Abstract
Colorectal cancer (CRC) screening has been demonstrated to reduce CRC incidence and mortality. However, besides such benefits, CRC screening is also associated with potential harmful effects. In an ideal world, screening would only be directed to the small proportion of the population that might potentially benefit. Risk‐based screening can be seen as a first step towards this ideal world, by redistributing screening resources from low‐risk to high‐risk individuals. In theory, this should result in scarce resources being used in individuals who benefit most, while intensity of screening is reduced in individuals who benefit less, hence improving the benefit‐harm ratio among all invitees. Available strategies that have been proposed for risk‐based CRC screening include using information on age, sex, prior screening history, lifestyle and/or genetic information. Implementation of risk‐based screening requires careful consideration of reliable risk prediction models, participation with screening and informed decision‐making. While it is important to recognise the limitations of current approaches, available evidence suggests that it might be feasible to start planning the introduction of tailored strategies within screening programmes. Implementing risk‐based screening based on age, sex and prior screening history alone would already represent a substantial improvement over current uniform screening approaches. We propose that it is time that screening programmes start there and continue striving towards more comprehensive approaches embedding primary prevention as an effective approach to lower risk for everyone.
Keywords: colorectal cancer, personalised medicine, screening
Abbreviations
- AUC
area under the curve
- CRC
colorectal cancer
- FIT
Faecal Immunochemical Test
- GP
general practitioner
- Hb
haemoglobin
- OR
odds ratio
- PRS
polygenic risk score
- RR
relative risk
- SES
socioeconomic status
- SNP
single nucleotide polymorphism
1. BACKGROUND
Colorectal cancer (CRC) is an important health problem worldwide. In 2017, 1.8 million new cases of CRC were diagnosed and almost 900 thousand people died from the disease. 1 The good news is that CRC is highly amenable to screening and several screening tests are available to prevent CRC incidence and/or mortality. 2 , 3 The potential for CRC screening to (efficiently) reduce the burden of CRC is recognised around the globe, evidenced from the large number of programmes (being) implemented worldwide. 4 There is considerable variation in the screening strategies being implemented, but virtually all CRC screening programmes in the world have one thing in common: they do not differentiate their screening strategy based on risk factors beyond age, and for some programmes, a family history of CRC. Exceptions include the CRC screening programmes of Finland and Stockholm‐Gotland (Sweden) using sex‐specific cut‐offs for the Faecal Immunochemical Test (FIT) 5 , 6 and the CRC programme in Germany using a sex‐specific starting age for screening colonoscopy since 2019 (Table 1).
TABLE 1.
Sex‐specific aspects of three national screening programmes using sex as a basis for differentiating colorectal cancer screening policy
| Programme characteristic | Finland | Sweden | Germany | |||
|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |
| Cut‐off for positive Faecal Immunochemical Test | 25 | 70 | 40 | 80 | ||
| Starting age for screening colonoscopy | 50 | 55 | ||||
Besides benefits such as CRC incidence and mortality reduction, CRC screening is also associated with harmful and negative effects, including anxiety, discomfort, false‐positive test results and its psychological impact, 7 false reassurance from false‐negative test results, complications from colonoscopy (which in rare cases might be fatal), overtreatment of adenomas and overdiagnosis and overtreatment of cancers (ie, treatment of adenomas and cancers that would not have given symptoms in the absence of screening). Screening should therefore only be implemented if its expected benefits outweigh the harms. Although this is considered to be the case for CRC screening overall, each year in Europe alone more than 9.6 million individuals are being screened for CRC, while significant disease is detected in only 5% to 10% of subjects undergoing endoscopy screening and less than 5% of subjects undergoing faecal testing, with rates further declining over subsequent screening rounds. In an ideal world, screening would only be directed to the small proportion of the population that might potentially benefit from it.
2. POTENTIAL ADVANTAGES OF RISK‐BASED SCREENING
Compared to the established age‐stratified approach, risk‐based CRC screening would provide an opportunity to tailor screening interval, modality or age range, to an individual's risk. 8 Subjects at lower risk might be invited for screening later and/or with longer screening intervals and/or less invasive tests, leading to a reduction of screening harms. Cost‐effectiveness is higher among high‐risk subjects, who are more likely to be detected with advanced neoplasia when undergoing screening. They could start screening earlier, with shorter intervals and/or using more invasive tests. 8 , 9 , 10 , 11 This way, risk‐based screening may also provide opportunities to detect cancers in younger at‐risk individuals, who are currently excluded from age‐based screening despite being at increased risk. 12 , 13 , 14 In theory, this should result in an improved balance between the benefits and harms and costs of screening: scarce resources are used in individuals that benefit most, increasing the benefits of screening. At the same time, intensity of screening is reduced in individuals that benefit less, reducing the harms of screening.
3. POSSIBILITIES FOR RISK‐BASED SCREENING
Two approaches are available to assess the necessary information to stratify the population into groups at varying levels of cancer risk. Risk stratification may be based on estimates of an individual's a priori probability of getting the disease over a certain time interval, taking into account their age, sex, genetic susceptibility profile, family history and level of exposure to modifiable environmental factors. An alternative approach is to stratify screenees into subgroups characterised by different levels of risk of being detected with or harbouring neoplasia, based on their screening history. Models combining the two approaches have also been developed.
3.1. Estimating background risk
CRC offers several starting points for deriving estimates of background risk. In addition to sex, age and family history, a range of environmental (eg, alcohol, obesity, physical activity, dietary habits) as well as genetic risk factors [single nucleotide polymorphisms (SNPs)] have been identified, 15 , 16 that increase, or decrease, disease specific risk. They could therefore be used as a first step towards the implementation of a risk‐based programme.
3.1.1. Sex
The risk of CRC is approximately 1.3‐fold higher in men than in women. 17 The age‐specific rates for women are nearly identical to those of 5 year younger men (ie, rates at age 60‐64 for women are similar to rates at age 55‐59 for men). 18 Moreover, CRC screening has been suggested to be less effective in women than in men. 19 These findings triggered the debate whether CRC screening should differ between men and women. 20 However, although screening was found to be more cost‐effective in men than in women, 21 decision analyses evaluating whether men and women should be screened differently, did not find any difference in optimal screening between men and women. 18 , 22 , 23 , 24
3.1.2. Race and ethnicity
Racial and ethnic disparities in CRC incidence and mortality are well known and widely documented. In the United States, for example, relative to non‐Hispanic whites, non‐Hispanic blacks tend to have higher rates of CRC incidence, 17 earlier age at diagnosis, 25 , 26 , 27 later stage at diagnosis 28 and worse stage‐specific CRC mortality. Hispanics and Asians and Pacific Islanders, on the other hand, have lower risk of CRC incidence and mortality. These disparities have resulted in calls for earlier initiation of CRC screening for black individuals, 29 especially black men. However, reasons for racial disparities are complex and likely reflect differences in lifestyle and access to screening and care, 17 rather than biological differences. This hypothesis is supported by the fact that CRC incidence between blacks and whites was similar before 1985, prior to the introduction of widespread screening for CRC, and that the observed trends in CRC mortality in the following decades are reflecting the pattern and timing of screening diffusion. These trends advocate for improving access‐to‐care and to implement risk‐based interventions based on lifestyle rather than race or ethnicity.
3.1.3. Lifestyle
Several lifestyle factors have been identified to be associated with CRC risk. According to the World Cancer Research Fund report, there is strong evidence that consumption of processed and red meat, alcoholic drinks, body fatness and adult attained height increase the risk of CRC, whereas physical activity, wholegrains, dairy products, calcium supplements and foods containing dietary fibre decrease this risk. 30 Yet the impact of each of these individual risk factors by itself is modest, with relative risk (RR) estimates varying from a 5% increase in risk per 5 kg/m2 increase in body fat to 12% increase per 100 g increase in red or processed meat consumption. Impact of protective factors was slightly larger, varying from 9% decrease in CRC risk with every 10 g/d increase in dietary fibre to a 20% lower risk between individuals in the highest category of physical activity compared to the lowest. Even smoking, the lifestyle factor with the largest impact on CRC risk, does not increase CRC risk by more than 40% in individuals who smoke 40 cigarettes per day compared to non‐smokers. Therefore, none of these lifestyle factors in itself warrants risk‐based screening. However combined together, their discriminatory performance improves and they could certainly be a candidate for risk stratification (see Section 3.1.5 for more details). Moreover, they offer the additional benefit of including interventions for primary prevention.
3.1.4. Single nucleotide polymorphisms
Genome‐wide association studies have shown that polygenic factors, such as common, low risk genetic variants or SNPs, play a significant role in defining CRC risk due to their relatively high prevalence in the population. Like lifestyle factors, in isolation, SNPs are only weakly associated with CRC risk; however, cumulatively they explain substantial variation in risk. 9 , 31 A polygenic test can be used to estimate someone's polygenic risk score (PRS) based on the absence or presence of specific risk alleles. Such a risk score can be used to identify individuals at several times lower and greater (0.49‐3.40) CRC risk than the average population. 15
A comprehensive whole‐genome sequencing study and meta‐analysis of genome‐wide association studies recently identified 40 new independent signals for CRC, bringing the number of known independent signals for CRC to about 100. 32 Together, these signals explain approximately 11% of the familial RR in US individuals. 32 As more genetic variants associated with CRC risk are detected, this percentage could potentially increase to 73%. 33 Another study suggested that if all variants were identified, at least 7.42% of all CRC cases would be explained by SNPs. 34
3.1.5. Risk prediction models
As described above, individual lifestyle factors and SNPs did not portray sufficient discriminatory performance to be used for risk stratification by themselves. To improve discriminatory performance, they were combined into risk prediction models. The first risk prediction models were solely based on clinical and lifestyle factors; one of the most famous being the National Cancer Institute's Colorectal Cancer Risk Assessment Tool. 35 Later, risk prediction models based on SNPs were developed, to assess an individual's PRS. More recently, risk prediction models are being developed that combine lifestyle information with SNPs. 36 , 37 Unfortunately, the ability of available models to predict who will develop cancer and who will not is still limited. A recent review systematically compared and externally validated 23 risk prediction models for CRC that included SNPs. The discriminatory performance [measured as the area under the curve (AUC)] of the models including only SNPs increased with the number of included SNPs. 37 The model with the highest number of SNPs (120) had an AUC of 0.62. 32 Adding lifestyle factors and age improved discriminatory performance, with the best performing models having AUCs between 0.64 and 0.67 in women and 0.67 and 0.71 in men. 37 Cost‐effectiveness analysis has shown that a discriminatory performance of at least 0.65 is required for risk‐based screening to be more cost‐effective than uniform screening (Figure 1). 38 With further discovery of SNPs and introduction of new machine learning techniques for estimating risk prediction models, we can soon hope to tip the balance in favour of risk‐based screening.
FIGURE 1.
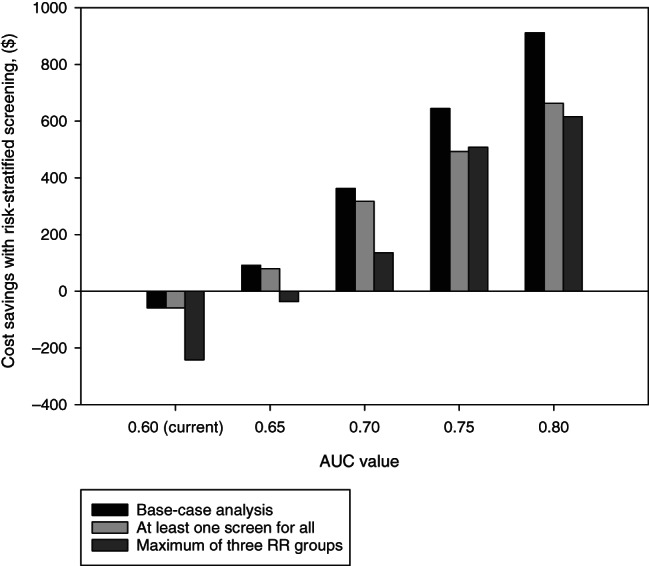
Cost savings of replacing uniform screening with risk‐stratified screening, when risk‐stratified screening yields (at least) as many QALYs as uniform screening. AUC, area under the receiver‐operating characteristic curve; QALY, quality‐adjusted life‐year; RR, relative risk. Source: Reference 38
3.2. Screening history
3.2.1. Faecal haemoglobin concentration
For FIT screening programmes specifically, there is an additional way of risk stratifying the target population: using the quantitative outcomes of prior screening results. Several studies have shown that faecal haemoglobin (Hb) concentrations in previous screening rounds are highly predictive for future detection of advanced neoplasia. 39 , 40 , 41 , 42 , 43 , 44 Individuals who tested just below the cut‐off for a positive test had an 8 to 38 higher odds or hazard of being detected with advanced neoplasia vs those without any detectable Hb, depending on the cut‐off used. The lower estimate comes from a study using a 10 μg Hb/g faeces cut‐off, while in the study with the higher estimate a cut‐off of 80 μg Hb/g faeces was used. Most countries currently operate with a cut‐off of 20 μg Hb/g faeces; odds ratios (ORs) in case of cut‐offs of 15 and 47 μg Hb/g faeces were 15 and 23, respectively. 42 , 44 Odds and hazard ratios became even more pronounced if results from multiple prior screening rounds were used. 45 , 46 , 47
For comparison, ORs for individual lifestyle factors most times do not exceed 1.4, and the risk difference between the OR of the 99th vs the first percentile of a risk prediction model combining genetic, environmental factors and family history was less than 14. 36 Given its high predictive value, Hb concentration has already been suggested as the key to risk‐based CRC screening. 48 In several programmes, Hb concentrations are already part of the standard documentation of screening registries, that is, the information is readily available for risk‐based screening.
Given the promising discriminatory performance of faecal Hb concentration at prior screening, now also risk prediction models are being developed combining this factor with demography, clinical and lifestyle information. 49 , 50
4. FEASIBILITY OF RISK‐BASED SCREENING
The idea of risk‐based CRC screening was already introduced more than 25 years ago. 51 Despite promising advances in risk prediction using genetic risk and lifestyle, 36 , 52 all CRC screening programmes in Europe still only use age for identifying the target population and no other factors. 53 Moreover, screening is the same for all individuals within the target age range with only a few exceptions.
We hypothesise that there are several important barriers to implementation of risk‐based cancer screening in practice. These include ethical, legal and communication issues that need to be resolved when planning to introduce risk‐based protocols, independent of the approach adopted for risk stratification. In addition, incorporating background risk estimates in the context of ongoing programmes adds new complexity to the screening organisation, requiring to address a number of economic, organisational and policy issues. In the here following paragraphs, we highlight the requirements that need to be met before widespread implementation.
4.1. Reliable risk prediction models
To accurately categorise individuals by risk, reliable risk prediction models are needed. Currently existing risk prediction models show a low discriminative accuracy, with AUCs of <65% for discriminating between cancer cases and other patients, and OR of maximum 14. 36 Although, for example, risk prediction models for cardiovascular disease are not substantially more accurate than current CRC risk prediction models, they are widely used in clinical practice and influence treatment decisions, for example, regarding statin therapy for primary prevention of cardiovascular disease.
Misclassification of risk can result in inappropriate recommendations. It has been estimated, for example, that more than 60% of women who developed CRC in the next 5 years would have been classified in the low‐risk group, receiving a weak recommendation against screening, when using the risk threshold recommended by a recent practice guideline. 54 Based on such evidence, clinicians might feel confident to recommend more intensive screening to those at high‐risk, but they might be hesitant to recommend reduced screening intensity for those at lower‐risk. Available evidence suggests that prediction models would allow identification of high‐risk younger individuals, currently ineligible for population‐based programmes, who might benefit from screening. On the other hand, using pre‐defined CRC risk thresholds to recommend screening also results in a large proportion of people starting screening at older ages than currently recommended, leading to a potential reduction of the preventive impact of screening. Even with reliable models, there is the concern of the prevention paradox that dictates that targeting high‐risk groups could result in exclusion of a large group of individuals at low‐moderate risk of CRC, where actually the majority of CRCs occur. 55
4.2. Translation from risk into actionable clinical information
Several gaps in current knowledge of the complex array of factors influencing CRC risk are not only limiting the accuracy, but also the clinical usefulness of risk predictions. Studies so far have focused exclusively on risk prediction and discriminating between low‐ and high‐risk individuals. Although it is obvious that low‐risk individuals should be screened less intensely and high‐risk people more intensely than the average, the optimal decrease or increase in intensity has never been studied. Decision models have proven their use for optimisation of screening strategies for a long time already 56 , 57 ; however their use in risk‐based screening has been limited, focusing on a single screening setting such as stop age, 58 or comparing only a limited number of risk groups. 59 The problem is that with the expanding number of risk groups, and the wide range of possibilities for varying screening (eg, age to start screening, age to stop screening, screening interval, screening test and the cut‐off value for a positive test), the number of possible strategies becomes endless which is even beyond the computational capacity of decision models to fully explore. First, smart algorithms need to be developed to efficiently determine optimal screening strategies by risk. Even then, models should only be used to extrapolate clinical evidence. We currently lack clinical studies evaluating risk‐based screening to allow valid extrapolation in this field. Therefore, it remains unclear whether differences in risk are related to the propensity to develop pre‐malignant lesions and/or to the rate of transition from polyp to invasive CRC (duration of the pre‐clinical detectable phase). It is also not clear whether cancers occurring in low‐risk vs high‐risk persons show the same characteristics and prognosis, which could influence the effectiveness of screening. Until we have this information, modelling can be used for hypothesis generation and indicate potential risk‐based strategies to explore first. These can be tested in clinical studies of which the results will shed light on the natural history of CRC by risk, and then we can validly use modelling to further optimise the choice of the test, as well as of the optimal screening interval.
4.3. Framework to collect risk factor information
Information about genetic risk profile, lifestyle and/or anthropometric measures is generally not available in population‐based screening programmes and needs to be collected. The logistics of data collection, including organisation of blood sampling and storage, delivery of life‐style questionnaires and measure of anthropometric parameters are posing new challenges to the screening organisation. The costs and the amount of resources needed to collect and manage risk information might be reduced in the near future, as a result of the adoption of validated simplified life‐style risk scores, as well as of advances in genetic profiling techniques, allowing for large scale genetic testing. Nevertheless, adding the costs of data collection to screening and treatment costs might still result in a less favourable cost‐effectiveness ratio of risk‐based, as compared to age‐stratified, screening. Also, the optimal timing to collect the information, which is most relevant to predict individual's level of risk, needs to be defined. Indeed, while genetic information can be considered fixed, individual's habits/lifestyle and anthropometric measures as well as family history are likely to undergo changes over time. Furthermore, the instruments to collect these data need to be standardised and optimised in order to minimise misclassification of self‐reported information.
4.4. Ensuring consistent levels of screening participation
Careful consideration should be given to the potential impact of risk‐based strategies on uptake, because benefits of population screening are largely dependent on participation. With many countries already experiencing suboptimal levels of participation in routine age‐based screening for CRC, 60 , 61 the impact of risk‐based screening on adherence requires careful monitoring. On the one hand, the increasing complexity of screening may confuse people to the extent that they no longer participate. If an invitation to cancer screening is accompanied by a lifestyle questionnaire, or a call for a blood test, this could put people off in participating. This may especially be a concern for groups with lower health literacy, such as lower socioeconomic status (SES) groups or ethnic minorities, potentially increasing disparities in screening uptake and thus disease burden. The fact that these groups often comprise a disproportionately high number of persons at higher CRC risk due to unhealthy lifestyle factors further adds to this concern. Acceptance might be particularly problematic for approaches that require genetic information due to data privacy concerns.
On the other hand, individuals at increased risk of CRC have been shown to be more compliant to screening guidelines than those at average risk, 62 suggesting that the provision of risk information may assist in screening uptake. 10 , 63 On the surface, a recent randomised controlled comparing a risk‐stratified screening arm with two arms offering colonoscopy or FIT only indeed showed a higher participation rate with colonoscopy in the risk‐stratified arm compared to the colonoscopy arm (Figure 2). 64 Nevertheless, even in this arm, still more than half of the population offered colonoscopy refused and participation with screening was highest in the FIT only arm. Yet, coupled with evidence that involvement of general practitioners (GPs) improves participation in CRC screening, 65 a simple risk assessment has the potential to positively impact screening participation. 66 However, those identified at lower risk may lose interest in screening altogether, or alternatively may not accept a reduction of the access to pre‐existing services and especially those with higher SES may search for more intensive screening elsewhere.
FIGURE 2.
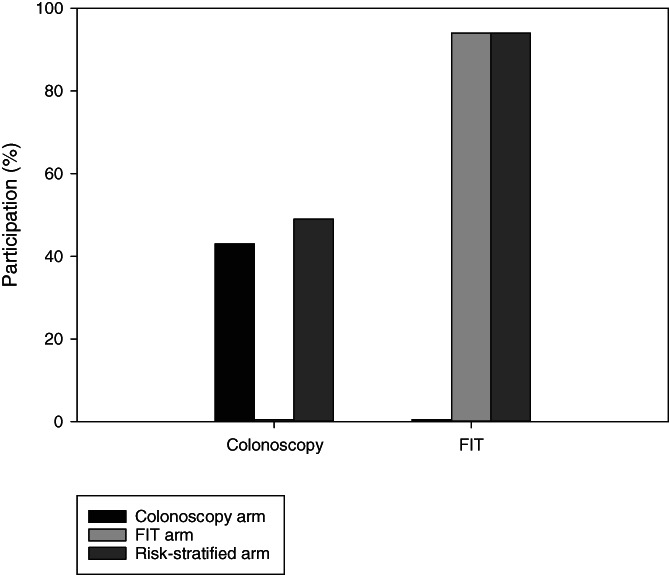
Participation with colonoscopy and Faecal Immunochemical Test (FIT) in a randomised controlled trial comparing (1) screening with colonoscopy only, (2) screening with FIT only and (3) risk‐stratified screening where colonoscopy was offered to higher‐risk individuals and FIT to those at lower risk. Based on: Chen et al 64
4.5. Enable informed decision‐making through information and communication
A challenge that is common to all approaches to risk stratification is to enable subjects targeted for screening to make an autonomous and informed decision about participation. On the one hand, it has been suggested that personalised risk communication may enhance informed choice. 63 However, communication strategies should be designed to convey clear and comprehensive information to help people targeted for screening to understand and to use risk estimates, while avoiding overload with complex information. Communicating risk in a way that can be understood by everybody, avoiding both the potential risk of inducing false reassurance and of causing psychological distress, requires careful consideration of invitees' information needs, concerns and preferences, as well as the adoption of different formats and channels to convey the relevant information.
4.6. Ensuring quality assurance
Monitoring, evaluation and other quality assurance measures should be inherent to any cancer screening programme. After implementation of risk‐stratification, it is important to carefully monitor the impact of risk‐based screening. Does risk‐stratification indeed lead to the anticipated increase in screen‐detected advanced neoplasia and a decrease in false‐positive test results, overtreatment and interval cancers? In particular, potential adverse effects of risk feedback on participation and on disparities in access to screening and care need to be monitored long term. Finally, new screening indicators need to be developed to monitor the psychological impact of risk‐stratification and communication on participants, as well as the impact on use of healthcare resources outside the screening programme from low‐risk individuals seeking higher‐intensity screening.
4.7. Protect against stigmatisation and discrimination
Labelling individuals with risk is inherent to any risk‐based intervention, but unexpected diagnosis labelling in screening has been shown to be associated with psychological harm. 67 Moreover, concerns have been raised about the potential risks of stigmatisation and discrimination when using information about individual's risk profile to modulate or implement medical interventions. 68 Several reports provided evidence that the belief that cancer is a self‐inflicted disease represents a major factor contributing to cancer stigma, 69 also among CRC patients. 70 This might lead to social stigmatisation of subjects in the high‐risk group, who may be perceived as partially responsible for their condition, which might undermine the principles of solidarity on which current screening programmes offering universal coverage have been established. In order to protect people enrolled in risk‐based screening interventions against the potential risk of discrimination, which appear especially high in the context of insurance, employment and social relationships, legislative measures are required, aimed to prohibit unauthorised access to sensitive information. However, the adoption of such measure might not be sufficient to completely alleviate fears of discrimination. 71 It was pointed out that even if data are protected, calls for risk‐based screening might be interpreted by third parties as a proxy of elevated risk and this information may be used to set higher insurance premiums. 68
5. FUTURE DIRECTIONS
This overview provides insight in the possibilities and challenges for risk‐based screening for CRC. Although the possibilities are plenty, there are still many organisational, societal and ethical issues that are understudied and/or need to be discussed and agreed upon. A first step is therefore to perform both qualitative and quantitative research investigating conditions for acceptability of risk‐based screening, and its impact on costs and organisation. Empirical evidence from large pilot studies is required to assess whether the expected benefits actually outweigh the observed harms. For example, it may be easier to change policy in the direction of what can be perceived as an addition rather than a reduction of the access to pre‐existing services. A study in breast cancer reported that 85% of the women were willing to participate in more frequent screening if they were at high genetic risk, while only 59% of the women were willing to participate in less frequent screening because they were at low genetic risk. 72 In fact, the majority of the challenges described are certainly not unique to CRC. It is therefore important to see these challenges in the broader light of risk‐based cancer screening in general and learn from experiences in other fields, such as for example the MyPeBS study, an international European Union‐funded clinical study that evaluates personalised breast cancer screening.
Collecting lifestyle information for risk‐based screening offers the additional possibility for adding lifestyle interventions to the screening programme. Indeed, an increase in the adherence score to healthy lifestyle recommendations among subjects aged 50 to 60 targeted for CRC screening was associated with a mortality reduction at 11‐year follow‐up, in a population cohort enrolled in a screening trial. 73 Moreover, a recent observational study showed that better adherence to a healthy life‐style score was associated with a substantial reduction in the absolute CRC risk in all subgroups of genetic risk, estimated based on a PRS, even among subjects who had undergone screening colonoscopy. 74
The implementation of risk‐based screening within ongoing population based programmes may ensure that adequate quality control measures are implemented and the impact of the intervention is carefully monitored. In particular, potential adverse effects of risk feedback on participation and on disparities in access to screening and care need to be monitored long term. In this way, concerns which have been raised about the potential for risk‐based screening to undermine the principles of solidarity on which current screening programmes offering universal coverage have been established might be mitigated.
From the discussion above, it is obvious that risk‐based screening inherently makes screening more complicated. We therefore propose to start with the low hanging fruit: introducing risk‐based screening based on already available information such as age, sex and screening history. Stratifying risk based on available information can tackle many of the more logistical barriers to risk‐based screening mentioned before. It is important to organise pilot experiences on risk‐based screening in order to support Health Technology Assessment evaluations and update of the guidelines.
In case of FIT‐based programmes, there are already programmes where information, such as sex, age and the quantitative FIT result (the faecal Hb concentration), is registered in existing databases for all screening participants, so additional questionnaires are not needed and no additional engagement is required for screenees in these programmes. Moreover, linking recommendations about screening intensity to the test results might have a limited impact on participation. Subjects referred for re‐screening at short interval would likely show a higher compliance, as it is the case for high‐risk subjects. A longer screening interval would be justified based on the longer duration of the protective effect of the test, given the observed results at previous tests, as opposed to a predicted low score of personal risk, which might reduce the risk of inducing a false sense of reassurance.
Of course, when starting with the low hanging fruit, it is important to build the infrastructure of the risk‐based programme in such a way that it can easily be adjusted to incorporate other types of risk factors, such as lifestyle and/or genetic information. IT infrastructure should be developed in a modular way, such that the module that assigns an individual's screening interval based on information on faecal Hb concentration from the screening database, can easily be replaced by a module that assigns screening interval based on that same information plus clinical information, for example, from GPs' electronic archives. Eventually such systems could even allow for individuals to opt for a different strategy than initially planned for them after informed decision‐making.
With all possibilities, it is important to recognise that risk‐stratification could always be further improved. However, the perfect should not become enemy of the good. Implementing risk‐based screening based on age, sex and prior screening history alone is a good improvement over current uniform screening approaches. Such an approach could involve the target population in the design and implementation of such a strategy to assess how risk‐based screening should be organised and communicated and this way help answer some of the prioritised questions identified by experts. 68 Perhaps it is time for the field to start there and continue improving towards more comprehensive risk‐based screening embedding primary prevention as an effective approach to lower risk for everyone. In other words, let us go from talking about risk‐based screening to implementing it, saving lives in optimal balance with harms and resources.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Lansdorp‐Vogelaar I, Meester R, de Jonge L, Buron A, Haug U, Senore C. Risk‐stratified strategies in population screening for colorectal cancer. Int. J. Cancer. 2022;150(3):397-405. doi: 10.1002/ijc.33784
REFERENCES
- 1. Correction to Lancet Gastroenterol Hepatol 2019; 4: 913–33. Lancet Gastroenterol Hepatol. 2020;5:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tinmouth J, Vella ET, Baxter NN, et al. Colorectal cancer screening in average risk populations: evidence summary. Can J Gastroenterol Hepatol. 2016;2016:2878149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lauby‐Secretan B, Vilahur N, Bianchini F, Guha N, Straif K, International Agency for Research on Cancer Handbook Working Group . The IARC perspective on colorectal cancer screening. N Engl J Med. 2018;378:1734‐1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64:1637‐1649. [DOI] [PubMed] [Google Scholar]
- 5. Blom J, Lowbeer C, Elfstrom KM, et al. Gender‐specific cut‐offs in colorectal cancer screening with FIT: increased compliance and equal positivity rate. J Med Screen. 2019;26:92‐97. [DOI] [PubMed] [Google Scholar]
- 6. Sarkeala T, Färkkilä M, Anttila A, et al. Piloting gender‐oriented colorectal cancer screening with a faecal immunochemical test: population‐based registry study from Finland. BMJ Open. 2021;11(2);e046667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Toft EL, Kaae SE, Malmqvist J, Brodersen J. Psychosocial consequences of receiving false‐positive colorectal cancer screening results: a qualitative study. Scand J Prim Health Care. 2019;37:145‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lello L, Raben TG, Yong SY, Tellier L, Hsu SDH. Genomic prediction of 16 complex disease risks including heart attack, diabetes, breast and prostate cancer. Sci Rep. 2019;9:15286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dunlop MG, Tenesa A, Farrington SM, et al. Cumulative impact of common genetic variants and other risk factors on colorectal cancer risk in 42,103 individuals. Gut. 2013;62:871‐881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hawken SJ, Greenwood CM, Hudson TJ, et al. The utility and predictive value of combinations of low penetrance genes for screening and risk prediction of colorectal cancer. Hum Genet. 2010;128:89‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khoury MJ, Janssens AC, Ransohoff DF. How can polygenic inheritance be used in population screening for common diseases? Genet Med. 2013;15:437‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974‐2013. J Natl Cancer Inst. 2017;109(8):djw322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Troeung L, Sodhi‐Berry N, Martini A, et al. Increasing incidence of colorectal cancer in adolescents and young adults aged 15‐39 years in Western Australia 1982‐2007: examination of colonoscopy history. Front Public Health. 2017;5:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vuik FE, Nieuwenburg SA, Bardou M, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut. 2019;68:1820‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jenkins MA, Makalic E, Dowty JG, et al. Quantifying the utility of single nucleotide polymorphisms to guide colorectal cancer screening. Future Oncol. 2016;12:503‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feng YL, Shu L, Zheng PF, et al. Dietary patterns and colorectal cancer risk: a meta‐analysis. Eur J Cancer Prev. 2017;26:201‐211. [DOI] [PubMed] [Google Scholar]
- 17. Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145‐164. [DOI] [PubMed] [Google Scholar]
- 18. Lansdorp‐Vogelaar I, van Ballegooijen M, Zauber AG, et al. Individualizing colonoscopy screening by sex and race. Gastrointest Endosc. 2009;70:96‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holme O, Loberg M, Kalager M, et al. Long‐term effectiveness of sigmoidoscopy screening on colorectal cancer incidence and mortality in women and men: a randomized trial. Ann Intern Med. 2018;168:775‐782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arana‐Arri E, Idigoras I, Uranga B, et al. Population‐based colorectal cancer screening programmes using a faecal immunochemical test: should faecal haemoglobin cut‐offs differ by age and sex? BMC Cancer. 2017;17:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Theuer CP, Taylor TH, Brewster WR, Anton‐Culver H. Gender and race/ethnicity affect the cost‐effectiveness of colorectal cancer screening. J Natl Med Assoc. 2006;98:51‐57. [PMC free article] [PubMed] [Google Scholar]
- 22. Meester RGS, Peterse EFP, Knudsen AB, et al. Optimizing colorectal cancer screening by race and sex: microsimulation analysis II to inform the American Cancer Society colorectal cancer screening guideline. Cancer. 2018;124:2974‐2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meulen MPV, Kapidzic A, Leerdam MEV, et al. Do men and women need to be screened differently with fecal immunochemical testing? A cost‐effectiveness analysis. Cancer Epidemiol Biomarkers Prev. 2017;26:1328‐1336. [DOI] [PubMed] [Google Scholar]
- 24. Wong MC, Ching JY, Chan VC, et al. Colorectal cancer screening based on age and gender: a cost‐effectiveness analysis. Medicine. 2016;95:e2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rahman R, Schmaltz C, Jackson CS, Simoes EJ, Jackson‐Thompson J, Ibdah JA. Increased risk for colorectal cancer under age 50 in racial and ethnic minorities living in the United States. Cancer Med. 2015;4:1863‐1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69:211‐233. [DOI] [PubMed] [Google Scholar]
- 27. Shavers VL. Racial/ethnic variation in the anatomic subsite location of in situ and invasive cancers of the colon. J Natl Med Assoc. 2007;99:733‐748. [PMC free article] [PubMed] [Google Scholar]
- 28. Mandelblatt J, Andrews H, Kao R, Wallace R, Kerner J. The late‐stage diagnosis of colorectal cancer: demographic and socioeconomic factors. Am J Public Health. 1996;86:1794‐1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Agrawal S, Bhupinderjit A, Bhutani MS, et al. Colorectal cancer in African Americans. Am J Gastroenterol. 2005;100:515‐523. [DOI] [PubMed] [Google Scholar]
- 30. World Cancer Research Fund/American Institute for Cancer Research Continuous Update Project Expert Report 2018. Diet, Nutrition, Physical Activity and Colorectal Cancer. 2018. dietandcancerreport.org. Accessed September 3, 2021.
- 31. Chatterjee N, Shi J, Garcia‐Closas M. Developing and evaluating polygenic risk prediction models for stratified disease prevention. Nat Rev Genet. 2016;17:392‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huyghe JR, Bien SA, Harrison TA, et al. Discovery of common and rare genetic risk variants for colorectal cancer. Nat Genet. 2019;51:76‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Law PJ, Timofeeva M, Fernandez‐Rozadilla C, et al. Association analyses identify 31 new risk loci for colorectal cancer susceptibility. Nat Commun. 2019;10:2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jiao S, Peters U, Berndt S, et al. Estimating the heritability of colorectal cancer. Hum Mol Genet. 2014;23:3898‐3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. National Cancer Institute The Colorectal Cancer Risk Assessment Tool. December 1, 2021. ccrisktool.cancer.gov. Accessed September 3, 2021.
- 36. Jeon J, Du M, Schoen RE, et al. Determining risk of colorectal cancer and starting age of screening based on lifestyle, environmental, and genetic factors. Gastroenterology. 2018;154:2152‐2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saunders CL, Kilian B, Thompson DJ, et al. External validation of risk prediction models incorporating common genetic variants for incident colorectal cancer using UK Biobank. Cancer Prev Res. 2020;13:509‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Naber SK, Kundu S, Kuntz KM, et al. Cost‐effectiveness of risk‐stratified colorectal cancer screening based on polygenic risk: current status and future potential. JNCI Cancer Spectr. 2020;4(1):pkz086. 10.1093/jncics/pkz086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Auge JM, Pellise M, Escudero JM, et al. Risk stratification for advanced colorectal neoplasia according to fecal hemoglobin concentration in a colorectal cancer screening program. Gastroenterology. 2014;147:628‐636. [DOI] [PubMed] [Google Scholar]
- 40. Chiu SY, Chuang SL, Chen SL, et al. Faecal haemoglobin concentration influences risk prediction of interval cancers resulting from inadequate colonoscopy quality: analysis of the Taiwanese Nationwide Colorectal Cancer Screening Program. Gut. 2017;66:293‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Digby J, Fraser CG, Carey FA, Diament RH, Balsitis M, Steele RJ. Faecal haemoglobin concentration is related to detection of advanced colorectal neoplasia in the next screening round. J Med Screen. 2017;24:62‐68. [DOI] [PubMed] [Google Scholar]
- 42. Kooyker AI, Toes‐Zoutendijk E, Opstal‐van Winden AWJ, et al. The second round of the Dutch colorectal cancer screening program: impact of an increased fecal immunochemical test cut‐off level on yield of screening. Int J Cancer. 2020;147:1098‐1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peng SM, Chiu HM, Jen HH, et al. Quantile‐based fecal hemoglobin concentration for assessing colorectal neoplasms with 1,263,717 Taiwanese screenees. BMC Med Inform Decis Mak. 2019;19:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van de Veerdonk W, Van Hal G, Peeters M, De Brabander I, Silversmit G, Hoeck S. Risk stratification for colorectal neoplasia detection in the Flemish colorectal cancer screening programme. Cancer Epidemiol. 2018;56:90‐96. [DOI] [PubMed] [Google Scholar]
- 45. Buron A, Roman M, Auge JM, et al. Changes in FIT values below the threshold of positivity and short‐term risk of advanced colorectal neoplasia: results from a population‐based cancer screening program. Eur J Cancer. 2019;107:53‐59. [DOI] [PubMed] [Google Scholar]
- 46. Grobbee EJ, Schreuders EH, Hansen BE, et al. Association between concentrations of hemoglobin determined by fecal immunochemical tests and long‐term development of advanced colorectal neoplasia. Gastroenterology. 2017;153:1251‐1259. [DOI] [PubMed] [Google Scholar]
- 47. Senore C, Zappa M, Campari C, et al. Faecal haemoglobin concentration among subjects with negative FIT results is associated with the detection rate of neoplasia at subsequent rounds: a prospective study in the context of population based screening programmes in Italy. Gut. 2020;69:523‐530. [DOI] [PubMed] [Google Scholar]
- 48. Cooper JA, Moss SM, Smith S, et al. FIT for the future: a case for risk‐based colorectal cancer screening using the faecal immunochemical test. Colorectal Dis. 2016;18:650‐653. [DOI] [PubMed] [Google Scholar]
- 49. Cooper JA, Parsons N, Stinton C, et al. Risk‐adjusted colorectal cancer screening using the FIT and routine screening data: development of a risk prediction model. Br J Cancer. 2018;118:285‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stegeman I, de Wijkerslooth TR, Stoop EM, et al. Combining risk factors with faecal immunochemical test outcome for selecting CRC screenees for colonoscopy. Gut. 2014;63:466‐471. [DOI] [PubMed] [Google Scholar]
- 51. Lieberman DA. Targeted colon cancer screening: a concept whose time has almost come. Am J Gastroenterol. 1992;87:1085‐1093. [PubMed] [Google Scholar]
- 52. Shieh Y, Hu D, Ma L, et al. Breast cancer risk prediction using a clinical risk model and polygenic risk score. Breast Cancer Res Treat. 2016;159:513‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ponti A, Anttila A, Ronco G, et al. Cancer Screening in the European Union. Report on the Implementation of the Council Recommendation on Cancer Screening. Lyon, France: International Agency for Research on Cancer; 2017. https://ec.europa.eu/health/sites/health/files/major_chronic_diseases/docs/2017_cancerscreening_2ndreportimplementation_en.pdf
- 54. Haug U, Senore C, Corley DA. Promises and potential pitfalls of shared decision making in cancer screening. Gastroenterology. 2020;158:802‐805. [DOI] [PubMed] [Google Scholar]
- 55. Rose G. Strategy of prevention: lessons from cardiovascular disease. Br Med J. 1981;282:1847‐1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016;315:2595‐2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. van Hees F, Zauber AG, van Veldhuizen H, et al. The value of models in informing resource allocation in colorectal cancer screening: the case of The Netherlands. Gut. 2015;64:1985‐1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. van Hees F, Saini SD, Lansdorp‐Vogelaar I, et al. Personalizing colonoscopy screening for elderly individuals based on screening history, cancer risk, and comorbidity status could increase cost effectiveness. Gastroenterology. 2015;149:1425‐1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cenin DR, Tinmouth J, Naber SK, et al. Calculation of stop ages for colorectal cancer screening based on comorbidities and screening history. Clin Gastroenterol Hepatol. 2021;19(3):547‐555. 10.1016/j.cgh.2020.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Senore C, Basu P, Anttila A, et al. Performance of colorectal cancer screening in the European Union Member States: data from the second European screening report. Gut. 2019;68:1232‐1244. [DOI] [PubMed] [Google Scholar]
- 61. Navarro M, Nicolas A, Ferrandez A, Lanas A. Colorectal cancer population screening programs worldwide in 2016: an update. World J Gastroenterol. 2017;23:3632‐3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rees G, Martin PR, Macrae FA. Screening participation in individuals with a family history of colorectal cancer: a review. Eur J Cancer Care. 2008;17:221‐232. [DOI] [PubMed] [Google Scholar]
- 63. Edwards AG, Naik G, Ahmed H, et al. Personalised risk communication for informed decision making about taking screening tests. Cochrane Database Syst Rev. 2013;2013(2):CD001865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chen H, Lu M, Liu C, et al. Comparative evaluation of participation and diagnostic yield of colonoscopy vs fecal immunochemical test vs risk‐adapted screening in colorectal cancer screening: interim analysis of a multicenter randomized controlled trial (TARGET‐C). Am J Gastroenterol. 2020;115:1264‐1274. [DOI] [PubMed] [Google Scholar]
- 65. Hewitson P, Ward AM, Heneghan C, Halloran SP, Mant D. Primary care endorsement letter and a patient leaflet to improve participation in colorectal cancer screening: results of a factorial randomised trial. Br J Cancer. 2011;105:475‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Church T. Colorectal cancer screening: will non‐invasive procedures triumph? Genome Med. 2014;6:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cotter AR, Vuong K, Mustelin L, et al. Do psychological harms result from being labelled with an unexpected diagnosis of abdominal aortic aneurysm or prostate cancer through screening? A systematic review. BMJ Open. 2017;7:e017565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chowdhury S, Dent T, Pashayan N, et al. Incorporating genomics into breast and prostate cancer screening: assessing the implications. Genet Med. 2013;15:423‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fijisawa D, Hagiwara N. Cancer stigma and its health consequences. Curr Breast Cancer Rep. 2015;7:143‐150. [Google Scholar]
- 70. Marlow LA, Waller J, Wardle J. Does lung cancer attract greater stigma than other cancer types? Lung Cancer. 2015;88:104‐107. [DOI] [PubMed] [Google Scholar]
- 71. Wauters A, Van Hoyweghen I. Global trends on fears and concerns of genetic discrimination: a systematic literature review. J Hum Genet. 2016;61:275‐282. [DOI] [PubMed] [Google Scholar]
- 72. Meisel SF, Pashayan N, Rahman B, et al. Adjusting the frequency of mammography screening on the basis of genetic risk: attitudes among women in the UK. Breast. 2015;24:237‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Berstad P, Botteri E, Larsen IK, et al. Lifestyle changes at middle age and mortality: a population‐based prospective cohort study. J Epidemiol Community Health. 2017;71:59‐66. [DOI] [PubMed] [Google Scholar]
- 74. Carr PR, Weigl K, Edelmann D, et al. Estimation of absolute risk of colorectal cancer based on healthy lifestyle, genetic risk, and colonoscopy status in a population‐based study. Gastroenterology. 2020;159:129‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]


