Abstract
Effective treatment of inflammatory diseases is often challenging owing to their heterogeneous pathophysiology. Understanding of the underlying disease mechanisms is improving and it is now clear that eosinophils play a complex pathophysiological role in a broad range of type 2 inflammatory diseases. Standard of care for these conditions often still includes oral corticosteroids (OCS) and/or cytotoxic immune therapies, which are associated with debilitating side effects. Selective, biological eosinophil‐reducing agents provide treatment options that improve clinical symptoms associated with eosinophilic inflammation and reduce OCS use. Mepolizumab is a humanized monoclonal antibody that binds to and neutralizes interleukin‐5, the major cytokine involved in eosinophil proliferation, activation, and survival. Mepolizumab is approved for the treatment of severe eosinophilic asthma, eosinophilic granulomatosis with polyangiitis and hypereosinophilic syndrome. Additionally, the efficacy of add‐on mepolizumab has been observed in patients with severe chronic rhinosinusitis with nasal polyposis and chronic obstructive pulmonary disease with an eosinophilic phenotype. Here, we review the development, approval, and real‐world effectiveness of mepolizumab for the treatment of patients with severe eosinophilic asthma, from the DREAM to REALITI‐A studies, and describe how knowledge from this journey extended to the use of mepolizumab and other biologics across a broad spectrum of eosinophilic diseases.
Keywords: clinical trials, eosinophilic diseases, interleukin‐5, mepolizumab, real‐world data
1. INTRODUCTION
Elevated eosinophil counts are implicated in several type 2 inflammatory diseases that occur at various sites throughout the body (Figure 1). 1 , 2 Over the last 20 years, our understanding of diseases driven by elevated eosinophil counts has advanced through the development of eosinophil‐depleting medicines. One such compound is mepolizumab, a humanized monoclonal antibody that binds to and neutralizes interleukin (IL)‐5, 3 , 4 the major cytokine involved in the proliferation, maturation, activation, recruitment, and survival of eosinophils (Figure 2). 2 Mepolizumab was first studied in patients with asthma in the late 1990s 5 and was approved as a first‐in‐class, add‐on treatment for adults with severe eosinophilic asthma in 2015 (Figure 3). 6 Since then, mepolizumab has been approved for pediatric and adult patients (aged ≥ 6 years) with severe eosinophilic asthma, for adult patients with eosinophilic granulomatosis with polyangiitis (EGPA), and for patients aged ≥ 12 years with hypereosinophilic syndrome (HES) (Figure 3). 4 Other eosinophil‐targeting monoclonal antibodies approved as add‐on therapies in patients with severe eosinophilic asthma include reslizumab (IL‐5 antagonist monoclonal antibody for patients aged ≥ 18 years) and benralizumab (IL‐5 receptor alpha‐directed cytolytic monoclonal antibody for patients aged ≥ 12 years) (Figure 2). 7 , 8 Furthermore, the IL‐4 receptor alpha antagonist, dupilumab, has been approved for use in moderate‐to‐severe asthma, chronic rhinosinusitis with nasal polyposis (CRSwNP) and moderate‐to‐severe atopic dermatitis, and the anti‐immunoglobulin (Ig) E antibody, omalizumab, has been approved for use in moderate‐to‐severe, persistent, allergic asthma, nasal polyps, and chronic idiopathic urticaria. 9 , 10
FIGURE 1.
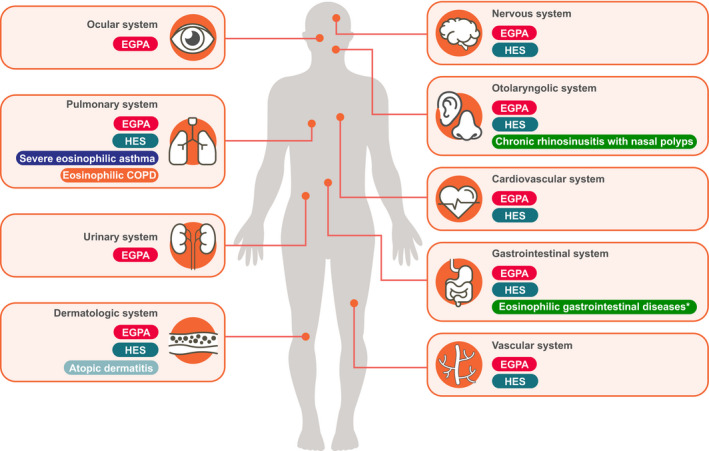
Sites of eosinophilic diseases. *Eosinophilic gastritis, eosinophilic gastroenteritis, eosinophilic colitis. COPD, chronic obstructive pulmonary disease; EGPA, eosinophilic granulomatosis with polyangiitis; HES, hypereosinophilic syndrome
FIGURE 2.
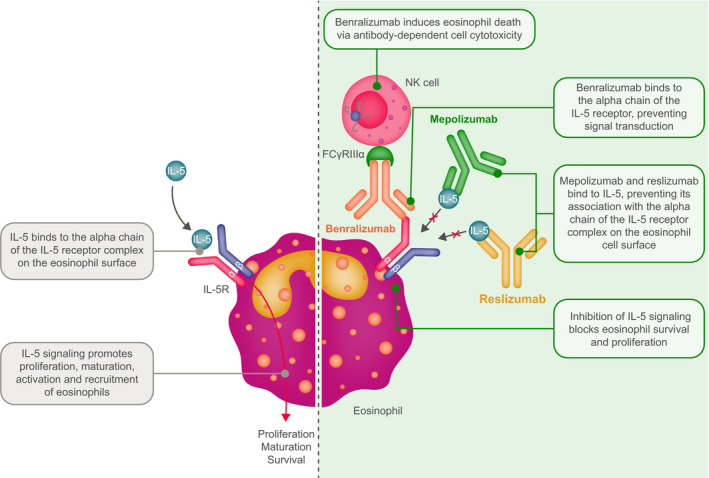
Mechanisms of action of anti‐IL‐5/anti‐IL‐5‐receptor antibodies. IL, interleukin
FIGURE 3.
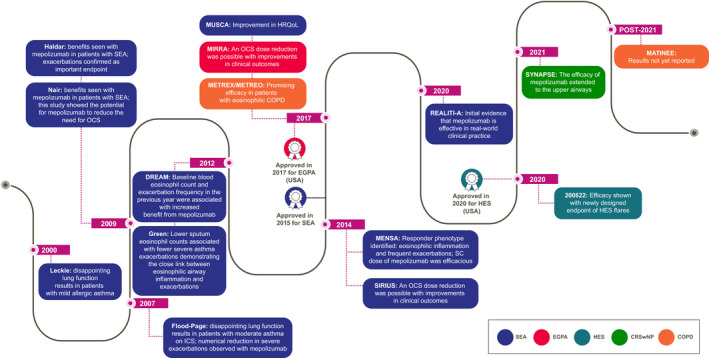
Timeline of the Phase III trials and approvals of mepolizumab in eosinophil‐driven diseases and the key takeaways. COPD, chronic obstructive pulmonary disease; CRSwNP, chronic rhinosinusitis with nasal polyposis; DREAM, Dose Ranging Efficacy And safety with Mepolizumab in severe asthma; EGPA, eosinophilic granulomatosis with polyangiitis; HES, hypereosinophilic syndrome; HRQoL, health‐related quality of life; ICS, inhaled corticosteroids; MATINEE, Mepolizumab as Add‐on Treatment IN participants with COPD characterized by frequent Exacerbations and Eosinophil level; MENSA, MEpolizumab as adjunctive therapy iN patients with Severe Asthma; METREO, MEpolizumab vs. placebo as add‐on TReatment for frequently exacerbating COPD patients characterized by EOsinophil level; METREX, MEpolizumab vs. placebo as add‐on TReatment for frequently EXacerbating COPD patients; MIRRA, Mepolizumab In Relapsing or Refractory EGPA; MUSCA, Mepolizumab adjUnctive therapy in subjects with Severe eosinophiliC Asthma; OCS, oral corticosteroids; REALITI‐A, REAL world effectiveness of mepolizumab In paTIent care—Asthma; SC, subcutaneous; SEA, severe eosinophilic asthma; SIRIUS, SteroId ReductIon with mepolizUmab Study; SYNAPSE, StudY in NAsal Polyps patients to assess the Safety and Efficacy of mepolizumab
Early studies demonstrated the association of blood and tissue eosinophilia with bronchial asthma, 11 and the relationship between elevated eosinophil counts in blood and sputum and disease severity. 12 This was supported during the clinical development of mepolizumab, which showed that mepolizumab induces sustained, significant reductions in blood eosinophil counts with improved clinical outcomes in patients with severe asthma who had an eosinophilic phenotype. 13 , 14 , 15 , 16 Furthermore, blood eosinophil count was identified as a treatable trait and was established as a validated pharmacodynamic and predictive biomarker for response to mepolizumab in patients with severe eosinophilic asthma. 13 , 17 , 18 , 19
Experience has shown that an understanding of pathobiology in one disease can be extended to other diseases, in which biological pathways overlap. Moreover, in‐depth knowledge of a disease with a relatively straightforward pathogenesis may benefit from understanding and identification of treatment options in diseases with more complex underlying mechanisms. Indeed, this has occurred in other fields. For example, tocilizumab, a humanized monoclonal antibody that targets the IL‐6 receptor, was first approved in Castleman disease and later approved for other IL‐6‐related diseases, such as rheumatoid arthritis, and is now being evaluated for the treatment of severe COVID‐19 pneumonia. 20 , 21 , 22 Overall, discovery of the importance of eosinophils during the development of mepolizumab for asthma, along with the confirmation that neutralizing IL‐5 results in eosinophil reduction and disease control in HES, paved the way for mepolizumab treatment in other eosinophilic disorders. 6 , 23
2. SEVERE EOSINOPHILIC ASTHMA WAS THE FIRST DISEASE APPROVED FOR ANTI‐IL‐5 TREATMENTS (MEPOLIZUMAB) THAT SPECIFICALLY REDUCE EOSINOPHIL COUNTS
Eosinophils are derived in the bone marrow and reside in a range of tissues in healthy individuals. 24 , 25 They are multifunctional cells involved in the modulation of innate and adaptive immune responses as well as tissue homeostasis, remodeling, and repair. 24 , 26 , 27 Eosinophils express several membrane receptors that are critical to their function, including IL‐5 receptor alpha, that binds IL‐5 to facilitate eosinophil differentiation, maturation, homing and survival, and CC chemokine receptor 3, which binds the eotaxins responsible for migration of eosinophils into target tissues. 28 , 29 , 30 In addition to cytokines, enzymes, and growth factors, eosinophils contain specific cytoplasmic granule proteins that are released into target tissues upon activation. 31
The role of eosinophils in asthma pathogenesis was highlighted in the 1980s, when it was noted that they secrete proteins that damage bronchial epithelium when in an activated state. 11 Blood and airway eosinophils in humans with asthma were later shown to correlate with asthma severity, 12 and treatment directed at normalizing sputum eosinophil counts was shown to markedly reduce severe asthma exacerbations. 32 Analyses of IL‐5 knockout mice and a mouse model of asthma also showed that IL‐5 and eosinophilia play a pivotal role in the development of lung inflammation in asthma 33 , 34 ; in vitro experiments confirmed IL‐5 as a useful target in eosinophil‐driven diseases. 23
Establishing the clinical efficacy of eosinophil‐reducing treatment in patients was not straightforward; while mepolizumab treatment was shown to reduce markers of airway remodeling in patients with mild atopic asthma, 35 early trials of mepolizumab in a mild allergic asthma population in 2000 and in a moderate asthma population in 2007 were considered unsuccessful in terms of clinical improvement 36 , 37 ; early trials of reslizumab in patients with severe persistent asthma were also unsuccessful. 38 Although mepolizumab reduced blood and sputum eosinophil counts in the two early studies, there were no significant clinical improvements in the endpoints commonly assessed in asthma at the time, such as lung function and asthma symptoms. 36 , 37 At that point in the development of mepolizumab, patients had been selected based on clinical and physiological characteristics, not on the presence of eosinophilic inflammation, and it is now clear that the outcomes in these studies were not closely associated with eosinophilic airway inflammation. 36 , 37 However, while there was no difference in lung function for patients treated with mepolizumab versus placebo in Flood‐Page et al., there was a numerical reduction in the percentage of patients with severe exacerbations with mepolizumab. 37 This suggested that mepolizumab might reduce exacerbations in patients with severe asthma.
Increasing knowledge surrounding the link between exacerbations, eosinophilic airway inflammation, and asthma symptoms led to the discovery that eosinophilic airway inflammation correlated more closely with asthma exacerbations and oral corticosteroid (OCS) responsiveness than with asthma symptoms and variable airflow limitation. 23 , 39 , 40 An exploratory investigation was then conducted with mepolizumab in patients with refractory asthma, sputum eosinophils >3% despite high‐dose inhaled corticosteroid treatment, and at least two exacerbations requiring rescue prednisolone treatment in the previous 12 months. 41 Reductions in blood and sputum eosinophil counts seen among patients corresponded with a 43% reduction (p = .02) in exacerbations versus placebo and a 0.35‐point improvement (p = .02) in asthma quality of life questionnaire (AQLQ) score; however, there were no statistically significant differences between the groups in lung function or asthma symptoms (measured monthly). 41 In another, small, exploratory study in patients with persistent sputum eosinophilia and continued symptoms despite maintenance OCS therapy, published concurrently, mepolizumab reduced blood and sputum eosinophil counts to within normal limits, versus placebo, and permitted a reduction in maintenance OCS dose without any increase in asthma exacerbations. 42 The development of mepolizumab therefore required a fundamental rethink of the way asthma was viewed. Of particular importance was the recognition that eosinophilic airway inflammation and airway reversibility to bronchodilators (ie, the definition of asthma) are relatively independent treatable traits, and the realization that eosinophilic airway inflammation is associated particularly with asthma exacerbations. 23
The target population that would benefit from mepolizumab treatment was identified in 2012 based on data from the DREAM (Dose Ranging Efficacy And safety with Mepolizumab in severe asthma; NCT01000506) clinical trial; this trial showed that mepolizumab was efficacious in patients with severe asthma, evidence of eosinophilic inflammation, and a history of exacerbations. 13 In addition, it was shown that baseline peripheral blood eosinophil count and exacerbation frequency in the previous year were associated with response to mepolizumab (Table 1; Figure 3). 13 The correlation between blood eosinophil count and mepolizumab response was confirmed in the Phase III MENSA (MEpolizumab as adjunctive therapy iN patients with Severe Asthma; NCT01691521) trial (Table 1; Figure 3), in which a direct relationship between baseline blood eosinophil count and reduction in exacerbation rates was shown. 14 Importantly, clinically relevant reductions in exacerbation rates (54% in DREAM and 53% in MENSA) were observed at baseline blood eosinophil counts of ≥150 cells/µl. 17 As patient stratification improved throughout the development of mepolizumab, there was more clear evidence of treatment benefits in lung function and asthma symptoms. 43 Taken together, in patients with severe eosinophilic asthma, mepolizumab treatment was shown to reduce exacerbations, increase forced expiratory volume in 1 s (FEV1) and improve both asthma control and quality of life in the DREAM, MENSA, and MUSCA (Mepolizumab adjUnctive therapy in subjects with Severe eosinophiliC Asthma; NCT02281318) trials, 13 , 14 , 43 and to have an OCS‐sparing effect in the SIRIUS (SteroId ReductIon with mepolizUmab Study; NCT01691508) trial (Table 1; Figure 3). 44 Clinical benefit with mepolizumab treatment has now been clearly demonstrated in randomized controlled trials in patients with a blood eosinophil count of ≥150 cells/µl despite standard of care treatment including inhaled and/or systemic corticosteroids. 14 , 17 , 44 With increasing use in clinical practice, the improvements with mepolizumab noted in clinical trials are now being confirmed in the real world. 45 , 46 , 47 , 48 For example, in an initial analysis from the large prospective REALITI‐A (REAL world effectiveness of mepolizumab In paTIent care—Asthma) study (n = 368), there was an 83% reduction in blood eosinophil count and a significant 69% reduction in the rate of clinically significant exacerbations in the 12 months following initiation of mepolizumab treatment. 45 Furthermore, a clinically meaningful 52% median reduction in daily maintenance OCS dose was shown in the 12 months post versus the 12 months pre‐mepolizumab treatment initiation.
TABLE 1.
Phase III a mepolizumab clinical trials, primary endpoints, and key inclusion/exclusion criteria across eosinophilic‐driven diseases
| Eosinophilic‐driven disease and studies | ITT/mITT/treated population, n | Key criteria for patient population (full details are in the publications) | Treatment dosages | Primary and secondary endpoint results |
|---|---|---|---|---|
| Severe eosinophilic asthma | ||||
|
DREAM 13 Pavord, et al. 2012 |
616 |
|
Mepolizumab 75 mg IV Mepolizumab 250 mg IV Mepolizumab 750 mg IV Placebo Every 4 weeks for 52 weeks Treatment was plus SoC |
Primary
Rate of clinically significant exacerbations/patient/year
b
: reduced vs. placebo by:
Secondary
Mean pre‐bronchodilator FEV1: improved vs. placebo by:
Mean ACQ score: improved vs. placebo by:
Mean AQLQ score: improved vs. placebo by:
Geometric mean FeNO: ratio to placebo:
|
|
MENSA 14 Ortega, et al. 2014 |
576 |
|
Mepolizumab 75 mg IV Mepolizumab 100 mg SC Placebo Every 4 weeks for 32 weeks Treatment was plus SoC |
Primary
Rate of clinically significant exacerbations/patient/year
b
: reduced vs. placebo by:
Secondary
Mean pre‐bronchodilator FEV1: improved vs. placebo by:
Mean ACQ score: improved vs. placebo by:
Mean SGRQ score: improved vs. placebo by:
|
|
SIRIUS 44 Bel, et al. 2014 |
135 |
|
Mepolizumab 100 mg SC Placebo Every 4 weeks for 20 weeks Treatment was plus SoC |
Primary Daily OCS dose reduction category (90–100%; 75–<90%; 50–<75%; >0–<50%): mepolizumab vs. placebo: overall OR of 2.39 (95% CI 1.25, 4.56; p = .008) Other Clinically significant exacerbations/patient/year b : reduced by 32% vs. placebo (RR 0.68 [95% CI 0.47, 0.99]; p = .04) Mean ACQ‐5 score: improved vs. placebo by −0.52 (95% CI −0.87, −0.17; p = .004) Mean SGRQ score: improved vs. placebo by −5.8 (95% CI −10.6, −1.0; p = .02) Mean pre‐bronchodilator FEV1: improved vs. placebo by 114 ml (p = .15) |
|
MUSCA 43 Chupp, et al. 2017 |
551 |
|
Mepolizumab 100 mg SC Placebo Every 4 weeks for 24 weeks Treatment was plus SoC |
Primary Mean SGRQ total score: improved vs. placebo by −7.7 (95% CI −10.5, −4.9; p < .0001) c Secondary Mean ACQ‐5 score: improved vs placebo by −0.4 (95% CI −0.6, −0.2; p < .0001) Mean pre‐bronchodilator FEV1 : improved vs placebo by 120 ml (95% CI 47, 192; p = .001) Rate of clinically significant exacerbations/patient/year: 58% reduction vs. placebo (RR 0.42 [95% CI 0.31, 0.56; p < .0001]) |
|
REALITI‐A a Harrison, et al. 2020 45 |
368 |
|
Mepolizumab 100 mg SC as prescribed in clinical practice (every 4 weeks) Treatment was plus SoC |
Rate of clinically significant asthma exacerbations/patient/year b during the 12‐month mepolizumab follow‐up period vs. the pre‐mepolizumab treatment period: relative reduction of 69% (RR 0.31 [95% CI 0.27, 0.35]; p < .001) Other Median daily maintenance OCS dose: the median percent reduction during treatment up to Weeks 53–56 was 52% (95% CI 50.0, 75.0) |
| EGPA | ||||
|
MIRRA 64 Wechsler, et al. 2017 |
136 |
|
Mepolizumab 300 mg SC Placebo Every 4 weeks for 52 weeks Treatment was plus SoC |
Co‐primary Total accrued weeks of remission e : 28% of the patients in the mepolizumab group vs. 3% in the placebo group had remission for ≥24 weeks Proportion of patients who had remission: 32% patients in the mepolizumab group vs. 3% patients in the placebo group had remission at both Week 36 and week 48 (OR 16.74 [95% CI 3.61, 77.56]; p < .001). |
| HES | ||||
|
200622 82 Roufosse, et al. 2020 |
108 |
|
Mepolizumab 300 mg SC Placebo Every 4 weeks for 32 weeks Treatment was in addition to existing background HES therapy (whether chronic or episodic) |
Primary Proportion of patients who experienced an HES flare g : was 50% lower for patients receiving mepolizumab vs. placebo (n = 15/54 [28%] vs. n = 30/54 [56%]; p = .002). |
| CRSwNP | ||||
|
SYNAPSE 101 Han, et al. 2021 |
407 |
|
Mepolizumab 100 mg SC Placebo Every 4 weeks (by safety syringe) for 52 weeks Treatment was plus SoC, including intranasal corticosteroids |
Co‐primary
Median change from baseline in total endoscopic NP score
h
:
Median change from baseline in nasal obstruction VAS score
h
:
Key secondary
Time to first nasal surgery up to Week 52:
|
| COPD | ||||
|
METREX/METREO 121 Pavord, et al. 2017 |
836/674 |
|
METREX: mepolizumab 100 mg SC or placebo METREO: mepolizumab 100 mg SC, mepolizumab 300 mg SC or placebo In both trials, treatment was administered every 4 weeks, for 52 weeks Treatment was plus SoC |
Primary Rate of moderate/severe exacerbations reduced vs. placebo by:
|
|
MATINEE 124 ClinicalTrials.gov, 2020. https://clinicaltrials.gov/ct2/show/NCT04133909 |
Target: 800 |
|
Mepolizumab 100 mg SC Placebo Every 4 weeks for 52 weeks Treatment s plus SoC (ICS plus 2 additional COPD medications [ie, ICS‐based triple therapy]) |
Primary Annualized rate of moderate/severe exacerbations Primary endpoint: rate of moderate or severe exacerbations Study not yet completed |
Abbreviations: BVAS, Birmingham Vasculitis Activity Score; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CRSwNP, chronic rhinosinusitis with nasal polyposis; ED, emergency department; EGPA, eosinophilic granulomatosis with polyangiitis; FeNO, an exhaled nitric oxide concentration; FEV1, forced expiratory volume in 1 s; HES, hypereosinophilic syndrome; HRQOL, health‐related quality of life; ICS, inhaled corticosteroids; ITT, intent‐to‐treat; IV, intravenous; LABA, long‐acting β2‐agonist; LAMA, long‐acting muscarinic‐receptor antagonist; MCID, minimal clinically important difference; mITT, modified intent‐to‐treat; NP, nasal polyposis; OCS, oral corticosteroids; OR, odds ratio; RR, rate ratio; SC, subcutaneous; SCS, systemic corticosteroid(s); SoC, standard of care; VAS, visual analog scale.
All trials are Phase III except for the REALITI‐A study, which was a real‐world study.
Clinically significant exacerbations were defined as the worsening of asthma requiring systemic corticosteroids for ≥3 days (or a doubling [or more] of the existing maintenance dose of OCS for ≥3 days if patients were on maintenance OCS) or an ED visit or hospital admission.
A reduction in SGRQ is indicative of improvement. The SGRQ is scored from 0 to 100, with higher scores indicating worse HRQOL. The MCID is a 4‐point reduction in score. 43
Criteria typical of EGPA included histopathological evidence of eosinophilic vasculitis, perivascular eosinophilic infiltration, or eosinophil‐rich granulomatous inflammation; neuropathy; pulmonary infiltrates; sinonasal abnormality; cardiomyopathy; glomerulonephritis; alveolar hemorrhage; palpable purpura; or antineutrophil cytoplasmic antibody positivity.
Defined as a BVAS of 0 and the receipt of prednisolone or prednisone ≤4.0 mg/day during the 52‐week period. The BVAS version 3 has a scale of 0–63, with higher scores indicating greater disease activity.
HES diagnosis was based on organ system involvement and/or dysfunction that could be directly related to a blood eosinophil count more than 1500 cells/µl on ≥2 occasions, and/or tissue eosinophilia, without a discernible secondary cause.
HES flares during the treatment period were defined as either of the following: physician‐documented change in clinical signs or symptoms of a HES‐related clinical manifestation that required an increase in maintenance OCS dose by ≥10 mg prednisone equivalent/day for 5 days or an increase in/addition of any cytotoxic and/or immunosuppressive HES therapy; 2 receipt of ≥2 courses of blinded OCS during the treatment period, blinded OCS was administered for approx. Two weeks if the blood eosinophil count exceeded a predefined threshold (2 × baseline value [randomization] or baseline value +2500 cells/µl).
Co‐primary endpoints. Total endoscopic NP score was the sum of left and right nostril scores ranging from 0 (no polyps) to 4 (large polyps causing complete obstruction of the inferior meatus) giving a total score of up to 8. VAS scores ranged from 0.0 to 10.0.
The clinical benefits associated with targeting the IL‐5 pathway in patients with asthma have been further supported by the results of clinical trials with intravenous (IV) reslizumab and subcutaneous (SC) benralizumab, both of which have shown reduced exacerbations and improved lung function and quality of life in patient populations similar to those included in the mepolizumab trials. 49 , 50 , 51 , 52 , 53 An OCS‐sparing effect has also been shown with reslizumab 54 and benralizumab. 55 Trials with benralizumab also confirmed the relationship between baseline blood eosinophil counts ≥ 150 cells/µl and response to treatment. 56
3. MEPOLIZUMAB TREATMENT IN THE RARE DISEASE EOSINOPHILIC GRANULOMATOSIS WITH POLYANGIITIS CONFIRMED THAT THE IL‐5 PATHWAY COULD BE TARGETED IN OTHER EOSINOPHIL‐DRIVEN DISEASES
EGPA (also known as Churg‐Strauss syndrome) is a rare, complex inflammatory disease defined by an eosinophil‐rich granulomatosis often involving the respiratory tract, and necrotizing eosinophilic vasculitis predominantly affecting small to medium‐sized vessels; it is associated with asthma, marked blood or tissue eosinophilia, sinusitis/nasal polyps, neuropathy, and in 30%–40% of patients, antineutrophil cytoplasmic antibodies (ANCA). 57 , 58 , 59 Along with T and B cells, eosinophils are key inflammatory cells involved in causing tissue damage in EGPA. 59 Historically, conventional treatment was with OCS, with or without additional immunosuppressive drugs, 59 but many patients experienced corticosteroid‐related side effects or relapses during OCS tapering. 60 As a result, healthcare resource utilization and burden of disease and treatment were high. 61
Since eosinophils contribute to the pathophysiology of EGPA, and with severe eosinophilic asthma being a key feature of the disease, 57 mepolizumab was postulated as a potential treatment option for patients with EGPA. In 2010, early data on the use of mepolizumab in patients with EGPA were published in a case report and in a pilot trial. 62 , 63 Following the promising early findings shown in these publications, which showed reductions in OCS and exacerbation rates with mepolizumab, recruitment of patients with relapsing or refractory EGPA (regardless of ANCA status) who were receiving standard of care therapy began in 2014 for the Phase III MIRRA (Mepolizumab In Relapsing or Refractory EGPA; NCT02020889) trial. 64 In this 136 patient study, add‐on mepolizumab therapy (300 mg SC), versus placebo, led to a reduction in blood eosinophil count and more accrued time in remission, reduced the rate of relapse, and allowed patients to decrease their OCS use (Table 1). 64 Based on these findings, mepolizumab 300 mg SC was approved for EGPA in the USA in 2017 (Figure 3). 65 Notably, not all patients in the mepolizumab group in MIRRA achieved protocol‐defined remission (a Birmingham Vasculitis Activity Score of 0 [scale: 0–63] and a prednisolone/prednisone dose of ≤4.0 mg/day over a 52‐week period). However, a post hoc analysis using a composite endpoint comprising remission, OCS reduction, and/or being relapse free for the treatment period showed that mepolizumab provided clinical benefit in up to 87% of patients with EGPA beyond the remission‐based primary endpoints. 66 Early evidence of efficacy for other anti‐IL‐5 treatments, reslizumab, and benralizumab has also been shown in recent small studies (≤10 patients). 67 , 68 , 69 A Phase III trial of benralizumab in EGPA is currently underway (MANDARA; NCT04157348). 70
Overall, the clinical benefits associated with eosinophil reduction in patients with EGPA confirmed that eosinophils contribute to EGPA disease pathology and supported the use of mepolizumab in eosinophil‐driven diseases beyond severe eosinophilic asthma.
4. MEPOLIZUMAB TREATMENT FOR THE RARE DISEASE HYPEREOSINOPHILIC SYNDROME HIGHLIGHTED THE IMPORTANCE OF IDENTIFYING THE APPROPRIATE ENDPOINT TO ASSESS EFFICACY
HES is a rare, heterogeneous group of disorders defined by the presence of persistent eosinophil counts in blood and/or tissues (≥1500 cells/μl in the blood), and evidence of a major role for eosinophils in end‐organ damage. 71 In the absence of validated biomarkers, an elevated blood eosinophil count is generally considered to be a surrogate marker for tissue eosinophilia and organ damage in patients with HES; a reduction in the blood eosinophil count is therefore a therapeutic objective, to reverse and prevent further damage. 72 , 73 , 74 Since the underlying mechanisms resulting in eosinophilia vary across the HES subtypes (reviewed elsewhere 6 ), differences in clinical presentation, prognosis, and responses to therapy occur among patients with HES. 71 , 75 , 76 , 77 , 78 Treatment options for HES are limited, with the standard of care generally comprising systemic corticosteroids and cytotoxic/immunosuppressive therapy, similar to patients with EGPA. 59 , 74
The potential of mepolizumab for the treatment of HES was shown as early as 2003/2004 in a small case series and an open‐label, non‐controlled trial, in which it was shown to reduce blood eosinophil counts and have an OCS‐sparing effect. 79 , 80 It subsequently became available for compassionate use in 2005 (NCT00244686). 73 , 81 In 2008, a randomized, double‐blind, placebo‐controlled, trial evaluating the efficacy of mepolizumab versus placebo in OCS‐dependent (20–60 mg/day) patients with HES without the FIP1L1‐PDGFRA fusion gene (GSK ID: MHE100185; NCT00086658) demonstrated that 4‐weekly mepolizumab infusions (750 mg IV) resulted in a higher proportion of patients able to taper to ≤10 mg/day prednisone (odds ratio 8.0; 95% CI 2.7, 23.8; p < .001) versus placebo. 72 Other endpoints assessed in this trial showed further benefits of mepolizumab treatment; there was a significantly lower daily prednisone dose at the end of the study (Week 36) with mepolizumab versus placebo (6.2 mg vs. 21.8 mg) and a significantly higher proportion of patients stopping prednisone completely during the treatment period with mepolizumab versus placebo (47% vs. 5%). 72
Despite the clinical success demonstrated in the NCT00086658 trial, the development of mepolizumab in HES was halted temporarily owing to absence of established efficacy endpoints deemed suitable by regulatory agencies; efficacy could not be based solely on reductions in OCS. 6 Given the heterogeneous range of symptoms experienced by patients with HES, the assessment of response and change in symptoms through an established clinical efficacy endpoint posed a challenge. 6 , 82
After further regulatory discussion, another trial was subsequently designed to investigate clinical improvements with mepolizumab in HES using a clinical endpoint based on disease flares, inspired by the benefits shown in the severe asthma and EGPA trials. The Phase III mepolizumab HES trial (GSK ID: 200622, NCT02836496) assessed the 300 mg SC dose, which was a lower dose and different administration route than assessed in the previous HES trial (750 mg IV). 82 In this trial, HES flares were defined as (1) a HES‐related clinical manifestation, based on a physician‐documented change in clinical signs or symptoms, requiring an increase in the maintenance OCS dose by ≥10 mg prednisone equivalent/day for 5 days or an increase in/addition of any cytotoxic and/or immunosuppressive HES therapy; or (2) receipt of ≥2 courses of blinded rescue OCS treatment (triggered by a predefined marked rise in the blood eosinophil count) during the treatment period. 82 This was the first trial to show that treatment (ie, mepolizumab) could reduce disease flares in addition to decreasing blood eosinophil count in patients with FIP1L1‐PDGFRA‐negative HES; there was a 50% reduction in the proportion of patients who experienced a flare or withdrew during the study (28% vs. 56%), a 66% reduction in risk of a first flare and a 66% reduction in annualized flare rate with mepolizumab versus placebo (Table 1; Figure 3). 82 The Phase III mepolizumab trial was also designed to assess daily symptoms that were self‐reported as most bothersome to patients using a HES daily symptom questionnaire, as an exploratory endpoint. 83 This pragmatic approach, reflecting the goals of individual patients to reduce the symptoms they found to be most disruptive to their lives, demonstrated that mepolizumab, versus placebo, improved the most bothersome HES symptoms as rated by patients.
A post hoc analysis of data from the mepolizumab HES compassionate use/expanded access program, collecting data over more than 10 years, suggested peak absolute eosinophil count, OCS sensitivity, pulmonary involvement, HES clinical subtype, and serum IL‐5 levels may be associated with a response to mepolizumab treatment in severe HES. 75 Whether these findings apply to the larger patient population for which mepolizumab is now approved at monthly 300 mg SC dosing remains to be explored. In terms of other anti‐IL‐5 therapies, a small Phase II trial (NCT02130882) has shown that 4‐weekly benralizumab (30 mg SC) was able to reduce blood eosinophil counts with associated improvements in clinical symptoms in patients with FIP1L1‐PDGFRA‐negative HES. 84
Overall, findings from the Phase III mepolizumab trial and subsequent approval for treatment of HES in the USA have provided physicians with a well‐tolerated efficacious treatment for this rare, debilitating disease.
5. MEPOLIZUMAB TREATMENT FOR CHRONIC RHINOSINUSITIS WITH NASAL POLYPS SHOWED THAT BENEFIT COULD BE EXTENDED TO THE UPPER AIRWAYS
CRSwNP is a heterogeneous disease of the upper airways characterized by chronic local eosinophilic inflammation 85 , 86 and symptoms of nasal blockage, loss of smell (anosmia), nasal discharge, facial pain/pressure, and sneezing as a result of nasal mucosal thickening and the formation of nasal polyps. 86 , 87 It often involves type 2 inflammation and is a frequent comorbidity in patients with severe eosinophilic asthma. 88 , 89 , 90 IL‐5 was implicated in the pathogenesis of NP in 1997 when it was found in significant amounts in the NP tissue samples from patients with asthma undergoing polypectomy. 91 Neutralization of IL‐5 led to a reduction in eosinophilia in the NP tissues. 91 Later, in 2010, IL‐5 was found to significantly predict comorbid asthma in patients with NP. 89 , 92 In addition, IL‐4/IL‐13 are now known to be involved in the differentiation of nasal polyp basal cells. 93
In patients with CRSwNP, treatment with systemic corticosteroids can temporarily reduce NP size and improve symptoms while on treatment, but is associated with adverse effects; additionally, patients who have surgery frequently experience recurrence of NPs. 87 , 94 Data from the Severe Asthma Network in Italy (SANI) registry have shown that patients with severe asthma and comorbid CRSwNP have worse outcomes in terms of number of exacerbations, number of days on OCS, and likelihood of a need for long‐term OCS use, compared with those patients with severe asthma without CRSwNP. 95 Similarly, the presence of asthma increases disease burden in patients with CRS. 96 , 97 In patients with severe eosinophilic asthma receiving anti‐IL‐5 therapies, improvements in nasal symptoms in addition to asthma outcomes have been shown, 98 , 99 , 100 and the presence of NPs has been shown to predict a positive response to mepolizumab in this population. 48 Based on this knowledge, mepolizumab was investigated as a treatment option in patients with recurrent, refractory severe CRSwNP who had a high symptom burden and previous NP surgeries and were eligible for repeat nasal surgery despite treatment with intranasal corticosteroids. 101 In the Phase III SYNAPSE (StudY in NAsal Polyps patients to assess the Safety and Efficacy of mepolizumab; NCT03085797) study, mepolizumab 100 mg SC reduced the occurrence of surgery and corticosteroid use and improved symptoms in this population, while also reducing the blood eosinophil count, when compared with placebo (Table 1). 101 These effects were not dissimilar to those seen in CRSwNP populations with dupilumab and omalizumab in the SINUS (NCT02912468 and NCT02898454) and POLYP (NCT03280550 and NCT03280537) studies. 90 , 102 Notably, these populations differed from the SYNAPSE population in their surgical history, with 63% and 60% of patients in SINUS and POLYP, respectively, having had ≥1 prior nasal surgery at enrollment compared with 100% of those in SYNAPSE. Both dupilumab and omalizumab have recently been approved for the treatment of patients with CRSwNP. 9 , 10 Additionally, there is early evidence for the efficacy of benralizumab in patients with CRSwNP, and the Phase III OSTRO (NCT03401229) and ORCHID (NCT04157335) studies are currently evaluating benralizumab in this population. 103 , 104 , 105
The identification of biomarkers, such as blood or tissue eosinophils, total immunoglobulin E (IgE), and fractional exhaled nitric oxide (FeNO), in patients with CRSwNP will help tailor treatment to endotype. 106 Interestingly, preliminary clinical research suggests that pre‐operative blood eosinophil count in patients with CRSwNP may have potential in clinical phenotyping, and help predict the recurrence of NP in this population. 107 , 108
6. MEPOLIZUMAB TREATMENT MAY ALSO PROVIDE CLINICAL BENEFIT TO PATIENTS WITH CHRONIC OBSTRUCTIVE PULMONARY DISEASE
COPD is another heterogenous respiratory disease, in which eosinophils may contribute to pathogenesis. 109 , 110 , 111 , 112 Approximately 55% of patients with COPD have eosinophil‐associated COPD and eosinophilic inflammation is associated with an increased risk of COPD exacerbations, which can be minimized by long term, consistent treatment with inhaled corticosteroids (ICS). 113 , 114 , 115 , 116 , 117 The use of ICS in patients with COPD may be guided by the recommended biomarker of baseline blood eosinophil count, 114 and the COPD Biomarker Qualification Consortium have proposed to the US Food and Drug Administration that blood eosinophil count should be assessed as a drug development tool. 115 , 118 , 119 An eosinophilic phenotype in patients with COPD therefore represents a treatable trait, 111 and such patients have shown a good response to treatment of acute exacerbations with OCS. 109 , 110 Furthermore, treatment directed at normalizing sputum eosinophil count reduces the number of severe exacerbations in patients with COPD. 120 Together, these findings suggest eosinophils play a pathogenic role in exacerbations of COPD and provide a strong rationale for therapies that specifically inhibit eosinophilic inflammation.
The Phase III METREX (MEpolizumab vs. placebo as add‐on TReatment for frequently EXacerbating COPD patients; NCT02105948) and METREO (MEpolizumab vs. placebo as add‐on TReatment for frequently exacerbating COPD patients characterized by EOsinophil level; NCT02105961) trials investigated the effect of mepolizumab in patients with COPD who had exacerbations despite receiving ICS‐based triple maintenance therapy; the primary endpoint was the annual rate of moderate or severe exacerbations. 121 Both trials examined outcomes in patients with COPD with an eosinophilic phenotype (defined as a blood eosinophil count of ≥150 cells/µl at screening or ≥300 cells/µl within the previous year). 121 In addition, the METREX trial included a cohort of patients without an eosinophilic phenotype. In METREX and METREO, in patients with an eosinophilic COPD phenotype, mepolizumab reduced the annual rate of moderate or severe exacerbations by 18% and 20%, respectively, for mepolizumab 100 mg SC versus placebo (Table 1). 121 This is consistent with results observed in the benralizumab Phase III COPD trials. 122 In contrast, COPD exacerbations were not significantly reduced versus placebo in the non‐eosinophilic populations in METREX. Of note, in METREX and METREO, there was a clear blood eosinophil count‐dependent suppressive effect on exacerbations treated with OCS but no effect on exacerbations treated with antibiotics alone. 123 Better stratification of exacerbation endpoints may be key to the successful clinical development of eosinophil‐targeting therapies in COPD. 121
Overall, the findings from the METREX and METREO trials suggested that mepolizumab treatment may represent precision treatment in the management of COPD and led to the identification of a potentially treatable trait, the COPD eosinophilic phenotype (Figure 3). 121 A new multicenter, randomized, placebo‐controlled, double‐blind, parallel‐group Phase III trial, MATINEE (Mepolizumab as Add‐on Treatment IN participants with COPD characterized by frequent Exacerbations and Eosinophil level; NCT04133909), began in 2019 and is currently recruiting patients with COPD (>40 years; former or current smokers) with an eosinophilic phenotype (blood eosinophil count ≥300 cells/µl at screening) who have experienced either ≥2 moderate COPD exacerbations in the previous year despite ICS‐based triple therapy or ≥1 severe COPD exacerbation requiring hospitalization. 124
7. MEPOLIZUMAB HAS A TOLERABLE AND ACCEPTABLE SAFETY PROFILE ACROSS THE EOSINOPHIL‐DRIVEN DISEASE SPECTRUM
Mepolizumab has demonstrated a consistent safety profile, broadly similar to that of placebo, in clinical trials of patients with a broad spectrum of eosinophil‐driven diseases (Table 2). 13 , 14 , 15 , 43 , 44 , 64 , 82 , 101 The most frequently reported adverse events with mepolizumab in these trials were nasopharyngitis and headache; the incidence of serious drug‐related adverse events was low (≤1%). 13 , 14 , 43 , 44 The favorable long‐term safety and tolerability profile of mepolizumab 100 mg SC in severe eosinophilic asthma has also been demonstrated in the COSMOS (NCT01842607), 125 COLUMBA (NCT01691859), 16 and COSMEX (COSMOS EXtension; NCT02135692) 15 extension studies, which followed patients for up to 4.8 years after starting treatment. These findings were consistent with previous randomized controlled trials that compared mepolizumab with placebo. 13 , 14 , 43 , 44 An open‐label extension study also demonstrated the long‐term safety of mepolizumab treatment for patients with HES, finding that mepolizumab 750 mg IV was well tolerated over a mean duration of 4.8 years of treatment. 126 Additionally, Kuang et al. confirmed that long‐term treatment with mepolizumab in patients with HES (≥5 years) does not increase the risk of malignancy and confers improvement in OCS‐related comorbidities. 75 Overall, the safety profiles for benralizumab and reslizumab are similar to the safety profile of mepolizumab. 49 , 50 , 51 , 52 , 53 , 55 , 67 , 68 , 69
TABLE 2.
Summary of safety data from long‐term asthma trials and other non‐asthma Phase III trials
| COSMOS (open label a ) 125 | COLUMBA (open label b ) 16 | COSMEX (open label c ) 15 | MIRRA 64 | 200622 82 | SYNAPSE 101 (safety population) | METREX 121 (safety population with an eosinophilic phenotype d ) | METREO 121 (safety population) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mepo 100 mg SC every 4 weeks for 52 weeks plus asthma SoC (N = 651) | Mepo 100 mg SC every 4 weeks plus asthma SoC (N = 347) | Mepo 100 mg SC every 4 weeks plus asthma SoC (N = 339) | Mepo 300 mg SC (N = 68) | Placebo (N = 68) | Mepo 300 mg SC (N = 54) | Placebo (N = 54) | Mepo 100 mg SC (N = 206) | Placebo (N = 201) | Mepo 100 mg SC (N = 233) | Placebo (N = 229) | Mepo 100 mg SC (N = 223) | Mepo 300 mg SC (N = 225) | Placebo (N = 226) | |
| Every 4 weeks for 52 weeks plus EGPA SoC | Every 4 weeks for 32 weeks plus HES SoC | Every 4 weeks for 52 weeks plus CRSwNP SoC | Every 4 weeks for 52 weeks plus COPD SoC | |||||||||||
| Any on‐treatment AEs | 558 (86) | 326 (94) | 315 (93) | 66 (97) | 64 (94) | 48 (89) | 47 (87) | 169 (82) | 168 (84) | 190 (82) | 189 (83) | 191 (86) | 196 (87) | 185 (82) |
| Treatment‐related AE | 123 (19) | 97 (28) | 51 (15) | 35 (51) | 24 (35) | 12 (22) | 7 (13) | 30 (15) | 19 (9) | – | – | – | – | – |
| Leading to study withdrawal | 11 (<2) | 19 (5) | 4 (1) | 2 (3) | 1 (1) | 1 (2) | 2 (4) | 0 | 1 (<1) | 7 (3) | 10 (4) | 7 (3) | 13 (6) | 18 (8) |
| Any on‐treatment SAE, n (%) | 94 (14) | 79 (23) | 84 (25) | 12 (18) | 18 (26) | 10 (19) | 8 (15) | 12 (6) | 13 (6) | 65 (28) | 80 (35) | 57 (26) | 60 (27) | 68 (30) |
| Treatment‐related SAE | 2 (<1) | 2 (<1) | 3 (0.9) | 3 (4) | 3 (4) | 0 | 0 | 0 | 1 (<1) | – | – | – | – | – |
| Any fatal SAE | 0 | 6 (2) e | 2 (<1) e | 1 (1) e | 0 | 1 (2) e | 0 | 0 | 1 (<1) | 6 (3) e | 8 (3) | 4 (2) e | 8 (4) e | 9 (4) |
| Any systemic reaction | 13 (2) | 9 (3) | 2 (0.6) | 4 (6) | 1 (1) | 1 (2) | 0 | 2 (<1) | 1 (<1) | 3 (1) | 4 (2) | 3 (1) | 5 (2) | 4 (2) |
| Local injection‐site reaction | 29 (4) | 42 (12) | 14 (4) | 10 (15) | 9 (13) | 4 (7) | 2 (4) | 5 (2) | 2 (<1) | 7 (3) | 7 (3) | 6 (3) | 11 (5) | 10 (4) |
| Immunogenicity (presence of anti‐mepolizumab antibodies) in patients tested | 31 (5) | 1/346 (8) | 6/335 (2) | 0 | 0 | 1 (<1) | 0 | 6 (3) | 3 (<1) | 14/395 (4) | 2/395 (2) | 13/220 (6) | 4/220 (2) | 3/217 (1) |
| Serious infections | – | 17 (5) | 20 (6) | – | – | – | – | – | – | – | – | – | – | – |
| Malignancies | – | 6 (2) | 8 (2) | – | – | 1 (2) | 0 | – | – | – | – | – | – | – |
Data are given as number (%) of patients unless otherwise noted.
Abbreviations: AE, adverse event; COPD, chronic obstructive pulmonary disease; CRSwNP, chronic rhinosinusitis with nasal polyposis; EGPA, eosinophilic granulomatosis with polyangiitis; HES, hypereosinophilic syndrome; IV, intravenous; mepo, mepolizumab; NP, nasal polyposis; SAE, serious adverse event; SC, subcutaneous; SoC, standard of care.
Eligible patients in the COSMOS open‐label extension study had completed either the MENSA or SIRIUS double‐blind studies and were treated with mepolizumab regardless of treatment allocation in the prior studies.
Eligible patients in the COLUMBA open‐label extension study had to have been randomized and received at least 2 doses of treatment (mepolizumab or placebo) in the DREAM study had received an asthma controller medication for ≥12 weeks before enrollment in COLUMBA. Mepolizumab treatment was administered until a protocol‐defined stopping criterion was met.
Patients were enrolled from the COSMEX open‐label extension study and had to have the most severe forms of severe eosinophilic asthma (eg, a history of life‐threatening or seriously debilitating asthma, the full definitions of which are noted in the COSMEX publication), have been receiving ICS controller therapy for the last 8 months, and had to have previously demonstrated a protocol‐defined clinical benefit with mepolizumab treatment. The study completed after all patients met a protocol‐defined discontinuation criterion.
The METREX safety population with an eosinophilic phenotype included patients with blood eosinophil count of ≥150 cells/µl at screening or ≥300 cells/µl within the previous year.
Fatalities were not considered related to mepolizumab treatment.
8. FUTURE DIRECTIONS
There are case reports and analyses in the literature on the varying impact of mepolizumab in several diseases, including allergic bronchopulmonary aspergillosis, Kimura's disease, chronic spontaneous urticaria, eosinophilic esophagitis, atopic dermatitis, idiopathic chronic eosinophilic pneumonia, and bronchiectasis not related to other pathologies. 127 , 128 , 129 , 130 , 131 , 132 , 133 Furthermore, genetic evidence associating eosinophil numbers and autoimmune diseases including rheumatoid arthritis and celiac disease may support investigation of IL‐5‐targeting treatments in these areas. 134 As such, anti‐IL‐5 therapies may have potential in other eosinophilic disorders beyond those discussed in this review.
Inflammatory diseases are heterogeneous in nature and pathophysiology is not always driven exclusively by eosinophils, as seen in asthma. 10 As such, prolonged OCS therapy remains a key component of the treatment of chronic eosinophil‐driven diseases. 112 , 135 , 136 , 137 However, there are significant risks associated with long‐term use of OCS 138 ; therefore, early initiation of targeted biologic treatment is essential to reduce OCS use, 44 , 64 , 82 , 101 and achieve the best results for patients. This is especially true for patients with EGPA 139 or HES, 140 since serious, irreparable damage, and remodeling may develop during the disease course. 141 This is also true for patients with severe asthma, as eosinophil‐driven changes to the airways are associated with reduced lung function. 142 Further analysis of treatable traits across the eosinophilic disease spectrum will help to facilitate early, targeted treatment. Although there are some real‐world reports on the use of mepolizumab during pregnancy and the post‐partum period, there are limited data on this subject. 126 , 143 Of note, an ongoing study (GSK ID: 200870) monitoring the outcome of exposure to mepolizumab during planned and unplanned pregnancies, expected to be completed in 2023, will provide much‐needed data on efficacy and safety among pregnant women.
Different dosing strategies across the different diseases could also be investigated in the future studies so that treatment can be personalized to the patient and their condition. Novel long‐acting IL‐5 monoclonal antibodies, such as Depemokimab, which is currently being investigated for the treatment of patients with severe eosinophilic asthma in the SWIFT (NCT04719832 and NCT04718103) and NIMBLE (NCT04718389) trials, 70 , 144 may be important in this regard. The impact of stopping versus continuing mepolizumab therapy after continuous treatment for ≥3 years in patients with severe eosinophilic asthma was investigated in asthma in the COMET study (GSK ID: 201810, NCT02555371). 145 This study showed that patients need to continue with biologic therapy in order to continue deriving clinical benefit and prevent worsening in clinical outcomes including lung function. This concept was supported by small, early trials of mepolizumab cessation in patients with EGPA, in which the majority of patients experienced relapses when switched to methotrexate therapy following induction of remission with mepolizumab. 146 , 147 This will be an important area of research as the use of mepolizumab expands into other eosinophilic diseases.
Further insights into the cost‐effectiveness of biologic treatments in asthma and other eosinophilic diseases are also needed. In asthma, it is suggested that biologic therapy is directed to those patients most likely to respond in order to improve cost‐effectiveness. 148 This would involve the use of biomarkers to identify such patients prior to treatment, as well as close monitoring of individuals following treatment initiation to assess treatment response. As work to identify biomarkers that are useful in this regard is ongoing, continued assessment of cost‐effectiveness is needed to support treatment decision‐making.
It is known that eosinophil biology is complex, with roles in both maintaining health and contributing to disease. 149 In a healthy population, the blood eosinophil count can vary; however, the “normal” blood eosinophil count in healthy individuals is lower than generally perceived, as several highly prevalent factors, such as atopy, allergy rhinitis, and smoking, can elevate counts. 150 In patients with mild asthma, there is now convincing and consistent evidence that high blood eosinophil counts are associated with increased exacerbation risk, even if they initially present with mild symptoms and normal lung function. 151 , 152 In addition, an association between blood eosinophilia and lung function decline has been identified, independent of asthma and smoking, meaning that eosinophilia is a risk factor for airflow obstruction even in individuals without disease symptoms. 151 Whether reducing eosinophils in these healthy individuals may be beneficial is an interesting consideration. In addition, the consequences of long‐term eosinophil reduction in patients with eosinophilic diseases will be an important topic for future research.
9. CONCLUSIONS
Inflammatory diseases often have heterogeneous pathogenic mechanisms and phenotypes. Several important lessons have been learned from the mepolizumab clinical development program. First, it has highlighted the importance of identifying target populations with the relevant phenotypes that will respond to a particular therapy, information that should be used in clinical practice to accurately phenotype patients and direct treatment accordingly. Second, it has emphasized the importance of using the appropriate endpoint to assess treatment efficacy; indeed, early studies in which mepolizumab did not demonstrate clinical benefits provided valuable information enabling the refinement of clinical study designs, which ultimately allowed the full clinical potential of mepolizumab and the other IL‐5 targeting antibodies to be uncovered. Persistence in the goal of identifying the appropriate target patient population was of the utmost importance in this regard. Finally, data on the safety and impact of mepolizumab treatment in clinical trials and real‐world settings demonstrate its clinical benefits across the eosinophil‐driven disease spectrum, providing valuable insights into the importance of eosinophils in these chronic inflammatory diseases. These insights may contribute to the further development of mepolizumab and extend to other rare/orphan eosinophilic‐driven diseases and provide a template of success for developing medicines in general.
CONFLICT OF INTEREST
Ian Pavord reports that he has received speaker's honoraria for sponsored meetings from AstraZeneca, Boehringer Ingelheim, Aerocrine, Almirall, Novartis, Teva, Chiesi, Sanofi/Regeneron, and GSK; and payments for organizing educational events from AstraZeneca, GSK, Sanofi/Regeneron, and Teva. He has received honoraria for attending advisory panels with Genentech, Sanofi/Regeneron, AstraZeneca, Boehringer Ingelheim, GSK, Novartis, Teva, Merck, Circassia, Chiesi, and Knopp; and payments to support FDA approval meetings from GSK. He has received sponsorship to attend international scientific meetings from Boehringer Ingelheim, GSK, AstraZeneca, Teva, and Chiesi. He has received a grant from Chiesi to support a Phase II clinical trial in Oxford. He is co‐patent holder of the rights to the Leicester Cough Questionnaire; and has received payments for its use in clinical trials from Merck, Bayer, and Insmed. In 2014–15, he was an expert witness for a patent dispute involving AstraZeneca and Teva. Elisabeth H Bel reports grants from GSK and Teva; and personal fees from AstraZeneca, GSK, Sanofi/Regeneron, Sterna Biologicals, and Chiesi. Arnaud Bourdin has received grants from Boehringer Ingelheim and AstraZeneca; has participated in clinical research projects (as an investigator) with GSK, AstraZeneca, Boehringer Ingelheim, Chiesi, Novartis, and Sanofi; and has received personal fees from GSK, AstraZeneca, Regeneron‐Sanofi, Novartis, and Chiesi. Robert Chan, Oliver N Keene, Jonathan Steinfeld, and Steven W Yancey are employees of GSK and own stocks/shares. Neil Martin is a former employee of GSK and owns stocks and shares. Joseph K Han has received consultancy fees from Sanofi Genzyme, Regeneron, Genentech, Novartis, AstraZeneca, GSK, and Gossamer Bio. Mark C Liu reports funding for clinical trials or personal fees for advisory board participation from AstraZeneca, Boehringer Ingelheim, Gossamer Bio, GSK, and MedImmune. Alberto Papi has received grants, personal fees and non‐financial support and other from AstraZeneca, Teva, Mundipharma, GSK, Chiesi, and Boehringer Ingelheim; has received personal fees and non‐financial support from Novartis, Menarini, and Zambon; and has received grants from Sanofi. Florence Roufosse reports consultancy fees from AstraZeneca and GSK; and royalties from UpToDate. Michael E Wechsler has research grants with the National Institute of Allergy and Infectious Diseases and the National Heart, Lung, and Blood Institute and is a consultant with GSK, Genentech, Sanofi, Regeneron, AstraZeneca, Teva, Novartis, Boehringer Ingelheim, Sentien, and Equillium.
AUTHORS’ CONTRIBUTIONS
RC, NM, JS, and SWY involved in the conception of the work. AB, FR, MEW, IP, JKH, EHB, AP, and MCL involved in the acquisition of data. All authors contributed to the analysis or interpretation of data, drafted the work or revised it critically for important intellectual content, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
ACKNOWLEDGMENTS
Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing and referencing) was provided by Roisin McCorkell MSc and Elizabeth Hutchinson PhD CMPP, at Fishawack Indicia Ltd, UK, part of Fishawack Health, and was funded by GlaxoSmithKline (GSK).
Pavord ID, Bel EH, Bourdin A, et al. From DREAM to REALITI‐A and beyond: Mepolizumab for the treatment of eosinophil‐driven diseases. Allergy.2022;77:778–797. 10.1111/all.15056
Neil Martin: Affiliation at the time of the work.
REFERENCES
- 1. Fulkerson PC, Rothenberg ME. Targeting eosinophils in allergy, inflammation and beyond. Nat Rev Drug Discov. 2013;12(2):117‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akuthota P, Weller PF. Eosinophils and disease pathogenesis. Semin Hematol. 2012;49(2):113‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Menzella F, Lusuardi M, Galeone C, Taddei S, Zucchi L. Profile of anti‐IL‐5 mAb mepolizumab in the treatment of severe refractory asthma and hypereosinophilic diseases. J Asthma Allergy. 2015;8:105‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. GSK . Mepolizumab US prescribing information. Updated 2020. https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Nucala/pdf/NUCALA‐PI‐PIL‐IFU‐COMBINED.PDF. Accessed October 07, 2020.
- 5. Zia‐Amirhosseini P, Walls C, Patel B, Cowley H, Minthorn E, Hottenstein CS. Pharmacokinetics and pharmacodynamics of SB‐240563, a humanized monoclonal antibody directed to human interleukin‐5, in mild asthmatics. Clin Pharmacol Ther 1999;65:147. [PubMed] [Google Scholar]
- 6. Roufosse F. Targeting the interleukin‐5 pathway for treatment of eosinophilic conditions other than asthma. Front Med. 2018;5:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Teva Pharmaceuticals . Reslizumab US highlights of prescribing information. Updated in 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/0761033s010lbl.pdf. Accessed 16 November 2020.
- 8. AstraZeneca . Benralizumab US highlights of prescribing information. Updated 2019. https://www.azpicentral.com/fasenra/fasenra.pdf#page=1. Accessed 16 November 2020.
- 9. Genentech . Omalizumab US prescribing information. https://www.gene.com/download/pdf/xolair_prescribing.pdf. Accessed 16 February 2021.
- 10. Regeneron . Dupilumab US prescribing information. https://www.regeneron.com/sites/default/files/Dupixent_FPI.pdf. Accessed 16 February 2021.
- 11. Frigas E, Gleich GJ. The eosinophil and the pathophysiology of asthma. J Allergy Clin Immunol. 1986;77(4):527‐537. [DOI] [PubMed] [Google Scholar]
- 12. Bousquet J, Chanez P, Lacoste JY, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323(15):1033‐1039. [DOI] [PubMed] [Google Scholar]
- 13. Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double‐blind, placebo‐controlled trial. Lancet. 2012;380(9842):651‐659. [DOI] [PubMed] [Google Scholar]
- 14. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198‐1207. [DOI] [PubMed] [Google Scholar]
- 15. Khurana S, Brusselle GG, Bel EH, et al. Long‐term safety and clinical benefit of mepolizumab in patients with the most severe eosinophilic asthma: the COSMEX study. Clin Ther. 2019;41(10):2041‐2056. [DOI] [PubMed] [Google Scholar]
- 16. Khatri S, Moore W, Gibson PG, et al. Assessment of the long‐term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2019;143(5):1742‐1751.e1747. [DOI] [PubMed] [Google Scholar]
- 17. Ortega HG, Yancey SW, Mayer B, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med. 2016;4(7):549‐556. [DOI] [PubMed] [Google Scholar]
- 18. Wagener AH, de Nijs SB, Lutter R, et al. External validation of blood eosinophils, FE(NO) and serum periostin as surrogates for sputum eosinophils in asthma. Thorax. 2015;70(2):115‐120. [DOI] [PubMed] [Google Scholar]
- 19. Yancey SW, Keene ON, Albers FC, et al. Biomarkers for severe eosinophilic asthma. J Allergy Clin Immunol. 2017;140(6):1509‐1518. [DOI] [PubMed] [Google Scholar]
- 20. Genentech . Tocilizumab US prescribing information. https://www.gene.com/download/pdf/actemra_prescribing.pdf. Accessed 10 May 2021.
- 21. ClinicalTrials.gov . Tocilizumab and Cytokine Release Syndrome (CRS) In Covid‐19 Pneumonia. 2021; https://clinicaltrials.gov.ct2/show/NCT04873141?term=tocilizumab&draw=2&rank=2. Accessed 10 May 2021.
- 22. Nishimoto N, Kanakura Y, Aozasa K, et al. Humanized anti‐interleukin‐6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106(8):2627‐2632. [DOI] [PubMed] [Google Scholar]
- 23. Pavord ID, Menzies‐Gow A, Buhl R, et al. Clinical development of mepolizumab for the treatment of severe eosinophilic asthma: On the path to personalized medicine. J Allergy Clin Immunol. 2020;9(3):1121‐1132.e7. [DOI] [PubMed] [Google Scholar]
- 24. Abdala‐Valencia H, Coden ME, Chiarella SE, et al. Shaping eosinophil identity in the tissue contexts of development, homeostasis, and disease. J Leukoc Biol. 2018;104(1):95‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blanchard C, Rothenberg ME. Biology of the eosinophil. Adv Immunol. 2009;101:81‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in health and disease: the LIAR hypothesis. Clin Exp Allergy. 2010;40(4):563‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Long H, Liao W, Wang L, Lu Q. A Player and Coordinator: The Versatile Roles of Eosinophils in the Immune System. Transfus Med Hemother. 2016;43(2):96‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu LY, Sedgwick JB, Bates ME, et al. Decreased expression of membrane IL‐5 receptor alpha on human eosinophils: I. Loss of membrane IL‐5 receptor alpha on airway eosinophils and increased soluble IL‐5 receptor alpha in the airway after allergen challenge. J Immunol. 2002;169(11):6452‐6458. [DOI] [PubMed] [Google Scholar]
- 29. Liu LY, Sedgwick JB, Bates ME, et al. Decreased expression of membrane IL‐5 receptor alpha on human eosinophils: II. IL‐5 down‐modulates its receptor via a proteinase‐mediated process. J Immunol. 2002;169(11):6459‐6466. [DOI] [PubMed] [Google Scholar]
- 30. Kitaura M, Nakajima T, Imai T, et al. Molecular cloning of human eotaxin, an eosinophil‐selective CC chemokine, and identification of a specific eosinophil eotaxin receptor, CC chemokine receptor 3. J Biol Chem. 1996;271(13):7725‐7730. [DOI] [PubMed] [Google Scholar]
- 31. Acharya KR, Ackerman SJ. Eosinophil granule proteins: form and function. J Biol Chem. 2014;289(25):17406‐17415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Green RH, Brightling CE, McKenna S, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360(9347):1715‐1721. [DOI] [PubMed] [Google Scholar]
- 33. Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996;183(1):195‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hogan SP, Koskinen A, Foster PS. Interleukin‐5 and eosinophils induce airway damage and bronchial hyperreactivity during allergic airway inflammation in BALB/c mice. Immunol Cell Biol. 1997;75(3):284‐288. [DOI] [PubMed] [Google Scholar]
- 35. Flood‐Page P, Menzies‐Gow A, Phipps S, et al. Anti‐IL‐5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112(7):1029‐1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leckie MJ, ten Brinke A, Khan J, et al. Effects of an interleukin‐5 blocking monoclonal antibody on eosinophils, airway hyper‐responsiveness, and the late asthmatic response. Lancet. 2000;356(9248):2144‐2148. [DOI] [PubMed] [Google Scholar]
- 37. Flood‐Page P, Swenson C, Faiferman I, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med. 2007;176(11):1062‐1071. [DOI] [PubMed] [Google Scholar]
- 38. Kips JC, O'Connor BJ, Langley SJ, et al. Effect of SCH55700, a humanized anti‐human interleukin‐5 antibody, in severe persistent asthma: a pilot study. Am J Respir Crit Care Med. 2003;167(12):1655‐1659. [DOI] [PubMed] [Google Scholar]
- 39. Pavord ID, Brightling CE, Woltmann G, Wardlaw AJ. Non‐eosinophilic corticosteroid unresponsive asthma. Lancet. 1999;353(9171):2213‐2214. [DOI] [PubMed] [Google Scholar]
- 40. Wenzel SE, Schwartz LB, Langmack EL, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999;160(3):1001‐1008. [DOI] [PubMed] [Google Scholar]
- 41. Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360(10):973‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nair P, Pizzichini MMM, Kjarsgaard M, et al. Mepolizumab for prednisone‐dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360(10):985‐993. [DOI] [PubMed] [Google Scholar]
- 43. Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add‐on therapy on health‐related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double‐blind, placebo‐controlled, parallel‐group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5(5):390‐400. [DOI] [PubMed] [Google Scholar]
- 44. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid‐sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189‐1197. [DOI] [PubMed] [Google Scholar]
- 45. Harrison T, Canonica GW, Chupp G, et al. Real‐world mepolizumab in the prospective severe asthma REALITI‐A study: initial analysis. Eur Respir J. 2020;56(4):2000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van Toor JJ, van der Mark SC, Kappen JH, In ’t Veen J, Braunstahl GJ. Mepolizumab add‐on therapy in a real world cohort of patients with severe eosinophilic asthma: response rate, effectiveness, and safety. J Asthma 2021;58(5):651‐658. [DOI] [PubMed] [Google Scholar]
- 47. Llanos JP, Ortega H, Bogart M, et al. Real‐world effectiveness of mepolizumab in patients with severe asthma: an examination of exacerbations and costs. J Asthma Allergy. 2020;13:77‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kavanagh JE, d’Ancona G, Elstad M, et al. Real‐world effectiveness and the characteristics of a "super‐responder" to mepolizumab in severe eosinophilic asthma. Chest 2020;158(2):491‐500. [DOI] [PubMed] [Google Scholar]
- 49. Bjermer L, Lemiere C, Maspero J, Weiss S, Zangrilli J, Germinaro M. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest 2016;150(4):789‐798. [DOI] [PubMed] [Google Scholar]
- 50. Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high‐dosage inhaled corticosteroids and long‐acting beta2‐agonists (SIROCCO): a randomised, multicentre, placebo‐controlled phase 3 trial. Lancet. 2016;388(10056):2115‐2127. [DOI] [PubMed] [Google Scholar]
- 51. Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double‐blind, randomised, placebo‐controlled, phase 3 trials. Lancet Respir Med. 2015;3(5):355‐366. [DOI] [PubMed] [Google Scholar]
- 52. Corren J, Weinstein S, Janka L, Zangrilli J, Garin M. Phase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. Chest 2016;150(4):799‐810. [DOI] [PubMed] [Google Scholar]
- 53. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti‐interleukin‐5 receptor alpha monoclonal antibody, as add‐on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet. 2016;388(10056):2128‐2141. [DOI] [PubMed] [Google Scholar]
- 54. Nair P, Bardin P, Humbert M, et al. Efficacy of intravenous reslizumab in oral corticosteroid‐dependent asthma. J Allergy Clin Immunol Pract. 2020;8(2):555‐564. [DOI] [PubMed] [Google Scholar]
- 55. Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid‐sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376(25):2448‐2458. [DOI] [PubMed] [Google Scholar]
- 56. Goldman M, Hirsch I, Zangrilli JG, Newbold P, Xu X. The association between blood eosinophil count and benralizumab efficacy for patients with severe, uncontrolled asthma: subanalyses of the Phase III SIROCCO and CALIMA studies. Curr Med Res Opin. 2017;33(9):1605‐1613. [DOI] [PubMed] [Google Scholar]
- 57. Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1‐11. [DOI] [PubMed] [Google Scholar]
- 58. Lyons PA, Peters JE, Alberici F, et al. Genome‐wide association study of eosinophilic granulomatosis with polyangiitis reveals genomic loci stratified by ANCA status. Nat Commun. 2019;10(1):5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Marvisi C, Sinico RA, Salvarani C, et al. New perspectives in eosinophilic granulomatosis with polyangiitis (EGPA): report of the first meeting of the European EGPA Study Group. Intern Emerg Med. 2019;14(8):1193‐1197. [DOI] [PubMed] [Google Scholar]
- 60. Furuta S, Iwamoto T, Nakajima H. Update on eosinophilic granulomatosis with polyangiitis. Allergol Int. 2019;68(4):430‐436. [DOI] [PubMed] [Google Scholar]
- 61. Gokhale M, Bell CF, Doyle S, Fairburn‐Beech J, Steinfeld J, Van Dyke MK. Prevalence of eosinophilic granulomatosis with polyangiitis and associated health care utilization among patients with concomitant asthma in US commercial claims database. J Clin Rheumatol. 2021;27(3):107‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kahn J‐E, Grandpeix‐Guyodo C, Marroun I, et al. Sustained response to mepolizumab in refractory Churg‐Strauss syndrome. J Allergy Clin Immunol. 2010;125(1):267‐270. [DOI] [PubMed] [Google Scholar]
- 63. Kim S, Marigowda G, Oren E, Israel E, Wechsler ME. Mepolizumab as a steroid‐sparing treatment option in patients with Churg‐Strauss syndrome. J Allergy Clin Immunol. 2010;125(6):1336‐1343. [DOI] [PubMed] [Google Scholar]
- 64. Wechsler ME, Akuthota P, Jayne D, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med. 2017;376(20):1921‐1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. GSK . Press release: GSK achieves approval for Nucala (mepolizumab) for the treatment of eosinophilic granulomatosis with polyangiitis (EGPA) for adults in the US. https://www.gsk.com/en‐gb/media/press‐releases/gsk‐achieves‐approval‐for‐nucala‐mepolizumab‐for‐the‐treatment‐of‐eosinophilic‐granulomatosis‐with‐polyangiitis‐egpa‐for‐adults‐in‐the‐us/. Accessed 18 January 2021.
- 66. Steinfeld J, Bradford ES, Brown J, et al. Evaluation of clinical benefit from treatment with mepolizumab for patients with eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol. 2019;143(6):2170‐2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Manka LA, Guntur VP, Denson JL, et al. Efficacy and safety of reslizumab in the treatment of eosinophilic granulomatosis with polyangiitis. Ann Allergy Asthma Immunol. 2021;126(6):696‐701. [DOI] [PubMed] [Google Scholar]
- 68. Kent BD, d'Ancona G, Fernandes M, et al. Oral corticosteroid‐sparing effects of reslizumab in the treatment of eosinophilic granulomatosis with polyangiitis. ERJ Open Res. 2020;6(1):00311‐2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Guntur VP, Manka LA, Denson JL, et al. Benralizumab as a steroid‐sparing treatment option in eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol Pract. 2021;9(3):1186‐1193. [DOI] [PubMed] [Google Scholar]
- 70. ClinicalTrials.gov . Placebo‐controlled efficacy and safety study of GSK3511294 in participants with severe asthma with an eosinophilic phenotype. 2021. https://www.clinicaltrials.gov/ct2/show/NCT04719832?term=GSK3511294&draw=2&rank=1. Accessed 10 May 2021.
- 71. Ogbogu PU, Bochner BS, Butterfield JH, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 2009;124(6):1319‐1325.e1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rothenberg ME, Klion AD, Roufosse FE, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med. 2008;358(12):1215‐1228. [DOI] [PubMed] [Google Scholar]
- 73. Duncan EA, Ortega H, Gleich G, Price R, Yancey S, Klion A. Observational experience describing the use of mepolizumab in patients with hypereosinophilic syndrome. Am J Respir Crit Care Med. 2015;191:Abstract A1365. [Google Scholar]
- 74. Shomali W, Gotlib J. World Health Organization‐defined eosinophilic disorders: 2019 update on diagnosis, risk stratification, and management. Am J Hematol. 2019;94(10):1149‐1167. [DOI] [PubMed] [Google Scholar]
- 75. Kuang FL, Fay MP, Ware JA, et al. Long‐term clinical outcomes of high‐dose mepolizumab treatment for hypereosinophilic syndrome. J Allergy Clin Immunol Pract. 2018;6(5):1518‐1527.e1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Valent P, Klion AD, Horny H‐P, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130(3):607‐612.e609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cools J, DeAngelo DJ, Gotlib J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348(13):1201‐1214. [DOI] [PubMed] [Google Scholar]
- 78. Roufosse F, Cogan E, Goldman M. Lymphocytic variant hypereosinophilic syndromes. Immunol Allergy Clin North Am. 2007;27(3):389‐413. [DOI] [PubMed] [Google Scholar]
- 79. Garrett JK, Jameson SC, Thomson B, et al. Anti‐interleukin‐5 (mepolizumab) therapy for hypereosinophilic syndromes. J Allergy Clin Immunol. 2004;113(1):115‐119. [DOI] [PubMed] [Google Scholar]
- 80. Plötz S‐G, Simon H‐U, Darsow U, et al. Use of an anti‐interleukin‐5 antibody in the hypereosinophilic syndrome with eosinophilic dermatitis. N Engl J Med. 2003;349(24):2334‐2339. [DOI] [PubMed] [Google Scholar]
- 81. ClinicalTrials.gov . Mepolizumab compassionate use study (NCT00244686). https://clinicaltrials.gov.ct2/show/record/NCT00244686?view=record. Accessed 23 November 2020.
- 82. Roufosse F, Kahn JE, Rothenberg ME, et al. Efficacy and safety of mepolizumab in hypereosinophilic syndrome: a phase III, randomized, placebo‐controlled trial. J Allergy Clin Immunol. 2020;146(6):1397‐1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Roufosse F, Butterfield JH, von Maltzahn R, et al. Impact of mepolizumab on symptom severity in patients with hypereosinophilic syndrome. J Allergy Clin Immunol. 2021;147(2 Suppl):AB139. [Google Scholar]
- 84. Kuang FL, Legrand F, Makiya M, et al. Benralizumab for PDGFRA‐Negative Hypereosinophilic Syndrome. N Engl J Med. 2019;380(14):1336‐1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Radabaugh JP, Han JK, Moebus RG, Somers E, Lam K. Analysis of histopathological endotyping for chronic rhinosinusitis phenotypes based on comorbid asthma and allergic rhinitis. Am J Rhinol Allergy. 2019;33(5):507‐512. [DOI] [PubMed] [Google Scholar]
- 86. Schleimer RP. Immunopathogenesis of chronic rhinosinusitis and nasal polyposis. Annu Rev Pathol. 2017;12:331‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(Suppl S29):1‐464. [DOI] [PubMed] [Google Scholar]
- 88. de Groot JC, Storm H, Amelink M, et al. Clinical profile of patients with adult‐onset eosinophilic asthma. ERJ Open Res. 2016;2(2):00100‐2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Han JK. Subclassification of chronic rhinosinusitis. Laryngoscope. 2013;123(Suppl 2):S15‐S27. [DOI] [PubMed] [Google Scholar]
- 90. Bachert C, Zhang N, Cavaliere C, Weiping W, Gevaert E, Krysko O. Biologics for chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2020;145(3):725‐739. [DOI] [PubMed] [Google Scholar]
- 91. Simon HU, Yousefi S, Schranz C, Schapowal A, Bachert C, Blaser K. Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. J Immunol. 1997;158(8):3902‐3908. [PubMed] [Google Scholar]
- 92. Bachert C, Zhang N, Holtappels G, et al. Presence of IL‐5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J Allergy Clin Immunol. 2010;126(5):962‐968, 968 e961–966. [DOI] [PubMed] [Google Scholar]
- 93. Ordovas‐Montanes J, Dwyer DF, Nyquist SK, et al. Allergic inflammatory memory in human respiratory epithelial progenitor cells. Nature. 2018;560(7720):649‐654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. DeConde AS, Mace JC, Levy JM, Rudmik L, Alt JA, Smith TL. Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Laryngoscope. 2017;127(3):550‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Canonica GW, Malvezzi L, Blasi F, et al. Chronic rhinosinusitis with nasal polyps impact in severe asthma patients: evidences from the Severe Asthma Network Italy (SANI) registry. Respir Med. 2020;166:105947. [DOI] [PubMed] [Google Scholar]
- 96. Batra PS, Tong L, Citardi MJ. Analysis of comorbidities and objective parameters in refractory chronic rhinosinusitis. Laryngoscope. 2013;123(Suppl 7):S1‐S11. [DOI] [PubMed] [Google Scholar]
- 97. Lin DC, Chandra RK, Tan BK, et al. Association between severity of asthma and degree of chronic rhinosinusitis. Am J Rhinol Allergy. 2011;25(4):205‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yilmaz I, Türk M, Nazik Bahçecioğlu S, Tutar N, Gülmez I. Efficacy of mepolizumab treatment in oral corticosteroid‐dependent severe eosinophilic asthma patients with chronic rhinosinusitis with nasal polyps: single center, real life study. Turkish J Med Sci. 2020;50(2):433‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Weinstein SF, Katial RK, Bardin P, et al. Effects of reslizumab on asthma outcomes in a subgroup of eosinophilic asthma patients with self‐reported chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract. 2019;7(2):589‐596.e583. [DOI] [PubMed] [Google Scholar]
- 100. Howarth P, Chupp G, Nelsen LM, et al. Severe eosinophilic asthma with nasal polyposis: a phenotype for improved sinonasal and asthma outcomes with mepolizumab therapy. J Allergy Clin Immunol. 2020;145(6):1713‐1715. [DOI] [PubMed] [Google Scholar]
- 101. Han JK, Bachert C, Fokkens W, et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Respir Med. 2021.S2213‐2600(21)00097‐7. 10.1016/s2213-2600(21)00097-7 [DOI] [PubMed] [Google Scholar]
- 102. Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS‐24 and LIBERTY NP SINUS‐52): results from two multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group phase 3 trials. Lancet. 2019;394(10209):1638‐1650. [DOI] [PubMed] [Google Scholar]
- 103. Lombardo N, Pelaia C, Ciriolo M, et al. Real‐life effects of benralizumab on allergic chronic rhinosinusitis and nasal polyposis associated with severe asthma. Int J Immunopathol Pharmacol. 2020;34:2058738420950851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Matsuno O, Minamoto S. Rapid effect of benralizumab for severe asthma with chronic rhinosinusitis with nasal polyps. Pulm Pharmacol Ther. 2020;64:101965. [DOI] [PubMed] [Google Scholar]
- 105. ClinicalTrials.gov . Efficacy and safety study of benralizumab for patients with severe nasal polyposis (OSTRO). 2021; https://clinicaltrials.gov.ct2/show/NCT03401229?term=benralizumab&cond=nasal+polyps&draw=2&rank=3. Accessed 10 May 2021.
- 106. Workman AD, Kohanski MA, Cohen NA. Biomarkers in chronic rhinosinusitis with nasal polyps. Immunol Allergy Clin North Am. 2018;38(4):679‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhong B, Yuan T, Du J, et al. The role of preoperative blood eosinophil counts in distinguishing chronic rhinosinusitis with nasal polyps phenotypes. Int Forum Allergy Rhinol. 2021;11(1):16‐23. [DOI] [PubMed] [Google Scholar]
- 108. Hu Y, Cao PP, Liang GT, Cui YH, Liu Z. Diagnostic significance of blood eosinophil count in eosinophilic chronic rhinosinusitis with nasal polyps in Chinese adults. Laryngoscope. 2012;122(3):498‐503. [DOI] [PubMed] [Google Scholar]
- 109. Bafadhel M, Davies L, Calverley PM, Aaron SD, Brightling CE, Pavord ID. Blood eosinophil guided prednisolone therapy for exacerbations of COPD: a further analysis. Eur Respir J. 2014;44(3):789‐791. [DOI] [PubMed] [Google Scholar]
- 110. Bafadhel M, McKenna S, Terry S, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo‐controlled trial. Am J Respir Crit Care Med. 2012;186(1):48‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. David B, Bafadhel M, Koenderman L, De Soyza A. Eosinophilic inflammation in COPD: from an inflammatory marker to a treatable trait. Thorax. 2020;76(2):188‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Global initiative for chronic obstructive lung disease (GOLD) . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2021. Accessed 10 May 2021.
- 113. Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662‐671. [DOI] [PubMed] [Google Scholar]
- 114. Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3(6):435‐442. [DOI] [PubMed] [Google Scholar]
- 115. Agusti A, Fabbri LM, Singh D, et al. Inhaled corticosteroids in COPD: friend or foe? Eur Respir J 2018;52(6):1801219. [DOI] [PubMed] [Google Scholar]
- 116. Magnussen H, Lucas S, Lapperre T, et al. Withdrawal of inhaled corticosteroids versus continuation of triple therapy in patients with COPD in real life: observational comparative effectiveness study. Respir Res. 2021;22(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wu HX, Zhuo KQ, Cheng DY. Prevalence and baseline clinical characteristics of eosinophilic chronic obstructive pulmonary disease: a meta‐analysis and systematic review. Front Med. 2019;6:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. [DOI] [PubMed] [Google Scholar]
- 119. Food and Drug Administration . DDT #000057 BQP qualification program cover letter. Submitted in 2019. https://www.fda.gov/media/130245/download. Accessed 19 January 2021.
- 120. Siva R, Green RH, Brightling CE, et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir J. 2007;29(5):906‐913. [DOI] [PubMed] [Google Scholar]
- 121. Pavord ID, Chanez P, Criner GJ, et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med. 2017;377(17):1613‐1629. [DOI] [PubMed] [Google Scholar]
- 122. Criner GJ, Celli BR, Singh D, et al. Predicting response to benralizumab in chronic obstructive pulmonary disease: analyses of GALATHEA and TERRANOVA studies. Lancet Respir Med. 2020;8(2):158‐170. [DOI] [PubMed] [Google Scholar]
- 123. Pavord I, Chapman K, Bafadhel M, et al. Mepolizumab for eosinophil‐associated COPD: analysis of METREX and METREO. Int J Chron Obstruct Pulmon Dis. 2021;16:1755‐1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. ClinicalTrials.gov . Mepolizumab as add‐on treatment in participants with COPD characterized by frequent exacerbations and eosinophil level (MATINEE). https://clinicaltrials.gov.ct2/show/NCT04133909. Accessed 3 February 2020.
- 125. Lugogo N, Domingo C, Chanez P, et al. Long‐term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi‐center, open‐label, phase IIIb study. Clin Ther. 2016;38(9):2058‐2070.e2051. [DOI] [PubMed] [Google Scholar]
- 126. Roufosse FE, Kahn J‐E, Gleich GJ, et al. Long‐term safety of mepolizumab for the treatment of hypereosinophilic syndromes. J Allergy Clin Immunol. 2013;131(2):461‐467.e461–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Rokkas T, Niv Y, Malfertheiner P. A network meta‐analysis of randomized controlled trials on the treatment of eosinophilic esophagitis in adults and children. J Clin Gastroenterol. 2021;55(5):400‐410. [DOI] [PubMed] [Google Scholar]
- 128. Al Shammari F, Nasiri A, Alkhathami M, Alawfi F, Alfifi M, Al OE. Mepolizumab as an effective treatment for Kimura's disease associated with ulcerative colitis: a case report. J Family Med Prim Care. 2019;8(9):3028‐3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Tolebeyan A, Mohammadi O, Vaezi Z, Amini A. Mepolizumab as possible treatment for allergic bronchopulmonary aspergillosis: a review of eight cases. Cureus. 2020;12(8):e9684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kang EG, Narayana PK, Pouliquen IJ, Lopez MC, Ferreira‐Cornwell MC, Getsy JA. Efficacy and safety of mepolizumab administered subcutaneously for moderate to severe atopic dermatitis. Allergy. 2020;75(4):950‐953. [DOI] [PubMed] [Google Scholar]
- 131. Magerl M, Terhorst D, Metz M, et al. Benefit of mepolizumab treatment in a patient with chronic spontaneous urticaria. J Dtsch Dermatol Ges. 2018;16(4):477‐478. [DOI] [PubMed] [Google Scholar]
- 132. Carpagnano GE, Scioscia G, Lacedonia D, Curradi G, Foschino Barbaro MP. Severe uncontrolled asthma with bronchiectasis: a pilot study of an emerging phenotype that responds to mepolizumab. J Asthma Allergy. 2019;12:83‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Brenard E, Pilette C, Dahlqvist C, et al. Real‐life study of mepolizumab in idiopathic chronic eosinophilic pneumonia. Lung. 2020;198(2):355‐360. [DOI] [PubMed] [Google Scholar]
- 134. Astle WJ, Elding H, Jiang T, et al. The allelic landscape of human blood cell trait variation and links to common complex disease. Cell. 2016;167(5):1415‐1429.e1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Mukhtyar C, Guillevin L, Cid MC, et al. EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann Rheum Dis. 2009;68(3):310‐317. [DOI] [PubMed] [Google Scholar]
- 136. Global Initiative For Asthma (GINA) . Global Strategy for Asthma Management and Prevention. Updated 2020. 2020; https://ginasthma.org/gina‐reports/. Accessed 19 January 2021.
- 137. Gotlib J. World Health Organization‐defined eosinophilic disorders: 2017 update on diagnosis, risk stratification, and management. Am J Hematol. 2017;92(11):1243‐1259. [DOI] [PubMed] [Google Scholar]
- 138. Rice JB, White AG, Scarpati LM, Wan G, Nelson WW. Long‐term systemic corticosteroid exposure: a systematic literature review. Clin Ther. 2017;39(11):2216‐2229. [DOI] [PubMed] [Google Scholar]
- 139. Ikeda T, Komatsu T, Yokoyama K, Takahashi K, Kawakami T. Early add‐on administration of mepolizumab and intravenous immunoglobulin effective in treating eosinophilic granulomatosis with polyangiitis. J Dermatol. 2021;48(4):529‐532. [DOI] [PubMed] [Google Scholar]
- 140. Schwartz LB, Sheikh J, Singh A. Current strategies in the management of hypereosinophilic syndrome, including mepolizumab. Curr Med Res Opin. 2010;26(8):1933‐1946. [DOI] [PubMed] [Google Scholar]
- 141. Aceves SS. Remodeling and fibrosis in chronic eosinophil inflammation. Dig Dis. 2014;32(1–2):15‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Dunican EM, Elicker BM, Gierada DS, et al. Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J Clin Invest. 2018;128(3):997‐1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kasuya A, Kitano S, Hoshino T, et al. Successful control of severe eosinophilic granulomatosis with polyangiitis in a pregnancy and perinatal period: a use of mepolizumab. J Dermatol. 2019;46(9):e309‐e311. [DOI] [PubMed] [Google Scholar]
- 144. ClinicalTrials.gov . A study of GSK3511294 in participants with severe asthma with an eosinophilic phenotype. 2021; https://www.clinicaltrials.gov/ct2/show/NCT04718103?term=GSK3511294&draw=2&rank=2. Accessed 10 May 2021.
- 145. Moore WC, Kornmann O, Humbert M, et al. Stopping versus continuing long‐term mepolizumab treatment in severe eosinophilic asthma (COMET study). Eur Respir J. 2021.2100396: 10.1183/13993003.00396-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Herrmann K, Gross WL, Moosig F. Extended follow‐up after stopping mepolizumab in relapsing/refractory Churg‐Strauss syndrome. Clin Exp Rheumatol. 2012;30(1 Suppl 70):S62‐S65. [PubMed] [Google Scholar]
- 147. Moosig F, Gross WL, Herrmann K, Bremer JP, Hellmich B. Targeting interleukin‐5 in refractory and relapsing Churg‐Strauss syndrome. Ann Intern Med. 2011;155(5):341‐343. [DOI] [PubMed] [Google Scholar]
- 148. Anderson WC 3rd, Szefler SJ. Cost‐effectiveness and comparative effectiveness of biologic therapy for asthma: to biologic or not to biologic? Ann Allergy Asthma Immunol. 2019;122(4):367‐372. [DOI] [PubMed] [Google Scholar]
- 149. Kim HJ, Jung Y. The emerging role of eosinophils as multifunctional leukocytes in health and disease. Immune network. 2020;20(3):e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kwon N, Pizzichini E, Bansal AT, et al. Factors that affect blood eosinophil counts in a non‐asthmatic population: post hoc analysis of data from Brazil. World Allergy Organ J. 2020;13(5):100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Hancox RJ, Pavord ID, Sears MR. Associations between blood eosinophils and decline in lung function among adults with and without asthma. Eur Respir J. 2018;51(4):1702536. [DOI] [PubMed] [Google Scholar]
- 152. Pavord ID, Holliday M, Reddel HK, et al. Predictive value of blood eosinophils and exhaled nitric oxide in adults with mild asthma: a prespecified subgroup analysis of an open‐label, parallel‐group, randomised controlled trial. Lancet Respir Med. 2020;8(7):671‐680. [DOI] [PubMed] [Google Scholar]


