Abstract
The “epithelial barrier hypothesis” proposes that the exposure to various epithelial barrier–damaging agents linked to industrialization and urbanization underlies the increase in allergic diseases. The epithelial barrier constitutes the first line of physical, chemical, and immunological defense against environmental factors. Recent reports have shown that industrial products disrupt the epithelial barriers. Innate and adaptive immune responses play an important role in epithelial barrier damage. In addition, recent studies suggest that epithelial barrier dysfunction plays an essential role in the pathogenesis of the atopic march by allergen sensitization through the transcutaneous route. It is evident that external factors interact with the immune system, triggering a cascade of complex reactions that damage the epithelial barrier. Epigenetic and microbiome changes modulate the integrity of the epithelial barrier. Robust and simple measurements of the skin barrier dysfunction at the point‐of‐care are of significant value as a biomarker, as recently reported using electrical impedance spectroscopy to directly measure barrier defects. Understanding epithelial barrier dysfunction and its mechanism is key to developing novel strategies for the prevention and treatment of allergic diseases. The aim of this review is to summarize recent studies on the pathophysiological mechanisms triggered by environmental factors that contribute to the dysregulation of epithelial barrier function.
Keywords: allergy, asthma, atopic dermatitis, barrier dysfunction, epithelium, rhinitis, sinusitis, type 2 immunity
Abbreviations
- AD
atopic dermatitis
- AJ
adherence junction
- ALI
air‐liquid interface
- AR
allergic rhinitis
- CRS
chronic rhinosinusitis
- DEP
diesel exhaust particles
- Der p1
Dermatophagoides pteronyssinus peptidase 1
- EI
electrical impedance
- HBEC
human bronchial epithelial cells
- HDACs
histone deacetylases
- HDM
house dust mite
- IL
interleukin
- ILC
innate lymphoid cell
- NEC
nasal epithelial cell
- NHEK
normal human keratinocytes
- NO2
nitrogen dioxide
- PAR
protease‐activated receptor
- PM
particulate matter
- ROS
reactive oxygen species
- TEWL
transepidermal water loss
- TJ
tight junctions
- TLR
Toll‐like receptor
- TSLP
thymic stromal lymphopoietin
- VOCs
volatile organic compounds
- ZO
zonula occluden
1. INTRODUCTION
Since the introduction of the “epithelial barrier hypothesis,” factors that damage the skin and mucosal barriers have become a major area of interest. 1 Epithelial barrier leakiness has been demonstrated in asthma, atopic dermatitis (AD), allergic rhinitis (AR), chronic rhinosinusitis (CRS), eosinophilic esophagitis, celiac disease, and inflammatory bowel disease. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 Leakiness of the gut epithelium is also implicated in systemic autoimmune and metabolic conditions such as obesity, diabetes, rheumatoid arthritis, multiple sclerosis, systemic lupus erythematosus, fatty liver, autoimmune hepatitis, and ankylosing spondylitis. Although further studies are needed, microbial dysbiosis and an impaired gut barrier are suspected in Alzheimer's disease, Parkinson's disease, chronic depression, and autism spectrum disorders. 1 The epithelium is a pivotal structure in host defense because it not only provides a physical barrier between the environment and the subepithelial region, but also contributes to the induction of an appropriate immune response against allergens, pathogens, and noxious stimuli that attenuate the epithelial barrier. Recent studies have shown that a defective epithelial barrier, with compromised tight junctions (TJs) and adherence junctions (AJs), is part of the underlying pathology in diseases such as AD, bronchial asthma, eosinophilic esophagitis, food allergy, CRS, and AR, which are discussed in this review. 3 , 7 , 9 , 10 The sequential development of these diseases starting from AD in infancy followed by food allergy, asthma and rhinitis, commonly known as the atopic march, has sharply increased. 11 , 12 , 13 The inner molecular mechanisms underlying the epithelial barrier dysfunction involve a type 2 immune response through type 2 cytokines produced by Th2 cells and type 2 innate lymphoid cells (ILC2s). 14 , 15 It is well‐known that lifestyle changes due to industrialization cause epithelial barrier dysfunction. 16 , 17 Factors that have been recognized to damage the epithelial barrier include allergens, pathogens, commercial detergents, surfactants, emulsifiers in processed food, cigarette smoke, particulate matter, diesel exhaust, ozone, nanoparticles, and microplastics. 1 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 These changes also compromise epithelial barrier function across multiple organ systems. 21 , 23 , 25 , 28 , 29 , 30 , 31 Quantitative skin barrier assessment in vivo can be beneficial for many clinical applications, including diagnosis, follow‐up, prevention, and monitoring the therapy of AD.
Supporting our “epithelial barrier hypothesis,” we discuss recent advances in our understanding of the mechanisms underlying epithelial barrier dysfunction triggered by environmental and other culprit factors. The latest technological developments to measure skin barrier dysfunction are reviewed.
1.1. Structure of the epithelial barrier
The epithelial barrier consists of a stratified epithelial cellular sheet that forms a physical barrier on the body surface. Four main components can be identified: (a) epithelial cells; (b) structural proteins, such as filaggrin, loricrin, involucrin, and proteins involved in the formation of TJs and AJs; (c) secreted epithelial products, such as mucus, antimicrobial peptides, and a lipid‐rich matrix composed of ceramides, cholesterol, and free fatty acids (FFAs); and (d) epithelial microbiota (Figure 1). 3 , 32
FIGURE 1.
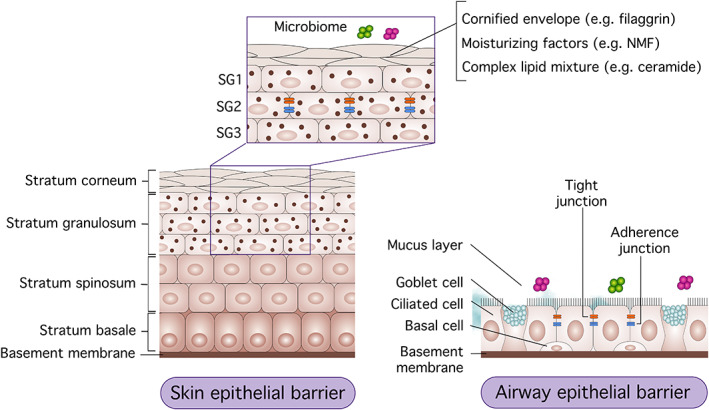
Skin and mucosal barrier structure. The epithelial barrier of the skin and airways consists of four main components. (a): The epithelial layers—the epidermis consists of four sub‐layers and the airway epithelial barrier is made of a pseudostratified epithelium. (b): Structural proteins, such as filaggrin, natural moisturizing factor (NMF), ceramide, tight junctions (TJs), and adherence junctions (AJs). (c): Secreted molecules, such as mucin, anti‐microbial peptides, and fatty acids. (d) Microbiota on the surface of the epithelial barrier
The epidermis has its original structure and is composed of four main layers: the stratum corneum, stratum granulosum, stratum spinosum, and the stratum basale. 3 The stratum corneum is the outermost layer, consisting of enucleated keratinocytes referred to as corneocytes. It is maintained by the complex interaction of the cornified envelope, intracytoplasmic moisturizing factors, and a complex lipid mixture in the extracellular space. Filaggrin is a structural protein important for the alignment of keratin filaments and the formation of the cornified envelope and natural moisturizing factor. 33 , 34 , 35
TJs are composed of transmembrane proteins, such as claudins, occludin, zonula occludens (ZOs), and junctional adhesion molecules, which regulate the paracellular permeability and are located in the stratum granulosum of the epidermis and mucosal epithelium. Allergens gain rapid entry across disrupted TJs, engage dendritic/Langerhans cells, and prime the systemic inflammatory response. 3 , 32 In intact skin, Langerhans cells have been found essential for immune tolerance in a murine ovalbumin (OVA)‐induced model. 36 Epicutaneous sensitization with allergen promotes a local and systemic type 2–predominant response, characterized by increased interleukin (IL)‐4, IL‐5, IL‐13, and immunoglobulin E (IgE) (Figure 2). 37 , 38
FIGURE 2.
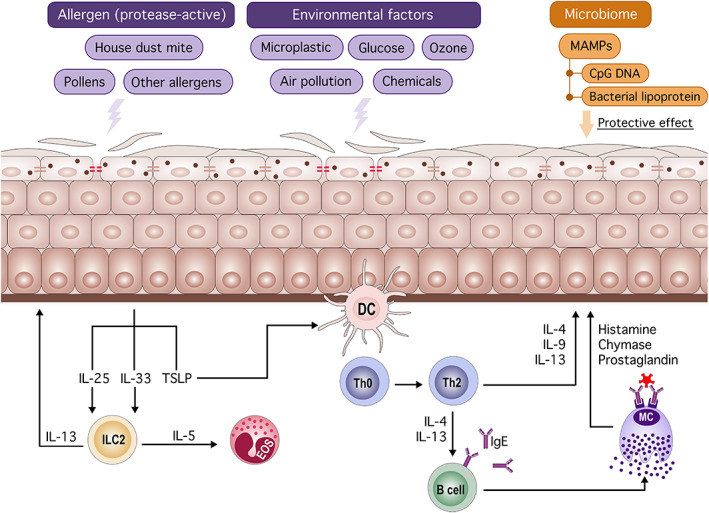
Endogenous and exogenous factors affecting epithelial barrier function. Allergens (eg, house dust mite) and noxious stimuli (eg, air pollution) attenuate the epithelial barrier function. An impaired skin barrier facilitates the entry of allergens and activation of the innate immune response. Damaged epithelial cells produce IL‐25, IL‐33, and TSLP, followed by activation of ILC2 and dendritic cells. Activated dendritic cells induce type 2 skewing and IgE production by B cells. Type 2 cytokines and degranulation of mast cells exacerbate the inflammation and further attenuates barrier function. Microbe‐associated molecular patterns (eg, CpG DNA) restore epithelial barrier integrity and maintain epithelial barrier function. DC: dendritic cell, ILC: innate lymphoid cell, EOS; eosinophil, MC: mast cell, TSLP: thymic stromal lymphopoietin, MAMP: microbe‐associated molecular pattern
Many microorganisms colonize the area above and within the stratum corneum, and mucosa, thereby maintaining immune homeostasis. The epithelial microbial dysbiosis contributes to the development and course of allergic disorders such as AD and asthma. 39 , 40 The mechanism of epithelial barrier dysfunction is not fully understood and further research is warranted.
1.2. Culprit factors for epithelial barrier dysfunction
1.2.1. Environmental factors
Environmental factors and lifestyle changes may be responsible for the increasing prevalence of allergic diseases. 16 , 17 The sum of external factors that an individual is exposed to throughout life is referred to as the exposome. 41 , 42 The combination of multiple factors, such as cigarette smoke, air pollutants, chemicals, aeroallergens, and diet, contributes to the development and exacerbation of asthma and other allergic diseases. 1 , 43 , 44 , 45 , 46 , 47
Oxidative stress and air pollution
Cigarette smoke and its reactive oxygen species (ROS) products impair the epithelial barrier function and increase epithelial permeability by triggering the RhoA/Rho‐associated protein kinase (ROCK) signaling pathway in which ROCK is activated after hyaluronan fragmentation via ROS, which leads to disruption of AJs through a decrease in E‐cadherin gene and protein expression in human bronchial epithelial cells (HBECs). 43 Exposure to microplastics and air pollutants, such as diesel exhaust particles (DEPs), ozone, and nitrogen dioxide, induces the production of ROS. 21 , 44 , 48 , 49 , 50 Particulate matter 2.5 (PM2.5), the main component of DEP, exacerbated AR through TJ disruption in a mouse model of allergic rhinitis. 51 , 52 A population‐based prospective study has shown that a 2.3 parts per billion increase in nitrogen dioxide (NO2) is associated with an increased risk of prevalent and atopic eczema in male adults. 47 Exposure to traffic‐related air pollutants, PM2.5, PM10, and oxides of nitrogen, is associated with increased odds of incident eczema, particularly PM2.5, and is more pronounced with nonatopic eczema in elderly women. 53 Moreover, DEP exposure induces IL‐25, IL‐33, and thymic stromal lymphopoietin (TSLP) expression in the lungs, leading to severe asthma by activation of the aryl hydrocarbon receptor. 28 , 54
Volatile organic compounds and ozone
Volatile organic compounds (VOCs) are composed of carbon‐based molecules such as formaldehyde, benzene, and toluene that affect the urban air quality and human health. VOCs are commonly found in cleaning products, wallpaper, furniture, and plastics, and are main contributors to indoor air pollution. 55 , 56 VOC exposure leads to proteasome inactivation and protein oxidation, DNA damage, and apoptosis in keratinocytes and human skin explants. 57 Exposure to formaldehyde for 1 hour increases transepidermal water loss (TEWL) in both healthy and AD children, supporting the role of short‐term exposure to airborne formaldehyde in impairment of the skin barrier. 58 VOCs can react with NO2 in the presence of sunlight to form ozone, 44 which causes airway hyperreactivity, airway inflammation, and bronchial epithelial barrier disruption by inducing ROS production. 21 , 48 , 59 IL‐33 plays a critical role in ozone‐induced neutrophilic rapid lung inflammation and bronchial epithelial barrier injury.
Microplastics and nanoplastics
The internalization of microplastics and nanoplastics into human lung epithelial cells has been recently demonstrated. 60 Microplastics can cause cytotoxic and inflammatory responses by suppressing ZO‐1 expression in HBECs through ROS. 49
Detergents, surfactants, and enzymes
Surfactants, a main constituent in detergents, can significantly damage the epithelial barrier because the detergent disrupts the lipid‐lipid and lipid‐protein interactions of the membrane. Our group showed that anionic surfactants can directly impair the TJ integrity of air‐liquid interface (ALI)–cultured normal human keratinocytes (NHEKs) and HBECs. 23 , 25 In addition, treatment with post‐laundry detergent residue was demonstrated to open the epithelial barrier in a dose‐dependent manner. RNA sequencing analysis indicates that exposure to 50 000 times diluted detergents upregulated gene expression associated with lipid metabolism, oxidative stress, and cell survival to compensate the damage caused by the detergents to the cell membrane lipids. Laundry detergents had no significant effect on chromatin accessibility and DNA methylation in HBECs. 23 Moreover, the marked increase in Bacillus species–derived proteolytic enzymes in commercial detergents was associated with the development of asthma and rhinitis. 61 , 62
Household cleaners, hand sanitizers, and disinfectants
Hand hygiene is one of the most important preventive measures for contact transmission of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). The World Health Organization recommended hand hygiene practices of using alcohol‐based sanitizers and/or handwashing with water and soap. An increased risk of hand eczema has been reported in children and health care workers during the coronavirus disease 2019 (COVID‐19) pandemic. 63 , 64
There is increasing evidence linking asthma to the use of respiratory irritant cleaning agents, mainly bleach, ammonia, and cleaning/degreasing sprays. In addition, exposure to low molecular weight agents/irritants in cleaning products and disinfectants is related to adult‐onset asthma. 65 , 66
Emulsifiers in processed foods
Emulsifiers are frequently used as additives in the food industry. There is evidence indicating that emulsifiers increase intestinal permeability, even at low concentrations. 67 , 68 Some commonly used emulsifiers, including polysorbate 80 and carboxymethyl cellulose, significantly increased bacterial translocation in Caco‐2 cells derived from a human colon carcinoma. 27
High glucose‐induced barrier defects
Thaiss et al showed that elevated glucose concentrations lead to intestinal barrier dysfunction and enhanced dissemination of enteric infection in a mouse model of type I diabetes mellitus induced by administering streptozotocin. 69 Similarly, Yu et al reported that high glucose levels disrupted the TJs by downregulation of connexin 43, which plays a critical role in maintaining the function of the airway epithelial barrier in the respiratory tract. 70
2. ALLERGENS
The protease activity of allergens, such as house dust mites (HDMs), Aspergillus fumigatus, and pollen, has been suggested to be involved in the pathogenesis of allergic diseases by increasing the epithelial permeability and causing barrier dysfunction (previously reviewed). 37 , 71 Proteolytically active allergens not only cleave the junction proteins but also activate epithelial cells and adaptive immune responses directly through protease‐activated receptors (PARs).
HDM allergens increase the mucosal permeability of ALI‐cultured nasal epithelial cells (NECs) from HDM‐induced AR patients. 10 In addition, HDM perturb N‐glycosylation of Toll‐like receptor (TLR) 3 and impair the interferon response, which may aggravate allergic lung inflammation. 72 Protease allergens, such as Dermatophagoides pteronyssinus peptidase 1 (Der p 1) derived from HDM, induce type 2 inflammation, thereby promoting alarmins (IL‐25, IL‐33, TSLP) in epithelial cells. 39 , 73 These cytokines activate ILC2 and promote type 2 helper cell differentiation. 14 , 15 Hiraishi et al showed that fungal‐associated proteases induce ILC‐mediated airway eosinophilia through the expression of IL‐25, IL‐33, and TSLP. 74 Unlike other protease allergens, Der f3 from Dermatophagoides farinae (Der f) induces pro‐inflammatory mediators (IL‐6, IL‐8, and granulocyte‐macrophage colony‐stimulating factor), but not IL‐25, IL‐33, and TSLP, and causes barrier dysfunction through PAR‐1 and PAR‐2. 75 Allergen immunotherapy has been used for the treatment of allergic diseases. 76 , 77 , 78 , 79 Yuan et al reported that allergen‐specific immunotherapy restored airway epithelial integrity, and attenuated IL‐25–induced epithelial endoplasmic reticulum stress and epithelial apoptosis in a Der f ‐induced airway inflammation model. 80
Pollen is also a well‐known trigger of respiratory allergies and asthma. Vinhas et al found that pollen proteases damage the epithelial barrier via PARs. 81 Consistently, Cleemput et al showed that pollen proteases selectively and irreversibly damage the integrity and anchorage of columnar respiratory epithelial cells. In addition, this partial loss of barrier function facilitates the invasion of alpha‐herpesviruses. 82
3. MICROBIOTA CHANGES
The human body is colonized by a diverse microbial flora that plays an essential role in human health and disease. As the outermost component, the skin microbiota is the first line of defense against pathogenic microorganisms. It is known that healthy skin microbiota limits pathogen colonization via competition for space and nutrients, altering skin pH, and producing antimicrobial peptides (AMPs). 83 Skin injury induced by tape‐stripping in mice disturbs the skin barrier and alters the skin microbiota and host genes. 84 It is well‐known that poor microbiome diversity is accompanied by an increase in Staphylococcus aureus (S. aureus) abundance, which is positively correlated with AD disease severity. 85 , 86 , 87 Recently we also demonstrated that S. aureus is significantly more abundant in lesional skin compared to nonlesional and healthy skin. 39 Moreover, when performing next‐generation sequencing on matched lesional and nonlesional skin tissue biopsy specimens from patients with AD and healthy subjects, we identified four TJs genes (CLDN4, CLDN5, TJP1, and TJP2) that are robustly correlated to microbiome dysbiosis in patients with AD. 39 , 88 Consistent with these results, Clausen et al reported that the microbiome alpha diversity is lower in patients with AD compared with healthy controls and is inversely correlated with disease severity, not only for lesional skin but also nonlesional skin. Filaggrin gene mutations were associated with microbiome composition in the nonlesional skin of patients with AD. 89 Of interest, we found that S. aureus and Staphylococcus epidermidis enhance the expression of TJs in healthy nasal tissue, but not in nasal polyps. 88 , 89 The microbiome may have a protective role as observed in healthy epithelial cells. S. aureus–secreted proteins may be contributing to the initiation and acute exacerbations of human asthma and CRS with nasal polyps. 90 Recent reports suggest that early life bacterial supplementation may prevent the onset and progression of allergic diseases. 91 On the other hand, Roßberg et al reported that applying bacterial lysates of heat‐killed gram‐negative Escherichia coli and gram‐positive Enterococcus faecalis in early infancy does not influence the development of AD, AR, asthma, and sensitization at school age. 92
It is widely accepted that the epithelial barrier is armed with its own protective mechanisms. Epithelial cells sense pathogen‐associated molecular patterns by expressing pattern‐recognition receptors, including TLR and PAR. It is known that TLR2 is essential for maintaining epithelial barrier function. 93 , 94 In line with these studies, Ruffner et al found that TLR2 stimulation induces claudin‐1 and ZO‐1 production in esophageal epithelial cells and increases histone 4 acetylation in claudin‐1 gene enhancer and promoter. 95 Decreased expression of TLR2 on Langerhans cells in AD skin impairs S. aureus–derived signals and contributes to the immune deviation from S. aureus clearance. 96 Microbial DNA sequences containing unmethylated CpG dinucleotides activate TLR9, restoring epithelial barrier integrity by modulating TJ expression. 97 Furthermore, probiotic bacteria, mostly lactobacilli, interact with the epithelial barrier and immune cells through pattern recognition receptors and promote the expression of TJs and AJs, restoring the epithelial barrier integrity. 98
S. aureus induces proinflammatory cytokines, such as tumor necrosis factor α (TNF‐α) in keratinocytes. We showed that TNF‐α and TNF‐like weak inducer of apoptosis (TWEAK) cooperate in the induction of apoptosis in primary keratinocytes and that a high TWEAK expression is observed in lesional AD skin. 99
3.1. Mechanism of barrier dysfunction
3.1.1. Cytokines and hormonal factors
It is well‐established that signature type 2 cytokines, such as IL‐4 and IL‐13, impair the epithelial barrier function, reducing the expression of TJs proteins and filaggrin. 7 , 38 , 100 For example, IL‐4 and IL‐13 downregulate claudin‐4, ZO‐1, and occludin in HBECs, which results in increased paracellular leakiness and respiratory hyper‐responsiveness. 101 On the other hand, Sweerus et al reported that IL‐13 downregulates the expression of claudin‐18 but not of claudin‐1, ‐4, and ‐7. 102 A recent systematic review showed that anti–IL‐4 and IL‐13 receptor monoclonal antibodies (dupilumab), anti–IL‐5 monoclonal antibodies (mepolizumab and reslizumab), and anti–IL‐5 receptor alpha monoclonal antibodies (benralizumab) are effective treatments for severe asthma and AD. 103 , 104 , 105 , 106 , 107 , 108 , 109 Type 2 cytokines can cause barrier dysfunction by downregulating filaggrin gene expression. Mitamura et al reported that IL‐24, a member of the IL‐20 family, mediates the IL‐13–induced downregulation of filaggrin gene expression. 110 Single‐cell analysis of biopsy samples and blisters showed that the frequencies of type 2 (IL13+)/type 22(IL22+) T cells are higher than those of type 1 (IFN‐γ+) in lesional AD, whereas this ratio is slightly reduced in nonlesional AD. 111 , 112 , 113
Epithelium‐derived alarmins, IL‐25, IL‐33, and TSLP, lead to systemic inflammation characterized by activation of ILC2, exacerbated eosinophilia and IgE/IgG1 production and induce an immune response toward inhaled noxious agents and allergens. 114 , 115 Xiong et al found that leukotriene B4 induces IL‐33 expression and ILC2 activation in the lungs via engagement of its receptor 1 (BLT1) in a papain‐induced lung inflammation mouse model. 116 ILC2s also play an essential role in disrupting the epithelial barrier. We demonstrated that IL‐13 produced by ILC2s induces keratinocyte and bronchial epithelial barrier disruption via downregulation of the expression of TJ components. 8 , 117
The common inflammatory cascade in allergic diseases is initiated by an IgE‐dependent mast cell degranulation with the release of histamine, among other mediators, and is further orchestrated by type 2 cytokines. 118 , 119 Gschwandtner et al first demonstrated that histamine aggravates the severity of AD and that anti‐histamines may have potential therapeutic value to restore skin barrier function in these patients. 119 Of interest, histamine directly inhibits the expression of filaggrin, loricrin, claudin‐1, claudin‐4, and occludin in keratinocytes. 119 Histamine and Th2 cells are critical factors in initiating and maintaining a leaky barrier in patients via histamine receptor 1 with an early phase allergic airway response. 120 Leukotriene E4, an arachidonic acid–derived bioactive molecule, mediates eosinophilic airway inflammation by decreasing the level of surfactant protein D. 121 On the other hand, prostaglandin D2, which is also a potent pro‐inflammatory lipid mediator, has been reported to have protective effects in acute lung injury 122 and later phase skin and airway inflammation. 123 Chymases are a family of serine proteases found primarily in mast cells. Zhou et al reported that chymase suppresses the expressions of occludin, claudin‐4, ZO‐1, E‐cadherin, focal adhesion kinase, and cytokeratin, resulting in dysfunction of the airway wall in the pathogenesis of asthma. 124 Moreover, an impaired epithelial barrier facilitates allergen passage and mast cell degranulation even in the absence of allergic inflammation. 125
4. GENETICS AND EPIGENETICS OF EPITHELIAL BARRIER LEAKINESS
Recent studies have shown that epigenetic mechanisms mediate the effect of environmental and inflammatory triggers associated with allergic diseases. 126 , 127 Histone acetylation induced by histone acetyltransferases is associated with gene transcription. On the other hand, histone deacetylases (HDACs) suppress gene transcription. Of interest, Nicodemus‐Johnson et al showed that exposure of HBEC to IL‐13 changes global DNA methylation patterns and results in long‐lasting epigenetic changes near asthma‐associated genes. 128 Moreover, we found that IL‐4 and IL‐13 increase HDAC activity, inducing barrier leakiness in HBECs from patients with allergic asthma. 100 Kaneko et al reported that HDAC inhibitors induce the expression of claudin‐1 and ‐4 and ciliogenesis via p63 in normal and diseased nasal epithelium tissues. 129 The role of HDAC in barrier dysfunction and potential treatments using HDAC inhibitors are yet to be elucidated.
CpG sites are regions of DNA consisting of guanine and cytosine. The methylation of CpG sites plays an important role in the regulation of gene expression. We recently reported that an increased global methylation level in HBEC from asthmatic individuals. Interestingly, the inhibition of CpG methylation restores leakiness that was shown in the asthmatic epithelium. 130
Filaggrin loss‐of‐function mutations are associated with higher total IgE levels, more sensitizations, and a more severe course of AD. 131 , 132 In humans, individuals with reduced filaggrin gene expression levels exhibit early onset AD that is closely associated with asthma and food allergy, compared to subjects with normal filaggrin gene expression levels. 133 , 134 Renert‐Yuval et al showed comparative skin biopsy profiles of AD across different age groups and found that only adults had a significant reduction in filaggrin expression. 135 The human filaggrin gene (FLG) is polymorphic with an intragenic copy number variation. Specifically, 10, 11, or 12 nearly identical tandem repeats can be present in exon 3. Not only loss‐of‐function mutation, but also low copy numbers of filaggrin affect AD risk. 136
4.1. Detection of skin barrier dysfunction
Quantitative skin barrier assessment in vivo has potential value in many clinical applications, including diagnosis, follow‐up, prevention, and therapy evaluation. Several invasive and non‐invasive methods have been developed to study the barrier function. The measurement of skin hydration, colorimetry, skin surface pH, and sebometry are all examples of non‐invasive methods. However, these methods provide only limited information on different skin characteristics and conditions and do not directly measure the skin barrier function. 137 TEWL is the amount of water that evaporates passively from the surface of the skin and its measurement allows for quantification of skin barrier dysfunction. 138 However, TEWL can be affected by several environmental factors. Indeed, its accuracy is dependent on humidity, temperature, and skin hydration level. In diseased skin, TEWL is higher due to damage in the skin barrier and/or alterations in keratinization. 138 , 139
Confocal Raman spectroscopy is another non‐invasive method to characterize the molecular composition of the skin in vivo by irradiating the sample with low‐power monochromatic laser light. After the incident light excites the molecules within the tissue, the light is reflected in a wavelength characteristic of each skin component. The scattered light is then captured and analyzed, thus allowing non‐invasive in vivo measurements at different depths within the tissue. 140 Several studies have suggested that the confocal Raman spectroscopy is useful for the detection of FLG‐related AD skin barrier by assessing the reduction of natural moisturizing factors, which are driven from the breakdown of FLGs. 141 , 142 , 143 A recent study used confocal Raman spectroscopy to quantify the amount of water, ceramide, and urocanic acid in the skin, and found reduced levels of these components in atopic skin and a decreased lipid‐to‐protein ratio, suggesting the potential use of this technique for the stratification of AD patients. 144
A promising non‐invasive tool for detecting skin barrier function in vivo is represented by electrical impedance spectroscopy (EIS). The electrical impedance of a tissue measured at various frequencies provides information about its structure and integrity, reflecting its pathophysiological status because normal and pathologic tissues differ in cell size, shape, orientation, compactness, and membrane structures. 145 , 146 , 147 Current EIS applications include the diagnosis of malignant tissues in melanoma, breast tissue, prostate, and skin, and the characterization of tissue changes after ischemia. 147 , 148 , 149 , 150 Recently, Rinaldi et al demonstrated EIS as a tool for the characterization of the epithelial barrier function in vivo. 151 , 152 In the first group of studies in mice, EIS appeared to be a useful and reliable tool for detecting skin barrier defects. After experimentally damaging the skin barrier by tape stripping and epicutaneous application of proteases, such as papain, trypsin, and cholera toxin, a significant reduction in skin electrical impedance was observed. The findings were inversely correlated with TEWL. 151 In a clinical study of AD patients, EIS was used to measure the skin barrier status with good specificity and sensitivity between controls, non‐lesional skin of AD, and lesional skin of AD patients. 152 EIS was also useful in assessing skin lesion healing in response to treatment, correlating with the disease score SCORAD and pruritus score, confirming that EIS has clinical potential for objective disease assessment and lesion follow‐up in AD. 152 Furthermore, EIS measurements on the non‐lesional skin of AD patients correlated with the number of tandem repeats in the FLG gene. EIS has many potential clinical applications (Figure 3). We consider that it may be useful for identifying infants with a high risk of developing AD in a simple, fast and non‐invasive manner. Preventive therapies can be recommended to strengthen the skin barrier function and simultaneously avoiding exposure to environmental factors. There is a current need to develop novel tools for monitoring skin in AD patients to predict exacerbations and lesion follow‐up in response to therapy.
FIGURE 3.
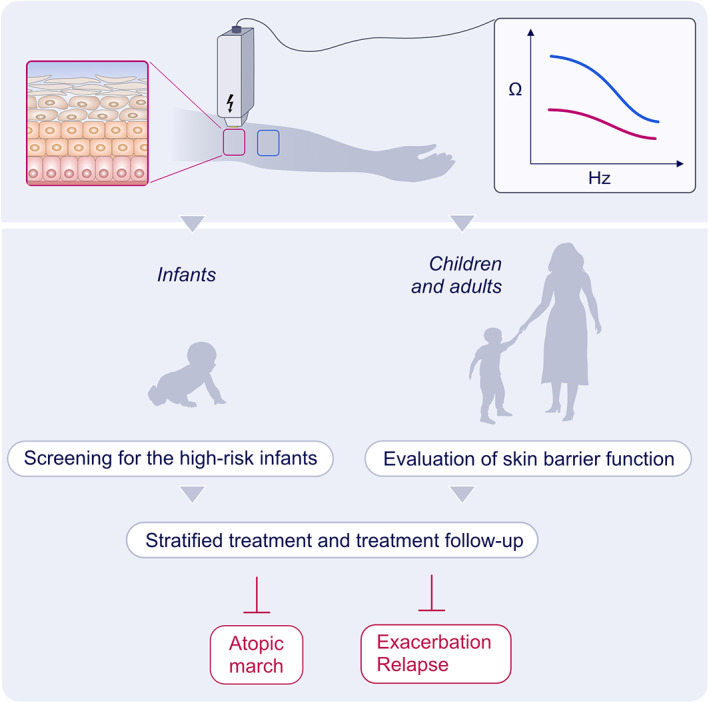
Detection of epithelial barrier function by electric impedance spectroscopy. Quantitative skin barrier assessment in vivo can provide valuable information for the prevention, diagnosis, and monitoring treatment response of AD patients. Electrical impedance spectroscopy (EIS) can be useful for identifying infants with a high risk of developing AD by measuring the integrity of the epithelial barrier. EIS can be a valuable tool for precision medicine to prevent the atopic march, exacerbation, and relapse of AD
5. CONCLUSION
A dysfunctional epithelial barrier might facilitate the uptake of allergens and exogenous particles, leading to lesional inflammation. Allergen entry through a leaky epithelial barrier may cause systemic atopic responses that contribute to the atopic march. Understanding the underlying mechanisms involved in epithelial dysfunction is essential for developing novel strategies for the prevention and treatment of allergic diseases.
There is a pressing need to improve our understanding of the factors and molecular mechanisms associated with “leaky epithelial barriers.” Experimental models and skin organoids should be developed to study the passage of allergens across a leaky epithelial barrier. There is strong evidence linking barrier leakiness and inflammatory skin diseases, which needs to be considered when developing novel strategies for its prevention, early intervention, and diagnosis. Numerous strategies target the epithelial barrier including avoidance and dose control of hazardous products together with the development of safer alternatives, clinical application of EIS for the identification of individuals with a leaky barrier, development of therapeutic approaches for restoring the epithelial barrier integrity, and interventions through diet and microbiome.
AUTHOR CONTRIBUTIONS
Yasutaka Mitamura: Conceptualization, methodology, visualization, writing – original draft (equal); writing – review and editing (equal). Ismail Ogulur: Conceptualization, methodology, visualization, writing – original draft, writing – review and editing. Yagiz Pat: Conceptualization, methodology, visualization, writing – original draft. Arturo Rinaldi: Conceptualization, methodology, visualization, writing – original draft. Ozge Ardicli: Conceptualization, methodology, visualization, writing – original draft. Lacin Cevhertas: Conceptualization, methodology, visualization, writing – original draft. Marie‐Charlotte Brüggen: Conceptualization, visualization, writing – review and editing. Claudia Traidl‐Hoffmann: Conceptualization, visualization, writing – review and editing. Mubeccel Akdis: Conceptualization, methodology, visualization, writing – review and editing. Cezmi Akdis: Conceptualization, methodology, supervision, visualization, writing – review and editing.
ACKNOWLEDGEMENTS
We would like to acknowledge Anna Głobińska for preparation of the figures. The authors have not received external grants for this work.
Open Access Funding provided by Universitat Zurich.
Mitamura Y, Ogulur I, Pat Y, et al. Dysregulation of the epithelial barrier by environmental and other exogenous factors. Contact Dermatitis. 2021;85(6):615-626. doi: 10.1111/cod.13959
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Akdis CA. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat Rev Immunol. 2021. [DOI] [PubMed] [Google Scholar]
- 2. Irvine AD, McLean WHI, DLeung DYM. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365(14):1315‐1327. [DOI] [PubMed] [Google Scholar]
- 3. De Benedetto A, Rafaels NM, McGirt LY, et al. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol. 2011;127(3):773‐786 e771‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Masterson JC, Biette KA, Hammer JA, et al. Epithelial HIF‐1alpha/claudin‐1 axis regulates barrier dysfunction in eosinophilic esophagitis. J Clin Invest. 2019;129(8):3224‐3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schoultz I, Keita AV. Cellular and molecular therapeutic targets in inflammatory bowel disease‐focusing on intestinal barrier function. Cells. 2019;8(2):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schumann M, Siegmund B, Schulzke JD, Fromm M. Celiac disease: role of the epithelial barrier. Cell Mol Gastroenterol Hepatol. 2017;3(2):150‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soyka MB, Wawrzyniak P, Eiwegger T, et al. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN‐gamma and IL‐4. J Allergy Clin Immunol. 2012;130(5):1087‐1096.e10. [DOI] [PubMed] [Google Scholar]
- 8. Sugita K, Altunbulakli C, Morita H, et al. Human type 2 innate lymphoid cells disrupt skin keratinocyte tight junction barrier by IL‐13. Allergy. 2019;74(12):2534‐2537. [DOI] [PubMed] [Google Scholar]
- 9. Xiao C, Puddicombe SM, Field S, et al. Defective epithelial barrier function in asthma. J Allergy Clin Immunol. 2011;128(3):549‐556 e541‐512. [DOI] [PubMed] [Google Scholar]
- 10. Steelant B, Farre R, Wawrzyniak P, et al. Impaired barrier function in patients with house dust mite‐induced allergic rhinitis is accompanied by decreased occludin and zonula occludens‐1 expression. J Allergy Clin Immunol. 2016;137(4):1043‐1053.e1045. [DOI] [PubMed] [Google Scholar]
- 11. Martin PE, Matheson MC, Gurrin L, et al. Childhood eczema and rhinitis predict atopic but not nonatopic adult asthma: a prospective cohort study over 4 decades. J Allergy Clin Immunol. 2011;127(6):1473‐1479.e1471. [DOI] [PubMed] [Google Scholar]
- 12. Davidson WF, Leung DYM, Beck LA, et al. Report from the National Institute of Allergy and Infectious Diseases workshop on “atopic dermatitis and the atopic march: mechanisms and interventions”. J Allergy Clin Immunol. 2019;143(3):894‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Borna E, Nwaru BI, Bjerg A, et al. Changes in the prevalence of asthma and respiratory symptoms in western Sweden between 2008 and 2016. Allergy. 2019;74(9):1703‐1715. [DOI] [PubMed] [Google Scholar]
- 14. Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL‐33‐responsive lineage‐ CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188(3):1503‐1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moro K, Yamada T, Tanabe M, et al. Innate production of T(H)2 cytokines by adipose tissue‐associated c‐kit(+)Sca‐1(+) lymphoid cells. Nature. 2010;463(7280):540‐544. [DOI] [PubMed] [Google Scholar]
- 16. Miller RL, Peden DB. Environmental effects on immune responses in patients with atopy and asthma. J Allergy Clin Immunol. 2014;134(5):1001‐1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bowatte G, Markevych I, Standl M, et al. Hygienic behavior and allergic sensitization in German adolescents. Allergy. 2018;73(9):1915‐1918. [DOI] [PubMed] [Google Scholar]
- 18. Caraballo JC, Yshii C, Westphal W, Moninger T, Comellas AP. Ambient particulate matter affects occludin distribution and increases alveolar transepithelial electrical conductance. Respirology. 2011;16(2):340‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jin Y, Lu L, Tu W, Luo T, Fu Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci Total Environ. 2019;649:308‐317. [DOI] [PubMed] [Google Scholar]
- 20. Leino MS, Loxham M, Blume C, et al. Barrier disrupting effects of alternaria alternata extract on bronchial epithelium from asthmatic donors. PLoS One. 2013;8(8):e71278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Michaudel C, Mackowiak C, Maillet I, et al. Ozone exposure induces respiratory barrier biphasic injury and inflammation controlled by IL‐33. J Allergy Clin Immunol. 2018;142(3):942‐958. [DOI] [PubMed] [Google Scholar]
- 22. Vita AA, Royse EA, Pullen NA. Nanoparticles and danger signals: oral delivery vehicles as potential disruptors of intestinal barrier homeostasis. J Leukoc Biol. 2019;106(1):95‐103. [DOI] [PubMed] [Google Scholar]
- 23. Wang M, Tan G, Eljaszewicz A, et al. Laundry detergents and detergent residue after rinsing directly disrupt tight junction barrier integrity in human bronchial epithelial cells. J Allergy Clin Immunol. 2019;143(5):1892‐1903. [DOI] [PubMed] [Google Scholar]
- 24. Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427‐434. [DOI] [PubMed] [Google Scholar]
- 25. Xian M, Wawrzyniak P, Ruckert B, et al. Anionic surfactants and commercial detergents decrease tight junction barrier integrity in human keratinocytes. J Allergy Clin Immunol. 2016;138(3):890‐893. e899. [DOI] [PubMed] [Google Scholar]
- 26. Gullikson GW, Cline WS, Lorenzsonn V, Benz L, Olsen WA, Bass P. Effects of anionic surfactants on hamster small intestinal membrane structure and function: relationship to surface activity. Gastroenterology. 1977;73(3):501‐511. [PubMed] [Google Scholar]
- 27. Roberts CL, Keita AV, Duncan SH, et al. Translocation of Crohn's disease Escherichia coli across M‐cells: contrasting effects of soluble plant fibres and emulsifiers. Gut. 2010;59(10):1331‐1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weng CM, Wang CH, Lee MJ, et al. Aryl hydrocarbon receptor activation by diesel exhaust particles mediates epithelium‐derived cytokines expression in severe allergic asthma. Allergy. 2018;73(11):2192‐2204. [DOI] [PubMed] [Google Scholar]
- 29. Kortekaas Krohn I, Seys SF, Lund G, et al. Nasal epithelial barrier dysfunction increases sensitization and mast cell degranulation in the absence of allergic inflammation. Allergy. 2019;75(5):1155‐1164. [DOI] [PubMed] [Google Scholar]
- 30. Simon D, Page B, Vogel M, et al. Evidence of an abnormal epithelial barrier in active, untreated and corticosteroid‐treated eosinophilic esophagitis. Allergy. 2018;73(1):239‐247. [DOI] [PubMed] [Google Scholar]
- 31. Khan SJ, Dharmage SC, Matheson MC, Gurrin LC. Is the atopic march related to confounding by genetics and early‐life environment? A systematic review of sibship and twin data. Allergy. 2018;73(1):17‐28. [DOI] [PubMed] [Google Scholar]
- 32. Zhu TH, Zhu TR, Tran KA, Sivamani RK, Shi VY. Epithelial barrier dysfunctions in atopic dermatitis: a skin‐gut‐lung model linking microbiome alteration and immune dysregulation. Br J Dermatol. 2018;179(3):570‐581. [DOI] [PubMed] [Google Scholar]
- 33. Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6(4):328‐340. [DOI] [PubMed] [Google Scholar]
- 34. Riethmuller C, McAleer MA, Koppes SA, et al. Filaggrin breakdown products determine corneocyte conformation in patients with atopic dermatitis. J Allergy Clin Immunol. 2015;136(6):1573‐1580.e1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Steinert PM, Marekov LN. The proteins elafin, filaggrin, keratin intermediate filaments, loricrin, and small proline‐rich proteins 1 and 2 are isodipeptide cross‐linked components of the human epidermal cornified cell envelope. J Biol Chem. 1995;270(30):17702‐17711. [DOI] [PubMed] [Google Scholar]
- 36. Luo Y, Wang S, Liu X, Wen H, Li W, Yao X. Langerhans cells mediate the skin‐induced tolerance to ovalbumin via Langerin in a murine model. Allergy. 2019;74(9):1738‐1747. [DOI] [PubMed] [Google Scholar]
- 37. Lambrecht BN, Hammad H, Fahy JV. The cytokines of asthma. Immunity. 2019;50(4):975‐991. [DOI] [PubMed] [Google Scholar]
- 38. Akdis CA, Arkwright PD, Brüggen MC, et al. Type 2 immunity in the skin and lungs. Allergy. 2020;75(7):1582‐1605. [DOI] [PubMed] [Google Scholar]
- 39. Altunbulakli C, Reiger M, Neumann AU, et al. Relations between epidermal barrier dysregulation and staphylococcus species‐dominated microbiome dysbiosis in patients with atopic dermatitis. J Allergy Clin Immunol. 2018;142(5):1643‐1647.e12. [DOI] [PubMed] [Google Scholar]
- 40. Breiteneder H, Peng YQ, Agache I, et al. Biomarkers for diagnosis and prediction of therapy responses in allergic diseases and asthma. Allergy. 2020;75(12):3039‐3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Agache I, Miller R, Gern JE, et al. Emerging concepts and challenges in implementing the exposome paradigm in allergic diseases and asthma: a Practall document. Allergy. 2019;74(3):449‐463. [DOI] [PubMed] [Google Scholar]
- 42. Stefanovic N, Flohr C, Irvine AD. The exposome in atopic dermatitis. Allergy. 2020;75(1):63‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aghapour M, Raee P, Moghaddam SJ, Hiemstra PS, Heijink IH. Airway epithelial barrier dysfunction in chronic obstructive pulmonary disease: role of cigarette smoke exposure. Am J Respir Cell Mol Biol. 2018;58(2):157‐169. [DOI] [PubMed] [Google Scholar]
- 44. Murrison LB, Brandt EB, Myers JB, Hershey GKK. Environmental exposures and mechanisms in allergy and asthma development. J Clin Invest. 2019;129(4):1504‐1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Venter C, Greenhawt M, Meyer RW, et al. EAACI position paper on diet diversity in pregnancy, infancy and childhood: novel concepts and implications for studies in allergy and asthma. Allergy. 2020;75(3):497‐523. [DOI] [PubMed] [Google Scholar]
- 46. Venter C, Meyer RW, Nwaru BI, et al. EAACI position paper: influence of dietary fatty acids on asthma, food allergy, and atopic dermatitis. Allergy. 2019;74(8):1429‐1444. [DOI] [PubMed] [Google Scholar]
- 47. Lopez DJ, Lodge CJ, Bui DS, et al. Association between ambient air pollution and development and persistence of atopic and non‐atopic eczema in a cohort of adults. Allergy. 2021;76(8):2524‐2534. [DOI] [PubMed] [Google Scholar]
- 48. Sokolowska M, Quesniaux VFJ, Akdis CA, Chung KF, Ryffel B, Togbe D. Acute respiratory barrier disruption by ozone exposure in mice. Front Immunol. 2019;10:2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dong CD, Chen CW, Chen YC, Chen HH, Lee JS, Lin CH. Polystyrene microplastic particles: in vitro pulmonary toxicity assessment. J Hazard Mater. 2020;385:121575. [DOI] [PubMed] [Google Scholar]
- 50. Zhu T, Zhang X, Chen X, et al. Nasal DNA methylation differentiates severe from non‐severe asthma in African‐American children. Allergy. 2020;76(6):1836‐1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fukuoka A, Matsushita K, Morikawa T, Takano H, Yoshimoto T. Diesel exhaust particles exacerbate allergic rhinitis in mice by disrupting the nasal epithelial barrier. Clin Exp Allergy. 2016;46(1):142‐152. [DOI] [PubMed] [Google Scholar]
- 52. Fukuoka A, Yoshimoto T. Barrier dysfunction in the nasal allergy. Allergol Int. 2018;67(1):18‐23. [DOI] [PubMed] [Google Scholar]
- 53. Hüls A, Abramson MJ, Sugiri D, et al. Nonatopic eczema in elderly women: effect of air pollution and genes. J Allergy Clin Immunol. 2019;143(1):378‐385.e379. [DOI] [PubMed] [Google Scholar]
- 54. Brandt EB, Bolcas PE, Ruff BP, Khurana Hershey GK. IL33 contributes to diesel pollution‐mediated increase in experimental asthma severity. Allergy. 2020;75(9):2254‐2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hendricks AJ, Eichenfield LF, Shi VY. The impact of airborne pollution on atopic dermatitis: a literature review. Br J Dermatol. 2020;183(1):16‐23. [DOI] [PubMed] [Google Scholar]
- 56. Farraia M, Cavaleiro Rufo J, Paciencia I, et al. Human volatilome analysis using eNose to assess uncontrolled asthma in a clinical setting. Allergy. 2020;75(7):1630‐1639. [DOI] [PubMed] [Google Scholar]
- 57. Dezest M, Le Bechec M, Chavatte L, et al. Oxidative damage and impairment of protein quality control systems in keratinocytes exposed to a volatile organic compounds cocktail. Sci Rep. 2017;7(1):10707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim J, Han Y, Ahn JH, et al. Airborne formaldehyde causes skin barrier dysfunction in atopic dermatitis. Br J Dermatol. 2016;175(2):357‐363. [DOI] [PubMed] [Google Scholar]
- 59. Uchida M, Anderson EL, Squillace DL, et al. Oxidative stress serves as a key checkpoint for IL‐33 release by airway epithelium. Allergy. 2017;72(10):1521‐1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Celebi Sözener Z, Cevhertas L, Nadeau K, Akdis M, Akdis CA. Environmental factors in epithelial barrier dysfunction. J Allergy Clin Immunol. 2020;145(6):1517‐1528. [DOI] [PubMed] [Google Scholar]
- 61. Adisesh A, Murphy E, Barber CM, Ayres JG. Occupational asthma and rhinitis due to detergent enzymes in healthcare. Occup Med. 2011;61(5):364‐369. [DOI] [PubMed] [Google Scholar]
- 62. Flindt ML. Pulmonary disease due to inhalation of derivatives of Bacillus subtilis containing proteolytic enzyme. Lancet. 1969;1(7607):1177‐1181. [DOI] [PubMed] [Google Scholar]
- 63. Erdem Y, Inal S, Sivaz O, et al. How does working in pandemic units affect the risk of occupational hand eczema in healthcare workers during the coronavirus disease‐2019 (COVID‐19) pandemic: a comparative analysis with nonpandemic units. Contact Dermatitis. 2021;85(2):215‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Simonsen AB, Ruge IF, Quaade AS, Johansen JD, Thyssen JP, Zachariae C. Increased occurrence of hand eczema in young children following the Danish hand hygiene recommendations during the COVID‐19 pandemic. Contact Dermatitis. 2021;84(3):144‐152. [DOI] [PubMed] [Google Scholar]
- 65. Dumas O, Wiley AS, Quinot C, et al. Occupational exposure to disinfectants and asthma control in US nurses. Eur Respir J. 2017;50(4):1700237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Folletti I, Siracusa A, Paolocci G. Update on asthma and cleaning agents. Curr Opin Allergy Clin Immunol. 2017;17(2):90‐95. [DOI] [PubMed] [Google Scholar]
- 67. Aguayo‐Patron SV, de la Barca AM C. Old fashioned vs. ultra‐processed‐based current diets: possible implication in the increased susceptibility to type 1 diabetes and celiac disease in childhood. Foods. 2017;6(11):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Roberts CL, Rushworth SL, Richman E, Rhodes JM. Hypothesis: increased consumption of emulsifiers as an explanation for the rising incidence of Crohn's disease. J Crohns Colitis. 2013;7(4):338‐341. [DOI] [PubMed] [Google Scholar]
- 69. Thaiss CA, Levy M, Grosheva I, et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science. 2018;359(6382):1376‐1383. [DOI] [PubMed] [Google Scholar]
- 70. Yu H, Yang J, Zhou X, Xiao Q, Lu Y, Xia L. High glucose induces dysfunction of airway epithelial barrier through down‐regulation of connexin 43. Exp Cell Res. 2016;342(1):11‐19. [DOI] [PubMed] [Google Scholar]
- 71. Heijink IH, Kuchibhotla VNS, Roffel MP, et al. Epithelial cell dysfunction, a major driver of asthma development. Allergy. 2020;75(8):1898‐1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Akbarshahi H, Menzel M, Ramu S, Mahmutovic Persson I, Bjermer L, Uller L. House dust mite impairs antiviral response in asthma exacerbation models through its effects on TLR3. Allergy. 2018;73(5):1053‐1063. [DOI] [PubMed] [Google Scholar]
- 73. Rouyar A, Classe M, Gorski R, et al. Type 2/Th2‐driven inflammation impairs olfactory sensory neurogenesis in mouse chronic rhinosinusitis model. Allergy. 2019;74(3):549‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hiraishi Y, Yamaguchi S, Yoshizaki T, et al. IL‐33, IL‐25 and TSLP contribute to development of fungal‐associated protease‐induced innate‐type airway inflammation. Sci Rep. 2018;8(1):18052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Li B, Zou Z, Meng F, et al. Dust mite‐derived Der f 3 activates a pro‐inflammatory program in airway epithelial cells via PAR‐1 and PAR‐2. Mol Immunol. 2019;109(2019):1‐11. [DOI] [PubMed] [Google Scholar]
- 76. Agache I, Lau S, Akdis CA, et al. EAACI guidelines on allergen immunotherapy: house dust mite‐driven allergic asthma. Allergy. 2019;74(5):855‐873. [DOI] [PubMed] [Google Scholar]
- 77. Jensen‐Jarolim E, Bachmann M, Bonini S, et al. State‐of‐the‐art in marketed adjuvants and formulations in allergen immunotherapy: a position paper of the European academy of allergy and clinical immunology (EAACI). Allergy. 2019;75(4):746‐760. [DOI] [PubMed] [Google Scholar]
- 78. Pfaar O, Agache I, de Blay F, et al. Perspectives in allergen immunotherapy: 2019 and beyond. Allergy. 2019;74(Suppl. 108):3‐25. [DOI] [PubMed] [Google Scholar]
- 79. van de Veen W, Akdis M. Tolerance mechanisms of allergen immunotherapy. Allergy. 2020;75(5):1017‐1018. [DOI] [PubMed] [Google Scholar]
- 80. Yuan X, Wang J, Li Y, et al. Allergy immunotherapy restores airway epithelial barrier dysfunction through suppressing IL‐25 ‐induced endoplasmic reticulum stress in asthma. Sci Rep. 2018;8(1):7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Vinhas R, Cortes L, Cardoso I, et al. Pollen proteases compromise the airway epithelial barrier through degradation of transmembrane adhesion proteins and lung bioactive peptides. Allergy. 2011;66(8):1088‐1098. [DOI] [PubMed] [Google Scholar]
- 82. Van Cleemput J, Poelaert KCK, Laval K, et al. Pollens destroy respiratory epithelial cell anchors and drive alphaherpesvirus infection. Sci Rep. 2019;9(1):4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Belkaid Y, Tamoutounour S. The influence of skin microorganisms on cutaneous immunity. Nat Rev Immunol. 2016;16(6):353‐366. [DOI] [PubMed] [Google Scholar]
- 84. Karisola P, Suomalainen A, Fortino V, et al. Tape‐stripping alters the microbe‐host correlations in mouse skin. Allergy. 2019;74(3):617‐621. [DOI] [PubMed] [Google Scholar]
- 85. Gong JQ, Lin L, Lin T, et al. Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: a double‐blind multicentre randomized controlled trial. Br J Dermatol. 2006;155(4):680‐687. [DOI] [PubMed] [Google Scholar]
- 86. Gonzalez T, Stevens ML, Baatyrbek Kyzy A, et al. Biofilm propensity of Staphylococcus aureus skin isolates is associated with increased atopic dermatitis severity and barrier dysfunction in the MPAACH pediatric cohort. Allergy. 2021;76(1):302‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ottman N, Barrientos‐Somarribas M, Fyhrquist N, et al. Microbial and transcriptional differences elucidate atopic dermatitis heterogeneity across skin sites. Allergy. 2021;76(4):1173‐1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Altunbulakli C, Costa R, Lan F, et al. Staphylococcus aureus enhances the tight junction barrier integrity in healthy nasal tissue, but not in nasal polyps. J Allergy Clin Immunol. 2018;142(2):665‐668.e668. [DOI] [PubMed] [Google Scholar]
- 89. Clausen ML, Agner T, Lilje B, Edslev SM, Johannesen TB, Andersen PS. Association of disease severity with skin microbiome and filaggrin gene mutations in adult atopic dermatitis. JAMA Dermatol. 2018;154(3):293‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Krysko O, Teufelberger A, Van Nevel S, Krysko DV, Bachert C. Protease/antiprotease network in allergy: the role of Staphylococcus aureus protease‐like proteins. Allergy. 2019;74(11):2077‐2086. [DOI] [PubMed] [Google Scholar]
- 91. Boutin RCT, Sbihi H, Dsouza M, et al. Mining the infant gut microbiota for therapeutic targets against atopic disease. Allergy. 2020;75(8):2065‐2068. [DOI] [PubMed] [Google Scholar]
- 92. Roßberg S, Keller T, Icke K, et al. Orally applied bacterial lysate in infants at risk for atopy does not prevent atopic dermatitis, allergic rhinitis, asthma or allergic sensitization at school age: follow‐up of a randomized trial. Allergy. 2020;75(8):2016‐2021. [DOI] [PubMed] [Google Scholar]
- 93. Kuo IH, Carpenter‐Mendini A, Yoshida T, et al. Activation of epidermal toll‐like receptor 2 enhances tight junction function: implications for atopic dermatitis and skin barrier repair. J Invest Dermatol. 2013;133(4):988‐998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ragupathy S, Esmaeili F, Paschoud S, Sublet E, Citi S, Borchard G. Toll‐like receptor 2 regulates the barrier function of human bronchial epithelial monolayers through atypical protein kinase C zeta, and an increase in expression of claudin‐1. Tissue Barriers. 2014;2:e29166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ruffner MA, Song L, Maurer K, et al. Toll‐like receptor 2 stimulation augments esophageal barrier integrity. Allergy. 2019;74(12):2449‐2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Iwamoto K, Numm TJ, Koch S, Herrmann N, Leib N, Bieber T. Langerhans and inflammatory dendritic epidermal cells in atopic dermatitis are tolerized toward TLR2 activation. Allergy. 2018;73(11):2205‐2213. [DOI] [PubMed] [Google Scholar]
- 97. Kubo T, Wawrzyniak P, Morita H, et al. CpG‐DNA enhances the tight junction integrity of the bronchial epithelial cell barrier. J Allergy Clin Immunol. 2015;136(5):1413‐1416.e1‐8. [DOI] [PubMed] [Google Scholar]
- 98. Martens K, Pugin B, De Boeck I, et al. Probiotics for the airways: potential to improve epithelial and immune homeostasis. Allergy. 2018;73(10):1954‐1963. [DOI] [PubMed] [Google Scholar]
- 99. Zimmermann M, Koreck A, Meyer N, et al. TNF‐like weak inducer of apoptosis (TWEAK) and TNF‐alpha cooperate in the induction of keratinocyte apoptosis. J Allergy Clin Immunol. 2011;127(1):200‐207.e1‐10. [DOI] [PubMed] [Google Scholar]
- 100. Wawrzyniak P, Wawrzyniak M, Wanke K, et al. Regulation of bronchial epithelial barrier integrity by type 2 cytokines and histone deacetylases in asthmatic patients. J Allergy Clin Immunol. 2017;139(1):93‐103. [DOI] [PubMed] [Google Scholar]
- 101. Saatian B, Rezaee F, Desando S, et al. Interleukin‐4 and interleukin‐13 cause barrier dysfunction in human airway epithelial cells. Tissue Barriers. 2013;1(2):e24333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sweerus K, Lachowicz‐Scroggins M, Gordon E, et al. Claudin‐18 deficiency is associated with airway epithelial barrier dysfunction and asthma. J Allergy Clin Immunol. 2017;139(1):72‐81.e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Agache I, Rocha C, Beltran J, et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab and omalizumab) for severe allergic asthma: a systematic review for the EAACI Guidelines ‐ recommendations on the use of biologicals in severe asthma. Allergy. 2020;75(5):1043‐1057. [DOI] [PubMed] [Google Scholar]
- 104. Agache I, Beltran J, Akdis C, et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines ‐ recommendations on the use of biologicals in severe asthma. Allergy. 2020;75(5):1023‐1042. [DOI] [PubMed] [Google Scholar]
- 105. Agache I, Song Y, Rocha C, et al. Efficacy and safety of treatment with dupilumab for severe asthma: a systematic review of the EAACI guidelines‐recommendations on the use of biologicals in severe asthma. Allergy. 2020;75(5):1058‐1068. [DOI] [PubMed] [Google Scholar]
- 106. Le Floc'h A, Allinne J, Nagashima K, et al. Dual blockade of IL‐4 and IL‐13 with dupilumab, an IL‐4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy. 2020;75(5):1188‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Agache I, Akdis CA, Akdis M, et al. EAACI Biologicals guidelines‐dupilumab for children and adults with moderate‐to‐severe atopic dermatitis. Allergy. 2021;76(4):988‐1009. [DOI] [PubMed] [Google Scholar]
- 108. Agache I, Song Y, Posso M, et al. Efficacy and safety of dupilumab for moderate‐to‐severe atopic dermatitis: a systematic review for the EAACI biologicals guidelines. Allergy. 2021;76(1):45‐58. [DOI] [PubMed] [Google Scholar]
- 109. Siegels D, Heratizadeh A, Abraham S, et al. Systemic treatments in the management of atopic dermatitis: a systematic review and meta‐analysis. Allergy. 2021;76(4):1053‐1076. [DOI] [PubMed] [Google Scholar]
- 110. Mitamura Y, Nunomura S, Nanri Y, et al. The IL‐13/periostin/IL‐24 pathway causes epidermal barrier dysfunction in allergic skin inflammation. Allergy. 2018;73(9):1881‐1891. [DOI] [PubMed] [Google Scholar]
- 111. He H, Suryawanshi H, Morozov P, et al. Single‐cell transcriptome analysis of human skin identifies novel fibroblast subpopulation and enrichment of immune subsets in atopic dermatitis. J Allergy Clin Immunol. 2020;145(6):1615‐1628. [DOI] [PubMed] [Google Scholar]
- 112. Rojahn TB, Vorstandlechner V, Krausgruber T, et al. Single‐cell transcriptomics combined with interstitial fluid proteomics defines cell type‐specific immune regulation in atopic dermatitis. J Allergy Clin Immunol. 2020;146(5):1056‐1069. [DOI] [PubMed] [Google Scholar]
- 113. Brüggen MC, Bauer WM, Reininger B, et al. In situ mapping of innate lymphoid cells in human skin: evidence for remarkable differences between normal and inflamed skin. J Invest Dermatol. 2016;136(12):2396‐2405. [DOI] [PubMed] [Google Scholar]
- 114. Doherty TA, Broide DH. Airway innate lymphoid cells in the induction and regulation of allergy. Allergol Int. 2019;68(1):9‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Yi L, Cheng D, Zhang K, et al. Intelectin contributes to allergen‐induced IL‐25, IL‐33, and TSLP expression and type 2 response in asthma and atopic dermatitis. Mucosal Immunol. 2017;10(6):1491‐1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Xiong Y, Cui X, Li W, et al. BLT1 signaling in epithelial cells mediates allergic sensitization via promotion of IL‐33 production. Allergy. 2019;74(3):495‐506. [DOI] [PubMed] [Google Scholar]
- 117. Sugita K, Steer CA, Martinez‐Gonzalez I, et al. Type 2 innate lymphoid cells disrupt bronchial epithelial barrier integrity by targeting tight junctions through IL‐13 in asthmatic patients. J Allergy Clin Immunol. 2018;141(1):300‐310.e311. [DOI] [PubMed] [Google Scholar]
- 118. Elieh Ali Komi D, Bjermer L. Mast cell‐mediated orchestration of the immune responses in human allergic asthma: current insights. Clin Rev Allergy Immunol. 2019;56(2):234‐247. [DOI] [PubMed] [Google Scholar]
- 119. Gschwandtner M, Mildner M, Mlitz V, et al. Histamine suppresses epidermal keratinocyte differentiation and impairs skin barrier function in a human skin model. Allergy. 2013;68(1):37‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Steelant B, Seys SF, Van Gerven L, et al. Histamine and T helper cytokine‐driven epithelial barrier dysfunction in allergic rhinitis. J Allergy Clin Immunol. 2018;141(3):951‐963.e958. [DOI] [PubMed] [Google Scholar]
- 121. Choi Y, Lee DH, Trinh HKT, et al. Surfactant protein D alleviates eosinophil‐mediated airway inflammation and remodeling in patients with aspirin‐exacerbated respiratory disease. Allergy. 2019;74(1):78‐88. [DOI] [PubMed] [Google Scholar]
- 122. Horikami D, Toya N, Kobayashi K, Omori K, Nagata N, Murata T. L‐PGDS‐derived PGD2 attenuates acute lung injury by enhancing endothelial barrier formation. J Pathol. 2019;248(3):280‐290. [DOI] [PubMed] [Google Scholar]
- 123. Rittchen S, Heinemann A. Therapeutic potential of hematopoietic prostaglandin D2 synthase in allergic inflammation. Cells. 2019;8(6):619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zhou X, Wei T, Cox CW, Jiang Y, Roche WR, Walls AF. Mast cell chymase impairs bronchial epithelium integrity by degrading cell junction molecules of epithelial cells. Allergy. 2019;74(7):1266‐1276. [DOI] [PubMed] [Google Scholar]
- 125. Kortekaas Krohn I, Seys SF, Lund G, et al. Nasal epithelial barrier dysfunction increases sensitization and mast cell degranulation in the absence of allergic inflammation. Allergy. 2020;75(5):1155‐1164. [DOI] [PubMed] [Google Scholar]
- 126. Stadhouders R, Li BWS, de Bruijn MJW, et al. Epigenome analysis links gene regulatory elements in group 2 innate lymphocytes to asthma susceptibility. J Allergy Clin Immunol. 2018;142(6):1793‐1807. [DOI] [PubMed] [Google Scholar]
- 127. Yang IV, Pedersen BS, Liu A, et al. DNA methylation and childhood asthma in the inner city. J Allergy Clin Immunol. 2015;136(1):69‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Nicodemus‐Johnson J, Naughton KA, Sudi J, et al. Genome‐wide methylation study identifies an IL‐13‐induced epigenetic signature in asthmatic airways. Am J Respir Crit Care Med. 2016;193(4):376‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kaneko Y, Kohno T, Kakuki T, et al. The role of transcriptional factor p63 in regulation of epithelial barrier and ciliogenesis of human nasal epithelial cells. Sci Rep. 2017;7(1):10935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wawrzyniak P, Krawczyk K, Acharya S, et al. Inhibition of CpG methylation improves the barrier integrity of bronchial epithelial cells in asthma. Allergy. 2020;76(6):1864‐1868. [DOI] [PubMed] [Google Scholar]
- 131. Baurecht H, Irvine AD, Novak N, et al. Toward a major risk factor for atopic eczema: meta‐analysis of filaggrin polymorphism data. J Allergy Clin Immunol. 2007;120(6):1406‐1412. [DOI] [PubMed] [Google Scholar]
- 132. Palmer CN, Irvine AD, Terron‐Kwiatkowski A, et al. Common loss‐of‐function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38(4):441‐446. [DOI] [PubMed] [Google Scholar]
- 133. Marenholz I, Kerscher T, Bauerfeind A, et al. An interaction between filaggrin mutations and early food sensitization improves the prediction of childhood asthma. J Allergy Clin Immunol. 2009;123(4):911‐916. [DOI] [PubMed] [Google Scholar]
- 134. Venkataraman D, Soto‐Ramirez N, Kurukulaaratchy RJ, et al. Filaggrin loss‐of‐function mutations are associated with food allergy in childhood and adolescence. J Allergy Clin Immunol. 2014;134(4):876‐882.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Renert‐Yuval Y, Del Duca E, Pavel AB, et al. The molecular features of normal and atopic dermatitis skin in infants, children, adolescents, and adults. J Allergy Clin Immunol. 2021;148(1):148‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Brown SJ, Kroboth K, Sandilands A, et al. Intragenic copy number variation within filaggrin contributes to the risk of atopic dermatitis with a dose‐dependent effect. J Invest Dermatol. 2012;132(1):98‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Antonov D, Schliemann S, Elsner P. Methods for the assessment of barrier function. Curr Probl Dermatol. 2016;49:61‐70. [DOI] [PubMed] [Google Scholar]
- 138. Berardesca E, Loden M, Serup J, Masson P, Rodrigues LM. The revised EEMCO guidance for the in vivo measurement of water in the skin. Skin Res Technol. 2018;24(3):351‐358. [DOI] [PubMed] [Google Scholar]
- 139. Cork MJ, Danby SG, Vasilopoulos Y, et al. Epidermal barrier dysfunction in atopic dermatitis. J Invest Dermatol. 2009;129(8):1892‐1908. [DOI] [PubMed] [Google Scholar]
- 140. Binder L, SheikhRezaei S, Baierl A, Gruber L, Wolzt M, Valenta C. Confocal Raman spectroscopy: in vivo measurement of physiological skin parameters ‐ a pilot study. J Dermatol Sci. 2017;88(3):280‐288. [DOI] [PubMed] [Google Scholar]
- 141. Gonzalez FJ, Alda J, Moreno‐Cruz B, et al. Use of Raman spectroscopy for the early detection of filaggrin‐related atopic dermatitis. Skin Res Technol. 2011;17(1):45‐50. [DOI] [PubMed] [Google Scholar]
- 142. O'Regan GM, Kemperman PM, Sandilands A, et al. Raman profiles of the stratum corneum define 3 filaggrin genotype‐determined atopic dermatitis endophenotypes. J Allergy Clin Immunol. 2010;126(3):574‐580.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Hoste E, Kemperman P, Devos M, et al. Caspase‐14 is required for filaggrin degradation to natural moisturizing factors in the skin. J Invest Dermatol. 2011;131(11):2233‐2241. [DOI] [PubMed] [Google Scholar]
- 144. Ho CJH, Yew YW, Dinish US, et al. Handheld confocal Raman spectroscopy (CRS) for objective assessment of skin barrier function and stratification of severity in atopic dermatitis (AD) patients. J Dermatol Sci. 2020;98(1):20‐25. [DOI] [PubMed] [Google Scholar]
- 145. Dean DA, Ramanathan T, Machado D, Sundararajan R. Electrical impedance spectroscopy study of biological tissues. J Electrostat. 2008;66(3–4):165‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Birgersson U, Birgersson E, Aberg P, Nicander I, Ollmar S. Non‐invasive bioimpedance of intact skin: mathematical modeling and experiments. Physiol Meas. 2011;32(1):1‐18. [DOI] [PubMed] [Google Scholar]
- 147. Mohr P, Birgersson U, Berking C, et al. Electrical impedance spectroscopy as a potential adjunct diagnostic tool for cutaneous melanoma. Skin Res Technol. 2013;19(2):75‐83. [DOI] [PubMed] [Google Scholar]
- 148. Kerner TE, Paulsen KD, Hartov A, Soho SK, Poplack SP. Electrical impedance spectroscopy of the breast: clinical imaging results in 26 subjects. IEEE Trans Med Imaging. 2002;21(6):638‐645. [DOI] [PubMed] [Google Scholar]
- 149. Halter RJ, Hartov A, Heaney JA, Paulsen KD, Schned AR. Electrical impedance spectroscopy of the human prostate. IEEE Trans Biomed Eng. 2007;54(7):1321‐1327. [DOI] [PubMed] [Google Scholar]
- 150. Beltran NE, Sacristan E. Gastrointestinal ischemia monitoring through impedance spectroscopy as a tool for the management of the critically ill. Exp Biol Med (Maywood). 2015;240(7):835‐845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Rinaldi AO, Morita H, Wawrzyniak P, et al. Direct assessment of skin epithelial barrier by electrical impedance spectroscopy. Allergy. 2019;74(10):1934‐1944. [DOI] [PubMed] [Google Scholar]
- 152. Rinaldi AO, Korsfeldt A, Ward S, et al. Electrical impedance spectroscopy for the characterization of skin barrier in atopic dermatitis. Allergy. 2021; Online ahead of print. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


