Short abstract
There is a growing need for diversity, equity, and inclusion (DEI) in cancer care, particularly with respect to equal access and accrual to clinical trials. This commentary describe steps taken to address disparities in the authors' own clinical practice and proposes actions at the patient, provider, community, and institution levels to improve DEI in clinical trials.
Keywords: cancer, clinical trials, diversity, equity, inclusion
Introduction
There is a growing need for diversity, equity, and inclusion (DEI) in cancer care. One area requiring immediate attention and solutions is equal access and accrual to clinical trials. Increasing DEI in clinical trials is identified as a high‐priority area by both the Institute of Medicine 1 and the National Cancer Institute (NCI); however, persistent underenrollment of Black, Indigenous, and People of Color (BIPOC) and socially disadvantaged populations represents an unresolved disparity in cancer medicine. Today, overall cancer clinical trial enrollment (CTE) in the United States is 8% (6.3%‐7.0% at community centers and 14.0%‐15.9% at academic centers), 2 , 3 , 4 and BIPOC patients represent only approximately 15% of that low overall participation. 5 , 6 With diverse ethnic and racial groups composing nearly 40% of the US population, 4 this staggering mismatch of racial and ethnic representation in cancer clinical trials must be addressed. Underrepresentation of minority patients in clinical trials compromises the generalizability of trial results, 7 , 8 , 9 may lead to miscalculations of disease‐free survival rates and to erroneous estimates of treatment efficacy, 10 and, as a result, may further exacerbate health disparities. 11
Proposed barriers to CTE in minority and low‐income populations operate at multiple levels (Fig. 1). At system levels, geographic access, trial availability, and insurance barriers may disproportionately affect minority patients. System‐level factors may include a lack of research or regulatory support in underresourced hospital settings; this effectively makes it impossible for these sites to open clinical trials. Patient‐level factors affecting CTE include (but are not limited to) health beliefs and sociocultural factors, logistical barriers related to work and social support, and mistrust. Non–English‐speaking patients may additionally be faced with language barriers that can lead to misunderstanding if communication with interpreters is not prioritized. Additionally, underenrollment of BIPOC patients in clinical trials may be the result of providers being less likely to offer trials to these populations for reasons related to their own biases or health beliefs (Fig. 1).
Figure 1.
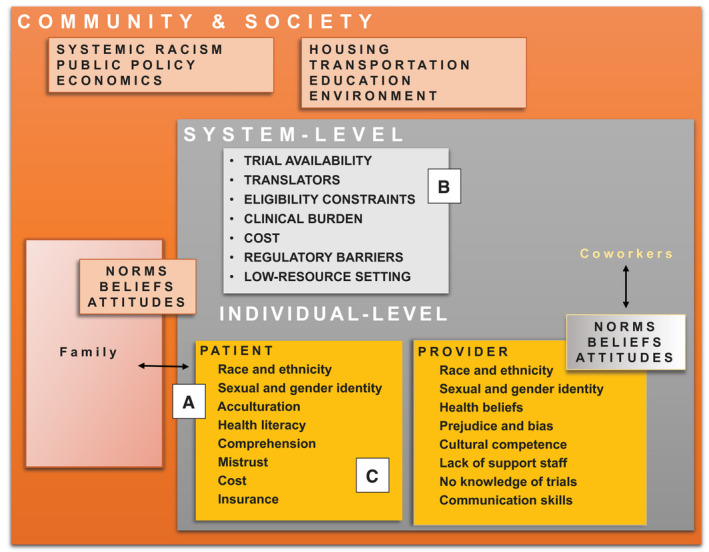
Patients interact with their families and carry a set of health beliefs that are informed by culture, background, and prior experiences in medicine (A). Providers work within a health care system that may have limited capacity to support clinical research, and even less capacity to support enrollment of diverse patients onto trials (B). Both patients and providers exist as part of a larger community and society and the norms, beliefs, and attitudes that exist through multiple levels collectively influence how patients and providers communicate about cancer therapy and clinical trials (C). Adapted with permission from Paskett et al. 29
Over the last decade, large‐scale efforts to increase DEI in clinical trials have included expanded eligibility criteria, centralized translation services, and remote consent/monitoring options, with all garnering robust support from clinical trial consortia, national cancer foundations, and, most recently, the US federal government. Unfortunately, rates of minority participation remain low despite these efforts and do not reflect the higher incidence of cancer observed in these populations. 12 , 13 , 14 In the commentary that follows, we reflect on the ongoing efforts to increase clinical trial diversity and consider whether, in addition to national and policy‐level interventions, a shift in care delivery and clinical practice at the local level may also be important.
Standardize the Collection of Sociodemographic Data in the Clinical Practice Setting
Despite the importance of demographic characteristics, socioeconomics, and language to cancer‐related outcomes, many centers do not routinely collect data on these variables from their patients. Collecting accurate and reliable data on race and ethnicity enables the measurement of care, quality, utilization, and outcomes in vulnerable populations (Fig. 2A). The US Census provides a minimum set of racial and ethnic categories for use during the collection of these data, and in 1997, the Office of Management and Budget 15 revised the Standards for Maintaining, Collecting, and Presenting Federal Data on Race and Ethnicity. 16 Drawing on stakeholder input, statistical analyses, and public guidance, the revision included 2 key provisions that inform how we are expected to collect these data today. First, the ethnicity category (Hispanic or not Hispanic) was separated from the race category. Second, respondents were given the option to select more than 1 racial designation, with instructions stating “mark one or more” and “select one or more.” 17 In presenting the option to select more than 1 racial category, the Office of Management and Budget established a precedent: To ensure accuracy, race categories should be assigned by self‐report. 5
Figure 2.
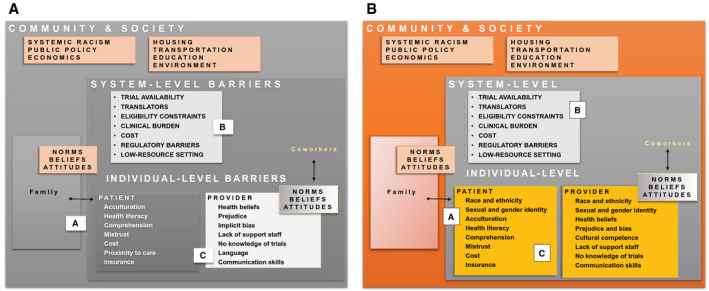
Multilevel interventions to address barriers and improve clinical trial access and enrollment. (A) Collecting sociodemographic data and patient‐reported social determinants of health. (B) Bolstering the research infrastructure, expanding access to interpreter services, and addressing logistical barriers in low‐resource settings. (C) Understanding how health beliefs, patient comprehension, communication, and perceptions of clinical research influence clinical trial enrollment decisions among diverse populations. At the community level, policy changes and interventions aimed at confronting systemic racism and its numerous sequelae are critical.
Similarly to racial and ethnic identity, sexual orientation and gender identity (SOGI) data should be self‐reported, and elements should follow guidelines from the US Health Resources and Services Administration. 18 The importance of incorporating SOGI data is that patients from sexual and gender minority groups similarly experience disadvantages in health and society that are related to bias and discrimination. Self‐reported sociodemographic data, when collected routinely, can be used to categorize populations, assess how well an institution's population reflects the catchment area, and identify diseases requiring focused study within the population. The data can also be used to inform local health initiatives, set milestones for staff DEI representation, and, when entered into the electronic health record, identify demographics of clinical trial participants. Thus, we propose that clinics consider how to best integrate assessment tools into existing workflow processes and that staff training protocols, uniform data system reporting guidelines, and clear procedures for entering demographic and SOGI data confidentially into electronic health records should be implemented.
Ask Patients About Their Preferred Language for Medical Conversations
The US Census Bureau's American Community Survey (2014‐2018; 5 years) reports that 21.6% of the US population speak a language other than English at home, and 8.4% speak English “less than very well.” 19 Patients with limited English proficiency (LEP) experience a breadth of health care inequities, including being underenrolled in clinical trials. Communication in patients' primary languages facilitates productive discussions about clinical trials, increases adherence to medical appointments, and reduces attrition for those with abnormal tests requiring further follow‐up (Fig. 2B). 20 , 21 Optimizing care for LEP populations requires system‐level capacity (ie, translated brochures and available interpreter services) and provider‐level commitment because conversations with interpreters necessarily require more time. Enrolling non–English‐speaking or LEP patients into clinical trials adds a layer of complexity to the clinical trial and treatment discussion, which may deter even the most proresearch providers from discussing trial participation with potentially eligible patients. 22
The Belmont report encourages equitable selection of study participants and mandates autonomy in the consenting process. 23 To ensure consenting autonomy and to avoid excluding patients with LEP, centers ideally should have translated study documents to match the languages spoken by the patients in their catchment area (eg, Spanish consent forms for patients in Washington Heights, New York, where more than 50% of the population is Spanish‐speaking). Certainly, it is not possible to have consent forms translated into all languages, and thus a short form consent should be available for almost all spoken languages from a center's institutional review board. For centers where a high proportion of patients speak Spanish or another common language, efforts should be made to have consent forms and surveys available in that language whenever possible. Translating English language consent forms, however, is a multistep process with high costs rarely covered in research budgets. These barriers may lead some centers, particularly those in low‐resource settings, to forgo translations; this makes it impossible to offer clinical trials to LEP populations. Recently, NCI‐sponsored protocols have facilitated translating consent forms into Spanish; however, the service does not uniformly cover the translation of all study materials (eg, surveys, ancillary studies, and assessments). Increasingly, industry‐sponsored trials are providing translated study documents on request. Additional investment from the NCI, institutions, foundations, or other study sponsors should be allocated to ensure that translated consent forms and study documents are available to centers enrolling a high proportion of LEP patients. Ideally, these translated forms could be provided at a low cost, and this would help to ensure the availability of translated documents when they are needed.
Identify Barriers Affecting CTE in the Real‐World Setting
A recent article by the NCI reported the results of a clinical trial screening tool administered across 46 NCI Community Oncology Research Program sites to 16,095 patients considering trial enrollment. 24 In total, 74% of those who participated in the screening tool assessment (83% of those approached) enrolled in a trial. Among the remaining 26%, the most common reasons for not enrolling included not meeting eligibility criteria (50%) and declining participation (47%). The most common reason for patient refusal was “no desire to participate in clinical research” (29%). Approximately 15% of patients refused for reasons related to potentially modifiable factors, including familial responsibilities, financial burdens, and insurance barriers. Interventions to reduce logistical barriers are certainly important for these populations that may be interested in participating but find the burden of extra visits or logistics impossible to reconcile with competing responsibilities. Of additional interest are the patients who simply endorse not wanting to participate in research. We propose that further work is needed to understand the reservations that these patients may have about participating. Often, educational interventions, more focused discussions, and the provision of additional information can allay some of the concerns about research that potential participants may have (Fig. 2C).
Collect Data on Social Determinants of Health for Clinical Trial Participants
Social determinants of health (SDOHs) are conditions in the places where people live, learn, work, and play that affect health risks and outcomes. They include upstream factors such as political, socioeconomic, and cultural constructs as well as place‐based factors such as access to health care, transportation, education systems, safe neighborhoods, and the availability of healthy foods. 25 Social and structural determinants, 26 which are largely related to systemic racism in the United States, influence not only peoples' interactions with the health care system 27 but also their epigenetic makeup, which is increasingly recognized as an important contributor to health disparities. 28 Despite mounting evidence linking SDOHs to cancer outcomes, the majority of consortium clinical trials do not collect these data from enrolled patients. Without these data to annotate clinical courses, biological specimens, or other study‐related end points, unmeasured factors affecting outcomes may be overlooked. Additionally, having uniform data collected from patients enrolled in clinical trials allows us to accurately characterize the populations that participate and to identify gaps in representation. We propose that all studies should collect a series of key baseline demographic and socioeconomic data from participants. For studies in which more detailed SDOH data are desired, additional consent forms and ancillary protocols may be warranted.
Recent work has demonstrated that it is feasible from a study perspective and acceptable from the patients' perspective to incorporate questions about SDOHs into clinical trials. A pilot trial was conducted in an Alliance trial (A191401) to determine the feasibility of collecting self‐reported data on demographics, health and health behaviors, and psychosocial constructs across academic and community sites. Demographic constructs included age, gender, race, ethnicity, languages spoken at home, education level, military service, marital status, sexual orientation, health insurance, number of household members, income, and home address. Health and health behaviors included tobacco and alcohol use, physical activity, diet, existing medical conditions, and self‐rated health. Psychosocial variables included quality of life, anxiety, fatigue, distress, depression, loneliness, pain, and social support. The study was completed within 38 months (from March 2015 to May 2018) at 9 sites (5 academic and 4 community) and included 200 participants. The refusal rate was 4%, and among those who participated, the rate of question completion was 97% to 100%. For most participants, the survey took 15 to 20 minutes to complete and was overwhelmingly considered acceptable in both length and question appropriateness.
This study's success demonstrates that it is both feasible and acceptable to collect self‐reported SDOH data from patients enrolling in clinical trials. To minimize the burden of additional data collection for clinical research teams, we propose that these data be incorporated into the enrollment procedure at the time of consent. For studies in which more extensive SDOHs are being collected, mechanisms to cover the cost of data collection and entry will be important.
The Accrual to Clinical Trials Framework
The CTE process involves more than simply the patient and the provider. We developed the Accrual to Clinical Trials framework, 29 which uses a multilevel lens through which to understand and address the individual, health system, and community‐level factors that influence a patient's decision about clinical trial participation. Patients interact with their families, providers interact with their colleagues, and each group lives as part of a larger community and society. The norms, beliefs, and attitudes that exist through these levels collectively influence how patients and providers communicate and collaborate when they interact within the health care system (Fig. 1).
To gain insight into CTE barriers at our institution, we established the Accrual Enhancement Protocol (AEP). The AEP relies on documentation of procedures and real‐time review and thus holds the system, providers, and support staff accountable as active participants in the trial enrollment process for every patient. The premise of the AEP is as follows: 1) all patients with cancer entering the system are screened for trial eligibility, with an emphasis on BIPOC patients, and 2) clinicians document the number of patients who are a) screened for eligibility, b) determined to be eligible, c) approached for enrollment, and d) enrolled. Additional documentation includes reasons for ineligibility, no contact, and nonenrollment. The team then 3) generates and reviews monthly reports of patient demographics, enrollment numbers, and documented reasons for ineligibility and refusals; 4) identifies areas where enrollment procedures meet resistance, including which step in the enrollment process and which clinics, providers, and patients need focused attention; and 5) assesses the impact of the AEP.
The AEP allows an institution to identify priorities for intervention at each level of the Accrual to Clinical Trials framework (Fig. 2B). 29 For example, if patients with certain diagnoses (eg, stage IV prostate cancer) are consistently ineligible for any open trial, then institutions can focus efforts on developing new trials in these areas. Alternatively, if certain clinics/providers/days of the week have lower rates of approaching potentially eligible patients for trials, qualitative work to identify and address barriers can be implemented. Lastly, if certain patient populations have higher refusal rates when approached to participate in clinical research, a focused study followed by targeted interventions (eg, navigators and education materials) can be initiated.
In conclusion, health care disparities exist in the context of historic and contemporary racism and serve as evidence of the persistent inequities rooted in many aspects of American life. The National Institutes of Health Protocol Worksheet, for example, which was developed in 2000 and based on the 2000 US Census, included the word Negro in the definition of Black race. Although this language is clearly unacceptable by today's standards, the systems supporting the form's development, approval, and dissemination just 20 years ago—and its continued use in some settings—remain solidly in place today. Fortunately, the National Institutes of Health acknowledges the need to end structural racism 14 ; however, concrete action and resources to support this acknowledgment are necessary. Simultaneously, the medical and scientific communities must work to dismantle the institutionalized barriers that pervade our clinical settings and must leverage the tools of critical inquiry to interrogate its role in perpetuating health disparities. We propose that 1) systematically collecting accurate data on race, ethnicity, SOGI, and language preference; 2) understanding how SDOHs influence trial participation; and 3) implementing procedures to improve communication and address accrual inequities through real‐time feedback at the individual, institutional, and community levels are the necessary first steps toward achieving this goal. Moreover, using protocols grounded in models will lead to success in ensuring DEI in trials and ultimately to the elimination of cancer outcome disparities.
Funding Support
Parts of the work described were funded by grants from the National Institutes of Health (P30CA016058 to Electra D. Paskett, Darrell M. Gray II, and Chasity M. Washington) and the Alliance NCI Community Oncology Research Program (UG1 CA189823 to Paskett and Jill M. Oliveri).
Conflict of Interest Disclosures
Justine M. Kahn has received grants from the Lymphoma Research Foundation and the National Center for Advancing Translational Sciences of the National Institutes of Health (KL2TR001874) for work performed outside the current study. Darrell M. Gray II reports consulting fees from Guardant Health and leadership on committees for the American College of Gastroenterology, the Association of Black Gastroenterologists and Hepatologists, and Fight CRC. Electra D. Paskett reports grants given to Ohio State University from Pfizer and the Merck Foundation for work not described here. The other authors made no disclosures.
Author Contributions
All authors contributed to the conceptualization, the writing of the draft, and the preparation of the manuscript.
Kahn JM, Gray DM II, Oliveri JM, Washington CM, DeGraffinreid CR, Paskett ED. Strategies to improve diversity, equity, and inclusion in clinical trials. Cancer.2022. 10.1002/cncr.33905
References
- 1. National Academies of Sciences, Engineering, and Medicine . Strategies for Ensuring Diversity, Inclusion, and Meaningful Participation in Clinical Trials: Proceedings of a Workshop. National Academies Press; 2016. [PubMed] [Google Scholar]
- 2. Barriers to Patient Enrollment in Therapeutic Clinical Trials for Cancer: A Landscape Report. American Cancer Society Cancer Action Network. Published April 11, 2018. Accessed May 15, 2021. https://www.fightcancer.org/sites/default/files/National%20Documents/Clinical‐Trials‐Landscape‐Report.pdf [Google Scholar]
- 3. Lara PN Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728‐1733. [DOI] [PubMed] [Google Scholar]
- 4. Unger JM, Vaidya R, Hershman DL, Minasian LM, Fleury ME. Systematic review and meta‐analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J Natl Cancer Inst. 2019;111:245‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duma N, Vera Aguilera J, Paludo J, et al. Representation of minorities and women in oncology clinical trials: review of the past 14 years. J Oncol Pract. 2018;14:e1‐e10. [DOI] [PubMed] [Google Scholar]
- 6. Niranjan SJ, Martin MY, Fouad MN, et al. Bias and stereotyping among research and clinical professionals: perspectives on minority recruitment for oncology clinical trials. Cancer. 2020;126:1958‐1968. [DOI] [PubMed] [Google Scholar]
- 7. Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112:228‐242. [DOI] [PubMed] [Google Scholar]
- 8. Hamel LM, Penner LA, Albrecht TL, Heath E, Gwede CK, Eggly S. Barriers to clinical trial enrollment in racial and ethnic minority patients with cancer. Cancer Control. 2016;23:327‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kwiatkowski K, Coe K, Bailar JC, Swanson GM. Inclusion of minorities and women in cancer clinical trials, a decade later: have we improved? Cancer. 2013;119:2956‐2963. [DOI] [PubMed] [Google Scholar]
- 10. Antman K, Amato D, Wood W, et al. Selection bias in clinical trials. J Clin Oncol. 1985;3:1142‐1147. [DOI] [PubMed] [Google Scholar]
- 11. Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Healthcare. National Academy Press; 2003. [PubMed] [Google Scholar]
- 12. Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race‐, sex‐, and age‐based disparities. JAMA. 2004;291:2720‐2726. [DOI] [PubMed] [Google Scholar]
- 13. Nazha B, Mishra M, Pentz R, Owonikoko TK. Enrollment of racial minorities in clinical trials: old problem assumes new urgency in the age of immunotherapy. Am Soc Clin Oncol Educ Book. 2019;39:3‐10. [DOI] [PubMed] [Google Scholar]
- 14. Stewart JH, Bertoni AG, Staten JL, Levine EA, Gross CP. Participation in surgical oncology clinical trials: gender‐, race/ethnicity‐, and age‐based disparities. Ann Surg Oncol. 2007;14:3328‐3334. [DOI] [PubMed] [Google Scholar]
- 15. Friedman DJ, Cohen BB, Averbach AR, Norton JM. Race/ethnicity and OMB Directive 15: implications for state public health practice. Am J Public Health. 2000;90:1714‐1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Durch JS, Madans JH. Methodological issues for vital rates and population estimates: 1997 OMB standards for data on race and ethnicity. Vital Health Stat 4. 2001;31:1‐30. [PubMed] [Google Scholar]
- 17. Valles SA, Bhopal RS, Aspinall PJ. Census categories for mixed race and mixed ethnicity: impacts on data collection and analysis in the US, UK and NZ. Public Health. 2015;129:266‐270. [DOI] [PubMed] [Google Scholar]
- 18. LGBT Policy Coordinating Committee . Advancing LGBT Health and Well‐Being. US Department of Health and Human Services. Published 2016. Accessed June 10, 2021. https://www.hhs.gov/sites/default/files/2016‐report‐with‐cover.pdf [Google Scholar]
- 19. 2014‐2018 American Community Survey 5‐year public use microdata samples [SAS data file]. US Census Bureau. Published 2020. Accessed June 15, 2021. https://www.census.gov/programs‐surveys/acs/data.html [Google Scholar]
- 20. Lee JS, Perez‐Stable EJ, Gregorich SE, et al. Increased access to professional interpreters in the hospital improves informed consent for patients with limited English proficiency. J Gen Intern Med. 2017;32:863‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ju M. Addressing health inequities for limited English proficiency patients: interpreter use and beyond. Pediatrics. 2021;147:e2020032383. [DOI] [PubMed] [Google Scholar]
- 22. Virnig BA, Morgan RO. Assessing capacity for clinical decisions and research for persons with low English proficiency: ethical and practical challenges. HEC Forum. 2002;14:235‐240. [DOI] [PubMed] [Google Scholar]
- 23. National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research . The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. US Government Printing Office; 1978. [PubMed] [Google Scholar]
- 24. St Germain DC, McCaskill‐Stevens W. Use of a clinical trial screening tool to enhance patient accrual. Cancer. 2021;127:1630‐1637. [DOI] [PubMed] [Google Scholar]
- 25. Kleinman DV, Pronk N, Gomez CA, et al. Addressing health equity and social determinants of health through Healthy People 2030. J Public Health Manag Pract. Published March 12, 2021. doi: 10.1097/PHH.0000000000001297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bona K, London WB, Guo D, Frank DA, Wolfe J. Trajectory of material hardship and income poverty in families of children undergoing chemotherapy: a prospective cohort study. Pediatr Blood Cancer. 2016;63:105‐111. [DOI] [PubMed] [Google Scholar]
- 27. Chambers BD, Erausquin JT, Tanner AE, Nichols TR, Brown‐Jeffy S. Testing the association between traditional and novel indicators of county‐level structural racism and birth outcomes among Black and White women. J Racial Ethn Health Disparities. 2018;5:966‐977. [DOI] [PubMed] [Google Scholar]
- 28. Lapp HE, Ahmed S, Moore CL, Hunter RG. Toxic stress history and hypothalamic‐pituitary‐adrenal axis function in a social stress task: genetic and epigenetic factors. Neurotoxicol Teratol. 2019;71:41‐49. [DOI] [PubMed] [Google Scholar]
- 29. Paskett ED, Katz ML, DeGraffinreid CR, Tatum CM. Participation in cancer trials: recruitment of underserved populations. Clin Adv Hematol Oncol. 2003;1:607‐613. [PubMed] [Google Scholar]


