CONFLICT OF INTEREST
Dr Paula A Benítez has received a Grant from Allergan Aesthetics, an AbbVie company, for covering the medical writing services.
AUTHOR CONTRIBUTIONS
All authors met the ICMJE authorship criteria. All authors contributed to the drafting and critical revision of the manuscript, commented on previous versions of the manuscript, and read and approved the final manuscript prior to submission.
ETHICAL STATEMENT
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
To the Editor
Hypertrophied trapezius muscle may result in an aesthetic problem that impacts on patient's attractiveness and beauty perception. 1 Botulinum toxin A (BoNTA) can create smooth and neat contours by volume reduction of trapezius muscle. 1
We retrospectively assessed the effectiveness of onabotulinumtoxin A (onabotA) in women aged ≥18 and ≤50 years with bilateral trapezius hypertrophy, who underwent treatment with BoNTA for aesthetic purposes.
1.
1.1. Injection technique
Fifty units of onabotA were injected into the most prominent strip of the upper portion of the trapezius muscle following the model (Figure 1).
FIGURE 1.
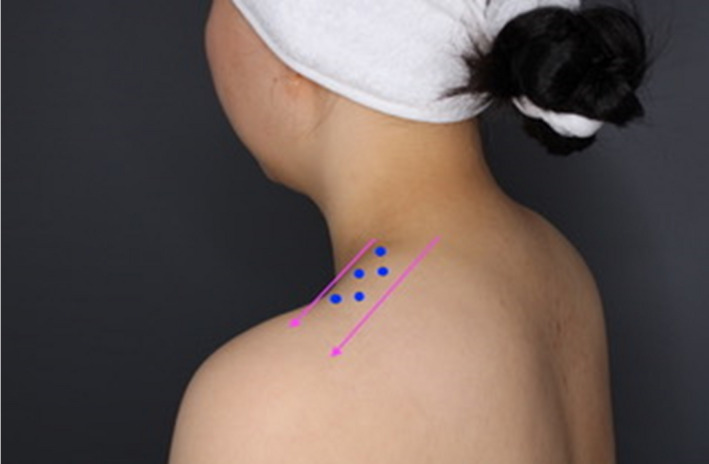
Technique for the delimitation of the trapezius muscle and treatment point strategy. Injection points were separated by a distance of 1.5–2.5 cm each. A 27‐gauge injection needle was used. For the treatment of the trapezius muscles, 10 U per selected point was administered [Color figure can be viewed at wileyonlinelibrary.com]
1.2. Measurements
With the patient looking straight ahead, we measured the distance between the inferior tip of the mastoid process to the base of the neck (MPNB) and between the shoulder and the base of the neck (SNB) (Figure S1).
1.3. Patient satisfaction
We used a 5‐point Likert scale (Strongly disagree; Disagree; Neither agree nor disagree; Agree; and Strongly agree) to evaluate the satisfaction of the patient. The scale consisted of two questions: Are you satisfied with the treatment results? Would you repeat the treatment?
2. RESULTS
Eleven patients were included in the analysis. The median (range) age was 34.0 (23.0 to 46.0) years, and all the patients were women. Median (range) administered dose of onabotA was 50.0 (35.0–60.0) U in the right side and 50.0 (15.0–60) in the left one.
There were no significant differences at baseline between the right and the left sides in either MPNB (p = 0.0859) or SNB (0.1929).
Median (95%CI) reductions in MPNB were 1.5 (1.0–1.9) cm, p = 0.0033 (Figure 2A), 1.5 (1.2–2.0) cm, p = 0.0033 (Figure 2B), in the right and left sides, respectively. Median increases in SNB were 0.8 (0.5–1.1) cm, p = 0.0051 (Figure 2C), and 1.0 (0.6–1.2) cm, p = 0.0051 (Figure 2D), in the right and left sides, respectively.
FIGURE 2.
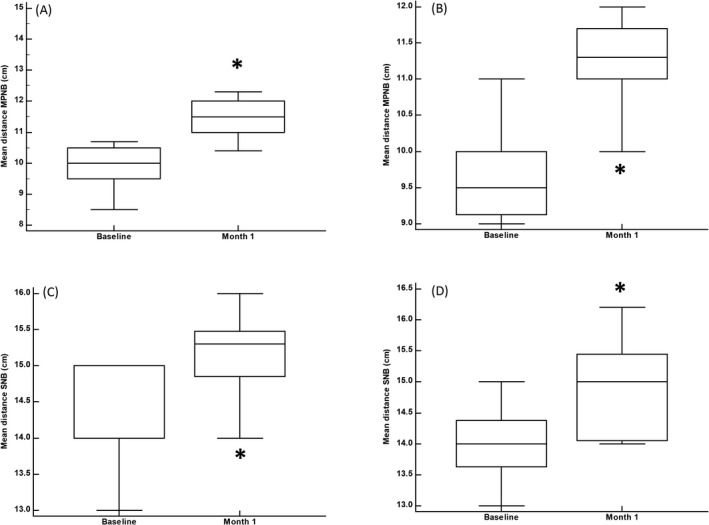
Overview of the different study measurements. The vertical bars represent the 95% confidence interval. A, MPNB in the right trapezius muscle. B, MPNB in the left trapezius muscle. C, SNB in the right trapezius muscle. D, SNB in the left trapezius muscle. MPNB: Mean distance from mastoid process to the base of the neck; SNB: mean distance from shoulder to the base of the neck. Statistical significance, as compared to baseline, was determined using the Wilcoxon test with the Hodges‐Lehmann estimate of location shift. *p < 0.01
Clinical results, assessed by means of photographs, have shown a significant aesthetic improvement (Figures S2 and S3).
Patients showed high satisfaction levels, with 9 (81.8%) patients reporting to be “Strongly agree” and 2 (18.2%) ones “agree,” with the treatment results. Regarding the question: Would you repeat the treatment? 72.7% (8/11) patients report to be “Strongly agree” and 3 (27.3%) ones “agree,” with the question.
Five (45.5%) patients reported pain at the time of injection, which was resolved spontaneously without treatment. Two patients experienced pectoral muscles soreness, due mainly to a mild weakness of the trapezius muscles, which was resolved spontaneously within 15 days.
3. COMMENT
The current study observed that onabotA was able to relax significantly the hypertrophied trapezius muscles creating smooth contours, which, in turn, helped to improve the neck length. Additionally, due to these optimal aesthetic results, patients showed a high satisfaction rating.
The aim of injecting BoNTA into the muscles is to produce a neurogenic atrophy, with muscle mass loss. 2 , 3
Minimally invasive aesthetic procedures, particularly BoNTA injections, have become increasingly popular over the last decade, with more than 6.2 million aesthetic treatments were performed in 2019 worldwide. 4 However, information about the effect of BoNTA in patients with bilateral trapezius hypertrophy for aesthetic purposes is very limited. 1
As compared to Zhou et al, 1 our injection technique was more precise and designed for minimizing potential adverse events derived from a possible diffusion of BoNTA. Moreover, the fact that Zhou et al did not mention the BoNTA they used makes it impossible to compare our results with theirs, since dosing and product performance of the different BoNTAs are not interchangeable, which may have relevant clinical implications in efficacy, safety, and duration of action. 5 , 6
Although this was a short‐term study with a limited number of patients, its results suggested that injecting 50 U of onabotA was an effective and safe aesthetic procedure in patients with bilateral trapezius hypertrophy.
INFORMED CONSENT
All patients were fully informed about the details of the study protocol. The need of informed consent was waived for this study.
FUNDING SOURCES
Medical writing services has been provided by Allergan Aesthetics, an AbbVie company. Allergan did not participate in either data collection, analysis, or redaction of the manuscript. Neither honoraria nor payments were made for authorship.
Supporting information
Fig S1
Fig S2
Fig S3
Supplementary Material
ACKNOWLEDGEMENTS
Medical writing and Editorial assistant services have been provided by Ciencia y Deporte S.L. and covered by a Grant from Allergan. Support for this assistance was funded by Allergan Aesthetics, an AbbVie company.
Benítez PA. Onabotulinumtoxin A for correcting trapezius muscle hypertrophy in Asian women. J Cosmet Dermatol. 2022;21:2677–2679. 10.1111/jocd.14423
DATA AVAILABILITY
Data available on request from the authors.
REFERENCES
- 1. Zhou RR, Wu HL, Zhang XD, et al. Efficacy and safety of botulinum toxin type A injection in patients with bilateral trapezius hypertrophy. Aesthetic Plast Surg. 2018;42(6):1664‐1671. [DOI] [PubMed] [Google Scholar]
- 2. Fortuna R, Vaz MA, Youssef AR, Longino D, Herzog W. Changes in contractile properties of muscles receiving repeat injections of botulinum toxin (Botox). J Biomech. 2011;44(1):39‐44. [DOI] [PubMed] [Google Scholar]
- 3. Stone AV, Ma J, Callahan MF, Smith BP, Garrett JP, Smith TL. Dose‐ and volume dependent‐response to intramuscular injection of botulinum neurotoxin‐A optimizes muscle force decrement in mice. J Orthop Res. 2011;29(11):1764‐1770. [DOI] [PubMed] [Google Scholar]
- 4.Authors not listed. The international study on aesthetic/cosmetic procedures performed in 2019. https://www.isaps.org/wp‐content/uploads/2020/12/Global‐Survey‐2019.pdf
- 5. Sundaram H, Signorini M, Liew S, et al. Global Aesthetics Consensus Group. Global aesthetics consensus: botulinum toxin type A‐evidence‐based review, emerging concepts, and consensus recommendations for aesthetic use, including updates on complications. Plast Reconstr Surg. 2016;137(3):518e‐529e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brin MF, James C, Maltman J. Botulinum toxin type A products are not interchangeable: a review of the evidence. Biologics. 2014;8:227‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Supplementary Material
Data Availability Statement
Data available on request from the authors.


