Abstract
Background
Recurrent head and neck squamous cell carcinoma (rHNSCC) represents a significant global health burden with an unmet medical need. In this study we determined the safety and efficacy of RM‐1929 photoimmunotherapy in patients with heavily pretreated rHNSCC.
Methods
RM‐1929 (anti‐EGFR–IR700 dye conjugate) was infused, followed by tumor illumination. We evaluated safety, tumor response, and pharmacokinetics.
Results
Nine patients were enrolled in Part 1 (dose‐finding) and 30 patients in Part 2 (safety and efficacy). No dose‐limiting toxicities were experienced in Part 1; 640 mg/m2 with fixed light dose (50 J/cm2 or 100 J/cm) was recommended for Part 2. Adverse events (AEs) in Part 2 were mostly mild to moderate but 19 (63.3%) patients had AE ≥Grade 3, including 3 (10.0%) with serious AEs leading to death (not treatment related). Efficacy in Part 2: unconfirmed objective response rate (ORR) 43.3% (95% CI 25.46%–62.57%); confirmed ORR 26.7% (95% CI 12.28%–45.89%); median overall survival 9.30 months (95% CI 5.16–16.92 months).
Conclusions
Treatment was well tolerated. Responses and survival following RM‐1929 photoimmunotherapy in heavily pretreated patients with rHNSCC were clinically meaningful and warrant further investigation.
Clinical Trial Information
Keywords: cetuximab–IR700 conjugate, light‐activatable dye (IRDye 700DX), recurrent head and neck squamous cell carcinoma (rHNSCC), RM‐1929 photoimmunotherapy, tumor‐targeted monoclonal antibody
1. INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) is the seventh most common cancer worldwide. 1 , 2 Recurrence is seen in 40%–65% of advanced cases and most often occurs locoregionally after primary therapy with surgery, radiation, chemotherapy, or combinations of these modalities. 2 Speech and swallowing dysfunction and disfigurement are common and frequently debilitating. 3 Many patients may not be candidates for curative therapy, and salvage treatments such as surgery and re‐irradiation carry a high risk of serious complications, including salivary fistula, carotid artery rupture, and airway emergencies. 4 , 5 , 6
Successful treatment of recurrent locoregional disease in advanced HNSCC improves disease‐free survival, symptoms, and long‐term disease control. 7 , 8 Treatment of recurrent or metastatic HNSCC often relies on platinum‐based chemotherapy and targeted therapies such as cetuximab, 9 , 10 , 11 but responses are limited. The introduction of immune checkpoint inhibitors has provided an additional therapeutic option, 12 , 13 , 14 although overall response rates to immunotherapy in recurrent HNSCC (rHNSCC) are low. 9 , 10 , 11 , 12 , 13 , 14 Given the poor treatment‐related outcomes, there is an urgent need for new therapeutic approaches to provide locoregional control in patients with rHNSCC.
Photoimmunotherapy utilizes tumor‐targeted monoclonal antibodies conjugated with a light‐activatable dye (IRDye® 700DX, abbreviated as IR700). 15 Preclinical studies demonstrate that activation of the dye with non‐thermal red light (690 nm) results in rapid anticancer activity mediated by biophysical processes that damage the membrane integrity of cells. 16 , 17 The requirement of antibody–antigen binding and light activation to induce cell killing enables cell selectivity of tumor cells expressing the antigen, while minimizing damage to surrounding tissue. 18 Preclinical cancer models indicate that photoimmunotherapy induces tumor necrosis and immunogenic cell death that can lead to local and systemic induction of innate and adaptive immunity. 19 , 20 , 21
RM‐1929 photoimmunotherapy is an investigational drug‐device combination treatment consisting of a cancer‐targeting drug (RM‐1929) and a laser device system, which is being developed on the Illuminox™ platform. 22 RM‐1929 (cetuximab sarotalocan) 23 comprises cetuximab, an antibody targeting epidermal growth factor receptor (EGFR) that is highly expressed in HNSCC, conjugated with IR700. RM‐1929 photoimmunotherapy requires two steps conducted in sequence: (a) intravenous (IV) infusion of the drug RM‐1929; and (b) light illumination of the tumor 24 ± 4 h after infusion (Figure 1).
FIGURE 1.
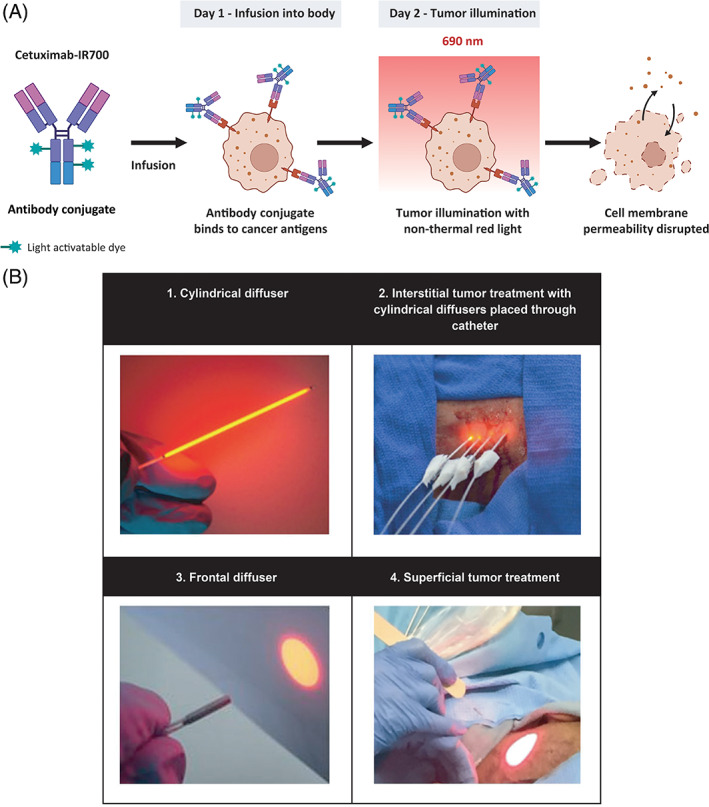
Mechanism of action and RM‐1929 photoimmunotherapy overview. (A) A tumor‐targeted antibody is conjugated with a light‐activatable dye. Following infusion into the body, the tumor is illuminated with non‐thermal red light (690 nm), leading to anticancer activity mediated by biophysical processes that damage the membrane integrity of cells. (B) Tumor illumination is performed 24 ± 4 h after antibody conjugate infusion. Cylindrical diffusers placed in needle catheters are used to treat interstitial tumors (B1, B2), while frontal diffusers are used to treat superficial tumors (B3, B4). Light illumination of the tumor is administered 24 ± 4 h after RM‐1929 infusion to allow for drug distribution within the tumor. The non‐thermal red light is applied to the tumor using a (1) frontal diffuser for superficial light illumination for tumors ≤1 cm from the skin or mucosal surface or a (2) cylindrical diffuser for interstitial illumination for tumors >1 cm from the skin or mucosal surface. The illumination time for frontal and cylindrical diffusers is 5 min for each treated area. For interstitial illumination, cylindrical diffusers are placed uniformly into the tumor 1.8 ± 0.2 cm apart using 17‐gauge closed‐tipped needle catheters under radiographic or ultrasound imaging
We report findings from a Phase 1/2a, multicenter, open‐label, dose‐escalation study conducted to determine the recommended dose, safety, pharmacokinetics (PK), immunogenicity, and preliminary efficacy of RM‐1929 photoimmunotherapy in patients with locoregional rHNSCC.
2. METHODS
2.1. Study oversight
The study was performed in accordance with the principles of the Declaration of Helsinki (1964), Good Clinical Practice guidelines, and applicable US Code of Federal Regulations (CFR), 21 CFR Parts 50 and 312. The study protocol, informed consent form, and any other appropriate documents were approved by the Institutional Review Board/Independent Ethics Committee for each participating center. All patients signed a written informed consent form. ClinicalTrials.gov identifier: NCT02422979.
2.2. Patients
Patient eligibility criteria were as follows: ≥18 years of age; histologically confirmed rHNSCC which, in the opinion of the treating physician, could not be satisfactorily treated with surgery, radiation, or platinum‐based chemotherapy; and an ECOG score of 0–2. Patients must have received prior systemic platinum‐based chemotherapy unless contraindicated. Patients were excluded if they had any of the following: tumors invading major blood vessels unless embolized, stented, or ligated; tumors not accessible with light illumination; impaired renal or hepatic function; or history of significant cetuximab infusion reactions. Distant metastatic disease was not a reason for exclusion.
2.3. Study design and endpoints
The primary objective of Part 1 of the study was to determine the recommended drug dose of RM‐1929 with a fixed light dose and the associated safety. Primary endpoints of Part 1 were to: determine maximum tolerated dose (MTD) or maximum feasible dose (MFD), whichever was lowest; determine the safety of combination of drug dose at the fixed light dose; and evaluate photosafety by determining the Minimal Erythema Dose (MED) following infusion of RM‐1929. The criteria for drug dose selection were set to reach either: (a) the MTD, defined as the dose level below which a dose limiting toxicity (DLT) is documented in more than one of six patients; or (b) the maximum feasible dose of RM‐1929 considered to be sufficient to saturate binding to EGFR in tumor tissue based on previous PK characterization of cetuximab. 24 Three dose‐escalation cohorts were included; the starting dose was 160 mg/m2, which was escalated to 320 and 640 mg/m2 according to a standard 3 + 3 design. Further details of DLT criteria can be found in the Appendix S1 (DLT criteria section). The criteria to select the light dose (fluence) was based on reaching either the MTD or achieving a dose of light that resulted in measurable anticancer response (defined as a significant tumor reduction after one treatment cycle). Other endpoints in Part 1 included tumor response, PK, and immunogenicity evaluation.
The co‐primary objectives of study Part 2 were: to determine the optimal light dose in combination with the Part 1 dose to achieve clinical response with an acceptable safety profile; and to document the safety profile of repeat treatment of up to a maximum of four cycles of therapy. Other endpoints of Part 2 were to determine the safety associated with repeat treatment cycles, PK, immunogenicity, and assessment of response.
2.4. Treatment: Part 1 dose escalation and Part 2 recommended dose
In Part 1, dose escalation continued until a protocol‐specified DLT was identified or until it was determined that adequate EGFR occupancy had been achieved in the tumor to achieve a positive response. Adequate EGFR saturation was expected to be achieved at a plasma concentration for RM‐1929 of 3000 μgh/mL or higher. Based on the safety and efficacy results from Part 1, the RM‐1929 drug dose for further evaluation in Part 2 was fixed at 640 mg/m2, and the light dose was fixed at 50 J/cm2 for superficial tumors and 100 J/cm fiber diffuser length for interstitial tumors.
RM‐1929 was infused intravenously over 2 h on Day 1. On Day 2, the tumor(s) was exposed to red light illumination with a wavelength of 690 nm applied at non‐thermal light doses using the laser device system, which include frontal and cylindrical light diffusers for superficial tumors (<1 cm thick) and interstitial tumors (≥1 cm deep), respectively (Figure 1B). For cylindrical diffusers, 17‐gauge needle catheters were placed uniformly into the tumor 1.8 ± 0.2 cm apart covering the entire tumor volume guided by imaging techniques such as ultrasound. The illumination time for frontal and cylindrical diffusers was 5 min for each treated region. Patients received one treatment cycle in Part 1 and up to four treatment cycles were allowed in Part 2.
2.5. Study assessments
Safety was assessed at regularly scheduled timepoints using standard assessments including treatment‐emergent AE (TEAE) monitoring, clinical laboratory tests, vital signs, electrocardiograms, physical exams, and head and neck exams. AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 18.0. The intensity of AEs was graded according to National Cancer Institute‐Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.03. In Part 1, patients were evaluated for skin photosafety at two separate areas on the arm exposed to a light dose of 45 J/cm2 for 10 min.
Tumor response evaluation was conducted by a central imaging reader and the investigator, although only central imaging results are reported here. CT and PET scans performed during the study were transferred to a central imaging reader and analyzed utilizing modified RECIST (mRECIST 1.1; see Appendix S1), PERCIST (Part 2), and Choi criteria (for tumor density/necrosis evaluation). Central radiology review used mRECIST 1.1 and Choi criteria to determine the unconfirmed objective response rate (ORR), which was defined as the proportion of patients with a confirmed or unconfirmed complete response (CR) or partial response (PR). Individual patient responses were recorded from the start of study treatment until patient follow‐up was completed or the patient went off study (the end of treatment, taking into account any requirement for confirmation). Per protocol, patients not receiving subsequent treatment cycles (either because they had a CR with no clinical recurrence, or had progressive disease) were not required to have additional scans for response confirmation; thus, both unconfirmed ORR as well as the confirmed ORR, defined as the proportion of patients with CR or PR that was subsequently confirmed by a second imaging assessment >4 weeks after the initial assessment, are reported.
2.6. Statistical methods
The safety and treated populations (determined separately for Part 1 and Part 2) comprised all patients who received ≥1 study infusion of RM‐1929. Overall survival (OS) was defined as the time from the first RM‐1929 administration to any cause of death. Time to event was analyzed by Kaplan–Meier product estimates and Clopper–Pearson's 95% confidence interval was used for frequency analysis. Post‐hoc analyses were conducted for efficacy in prior/naïve anti‐PD‐1 subgroups, duration of response (DOR) and long‐term survival. All other analyses were preplanned in the statistical analysis plan (SAP) before database lock. SAS® version 9.4 was used for efficacy and safety analyses. The database lock was March 12, 2019. Additional long‐term survival data were collected through August 8, 2019. Details on PK and immunogenicity assessments, and evaluable populations are shown in Appendix S1.
3. RESULTS
A total of 38 patients received RM‐1929 photoimmunotherapy (one patient was enrolled in both Parts 1 and 2). Median age was 66.0 years, and previous treatment included radiotherapy (all patients), surgery (97.4% of patients) and chemotherapy (73.7% of patients). In Part 2, 36.7% of patients received prior immunotherapy and 23.3% received prior cetuximab (Table 1). Patients were generally heavily pretreated, and subsites of head and neck disease were broadly represented. Prior therapies were determined by the enrolling investigator and included systemic therapy, surgery, and radiation. Tumor locations are summarized in Table 1. Eight patients across study Parts 1 and 2 had distant metastatic disease at study entry. Three patients had lung metastases (all in study Part 2), and seven patients had dermal metastases (Part 1, n = 1; Part 2, n = 6).
TABLE 1.
Baseline demographics and disease characteristics—study Part 1 and Part 2
| Characteristic | Part 1 | Part 2 |
|---|---|---|
| (n = 9) | (n = 30) a | |
| Age | ||
| Median (range), years | 58.0 (52–86) | 68.5 (39–86) |
| <65 years, n (%) | 6 (66.7) | 12 (40.0) |
| ≥65 years, n (%) | 3 (33.3) | 18 (60.0) |
| Gender, n (%) | ||
| Female | 2 (22.2) | 6 (20.0) |
| Male | 7 (77.8) | 24 (80.0) |
| Race, n (%) | ||
| White | 9 (100) | 24 (80.0) |
| Asian | 0 | 2 (6.7) |
| American Indian/Alaska Native | 0 | 1 (3.3) |
| Other | 0 | 4 (13.3) b |
| ECOG PS, n (%) | ||
| 0 | 1 (11.1) | 8 (26.7) |
| 1 | 6 (66.7) | 17 (56.7) |
| 2 | 2 (22.2) | 5 (16.7) |
| Recurrent tumor location, n (%) c | ||
| Neck | 4 (44.4) | 13 (43.3) |
| Oral cavity | 2 (22.2) | 9 (30.0) |
| Oropharynx | 5 (55.6) | 7 (23.3) |
| Skin | 0 | 3 (10.0) |
| Hypopharynx | 0 | 2 (6.7) |
| Sinus | 0 | 2 (6.7) |
| Nasal cavity | 0 | 1 (3.3) |
| Larynx | 0 | 0 |
| Other d | 4 (44.4) | 1 (3.3) |
| Prior lines of therapy, n (%) e | ||
| 1 | 1 (11.1) | 3 (10.0) |
| 2 | 2 (22.2) | 16 (53.3) |
| 3 | 1 (11.1) | 7 (23.3) |
| ≥4 | 5 (55.6) | 4 (13.3) |
| Prior therapy, n (%) | ||
| Cancer‐related surgery f | 8 (88.9) | 30 (100) |
| Radiotherapy | 9 (100) | 30 (100) |
| Chemotherapy | 7 (77.8) | 21 (70.0) |
| Immunotherapy g | 3 (33.3) | 11 (36.7) |
| Pembrolizumab | 0 | 6 (20.0) |
| Nivolumab | 3 (33.3) | 4 (13.3) |
| Other h | 0 | 2 (6.7) |
| Biological g /hormonal g /other g | 5 (55.6) | 8 (26.7) |
| Cetuximab | 5 (55.6) | 7 (23.3) |
| Other i | 1 (11.1) | 1 (3.3) |
Abbreviations: ECOG, Eastern Co‐operative Oncology Group; PS, performance status.
Thirty‐one patients enrolled but one patient did not receive RM‐1929 photoimmunotherapy due to cetuximab reaction observed during a test dose.
One patient was mixed race and was counted twice.
Some patients had more than one recurrent tumor locations.
Other: nasopharynx (2), parotid (2), occipital.
Lines of therapy were determined by a sponsor clinical expert and were based on the prior treatments reported on study (includes systemic therapy, surgery, and radiotherapy).
Surgery for reconstruction or biopsy not included here.
Categories of prior therapy were determined by a sponsor clinical expert based on reported prior treatment report on study.
Other: Rituximab and MEDI0562.
Other: BKM120 and RM‐1929 photoimmunotherapy.
3.1. Part 1
Nine patients were treated in study Part 1, with three patients in each dosing cohort, as described in the methods. Baseline characteristics are shown in Table 1. A summary of the PK parameters for these patients is shown in Table S1.
No protocol‐specified DLTs were observed at any dose level. Evidence of low‐grade, reversible skin photosensitivity was observed in two patients as assessed by MED testing. TEAEs observed in patients treated during Part 1 are summarized in Table S2. TEAEs ≥Grade 3 occurring in more than one patient were oral pain and application site pain. No patients experienced a TEAE that led to discontinuation of study treatment.
At the 640 mg/m2 dose level of RM‐1929 photoimmunotherapy, one of three patients achieved a CR and one patient experienced SD. Furthermore, seven of the nine (77.8%) patients included in Part 1 achieved disease control (unconfirmed CR or PR or SD). All three patients in the 160 mg/m2 dosing cohort and two of the three patients in the 320 mg/m2 dosing cohort demonstrated SD. Tumor reduction by clinical or radiologic assessment was seen in patients at all doses. In addition, the average AUC0–∞ (μg h/mL) of RM‐1929 at 640 mg/m2 was 13 400 μg h/mL, which exceeded the required AUC0–∞ of approximately 12 000 μg h/mL to achieve EGFR saturation demonstrated by cetuximab alone. 23 Based on these findings, 640 mg/m2 was recommended as the Part 2 dosing regimen and additional dose escalation with Cohort 4 was discontinued.
The optimal light dose was determined to be one that resulted in a satisfactory anticancer response (defined as a significant tumor reduction at 1 month after treatment) at the RM‐1929 MFD. All dose levels of RM‐1929 (160, 320, and 640 mg/m2) resulted in tumor reduction by clinical or radiologic assessment with 50 J/cm2 for superficial illumination and 100 J/cm fiber diffuser length for interstitial illumination. Therefore, the established drug dose of 640 mg/m2 with light dose of 50 J/cm2 for superficial lesions and 100 J/cm fiber diffuser length for interstitial lesions was determined to be the recommended drug dose and optimal light dose for Part 2, and no further light doses were evaluated in Part 2 of the study.
3.2. Part 2
Thirty‐one patients were enrolled between June 2015 and December 2017. Of these, 30 received RM‐1929 photoimmunotherapy and were included in the safety and treated populations. Overall, the treated patients received 65 cycles of RM‐1929 photoimmunotherapy at a median of 2 cycles (range 1–4).
3.3. Safety
All of the 30 patients who received RM‐1929 photoimmunotherapy experienced at least one TEAE. The most frequent all‐grade TEAEs were fatigue, dysphagia, constipation, erythema, and peripheral edema (Table 3). Nineteen (63.3%) patients reported a TEAE of ≥Grade 3 during study participation (Table 3). TEAEs ≥Grade 3 reported in at least two patients included anemia, dysphagia, oral pain, pneumonia, application site pain, localized edema, hyponatremia, tumor hemorrhage, and tumor pain. Additional safety tables are provided in the Appendix S1.
TABLE 3.
Treatment‐emergent adverse events (TEAEs) in >10% of patients in Part 2 of the study (n = 30)
| Preferred term | All grades, n (%) | Grade ≥ 3, n (%) a |
|---|---|---|
| Any TEAE | 30 (100) | 19 (63.3) |
| Fatigue | 10 (33.3) | – |
| Dysphagia | 7 (23.3) | 2 (6.7) |
| Constipation | 6 (20.0) | – |
| Erythema | 6 (20.0) | – |
| Peripheral edema | 6 (20.0) | – |
| Anemia | 5 (16.7) | 3 (10.0) |
| Dehydration | 5 (16.7) | – |
| Facial edema | 5 (16.7) | 1 (3.3) |
| Rash | 5 (16.7) | – |
| Facial pain | 4 (13.3) | – |
| Oral pain | 4 (13.3) | 2 (6.7) |
| Tumor pain | 4 (13.3) | 2 (6.7) |
| Tongue edema | 4 (13.3) | – |
| Local swelling | 4 (13.3) | – |
| Cough | 4 (13.3) | – |
| Oropharyngeal pain | 4 (13.3) | – |
| Pneumonia | 4 (13.3) | 2 (6.7) |
| Weight decreased | 4 (13.3) | – |
Abbreviation: TEAE, treatment‐emergent adverse event.
Other Grade ≥ 3 TEAEs occurring in two patients (6.7%): application site pain, localized edema, hyponatremia, and tumor hemorrhage.
Five (16.7%) patients discontinued RM‐1929 photoimmunotherapy due to TEAEs; however, only in one patient was the TEAE considered possibly related to treatment by the investigator (blood creatinine increase), and no further treatments were performed. A total of 13 (43.3%) patients reported a serious TEAE, and three were considered treatment related (tumor pain, oral pain, and airway obstruction). Three (10%) patients experienced SAEs within 5 weeks of RM‐1929 photoimmunotherapy that led to an outcome of death (tumor hemorrhage, arterial hemorrhage, and pneumonia). These events, which occurred 19 days or more after last treatment, were considered by investigators to be associated primarily with tumor response to treatment or tumor encroachment to vessels, and were not related to the procedure itself. A patient staged as recurrent T3N0M0 in the posterior tongue and pre‐epiglottic space died due to cervical vessel rupture with progressive disease (PD) 19 days after the last treatment. A patient with laryngectomy stomal recurrence died 32 days after last treatment due to arterial hemorrhage from an exposed carotid artery. A patient with recurrent T2N0M0 at the posterior pharyngeal wall died 29 days after the last treatment due to pneumonia.
3.4. Efficacy
Efficacy outcomes among patients in study Part 2 are shown in Table 2 and Figure 2. The unconfirmed ORR was 43.3% (95% CI 25.46%–62.57%). Four (13.3%) patients achieved a CR, and nine (30.0%) patients achieved a PR with disease control observed in 24 (80.0%) patients (95% CI 61.43%–92.29%). The confirmed ORR was 26.7% (95% CI 12.28%–45.89%). Figure 2A represents the percentage best change in size of target lesions following RM‐1929 photoimmunotherapy. Radiographic changes in tumor density were also evaluated. Using Choi criteria, which quantifies CT changes in tumor volume and density, 25 the response rate was 66.7% (95% CI 47.19%–82.71%; Table S3). Among patients in study Part 2, the median OS was 9.30 months (95% CI 5.16%–16.92 months; Figure 2B). Figure S2 demonstrates survival outcomes among all patients enrolled in study Parts 1 and 2 (n = 38).
TABLE 2.
Response rates—study Part 2
| Characteristic | RM‐1929 photoimmunotherapy 640 mg/m2 |
|---|---|
| (n = 30) | |
| Objective response, confirmed, n (%) | 8 (26.7) |
| (95% CI) | (12.28–45.89) |
| Duration of response for confirmed responders, a months | 2.1–12.2 |
| Objective response, b confirmed and unconfirmed, n (%) | 13 (43.3) |
| (95% CI) | (25.46–62.57) |
| Complete response, c n (%) | 4 (13.3) |
| (95% CI) | (3.76–30.72) |
| Partial response, c n (%) | 9 (30.0) |
| (95% CI) | (14.73–49.40) |
| Disease control (CR + PR + SD), d n (%) | 24 (80.0) |
| (95% CI) | (61.43–92.29) |
Abbreviations: CI, confidence interval; CR, complete response; PR, partial response; SD, stable disease.
Duration of response reflects when patients were censored at the last evaluable post‐baseline scan if new anticancer therapy was reported.
According to mRECIST 1.1 by central radiology review.
Best response across all time points.
Disease control is the number of patients who reported either CR, PR, or SD of any duration between Cycle 1 Day 1 and PD or death or date of their last evaluable tumor assessment.
FIGURE 2.
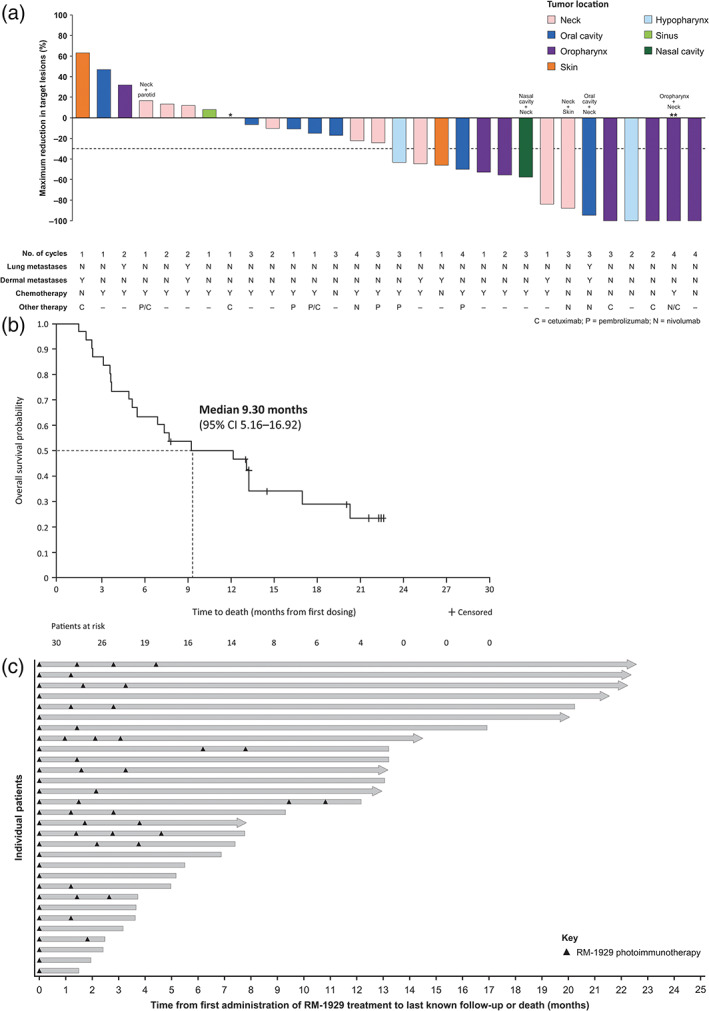
Efficacy outcomes following RM‐1929 photoimmunotherapy in study Part 2 patients (n = 30) with locoregional rHNSCC. (A) Waterfall plot of best change in sum of diameters of target lesions from baseline by central radiology assessment; (B) Kaplan–Meier plot of overall survival; and (C) Swimmer plot of individual patient survival. Arrowed bars represent patients who were censored at last contact date. *One enrolled patient did not have post‐baseline scans. **Lesions completely resolved, and lymph nodes were <10 mm. Target lesions were defined as lesions identified by the investigator that were light illuminated. Non‐target lesions, including lung metastases, did not undergo light illumination and further discussion on change in tumor size of non‐target lesions can be found in Appendix S1. Tumor locations were determined by a sponsor clinical expert based on target and non‐target lesions reported in the study
Of the 30 patients enrolled in Part 2 of the study, 10 had received prior anti‐PD‐1 therapy with documented disease progression (six received pembrolizumab and four received nivolumab, disease progression on anti‐PD‐1 monotherapy was documented prior to study entry), and 20 were anti‐PD‐1 naïve. The ORR was 30% (95% CI 6.67%–65.25%) and 25% (95% CI 8.66%–49.10%) in patients with and without prior anti‐PD‐1 exposure, respectively. Of the 10 patients with prior anti‐PD‐1 exposure and documented progression, four received concurrent anti‐PD‐1 therapy during treatment with RM‐1929 photoimmunotherapy per the decision of the treating physician. Best responses observed in these four patients were two PRs, one SD, and one PD.
Survival rates were estimated as 49.8% (number at risk, n = 14) at 12 months and 29.1% (number at risk, n = 6) at 18 months, based on protocol‐planned survival data collected at database lock (March 12, 2019) for patients enrolled in Part 2 of the study (n = 30; Figure 2B,C). The survival rate at 24 months was estimated as 27.9% (number at risk, n = 9) based on additional survival data collected as ad hoc analysis among all treated patients in Parts 1 and 2 of the study (n = 38). Notably, four patients remained treatment free and are still alive as of last long‐term follow‐up (August 8, 2019 or earlier; range of follow‐up time, 22.18–32.33 months). Of these, none had lung metastases or untreated non‐target lesions. Three of the four patients received prior immunotherapy, and none were treated with concurrent anti‐PD‐1 therapy. In 25 patients with pretreatment biopsies, no trend was observed between EGFR expression and tumor response. 26
3.5. PK and immunogenicity
PK and immunogenicity data are shown in Tables S1 and S4. At a dose of 640 mg/m2 the exposure of RM‐1929 (AUC0–∞) was 10 600 ± 3120 μg h/mL in Cycle 1 Part 2. This exposure level is comparable to that of cetuximab single dose at 250 mg/m2, which is expected to achieve full saturation of EGFR in tissues. 24 Hence, a dose of 640 mg/m2 should achieve binding saturation of EGFR in the tumor. Overall, immunogenicity as measured by anti‐drug antibodies had no impact on the single‐dose or multiple‐dose PK evaluated.
4. DISCUSSION
Our results using a novel photoimmunotherapy approach show evidence of clinically meaningful activity with an unconfirmed ORR of 43.3% and confirmed ORR of 26.7% in heavily pretreated rHNSCC patients with locoregional disease who had failed standard of care. RM‐1929 photoimmunotherapy was associated with longer median OS (9.3 months), with four patients achieving CRs (13%). These results are encouraging, suggesting that RM‐1929 photoimmunotherapy has the potential to provide significant benefit in patients who have failed multiple prior lines of therapy. This study also suggests that RM‐1929 photoimmunotherapy may offer the potential for improved locoregional control in rHNSCC. This is important as it has been shown that achieving locoregional control results in increased survival in patients with head and neck cancer. 7 , 8
The benefits of improved local control are more evident when noting that existing treatment outcomes for patients with unresectable recurrent/metastatic head and neck cancer remain poor, with essentially no curative options and very low response rates to available therapies. 9 , 10 , 11 , 12 , 13 , 14 While pembrolizumab was recently approved for unresectable recurrent and metastatic HNSCC, response rates are durable but low. 27 In KEYNOTE‐048, pembrolizumab alone resulted in an ORR of 19% and a median OS of 12.3 months in patients with rHNSCC whose tumors express programmed death ligand 1 (CPS ≥1). The median OS ranged from 10.7 to 13.0 months, regardless of PD‐L1 expression, and the ORR was ≤36% in the chemotherapy‐containing arms, with or without pembrolizumab. 14 , 27 Other approved first‐line treatments include platinum‐based chemotherapy with or without cetuximab with response rates of 20%–36% and median OS of 7.4–10.7 months. 9 , 10 , 11 For patients whose disease recurs after first‐line systemic treatment, approved second‐line options include single‐agent cetuximab, pembrolizumab, or nivolumab. None of the second‐line systemic treatments yield ORRs higher than 16% and median OS ranges from 6 to 8 months. 27 , 28 After platinum‐based chemotherapy combinations and immunotherapy, available treatment options for these patients are limited. Thus, there is an urgent need to develop new therapeutic approaches for patients with rHNSCC.
The majority of TEAEs observed in this study were localized primarily to the treatment site, were mild to moderate in severity, and resolved. It is important to recognize that RM‐1929 photoimmunotherapy is a procedural intervention when assessing safety. The majority of TEAEs related to tumor pain, swelling, and local site bleeding are not unexpected given the type of procedure and the rapid degree of tumor necrosis resulting from this treatment (see Figure 3, Patient 2). Furthermore, in one patient, tissue swelling resulted in airway obstruction which required temporary tracheostomy. Two patients had bleeding related to vessel exposure or tumor bleeding resulting in death, which were both assessed by the investigator as unrelated to the treatment itself but instead related to tumor response. Photoimmunotherapy is less invasive than a surgical intervention such as oncologic resection, yet the importance of patient selection, assessment of tumor involvement with large vessels, and postoperative wound care should not be underestimated. With the potential for posttreatment sequelae, hospital observation or admission, narcotic pain management, and supportive measures may be needed. Hemorrhage from tumor or rupture of vessels is not uncommon in the natural course of progressive HNSCC, 29 , 30 , 31 and such events may be more likely in cases where a tumor response leaves a large unreconstructed defect. The two patients who died during the study due to bleeding sequelae had significant end‐stage disease; one had recurrent base of tongue cancer assessed as disease progression and the other had a large stomal recurrence assessed as stable disease at the time of last scan. Caution should be observed with tumors involving large blood vessels and measures such as preoperative embolization may be required. In the case of airway compromise, swelling around the tumor site necessitated urgent management of the airway. Difficult airways are not uncommon in patients with head and neck cancer 32 for numerous reasons and protection of the airway must always be considered with procedural interventions.
FIGURE 3.
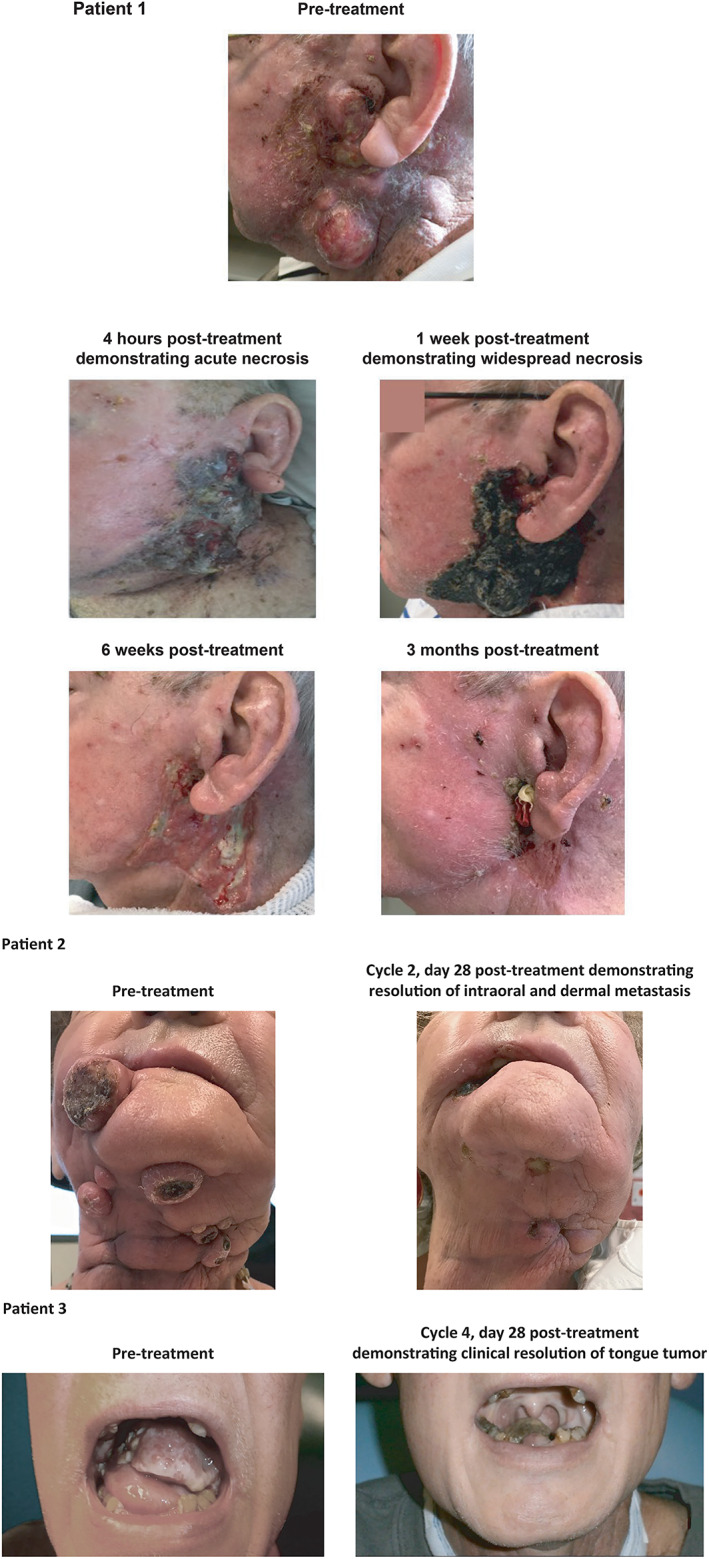
Clinical course of patients with locoregional rHNSCC treated with RM‐1929 photoimmunotherapy: Patient 1 with recurrent left facial cancer; Patient 2 with bilateral intraoral recurrent tumors and neck dermal metastases; and Patient 3 with a tongue tumor
The precision of RM‐1929 photoimmunotherapy, driven by the tumor‐targeted antibody dye conjugate and localized light activation, aims to achieve locoregional tumor control with minimal side effects to surrounding normal tissue compared with other light therapies such as non‐targeted photodynamic therapy. Of note, photosensitivity was reported in two patients in Part 1 of the study (Grade 1 photophobia and Grade 2 skin photosensitivity) and in one patient in Part 2 of the study (Grade 1 skin photosensitivity), which contrasts markedly with the greater severity and frequency of photosensitivity reactions typically seen with photodynamic therapy. 33 , 34
Limitations of this study include the lack of a control arm, low patient numbers, and robustness of repeat imaging assessments. Patients who did not receive additional treatment cycles, either because of CR with no clinical recurrence or PD, were not required to have further scans for response confirmation and additional imaging was only obtained per clinical judgment. As a result, it was challenging to appropriately assess duration of response (DOR). However, the DOR for confirmed responders (n = 8) ranged from 2.1 to 12.2 months when patients were censored at the last evaluable post‐baseline scan. The response rate is compelling when one considers the OS observed in these patients. Of the patients in Parts 1 and 2, ad hoc analysis estimated the survival rate at 24 months as 27.9% (number at risk, n = 9). We acknowledge that the OS of patients is affected by subsequent post‐study treatments received, and a limitation of this study is the lack of collection of detailed anticancer treatments received to assess if subsequent treatments could have impacted OS outcomes. Nevertheless, of the patients with long‐term survival, four were alive without additional anticancer treatment (in remission) as of last follow up (≥22 months), demonstrating a response to RM‐1929 photoimmunotherapy despite previous standard‐of‐care therapy administration. Taken in totality, these results suggest the potential for RM‐1929 photoimmunotherapy leading to durable responses that may translate into improvements in OS.
In our exploratory analysis involving prior anti‐PD‐1 treated and anti‐PD‐1 naïve subgroups, 33.3% of patients in Part 2 received prior pembrolizumab or nivolumab. Patients who received prior anti‐PD‐1 were more heavily pretreated with a median of three prior lines of therapy. Regardless of prior anti‐PD‐1 treatment, responses were observed in both groups and the ORR in each subgroup was comparable to the treated population (n = 30). Anti‐PD‐1 therapy has become standard of care in head and neck cancer based on several practice‐changing studies. However, in the recurrent and metastatic setting, response rates remain low at approximately 13%–17%. 12 , 14 Therefore, an urgent need to find additional therapies to augment anti‐PD‐1 monotherapy has prompted numerous combinatorial strategies. These studies have failed to produce meaningful gains with the exception of the combination with chemotherapy as in KEYNOTE‐048. 14 The limited data from this cohort may suggest a promising potential combination for photoimmunotherapy and anti‐PD‐1 therapy, which has prompted the design of a combination trial targeting rHNSCC and cutaneous squamous cell carcinoma (ClinicalTrials.gov identifier: NCT04305795).
Locoregional progression is a key contributor to morbidity and mortality in HNSCC, which may be due to the tumors affecting vital functions including breathing, swallowing, speaking and cranial nerve function, as well as encroachment on blood vessels that can result in lethal hemorrhage. 35 The physical improvements experienced by the patients on this study were notable (Figure 3), which is an important finding as the function and appearance of the head and neck region are crucial to self‐image and health‐related quality of life (HRQoL). 3 Although the impact of HRQoL was not evaluated in the current study, it will be important to include patient‐reported outcomes in any future evaluations of this novel treatment.
In conclusion, RM‐1929 photoimmunotherapy has a tolerable and manageable safety profile, although patient numbers were limited in this study. The preliminary efficacy of RM‐1929 photoimmunotherapy in patients with rHNSCC was promising with response and survival rates favorable for this subset of heavily pretreated patients. Given the poor prognosis of the patients enrolled in the study, there is a critical unmet need for new therapeutic modalities that have limited systemic side effects and provide improved tumor response and locoregional control. Such therapies could result in improvement in OS and QoL for these patients. Further investigation of RM‐1929 photoimmunotherapy is warranted given the CR and ORR observed in this study. A global Phase 3 clinical trial evaluating photoimmunotherapy in locoregional rHNSCC patients is currently enrolling (ClinicalTrials.gov identifier: NCT03769506).
CONFLICT OF INTEREST
David M. Cognetti: Honoraria (Intuitive Surgical); Consulting or Advisory Role (Rakuten Medical). Jennifer M. Johnson: Consulting or Advisory Role (Bristol‐Myers Squibb; Foundation Medicine; Rakuten Medical); Research Funding (AstraZeneca; Bristol‐Myers Squibb; Merck). Joseph M. Curry: Consulting or Advisory Role (Rakuten Medical); Research Funding (AstraZeneca; Castle Biosciences). Samith T. Kochuparambil: none. Darren McDonald: Consulting or Advisory Role (Rakuten Medical). Frank Mott: none. Mary J. Fidler: Honoraria (AstraZeneca); Consulting or Advisory Role (AstraZeneca; Genentech; G1 Therapeutics, Rakuten Medical); Speakers' Bureau (Merck; Genentech); Research Funding (Biodesix; Pfizer). Kerstin Stenson: Stock or Other Ownership (Abbott; Abbvie; Biogen; Bristol‐Myers Squibb; Celgene); Honoraria (UpToDate); Travel, Accommodations, Expenses (Rakuten Medical). Nilesh R. Vasan: Employment (Adroit Surgical LLC); Leadership (Adroit Surgical LLC); Stock or Other Ownership (Adroit Surgical LLC); Patents, Royalties, Other Intellectual Property (Adroit Surgical LLC; University of Oklahoma Board of Regents). Mohammad A. Razaq: Honoraria (AstraZeneca); Speakers' Bureau (Merck & Co); Stock or Other Ownership (Amgen); Travel, Accommodation, Expenses (Merck). John Campana: Travel, Accommodations, Expenses (Axogen). Patrick Ha: Consulting or Advisory Role (Bayer/LOXO Oncology, Rakuten Medical); Travel, Accommodations, Expenses (Genentech). Educational funding (Stryker, Johnson & Johnson, Medtronic, Axogen). Grace Mann: Employment (Rakuten Medical); Stock or Other Ownership (Rakuten Medical). Kosuke Ishida: Employment (Rakuten Medical; Boehringer Ingelheim). Miguel Garcia‐Guzman: Employment (Rakuten Medical); Leadership (Rakuten Medical); Stock or Other Ownership (Vertex Pharma; Gilead); Patents, Royalties, Other Intellectual Property (UC Irvine; Rakuten Medical). Merrill Biel: Employment (Rakuten Medical); Leadership (Rakuten Medical); Stock and Other Ownership Interests (Rakuten Medical); Travel, Accommodation, Expenses (Rakuten Medical). Ann M. Gillenwater: Consulting or Advisory Role (Rakuten Medical Inc., Masimo Corporation).
AUTHOR CONTRIBUTIONS
Conception and design: Merrill Biel, Miguel Garcia‐Guzman, David M. Cognetti, Ann M. Gillenwater. Administrative support: None. Provision of study materials or patients: All authors. Collection and assembly of data: David M. Cognetti, Jennifer M. Johnson, Joseph M. Curry, Samith T. Kochuparambil, Darren McDonald, Frank Mott, Mary J. Fidler, Kerstin Stenson, Nilesh R. Vasan, Mohammad A. Razaq, John Campana, Patrick Ha, Miguel Garcia‐Guzman, Merrill Biel, Ann M. Gillenwater. Data analysis and interpretation: Merrill Biel, Miguel Garcia‐Guzman, Ann M. Gillenwater, David M. Cognetti, Grace Mann, and Kosuke Ishida. Manuscript writing: All authors. Final approval of manuscript: All authors. Accountable for all aspects of the work: All authors.
Supporting information
Appendix S1: Supporting Information
Figure S1 Progression‐free survival in study Part 2 (n = 30). Progression‐free survival (PFS) is defined as the time from the first RM‐1929 photoimmunotherapy administration to progressive disease or death. Although PFS was assessed for the treated population in Part 2, the results should be interpreted with caution due to the lack of subsequent radiological imaging for disease assessment after completion of treatment.
Figure S2 Integrated survival analysis of patients enrolled in study Parts 1 and 2 (n = 38). (A) Overall Kaplan–Meier analysis for patients enrolled in study Parts 1 and 2 (n = 38). For the patient that joined both Parts 1 and 2, censor time is defined as time from the treatment in Part 1 to last known date alive. (B) Swimmer plot demonstrating individual survival outcomes among Part 1 and 2 patients (n = 38). One patient participated in both Part 1 (160 mg/m2) and Part 2 of the study. For this patient, censor time is defined as time from the treatment in Part 1 to last known date alive in Part 2. The swimmer plot shows a total of 38 patients, as this patient was counted only once. Arrowed bars represent patients who were censored at last contact date.
ACKNOWLEDGMENTS
The authors would like to thank all patients and their families for participating in this study. The authors would also like to thank the following individuals who played a role in the management of this study and/or support of the publication: Dawn Poller (program manager at Thomas Jefferson University); Stephanie Erickson (senior research nurse at Virginia Piper Cancer Institute); Julia Neuenschwander (University of Oklahoma Health Sciences Center); Lawrence E. Ginsberg, MD (Professor, Neuroradiology Department, UT M. D. Anderson Cancer Center); Heather J. Jamieson (RN, UT M. D. Anderson Cancer Center); Audrey Humphries (senior clinical research coordinator at University of California, San Francisco); Jaehyung Hong, Lola Fong, Jessica Berrett, Jeannie Hou, Doreen Gousset, Eileen Sun, Theresa Operaña, Orapim Tulyathan, and Laura Spuhler (Rakuten Medical); Lee Miller and Lucy Kanan (Miller Medical Communications) for medical writing/editing support of the manuscript (funded by Rakuten Medical). Funded by Rakuten Medical.
Cognetti DM, Johnson JM, Curry JM, et al. Phase 1/2a, open‐label, multicenter study of RM‐1929 photoimmunotherapy in patients with locoregional, recurrent head and neck squamous cell carcinoma. Head & Neck. 2021;43(12):3875‐3887. doi: 10.1002/hed.26885
Prior presentation: Presented, in part, at the 2019 American Society of Clinical Oncology Annual Meeting, May 31 to June 4, 2019, Chicago, IL, USA.
Section Editor: Jose Zevallos
Funding information Rakuten Medical
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are not publicly available due to privacy or ethical restrictions. Requests to access the data should be directed to medinfo@rakuten-med.com.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Chow LQM. Head and neck cancer. N Engl J Med. 2020;382:60‐72. [DOI] [PubMed] [Google Scholar]
- 3. Ojo B, Genden EM, Teng MS, Milbury K, Misiukiewicz KJ, Badr H. A systematic review of head and neck cancer quality of life assessment instruments. Oral Oncol. 2012;48:923‐937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hamoir M, Schmitz S, Suarez C, et al. The current role of salvage surgery in recurrent head and neck squamous cell carcinoma. Cancers (Basel). 2018;10:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Temam S, Pape E, Janot F, et al. Salvage surgery after failure of very accelerated radiotherapy in advanced head‐and‐neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2005;62:1078‐1083. [DOI] [PubMed] [Google Scholar]
- 6. Khan N, Clemens M, Liu J, et al. The role of salvage surgery with interstitial brachytherapy for the management of regionally recurrent head and neck cancers. Cancers Head Neck. 2019;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee N, Chan K, Bekelman JE, et al. Salvage re‐irradiation for recurrent head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;68:731‐740. [DOI] [PubMed] [Google Scholar]
- 8. Patel TD, Marchiano E, Chin OY, et al. Utility of surgery/radiotherapy in distant metastatic head and neck squamous cell carcinoma: a population‐based approach. Otolaryngol Head Neck Surg. 2016;154:868‐874. [DOI] [PubMed] [Google Scholar]
- 9. Vermorken JB, Mesia R, Rivera F, et al. Platinum‐based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116‐1127. [DOI] [PubMed] [Google Scholar]
- 10. Gibson MK, Li Y, Murphy B, et al. Randomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): an intergroup trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2005;23:3562‐3567. [DOI] [PubMed] [Google Scholar]
- 11. Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA, Eastern Cooperative Oncology Group . Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23:8646‐8654. [DOI] [PubMed] [Google Scholar]
- 12. Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous‐cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856‐1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE‐012): an open‐label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956‐965. [DOI] [PubMed] [Google Scholar]
- 14. Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE‐048): a randomised, open‐label, phase 3 study. Lancet. 2019;394:1915‐1928. [DOI] [PubMed] [Google Scholar]
- 15. Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Cancer cell‐selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat Med. 2011;17:1685‐1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kishimoto S, Bernardo M, Saito K, et al. Evaluation of oxygen dependence on in vitro and in vivo cytotoxicity of photoimmunotherapy using IR‐700‐antibody conjugates. Free Radic Biol Med. 2015;85:24‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sato K, Ando K, Okuyama S, et al. Photoinduced ligand release from a silicon phthalocyanine dye conjugated with monoclonal antibodies: a mechanism of cancer cell cytotoxicity after near‐infrared photoimmunotherapy. ACS Cent Sci. 2018;4:1559‐1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mitsunaga M, Nakajima T, Sano K, Kramer‐Marek G, Choyke PL, Kobayashi H. Immediate in vivo target‐specific cancer cell death after near infrared photoimmunotherapy. BMC Cancer. 2012;12:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Magalhaes Filho CM, Hsu MA, Okamura SM, et al. In: Proceedings from the American Association for Cancer Research Annual Meeting 2020; June 22–24; Virtual Meeting II. Abstract nr 949.
- 20. Hsu MA, Okamura SM, Bergeron DM, et al. Abstract 3734: Cancer cell‐targeted photoimmunotherapy elicits immunogenic cell death and activates the innate and adaptive immune response in the tumor microenvironment. Cancer Res. 2019;79(13):3734. [Google Scholar]
- 21. Ogawa M, Tomita Y, Nakamura Y, et al. Immunogenic cancer cell death selectively induced by near infrared photoimmunotherapy initiates host tumor immunity. Oncotarget. 2017;8:10425‐10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. https://rakuten-med.com/us/illuminox/. Accessed May 21, 2020.
- 23. https://chem.nlm.nih.gov/chemidplus/rn/2166339-33-7. Accessed May 21, 2020.
- 24. Fracasso PM, Burris H 3rd, Arquette MA, et al. A phase 1 escalating single‐dose and weekly fixed‐dose study of cetuximab: pharmacokinetic and pharmacodynamic rationale for dosing. Clin Cancer Res. 2007;13:986‐993. [DOI] [PubMed] [Google Scholar]
- 25. Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753‐1759. [DOI] [PubMed] [Google Scholar]
- 26. Bui JD, Suslov N, Yadav D, et al. Intratumoral and peripheral exploratory biomarker analysis in patients with locoregional, recurrent head and neck squamous cell carcinoma (rHNSCC) treated with RM‐1929 photoimmunotherapy. Ann Oncol. 2019;30:v470. [Google Scholar]
- 27. Keytruda Prescribing Information . Accessed May 21, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125514s066lbl.pdf
- 28. Cohen EEW, Soulières D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head‐and‐neck squamous cell carcinoma (KEYNOTE‐040): a randomised, open‐label, phase 3 study. Lancet. 2019;393:156‐167. [Erratum in: Lancet 2019;393:132]. [DOI] [PubMed] [Google Scholar]
- 29. Suárez C, Fernández‐Alvarez V, Hamoir M, et al. Carotid blowout syndrome: modern trends in management. Cancer Manag Res. 2018;10:5617‐5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Storck K, Kreiser K, Hauber J, et al. Management and prevention of acute bleedings in the head and neck area with interventional radiology. Head Face Med. 2016;23:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Forbes K. Palliative care in patients with cancer of the head and neck. Clin Otolaryngol Allied Sci. 1997;22:117‐122. [DOI] [PubMed] [Google Scholar]
- 32. Luo Y, Chen J, Huang K, et al. Early evaluation of sunitinib for the treatment of advanced gastroenteropancreatic neuroendocrine neoplasms via CT imaging: RECIST 1.1 or Choi criteria? BMC Cancer. 2017;17:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morton CA. A synthesis of the world's guidelines on photodynamic therapy for non‐melanoma skin cancer. G Ital Dermatol Venereol. 2018;153:783‐792. [DOI] [PubMed] [Google Scholar]
- 34.PhoTofrin® prescribing information. Accessed May 21, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020451s020lbl.pdf.
- 35. Zumsteg ZS, Luu M, Yoshida EJ, et al. Combined high‐intensity local treatment and systemic therapy in metastatic head and neck squamous cell carcinoma: an analysis of the National Cancer Data Base. Cancer. 2017;123:4583‐4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Figure S1 Progression‐free survival in study Part 2 (n = 30). Progression‐free survival (PFS) is defined as the time from the first RM‐1929 photoimmunotherapy administration to progressive disease or death. Although PFS was assessed for the treated population in Part 2, the results should be interpreted with caution due to the lack of subsequent radiological imaging for disease assessment after completion of treatment.
Figure S2 Integrated survival analysis of patients enrolled in study Parts 1 and 2 (n = 38). (A) Overall Kaplan–Meier analysis for patients enrolled in study Parts 1 and 2 (n = 38). For the patient that joined both Parts 1 and 2, censor time is defined as time from the treatment in Part 1 to last known date alive. (B) Swimmer plot demonstrating individual survival outcomes among Part 1 and 2 patients (n = 38). One patient participated in both Part 1 (160 mg/m2) and Part 2 of the study. For this patient, censor time is defined as time from the treatment in Part 1 to last known date alive in Part 2. The swimmer plot shows a total of 38 patients, as this patient was counted only once. Arrowed bars represent patients who were censored at last contact date.
Data Availability Statement
The data that support the findings of this study are not publicly available due to privacy or ethical restrictions. Requests to access the data should be directed to medinfo@rakuten-med.com.


