z, Gooley T, Applebaum FR, Deeg HJ. Splenectomy and hemopoietic stem cell transplantation for myelofibrosis. Blood. 2001;97(7):2180‐2181. doi: 10.1182/10.1182/blood.v97.7.2180 [DOI] [PubMed] [Google Scholar]
- 12. Hart C, Klatt S, Barop J, et al. Splenic pooling and loss of VCAM‐1 causes an engraftment defect in patients with myelofibrosis after allogeneic hematopoietic stem cell transplantation. Haematologica. 2016;101(11):1407‐1416. doi: 10.3324/haematol.2016.146811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Polverelli N, Mauff K, Kröger N, et al. Impact of spleen size and splenectomy on outcomes of allogeneic hematopoietic cell transplantation for myelofibrosis: a retrospective analysis by the chronic malignancies working party on behalf of European society for blood and marrow transplantation (EBMT). Am J Hematol. 2021;96(1):69‐79. doi: 10.1002/10.1002/ajh.26020 [DOI] [PubMed] [Google Scholar]
- 14. Atilla E, Ataca Atilla P, Demirer T. A review of Myeloablative vs reduced intensity/non‐myeloablative regimens in allogeneic hematopoietic stem cell transplantations. Balkan Med J. 2017;34(1):1‐9. doi: 10.4274/10.4274/balkanmedj.2017.0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robin M, Porcher R, Wolschke C, et al. Outcome after transplantation according to reduced‐intensity conditioning regimen in patients undergoing transplantation for myelofibrosis. Biol Blood Marrow Transplant. 2016;22(7):1206‐1211. doi: 10.1016/10.1016/j.bbmt.2016.02.019 [DOI] [PubMed] [Google Scholar]
- 16. Ciurea SO, Kongtim P, Varma A, et al. Is there optimal conditioning for older patients with AML receiving allogeneic hematopoietic cell transplantation? Blood. 2020;135(6):449‐452. doi: 10.1182/10.1182/blood.2019003662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tefferi A. Primary myelofibrosis: 2021 update on diagnosis, risk‐stratification, and management. Am J Hematol. 2021;96:145‐162. [DOI] [PubMed] [Google Scholar]
- 18. Scott BL, Gooley TA, Sorror ML, et al. The dynamic international prognostic scoring system for myelofibrosis predicts outcomes after hematopoietic cell transplantation. Blood. 2012;119(11):2657‐2664. doi: 10.1182/blood-2011-08-372904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Samuelson Bannow BT, Salit RB, Storer BE, et al. Hematopoietic cell transplantation for myelofibrosis: the dynamic international prognostic scoring system plus risk predicts post‐transplant outcomes. Biol Blood Marrow Transplant. 2018;24(2):386‐392. doi: 10.1016/10.1016/j.bbmt.2017.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ali H, Aldoss I, Yang D, et al. MIPSS70+ v2.0 predicts long‐term survival in myelofibrosis after allogeneic HCT with the Flu/Mel conditioning regimen. Blood Adv. 2019;3(1):83‐95. doi: 10.1182/10.1182/bloodadvances.2018026658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gagelmann N, Ditschkowski M, Bogdanov R, et al. Comprehensive clinical‐molecular transplant scoring system for myelofibrosis undergoing stem cell transplantation. Blood. 2019;133(20):2233‐2242. doi: 10.1182/blood-2018-12-890889 [DOI] [PubMed] [Google Scholar]
- 22. Bacigalupo A, Soraru M, Dominietto A, et al. Allogeneic hemopoietic SCT for patients with primary myelofibrosis: a predictive transplant score based on transfusion requirement, spleen size and donor type. Bone Marrow Transplant. 2010;45(3):458‐463. doi: 10.1038/10.1038/bmt.2009.188 [DOI] [PubMed] [Google Scholar]
- 23. Gupta V, Malone AK, Hari PN, et al. Reduced‐intensity hematopoietic cell transplantation for patients with primary myelofibrosis: a cohort analysis from the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2014;20(1):89‐97. doi: 10.1016/10.1016/j.bbmt.2013.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kunte SJ, Rybicki L, Viswabandya A, et al. Haploidentical allogeneic hematopoietic cell transplantation with post‐transplant cyclophosphamide in patients with myelofibrosis: a multi‐institutional experience. Blood. 2020;136(suppl 1):33‐34. [Google Scholar]
- 25. Raj K, Eikema DJ, McLornan DP, et al. Family mismatched allogeneic stem cell transplantation for myelofibrosis: report from the chronic malignancies working party of European Society for blood and marrow transplantation. Biol Blood Marrow Transplant. 2019;25(3):522‐528. doi: 10.1016/10.1016/j.bbmt.2018.10.017 [DOI] [PubMed] [Google Scholar]
- 26. Olsson R, Remberger M, Schaffer M, et al. Graft failure in the modern era of allogeneic hematopoietic SCT. Bone Marrow Transplant. 2013;48(4):537‐543. doi: 10.1038/10.1038/bmt.2012.239 Erratum: Bone Marrow Transplant. 2013;48(4):616. [DOI] [PubMed] [Google Scholar]
- 27. Rajantie J, Sale GE, Deeg HJ, et al. Adverse effect of severe marrow fibrosis on hematologic recovery after chemoradiotherapy and allogeneic bone marrow transplantation. Blood. 1986;67(6):1693‐1697. [PubMed] [Google Scholar]
- 28. Barbui T, Tefferi A, Vannucchi AM, et al. Barosi chromosome‐negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32(5):1057‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ecsedi M, Schmohl J, Zeiser R, et al. Anti‐thymocyte globulin‐induced hyperbilirubinemia in patients with myelofibrosis undergoing allogeneic hematopoietic cell transplantation. Ann Hematol. 2016;95(10):1627‐1636. [DOI] [PubMed] [Google Scholar]
- 30. Salas MQ, Lam W, Law AD, et al. Reduced‐intensity conditioning allogeneic transplant with dual T‐cell depletion in myelofibrosis. Eur J Haematol. 2019;103(6):597‐606. doi: 10.1111/10.1111/ejh.13327 [DOI] [PubMed] [Google Scholar]
- 31. Morozova EV, Barabanshikova MV, Moiseev I, et al. A prospective pilot study of graft‐versus‐host disease prophylaxis with post‐transplantation cyclophosphamide and ruxolitinib in patients with myelofibrosis. Acta Haematol. 2021;144:158‐165. doi: 10.1159/10.1159/000506758 [DOI] [PubMed] [Google Scholar]
- 32. Kröger N, Abd Kadir SSS, Zabelina T, et al. Peritransplantation ruxolitinib prevents acute graft‐versus‐host disease in patients with myelofibrosis undergoing allogenic stem cell transplantation. Biol Blood Marrow Transpl. 2018;24:2152‐2156. [DOI] [PubMed] [Google Scholar]
- 33. Snyder DS, Palmer J, Gaal K, et al. Improved outcomes using tacrolimus/sirolimus for graft‐versus‐host disease prophylaxis with a reduced‐intensity conditioning regimen for allogeneic hematopoietic cell transplant as treatment of myelofibrosis. Biol Blood Marrow Transpl. 2010;16:281‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patriarca F, Masciulli A, Bacigalupo A, et al. Busulfan‐ or Thiotepa‐based conditioning in Myelofibrosis: a phase II multicenter randomized study from the GITMO group. Biol Blood Marrow Transplant. 2019;25(5):932‐940. doi: 10.1016/10.1016/j.bbmt.2018.12.064 [DOI] [PubMed] [Google Scholar]
- 35. Larocca A, Piaggio G, Podestà M, et al. Boost of CD34+ ‐selected peripheral blood cells without further conditioning in patients with poor graft function following allogeneic stem cell transplantation. Haematologica. 2006;91(7):935‐940. [PubMed] [Google Scholar]
- 36. Giammarco S, Sica S, Chiusolo P, et al. Eltrombopag for the treatment of poor graft function following allogeneic stem cell transplant: a retrospective multicenter study. Int J Hematol. 2021;114:228‐234. doi: 10.1007/s12185-021-03153-3 [DOI] [PubMed] [Google Scholar]
- 37. Srour SA, Olson A, Ciyre S, et al. Mixed myeloid chimerism and relapse of myelofibrosis after allogeneic stem cell transplantation. Haematologica. 2019;106:1988‐1990. doi: 10.3324/10.3324/haematol.2019.223503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shouval R, Vega Y, Fein JA, et al. Allogeneic hematopoietic stem cell transplantation with fludarabine, busulfan, and thiotepa conditioning is associated with favorable outcomes in myelofibrosis. Bone Marrow Transpl. 2019;55:147‐156. [DOI] [PubMed] [Google Scholar]
- 39. Chiusolo P, Bregante S, Giammarco S, et al. Full donor chimerism after allogeneic hematopoietic stem cells transplant for myelofibrosis: the role of the conditioning regimen. Am J Hematol. 2021;96(2):234‐240. doi: 10.1002/10.1002/ajh.26042 [DOI] [PubMed] [Google Scholar]
- 40. Jain T, Kunze KL, Temkit M, et al. Comparison of reduced intensity conditioning regimens used in patients undergoing hematopoietic stem cell transplantation for myelofibrosis. Bone Marrow Transplant. 2019;54(2):204‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McLornan D, Eikema DJ, Czerw T, et al. Trends in allogeneic haematopoietic cell transplantation for myelofibrosis in Europe between 1995 and 2018: a CMWP of EBMT retrospective analysis. Bone Marrow Transplant. 2021;56:2160‐2172. doi: 10.1038/s41409-021-01305-x [DOI] [PubMed] [Google Scholar]
- 42. Kröger N, Sbianchi G, Sirait T, et al. Impact of prior JAK‐inhibitor therapy with ruxolitinib on outcome after allogeneic hematopoietic stem cell transplantation for myelofibrosis: a study of the CMWP of EBMT. Leukemia. 2021. doi: 10.1038/s41375-021-01276-4. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McLornan DP, Boluda JCH, Czerw T, et al. Allogeneic haematopoietic cell transplantation for myelofibrosis: proposed definitions and management strategies for graft failure, poor graft function and relapse: best practice recommendations of the EBMT chronic malignancies working party. Leukemia. 2021;35:2445‐2459. doi: 10.1038/s41375-021-01294-2 [DOI] [PubMed] [Google Scholar]
- 44. Warlik ED, DeFor T, Blazar BR, et al. Successful remission rates and survival after Lymphodepleting chemotherapy and donor lymphocyte infusion for relapsed hematologic malignancies Postallogeneic hematopoietic cell transplantation. Biology of Blood and Marrow Transplantation. 2012;18:480‐486. doi: 10.1016/10.1016/j.bbmt.2011.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]


