Abstract
Objectives/Hypotheses
Children with unilateral sensory hearing loss (UHL) struggle to understand speech in noise and locate the origin of sound and have reduced quality of hearing. This clinical trial will determine the benefits of cochlear implantation in children with UHL.
Study Design
Prospective clinical trial.
Methods
Twenty children with at least moderate to profound sensory hearing loss and poor speech perception (word score <30%) in one ear and normal hearing in the contralateral ear participated in a Food and Drug Administration‐approved clinical trial. Subjects were evaluated for speech perception in quiet, speech perception in noise, sound localization, and subjective benefits after implantation.
Results
CNC word score perception in quiet significantly improved (1% to 50%, P < .0001) by 12 months after activation. Speech perception in noise by BKB‐SIN significantly improved in all three noise configurations; there was a 3.6 dB advantage in head shadow (P < .0001), a 1.6 dB advantage in summation (P = .003), and a 2.5 dB advantage in squelch (P = .0001). Localization improved by 26° at 9 months (P < .0001). Speech, Spatial, and Qualities (SSQ) demonstrated significant improvements in speech (5.2 to 7.4, P = .0012), qualities of hearing (5.9 to 7.5, P = .0056), and spatial hearing (2.7 to 6.6, P < .0001). SSQ subscales associated with binaural hearing were significantly improved, as was listening effort (P = .0082). Subjects demonstrated a non‐significant improvement in fatigue.
Conclusions
This study demonstrates that children with UHL significantly benefit from cochlear implantation.
Level of Evidence
Level 3 Laryngoscope, 132:S1–S18, 2022
Keywords: Unilateral hearing loss, single sided deafness, pediatrics, cochlear implantation
INTRODUCTION
Unilateral sensory hearing loss (UHL) is thought to affect approximately 1 in 1,000 newborns with congenital loss and up to 3% to 6% of school‐aged children. 1 , 2 , 3 , 4 , 5 , 6 It has become well‐established that there are significant ramifications for multiple aspects of child development, education, and well‐being when UHL is present. 7 , 8 As such, efforts have been focused on identifying children with UHL, providing educational accommodations (speech and language services) necessary to optimize their outcomes, and fitting appropriate hearing technology. 8 , 9 , 10 Many of these children have or will develop a degree of hearing loss that is inadequately rehabilitated with a traditional hearing aid, 9 and as such are unable to take advantage of the critical benefits of binaural hearing. These patients with substantial UHL, or single‐sided deafness (SSD), can be defined as having moderate‐to‐profound sensorineural hearing loss with limited speech perception in one ear and normal to near‐normal hearing in the contralateral ear.
Improved binaural auditory function occurs when people with normal hearing listen with both ears, and when those with hearing loss utilize bilateral hearing aids or cochlear implants (CI). 11 , 12 Conversely, amplification or implantation of only one side in patients with bilateral hearing loss is associated with reduced auditory function as compared with bilateral input. 12 , 13 , 14 , 15 Three primary effects on auditory perception have been identified in binaural hearing: the head shadow effect, the binaural squelch effect, and the binaural summation effect. 16 , 17
The head shadow effect occurs when the target speech and masker are spatially separated. For example, a masker on the right side of the listener would interfere with the right ear, but the head would block the masker (create an acoustic shadow) for the left ear. Thus, the head shadow effect would result in a better target‐to‐masker ratio in the left ear. A listener is able to selectively attend to the ear with the better target‐to‐masker ratio for improved speech intelligibility. 18
The binaural squelch effect also occurs when the masker is spatially separated from the target and the brain interprets different inputs from each ear. Unlike the purely physical head shadow, however, binaural squelch requires central auditory processing to integrate the signals from each ear so that a clearer signal is received by the auditory cortex than could be possible from either ear alone. 19 The brainstem auditory nuclei process differences in timing, amplitude, and spectral signals coming from the two ears, resulting in improved separation of the target speech and masker, and consequent improved speech intelligibility.
Binaural summation also refers to a central processing effect, but it is thought to occur when both ears are presented with a similar signal. The combined signals from the two ears are perceived as louder by up to 3 dB compared with monaural listening to the same signal. 20 This doubling of perceptual loudness is accompanied by increased sensitivity to differences in intensity and frequency and can lead to improvements in speech intelligibility under both quiet conditions and when exposed to noise.
Another benefit of hearing with both ears is sound source localization. Depending on the location of the sound source, sound will arrive at one ear before the other. There will also be a difference in amplitude, as sound will arrive at a higher amplitude at the ear closer to the source than at the contralateral ear. Mechanisms of localization differ for low‐ and high‐frequency sounds. 17 For low‐frequency sounds, the primary cue for localizing is timing (interaural time difference); that is, the difference in time of arrival of a sound at the closer and farther ears. For higher frequency sounds, the primary cue for localization is differences in intensity (interaural level difference). As both of these effects require comparing information between the two ears, binaural hearing is required to enable this critical advantage.
An example of the benefits of binaural hearing is especially evident in studies in which adult subjects have undergone cochlear implantation bilaterally. 12 In a prospective multi‐center trial, subjects demonstrated improved speech perception in quiet and improved speech perception in noise when listening with bilateral implants as opposed to a monaural implant condition. 20 This benefit was present for all three binaural effects: head shadow, squelch, and summation. 20 These subjects also reported better hearing when listening with both implants. 20 Bilaterally implanted subjects were also found to have a significant improvement in their ability to localize sound in a horizontal plane, with only one implant their ability to localize sound was effectively at chance. 21 While binaural skills develop over time in children, pediatric bilateral CI recipients have demonstrated similar benefits, although they may be greatest when cochlear implantation is performed early. 22 , 23 , 24 , 25 Together these data demonstrate that bilateral cochlear implants use can provide binaural benefits for both speech perception in noise and localization.
A cochlear implant can provide binaural benefits when utilized with a contralateral cochlear implant. It is tempting therefore to suspect that patients with UHL too severe for effective hearing‐aid amplification could perform better with a cochlear implant. These patients are identified as having substantial sensory UHL or SSD. They may be defined as moderate‐to‐profound sensorineural hearing loss with limited speech perception (<60% on sentence testing in noise) in one ear and normal to near‐normal hearing in the contralateral ear. These patients would meet historic Food and Drug Administration (FDA) criteria for cochlear implantation for bilateral hearing loss, but in only one ear. 26 These patients experience many of the predictable difficulties based on known benefits of binaural hearing. Despite the normal to near‐normal hearing sensitivity in one ear, patients with UHL experience poor speech understanding, especially in noise, 27 , 28 and variable ability at localizing the source of sounds in their environment. 29
Contemporary treatment options for UHL include conventional hearing aids, contralateral routing of the signal (CROS) hearing aids, and bone‐conduction devices. Patients with substantial UHL typically do not benefit from a conventional hearing aid due to the severity of their hearing loss and poor speech discrimination abilities in the affected ear. Alternatively, CROS hearing aids and bone‐conduction devices route the acoustic signal from the poorer hearing ear to the normal hearing ear. A CROS hearing aid is a two‐part system that includes a microphone/transmitter on the poorer hearing ear and a receiver on the normal hearing ear. The microphone/transmitter sends the acoustic signal from the poorer hearing ear to the receiver, which presents the signal to the normal hearing ear. Bone‐conduction devices use an implanted abutment and vibrating transducer to send the acoustic signal from the poorer hearing ear to the normal hearing ear. While CROS hearing aids and bone‐conduction devices provide the listener with access to the sound from the poorer hearing ear, they do not restore binaural hearing, as the information is still processed through one auditory pathway. 30 The listener is unable to benefit from interaural timing and level difference cues, and as such speech understanding in noise is variable. 31 Localization abilities with these devices have been found to be at chance or poorer than unaided performance. 32 , 33 , 34
As outcomes with contralateral routing of sound by CROS or bone conduction are unsatisfactory, interest in cochlear implants, which have the potential to provide true binaural hearing, has increased. One concern that has been raised is how auditory processing centers will integrate both an acoustic signal and an electric signal from a cochlear implant together. Will the two signals sound so significantly different that the brain will be unable to combine the two signals in a beneficial manner to permit binaural hearing? Ample evidence suggests that the brain can incorporate both electric and acoustic signals, at least from the same ear. Cochlear implant recipients with low‐frequency hearing preservation in the implanted ear demonstrate significant benefit when listening with the combination of acoustic and electric stimulation in the same ear. 35 , 36 , 37 In this situation, a longer array may be partially inserted or a shorter array fully inserted into the cochlea to permit hearing preservation. Due to the tonotopic organization of the cochlea, patients hear both acoustically with their residual low‐frequency hearing and electrically in the mid‐ and high‐frequency ranges with their cochlear implant. The benefit of combined electric and acoustic hearing has also been well‐demonstrated in children in whom low‐frequency hearing is preserved. 38 , 39 , 40 In addition, the benefit of the combination of acoustic hearing and a cochlear implant has also been well‐demonstrated with bimodal hearing in which patients with a cochlear implant benefit from a hearing aid in the contralateral, poor hearing ear. 41 , 42 Together these data suggest that auditory processing centers can integrate both electric and acoustic signals for an overall greater benefit for speech perception in both adults and children.
If the brain can combine information from acoustic hearing and a cochlear implant in the same ear, and from a CI and hearing aid on opposite ears, it seems reasonable to expect that information from a cochlear implant could be integrated with normal hearing from an opposite ear as well. Initial studies on subjects with UHL focused on treating the potentially disabling tinnitus associated with the hearing loss with a cochlear implant. 43 Cochlear implantation was highly successful in this initial study for reducing the symptoms of tinnitus in patients with UHL, with a reduction in tinnitus severity on a visual analog scale from 8.6 to 2.2. 44 This same cohort of cochlear implant recipients were then also examined for benefits to speech perception in noise and were found to have substantial gains in speech perception in both head shadow and squelch configurations. 45
Since these initial exciting findings, there have been a number of case series that have evaluated the benefit of cochlear implantation in adults with UHL. These have shown variable degrees of improved speech perception in quiet, speech perception in noise, localization, and quality of hearing/quality of life. 45 , 46 , 47 As there has been some heterogeneity in results from retrospective investigations, a number of well‐performed prospective clinical trials have subsequently been performed and demonstrated consistent benefits of cochlear implantation in adults with UHL. Buss et al. demonstrated a mean increase in CNC word recognition in quiet from an average of 4% to a mean of 55% with the cochlear implant alone at 12 months after implantation. 48 Firszt et al. found an improvement in sentence perception in quiet with multiple talkers (TIMITQ) from 55% to 75% correct when both the acoustic hearing ear and the cochlear implant ear were used together. 49 As much of human existence occurs in work and social settings in which noise is a constant attendant, speech perception in noise is a more critical metric to determine how subjects are performing in the real world. Data from prospective clinical trials support improved speech perception in noise when listening with the cochlear implant plus the normal‐hearing ear, particularly when taking advantage of head shadow. Buss et al. demonstrated that a CI improves speech perception on AzBio sentences by an average of 36 percentage points 48 when the target speech is presented in front of the subject and the masker is separated to the side of the normal‐hearing ear. A marked improvement in masked speech perception was also seen using adaptive tests. These measure the signal‐to‐noise ratio (SNR) at which half of speech is perceptible; hence, a lower score indicates improvement. SNR was reduced from 4 to 1 dB in R‐space environments and from 5 to 2.5 dB on tests of sentences in 4 talker babble (BKB‐SIN). 49 A similar improvement in adaptive SNR was seen in Tavora‐Viera et al. in which addition of the cochlear implant improved SNR by 3.5 dB. 50 Collectively these data demonstrate marked, consistent improvements in masked speech perception with the cochlear implant in comparison to without the implant in adults.
Localization is also markedly improved with cochlear implant use in cases of substantial UHL. Sound source localization is the ability of an individual to determine where a sound originates. This allows the individual to find a talker in a noisy room, identify the location of a ringing cell phone, or determine the direction of a honking horn before crossing traffic. Root‐means‐squared (RMS) error is a metric that identifies the accuracy with which an individual can determine the location of the sound source, with lower values reflecting greater accuracy. All of these prospective studies evaluated changes in localization ability following cochlear implantation for UHL. Data from one prospective trial demonstrated an improvement in localization from an average of 64° RMS error to 25° with medium (62 dB SPL) levels of sound presented; this persisted out to 1 year post‐activation. 51 Data from a separate multicenter trial demonstrated a similar improvement from 60° RMS error to 22° using the same MED‐EL system. 50 Firszt et al. also showed consistent, significant improvement with an average RMS error improving from 50° to 30° RMS error. 49 All of these studies conclusively and consistently demonstrate that cochlear implant recipients with UHL experience improved sound source localization with cochlear implant use.
As mentioned earlier, the initial motivation for cochlear implantation in UHL was for the treatment of the associated disabling tinnitus. 43 Although speech perception and sound localization have emerged as greater benefits, the effect on tinnitus is still significant and well documented. In the FDA clinical trial, tinnitus severity was noted to drop significantly and dramatically following cochlear implantation to nearly undetectable levels by one month after cochlear implant activation. 52 These data demonstrate that tinnitus can be significantly improved following cochlear implantation for UHL.
With all of these benefits reported, it is of substantial interest to determine how CI recipients with UHL perceive changes in the attributes of sound and quality of life. All of these major studies have evaluated this. The multicenter trial evaluated subjects before and after implantation using the short form of the Speech, Spatial, and Qualities (SSQ12) form. This demonstrated a marked improvement in the SSQ12 score from 4 to 6 after 5 years of use. 50 Firszt et al. used the full SSQ questionnaire and demonstrated marked, statistically significant improvements in all three domains of the SSQ including speech, spatial hearing, and qualities of hearing. 49 They also utilized the Glasgow benefit inventory to determine the overall benefit and determined a benefit of a cochlear implant of 37.9 (score of zero would indicate no benefit). The other prospective trial also demonstrated significant benefit of a cochlear implant in the Abbreviated Profile of Hearing Aid Benefit test, most notably showing benefit in situations of background and reverberant noise. 52 Similar marked and significant benefits were seen with speech, spatial, and qualities of hearing. 52 These data conclusively demonstrate substantial and consistent benefits in multiple well‐performed prospective clinical trials for improving quality of hearing and quality of life in adult subjects receiving a cochlear implant for UHL.
The fusion of auditory information from the cochlear implant and the normal hearing ear is likely critical to early and consistent success. It is therefore of paramount importance to select an electrode array that most closely replicates natural cochlear tonotopicity. Cochlear implants are designed to filter incoming sound signals into distinct frequency bands and then present the envelope information of individual channels to specific electrode contacts along the array. The electric stimulation within specific regions of the cochlea results in specific frequency percepts, with high‐frequency sound resolved in the basal portion of the cochlea and low‐frequency sounds in the apical portion of the cochlea. 53 Frequency–place matching refers to when the electric filter frequencies of an individual channel match the cochlear place frequencies corresponding to the electrode contact. 54 A frequency–place mismatch occurs when the electric filter frequencies are shifted relative to the cochlear place frequency. Frequency–place mismatch, coupled with spectral degradation (due to limited number of channels in an electrode array), are two of the major sources of signal degradation that occur as cochlear implants attempt to replicate speech. 54 Shorter cochlear implant electrode arrays that incompletely cover the cochlea characteristically create frequency–place mismatches that are exaggerated in the apical region of the cochlea, as the implant does not penetrate deeply enough to directly stimulate this region. 55 , 56 , 57 , 58
Despite these potential shortcomings, subjects with bilateral sensory hearing loss implanted unilaterally perform quite well with shorter electrode arrays. This has been suggested to be due to a process referred to as cortical tonotopic re‐mapping. 59 In this process, frequency–place mismatches in the cochlea are resolved by tonotopic remapping, thought to be mainly occurring at the level of the auditory cortex. 60 , 61 The amount of remapping that can occur can be quite impressive. Acclimatization with shorter electrode arrays has been reported up to three octaves. 59 Many factors may influence how effective this process of tonotopic remapping occurs. Some factors may include age, cognitive ability, electrode array position in the cochlea, and consistency of device usage. 59 All subjects may therefore not have the same capacity to adjust to frequency mismatches. The process of cortical tonotopic remapping may vary across cochlear implant recipients as well. Acclimatization of frequency–place mismatches may take only months, may take multiple years, or may never fully occur following cochlear implant activation. 62
The ultimate impact of frequency–place mismatches on speech perception has begun to be addressed. Long electrode arrays have demonstrated a close approximation with pitch presented in the cochlear implant ear or the contralateral normal hearing ear, 63 , 64 , 65 suggesting minimal place‐pitch mismatch. Vocoder simulations of speech have suggested greater clarity of speech with longer arrays that provide better place‐pitch matching. 66 In a prospective, randomized trial that evaluated speech perception outcomes following implantation with either a medium (24 mm) or a long (31.5 mm) array, direct comparisons were made using the same processing strategy and device from the same manufacturer. 67 Substantial differences were noted early in the study between medium array recipients and long array recipients, necessitating early discontinuation of the trial, and retrospective recruitment of more long array recipients. At 1 year, subjects receiving the longer array performed at significantly higher levels for speech perception in quiet and noise. The significant benefit of a longer array persisted out to 4 years, 67 suggesting that cognitive remapping of cochlear place‐pitch may remain incomplete in medium array recipients to the detriment of their ultimate speech perception.
Although the effect of place‐pitch mismatch between shorter and longer electrodes has not been compared directly for perception and localization outcomes in cases of UHL, differences have been seen between different array designs that have different angular insertion depths. One of the earlier retrospective studies evaluating the benefits of cochlear implantation in UHL demonstrated an average improvement in CNC word scores of 28% and an average improvement in AzBio sentence scores of 40% (in quiet only) with predominantly perimodiolar electrode arrays. 47 Localization was not completely reported, but demonstrated a non‐significant improvement of approximately 10° in RMS error at 12 months post‐activation. 47 In contrast, a prospective study utilizing a 31.5 mm electrode demonstrated an improvement in CNC words scores of 51% at 12 months post‐activation and a 42% improvement in AzBio scores in a more challenging speech in noise condition (head shadow effect). 48 Localization demonstrated a marked improvement in RMS error that immediately and significantly improved by an average of 31° at medium presentation levels, improving by an average of 41° at 6 months. 51 These accelerated and greater benefits suggest that there are greater advantages in localization and speech perception in subjects implanted with longer arrays. Together these data suggest that long arrays which permit the closest approximation of natural cochlear place may be optimal for patients undergoing cochlear implantation for UHL.
As data emerged demonstrating marked improvement in multiple outcome measures for speech perception, localization, and quality of life in adults, and evidence to suggest the optimal electrode arrays for UHL has begun to emerge, attention has begun to shift toward the benefits of cochlear implant use for children with UHL. There are, however, important differences between children and adults that must be taken into consideration before proceeding with cochlear implantation. The first of these considerations is that the etiology of hearing loss in children with UHL is very different than the etiology in adults. Overall, the causes of UHL in adults and children include sudden sensorineural hearing loss (SSNHL), inner ear malformations, acoustic neuroma, cochlear nerve deficiency, mumps, congenital cytomegalovirus (CMV) infection, meningitis and auditory neuropathy. 68 , 69 The distribution of causes between adults and children is quite different and has significant indications for outcomes with cochlear implantation. These differences have been evaluated by comparing pre‐lingual and post‐lingual causes of UHL. In 197 cases of post‐lingual UHL (both SSD and asymmetric hearing loss), the major causes were idiopathic SSNHL (58%) followed by chronic otitis media/cholesteatoma (30%), and cerebellopontine angle tumor (9%). 70 The etiology in cases of pre‐lingual hearing loss was substantially different. In pre‐lingual hearing loss (combined SSD and asymmetric hearing loss) the major cause was cochlear nerve deficiency (44% of subjects undergoing imaging) followed by CMV (6%), mumps (6%), and inner ear malformations (4%). 70 Malformations may be under‐represented in this cohort as other studies have indicated up to 41% of cases have identifiable inner ear malformations. 71 Etiologies will therefore be quite different between adults and children. This is of enormous importance, as a good outcome with a cochlear implant will depend upon the severity of inner ear malformations and/or the presence of cochlear nerve deficiency. 72 This may be especially true in cases of UHL as a distorted cochlear implant signal from a severely malformed ear (more severe than incomplete partition 2) or hypoplastic/atretic cochlear nerve may preclude functional outcomes. The cochlear implant signal may never synergize with the contralateral acoustic hearing ear. The risk and expense of surgery would therefore be unwarranted and inappropriate in these cases of severely malformed ears or hyoplastic or atretic cochlear nerves.
If the origins of UHL are different in children, it would seem likely that there are different effects of UHL in children as well. The psychoacoustic effects of loss of binaural hearing are also seen in children. Children with UHL have greater difficulty than their normal hearing peers with speech perception in quiet and in a dynamic listening environment when the target speech is in front and the masker is directed to the normal hearing ear and in co‐located conditions. 73 They also have significantly more difficulty localizing the source of sounds than their normal hearing peers. 74 Children with UHL also report a poorer quality of life than their peers with normal hearing. 73 Together these data demonstrate children suffer similar problems as adults due to their loss of binaural hearing. As children though, they are still developing, learning language, and cultivating a sense of self, so their needs may be greater than adults.
Unlike adults who have matured their lexicon, children are still learning language and require a more favorable SNR to access spoken language. 75 Absence of binaural hearing, especially in a noisy environment, can therefore compromise a child's ability to learn language. Not surprisingly, children with UHL have been shown to have poorer language scores than their normal hearing peers, and the delay persists at least into adolescence. 76 , 77 Additionally, children are building their educational foundation in consistently challenging listening environments. The noise to signal levels in a typical classroom can exceed −6 dB SNR, 78 and the cognitive effort that is required to decipher speech in this environment can be significant. Over the course of a typical school day, it could lead to fatigue that may have a negative effect on learning, especially in a child trying to hear with only one ear. 79 , 80 Finally, children are developing a sense of self‐worth and esteem early in education that promotes confidence later. Difficulties with education and problems interacting with peers could damage a child's sense of self‐esteem. Previous studies have suggested higher rates of behavioral issues in children with UHL. 9 , 10 Other studies have demonstrated that children with hearing loss can have lower levels of self‐esteem in the social domains, 81 , 82 and that those who receive early intervention have higher levels of self‐esteem than their peers. 83 All of these unique concerns, if unaddressed, could have substantial ramifications throughout childhood and into adulthood. Treatment decisions made early in a child's life could therefore have the potential to resonate throughout their lifetime.
The currently approved treatment options for children with UHL are the same as adults, namely utilizing technology to route an acoustic signal from the poorer hearing ear to the normal‐hearing ear. These technologies include both the CROS hearing system and bone conduction systems. Both systems have shown a modest benefit for speech perception in noise if the noise is not focused on the aided/implanted ear. 31 , 84 , 85 , 86 , 87 , 88 , 89 Bone conduction aids do not improve localization as they do not restore true binaural hearing 33 , 88 , 89 , 90 and may in fact worsen localization abilities. 91 Current treatment options are therefore inadequate for treating all the difficulties children with UHL experience.
As the current options are unsatisfactory, a number of retrospective studies and case series have evaluated outcomes in children receiving cochlear implants off‐label. These have variably demonstrated improved speech in quiet, 92 , 93 improved speech in noise, 94 , 95 and better localization of sound. 96 , 97 , 98 , 99 An improvement in subjective measures of speech, spatial, and qualities of hearing has also been seen. 96 Although these studies have suggested a benefit of cochlear implantation for UHL in children, a carefully controlled prospective clinical trial determining the benefits of cochlear implantation in children with UHL has not been performed. The aims of the present prospective, longitudinal clinical trial were to determine the objective and subjective benefits of cochlear implantation in children with moderate to profound UHL that are unable to benefit from traditional hearing aid amplification. This prospective clinical trial, used rigorous inclusion criteria, and fixed data collection points spanning the 12‐month post‐activation period. We hypothesized that children would experience improvements in word recognition in the implanted ear, speech perception in spatially separated noise, sound localization, and subjective perception of hearing with a cochlear implant. Together these data will provide the impetus to hearing health care providers to offer the option of cochlear implantation to appropriate pediatric candidates with UHL. It will also help compel government and private insurance carriers to cover cochlear implantation for this critical need in these children.
METHODS
This study was investigator‐initiated and industry‐sponsored. It was approved by the local Institutional Review Board and was completed under an Investigational Device Exemption from the FDA. The parents/guardians of the subjects provided parental consent prior to cochlear implantation.
Subjects
Twenty subjects were enrolled and received a cochlear implant. To be considered, potential participants needed at least a moderate‐to‐profound sensorineural hearing loss in one ear and normal hearing (based on pure tone average) in the contralateral ear. Speech perception in the ear to be implanted had to be less than 30%. Subjects were required to be between 3.5 and 6.5 years of age at the time of cochlear implantation. This age was chosen to permit rigorous preoperative testing, as younger children may have struggled to complete all of the audiologic testing required. Two subjects exceeded these criteria and were included under compassionate use as approved by the FDA. Both of the children were older than 6.5 years (7.0 and 12.7 years), but they reported sudden hearing losses and short durations of deafness (2.3 years each). All participants were screened for normal cognition with the Leiter‐R Brief IQ subscale. Exclusion criteria included evidence of a cochlear malformation more significant than an incomplete partition type II (IP‐II), 100 ossification, CND based on MRI, and an inability to complete the test protocol.
Interest in the study was high and the parents of 46 additional potential participants contacted the study team for consideration. They were invited for in‐person consent and screening if initial review of medical records and imaging suggested potential candidacy, and they continued to be interested in the study.
Devices and Mapping
Nineteen of the participants received a MED‐EL SYNCHRONY device with a FLEX28 electrode array, and one subject with an IP‐II malformation received a FLEX24 array. The electrode array was fully inserted in all of the cases, with the exception of one FLEX28 recipient with an IP‐II malformation who had two extra cochlear electrode contacts.
Activation of the external processor occurred approximately 2 weeks post‐operatively. All participants were fit with the SONNET processor and were programmed with omni‐directional microphone settings and a frequency range of 100 to 8,500 Hz. Subjects were either programmed with behavioral methods, scaling Most Comfortable Levels (MCLs) to “comfortable but loud” or objectively with Electrical Stapedial Reflex Thresholds depending on what was most developmentally appropriate. MCL levels were loudness balanced globally to the normal‐hearing ear when possible. Electric threshold levels were measured behaviorally using conditioned play or conventional methods and were set below audibility.
Aided Speech Perception
Aided speech perception testing was completed in either a single‐ or double‐walled sound attenuating booth. A Grason‐Stadler GSI‐61 audiometer was used for audiometry and speech perception testing. Speech perception stimuli were all recorded, and the computer housing the sound files was coupled to the audiometer for speech perception testing in the soundfield. Subjects were seated approximately 1‐m from the speaker for soundfield testing.
Aided Speech Perception: Word Recognition in Quiet
Pre‐operatively, word recognition was assessed via the standard version of the Early Speech Perception test (ESP), and the 50‐item CNC word list. Stimuli were presented at 60 dB SPL in the soundfield. Subjects were evaluated using the normal hearing ear alone, and with a conventional hearing aid set to desired sensation level (v5) targets. 101 The contralateral ear was masked with 40 dB HL of speech shaped noise during aided testing.
The closed‐set ESP task contains four subtests aimed to detect whether subjects are unable to perceive patterns in speech (category 1), able to identify patterns in speech (category 2), able to identify some words (category 3), or able to consistently identify words through the use of vowels (category 4). For each subtest, a target word is presented, and subjects are instructed to point to a picture in a set of 12 that corresponds to the word they heard. Each word is presented twice in each subtest and stimuli were randomized. With the CNC word list, subjects are presented with a carrier word “ready” followed by the three‐phoneme target word. They are asked to repeat the target and their response is scored as correct or incorrect. A full list of 50 words was completed in each condition. List presentation was randomized.
Aided ESP results indicated that 17 of the subjects were not able to discriminate patterns of speech in the affected ear with the hearing aid alone (category 1). Testing was discontinued and the CNC word score was recorded as 0%. Three subjects had some or consistent word identification skills (category 3 or 4). Testing continued with the CNC word list in the aided condition and scored as percent correct.
Post‐operatively, the ESP and CNC tests were completed with a recorded stimuli at the 3, 6, 9, and 12 month post‐activation intervals via direct connect testing. This method has been found to be comparable to standard sound field testing while avoiding the impact of central masking in this population. 102 First, a splitter with separate volume controls was connected to the audio jack of the test computer. The audiologist wore a wired bone conduction headset plugged into one side of the splitter. The volume on this side of the splitter was lowered to just audible levels to ensure that the subject could not hear any stimuli from their normal hearing ear, but the tester could track where they were in the list. Second, the subject's SONNET processor was connected to the other side of the splitter using a 90/10 cable and FM battery sleeve. This attenuated the microphone of the processor to 10% and allowed for direct streaming of the stimulus to the cochlear implant. Subjects listened to practice stimuli to adjust the volume control to a comfortable level before testing began. One‐way analysis of variances (ANOVAs) with Tukey's multiple comparisons were performed on CNC data from each test battery utilizing Graph Pad Prism software version 8.0 for Mac, GraphPad Software, San Diego, CA. This statistical analysis package was used throughout the study.
Aided Speech Perception: Masked Sentence Recognition
The masked sentence recognition assessment was completed at the 6‐ and 12‐month post‐activation intervals using the BKB‐SIN. 103 The target sentence was presented at 60 dB SPL and the 4‐talker masker level increased over the course of each list. Subjects were tested with and without their processor in three conditions: Target and masker co‐located in front (summation), target in front and the masker to the side of the normal‐hearing ear (head shadow), and target in front and the masker to the side of the affected ear (squelch). An SNR‐50 (SNR level at which subject was able to repeat back 50% of sentences correctly) was computed for each of the 6 test conditions. Two list pairs of 10 sentences were used in each condition and both lists and test order were randomized. Wilcoxon matched pair signed rank test was performed to evaluate for differences between implant off and implant on conditions for each of the test intervals.
Sound Source Localization
Subjects completed localization testing at the 3‐ and 9‐month post‐activation intervals in both device on and device off conditions. They were seated in a double‐walled sound booth facing a 180° arc of speakers. Eleven ear‐level speakers were evenly spaced at 18° intervals approximately 1‐m from the listener (−90° to 90°). Above each speaker was a picture of an animal. The subjects wore a small headlamp and were instructed to turn their head toward the sound to light the animal above the speaker that produced the sound, and then return to midline. The stimulus was a 200‐ms speech shaped noise burst randomly presented from one of the 11 speakers at 70‐dB SPL. Each speaker was used 4 times during a block of 44 trials. One block was completed for each condition. A test assistant sat in the booth below the middle speaker to help center the subject between presentations, maintain appropriate head position, and provide encouragement. Subjects were provided with several practice trials until the researcher and assistant agreed that the child understood the task.
Performance was analyzed for each condition at each test interval by computing the root‐mean‐squared error (RMSerr) as previously described. 21 , 48 Wilcoxon matched pair signed rank test was performed to evaluate for differences with implant on and implant off conditions at each interval.
Subjective Assessment
A modified version of the adult Bern Benefit in SSD Questionnaire was utilized to evaluate subjects' perceived benefit of a cochlear implant. 104 This 10‐item questionnaire was designed to be used by adults with UHL. Subjects are asked to rate perceived benefit of hearing technology in specific situations on a Likert scale, with −5 indicating hearing is much easier without the processor/hearing aid, and 5 indicating it is much easier with the processor/hearing aid. A modified version of this test was used where questions were rephrased to allow parents to respond as proxy for their child.
The SSQ questionnaire assesses subjective performance in three domains, hearing speech in quiet and noisy environments, spatial or directional hearing, and sound qualities. 105 Each item is rated on a 10‐point Likert scale. Domain scores represent an average of item ratings. The Speech, Spatial, and Qualities of Hearing Scale for Children with Impaired Hearing used in this study was created in 2013 and based on the adult version of the questionnaire. 106 A Friedman test for non‐parametric data was performed with post‐hoc Dunn's multiple comparisons where appropriate.
Pragmatic subscales of the SSQ can be examined by scoring specific test items as described by Gatehouse & Akeroyd. 107 Subscales related to speech in quiet, speech in noise, listening effort, localization, identification of sound and objects, segregation of sounds, speech in speech contexts, distance and movement, and multiple speech stream processing and switching have carried over to the pediatric version of the questionnaire and were calculated for each test interval. A Kruskal–Wallis test was performed for each of the subscales over the entire interval.
Fatigue in children was assessed using the PedsQL Multidimensional Fatigue Scale. This is a validated scale for determining fatigue in young children, including general fatigue, sleep/rest fatigue, and cognitive fatigue. 108 Scores from this test have been previously demonstrated to be substantially affected by hearing loss in children. 79 Higher scores indicate less fatigue. Scores were not anchored to prior responses. A one‐way ANOVA was performed to evaluate for differences between preoperative fatigue and fatigue after cochlear implantation over the 12‐month study period.
RESULTS
Patient demographics are shown in Table I. The most common, known etiologies were congenital CMV (n = 3), malformation (IP2) (n = 2), Waardenburg syndrome (n = 1), infection (n = 1), and trauma (n = 1). Twelve of the subjects had an unknown etiology. All 20 subjects had MRI imaging with no evidence of cochlear nerve deficiency. The average age at surgery was 5.5 years (SD = 2.0) and the average duration of deafness was 3.3 years (SD = 1.7). All subjects had normal hearing thresholds on their contralateral ear (x̅ pure tone average [PTA] = 9.6 dB HL, SD = 6.3) and severe‐to‐profound hearing loss in their implanted ear (x̅ PTA = 108.1 dB, SD = 14.9). The mean length of deafness at the time of initial CI activation was estimated at 3.3 years (SD = 1.7).
Table I.
Subject Demographics.
| Subject ID | Age at Surgery (yr) | Age at Activation (yr) | Length of Deafness (yr) | Age Hearing Aid | Age at Diagnosis | NBHS | Ethnicity | Sex | Etiology | Stability | Affected Ear | Pre‐Op Pure Tone Average CI (dB) | Pre‐Op Pure Tone Average Contra (dB) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 01SAHO | 6.42 | 6.44 | 1.85 | 4 yr | 4 yr | Pass | White | Male | Infection | Known sudden | Right | 112 | 2 |
| 02GAGA | 6.33 | 6.35 | 1.35 | Never aided | 5 yr | Pass | Mixed Race | Female | Unknown | Reported sudden | Right | 88 | 15 |
| 03ELHA | 4.52 | 4.56 | 1.39 | Never aided | 3 yr | Pass | Mixed Race | Female | Trauma | Known sudden | Right | 120 | 3 |
| 04SHKA | 6.05 | 6.09 | 6.09 | 2 yr | 2 yr | Fail, pass on rescreen | White | Female | Malformation | Suspected congenital | Left | 85 | 20 |
| 05TUWH | 4.67 | 4.72 | 4.72 | Never aided | 2 mo | Failed unilateral | White | Male | Waardenburg | Known congenital | Right | 118 | 20 |
| 06AUSI | 12.67 | 12.70 | 2.25 | Never aided | 10 yr | Pass | White | Male | Unknown | Reported sudden | Left | 97 | 8 |
| 07ZAJA | 3.95 | 3.98 | 3.98 | 22 mo | 8 mo | Failed unilateral | White | Male | Malformation | Known congenital | Right | 82 | 10 |
| 08HALA | 6.49 | 6.55 | 4.55 | Never aided | 3 yr | Pass | White | Male | Unknown | Progressive suspected | Right | 120 | 7 |
| 09ALGU | 6.47 | 6.52 | 6.52 | Never aided | 6 yr | Fail, pass on rescreen | White | Female | Unknown | Suspected congenital | Left | 110 | 8 |
| 10SYRO | 6.99 | 7.02 | 2.34 | Never aided | 4 yr | Pass | African American | Female | cCMV | Reported sudden | Left | 95 | 7 |
| 11LYMA | 6.08 | 6.13 | 3.50 | Never aided | 5 yr | Pass | White | Female | Unknown | Progressive suspected | Right | 120 | 2 |
| 12KAPL | 3.91 | 3.99 | 1.76 | Never aided | 2 mo | Failed unilateral | Mixed Race | Female | cCMV | Known progressive | Left | 113 | 22 |
| 13RAWI | 4.83 | 4.91 | 4.16 | Never aided | 2 yr | Failed unilateral | White | Male | Unknown | Known progressive | Right | 120 | 8 |
| 14WYJO | 5.39 | 5.46 | 1.25 | Never aided | 4 yr | Pass | African American | Male | Unknown | Reported sudden | Left | 118 | 2 |
| 15NIRA | 5.50 | 5.53 | 0.78 | 4 yr | 3 yr | Was not tested | Asian | Male | Unknown | Known progressive | Left | 77 | 8 |
| 16SOHE | 3.74 | 3.78 | 3.78 | Never aided | 2 yr | Fail, pass on rescreen | White | Female | Unknown | Suspected congenital | Right | 120 | 8 |
| 17SECO | 5.37 | 5.42 | 5.42 | Never aided | 1 mo | Failed unilateral | White | Male | Unknown | Known congenital | Left | 117 | 8 |
| 18WYBA | 3.53 | 3.58 | 3.58 | 3 mo | 2 mo | Failed unilateral | White | Male | Unknown | Known congenital | Left | 120 | 15 |
| 19CAGA | 3.87 | 3.91 | 3.91 | Never aided | 3 yr | Fail, pass on rescreen | White | Male | Unknown | Suspected congenital | Left | 110 | 15 |
| 20NODO | 3.56 | 3.60 | 3.60 | 3 yr | 1 mo | Failed unilateral | White | Male | cCMV | Known congenital | Right | 120 | 3 |
CND = cochlear nerve deficiency; NBHS = new born hearing screen.
Of the 46 additional potential subjects who underwent screening either remotely or in person (Table II), the majority were ineligible due to age. Approximately one third of these potential subjects had imaging reviewed by the principal investigator prior to trial consideration. Of these 16 children, 38% (n = 6) were determined to have cochlear nerve deficiency that was previously unknown to the family. This highlights the importance of careful review of preoperative MRI imaging, as has been previously published. 109
Table II.
Screen Failures.
| Screen ID | Seen for a Study Screening Appointment? | Age | Did We Review Imaging? | Concern Expressed by Family | Criteria Not Met |
|---|---|---|---|---|---|
| Screen01 | No | 0.2 | No | None | Age |
| Screen02 | No | 0.2 | No | None | Age |
| Screen03 | No | 0.6 | No | Travel (Korea) | Age |
| Screen04 | No | 0.7 | No | None | Age |
| Screen05 | No | 0.8 | No | None | Age |
| Screen06 | No | 0.9 | No | None | Age |
| Screen07 | No | 1.2 | No | None | Age |
| Screen08 | No | 1.25 | Yes | None | Age |
| Screen09 | No | 2.4 | Yes | Dad didn't want | None that we knew of |
| Screen10 | No | 2.5 | No | Lack of pediatric outcome data | Age |
| Screen11 | No | 2.6 | No | None | Age |
| Screen12 | No | 2.8 | Yes | None | Age and bilateral loss |
| Screen13 | No | 2.9 | No | Ability to afford travel | Age |
| Screen14 | No | 3 | No | None | Study full |
| Screen15 | No | 3.3 | No | Travel | None that we knew of |
| Screen16 | No | 3.5 | No | None | Ossification |
| Screen17 | No | 3.5 | Yes | Travel Cost | None that we knew of |
| Screen18 | Yes | 3.6 | Yes | None | Could not complete battery, commitment to procedures |
| Screen19 | No | 3.8 | No | Imaging | CND |
| Screen20 | No | 4 | No | Travel (west coast family) | None that we knew of |
| Screen21 | No | 4 | Yes | None | CND |
| Screen22 | No | 4 | Yes | Travel Cost | None that we knew of |
| Screen23 | No | 4.3 | No | None | None that we knew of |
| Screen24 | No | 4.8 | Yes | None | CND |
| Screen25 | No | 5 | Yes | None | CND, Too much hearing in poorer ear (pure tone average 48, poor word rec, though) |
| Screen26 | No | 5 | No | None | Study full |
| Screen27 | No | 5 | No | None | Cognitive delay |
| Screen28 | No | 5.4 | Yes | Unknown outcomes | CND |
| Screen29 | No | 5.4 | Yes | Travel Cost, want child to play football | None that we knew of |
| Screen30 | Yes | 5.8 | Yes | None | CND |
| Screen31 | No | 5.9 | Yes | Travel | Cognitive delay |
| Screen32 | No | 6 | No | None | Bilateral Hearing Loss |
| Screen33 | No | 6.1 | No | Travel Cost | None that we knew of |
| Screen34 | Yes | 6.3 | Yes | None | CND |
| Screen35 | No | 6.3 | Yes | Travel Cost | None that we knew of |
| Screen36 | No | 6.5 | No | None | Age |
| Screen37 | No | 6.5 | Yes | None | Ossification |
| Screen38 | No | 6.6 | No | None | Age |
| Screen39 | No | 6.8 | No | None | Age |
| Screen40 | No | 7.3 | No | None | Age |
| Screen41 | No | 9.1 | No | Age | Age |
| Screen42 | No | 10 | No | None | Age |
| Screen43 | No | 12 | No | Age | Age |
| Screen44 | No | DNK | No | None | Study full |
| Screen45 | No | DNK | No | Travel | None that we knew of |
| Screen46 | No | DNK | No | Age | Age? |
Aided Speech Perception: Word Recognition in Quiet
Speech perception in quiet was assessed utilizing the standard version of the ESP and when appropriate with CNC word list. 110 , 111 Pre‐operatively, 17 subjects only achieved category 1 on the ESP when wearing an appropriately fit hearing aid, indicating no perception of speech patterns in the affected ear. These subjects were given a CNC score of zero, as they were unable to complete open set testing. Three subjects with some or consistent aided word recognition underwent aided CNC word testing. All subjects completed CNC testing at all post‐activation intervals.
Results of ESP testing demonstrated rapid improvement in early auditory skills. At 3 months, only 5 subjects remained in category 1, by 9 months all subjects had at least some word identification (category 3), although one suspected non‐user relapsed to category 1 at 12 months. The normal hearing ear in all cases began and remained in category 4 (Fig. 1A).
Fig 1.
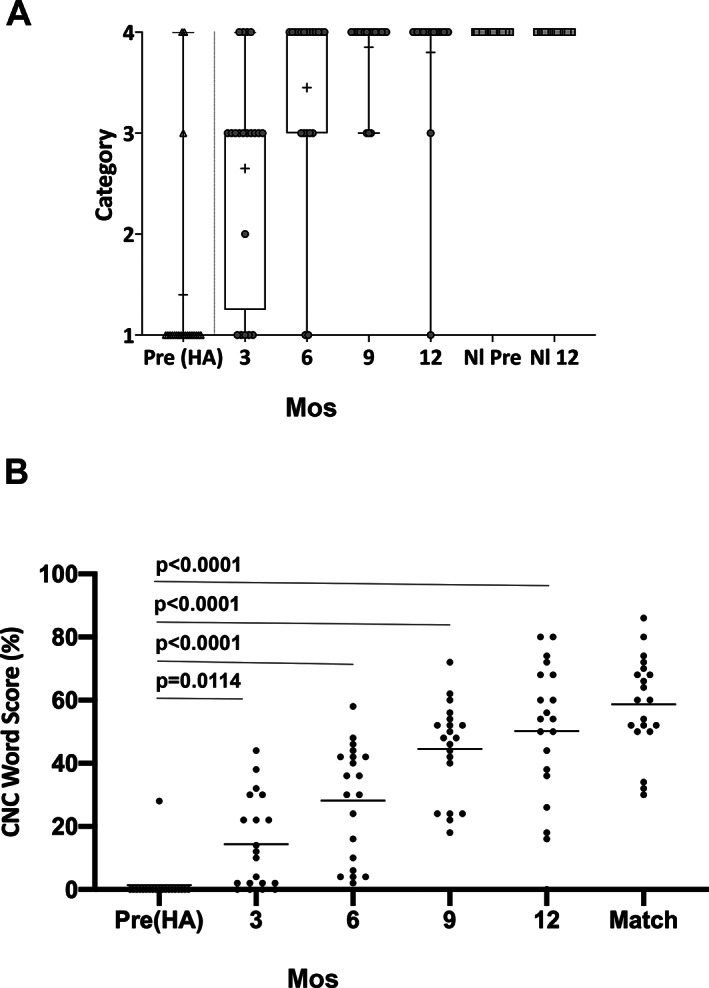
Speech perception in quiet improves following cochlear implantation in children with unilateral sensory hearing loss. Children undergoing cochlear implantation for unilateral sensory hearing loss were tested for speech perception both before surgery (pre) with a hearing aid (HA) and at 3, 6, 9, and 12 months post‐activation with both (A) ESP category testing and (B) CNC word testing by direct connection through their cochlear implant. For CNC word testing, a one‐tailed ANOVA was performed with P < .0001. Tukey's multiple comparisons were performed post hoc between groups and are demonstrated. CNC word scores are plotted as a scatter dot plot of all values with mean shown (horizontal bar). CI‐recipient controls matched on age at implantation, duration of deafness, length of device usage, and spoken language abilities (match) are shown for comparison.
CNC word testing demonstrated a significant improvement over time (F[4,19] = 55.65, P < .0001) from a mean pre‐operative 1% CNC word score (SD = 6.2) to 14% at 3 months (SD = 14.5), rising to a 50% CNC mean word score at 12 months (SD = 22.3) (Fig. 1B). Scores improved from the pre‐operative condition after only 3‐months of use (P = .008) and improved further between the 3 and 6 month (P = .0004), and 6 and 9 month intervals (P = .0009). There was no significant improvement between the 9‐ and 12‐month conditions indicating stabilization of performance (P = .5462) (Fig. 1B).
The mean CI alone average at 12 months compares favorably with a group of bilateral cochlear implant recipients drawn from the clinic database matched by age, length of deafness, length of CI use, and language skills who had an average CNC word score of 59% (SD = 15.30). This comparison demonstrated a non‐significant difference by unpaired t‐test (P = .1674). Together these data suggest that pediatric subjects with SSD can perform on average as typical cochlear implant recipients and do so within a similar time frame of 12 months.
Aided Speech Perception: Masked Sentence Recognition
Although the gains in speech perception in quiet with a cochlear implant are impressive, much of a child's educational and social existence will occur in a background of noise. 112 It is critical to determine how a child's acoustic hearing ear and cochlear implant ear are working together, and to determine how they are perceiving speech in noise after cochlear implantation. Results of BKB‐SIN testing demonstrated early advantages for speech perception in noise with a cochlear implant on vs. off (Fig. 2).
Fig 2.
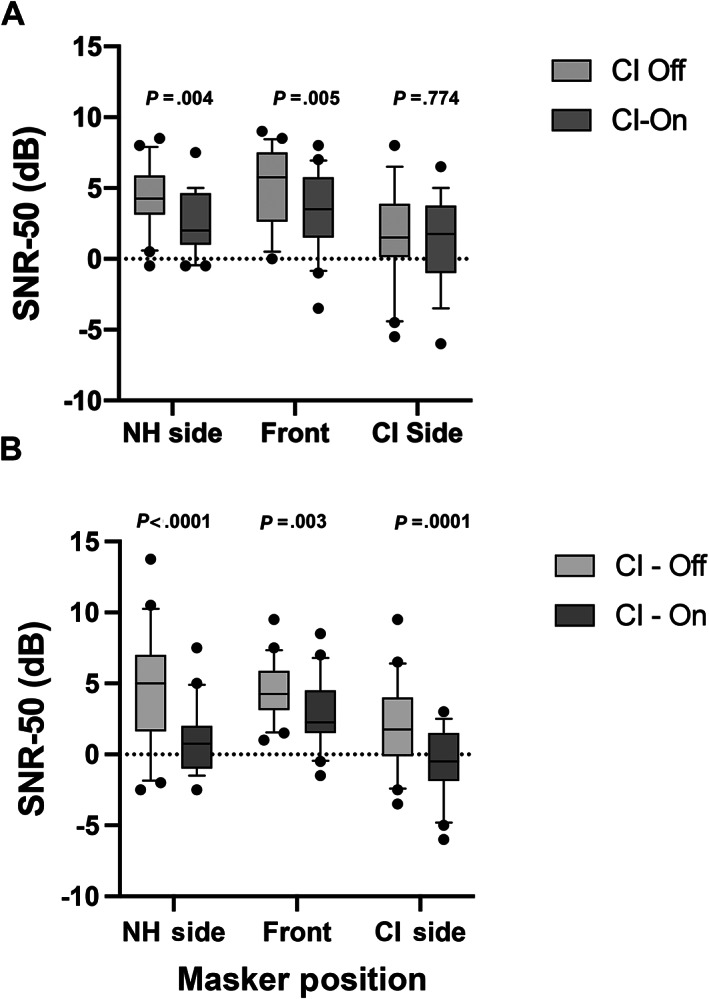
Speech perception in noise improves in all three noise conditions by 12 months following cochlear implantation in children with unilateral sensory hearing loss. Children undergoing cochlear implantation for unilateral sensory hearing loss were tested for speech perception in noise utilizing the BKB‐SIN to determine the signal to noise ratio at which subjects were able to understand 50% of sentences. Subjects were tested in three noise configurations: NH side = speech front, noise to normal ear (head shadow), Front = speech front, noise front (summation), and CI side = speech front, noise CI side (squelch). A lower number demonstrates better speech perception in noise. Subjects were tested at (A) 6 months post‐activation and (B) 12 months post‐activation. A Wilcoxon matched pair signed rank test was performed to compare device on and device off for each of the noise configurations. Data are plotted as box and whisker (10–90th percentile).
Comparisons between implant off and implant on by Wilcoxon matched pair signed rank test demonstrated an average significant gain at 6 months in SNR‐50 of approximately 2 dB (P = .005) when the noise/masker was directed at the normal hearing ear (head shadow) and 1.8 dB advantage when sound and the noise/masker was at front (summation) (P = .004). There was no statistically significant advantage when the noise/masker was presented to the cochlear implant ear (squelch) (P = .7740) at 6 months. All conditions demonstrated an advantage by 12 months with the cochlear implant with improvements in the SNR‐50 advantage. There was an even greater 3.6 dB advantage at 12 months when the noise/masker was presented to the normal hearing ear (head shadow) (P < .0001) and again a 1.6 dB advantage when the noise/masker was presented at front (summation) (P = .003). A significant squelch effect had now developed at 12 months as well with a 2.5 dB difference in SNR‐50 (P = .0002). These data together demonstrate that children are able to integrate information from their acoustic hearing ear and cochlear implant ear, ultimately in all noise conditions (head shadow, squelch and summation). Head shadow and summation benefits were seen earliest at 6 months, and a squelch effect was seen at 12 months. Head shadow effects continued to grow between 6 and 12 months. (Fig. 2)
Sound Source Localization
If binaural benefits are present for speech perception in noise with a cochlear implant, it seems likely that binaural cues would also be present with a cochlear implant for localization. Localization with and without an implant was therefore evaluated in an 11 speaker array as previously described with slight modifications for children as described in Section 2. 51 RMS error was then computed (Fig. 3). Comparison by a Wilcoxon matched pair signed rank test demonstrated an immediate improvement in localizing ability with implant on. This was a 17‐degree improvement on average at 3 months following activation (P = .0009). This improvement in localizing ability with implant on grew over the ensuing 6 months with subjects improving in localization on average by 26° at 9 months following activation (P < .0001). These data demonstrate that subjects are able to utilize binaural cues from their cochlear implant to more accurately determine the sound source. This ability appears to improve over time, at least up to 9 months following activation of their cochlear implant.
Fig 3.
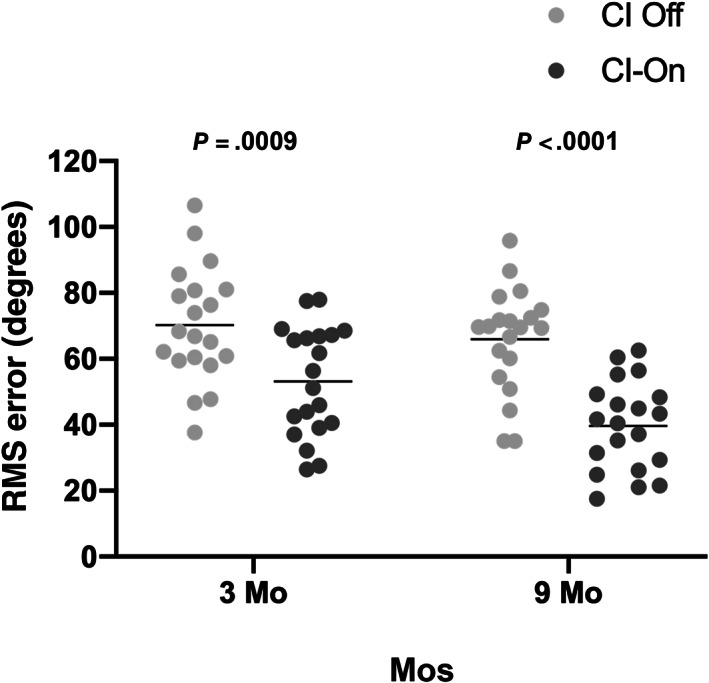
Localization rapidly improves following cochlear implantation in children with unilateral sensory hearing loss. Children undergoing cochlear implantation for unilateral sensory hearing loss were evaluated for sound localization by testing in an 11 speaker, 180‐degree array. The stimulus was a 200‐ms speech shaped noise burst randomly presented from one of the 11 speakers at 70 dB SPL. Each speaker was used 4 times during a block of 44 trials for both the device on and the device off conditions. Root‐means‐squared error was computed for each trial with each subject for 3‐ and 9‐month timepoints. A Wilcoxon matched pair signed rank test was performed at each timepoint to determine differences between device on and device off situations. Data are plotted as scatterplot with box and whiskers (10–90th percentile)
Subjective Assessment
Although the improvement in speech perception in quiet and noise and localization are critical demonstrations of the benefits of cochlear implantation for UHL, it is essential to also demonstrate that subjects perceive they are hearing better with their cochlear implant. Results of the Bern Benefit in SSD Questionnaire indicated that nineteen of the twenty subjects found hearing to be much easier with their cochlear implant than without. One subject was equivocal. This suggests that not only do subjects have objective benefits, but that they also subjectively feel that they are hearing more easily with a cochlear implant than without (Fig. 4).
Fig 4.
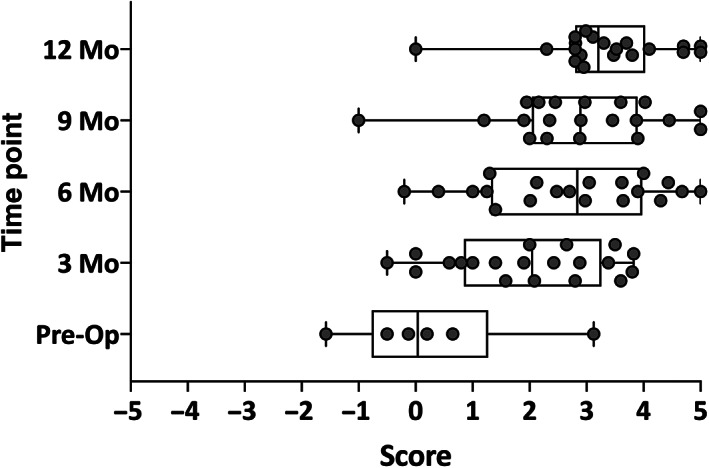
Pediatric subjects with unilateral sensory hearing loss subjectively perceive hearing to be easier with a cochlear implant than without. Subjects underwent evaluation by parent proxy using a modified Bern Benefit Inventory questionnaire to determine the benefit of their hearing device. Preoperatively the six subjects who used hearing aids were assessed. Post‐operatively all 20 subjects were assessed with their cochlear implant. Parents are asked to rate perceived benefit of hearing technology in specific situations on a Likert scale, with −5 indicating hearing is much easier without the aid in that condition, and 5 indicating it is much easier with the aid. Results are shown at 3, 6, 9, and 12 months following activation of the cochlear implant.
Subjects report improvements in their ease of hearing, but improvements in hearing could potentially occur from a number of sources. It could be the ability to understand speech in quiet or noise, it could be spatial or directional hearing, or it could be the quality of hearing. The SSQ helps differentiate these sources of improvement. Results demonstrated compelling improvements in all three domains of speech, spatial, and qualities of hearing (Fig. 5). Speech hearing scores improved significantly from an average score of 5.2 pre‐operatively (SD = 1.8) to 7.4 (SD = 1.3) at 12 months following implant activation (P = .0012). Qualities of hearing also significantly improved over the study intervals, increasing from an average score of 5.9 (SD = 2.2) to 7.5 (SD = 1.1) (P = .0056). Spatial hearing showed the largest gain in scores, improving from an average pre‐operative score of 2.7 (SD = 2.3) to a 12‐month score of 6.6 (SD = 1.7) (P < .0001). These data demonstrate that subjects significantly benefit in all three scales of the SSQ, indicating that their subjective perception is markedly improved.
Fig 5.
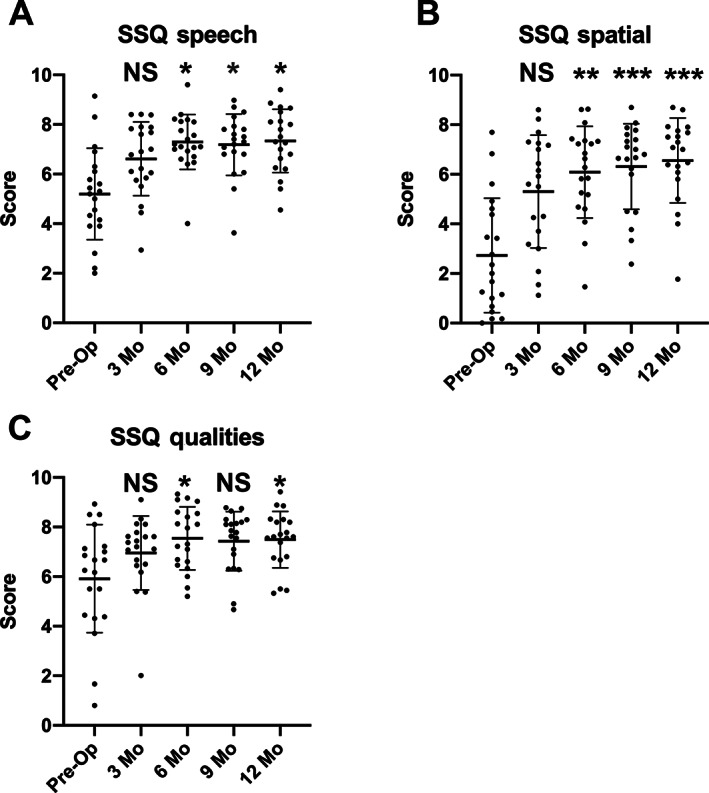
Pediatric subjects with unilateral sensory hearing loss have improved perception of speech in quiet and noise, improved perception of spatial or directional hearing and improved sound quality following cochlear implantation. Subjects undertook the pediatric version of the SSQ. The SSQ questionnaire assesses subjective performance in three domains, hearing speech in quiet and noisy environments, spatial or directional hearing, and sound qualities. Each item is rated on a 10‐point Likert scale. Domain scores represent an average of item ratings. The questionnaire was completed at the pre‐operative interval and the 3, 6, 9, and 12‐month post‐activation intervals. A Friedman test for non‐parametric data (P = .0010 for speech, P < .0001 for spatial and P = .0030 for qualities of hearing) with post hoc Dunn's multiple comparisons was performed to compare changes at the different intervals tested. Each interval is compared with preoperative values. Significance levels: *<0.05, **<0.001, ***<0.0001, NS = non‐significant.
Additional detail can be gained by performing a pragmatic subscale analysis of the SSQ. These data demonstrate that the greatest gains (which reached significance over time by Kruskal–Wallis test) were speech in noise (P = .0001), speech in speech (P = .0008), listening effort (P = .0082), localization (P < .0001), distance and movement of sound (P < .0001), and processing multiple speech streams (P = .0187) (Fig. 6A–I). Speech in quiet (P = .2738), identification of the sound (P = .8904), and segregation of sound (P = .2025) were not significantly affected. These data suggest that the specific aspects of hearing which require binaural hearing are most positively affected by the addition of a cochlear implant in subjects with UHL.
Fig 6.
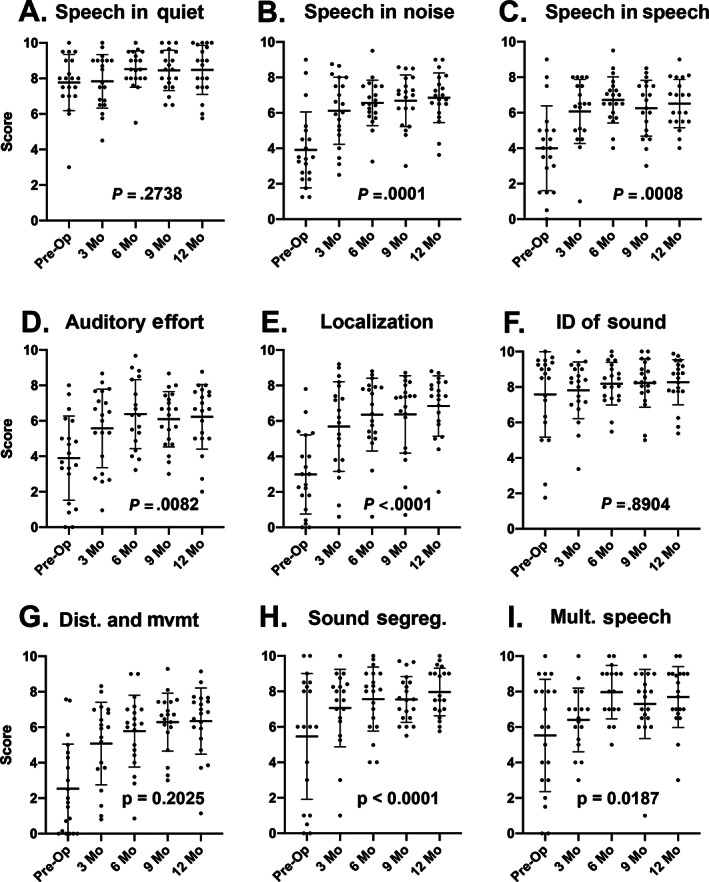
(A–I) Pediatric subjects with unilateral sensory hearing loss have improved perception on pragmatic subscales of speech, spatial, and qualities (SSQ) associated with binaural hearing following cochlear implantation. Subjects completed the pediatric version of the SSQ. Sub‐scales were computed for speech in quiet, speech in noise, listening effort, localization, identification of sound and objects, segregation of sounds, speech in speech contexts, distance and movement, and multiple speech stream processing and switching. Scores were calculated for each test interval at 3, 6, 9, and 12‐months post‐activation. A Kruskal–Wallis test was performed to determine if scores changed over time. P values are shown on each graph.
A number of studies have suggested that hearing loss can lead to fatigue in adults and children. 79 , 113 Recent data have also specifically suggested that children with unilateral hearing loss are at an increased risk of listening‐related fatigue. 80 The parent version of the PedsQL Multidimensional fatigue scale was used to determine if our cohort of children with UHL were suffering from fatigue as has been described, and what effect correction of the UHL loss by placement of a cochlear implant had on their fatigue. Scores were not anchored to prior responses. Overall children with UHL demonstrated mean levels of fatigue between levels previously reported for children with no hearing loss and children with congenital hearing loss. 79 Children demonstrated a non‐significant improvement in fatigue in all three subscales (Fig. 7). For general fatigue, scores increased from 73 (SD = 21.18) to 81 (SD = 16.47) over the 12‐month period (F[4,19] = 2.175, P = .1039). For sleep fatigue, scores increased 80 (SD = 23.37) to 86 (SD = 13.61) over the 12‐month period (F[4,19] = 2.389, P = .0967). For cognitive fatigue, scores increased from 61 (SD = 24.04) to as high as 72 at 6 months (SD = 17.93) before dropping back to 66 at 12 months (SD = 13.98) (F[4,19] = 2.038, P = .1309). Together these data suggest a trend for improvement in fatigue with cochlear implant use, though this does not reach significance.
Fig 7.
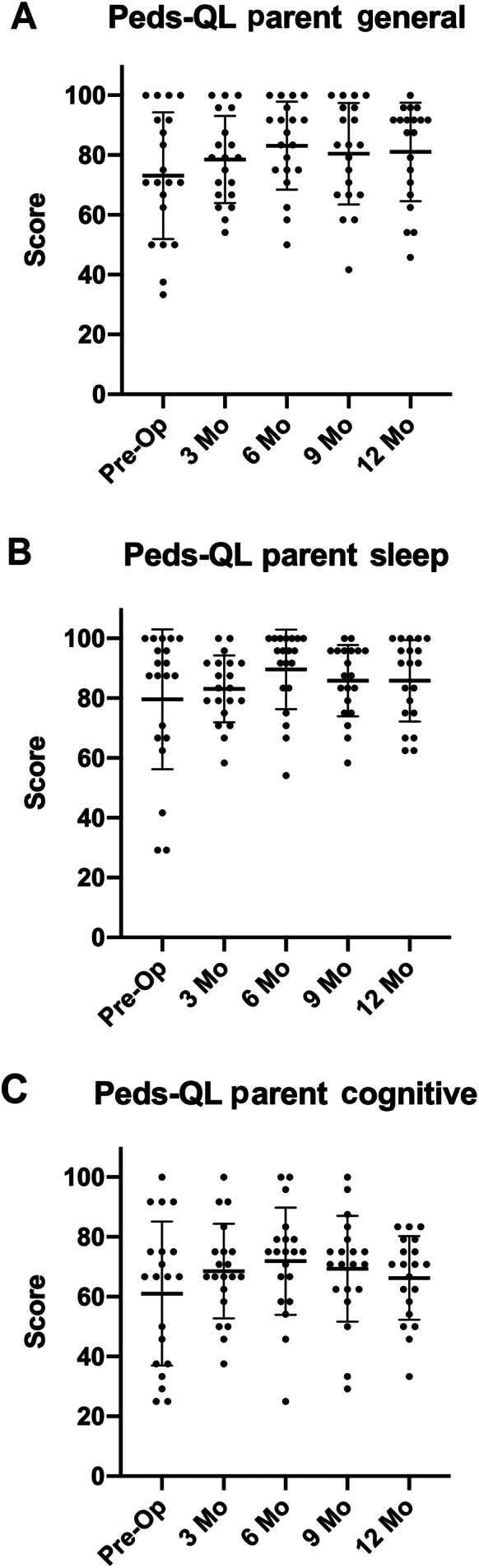
Pediatric subjects with unilateral sensory hearing loss do not significantly improve their fatigue score following cochlear implantation. Subject's parents undertook the PedsQL to assess fatigue over scales of (A) general fatigue, (B) sleep fatigue, and (C) cognitive fatigue. Responses were determined at 3, 6, 9, and 12 months following activation. An ANOVA was performed to determine if significant differences were present. Results were as follows (general P = .1039, sleep P = .0967, cognitive P = .1309).
DISCUSSION
Data from adult subjects have demonstrated substantial benefits of cochlear implantation for UHL including improved speech perception in quiet, improved speech perception in noise, improved sound source localization, suppression of tinnitus, and improved quality of hearing and quality of life. 48 , 50 , 51 , 52 , 98 These adult data have led to recent FDA approval of the MED‐EL Corporation cochlear implant system for treatment of substantial UHL in adults and children 5 years of age and older. Preliminary data from case series in children have shown similar, albeit varied outcomes. 92 , 93 , 94 , 96 , 97 , 98 , 99 We aimed to demonstrate, in a prospective clinical trial with explicit inclusion criteria and rigorous data collection, the benefits of cochlear implantation for children on measures of speech perception, localization, and quality of hearing/quality of life.
The age of inclusion for this trial was selected based on ability to undergo masked audiometry, perform open set speech perception, and complete localization tasks consistently. There have been reports suggesting that later implantation of children with congenital hearing loss may be associated with poorer outcomes, 114 although some case series have had success implanting children almost 9 years old with congenital UHL. It would ultimately be a goal to implant children with substantial UHL, and no anatomic contraindications, in a manner similar to typical bilateral cochlear implant candidates by 1 year of age. This would help prevent deleterious cortical reorganization associated with unilateral hearing loss (so called aural preference syndrome) and loss of ability to integrate binaural cues appropriately. 115
Trial subjects had either normal cochlea, or in two cases had incomplete partition 2 unilaterally, and all had normal cochlear nerves. This is a critical assessment, as numerous studies have highlighted the high incidence of cochlear nerve deficiency in this population. 70 Patients with cochlear nerve deficiency accounted for 38% of our screen failures that had imaging already performed (Table II), and this should be a significant caution for proper MRI imaging of the cochlear nerve with personnel qualified to determine presence of a cochlear nerve. It should be noted that it is tempting to offer the option of cochlear implantation in UHL to hopeful parents in cases of more substantial cochlear anomalies or cochlear nerve deficiency. In some cases of severely malformed cochlea and/or cochlear nerve deficiency there may even be measurable hearing. There are no data though that signal representation by a cochlear implant through a significantly deformed cochlea, or deficient cochlear nerve is sufficiently similar to acoustic hearing to enable binaural benefits. Patients with incomplete partition 2 have outcomes similar to standard cochlear implant candidates, 72 so it was reasonable to implant these individuals. With the exception of one IP2 subject, all patients received Flex‐28 devices, which at the time was the longest flexible lateral wall array produced by the company. Optimal place‐pitch consistency may enhance acceptance of the device in children as the implant will be coherent with pitch they are hearing in the normal ear. This may, similar to adults, permit earlier and/or greater speech perception benefits.
Outcomes for speech perception in children with cochlear implants for UHL demonstrated a rapid and consistent increase in ability to perceive speech in quiet (Fig. 1). This is consistent with results seen in adult patients and is also consistent with typical speech perception outcomes in matched cochlear implant peers receiving implants for bilateral hearing loss. 48 Interestingly, results are different for children in their ability to perceive speech in noise. Children by 6 months post‐activation were already demonstrating not only a head shadow benefit with their cochlear implant, but they were also demonstrating a significant summation effect (Fig. 2A) as well which did not develop as extensively in adults. 48 Children had also developed a significant squelch benefit of their cochlear implant by 12 months post‐activation which has not been observed consistently in adults (Fig. 2B). This may reflect increased cognitive plasticity in children that enables them to more rapidly integrate binaural cues to improve speech perception in noise. Children also developed improved sound localization at levels consistent with adults by 9 months post‐activation, also supporting their ability to integrate binaural cues (Fig. 3). Together these data demonstrate that children are able to use binaural cues from their cochlear implant to hear better in noise and localize sound more effectively. Long‐term follow‐up will permit a determination of whether these benefits continue to grow over time.
Objectively, this cohort of children is performing better with their cochlear implant than without, but as important is how children feel their cochlear implant is improving hearing. Results of the Bern Benefit Inventory demonstrate that they felt that the cochlear implant was more of a benefit to their ability to hear easily as opposed to a hindrance (Fig. 4). In addition, there were global improvements in the SSQ for all three domains of speech, spatial, and qualities of hearing, all of these significantly improving 12‐months post‐activation (Fig. 5). Of these, spatial hearing appeared to be the most negatively affected by lack of hearing in one ear pre‐operatively and had the greatest increase in score following cochlear implant activation. This is consistent with data seen in adult trials as well. 52 When SSQ pragmatic subscales were evaluated, these benefits appeared to be developing from predominantly binaural hearing benefits, namely speech perception in noise, speech perception in speech, sound segregation, processing multiple speech streams and identifying distance of sound and movement (Fig. 6). Benefits of monaural hearing such as speech perception in quiet and identification of a type of sound were not significantly improved. This is likely due to an ability to perform this adequately with the normal‐hearing ear alone.
Listening effort was also significantly improved (Fig. 6). Listening effort reflects the cognitive effort required to perceive and process speech, and it has been determined that children with UHL expend greater auditory effort when processing speech in noise. 116 Increased listening effort, especially over the course of a day in complex listening environments, is thought to be a key contributor to fatigue in children. 79 , 80 Accumulated fatigue over the course of the day may compromise attention, memory, and overall enthusiasm for learning in children with UHL. Children in school have to work in a dynamic classroom. They need to listen to peers and their teacher. Noise rarely originates from only one side of a listener in a classroom; therefore, the ability of a child to modify their position to enhance SNR may be compromised. 112 A cochlear implant is able to improve speech perception in noise regardless of the listening condition, including when noise is directed at the cochlear implant, a significant advantage over other technologies.
We wished to determine the effect of UHL on fatigue in our cohort, and also determine if cochlear implantation could improve that fatigue. Hornsby et al. demonstrated that children with congenital bilateral hearing loss have higher degrees of fatigue in comparison with normal hearing peers with average scores of 55, 53, and 53 for general, sleep, and cognitive fatigue, respectively, while normal hearing subjects had scores of 85, 73, and 71, respectively. 79 Our cohort pre‐operatively had scores of 73, 80, and 61, suggesting they are most prominently affected by cognitive fatigue (Fig. 7). Subject fatigue scores improved following cochlear implantation, but the improvement did not reach significance. Our study was not powered to detect a difference this small with such high variance. In addition, the responses were not anchored to prior responses; therefore, parents may have over‐scored fatigue at subsequent visits, especially as their children began attending pre‐school and primary school. For now, the effect of cochlear implantation on fatigue in children with unilateral hearing loss remains tantalizingly unanswered.
In this study, we had one child who was suspected of not using their cochlear implant. Although wear time appeared appropriate, it was determined that this participant had mastered taking the cochlear implant off but keeping the processor turned on to keep the appearance of wearing the device. Teachers at school had remarked as much. Although this is a caution, and negatively affected results in this study, it is meaningful to also highlight that 95% of children in the study are consistent users of their cochlear implant. A longer electrode array that provides better place‐pitch matching in comparison with the contralateral ear may help with their acceptance. Other studies have also demonstrated a high adherence to device usage, suggesting that children quickly realize the benefits of binaural hearing and, despite potential social stigma, choose to use their cochlear implant. 117 This helps ameliorate concerns that children may choose to reject their device, as they age into adolescence and teenage years.
As is the case with any clinical trial offering a unique technology option, parents choosing to travel from around the country to have their child implanted were typically well educated, affluent and attentive to their child's needs. All of these may favor better outcomes observed in this cohort in comparison with the general population.
CONCLUSIONS
The impact of treatment decisions for UHL in young children has the potential to reverberate throughout their lifetimes. We demonstrate at 12 months in this clinical trial that children implanted for UHL have marked and significant improvements in their speech perception in quiet. They have significant improvements in their speech perception in noise in three masker positions (head shadow, squelch, and summation), and they also have significant improvement in their localization abilities. Furthermore, they feel it is easier to hear with their device on, and they subjectively perceive better hearing, especially when the hearing task requires binaural cues. As cognitive plasticity in children may be greater than adults, it will be exceedingly exciting to determine if further improvements will occur, as these children are tested in the future. Together these data overwhelmingly support unilateral moderate to profound sensory hearing loss as an indication for cochlear implantation in children.
ACKNOWLEDGEMENTS
We would like to thank MED‐EL Corporation for support of this clinical trial. We would also like to thank and acknowledge the efforts of the staff of the Children's Cochlear Implant Center at the University of North Carolina, especially past faculty Holly Teagle for their invaluable assistance and work with these children. This project was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health through grant award number UL1TR002489.
Editor's Note: This Manuscript was accepted for publication on August 22, 2021.
This article was presented at the Combined Otolaryngology Spring Meeting, Triological Society, Virtual Meeting, April 7th–11th, 2021. It was submitted as a Triological Thesis by author k.d.b.
Dr k.d.b. is on the Surgical Advisory Board of MED‐EL Corporation. Drs k.d.b., m.t.d., and l.r.p. received research support from MED‐EL Corporation.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
REFERENCES
- 1. Everberg G. Etiology of unilateral total deafness studied in a series of children and young adults. Ann Otol Rhinol Laryngol 1960;69:711–730. [DOI] [PubMed] [Google Scholar]
- 2. Mehl AL, Thomson V. Newborn hearing screening: the great omission. Pediatrics 1998;101:E4. [DOI] [PubMed] [Google Scholar]
- 3. Niskar AS, Kieszak SM, Holmes A, Esteban E, Rubin C, Brody DJ. Prevalence of hearing loss among children 6 to 19 years of age: the Third National Health and Nutrition Examination Survey. JAMA 1998;279:1071–1075. [DOI] [PubMed] [Google Scholar]
- 4. Widen JE, Folsom RC, Cone‐Wesson B, et al. Identification of neonatal hearing impairment: hearing status at 8 to 12 months corrected age using a visual reinforcement audiometry protocol. Ear Hear 2000;21:471–487. [DOI] [PubMed] [Google Scholar]
- 5. Barsky‐Firkser L, Sun S. Universal newborn hearing screenings: a three‐year experience. Pediatrics 1997;99:E4. [DOI] [PubMed] [Google Scholar]
- 6. Ross DS, Visser SN, Holstrum WJ, Qin T, Kenneson A. Highly variable population‐based prevalence rates of unilateral hearing loss after the application of common case definitions. Ear Hear 2010;31:126–133. [DOI] [PubMed] [Google Scholar]
- 7. Lieu JE. Speech‐language and educational consequences of unilateral hearing loss in children. Arch Otolaryngol Head Neck Surg 2004;130:524–530. [DOI] [PubMed] [Google Scholar]
- 8. Lieu JE. Unilateral hearing loss in children: speech‐language and school performance. B‐ENT 2013;21:107–115. [PMC free article] [PubMed] [Google Scholar]
- 9. Lieu JEC. Permanent unilateral hearing loss (UHL) and childhood development. Curr Otorhinolaryngol Rep 2018;6:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lieu JE, Tye‐Murray N, Fu Q. Longitudinal study of children with unilateral hearing loss. Laryngoscope 2012;122:2088–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ricketts T, Lindley G, Henry P. Impact of compression and hearing aid style on directional hearing aid benefit and performance. Ear Hear 2001;22:348–361. [DOI] [PubMed] [Google Scholar]
- 12. Brown KD, Balkany TJ. Benefits of bilateral cochlear implantation: a review. Curr Opin Otolaryngol Head Neck Surg 2007;15:315–318. [DOI] [PubMed] [Google Scholar]
- 13. Gatehouse S. The time course and magnitude of perceptual acclimatization to frequency responses: evidence from monaural fitting of hearing aids. J Acoust Soc Am 1992;92:1258–1268. [DOI] [PubMed] [Google Scholar]
- 14. Gelfand SA, Silman S. Apparent auditory deprivation in children: implications of monaural versus binaural amplification. J Am Acad Audiol 1993;4:313–318. [PubMed] [Google Scholar]
- 15. Hattori H. Ear dominance for nonsense‐syllable recognition ability in sensorineural hearing‐impaired children: monaural versus binaural amplification. J Am Acad Audiol 1993;4:319–330. [PubMed] [Google Scholar]
- 16. Stern RM Jr, Colburn HS. Theory of binaural interaction based in auditory‐nerve data. IV. A model for subjective lateral position. J Acoust Soc Am 1978;64:127–140. [DOI] [PubMed] [Google Scholar]
- 17. Akeroyd MA. The psychoacoustics of binaural hearing. Int J Audiol 2006;45:S25–S33. [DOI] [PubMed] [Google Scholar]
- 18. Tyler RS, Dunn CC, Witt SA, Preece JP. Update on bilateral cochlear implantation. Curr Opin Otolaryngol Head Neck Surg 2003;11:388–393. [DOI] [PubMed] [Google Scholar]
- 19. Zurek PM. A note on onset effects in binaural hearing. J Acoust Soc Am 1993;93:1200–1201. [DOI] [PubMed] [Google Scholar]
- 20. Litovsky R, Parkinson A, Arcaroli J, Sammeth C. Simultaneous bilateral cochlear implantation in adults: a multicenter clinical study. Ear Hear 2006;27:714–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grantham DW, Ashmead DH, Ricketts TA, Labadie RF, Haynes DS. Horizontal‐plane localization of noise and speech signals by postlingually deafened adults fitted with bilateral cochlear implants. Ear Hear 2007;28:524–541. [DOI] [PubMed] [Google Scholar]
- 22. Grieco‐Calub TM, Litovsky RY. Sound localization skills in children who use bilateral cochlear implants and in children with normal acoustic hearing. Ear Hear 2010;31:645–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implants: implications for age of implantation. Ear Hear 2002;23:532–539. [DOI] [PubMed] [Google Scholar]
- 24. Peters BR, Litovsky R, Parkinson A, Lake J. Importance of age and postimplantation experience on speech perception measures in children with sequential bilateral cochlear implants. Otol Neurotol 2007;28:649–657. [DOI] [PubMed] [Google Scholar]
- 25. Kocdor P, Iseli CE, Teagle HF, et al. The effect of interdevice interval on speech perception performance among bilateral, pediatric cochlear implant recipients. Laryngoscope 2016;126:2389–2394. [DOI] [PubMed] [Google Scholar]
- 26. Carlson ML, Sladen DP, Gurgel RK, Tombers NM, Lohse CM, Driscoll CL. Survey of the American Neurotology Society on cochlear implantation: Part 1, candidacy assessment and expanding indications. Otol Neurotol 2018;39:e12–e19. [DOI] [PubMed] [Google Scholar]
- 27. Welsh LW, Welsh JJ, Rosen LF, Dragonette JE. Functional impairments due to unilateral deafness. Ann Otol Rhinol Laryngol 2004;113:987–993. [DOI] [PubMed] [Google Scholar]
- 28. Rothpletz AM, Wightman FL, Kistler DJ. Informational masking and spatial hearing in listeners with and without unilateral hearing loss. J Speech Lang Hear Res 2012;55:511–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Slattery WH 3rd, Middlebrooks JC. Monaural sound localization: acute versus chronic unilateral impairment. Hear Res 1994;75:38–46. [DOI] [PubMed] [Google Scholar]
- 30. Peters JP, Smit AL, Stegeman I, Grolman W. Review: bone conduction devices and contralateral routing of sound systems in single‐sided deafness. Laryngoscope 2015;125:218–226. [DOI] [PubMed] [Google Scholar]
- 31. Kunst SJ, Leijendeckers JM, Mylanus EA, Hol MK, Snik AF, Cremers CW. Bone‐anchored hearing aid system application for unilateral congenital conductive hearing impairment: audiometric results. Otol Neurotol 2008;29:2–7. [DOI] [PubMed] [Google Scholar]
- 32. Bosman AJ, Hol MK, Snik AF, Mylanus EA, Cremers CW. Bone‐anchored hearing aids in unilateral inner ear deafness. Acta Otolaryngol 2003;123:258–260. [DOI] [PubMed] [Google Scholar]
- 33. Grantham DW, Ashmead DH, Haynes DS, Hornsby BW, Labadie RF, Ricketts TA. Horizontal plane localization in single‐sided deaf adults fitted with a bone‐anchored hearing aid (Baha). Ear Hear 2012;33:595–603. [DOI] [PubMed] [Google Scholar]
- 34. Hol MK, Kunst SJ, Snik AF, Cremers CW. Pilot study on the effectiveness of the conventional CROS, the transcranial CROS and the BAHA transcranial CROS in adults with unilateral inner ear deafness. Eur Arch Otorhinolaryngol 2010;267:889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roland JT Jr, Gantz BJ, Waltzman SB, Parkinson AJ. Long‐term outcomes of cochlear implantation in patients with high‐frequency hearing loss. Laryngoscope 2018;128:1939–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pillsbury HC 3rd, Dillon MT, Buchman CA, et al. Multicenter US clinical trial with an electric‐acoustic stimulation (EAS) system in adults: final outcomes. Otol Neurotol 2018;39:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dunn CC, Oleson J, Parkinson A, Hansen MR, Gantz BJ. Nucleus hybrid S12: multicenter clinical trial results. Laryngoscope 2020;130:E548–E558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carlson ML, Patel NS, Tombers NM, et al. Hearing preservation in pediatric cochlear implantation. Otol Neurotol 2017;38:e128–e133. [DOI] [PubMed] [Google Scholar]
- 39. Selleck AM, Park LR, Choudhury B, et al. Hearing preservation in pediatric recipients of cochlear implants. Otol Neurotol 2019;40:e277–e282. [DOI] [PubMed] [Google Scholar]
- 40. Gantz BJ, Dunn C, Walker E, Van Voorst T, Gogel S, Hansen M. Outcomes of adolescents with a short electrode cochlear implant with preserved residual hearing. Otol Neurotol 2016;37:e118–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dorman MF, Cook S, Spahr A, et al. Factors constraining the benefit to speech understanding of combining information from low‐frequency hearing and a cochlear implant. Hear Res 2015;322:107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Loiselle LH, Dorman MF, Yost WA, Gifford RH. Sound source localization by hearing preservation patients with and without symmetrical low‐frequency acoustic hearing. Audiol Neurootol 2015;20:166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Van de Heyning P, Vermeire K, Diebl M, Nopp P, Anderson I, De Ridder D. Incapacitating unilateral tinnitus in single‐sided deafness treated by cochlear implantation. Ann Otol Rhinol Laryngol 2008;117:645–652. [DOI] [PubMed] [Google Scholar]
- 44. Punte AK, Vermeire K, Hofkens A, De Bodt M, De Ridder D, Van de Heyning P. Cochlear implantation as a durable tinnitus treatment in single‐sided deafness. Cochlear Implants Int 2011;12:S26–S29. [DOI] [PubMed] [Google Scholar]
- 45. Vermeire K, Van de Heyning P. Binaural hearing after cochlear implantation in subjects with unilateral sensorineural deafness and tinnitus. Audiol Neurootol 2009;14:163–171. [DOI] [PubMed] [Google Scholar]
- 46. Sladen DP, Frisch CD, Carlson ML, Driscoll CL, Torres JH, Zeitler DM. Cochlear implantation for single‐sided deafness: a multicenter study. Laryngoscope 2017;127:223–228. [DOI] [PubMed] [Google Scholar]
- 47. Hansen MR, Gantz BJ, Dunn C. Outcomes after cochlear implantation for patients with single‐sided deafness, including those with recalcitrant Meniere's disease. Otol Neurotol 2013;34:1681–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Buss E, Dillon MT, Rooth MA, et al. Effects of cochlear implantation on binaural hearing in adults with unilateral hearing loss. Trends Hear 2018;22:2331216518771173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Firszt JB, Reeder RM, Holden LK, Dwyer NY, Asymmetric Hearing Study T. Results in adult cochlear implant recipients with varied asymmetric hearing: a prospective longitudinal study of speech recognition, localization, and participant report. Ear Hear 2018;39:845–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tavora‐Vieira D, Rajan GP, Van de Heyning P, Mertens G. Evaluating the long‐term hearing outcomes of cochlear implant users with single‐sided deafness. Otol Neurotol 2019;40:e575–e580. [DOI] [PubMed] [Google Scholar]
- 51. Dillon MT, Buss E, Anderson ML, et al. Cochlear implantation in cases of unilateral hearing loss: initial localization abilities. Ear Hear 2017;38:611–619. [DOI] [PubMed] [Google Scholar]
- 52. Dillon MT, Buss E, Rooth MA, et al. Effect of cochlear implantation on quality of life in adults with unilateral hearing loss. Audiol Neurootol 2017;22:259–271. [DOI] [PubMed] [Google Scholar]
- 53. Hochmair I, Hochmair E, Nopp P, Waller M, Jolly C. Deep electrode insertion and sound coding in cochlear implants. Hear Res 2015;322:14–23. [DOI] [PubMed] [Google Scholar]
- 54. Svirsky MA, Talavage TM, Sinha S, Neuburger H, Azadpour M. Gradual adaptation to auditory frequency mismatch. Hear Res 2015;322:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McDermott H, Sucher C, Simpson A. Electro‐acoustic stimulation. Acoustic and electric pitch comparisons. Audiol Neurootol 2009;14:2–7. [DOI] [PubMed] [Google Scholar]
- 56. Harnsberger JD, Svirsky MA, Kaiser AR, Pisoni DB, Wright R, Meyer TA. Perceptual "vowel spaces" of cochlear implant users: implications for the study of auditory adaptation to spectral shift. J Acoust Soc Am 2001;109:2135–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Carlyon RP, Macherey O, Frijns JH, et al. Pitch comparisons between electrical stimulation of a cochlear implant and acoustic stimuli presented to a normal‐hearing contralateral ear. J Assoc Res Otolaryngol 2010;11:625–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zeng FG, Tang Q, Lu T. Abnormal pitch perception produced by cochlear implant stimulation. PLoS One 2014;9:e88662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Reiss LA, Turner CW, Karsten SA, Gantz BJ. Plasticity in human pitch perception induced by tonotopically mismatched electro‐acoustic stimulation. Neuroscience 2014;256:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Svirsky MA, Silveira A, Suarez H, Neuburger H, Lai TT, Simmons PM. Auditory learning and adaptation after cochlear implantation: a preliminary study of discrimination and labeling of vowel sounds by cochlear implant users. Acta Otolaryngol 2001;121:262–265. [DOI] [PubMed] [Google Scholar]
- 61. Reiss LA, Turner CW, Erenberg SR, Gantz BJ. Changes in pitch with a cochlear implant over time. J Assoc Res Otolaryngol 2007;8:241–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Svirsky MA, Silveira A, Neuburger H, Teoh SW, Suarez H. Long‐term auditory adaptation to a modified peripheral frequency map. Acta Otolaryngol 2004;124:381–386. [PubMed] [Google Scholar]
- 63. Dillon MT, Buss E, Rooth MA, King ER, Pillsbury HC, Brown KD. Low‐frequency pitch perception in cochlear implant recipients with normal hearing in the contralateral ear. J Speech Lang Hear Res 2019;62:2860–2871. [DOI] [PubMed] [Google Scholar]
- 64. Prentiss S, Staecker H, Wolford B. Ipsilateral acoustic electric pitch matching: a case study of cochlear implantation in an up‐sloping hearing loss with preserved hearing across multiple frequencies. Cochlear Implants Int 2014;15:161–165. [DOI] [PubMed] [Google Scholar]
- 65. Schatzer R, Vermeire K, Visser D, et al. Electric‐acoustic pitch comparisons in single‐sided‐deaf cochlear implant users: frequency‐place functions and rate pitch. Hear Res 2014;309:26–35. [DOI] [PubMed] [Google Scholar]
- 66. Canfarotta MW, Dillon MT, Buss E, Pillsbury HC, Brown KD, O'Connell BP. Frequency‐to‐place mismatch: characterizing variability and the influence on speech perception outcomes in cochlear implant recipients. Ear Hear 2020;41:1349–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Canfarotta M, Dillon MT, Buchman CA, et al. Speech recognition benefit with longer lateral wall electrode arrays: long‐term follow‐up of a prospective randomized Trial. Laryngoscope 2020;131:892–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vila PM, Lieu JE. Asymmetric and unilateral hearing loss in children. Cell Tissue Res 2015;361:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Voelker CC, Chole RA. Unilateral sensorineural hearing loss in adults: etiology and management. Semin Hear 2010;31:313–325. [Google Scholar]
- 70. Usami SI, Kitoh R, Moteki H, et al. Etiology of single‐sided deafness and asymmetrical hearing loss. Acta Otolaryngol 2017;137:S2–S7. [DOI] [PubMed] [Google Scholar]
- 71. Paul A, Marlin S, Parodi M, et al. Unilateral sensorineural hearing loss: medical context and etiology. Audiol Neurootol 2017;22:83–88. [DOI] [PubMed] [Google Scholar]
- 72. Buchman CA, Teagle HF, Roush PA, et al. Cochlear implantation in children with labyrinthine anomalies and cochlear nerve deficiency: implications for auditory brainstem implantation. Laryngoscope 2011;121:1979–1988. [DOI] [PubMed] [Google Scholar]
- 73. Griffin AM, Poissant SF, Freyman RL. Speech‐in‐noise and quality‐of‐life measures in school‐aged children with normal hearing and with unilateral hearing loss. Ear Hear 2019;40:887–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Reeder RM, Cadieux J, Firszt JB. Quantification of speech‐in‐noise and sound localisation abilities in children with unilateral hearing loss and comparison to normal hearing peers. Audiol Neurootol 2015;20:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hall JW 3rd, Grose JH, Buss E, Dev MB. Spondee recognition in a two‐talker masker and a speech‐shaped noise masker in adults and children. Ear Hear 2002;23:159–165. [DOI] [PubMed] [Google Scholar]
- 76. Fischer C, Lieu J. Unilateral hearing loss is associated with a negative effect on language scores in adolescents. Int J Pediatr Otorhinolaryngol 2014;78:1611–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lieu JE, Tye‐Murray N, Karzon RK, Piccirillo JF. Unilateral hearing loss is associated with worse speech‐language scores in children. Pediatrics 2010;125:e1348–e1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jamieson DG, Kranjc G, Yu K, Hodgetts WE. Speech intelligibility of young school‐aged children in the presence of real‐life classroom noise. J Am Acad Audiol 2004;15:508–517. [DOI] [PubMed] [Google Scholar]
- 79. Hornsby BW, Werfel K, Camarata S, Bess FH. Subjective fatigue in children with hearing loss: some preliminary findings. Am J Audiol 2014;23:129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bess FH, Davis H, Camarata S, Hornsby BWY. Listening‐related fatigue in children with unilateral hearing loss. Lang Speech Hear Serv Sch 2020;51:84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Theunissen SC, Rieffe C, Netten AP, et al. Self‐esteem in hearing‐impaired children: the influence of communication, education, and audiological characteristics. PLoS One 2014;9:e94521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kuppler K, Lewis M, Evans AK. A review of unilateral hearing loss and academic performance: is it time to reassess traditional dogmata? Int J Pediatr Otorhinolaryngol 2013;77:617–622. [DOI] [PubMed] [Google Scholar]
- 83. Warner‐Czyz AD, Loy BA, Evans C, Wetsel A, Tobey EA. Self‐esteem in children and adolescents with hearing loss. Trends Hear 2015;19:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Christensen L, Richter GT, Dornhoffer JL. Update on bone‐anchored hearing aids in pediatric patients with profound unilateral sensorineural hearing loss. Arch Otolaryngol Head Neck Surg 2010;136:175–177. [DOI] [PubMed] [Google Scholar]
- 85. Kim G, Ju HM, Lee SH, Kim HS, Kwon JA, Seo YJ. Efficacy of bone‐anchored hearing aids in single‐sided deafness: a systematic review. Otol Neurotol 2017;38:473–483. [DOI] [PubMed] [Google Scholar]
- 86. Leterme G, Bernardeschi D, Bensemman A, et al. Contralateral routing of signal hearing aid versus transcutaneous bone conduction in single‐sided deafness. Audiol Neurootol 2015;20:251–260. [DOI] [PubMed] [Google Scholar]
- 87. Lin LM, Bowditch S, Anderson MJ, May B, Cox KM, Niparko JK. Amplification in the rehabilitation of unilateral deafness: speech in noise and directional hearing effects with bone‐anchored hearing and contralateral routing of signal amplification. Otol Neurotol 2006;27:172–182. [DOI] [PubMed] [Google Scholar]
- 88. Niparko JK, Cox KM, Lustig LR. Comparison of the bone anchored hearing aid implantable hearing device with contralateral routing of offside signal amplification in the rehabilitation of unilateral deafness. Otol Neurotol 2003;24:73–78. [DOI] [PubMed] [Google Scholar]
- 89. Snapp HA, Holt FD, Liu X, Rajguru SM. Comparison of speech‐in‐noise and localization benefits in unilateral hearing loss subjects using contralateral routing of signal hearing aids or bone‐anchored implants. Otol Neurotol 2017;38:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wazen JJ, Ghossaini SN, Spitzer JB, Kuller M. Localization by unilateral BAHA users. Otolaryngol Head Neck Surg 2005;132:928–932. [DOI] [PubMed] [Google Scholar]
- 91. Agterberg MJH, Snik AFM, Van de Goor RMG, Hol MKS, Van Opstal AJ. Sound‐localization performance of patients with single‐sided deafness is not improved when listening with a bone‐conduction device. Hear Res 2019;372:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Plontke SK, Heider C, Koesling S, et al. Cochlear implantation in a child with posttraumatic single‐sided deafness. Eur Arch Otorhinolaryngol 2013;270:1757–1761. [DOI] [PubMed] [Google Scholar]
- 93. Greaver L, Eskridge H, Teagle HFB. Considerations for pediatric cochlear implant recipients with unilateral or asymmetric hearing loss: assessment, device fitting, and habilitation. Am J Audiol 2017;26:91–98. [DOI] [PubMed] [Google Scholar]
- 94. Deep NL, Gordon SA, Shapiro WH, Waltzman SB, Roland JT Jr, Friedmann DR. Cochlear implantation in children with single‐sided deafness. Laryngoscope 2020;131:E271–E277. [DOI] [PubMed] [Google Scholar]
- 95. Park LR, Dillon MT, Buss E, O'Connell BP, Brown KD. Spatial release from masking in pediatric cochlear implant recipients with single‐sided deafness. Am J Audiol 2021;30:443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Beck RL, Aschendorff A, Hassepass F, et al. Cochlear implantation in children with congenital unilateral deafness: a case series. Otol Neurotol 2017;38:e570–e576. [DOI] [PubMed] [Google Scholar]
- 97. Hassepass F, Aschendorff A, Wesarg T, et al. Unilateral deafness in children: audiologic and subjective assessment of hearing ability after cochlear implantation. Otol Neurotol 2013;34:53–60. [DOI] [PubMed] [Google Scholar]
- 98. Tavora‐Vieira D, Rajan GP. Cochlear implantation in children with congenital and noncongenital unilateral deafness: a case series. Otol Neurotol 2015;36:235–239. [DOI] [PubMed] [Google Scholar]
- 99. Zeitler DM, Sladen DP, DeJong MD, Torres JH, Dorman MF, Carlson ML. Cochlear implantation for single‐sided deafness in children and adolescents. Int J Pediatr Otorhinolaryngol 2019;118:128–133. [DOI] [PubMed] [Google Scholar]
- 100. Sennaroglu L, Bajin MD. Classification and current management of inner ear malformations. Balkan Med J 2017;34:397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bagatto M, Moodie S, Scollie S, et al. Clinical protocols for hearing instrument fitting in the Desired Sensation Level method. Trends Amplif 2005;9:199–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Park LR, Preston E, Noxon AS, Dillon MT. Comparison of test methods to assess the implanted ear alone for pediatric cochlear implant recipients with single‐sided deafness. Cochlear Implants Int 2021;22:283–290. [DOI] [PubMed] [Google Scholar]
- 103. Bench J, Kowal A, Bamford J. The BKB (Bamford‐Kowal‐Bench) sentence lists for partially‐hearing children. Br J Audiol 1979;13:108–112. [DOI] [PubMed] [Google Scholar]
- 104. Kompis M, Pfiffner F, Krebs M, Caversaccio MD. Factors influencing the decision for Baha in unilateral deafness: the Bern benefit in single‐sided deafness questionnaire. Adv Otorhinolaryngol 2011;71:103–111. [DOI] [PubMed] [Google Scholar]
- 105. Gatehouse S, Noble W. The speech, spatial and qualities of hearing scale (SSQ). Int J Audiol 2004;43:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Galvin KL, Noble W. Adaptation of the speech, spatial, and qualities of hearing scale for use with children, parents, and teachers. Cochlear Implants Int 2013;14:135–141. [DOI] [PubMed] [Google Scholar]
- 107. Gatehouse S, Akeroyd M. Two‐eared listening in dynamic situations. Int J Audiol 2006;45:S120–S124. [DOI] [PubMed] [Google Scholar]
- 108. Varni JW, Limbers CA, Burwinkle TM. How young can children reliably and validly self‐report their health‐related quality of life? An analysis of 8,591 children across age subgroups with the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes 2007;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Shah J, Pham GN, Zhang J, et al. Evaluating diagnostic yield of computed tomography (CT) and magnetic resonance imaging (MRI) in pediatric unilateral sensorineural hearing loss. Int J Pediatr Otorhinolaryngol 2018;115:41–44. [DOI] [PubMed] [Google Scholar]
- 110. Moog JS, Popelka GR, Geers AE, Russo MH Early Speech Perception Test for Profoundly Hearing‐Impaired Children. St. Louis MO: Central Institute for the Deaf; 1990. [Google Scholar]
- 111. Peterson GE, Lehiste I. Revised CNC lists for auditory tests. J Speech Hear Disord 1962;27:62–70. [DOI] [PubMed] [Google Scholar]
- 112. Ricketts TA, Picou EM, Galster J. Directional microphone hearing aids in school environments: working toward optimization. J Speech Lang Hear Res 2017;60:263–275. [DOI] [PubMed] [Google Scholar]
- 113. Hornsby BW, Naylor G, Bess FH. A taxonomy of fatigue concepts and their relation to hearing loss. Ear Hear 2016;37:136S–144S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Arndt S, Prosse S, Laszig R, Wesarg T, Aschendorff A, Hassepass F. Cochlear implantation in children with single‐sided deafness: does aetiology and duration of deafness matter? Audiol Neurootol 2015;20:21–30. [DOI] [PubMed] [Google Scholar]
- 115. Gordon K, Henkin Y, Kral A. Asymmetric hearing during development: the aural preference syndrome and treatment options. Pediatrics 2015;136:141–153. [DOI] [PubMed] [Google Scholar]
- 116. Lewis D, Schmid K, O'Leary S, Spalding J, Heinrichs‐Graham E, High R. Effects of noise on speech recognition and listening effort in children with normal hearing and children with mild bilateral or unilateral hearing loss. J Speech Lang Hear Res 2016;59:1218–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Polonenko MJ, Papsin BC, Gordon KA. Children with single‐sided deafness use their cochlear implant. Ear Hear 2017;38:681–689. [DOI] [PubMed] [Google Scholar]


