Abstract
Aims
Finerenone significantly reduced the risk of kidney and cardiovascular (CV) outcomes in patients with chronic kidney disease and type 2 diabetes in the FIDELIO‐DKD trial (NCT02540993). This exploratory subgroup analysis investigates the effect of glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) use on the treatment effect of finerenone.
Materials and Methods
Patients with type 2 diabetes, urine albumin‐to‐creatinine ratio (UACR) 30‐5000 mg/g and estimated glomerular filtration rate 25‐<75 ml/min per 1.73 m2 receiving optimized renin‐angiotensin system blockade were randomized to finerenone or placebo.
Results
Of the 5674 patients analysed, overall, 394 (6.9%) received GLP‐1RAs at baseline. A reduction in UACR with finerenone was observed with or without baseline GLP‐1RA use; ratio of least‐squares means 0.63 (95% confidence interval 0.56, 0.70) with GLP‐1RA use and 0.69 (95% confidence interval 0.67, 0.72) without GLP‐1RA use (p value for interaction .20). Finerenone also significantly reduced the primary kidney (time to kidney failure, sustained decrease in estimated glomerular filtration rate ≥40% from baseline, or renal death) and key secondary CV outcomes (time to CV death, non‐fatal myocardial infarction, non‐fatal stroke, or hospitalization for heart failure) versus placebo, with no clear difference because of GLP‐1RA use at baseline (p value for interaction .15 and .51 respectively) or any time during the trial. The safety profile of finerenone was similar between subgroups.
Conclusions
This exploratory subgroup analysis suggests that finerenone reduces UACR in patients with or without GLP‐1RA use at baseline, and the effects on kidney and CV outcomes are consistent irrespective of GLP‐1RA use.
Keywords: chronic kidney disease, finerenone, glucagon‐like peptide‐1 receptor agonist, mineralocorticoid receptor antagonist, type 2 diabetes
1. INTRODUCTION
Chronic kidney disease (CKD) in patients with type 2 diabetes (T2D) is a leading cause of morbidity and mortality, 1 , 2 , 3 increasing the risk of cardiovascular (CV) disease, hypertension and death. 1 , 2 , 3 , 4 Given that the prevalence of CKD in T2D is predicted to increase over the next 20 years, 1 additional treatment strategies that offer kidney protection will be critical for this patient population. 2 Therapeutic agents that showed CV and/or kidney benefits are approved for use in patients with T2D, including treatments from the glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) class. 5 CV outcomes trials, including LEADER, REWIND, SUSTAIN‐6 and AWARD‐7, 6 , 7 , 8 , 9 , 10 , 11 have proven the CV benefits and reported a kidney protective effect of GLP‐1RAs, in addition to glucose and weight‐reducing effects. Notably, the LEADER trial included patients with a mean estimated glomerular filtration rate (eGFR) of 80 ml/min/1.73 m2 (~75% of patients had an eGFR of ≥60 ml/min/1.73 m2), and showed a reduction in blood glucose and body weight with liraglutide that was independent of eGFR at baseline, with subgroup analyses suggesting the potential for greater benefit with respect to CV outcomes in patients with a lower eGFR. 6 , 7 CV benefits were also observed with injectable semaglutide in the SUSTAIN‐6 trial in which >70% of patients had normal kidney function or mild kidney impairment at baseline. 10 Secondary outcomes and analyses from GLP‐1RA trials have suggested that these agents may also have kidney protective effects, but these observations need to be confirmed in dedicated trials with a primary kidney disease outcome, such as the ongoing FLOW trial of injectable semaglutide versus placebo. 7 , 8 , 12 , 13 , 14 , 15 In patients with CKD and T2D, the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines of 2020 recommends a GLP‐1RA with a proven CV benefit for those who have not achieved glycaemic targets following therapeutic management with metformin and a sodium‐glucose co‐transporter‐2 inhibitor. 16
Finerenone, a novel, selective, non‐steroidal mineralocorticoid receptor antagonist, significantly reduced the risk of kidney and CV events in patients with CKD and T2D compared with placebo in the phase III FIDELIO‐DKD (FInerenone in reducing kiDnEy faiLure and dIsease prOgression in Diabetic Kidney Disease) trial. 17 , 18 Finerenone inhibits the overactivation of the mineralocorticoid receptor, which drives inflammation and fibrosis in patients with CKD and T2D, leading to progressive kidney and CV disease. 19 , 20 , 21 , 22 , 23 The aim of this prespecified exploratory subgroup analysis was to investigate further the effect of finerenone in patients according to GLP‐1RA treatment. The kidney‐ and CV‐protective mechanisms of GLP‐1RAs have yet to be fully elucidated, so it is relevant to investigate whether GLP‐1RA in combination with finerenone, an agent that showed organ protection in animal models via its anti‐inflammatory and antifibrotic effects, had any impact on the efficacy and safety of finerenone observed in the FIDELIO‐DKD trial.
2. MATERIALS AND METHODS
2.1. Study design and patient population
The study design of FIDELIO‐DKD (NCT02540993) has been described in detail previously (Figure S1). 17 , 24 Eligible patients were ≥18 years of age with T2D and a clinical diagnosis of CKD defined as either moderately increased albuminuria [urine albumin‐to‐creatinine ratio (UACR) ≥30 to <300 mg/g], an eGFR ≥25 to <60 ml/min/1.73 m2, and a history of diabetic retinopathy, or severely increased albuminuria (UACR ≥300 to ≤5000 mg/g) and an eGFR ≥25 to <75 ml/min/1.73 m2. Included patients received the maximum tolerated labelled dose of an angiotensin‐converting enzyme inhibitor or an angiotensin receptor blocker for ≥4 weeks before the screening visit and had a serum potassium concentration of ≤4.8 mmol/L at both the run‐in and screening visits. Key exclusion criteria included glycated haemoglobin >12% (>108 mmol/L) and chronic symptomatic heart failure with reduced ejection fraction [New York Heart Association (NYHA) Class II‐IV].
For FIDELIO‐DKD, signed informed consent was obtained from all patients before enrolment. The trial conformed to the Declaration of Helsinki and the protocol was approved by relevant regulatory authorities and ethics committees at each trial site. The study was registered with the European Union Clinical Trials Register (EudraCT 2015‐000990‐11) and ClinicalTrials.gov (NCT02540993).
2.2. Procedures and outcomes
In FIDELIO‐DKD, patients were randomized 1:1 to receive oral finerenone (10 or 20 mg) or matching placebo once daily (od). All participants and study personnel (except for the independent data monitoring committee) were masked to treatment allocation. Urinalysis was performed centrally during run‐in and screening visits as well as at baseline, month 4, month 12 and then every 12 months thereafter. Central laboratory values, including serum potassium and serum creatinine, were obtained at all study visits.
The key outcome assessed in this exploratory analysis was change in UACR from baseline to month 4. Composite kidney and CV outcomes were also evaluated; the primary composite kidney outcome of the FIDELIO‐DKD trial was time to kidney failure [defined as end‐stage kidney disease (initiation of chronic dialysis that lasts for ≥90 days or kidney transplantation) or sustained eGFR <15 ml/min/1.73 m2], sustained decrease of at least 40% in the eGFR from baseline over a period of ≥4 weeks, or renal death. The key secondary CV outcome was time to CV death, non‐fatal myocardial infarction, non‐fatal stroke, or hospitalization for heart failure. Other secondary outcomes assessed in this analysis included a secondary composite kidney outcome that included kidney failure, a sustained decrease of ≥57% in the eGFR from baseline over a period of ≥4 weeks, or renal death. Findings for the primary and secondary efficacy and safety outcomes have been previously reported. 17 , 18
The present exploratory subgroup analysis aims to investigate the effect of finerenone on kidney and CV outcomes and safety in subgroups of patients according to GLP‐1RA treatment at baseline or at any time in the FIDELIO‐DKD trial. GLP‐1RA use at baseline was determined at randomization and GLP‐1RA use at any time included patients with GLP‐1RA treatment at baseline and any patients who initiated GLP‐1RA treatment during the trial. There were no restrictions regarding initiation of GLP‐1RA treatment during the trial and the incidence of GLP‐1RA use was unknown before trial entry/randomization.
2.3. Statistical analysis
Statistical analysis methods for the primary and secondary outcomes in FIDELIO‐DKD study have been published previously. 17 This exploratory analysis for the primary and secondary efficacy outcomes from FIDELIO‐DKD was prespecified for subgroups by the use of a GLP‐1RA at baseline and was performed in the full analysis set. Change in UACR from baseline to month 4 was tested using an analysis of covariance model adjusted for treatment group, stratification factors and baseline value. Change in UACR throughout the trial was analysed using a mixed model approach that incorporated additional variables to account for differences in baseline characteristics between GLP‐1RA baseline subgroups. The mixed model included treatment group, stratification factors (region, albuminuria category at screening and eGFR category at screening), time, treatment over time, log‐transformed baseline value nested within type of albuminuria at screening and log‐transformed baseline value over time as covariates. Covariance patterns were estimated within patients to adjust for the within‐patient variation. For each treatment group a separate covariance pattern was estimated based on unstructured covariance. Treatment effects for time‐to‐event outcomes in patients stratified by GLP‐1RA use at baseline [expressed as the hazard ratio (HR) with corresponding 95% confidence intervals (CIs) and p values for GLP‐1RA use at baseline‐by‐treatment interaction] were derived from a stratified Cox proportional hazards model including treatment (finerenone vs. placebo), subgroup of GLP‐1RA use at baseline and subgroup of GLP‐1RA use at baseline‐by‐treatment interaction term as fixed effects.
A time‐to‐event analysis was also performed to estimate the treatment effect of finerenone on the primary and key secondary outcomes accounting for use of GLP‐1RA at any point during the trial. Stepwise selection methods were employed to account for confounding factors that may have influenced initiation of GLP‐1RA as well as the outcome of interest. Further details are provided in the Supplementary Appendix. Safety analyses were performed in the safety analysis set, consisting of all randomized patients without critical Good Clinical Practice violations who took at least one dose of the study drug. All analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
3. RESULTS
3.1. Patient population
In total, 5734 patients were randomized in the FIDELIO‐DKD trial. After the exclusion of patients with critical Good Clinical Practice violations, 5674 were assessed in the full analysis set. 17 The trial concluded after a median follow‐up of 2.6 years [interquartile range (IQR), 2.0‐3.4 years] with vital status available for 99.7% of patients. Of the patients included in this analysis, 394 (6.9%) were treated with GLP‐1RAs at baseline [189 of 2833 (6.7%) patients in the finerenone group, and 205 of 2841 (7.2%) patients in the placebo group].
In this subgroup, most patients [243 of 394 (62%)] received liraglutide at baseline (Table S1). In total, 368 patients initiated GLP‐1RA treatment during the trial and were not receiving this therapy at baseline [189 of 2833 (6.7%) with finerenone and 179 of 2841 (6.3%) with placebo; Table S2], resulting in a total of 762 patients who were treated with a GLP‐1RA at any time during the study (baseline and post‐baseline). Median time on GLP‐1RA treatment was 4.1 years (IQR, 2.9‐5.6 years) for patients receiving GLP‐1RA treatment at baseline and 2.3 years (IQR, 1.0‐4.2 years) for patients receiving GLP‐1RA treatment at any time.
Overall, most baseline characteristics were similar between the groups but there were some key differences (Table 1). Compared with those who did not, patients who received GLP‐1RAs at baseline had a longer duration of T2D, higher mean body mass index, higher mean waist circumference and lower median UACR but similar mean eGFR, and fewer patients had a history of CV disease. Regarding baseline medication use, a higher use of statins, diuretics and other glucose‐lowering therapies was observed in the GLP‐1RA subgroup at baseline. Additional baseline characteristics as well as individual data for finerenone and placebo groups, with or without GLP‐1RA treatment at baseline, are provided in Table S3. Baseline characteristics by GLP‐1RA treatment at any time are presented in Table S4; characteristics were similar to patients by GLP‐1RA use at baseline, with the exception of a higher baseline UACR for patients in the placebo arm versus the finerenone arm who used GLP‐1RA treatment at any time.
TABLE 1.
Baseline characteristics according to baseline GLP‐1RA use
| Characteristic | GLP‐1RA treatment at baseline (n = 394) | No GLP‐1RA treatment at baseline (n = 5280) |
|---|---|---|
| Age, years, mean ± SD | 63.8 ± 8.3 | 65.7 ± 9.1 |
| Sex, male, n (%) | 270 (68.5) | 3713 (70.3) |
| Race, n (%) | ||
| White | 288 (73.1) | 3304 (62.6) |
| Black/African American | 26 (6.6) | 238 (4.5) |
| Asian | 65 (16.5) | 1375 (26.0) |
| Duration of T2D, years, mean ± SD | 18.2 ± 8.1 | 16.4 ± 8.8 b |
| HbA1c, %, mean ± SD | 7.9 ± 1.2 a | 7.7 ± 1.4 a |
| BMI, kg/m2, mean ± SD | 34.2 ± 5.8 a | 30.9 ± 6.0 a |
| Waist circumference, cm, mean ± SD | 114.2 ± 14.6 a | 106.2 ± 15.1 a |
| Systolic blood pressure, mmHg, mean ± SD | 138.5 ± 13.5 | 138.0 ± 14.4 a |
| History of CVD, n (%) | 166 (42.1) | 2439 (46.2) |
| CAD | 120 (30.5) | 1582 (30.0) |
| Cerebrovascular disease b | 33 (8.4) | 656 (12.4) |
| PAD | 50 (12.7) | 873 (16.5) |
| eGFR, ml/min/1.73 m2, mean ± SD | 45.4 ± 11.9 | 44.3 ± 12.6 a |
| Distribution, n (%) | ||
| <25 | 5 (1.3) | 130 (2.5) |
| 25 to <45 | 196 (49.7) | 2785 (52.7) |
| 45 to <60 | 147 (37.3) | 1753 (33.2) |
| ≥60 | 46 (11.7) | 610 (11.6) |
| UACR, mg/g, median (IQR) | 717 (409‐1575) | 860 (452‐1635) a |
| Distribution, n (%) | ||
| <30 | 2 (0.5) | 21 (0.4) |
| 30‐<300 | 50 (12.7) | 635 (12.0) |
| ≥300 | 342 (86.8) | 4621 (87.5) |
| Serum potassium, mmol/L, mean ± SD | 4.32 ± 0.43 | 4.38 ± 0.46 a |
| Baseline medications, n (%) | ||
| ACE inhibitors | 123 (31.2) | 1819 (34.5) |
| ARBs | 270 (68.5) | 3455 (65.4) |
| Beta blockers | 226 (57.4) | 2742 (51.9) |
| Diuretics | 252 (64.0) | 2962 (56.1) |
| Statins | 327 (83.0) | 3888 (73.6) |
| Potassium supplements | 20 (5.1) | 150 (2.8) |
| Potassium‐binding agents | 7 (1.8) | 129 (2.4) |
| Glucose‐lowering therapies | 394 (100) | 5130 (97.2) |
| Insulin and analogues | 283 (71.8) | 3354 (63.5) |
| Metformin | 213 (54.1) | 2277 (43.1) |
| SGLT‐2 inhibitors | 48 (12.2) | 211 (4.0) |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CAD, coronary artery disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, glycated haemoglobin; IQR, interquartile range; PAD, peripheral artery disease; SD, standard deviation; SGLT‐2, sodium‐glucose co‐transporter‐2; T2D, type 2 diabetes; UACR, urine albumin‐to‐creatinine ratio.
Stroke or transient ischaemic attack.
Missing data from n ≤ 18 patients.
3.2. Effect of finerenone on markers of kidney disease by baseline glucagon‐like peptide‐1 receptor agonist use
Of the 5451 patients who had UACR determined at baseline and month 4, finerenone was associated with a 31% greater reduction in UACR from baseline to month 4 versus placebo (HR 0.69; 95% CI 0.66, 0.71) in the overall population. 17 A similar reduction in UACR following treatment with finerenone from baseline to month 4 was observed in both patient subgroups, and the effect was similar in patients with or without GLP‐1RA use at baseline (ratio of least‐squares means 0.63; 95% CI 0.56, 0.70 with GLP‐1RA and 0.69; 95% CI 0.67, 0.72 without GLP‐1RA; p value for interaction .20). The lower mean UACR observed with finerenone compared with placebo at month 4 was maintained for the duration of the study (Figure 1). Change in UACR in the placebo arms for those with or without a GLP‐1RA at baseline was similar, suggesting that the GLP‐1RA treatment alone did not affect albuminuria during the trial (6% and 4% increase in UACR at month 36, respectively) and that any reduction in UACR because of GLP‐1RA had stabilized before the trial. Change in eGFR over time, including eGFR slope, are included in Figure S2. The effect of finerenone on eGFR over time, with an acute dip and a reduced chronic slope, was very similar in patients with or without GLP‐1RA at baseline, consistent with the other kidney endpoints.
FIGURE 1.
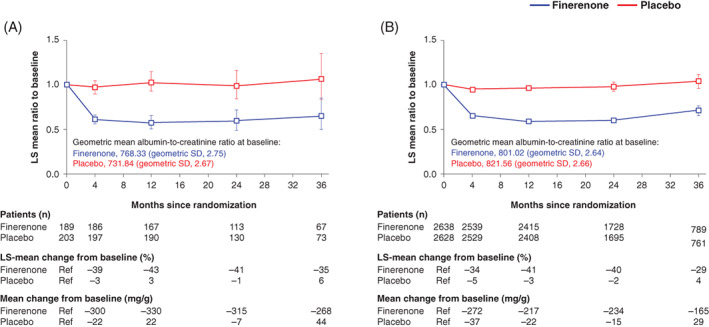
Effect on albuminuria over time by baseline GLP‐1RA use. Mixed model analysis of UACR levels in patients, A, with or, B, without GLP‐1RA at baseline. Analysis included the following covariates: treatment group, stratification factors (region, albuminuria category at screening and eGFR category at screening), time, treatment over time, log‐transformed baseline value nested within type of albuminuria at screening and log‐transformed baseline value over time. eGFR, estimated glomerular filtration rate; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; LS, least‐squares; Ref, reference; UACR, urine albumin‐to‐creatinine ratio
3.3. Effect of finerenone on primary and secondary composite outcomes by glucagon‐like peptide‐1 receptor agonist use
As previously reported, the incidence of the primary composite kidney outcome was significantly lower with finerenone versus placebo in the overall population of FIDELIO‐DKD (HR 0.82; 95% CI 0.73, 0.93; p = .001). 17 In this analysis, 64 (of 394; 16.2%) patients with GLP‐1RA treatment at baseline and 1040 (of 5280; 19.7%) of patients without GLP‐1RA treatment at baseline experienced a primary kidney event; with no clear difference in the effect of finerenone on the incidence of the primary composite kidney outcome because of baseline GLP‐1RA use (p value for interaction .15; Figure 2).
FIGURE 2.
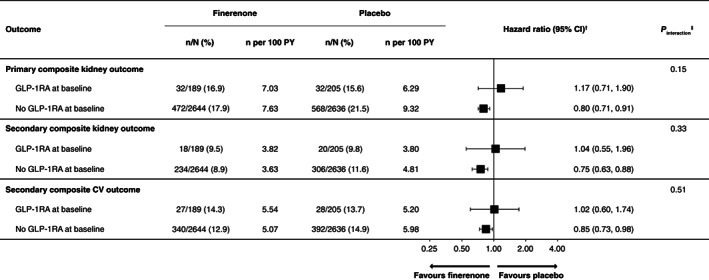
Primary and secondary composite outcomes by baseline GLP‐1RA use. †HR (95% CI) values based on the stratified Cox proportional hazards model estimated within each level of the subgroup variable. ‡ p value (two‐sided) for the interaction of treatment group and each baseline subgroup based on the Cox proportional hazards model, including the terms treatment group, baseline subgroup and their interaction. CI, confidence interval; CV, cardiovascular; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HR, hazard ratio; PY, patient‐years
The key secondary composite CV outcome was lower with finerenone compared with placebo in the overall population (HR 0.86; 95% CI 0.75, 0.99; p = .03). 17 In the present analysis, the key secondary composite CV outcome was reported by 55 (of 394; 14.0%) patients with GLP‐1RA treatment at baseline and 732 (of 5280; 13.9%) patients without GLP‐1RA treatment at baseline. The incidence of this outcome was similar, with no difference observed in patients with GLP‐1RA treatment at baseline (p value for interaction .51; Figure 1). For the secondary composite kidney outcome; in the overall population of FIDELIO‐DKD, the incidence was lower with finerenone versus placebo (HR 0.76; 95% CI 0.65, 0.90), 17 with no evidence of modification because of use of GLP‐1RA at baseline (p value for interaction .33; Figure 1).
The effect of GLP‐1RA use at any time during the trial was also investigated, including GLP‐1RA use a time‐varying covariate to account for initiation of GLP‐1RA use post‐baseline or GLP‐1RA treatment cessation. The benefit of finerenone for either the primary composite kidney or key secondary composite CV outcome with no significant differences because of GLP‐1RA use at any time (Wald test p value for interaction .31 and .86 respectively; Figures S3 and S4).
3.4. Safety
The incidences of any treatment‐emergent adverse event (AE) or treatment‐emergent serious AEs were similar between treatment groups, irrespective of GLP‐1RA use at baseline (Table 2). Treatment‐emergent AEs occurring in the placebo or finerenone groups did not indicate a lower tolerability for the combined use of finerenone and a GLP‐1RA (Table S5). Gastrointestinal side effects such as diarrhoea, nausea and vomiting were slightly more frequent in patients with GLP‐1RA treatment at baseline, but incidence was not modified by the use of finerenone (Table S4). The number of hypovolaemia or pancreatitis (acute or chronic) events was low (≤0.5% of patients who were treated with finerenone or placebo, with or without GLP‐1RA use). No cases of medullary thyroid carcinoma were recorded.
TABLE 2.
Safety outcomes according to baseline GLP‐1RA use
| GLP‐1RA at baseline | No GLP‐1RA at baseline | |||
|---|---|---|---|---|
| n (%) | Finerenone (N = 189) | Placebo (N = 203) a | Finerenone (N = 2638) | Placebo (N = 2628) |
| Any AE | 176 (93.1) | 190 (93.6) | 2292 (86.9) | 2288 (87.1) |
| Related to study drug | 52 (27.5) | 35 (17.2) | 594 (22.5) | 414 (15.8) |
| Leading to discontinuation | 19 (10.1) | 16 (7.9) | 188 (7.1) | 152 (5.8) |
| Any serious AE | 67 (35.4) | 69 (34.0) | 835 (31.7) | 902 (34.3) |
| Related to study drug | 4 (2.1) | 2 (1.0) | 44 (1.7) | 32 (1.2) |
| Leading to discontinuation | 5 (2.6) | 4 (2.0) | 70 (2.7) | 74 (2.8) |
| AE with outcome death | 0 (0.0) | 1 (0.5) | 31 (1.2) | 50 (1.9) |
| Hyperkalaemia‐related events | ||||
| Any AE | 36 (19.0) | 20 (9.9) | 480 (18.2) | 235 (8.9) |
| Related to study drug | 24 (12.7) | 9 (4.4) | 309 (11.7) | 126 (4.8) |
| Leading to discontinuation | 6 (3.2) | 3 (1.5) | 58 (2.2) | 22 (0.8) |
| Any serious AE | 3 (1.6) | 1 (0.5) | 41 (1.6) | 11 (0.4) |
| Related to study drug | 2 (1.1) | 0 (0.0) | 24 (0.9) | 5 (0.2) |
| Leading to discontinuation | ‐ | ‐ | 5 (0.2) | 1 (<0.1) |
| Reported as life‐threatening | ‐ | ‐ | 3 (0.1) | 3 (0.1) |
| Leading to hospitalization | 2 (1.1) | 1 (0.5) | 38 (1.4) | 7 (0.3) |
Abbreviations: AE, adverse event; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist.
2 patients from the full analysis set did not receive a dose of study drug and therefore were excluded from the safety analysis set.
The incidence of treatment‐emergent hyperkalaemia‐related AEs was higher in patients receiving finerenone compared with placebo in both subgroups with an approximate two‐fold increase with finerenone, but no differences were observed because of GLP‐1RA use at baseline (Table 2). Serious treatment‐emergent hyperkalaemia‐related events were rare and there were few events that led to treatment discontinuation (Table 2).
4. DISCUSSION
In the overall population of FIDELIO‐DKD, the largest study to date designed to investigate specifically the progression of kidney disease in patients with CKD and T2D receiving optimized renin‐angiotensin system inhibitor therapy, a 31% reduction in UACR was observed with finerenone versus placebo. 17 This subgroup analysis suggests a consistent reduction in UACR with finerenone irrespective of GLP‐1RA use at baseline (37% UACR reduction in patients with GLP‐1RA at baseline versus 31% in patients without GLP‐1RA at baseline); this suggests that finerenone has a kidney protective effect in patients who are already being treated with a GLP‐1RA, a treatment that reduces UACR. 7 , 8 , 11
A reduction in UACR is associated with improvement in hard kidney outcomes, particularly in patients with baseline albuminuria >30 mg/g as per the inclusion criteria for FIDELIO‐DKD. 17 , 24 , 25 Change in albuminuria has been used as a surrogate marker for kidney disease progression in several phase II trials including the ARTS‐DN study with finerenone, 26 and albuminuria is an independent risk factor for CV disease and mortality. 27 , 28 Although patients in the GLP‐1RA subgroup only represent 6.9% of the total FIDELIO‐DKD population (n = 394/5674), the number of patients included in the analysis is similar to or greater than group sizes included in several phase II studies that have assessed UACR as the primary endpoint; the treatment duration was also longer in FIDELIO‐DKD. 26 , 29 , 30 , 31 Based on the initial findings in this subgroup analysis, it may be reasonable to hypothesize that the reduction in UACR on top of GLP‐1RA treatment may be because of the combined activity of finerenone and GLP‐1RAs, acting either together or via discrete mechanisms; however, further investigation is required. Future subgroup analyses of patients according to GLP‐1RA treatment in the FIGARO‐DKD study, 32 a trial investigating finerenone in patients with stage 1‐4 CKD and T2D, as well as dedicated combination studies may provide further insights.
Finerenone also reduced the relative risk of the primary kidney composite outcome by 18% and the key secondary CV composite outcome by 14% in the FIDELIO‐DKD study. 17 This exploratory subgroup analysis suggests that the effects are consistent irrespective of GLP‐1RA use at baseline. The relatively small subgroup of patients receiving a GLP‐1RA at baseline as well as the low number of kidney and CV events observed in this group limits the interpretation of these results. Irrespective of these observations, there was no indication of treatment heterogeneity between the groups based on the p values for interaction and similar findings were observed when considering the use of GLP‐1RAs at any time during the study. The use of GLP‐1RA therapy did not appear to alter the safety profile observed with finerenone, and overall, combined use appeared to be well tolerated.
As expected, patients who were treated with GLP‐1RA at baseline had higher glycated haemoglobin, a longer duration of T2D, and more concomitant medications at baseline, which were suggestive of more advanced disease and/or patients being more obese or insulin resistant. The higher incidence of treatment‐emergent adverse events such as acute kidney injury, dyspnoea and elevations in blood creatinine and potassium may also have resulted because of more advanced disease in the GLP‐1RA at baseline subgroup. Patients treated with GLP‐1RA at baseline also had slightly lower baseline levels of albuminuria, which may be because of the albuminuria‐reducing effects of GLP‐1RAs. 7 , 8 , 13 , 15
Although GLP‐1RAs have shown a kidney protective effect in several CV outcomes trials or secondary analyses from CV outcomes trials, 6 , 7 , 8 , 9 , 10 , 11 results from dedicated kidney outcome trials are awaited 12 , 14 and the mechanism of action for the putative kidney protection has yet to be fully elucidated. 2 , 4 Possible protective mechanisms independent of, or in addition to, the glucose‐lowering effects of GLP‐1RAs include: tubular effects due the natriuretic and diuretic properties, improvement in haemodynamic function, reduction in body weight, reduction in angiotensin II and modulation of inflammation to reduce oxidative stress and tissue damage in the kidney. 2 , 4 , 12 , 33
This exploratory subgroup analysis had a number of limitations, including lack of statistical power for the composite kidney and CV outcomes because of the relatively small number of patients receiving GLP‐1RAs at baseline in the trial overall and the small number of clinical events in this group. A pooled analysis of data from the FIDELIO‐DKD and FIGARO‐DKD trials is planned. It must be acknowledged that patients receiving GLP‐1RAs at baseline had a longer duration of T2D and might have had a different rate of CKD progression than those not receiving a GLP‐1RA. Although some patients received GLP‐1RAs post‐baseline (n = 368/5674) and some patients receiving GLP‐1RAs at baseline may have stopped treatment during the trial, findings from the analysis including GLP‐1RA use at any time during the trial suggest that this did not significantly confound the results. Furthermore, the number of patients who initiated treatment with GLP‐1RA during the trial was similar in the finerenone and placebo arms of the overall population (6.7% vs 6.3%, respectively), suggesting that GLP‐1RA was not initiated more frequently in the placebo arm to target declining kidney function. The slight imbalance in baseline UACR between patients receiving a GLP‐1RA versus those who did not increased the potential for bias. In addition, an imbalance in sodium‐glucose co‐transporter‐2 inhibitor treatment between GLP‐1RA and no GLP‐1RA at baseline groups (~12% vs. ~4%, respectively) may also have confounded the results observed because the known UACR‐lowering effects of these agents; however, numbers were small, and no conclusions can be drawn. One other limitation was that adequate data on the duration of GLP‐1RA use before enrolment in the study were not available, so any impact of treatment duration on outcomes cannot be determined.
GLP‐1RAs are recommended for use in patients with T2D with elevated albuminuria to reduce their CV risk. 34 This includes an overlapping population to the clinical indication for finerenone, to reduce the risk of CV and kidney events in adults with CKD associated with T2D 35 ; therefore, these results provide reassurance that the clinical benefits and safety profile of finerenone appear to be unaffected by concomitant GLP‐1RA treatment.
In conclusion, in patients with CKD and T2D receiving optimized renin‐angiotensin system inhibitor therapy in the FIDELIO‐DKD study, the addition of finerenone to a GLP‐1RA resulted in further reduction in albuminuria. In addition, finerenone appeared to reduce kidney disease progression and CV events compared with placebo regardless of GLP‐1RA treatment at baseline or during the trial, further investigation is warranted to confirm these findings.
CONFLICT OF INTEREST
PR reports personal fees from Bayer, during the conduct of the study; he has received research support and personal fees from AstraZeneca and Novo Nordisk, and personal fees from Eli Lilly and Company, Boehringer Ingelheim, Astellas Pharma Inc., Gilead, Merck, Merck Sharp and Dohme, Mundipharma, Sanofi and Vifor Pharma. All fees are given to Steno Diabetes Center Copenhagen. RA reports personal fees and non‐financial support from Bayer Healthcare Pharmaceuticals Inc., during the conduct of the study; he also reports personal fees and non‐financial support from Akebia Therapeutics, Janssen, Relypsa Inc., Vifor Pharma, Boehringer Ingelheim, Sanofi, Eli Lilly and Company, AstraZeneca and Fresenius; he has received personal fees from Ironwood Pharmaceuticals, Merck & Co., Lexicon and Reata Pharmaceuticals, and non‐financial support from Otsuka America Pharmaceutical Inc., OPKO Health, Inc. and E. R. Squibb & Sons; he is a member of data safety monitoring committees for Amgen, AstraZeneca, and Celgene; a member of steering committees of randomized trials for Akebia Therapeutics, Bayer, Janssen and Relypsa Inc.; a member of adjudication committees for AbbVie, Bayer, Boehringer Ingelheim and Janssen; he has served as associate editor of the American Journal of Nephrology and Nephrology Dialysis and Transplantation and has been an author for UpToDate; and he has received research grants from the US Veterans Administration and the National Institutes of Health. SDA has received research support from Abbott Vascular and Vifor Pharma, and personal fees from Abbott Vascular, Boehringer Ingelheim, Bayer, BRAHMS, Novartis, Servier, Vifor Pharma, Impulse Dynamics and Cardiac Dimensions. GF reports lectures fees and/or that he is a committee member of trials and registries sponsored by Bayer, Novartis, Vifor Pharma, Medtronic, Servier, Amgen and Boehringer Ingelheim. He is a senior consulting editor for JACC Heart Failure, and he has received research support from the European Union. BP reports consultant fees for Bayer, AstraZeneca, Sanofi/Lexicon, scPharmaceuticals, SQ Innovation, G3 Pharmaceuticals, Sarfez Pharmaceutical, Inc., PhaseBio, Vifor Pharma/Relypsa, Inc., Cereno Scientific, Ardelyx, KBP Biosciences, Boehringer Ingelheim, Brainstorm Medical and Tricida; he has stock options for Ardelyx, KBP Biosciences, SQ Innovation, Sarfez Pharmaceutical, Inc., scPharmaceuticals, Cereno Scientific G3 Pharmaceuticals, Vifor Pharma/Relypsa, Inc., Brainstorm Medical and Tricida; he also holds a patent for site‐specific delivery of eplerenone to the myocardium (US patent no. 9931412) and a provisional patent for histone‐acetylation‐modulating agents for the treatment and prevention of organ injury (provisional patent US 63/045784). LMR has no disclosures. AA has served on advisory boards or consulted for Aspen Pharmacare, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck SA, Novo Nordisk and Servier. MM is a consultant for the Novo Nordisk Algerian subsidiary and has received personal fees from Bayer AG, Novo Nordisk, Merck, Sharp and Dohme and Servier during the past 3 years. AJ is a full‐time employee of Bayer AG, Division Pharmaceuticals, Germany. AL is a full‐time employee of Bayer SA, Division Pharmaceuticals, Brazil. CS is a full‐time employee of Bayer PLC, Division Pharmaceuticals, United Kingdom. GLB reports research funding, paid to the University of Chicago Medicine, from Bayer, during the conduct of the study; he also reports research funding, paid to the University of Chicago Medicine, from Novo Nordisk and Vascular Dynamics; he acted as a consultant and received personal fees from for Merck, Relypsa, Inc. and Alnylam; he is an editor of American Journal of Nephrology, Nephrology and Hypertension, and section editor of UpToDate; and he is an associate editor of both Diabetes Care and Hypertension Research.
AUTHOR CONTRIBUTIONS
The FIDELIO‐DKD trial was conducted and funded by Bayer AG. The sponsor participated in study design, data collection, data analysis, data interpretation and approval of the manuscript. Analyses were conducted by the sponsor, and all authors had access to and participated in the interpretation of the data. Peter Rossing wrote the first draft of the report. All authors were involved in drafting and critically revising the report. All authors had access to study results and the first and corresponding author assumes responsibility for the integrity and accuracy of the data reported. All authors reviewed and approved the final submitted version of the report.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14558.
Supporting information
Appendix S1. Supporting information
ACKNOWLEDGMENTS
We are indebted to the patients who have participated in this trial, the FIDELIO‐DKD study investigators, the study centres who supported the trial, and the study teams. Medical writing assistance was provided by Kate Weatherall, PhD, of Chameleon Communications International, and was funded by Bayer AG.
Rossing P, Agarwal R, Anker SD, et al. Efficacy and safety of finerenone in patients with chronic kidney disease and type 2 diabetes by GLP‐1RA treatment: A subgroup analysis from the FIDELIO‐DKD trial. Diabetes Obes Metab. 2022;24(1):125-134. doi:10.1111/dom.14558
Funding information Bayer AG
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are not currently available in a public repository
REFERENCES
- 1. Gorriz JL, Soler MJ, Navarro‐Gonzalez JF, et al. GLP‐1 receptor agonists and diabetic kidney disease: a call of attention to nephrologists. J Clin Med. 2020;9(4):947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Greco EV, Russo G, Giandalia A, Viazzi F, Pontremoli R, de Cosmo S. GLP‐1 receptor agonists and kidney protection. Medicina (Kaunas). 2019;55(6):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kawanami D, Takashi Y. GLP‐1 receptor agonists in diabetic kidney disease: from clinical outcomes to mechanisms. Front Pharmacol. 2020;11:967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yin WL, Bain SC, Min T. The effect of glucagon‐like peptide‐1 receptor agonists on renal outcomes in type 2 diabetes. Diabetes Ther. 2020;11(4):835‐844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schernthaner G, Shehadeh N, Ametov AS, et al. Worldwide inertia to the use of cardiorenal protective glucose‐lowering drugs (SGLT2i and GLP‐1 RA) in high‐risk patients with type 2 diabetes. Cardiovasc Diabetol. 2020;19(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mann JFE, Orsted DD, Brown‐Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377(9):839‐S848. [DOI] [PubMed] [Google Scholar]
- 8. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo‐controlled trial. Lancet. 2019;394(10193):131‐138. [DOI] [PubMed] [Google Scholar]
- 9. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double‐blind, randomised placebo‐controlled trial. Lancet. 2019;394(10193):121‐130. [DOI] [PubMed] [Google Scholar]
- 10. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834‐1844. [DOI] [PubMed] [Google Scholar]
- 11. Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate‐to‐severe chronic kidney disease (AWARD‐7): a multicentre, open‐label, randomised trial. Lancet Diabetes Endocrinol. 2018;6(8):605‐617. [DOI] [PubMed] [Google Scholar]
- 12. Alicic RZ, Cox EJ, Neumiller JJ, Tuttle KR. Incretin drugs in diabetic kidney disease: biological mechanisms and clinical evidence. Nat Rev Nephrol. 2021;17(4):227‐244. [DOI] [PubMed] [Google Scholar]
- 13. Mann JFE, Hansen T, Idorn T, et al. Effects of once‐weekly subcutaneous semaglutide on kidney function and safety in patients with type 2 diabetes: a post‐hoc analysis of the SUSTAIN 1‐7 randomised controlled trials. Lancet Diabetes Endocrinol. 2020;8(11):880‐893. [DOI] [PubMed] [Google Scholar]
- 14. Mann JFE, Muskiet MHA. Incretin‐based drugs and the kidney in type 2 diabetes: choosing between DPP‐4 inhibitors and GLP‐1 receptor agonists. Kidney Int. 2021;99(2):314‐318. [DOI] [PubMed] [Google Scholar]
- 15. Gerstein HC, Sattar N, Rosenstock J, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med. 2021;385:896‐907. doi: 10.1056/10.1056/NEJMoa2108269 [DOI] [PubMed] [Google Scholar]
- 16. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group . KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98(45):S1‐S115. [DOI] [PubMed] [Google Scholar]
- 17. Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383:2219‐2229. [DOI] [PubMed] [Google Scholar]
- 18. Filippatos G, Anker SD, Agarwal R, et al. Finerenone and cardiovascular outcomes in patients with chronic kidney disease and type 2 diabetes. Circulation. 2021;143(6):540‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barrera‐Chimal J, Estrela GR, Lechner SM, et al. The myeloid mineralocorticoid receptor controls inflammatory and fibrotic responses after renal injury via macrophage interleukin‐4 receptor signaling. Kidney Int. 2018;93(6):1344‐1355. [DOI] [PubMed] [Google Scholar]
- 20. Barrera‐Chimal J, Girerd S, Jaisser F. Mineralocorticoid receptor antagonists and kidney diseases: pathophysiological basis. Kidney Int. 2019;96(2):302‐319. [DOI] [PubMed] [Google Scholar]
- 21. Buonafine M, Bonnard B, Jaisser F. Mineralocorticoid receptor and cardiovascular disease. Am J Hypertens. 2018;31(11):1165‐1174. [DOI] [PubMed] [Google Scholar]
- 22. Guo C, Martinez‐Vasquez D, Mendez GP, et al. Mineralocorticoid receptor antagonist reduces renal injury in rodent models of types 1 and 2 diabetes mellitus. Endocrinology. 2006;147(11):5363‐5373. [DOI] [PubMed] [Google Scholar]
- 23. Agarwal R, Kolkhof P, Bakris G, et al. Steroidal and non‐steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2021;42(2):152‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bakris GL, Agarwal R, Anker SD, et al. Design and baseline characteristics of the Finerenone in reducing kidney failure and disease progression in diabetic kidney disease trial. Am J Nephrol. 2019;50(5):333‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heerspink HJL, Greene T, Tighiouart H, et al. Change in albuminuria as a surrogate endpoint for progression of kidney disease: a meta‐analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol. 2019;7(2):128‐139. [DOI] [PubMed] [Google Scholar]
- 26. Bakris GL, Agarwal R, Chan JC, et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314(9):884‐894. [DOI] [PubMed] [Google Scholar]
- 27. Solomon SD, Lin J, Solomon CG, et al. Influence of albuminuria on cardiovascular risk in patients with stable coronary artery disease. Circulation. 2007;116(23):2687‐2693. [DOI] [PubMed] [Google Scholar]
- 28. Gansevoort RT, Correa‐Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339‐352. [DOI] [PubMed] [Google Scholar]
- 29. Parving HH, Lehnert H, Brochner‐Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345(12):870‐878. [DOI] [PubMed] [Google Scholar]
- 30. Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358(23):2433‐2446. [DOI] [PubMed] [Google Scholar]
- 31. de Zeeuw D, Coll B, Andress D, et al. The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J Am Soc Nephrol. 2014;25(5):1083‐1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ruilope LM, Agarwal R, Anker SD, et al. Design and baseline characteristics of the Finerenone in reducing cardiovascular mortality and morbidity in diabetic kidney disease trial. Am J Nephrol. 2019;50(5):345‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Skov J, Pedersen M, Holst JJ, et al. Short‐term effects of liraglutide on kidney function and vasoactive hormones in type 2 diabetes: a randomized clinical trial. Diabetes Obes Metab. 2016;18(6):581‐589. [DOI] [PubMed] [Google Scholar]
- 34. Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of diabetes (EASD). Diabetes Care. 2020;2:487‐493. doi: 10.2337/10.2337/dci19-0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. KERENDIA . Prescribing Information. Whippany, NJ: Bayer Healthcare Pharmaceuticals Inc; 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information
Data Availability Statement
The data supporting the findings of this study are not currently available in a public repository


