Abstract
Background
Systemic sclerosis (SSc) is a multiorgan autoimmune disease characterized by inflammation, vascular modification, and progressive fibrosis of the skin and several visceral organs. Innate and adaptive immune cells, including myeloid, B and T cells, are believed to be central to the pathogenesis of SSc. However, the role and functional state of neutrophil granulocytes (neutrophils) are ill‐defined in SSc.
Methods
We performed a prospective study of neutrophils freshly isolated from SSc patients and healthy donors (HD) by measuring in these neutrophils (i) functional cell surface markers, including CD16, CD62L, CD66b, CD66c, CXCR1, CXCR2, and CXCR4; (ii) cytokine‐activated intracellular signal transducer and activator of transcription (STAT) pathways, such as phosphorylated STAT3 (pSTAT3), pSTAT5, and pSTAT6; (iii) production of neutrophil extracellular traps (NET) and intracellular myeloperoxidase (MPO); and (iv) phagocytosis of bacteria by the neutrophils.
Results
Neutrophils of SSc patients expressed lower CD16 and CD62L and higher pSTAT3 and pSTAT6 compared to HD. Moreover, neutrophils of SSc patients lacked CXCR1 and CXCR2, the receptors responding to the potent neutrophil chemoattractant CXCL8. Neutrophils of SSc patients were also deficient in MPO levels, NET formation, and phagocytosis of bacteria.
Conclusions
Neutrophils of patients with SSc display several functional defects affecting cell migration, NET formation, and phagocytosis of bacteria.
Keywords: IL‐4 receptor, inflammation, innate immunity, neutrophil, systemic sclerosis
Neutrophils of patients with systemic sclerosis (SSc) display a distinct phenotype. Systemic sclerosis neutrophils carry reduced chemokine receptors for CXCL8, which is important for migration to inflamed tissues. Systemic sclerosis neutrophils show deficient neutrophil extracellular trap formation and phagocytosis of bacteria.
Abbreviations: CXCR1/2, C‐X‐C motif chemokine receptor 1 and 2; NET, neutrophil extracellular trap; Neu, neutrophil; SSc, systemic sclerosis
1. INTRODUCTION
Systemic sclerosis (SSc) is an autoimmune disease characterized by damage to the skin and visceral organs due to inflammation, fibrosis, and vascular modification. 1 Commonly affected organs are the skin, lungs, heart, gastrointestinal tract, and kidneys, which determine the morbidity and mortality of SSc patients.
Several lines of evidence suggest that dysregulated innate and adaptive immune responses are central to the pathogenesis of SSc. 2 Autoimmune B‐cell responses result in the production of autoantibodies directed at different self‐antigens in the nucleus, targeting topoisomerase I, centromeres, RNA polymerase III, fibrillarin, and the nucleolus. 1 Several T helper (Th) cell subsets have been reported to be increased or activated in the peripheral blood of SSc patients, including interleukin (IL)‐4–secreting type 2 Th (Th2) and CD4+ CD8+ double‐positive T cells as well as IL‐17– and IL‐22–producing Th17 and Th22 cells. 3 , 4 , 5 , 6 However, other groups have also obtained different results for these Th cell subsets. 7 More recently, monocytes, macrophages, and dendritic cells have been implicated in the pathogenesis of SSc. 2 However, the functional state and role of neutrophils in SSc are presently ill‐defined and unclear.
Only a few publications have previously assessed neutrophils in SSc, and they suggested that, in comparison with their counterparts from healthy donors (HD), freshly isolated neutrophils from SSc patients produced less reactive oxygen species (ROS), whereas SSc neutrophils activated in vitro produced either more or less ROS. 8 , 9 , 10 Furthermore, they showed unaffected phagocytosis and reduced chemotaxis to zymosan‐induced serum in neutrophils of SSc patients. 8 , 11
We have recently identified a regulatory mechanism in mouse and human neutrophils resulting in the inhibition of effector functions in neutrophils receiving IL‐4 receptor (IL‐4R) signaling. 12 , 13 , 14 Although neutrophils are typically absent in IL‐4R signaling‐driven type 2 immune responses, neutrophils have been observed and implicated in the early stages of type 2 immunity. 15 , 16 Thus, as in type 1 and 3 immunity, also in type 2 immune responses, neutrophils are the first non‐resident immune cells migrating to a site of inflammation or infection, which is associated with the release of granulocyte colony‐stimulating factor (G‐CSF, also known as colony‐stimulating factor 3). Sensing of G‐CSF by neutrophils resulted in the activation of signal transducer and activator of transcription 3 (STAT3) and upregulation of IL‐4Rα (CD124), IL‐2Rγ (CD132), and IL‐13Rα1 (CD213a1), 12 , 13 which form the type I (CD124–CD132) and type II (CD124–CD213a1) IL‐4R. 16 Subsequent stimulation of neutrophils by IL‐4 (binds to both type I and II IL‐4Rs) or IL‐13 (associates only with type II IL‐4Rs) caused signaling through STAT6 followed by decreased surface abundance of C‐X‐C chemokine receptors CXCR1 (in human) and CXCR2 (in mouse and human). 12 , 13 , 14 As CXCR1 and CXCR2 enable human neutrophils to sense the potent neutrophil chemoattractant CXCL8 (previously called IL‐8), IL‐4R–activated neutrophils were deficient in migrating toward CXCL8 in vitro and in vivo. 12 , 13 , 14 Similarly, in mouse models of inflammatory arthritis, treatment with IL‐4 inhibited myeloid immune cells in vivo, including neutrophils. 17 , 18 , 19 Moreover, stimulation of neutrophils via the IL‐4R–STAT6 pathway reduced their capacity to release neutrophil extracellular traps (NET), the latter of which constitutes a key effector mechanism of neutrophils. 13 These results suggest that even though neutrophils appear to be present and functional in the initial phases of type 2 immunity and help recruit other immune cells, once the type 2 immune cytokines IL‐4 and IL‐13 predominate the inflammatory milieu, neutrophil effector functions become rapidly subdued. 16 Whether neutrophils stimulated via their IL‐4Rs display also other functional deficits, such as decreased phagocytosis of bacteria, is currently unknown. 16
As aforementioned, SSc patients have been reported to show increased IL‐17, which is known to induce G‐CSF‐mediated neutrophilia. 20 Conversely, human and preclinical data have suggested that SSc might feature increased IL‐4R‐mediated immune responses, 3 , 4 , 5 , 6 , 21 which can dampen neutrophil functions, as outlined above. Thus, we set out to conduct a detailed assessment of phenotypic and functional properties of primary human neutrophils freshly isolated from SSc patients vs. HDs.
2. METHODS
2.1. Human subjects and patient partnership
Following written informed consent, volunteers were recruited for donating blood, which was immediately processed to isolate neutrophils and serum. SSc patients fulfilled the 2013 American College of Rheumatology and European League Against Rheumatism classification criteria. 22 Their characteristics are given in Tables 1 and 2, and Table S1. The study was approved by the Cantonal Ethics Committee (#2016‐01440).
TABLE 1.
Overview of SSc patients included in this study
| Systemic sclerosis (SSc)–no. | 24 |
|---|---|
| Age–years | |
| Median age (range) | 60 (31–87) |
| Average age ± SD | 61.8 ± 11.9 |
| Gender–no. (%) | |
| Female | 5 (20.8) |
| Male | 19 (79.2) |
| SSc subtype–no. (%) | |
| Limited cutaneous | 18 (75) |
| Diffuse cutaneous | 3 (12.5) |
| Very early | 3 (12.5) |
| Type of autoantibodies–no. (%) | |
| ANA positive | 24 (100) |
| ACA positive | 11 (45.8) |
| Scl70 positive | 5 (20.8)* |
| U1‐RNP positive | 1 (4.2)* |
| RNA polymerase III positive | 2 (8.3)* |
| Clinical information and immunosuppression–no. (%) | |
| Infection in past 4 weeks | 3 (12.5) |
| Immunosuppressive treatment | 0 (0) |
The asterisks indicate the information of these autoantibodies was missing for one patient.
Abbreviations: ACA, anti‐centromer antibodies; ANA, anti‐nuclear antibodies; Scl70, anti‐Scl70 antibodies (also termed anti‐topoisomerase I antibodies); SSc, systemic sclerosis; U1‐RNP, anti‐U1 ribonucleoprotein antibodies.
TABLE 2.
Detailed characteristics of SSc patients included in this study
| ID | SSc subtype | Age | Gender | CRP | Neutrophils | Infection in past 4 weeks | Immunosuppression 4 months before and during visit | AutoAbs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| f | m | % | # | ANA pos | ACA pos | Scl70 pos | U1‐RNP pos | RNA polymerase III pos | ||||||
| SSc‐001 | Limited cutaneous SSc | 75 | x | 2.10 | 57.60 | 2.72 | No | No | Yes | No | No | No | Yes | |
| SSc‐002 | Diffuse cutaneous SSc | 58 | x | < 0.3 | 69.40 | 4.34 | No | No | Yes | No | Yes | No | No | |
| SSc‐003 | Limited cutaneous SSc | 60 | x | 89 | 83.2 | 13.8 | Yes | No | Yes | No | No | No | No | |
| SSc‐004 | Limited cutaneous SSc | 78 | x | 2.4 | 81.3 | 5.46 | No | No | Yes | Yes | No | No | No | |
| SSc‐005 | Limited cutaneous SSc | 64 | x | 1.3 | 67.2 | 2.14 | No | No | Yes | No | No | Yes | No | |
| SSc‐006 | Limited cutaneous SSc | 60 | x | 3.7 | 83.2 | 9.08 | No | No | Yes | No | Yes | No | No | |
| SSc‐007 | Very early SSc | 58 | x | 15 | 50.9 | 3.19 | No | No | Yes | No | No | No | No | |
| SSc‐008 | Very early SSc | 49 | x | 2.3 | 62.6 | 5.06 | No | No | Yes | Yes | No | No | No | |
| SSc‐009 | Limited cutaneous SSc | 87 | x | 18 | 75.7 | 4.65 | No | No | Yes | Yes | No | No | No | |
| SSc‐010 | Limited cutaneous SSc | 75 | x | 2.4 | 65.1 | 4.48 | No | No | Yes | Yes | No | No | No | |
| SSc‐011 | Very early SSc | 55 | x | 4.6 | 77.4 | 5.16 | No | No | Yes | Yes | No | No | No | |
| SSc‐012 | Limited cutaneous SSc | 59 | x | 52 | 85.9 | 4.96 | Yes | No | Yes | No | No | No | No | |
| SSc‐013 | Limited cutaneous SSc | 67 | x | 5.5 | 51.3 | 3.63 | No | No | Yes | Yes | No | No | No | |
| SSc‐014 | Diffuse cutaneous SSc | 68 | x | 5.1 | 79.4 | 5.66 | No | No | Yes | No | No | No | Yes | |
| SSc‐015 | Limited cutaneous SSc | 59 | x | 1.2 | 43 | 2.37 | No | No | Yes | Yes | No | No | No | |
| SSc‐016 | Limited cutaneous SSc | 50 | x | 1.8 | 64.3 | 3.06 | No | No | Yes | Yes | No | No | No | |
| SSc‐017 | Limited cutaneous SSc | 80 | x | 2.3 | 59.5 | 2.77 | No | No | Yes | No | No | No | No | |
| SSc‐018 | Limited cutaneous SSc | 31 | x | 0.9 | 61.6 | 3.67 | No | No | Yes | No | Yes | No | No | |
| SSc‐019 | Limited cutaneous SSc | 63 | x | 2.1 | 64.7 | 2.81 | No | No | Yes | Yes | Unknown | Unknown | Unknown | |
| SSc‐020 | Limited cutaneous SSc | 64 | x | 1.6 | 62.8 | 4.12 | No | No | Yes | Yes | No | No | No | |
| SSc‐021 | Diffuse cutaneous SSc | 56 | x | 1.6 | 74.3 | 3.89 | No | No | Yes | No | Yes | No | No | |
| SSc‐022 | Limited cutaneous SSc | 51 | x | 2 | 64 | 5.55 | Yes | No | Yes | No | Yes | No | No | |
| SSc‐023 | Limited cutaneous SSc | 51 | x | 1.7 | 65.1 | 4.03 | No | No | Yes | No | No | No | No | |
| SSc‐024 | Limited cutaneous SSc | 64 | x | 5.2 | 60.6 | 5.3 | No | No | Yes | Yes | No | No | No | |
Extended data from Table 1. Red font highlights those patients that showed signs of active infection in the 4 weeks prior to blood sampling, which were excluded from our analysis. Neutrophil percentages (%) and counts (#) are shown, with counts being expressed as number of cells * 106. Unknown denotes the information of these autoantibodies was missing for the indicated patient.
Abbreviations: AutoAbs, autoantibodies; CRP, C‐reactive protein (in mg/ml); f, female; m, male; pos, positive.
2.2. Isolation of neutrophils
Neutrophils were purified as previously described, 13 achieving more than 97% cell viability (Figure S1) and more than 96% purity. In brief, venous blood samples were collected in ethylene‐diamine‐tetraacetic acid tubes (BD Vacutainer). Neutrophils were purified with HetaSep (Stemcell Technologies), followed by negative magnetic selection using the EasySep Direct Human Neutrophil Isolation Kit (Stemcell Technologies). Neutrophils were resuspended in RPMI 1640 supplemented with 1% fetal bovine serum (FBS; Thermo Fisher) before use in experiments. Neutrophil counts and percentages were determined in full blood by using an automated system (Abbott Diagnostics).
2.3. In vitro stimulation of neutrophils
Freshly isolated neutrophils were stimulated as previously published. 13 Briefly, neutrophils were resuspended in RPMI 1640 supplemented with 1% FBS. Where indicated, neutrophils were stimulated with recombinant human G‐CSF (150 ng/ml) for 6 h. Viability was assessed using the Annexin V Apoptosis Detection kit (BD Biosciences) in combination with propidium iodide (PI). Samples were acquired on a BD LSR Fortessa and analyzed using FlowJo software (Tristar).
2.4. Transwell migration assay
Transwell migration assays were performed as previously published. 13 Briefly, freshly isolated neutrophils were incubated for 2 h at 37°C in 5% CO2 with medium (RPMI 1640 containing 1% of FBS) supplemented with serum from HD or SSc. Subsequently, neutrophils, seeded into the upper chamber of a 3 μm transwell (Corning Costar), migrated toward CXCL8 (PeproTech), which was added to the lower chamber. Migration was determined after 120 min by counting the neutrophils in the lower chamber using flow cytometry counting beads (123count eBeads, ThermoFisher).
2.5. Flow cytometry
Single‐cell suspensions of neutrophils were processed for analysis of extracellular and intracellular markers by flow cytometry, as previously described. 13 , 23 Briefly, isolated neutrophils were stained for using fluorochrome‐conjugated mAbs directed against the following human antigens (from BioLegend unless otherwise stated; clone names in parentheses): CD11b (ICRF 44), CD16 (3G8), CD35 (E11), CD61 (VI‐PL2), CD63 (H5C6), CD66b (G10F5), CD62L (DREG‐56), CD66c (B6.2), CD114 (LMM741), CD124 (G077F6), CD132 (TUGh4), CD162 (KPL‐1), CD213a1 (SS12B), CD213a2 (SHM38), CXCR1 (8F1/CXCR1), CXCR2 (5E8/CXCR2), and CXCR4 (12G5). For intracellular staining, neutrophils were fixed with Fix Buffer I and permeabilized with Perm Buffer III (BD Phosflow, BD Bioscience), followed by intracellular staining for myeloperoxidase (MPO421‐8B2), phospho‐Y705 of STAT3 (pSTAT3; 13A3‐1), phospho‐Y694 of STAT5 (pSTAT5; SRBCZX, ThermoFisher), and phospho‐Y641 of STAT6 (pSTAT6; CHI2S4, ThermoFisher), as previously established. 13 , 24 Cell viability was analyzed using Annexin V Apoptosis Detection Kit (eBioscience). Samples were acquired on a BD LSR Fortessa and analyzed using FlowJo software (Tristar).
2.6. Neutrophil extracellular traps formation and phagocytosis
Neutrophil extracellular traps were stained and quantified as published. 13 In brief, 105 neutrophils were seeded on glass coverslips treated with polylysine (Sigma) in 24‐well plates and incubated for 2 h at 37°C in 5% CO2 with medium (RPMI 1640 with 1% of FBS) with 100 nM phorbol 12‐myristate 13‐acetate (PMA; Sigma) to induce NET formation. Cells were then fixed with 4% paraformaldehyde for 10 min at room temperature and mounted using ProLong Gold AntiFade with 4′,6‐diamidino‐2‐phenylindole dihydrochloride (DAPI; Life Technologies). Cells were imaged using a 20‐fold magnification 1.25 NA with an inverted CSLM Leica SP5 confocal microscope. Every picture consisted of an overlay of 15 stacks of 20 μm. Quantification of NET+ neutrophils was done automatically using ImageJ software with the DANA plug‐in, as described. 13 To determine phagocytosis, 105 of freshly isolated neutrophils were plated into 96‐well plates and incubated for 1 h with pHrodo®‐expressing E. coli red bioparticles (phagocytosis kit for flow cytometry; ThermoFisher) at 37°C in 5% of CO2 with medium alone or with cytochalasin D (Sigma) to inhibit phagocytosis. After incubation, cells were washed and analyzed by flow cytometry.
2.7. Cytokines
Serum was processed for measuring IL‐1β, IL‐2, IL‐4, IL‐5, IL‐6, IL‐10, IL‐12p70, IL‐13, IL‐17A, IL‐23, interferon (IFN)‐γ, and tumor necrosis factor (TNF) by commercially available cytokine multiple arrays on a MagPix platform (Luminex Corporation) with a Human Mag Luminex Performance Assay Base Kit, HS Cytokine A or HS Cytokine B (LHSCM000 or LBHS000, respectively; R&D Systems, Bio‐Techne). C‐reactive protein (CRP) concentrations were determined by an enzyme immunoassay (EMIT, Merck Diagnostica).
2.8. Statistics
The number of samples and subjects used in each experiment are indicated in the figure legends. Data are presented as mean ± standard deviations (SD). p‐values were calculated using Student's t‐test and two‐way ANOVA. Correlations were performed running a Spearman test. Statistical analyses were performed with Graph‐Pad Prism, and statistical significance was established at p < .05.
3. RESULTS
3.1. Steady‐state phenotype differs in HD and SSc neutrophils
Absolute counts of neutrophils in peripheral blood were comparable between HDs and SSc patients (Figure 1A). However, freshly isolated neutrophils of SSc patients showed a slight but significant reduction of CD16 (p = .0012; Figure 1B) and of CD62L (p = .0337; Figure 1C) when compared to HD neutrophils. Conversely, the surface abundance of CD11b, CD35, CD63, CD66b, and CD66c was comparable in neutrophils freshly isolated from HDs or SSc patients (Figures S2 and S3A, and data not shown).
FIGURE 1.
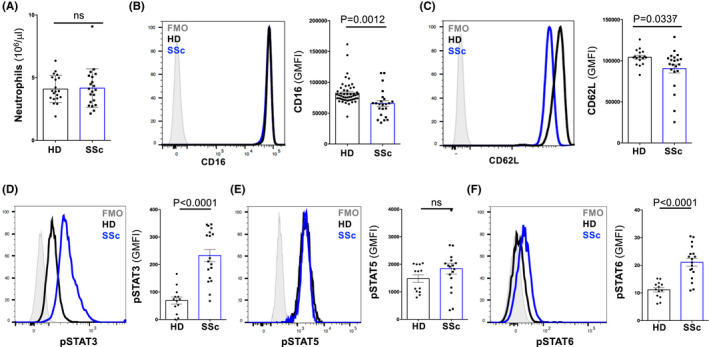
Neutrophils of patients with systemic sclerosis show signs of cytokine activation. (A) Counts of neutrophils in peripheral blood of healthy donors (HD; n = 20) and patients with systemic sclerosis (SSc; n = 21). (B–F) Representative histograms of CD16 (B; HD n = 52; SSc n = 21), CD62L (C; HD n = 17; SSc n = 21), phosphorylated STAT3 (pSTAT3; D), pSTAT5 (E), and pSTAT6 (F) in freshly isolated neutrophils of HDs (n = 13) and SSc (n = 18). Fluorescence minus one (FMO) values are represented by histograms filled with gray color. Bars represent geometric mean fluorescence intensity (GMFI) of indicated markers. Data are shown as mean ± SD. Each dot represents an independent and unrelated donor. Significance of differences between groups was calculated using Student's t‐test.; ns, not significant
3.2. Systemic sclerosis neutrophils show signs of chronic cytokine activation
The lower surface abundance of CD16 on SSc neutrophils was reminiscent of neutrophils isolated from patients with allergies that are known be exposed to increased concentrations of Th2 cytokines. 13 To assess whether SSc neutrophils displayed signs of cytokine‐mediated activation, we performed intracellular staining of phosphorylated STATs (pSTAT) in freshly isolated neutrophils. Compared to HD neutrophils, SSc neutrophils expressed significantly increased pSTAT3 (p < .0001) and pSTAT6 (p < .0001) levels, whereas abundance of pSTAT5 was similar between SSc and HD neutrophils (Figure 1D–F).
As our pSTAT data pointed to cytokine‐mediated activation of SSc neutrophils, we analyzed the concentrations of serum cytokines in SSc patients compared to HDs. At steady state, serum concentrations of certain cytokines are typically below the detection limit. 13 , 25 , 26 Nevertheless, we found several cytokines associated with inflammation and Th2 skewing to be elevated in the serum of SSc patients. Thus, IL‐1β (p = .0483), IL‐5 (p = .0046), IL‐6 (p = .0015), IL‐10 (p = .0218; Figure 2A), and TNF (p = .0046; Figure S4) were increased in the serum of SSc patients compared to HDs (Figures 2A and S4). We were able to validate the higher IL‐6 levels by measuring higher CRP concentrations in SSc serum (p = .0025; Figures 2B and S5), thus confirming a previous publication. 6 However, we were unable to detect serum G‐CSF, granulocyte‐macrophage colony‐stimulating factor (GM‐CSF, also known as colony‐stimulating factor 2), IFN‐γ, IL‐2, IL‐4, IL‐12p70, IL‐13, IL‐17, and G‐CSF (Figures 2A, S4 and S6), similar to previous reports that attempted at measuring these cytokines in HDs and individuals with SSc or allergic diseases. 6 , 13 , 25 , 26
FIGURE 2.
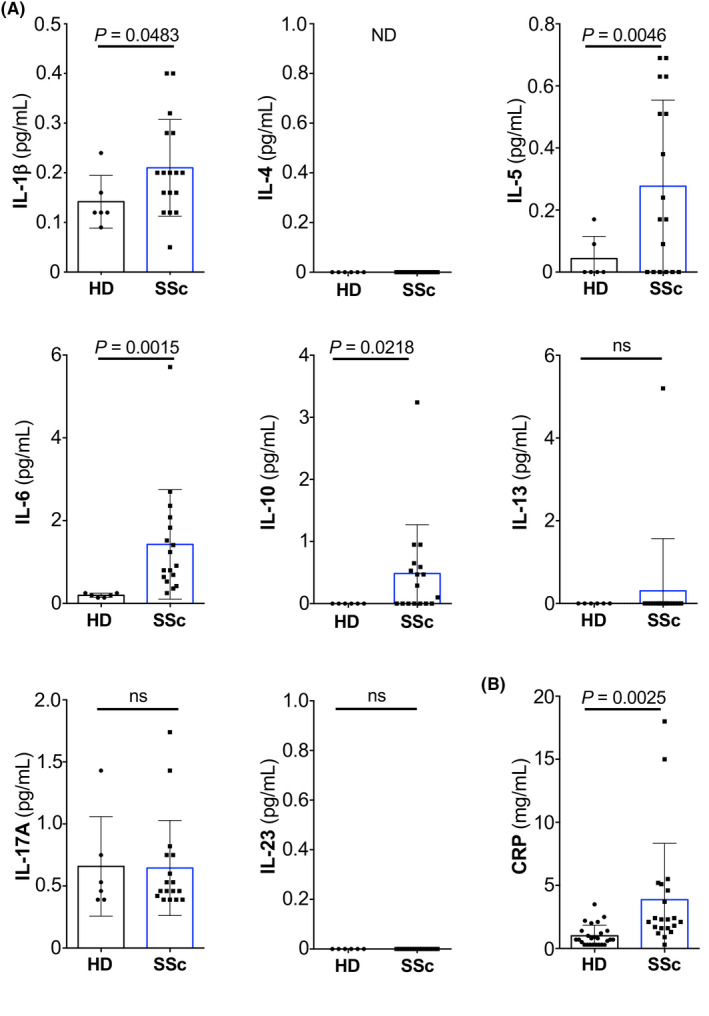
Concentrations of different cytokines and CRP in serum of HDs and SSc patients. (A and B) Levels of indicated cytokines (A; in pg/ml; HD, n = 6; SSc, n = 17) and CRP (B; in mg/ml) in the serum of HDs and SSc patients (HD, n = 26; SSc, n = 21). Data are presented as mean ± SD of independent and unrelated donors. Each dot represents an individual. Significance of differences between groups was calculated using Student's t‐test; ns, not significant. Abbreviations used: IL, interleukin; ND, not detectable; CRP, C‐reactive protein
Surface abundance of the IL‐4R subunits CD124, CD132, CD213a1, and the decoy IL‐13Rα2 (CD213a2) receptor was comparable between neutrophils of HDs and SSc patients (Figure S3B–E). Interestingly, the levels of G‐CSF receptor (G‐CSFR) were slightly reduced on SSc neutrophils compared to HD neutrophils (Figure S3F). The lower surface abundance of G‐CSFR on SSc neutrophils could have several causes, including ex vivo culture of neutrophils or contact with G‐CSF. Accordingly, stimulation of freshly isolated HD neutrophils in vitro with G‐CSF resulted in downregulation of surface G‐CSFR (Figure S3G).
3.3. Intermediary chemokine receptors are reduced in SSc neutrophils
IL‐4 receptor signaling on HD neutrophils reduces their surface expression of CXCR1 and CXCR2, but not CXCR4. 13 Compared to HDs, freshly isolated neutrophils from SSc patients exhibited significant downregulation of CXCR1 (p = .0002) and CXCR2 (p < .0001), whereas CXCR4 was not different between HD and SSc neutrophils (Figure 3A–F).
FIGURE 3.
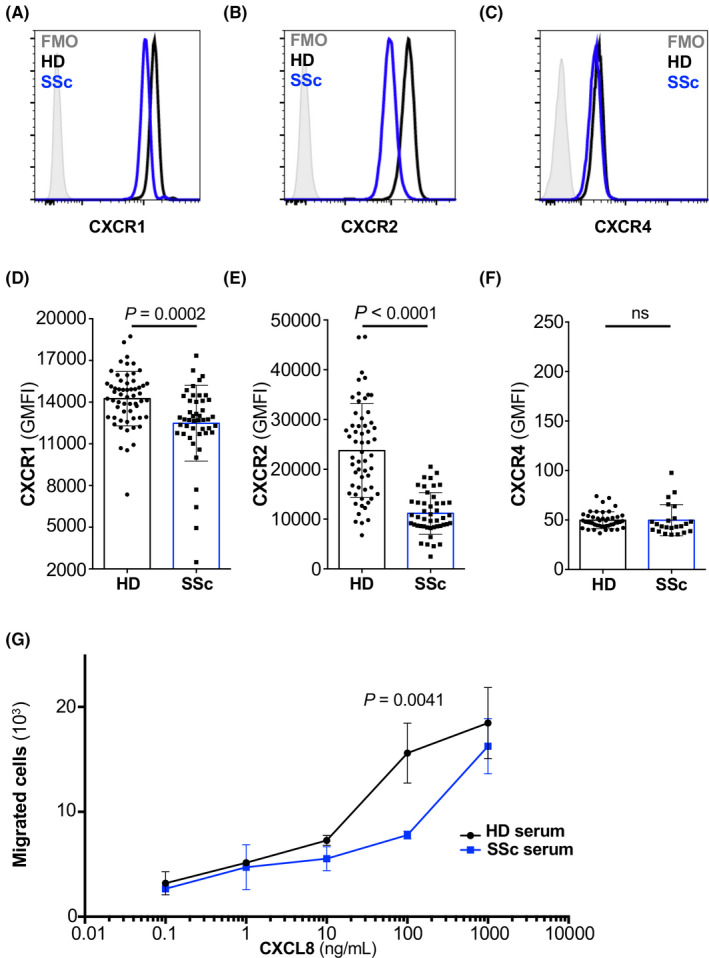
Systemic sclerosis neutrophils lack CXCR1 and CXCR2. (A–C) Representative histogram of the expression of CXCR1 (A), CXCR2 (B), and CXCR4 (C) on freshly isolated neutrophils of HDs (black line) and SSc (blue line). (D–F) Bar histogram of the geometric mean fluorescence (GMFI) of CXCR1 (D), CXCR2 (E), and CXCR4 (F) on freshly isolated neutrophils of HDs (n = 58 for CXCR1 and CXCR2, and n = 45 for CXCR4) and SSc (n = 46 for CXCR1 and CXCR2, and n = 23 for CXCR4). Data are shown as mean ± standard deviation (SD) of several donors. Each dot represents an independent and unrelated donor. (G) Chemotaxis of HD neutrophils toward titrated concentrations of CXCL8 (in ng/ml) following stimulation for 2 h with HD serum (10% in RPMI 1640; black line) or SSc serum (10% in RPMI 1640; blue line). Significance of differences between groups was calculated using Student's t‐test (A–F) or two‐way ANOVA (G); ns, not significant
To functionally link these surface CXCR abundance data on SSc neutrophils to our results obtained with serum of SSc patients, we performed migration assays using neutrophils incubated with HD vs. SSc serum. These experiments revealed that HD neutrophils incubated with SSc serum migrated less efficiently toward CXCL8 compared to HD neutrophils incubated with HD serum (p = .0041 for 100 ng/ml CXCL8; Figure 3G).
3.4. Neutrophil extracellular traps formation by SSc neutrophils is severely reduced
Neutrophil extracellular traps formation can be hampered by contact of neutrophils with different signals, such as by STAT6 activation in neutrophils by IL‐4 and IL‐13. 13 As pSTAT6 abundance was increased in neutrophils from SSc patients compared to HDs, we sought to assess stimulated NET formation in these neutrophils. Upon purification of neutrophils from HDs and SSc patients and stimulation with PMA, we evaluated the ability of neutrophils to form NETs. Interestingly, NET formation by SSc neutrophils was reduced on average by over 63% compared to HD neutrophils (p < .0001; Figure 4A,B).
FIGURE 4.
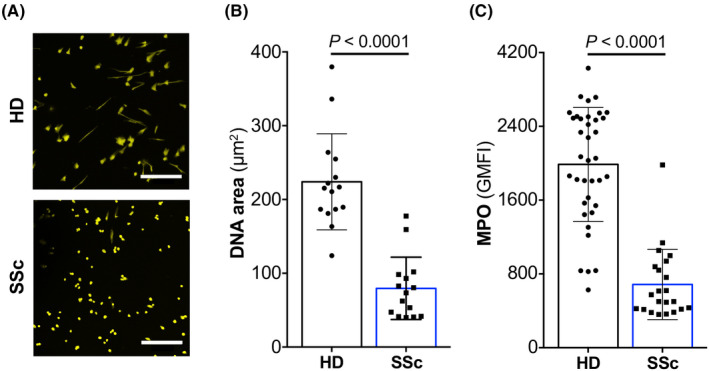
Deficient NET formation of SSc neutrophils. (A) Freshly isolated blood neutrophils of HDs (top) and SSc (bottom) were stimulated for 2 h with phorbol 12‐myristate 13‐acetate (PMA), followed by staining with 4′,6‐diamidino‐2‐phenylindole dihydrochloride (DAPI) and analyzed by confocal microscopy at 20‐fold magnification. Scale bar 200 μm. (B) DAPI‐stained neutrophils were analyzed by confocal microscopy, followed by automatic quantification of DNA area (μm2) using DANA plug‐in for ImageJ. Data are shown as mean ± standard deviation (SD) of HDs (n = 15) and SSc patients (n = 15). (C) Expression of intracellular myeloperoxidase (MPO) in freshly isolated neutrophils of HDs (n = 37) and SSc (n = 22). Data are shown as mean ± SD of GMFI. Significance of differences between groups was calculated using Student's t‐test
As MPO contributes to NET formation, 27 we determined intracellular MPO concentration in neutrophils. Matching our results on NET formation, neutrophils of SSc patients contained significantly reduced (p < .0001) intracellular MPO levels compared to HDs (Figure 4C).
3.5. Systemic sclerosis neutrophils show deficient phagocytosis of bacteria
We next tested whether the efficacy of SSc neutrophils to phagocytose bacteria was compromised. To this end, we incubated freshly isolated neutrophils with pHrodo®‐expressing E. coli red bioparticles, in the presence or absence of cytochalasin D to inhibit or allow phagocytosis, respectively (Figure 5A). Following a 1‐h incubation, neutrophils were washed and analyzed by flow cytometry for pHrodo®‐specific fluorescence in the phycoerythrin channel. Compared to their HD counterparts, neutrophils of SSc patients showed a significant (p = .0007) decrease in their ability to phagocytose pHrodo®‐expressing E. coli particles (Figure 5A,B). This effect resulted in a reduction of 10%–15% of phagtocytic activity of SSc neutrophils compared to HD neutrophils (Figure 5B).
FIGURE 5.
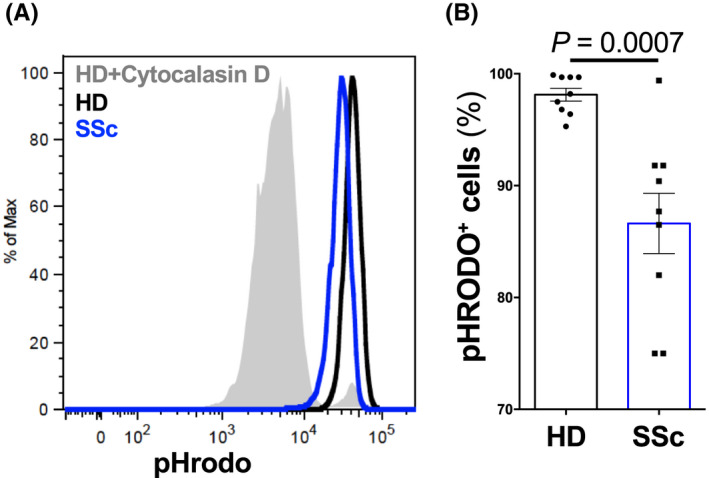
Impaired phagocytosis of SSc neutrophils. (A and B) Phagocytosis of HD or SSc neutrophils was analyzed by flow cytometry using pHrodo®‐expressing E. coli. (A) Representative histogram of pHrodo®‐positive neutrophils in a HD (black line) and SSc (blue line) after incubation with pHrodo®‐expressing E. coli. HD neutrophils incubated with cytochalasin D (gray filled line), a potent inhibitor of actin polymerization, served as control. (B) Quantification of pHrodo®‐positive neutrophils. Data are shown as mean ± standard deviation (SD) of different donors (HD n = 9; SSc n = 9). Each dot represents an unrelated donor. Significance of differences between groups was calculated using Student's t‐test
4. DISCUSSION
In this study, we have demonstrated that neutrophils of SSc patients show several phenotypic changes and functional deficits. Thus, compared to their counterparts from HDs, SSc neutrophils displayed elevated phosphorylation of STAT3 and STAT6, and they expressed lower CD16 and CD62L on their surface. In addition to their phenotypic differences, neutrophils from SSc patients showed several functional abnormalities, including a lack of the typical chemokine receptors CXCR1 and CXCR2, which regulate migration toward the potent neutrophil chemoattractant CXCL8. SSc neutrophils also contained lower intracellular MPO and produced less NETs upon activation. In addition lastly, we found that SSc neutrophils were less efficient in phagocytosing bacterial bioparticles. Of note, the observed changes in SSc neutrophils were not due to increased apoptosis or decreased viability.
To our knowledge, the data presented in this study provide the first evidence that freshly isolated neutrophils from SSc patients display several deficits. We think these phenotypic and functional changes in SSc neutrophils are due to chronic stimulation of the neutrophils by certain cytokines, possibly IL‐6 and G‐CSF (signaling via STAT3) and IL‐4 and IL‐13 (signaling through STAT6). 13 , 28 In line with this suggestion, SSc neutrophils showed increased phosphorylation of STAT6 directly ex vivo and several functional defects that have been observed in HD neutrophils stimulated with IL‐4, IL‐13 or serum of allergic patients. 13 Thus, a short‐term culture of HD neutrophils in serum of SSc patients decreased the cells' ability to migrate toward CXCL8. We tried to link these phenotypic and functional features of SSc neutrophils to IL‐4 or IL‐13. However, IL‐4 and IL‐13 concentrations in serum samples of SSc were below the detection limit, which is consistent with previous attempts to measure these cytokines in the serum of allergic patients and HDs. 13 Conversely, Th2 cytokines other than IL‐4 and IL‐13 as well as pro‐inflammatory cytokines were elevated in the serum of SSc patients, such as IL‐5, IL‐1β, IL‐6, and TNF, which constitute cytokines that are easier to measure in human serum. 25
When considering how the Th2 cytokines IL‐4 and IL‐13 affect neutrophil responses, it is worth also reflecting on the temporal aspect of immune responses. 16 Several studies using mouse models dominated by Th2 cytokines, such as models of helminth infection, showed that neutrophils were beneficial during the early stages, whereas the presence of abundant neutrophils at later stages resulted in increased tissue damage. 29 , 30 , 31 Thus, as in type 1 and type 3 immunity, also in type 2 immune responses neutrophils appear to be the first non‐resident immune cells to arrive at a site of inflammation or infection. This helps limit the spread of pathogens and attract other innate and adaptive immune cells. However, as the Th2 cytokines IL‐4 and IL‐13 become predominant, neutrophil effector functions are modulated and curtailed to prevent tissue damage. 16 Such modulation of neutrophils has likely emerged in evolution together with the emergence of type 2 immune responses and the IL‐4 and IL‐13 genes. 32 Notably, IL‐4–/IL‐13–mediated modulation of neutrophils is also shaped by the concomitant production of other factors in the tissue microenvironment, including other cytokines, chemokines, and anti‐microbial proteins, such as chitinase‐like proteins, eosinophil‐associated ribonucleases, and eosinophil‐associated cationic proteins. 31 , 33
Previous publications described several different IL‐4– and IL‐13–producing T‐cell subsets in SSc patients, 3 , 4 , 5 , 6 , 34 and they demonstrated the beneficial effects of blocking IL‐4 in the tight‐skin mouse models of scleroderma. 35 , 36 , 37 These data suggest that the type 2 cytokines IL‐4 and IL‐13 might be produced at increased concentrations in the tissues of SSc patients. Moreover, a recent phase II clinical trial in 97 patients with diffuse cutaneous SSc showed that romilkimab, a bispecific anti‐IL‐4 and anti‐IL‐13 antibody, was able to improve disease in these patients as measured by the modified Rodnan skin score. 38 Several other randomized clinical trials interfering with IL‐4R and its downstream signaling in SSc patients are currently ongoing. 39 , 40 , 41
Our data presented in this study demonstrate that neutrophils from SSc patients lack several effector molecules and effector properties, including key chemokines receptors, NET formation, and phagocytosis of bacterial particles. These functional deficits in neutrophils in SSc patients might contribute to disturbed tissue remodeling, inflammatory processes, and anti‐microbial defense by the neutrophils. 16 Whether the inefficient effector functions of neutrophils from SSc patients can be linked to increased IL‐4 and/or IL‐13 signaling in these cells, as suggested by their increased pSTAT6 abundance, remains correlative at this stage and needs to be assessed in future studies.
Notably, similar functional defects have been observed in neutrophils from patients with allergic disorders where the role of type 2 cytokine signals is well established. 42 Moreover, SSc is characterized by vascular abnormalities, and CD49d+ CXCR4+ neutrophils expressing vascular endothelial growth factor receptor 1 (VEGFR1) have been connected to angiogenesis. 43 We did not observe any significant changes in CXCR4 surface abundance of neutrophils from SSc patients compared to HDs; however, we did not measure VEGFR1 expression, which should be done in future studies to assess a possible link between the neutrophil phenotype and the vasculopathy in SSc.
Collectively, we find that ex vivo neutrophils of SSc patients display several phenotypic changes and functional deficits. These alternatively‐activated neutrophils are, at least in certain aspects, reminiscent of their counterparts in allergic individuals. The phenotypic changes of SSc neutrophils could potentially serve as a biomarker. Whether and how these dysfunctional neutrophils contribute to the pathogenesis of SSc is an interesting question to be addressed by future studies.
CONFLICT OF INTEREST
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
D.I. designed and performed the experiments, and analyzed the data. C.E. designed and performed the experiments, and analyzed the data. A.V. performed experiments and analyzed the data. O.D. provided patient material and analyzed the data. O.B. conceived the project, designed and analyzed experiments, and wrote the first draft of the manuscript. All authors edited and approved the final draft of the article.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We thank the blood donors and patients, as well as Maddalena Marconato, Patrick Taeschler, and Yves Zurbuchen for helpful discussion. Open Access Funding provided by Universitat Zurich.
Impellizzieri D, Egholm C, Valaperti A, Distler O, Boyman O. Patients with systemic sclerosis show phenotypic and functional defects in neutrophils. Allergy.2022;77:1274–1284. 10.1111/all.15073
Funding information
This work was funded by the Swiss National Science Foundation (310030‐172978, 310030‐200669, and CRSII5‐189950), the Rare Disease Initiative Zurich (radiz), the Hochspezialisierte Medizin Schwerpunkt Immunologie (HSM‐2‐Immunologie), and the Clinical Research Priority Program of the University of Zurich for the CRPP CYTIMM‐Z (all to O.B.).
REFERENCES
- 1. Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med. 2009;360(19):1989‐2003. [DOI] [PubMed] [Google Scholar]
- 2. Kania G, Rudnik M, Distler O. Involvement of the myeloid cell compartment in fibrogenesis and systemic sclerosis. Nat Rev Rheumatol. 2019;15(5):288‐302. [DOI] [PubMed] [Google Scholar]
- 3. Scaletti C, Vultaggio A, Bonifacio S, et al. Th2‐oriented profile of male offspring T cells present in women with systemic sclerosis and reactive with maternal major histocompatibility complex antigens. Arthritis Rheum. 2002;46(2):445‐450. [DOI] [PubMed] [Google Scholar]
- 4. Parel Y, Aurrand‐Lions M, Scheja A, et al. Presence of CD4+CD8+ double‐positive T cells with very high interleukin‐4 production potential in lesional skin of patients with systemic sclerosis. Arthritis Rheum. 2007;56(10):3459‐3467. 10.1002/art.22927 [DOI] [PubMed] [Google Scholar]
- 5. Truchetet ME, Brembilla NC, Montanari E, Allanore Y, Chizzolini C. Increased frequency of circulating Th22 in addition to Th17 and Th2 lymphocytes in systemic sclerosis: association with interstitial lung disease. Arthritis Res Ther. 2011;13(5):R166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Radstake TR, van Bon L, Broen J, et al. The pronounced Th17 profile in systemic sclerosis (SSc) together with intracellular expression of TGFbeta and IFNgamma distinguishes SSc phenotypes. PLoS One. 2009;4(6):e5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Papp G, Horvath IF, Barath S, et al. Altered T‐cell and regulatory cell repertoire in patients with diffuse cutaneous systemic sclerosis. Scand J Rheumatol. 2011;40(3):205‐210. [DOI] [PubMed] [Google Scholar]
- 8. Czirjak L, Danko K, Sipka S, Zeher M, Szegedi G. Polymorphonuclear neutrophil function in systemic sclerosis. Ann Rheum Dis. 1987;46(4):302‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Foerster J, Storch A, Fleischanderl S, et al. Neutrophil respiratory burst is decreased in scleroderma and normalized by near‐infrared mediated hyperthermia. Clin Exp Dermatol. 2006;31(6):799‐806. [DOI] [PubMed] [Google Scholar]
- 10. Barnes TC, Anderson ME, Edwards SW, Moots RJ. Neutrophil‐derived reactive oxygen species in SSc. Rheumatology. 2012;51(7):1166‐1169. [DOI] [PubMed] [Google Scholar]
- 11. Maugeri N, Capobianco A, Rovere‐Querini P, et al. Platelet microparticles sustain autophagy‐associated activation of neutrophils in systemic sclerosis. Sci Transl Med. 2018;10(451):eaao3089. [DOI] [PubMed] [Google Scholar]
- 12. Woytschak J, Keller N, Krieg C, et al. Type 2 Interleukin‐4 receptor signaling in neutrophils antagonizes their expansion and migration during infection and inflammation. Immunity. 2016;45(1):172‐184. [DOI] [PubMed] [Google Scholar]
- 13. Impellizzieri D, Ridder F, Raeber ME, et al. IL‐4 receptor engagement in human neutrophils impairs their migration and extracellular trap formation. J Allergy Clin Immunol. 2019;144(1):267‐279 e264. [DOI] [PubMed] [Google Scholar]
- 14. Grigolato F, Egholm C, Impellizzieri D, Arosio P, Boyman O. Establishment of a scalable microfluidic assay for characterization of population‐based neutrophil chemotaxis. Allergy. 2020;75(6):1382‐1393. [DOI] [PubMed] [Google Scholar]
- 15. Heeb LEM, Egholm C, Impellizzieri D, Ridder F, Boyman O. Regulation of neutrophils in type 2 immune responses. Curr Opin Immunol. 2018;54:115‐122. [DOI] [PubMed] [Google Scholar]
- 16. Egholm C, Heeb LEM, Impellizzieri D, Boyman O. The regulatory effects of interleukin‐4 receptor signaling on neutrophils in type 2 immune responses. Front Immunol. 2019;10:2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hemmerle T, Doll F, Neri D. Antibody‐based delivery of IL4 to the neovasculature cures mice with arthritis. Proc Natl Acad Sci U S A. 2014;111(33):12008‐12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schmid AS, Hemmerle T, Pretto F, Kipar A, Neri D. Antibody‐based targeted delivery of interleukin‐4 synergizes with dexamethasone for the reduction of inflammation in arthritis. Rheumatology. 2018;57(4):748‐755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Panda SK, Wigerblad G, Jiang L, et al. IL‐4 controls activated neutrophil FcgammaR2b expression and migration into inflamed joints. Proc Natl Acad Sci U S A. 2020;117(6):3103‐3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Capitano ML, Nemeth MJ, Mace TA, et al. Elevating body temperature enhances hematopoiesis and neutrophil recovery after total body irradiation in an IL‐1‐, IL‐17‐, and G‐CSF‐dependent manner. Blood. 2012;120(13):2600‐2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nie Y, Sun L, Wu Y, et al. AKT2 regulates pulmonary inflammation and fibrosis via modulating macrophage activation. J Immunol. 2017;198(11):4470‐4480. [DOI] [PubMed] [Google Scholar]
- 22. van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. 2013;72(11):1747‐1755. [DOI] [PubMed] [Google Scholar]
- 23. Bouchaud G, Gehrke S, Krieg C, et al. Epidermal IL‐15Ralpha acts as an endogenous antagonist of psoriasiform inflammation in mouse and man. J Exp Med. 2013;210(10):2105‐2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Karakus U, Sahin D, Mittl PRE, et al. Receptor‐gated IL‐2 delivery by an anti‐human IL‐2 antibody activates regulatory T cells in three different species. Sci Transl Med. 2020;12(574):eabb9283. [DOI] [PubMed] [Google Scholar]
- 25. Fellmann F, Angelini F, Wassenberg J, et al. IL‐17 receptor A and adenosine deaminase 2 deficiency in siblings with recurrent infections and chronic inflammation. J Allergy Clin Immunol. 2016;137(4):1189‐1196 e1182. [DOI] [PubMed] [Google Scholar]
- 26. Raeber ME, Rosalia RA, Schmid D, Karakus U, Boyman O. Interleukin‐2 signals converge in a lymphoid‐dendritic cell pathway that promotes anticancer immunity. Sci Transl Med. 2020;12(561):eaba5464. [DOI] [PubMed] [Google Scholar]
- 27. Metzler KD, Fuchs TA, Nauseef WM, et al. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood. 2011;117(3):953‐959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fielding CA, McLoughlin RM, McLeod L, et al. IL‐6 regulates neutrophil trafficking during acute inflammation via STAT3. J Immunol. 2008;181(3):2189‐2195. [DOI] [PubMed] [Google Scholar]
- 29. Bonne‐Annee S, Kerepesi LA, Hess JA, et al. Human and mouse macrophages collaborate with neutrophils to kill larval strongyloides stercoralis. Infect Immun. 2013;81(9):3346‐3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen F, Liu Z, Wu W, et al. An essential role for TH2‐type responses in limiting acute tissue damage during experimental helminth infection. Nat Med. 2012;18(2):260‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sutherland TE, Logan N, Ruckerl D, et al. Chitinase‐like proteins promote IL‐17‐mediated neutrophilia in a tradeoff between nematode killing and host damage. Nat Immunol. 2014;15(12):1116‐1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heeb LEM, Egholm C, Boyman O. Evolution and function of interleukin‐4 receptor signaling in adaptive immunity and neutrophils. Genes Immun. 2020;21(3):143‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Panova V, Gogoi M, Rodriguez‐Rodriguez N, et al. Group‐2 innate lymphoid cell‐dependent regulation of tissue neutrophil migration by alternatively activated macrophage‐secreted Ear11. Mucosal Immunol. 2021;14(1):26‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fuschiotti P, Larregina AT, Ho J, Feghali‐Bostwick C, Medsger TA Jr. Interleukin‐13‐producing CD8+ T cells mediate dermal fibrosis in patients with systemic sclerosis. Arthritis Rheum. 2013;65(1):236‐246. 10.1002/art.37706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McGaha T, Saito S, Phelps RG, et al. Lack of skin fibrosis in tight skin (TSK) mice with targeted mutation in the interleukin‐4R alpha and transforming growth factor‐beta genes. J Invest Dermatol. 2001;116(1):136‐143. [DOI] [PubMed] [Google Scholar]
- 36. Ong C, Wong C, Roberts CR, Teh HS, Jirik FR. Anti‐IL‐4 treatment prevents dermal collagen deposition in the tight‐skin mouse model of scleroderma. Eur J Immunol. 1998;28(9):2619‐2629. [DOI] [PubMed] [Google Scholar]
- 37. Gasparini G, Cozzani E, Parodi A. Interleukin‐4 and interleukin‐13 as possible therapeutic targets in systemic sclerosis. Cytokine. 2020;125:154799. [DOI] [PubMed] [Google Scholar]
- 38. Allanore Y, Wung P, Soubrane C, et al. A randomised, double‐blind, placebo‐controlled, 24‐week, phase II, proof‐of‐concept study of romilkimab (SAR156597) in early diffuse cutaneous systemic sclerosis. Ann Rheum Dis. 2020;79(12):1600‐1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eming S. Clinical trial to evaluate efficacy and safety of dupilumab in localized scleroderma (DupiMorph). 2021; https://clinicaltrials.gov/ct2/show/NCT04200755
- 40. Jego P. Systemic sclerosis and Jak inhibitors: emphasis on macrophages (SCLERO. JAK). 2021; https://clinicaltrials.gov/ct2/show/NCT04206644
- 41. Khanna D. Evaluation of tofacitinib in early diffuse cutaneous systemic sclerosis (dcSSc) (TOFA‐SSc). 2020; https://www.clinicaltrials.gov/ct2/show/record/NCT03274076
- 42. Boyman O, Kaegi C, Akdis M, et al. EAACI IG biologicals task force paper on the use of biologic agents in allergic disorders. Allergy. 2015;70(7):727‐754. [DOI] [PubMed] [Google Scholar]
- 43. Massena S, Christoffersson G, Vagesjo E, et al. Identification and characterization of VEGF‐A‐responsive neutrophils expressing CD49d, VEGFR1, and CXCR4 in mice and humans. Blood. 2015;126(17):2016‐2026. 10.1182/blood-2015-03-631572 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


