Abstract
Aim
To assess the accuracy of self‐reported gingival bleeding on brushing (GBoB) for differentiating between periodontal health and disease and explore the optimal haemoglobin concentration that enables visual detection of GBoB.
Materials and methods
Self‐assessment of GBoB was conducted in supervised sessions for 408 consecutive adults. The haemoglobin levels in saliva/toothpaste slurry (TPS) were analysed, followed by a full‐mouth periodontal examination. Periodontal diagnoses were made based on the 2017 classification of periodontal diseases. Gingival inflammation was defined as presence of at least 10% of sites with bleeding on probing (BOP). Logistic regression and area under the receiver operating characteristic curve (AUROC) analyses were applied to assess the accuracy of GBoB.
Results
Overall, 37.1% of the subjects claimed self‐reported GBoB, and they had higher values of BOP (median: 25.0%; interquartile range (IQR): 16.0%–37.5%) than those without GBoB (median: 13.5%; IQR: 8.0%–24.8%, p < .001). The concentration/total amount of haemoglobin in TPS was positively correlated with the number of bleeding sites (r = .409/r = .520, p < .001). Haemoglobin concentration of 90.58 μg/ml or 0.51 μl blood volume enabled visual detection of GBoB with an AUROC of 0.848. Self‐reported GBoB exhibited significantly increased values of diagnostic odds ratios (3–8) for varying degrees of gingival inflammation and periodontal disease (gingivitis and periodontitis). It showed low to moderate accuracy for discriminating periodontitis and gingivitis from periodontal health, with a sensitivity of 37.1% and 61.3% and a specificity of 84.8% and 84.4%, respectively. Absence of self‐reported GBoB and low levels of haemoglobin had 93%–98% predictive values for periodontal health.
Conclusions
Despite its low sensitivity for the discrimination of periodontitis, self‐reported GBoB is a promising sentinel sign for periodontal health and disease, and gingival inflammation in particular. It is visually detectable after minor blood loss. After validation in an independent population, identification of GBoB may promote earlier detection and better prevention and treatment of periodontal disease, thereby eventually reducing the global burden of the disease.
Keywords: diagnosis, gingival inflammation, periodontal disease, screening, sensitivity and specificity
Clinical Relevance.
Scientific rationale for study: Gingival bleeding is an early and objective sign of periodontal disease. If appropriately validated, self‐detected gingival bleeding during toothbrushing, a daily home care routine, has the potential to become a useful warning signal for periodontal health and disease. Currently, a clear understanding of the utility of gingival bleeding on brushing (GBoB) in periodontal diagnostics, through both visual self‐assessment and objective haemoglobin analysis, is lacking.
Principal findings: Quantitative analyses of Hb in TPS performed better than the qualitative assessment of GBoB in the discrimination of periodontal health and disease with moderate accuracy. Loss of 0.51 μl of blood enabled visual detection of GBoB with an AUROC of 0.848. Presence of self‐reported GBoB had increased diagnostic odds ratios for gingival inflammation, and the absence of self‐reported GBoB had high predictive value for periodontal health.
Practical implications: Gingival bleeding on brushing as a sentinel sign of gingival inflammation is visually detectable after minor blood loss, and it shows great potential to promote self‐monitoring of periodontal health and early detection of periodontal disease in home settings.
1. INTRODUCTION
The recent international workshop on the classification of periodontal diseases and conditions defined periodontal health as the absence of attachment loss and gingival inflammation limited to less than 10% of sites (Caton et al., 2018). Further, bleeding on probing (BOP) has been accepted as the gold standard for clinical assessment of gingival inflammation (Chapple et al., 2018), with 30% BOP being the cut‐off differentiating localized from generalized cases. Presence of gingival inflammation is considered the key in the dysbiotic changes leading to periodontitis (Kilian et al., 2016). The global burden of periodontitis remains high (GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018). Most of it remains undetected, and early diagnosis and effective management remain elusive. To tackle it, a key action recommended by the 11th European Workshop on Prevention of Periodontal Disease has been to communicate to the public the critical importance of gingival bleeding as an early sign of disease (Tonetti et al., 2015). Such action has been endorsed by more than 40 national and international learned societies in the field of periodontology (Tonetti et al., 2017).
While the pathology of periodontal bleeding is well understood (Greenstein et al., 1981; Davenport Jr. et al., 1982; Abrams et al., 1984), little is known about the diagnostic utility of self‐reported gingival bleeding, and gingival bleeding on brushing (GBoB) in particular. GBoB has the potential to promote self‐awareness and early detection of periodontal disease as a valuable sentinel sign in home settings, since it incorporates the identification of early warning signals into daily routine. Only a few studies have explored the association of GBoB with periodontal health (Kallio et al., 1990; 1994; Kallio, 1996; Buhlin et al., 2002; Taani & Alhaija, 2003; Dietrich et al., 2005; Mariath et al., 2009; Weintraub et al., 2013; Chatzopoulos et al., 2018; Romano et al., 2020; Goulão et al., 2021). Despite a significant association between GBoB and periodontal parameters, the above‐mentioned studies have two major limitations: (1) the self‐reported past bleeding experience through questionnaire or interview is at risk of recall bias, thereby resulting in possible misinterpretation of the validity, and (2) there is a tendency to neglect subtle and invisible gingival bleeding.
Recent preliminary observations using mobile health applications for recording GBoB events have shown a significant association between self‐reported GBoB and prediction of BOP, implying a promising utility of GBoB to identify subjects at risk of periodontal disease (Tonetti et al., 2020). Furthermore, haemoglobin (Hb) analysis in oral fluids can be regarded as a supplementary assessment of undetectable gingival bleeding (Ito et al., 2016). A recent systematic review has revealed that Hb concentrations in unstimulated and paraffin‐stimulated saliva have good performance to detect periodontitis (Arias‐Bujanda et al., 2020). In the included studies, however, toothbrushing has not been used to elicit gingival bleeding.
This study aimed to (1) assess the diagnostic accuracy of GBoB for differentiating between periodontal health and disease, and (2) define the optimal Hb concentration that enables self‐detection of GBoB. The study hypothesis was that self‐reported GBoB might contribute to screening subjects with gingival inflammation.
2. MATERIALS AND METHODS
2.1. Study design and population
This was a cross‐sectional diagnostic accuracy study utilizing data of the first toothbrushing session from an original study with a randomized cross‐over design, involving a convenience sample of 408 consecutive subjects recruited from the Prince Philip Dental Hospital, Hong Kong, from July 2019 to August 2020. The details of the population have been reported (Deng et al., 2021). Briefly, 408 consecutive adult patients with the ability to brush their teeth were recruited. The exclusion criteria included periodontal treatment (other than supragingival cleaning) within the previous 12 months.
2.2. GBoB index test
The GBoB test was performed prior to the full‐mouth periodontal examination, with a manual toothbrush (Sanxiao toothbrush 998T, Colgate Sanxiao) and a powered one (iO Pre‐series 1.0 handles and iO Clean brush heads, Oral‐B Braun) using a standard toothpaste that did not interfere with Hb colorimetric assays (Sensitive Pro‐Relief, Colgate). Participants were asked to brush their teeth as they usually did at home, without any disturbance and time restriction, in a separate room with a mirror and washbasin prior to the clinical examination. During and after the brushing session, the subject spit the saliva/toothpaste slurry (TPS) into a 50‐ml transparent collection container. At the end of the brushing session, participants were asked to self‐assess the presence or absence of blood in the TPS (GBoB). The TPS samples were collected and frozen at −70°C until analysis.
2.3. Quantification of Hb in the TPS samples
Frozen TPS samples were thawed at room temperature and centrifuged at 10,000 rpm for 10 min at 4°C for quantitative analysis. The volume of the supernatant of TPS was measured, and the concentration of Hb in the TPS was determined by ultraviolet–visible (UV–vis) spectroscopy at an absorbance peak at 406 nm specific for methemoglobin (van Kampen & Zijlstra, 1983). For the calibration curve, an Hb stock was prepared by dissolving lyophilized human haemoglobin (Sigma Co.) in deionized water at 5 mg/ml. Serial dilutions were then performed to create standards at 500, 400, 200, 100, 50, and 25 μg/ml. The absorbance of the standard solution and TPS samples were measured at 406 nm by a microplate reader (SpectraMax M2 Microplate Reader, Molecular Devices), and the concentration of Hb was then determined based on the standard curve. The total amount of Hb and the volume of blood lost were calculated based on the Hb concentration, sample volume, and a normal Hb concentration in blood of 15 g/dl (Bain, 2006). The UV–vis method had been validated in a pilot study, and the coefficient of determination (R 2) was >.999 (data not shown).
2.4. Periodontal examination and case definition—Reference standard
The clinical periodontal measurements, including probing pocket depth (PPD), BOP, clinical attachment level (CAL), furcation involvement (FI), tooth mobility, and the number of teeth lost attributed to periodontitis, were assessed by a single calibrated examiner (KD) who was blind to the results of the GBoB test. Details of the examination protocol and reproducibility of the examiner have been reported recently (Deng et al., 2021).
The cases of periodontal health, gingivitis, and different stages of periodontitis were diagnosed according to the 2017 classification of periodontal diseases (Chapple et al., 2018; Papapanou et al., 2018; Tonetti et al., 2018; Trombelli et al., 2018). Here, periodontal disease is referred to as plaque‐induced gingivitis or periodontitis as previously described (Deng et al., 2021). A gingivitis case was further classified as localized (10% ≤ BOP% ≤ 30%) or generalized (BOP% > 30%) based on the percentage of bleeding sites (Trombelli et al., 2018). To test the study hypothesis, gingival inflammation was defined by the presence of at least 10% bleeding sites (BOP% ≥ 10%) without regard to the CAL. A case with localized gingival inflammation was defined as 10%–30% bleeding sites (10% ≤ BOP% ≤ 30%), and a case with generalized gingival inflammation had more than 30% bleeding sites (BOP% > 30%). Cases with minimal gingival inflammation were defined as less than 10% bleeding sites (BOP% < 10%).
2.5. Data analysis
Frequencies were used to describe the distribution of self‐reported GBoB, and Chi‐square tests were employed to compare the inter‐group differences. Medians with interquartile range (IQR) were used to describe the distribution of Hb levels, and Mann–Whitney U tests or Kruskal–Wallis tests were used to assess differences across groups. To explore the optimal Hb concentration that enables visually noticeable GBoB, receiver operating characteristic (ROC) analysis was conducted. The cut‐off value was chosen by optimizing the sum of sensitivity and specificity from the ROC curves.
Pearson's correlation coefficient analyses were performed to investigate the correlation between Hb and periodontal clinical parameters. Logistic regression analyses assessed the unadjusted association of the GBoB test with periodontal status in univariate analyses. The diagnostic accuracies of self‐reported GBoB, Hb concentration, and the combination of self‐reported GBoB and Hb concentration were assessed based on logistic regression models. The area under the ROC curve (AUROC), diagnostic odds ratios (DORs), sensitivity, specificity, positive predictive values, and negative predictive values were calculated. The standard nomenclature for defining low, moderate, and high levels of diagnostic accuracy was previously reported (Deng et al., 2021). p‐Values <.05 were considered to be statistically significant. All analyses were performed with the SPSS software, version 26.0 (IBM Corp.).
3. RESULTS
The subject characteristics and disease distribution of the study population have been recently reported (Deng et al., 2021). The STARD flowchart of this study is displayed in Figure 1. There were 407 samples valid for Hb concentration measurements except for one low‐volume saliva/toothpaste slurry sample from a Stage IV periodontitis patient. A total of 152 (37.1%) subjects reported GBoB. The Hb concentration ranged from 5.90 to 5978.06 μg/ml with a median of 85.13 μg/ml for the entire population, while the total amount of Hb ranged from 1.823 to 2379.27 μg with a median of 79.36 μg corresponding to a median volume of blood loss of 0.53 μl (range 0.1–15.9 μl).
FIGURE 1.
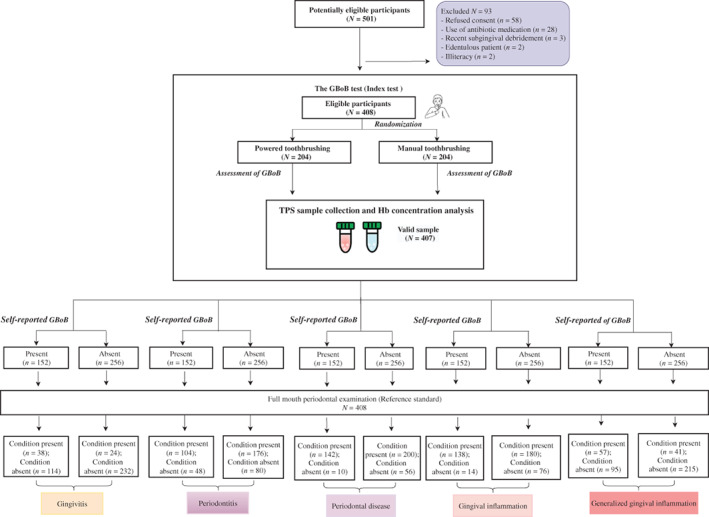
Standards for Reporting Diagnostic Accuracy (STARD) flow diagram of the study for various periodontal conditions
3.1. Relation of self‐reported GBoB to Hb levels
As shown in Figure 2, the Hb concentration from subjects with self‐reported GBoB (median: 205.69 μg/ml; IQR: 105.79–463.15 μg/ml) was significantly higher than those without self‐reported GBoB (median: 62.98 μg/ml; IQR: 40.96–98.45 μg/ml; p < .001). The ROC analysis demonstrated that an Hb concentration of 90.58 μg/ml enabled self‐detection of bleeding in the TPS samples (sensitivity: 80.1%; specificity: 71.1%; AUROC: 0.848). The same analysis for total amount of Hb or blood volume revealed a cut‐off value of 77.05 μg or 0.51 μl (sensitivity: 83.4%; specificity 68.4%, AUROC: 0.848, Appendix Figure S1).
FIGURE 2.
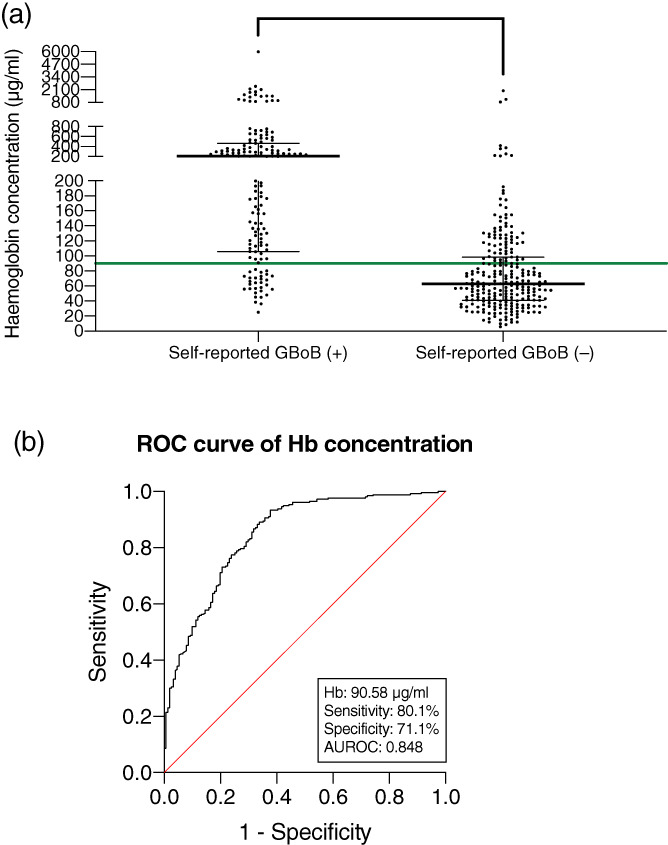
Relation of self‐reported GBoB to Hb concentrations in the TPS samples. (a) Distribution of Hb concentrations in subjects with or without self‐reported GBoB. Each point denotes the value for a participant. The horizontal bars display the medians and interquartile ranges (IQR). The horizontal green line denotes the threshold value for GBoB being self‐detected (Hb ≥90.58 μg/ml). (b) ROC analysis of Hb, comparing concentrations in subjects with self‐reported GBoB to those without GBoB
3.2. Association of GBoB with periodontal clinical parameters
Subjects with self‐reported GBoB had significantly higher values of BOP% (median: 25.0%; IQR: 16.0%–37.5%) compared with those without GBoB (median: 13.5%; IQR: 8.0%–24.8%; p < .001). Moreover, subjects had significantly greater numbers of periodontal pockets ≥4 mm (median: 19; IQR: 6–41) than those without GBoB (median: 11; IQR: 4–25; p < .001). They also had significantly greater numbers of bleeding pockets of ≥4 mm (median: 11; IQR: 3–25) than those without GBoB (median: 5; IQR: 1–13; p < .001). Despite the small number of periodontal pockets of ≥6 mm, subjects with self‐reported GBoB still had significantly greater numbers (median: 0.5; IQR: 0–4.8) than those without self‐reported GBoB (median: 0; IQR: 0–2; p = .012). The concentration and total amount of Hb were positively correlated with the number of bleeding sites, number of periodontal pockets ≥4 mm, number of deep pockets ≥6 mm, and number of bleeding pockets (Figure 3). Among these clinical variables, the number of bleeding pockets ≥6 mm had the highest correlation with the Hb concentration (r = .538, p < .001), while the number of bleeding sites showed the highest correlation with the total amount of Hb (r = .520, p < .001).
FIGURE 3.
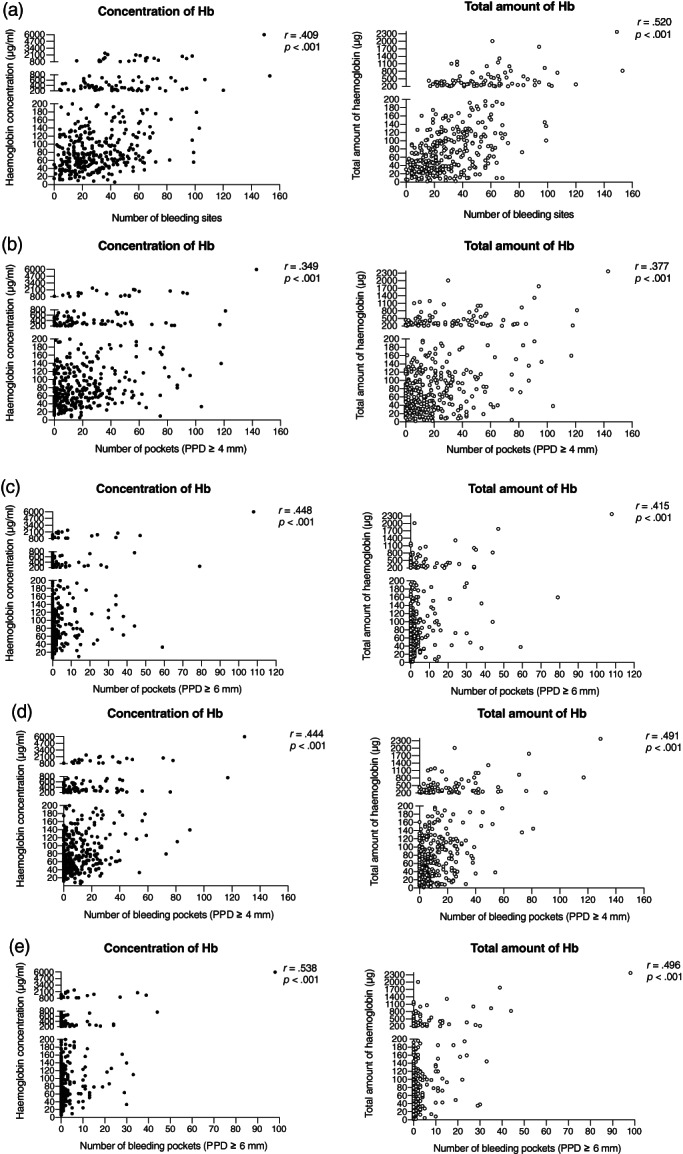
Pearson's correlation coefficient analyses of the concentration and total amount of Hb with periodontal clinical parameters. (a) Concentration and total amount of Hb with the number of bleeding sites. (b) Concentration and total amount of Hb with number of periodontal pockets ≥4 mm. (c) Concentration and total amount of Hb with number of deep pockets ≥6 mm. (d) Concentration and total amount of Hb with number of bleeding pockets ≥4 mm. (e) Concentration and total amount of Hb with number of bleeding pockets ≥6 mm. Each point denotes a subject in the population (n = 408); r, correlation coefficient
3.3. GBoB and periodontal case diagnosis
Table 1 shows the distribution of self‐reported GBoB and Hb levels by periodontal status. Gingivitis patients self‐detected GBoB more frequently than periodontitis and periodontal health cases (gingivitis > periodontitis > periodontal health, p < .001). The concentration and total amount of Hb were significantly higher in subjects with periodontitis and gingivitis than periodontally healthy ones (Figure 4). Although subjects with gingivitis reported more frequently GBoB than those with periodontitis, there was no significant difference in Hb levels in their TPS samples. Regarding the stages of periodontitis, there was no significant difference in the relative proportion of self‐reported GBoB among subjects with Stages I–IV periodontitis (Table 1). Likewise, no significant difference in the concentration and total amount of Hb across different stages of periodontitis was identified (Figure 4).
TABLE 1.
Prevalence of self‐reported GBoB, concentrations, and total amounts of Hb according to different periodontal statuses
| GBoB estimates | (a) Periodontal case definitions | |||
|---|---|---|---|---|
| Periodontal health (n = 66) | Gingivitis (n = 62) | Periodontitis (n = 280) | p‐Value | |
| Self‐reported GBoB | 10 (15.6%)a | 38 (61.3%)b | 104 (37.1%)c | <.001 |
| Hb concentration (μg/ml) | 56.71a (34.48–117.70) | 104.91b (57.72–239.56) | 90.18b (55.17–176.56) | <.001 |
| >Detection limit (90.08 μg/ml) | 21 (31.8%)a | 35 (56.5%)b | 139 (49.8%)b | .011 |
| Total amount of Hb (μg) | 35.99a (24.41–60.27) | 119.37b (75.49–246.97) | 93.06b (38.28–176.70) | <.001 |
| >Detection limit (70.05 μg) | 9 (13.6%)a | 44 (71.0%)b | 154 (55.2%)b | <.001 |
| GBoB estimates | (b) Stages of periodontitis | ||||
|---|---|---|---|---|---|
| Stage I (n = 65) | Stage II (n = 65) | Stage III (n = 121) | Stage IV (n = 29) | p‐Value | |
| Self‐reported GBoB | 23 (35.4%) | 25 (35.8%) | 43 (35.5%) | 12 (42.9%) | .884 |
| Hb concentration (μg/ml) | 79.60 (54.12–200.97) | 103.14 (59.91–201.48) | 80.15 (56.56–165.83) | 90.95 (47.99–273.88) | .784 |
| >Detection limit (90.08 μg/ml) | 31 (47.7%) | 37 (56.9%) | 57 (47.1%) | 14 (50.0%) | .618 |
| Total amount of Hb (μg) | 120.74 (41.07–201.60) | 82.69 (42.99–161.11) | 84.48 (34.53–158.47) | 100.91 (36.10–318.46) | .328 |
| >Detection limit (70.05 μg) | 38 (58.8%) | 35 (53.8%) | 64 (52.9%) | 17 (60.7%) | .818 |
| GBoB estimates | (c) Gingival inflammatory status | |||
|---|---|---|---|---|
| BOP% < 10% (n = 90) | 10% ≤ BOP% ≤ 30% (n = 220) | BOP% > 30% (n = 98) | p‐Value | |
| Self‐reported GBoB | 14 (15.6%)a | 81 (36.8%)b | 57 (58.2%)c | <.001 |
| Hb concentration (μg/ml) | 56.71a (34.49–108.63) | 76.59b (52.49–143.59) | 168.90c (80.06–370.52) | <.001 |
| >Detection limit (90.08 μg/ml) | 28 (31.1%)a | 99 (45.2%)a | 68 (69.4%)b | <.001 |
| Total amount of Hb (μg) | 34.39a (23.56–52.90) | 82.40b (38.43–133.86) | 187.13c (93.29–376.32) | <.001 |
| >Detection limit (70.05 μg) | 11 (12.2%)a | 116 (50.3%)b | 80 (81.6%)c | <.00 |
Note: Data are presented as either median, interquartile range (IQR), or n (%). Chi‐square tests (for categorical data) and Kruskal–Wallis tests (for continuous data) were used to assess differences across groups. If group differences are indicated (p < .05), then pair‐wise comparisons between the groups were conducted using a Bonferroni method to adjust the significance level for the multiple comparisons. Values with different superscripts (e.g., a, b, and c) are significantly different. Stage I periodontitis; II, Stage II periodontitis; III, Stage III periodontitis; IV, Stage IV periodontitis.
FIGURE 4.
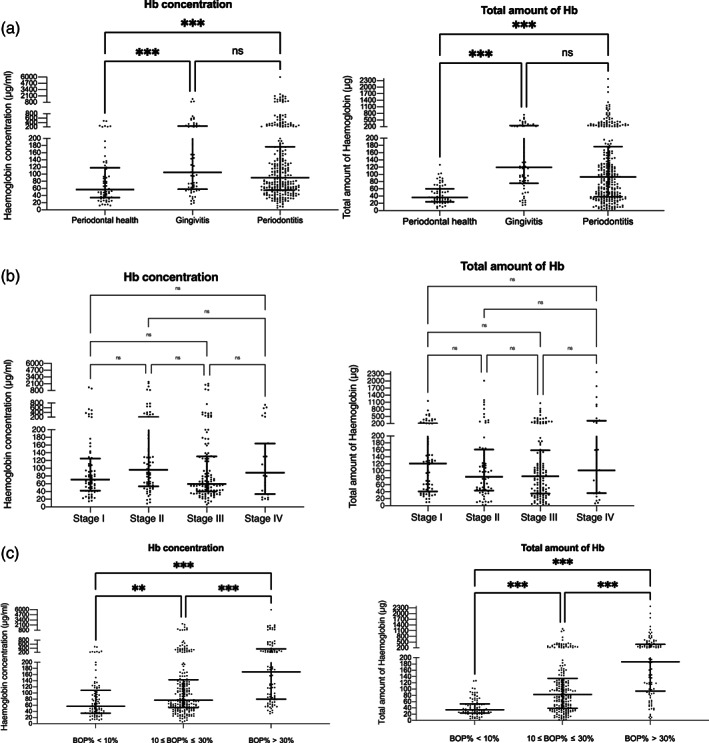
Distribution of the concentration and total amount of Hb among different periodontal statuses. (a) Concentration and total amount of Hb with periodontal case definitions. (b) Concentration and total amount of Hb with different stages of periodontitis. (c) Concentration and total amount of Hb with gingival inflammatory status. Each dot represents one participant; the horizontal bars in each graph display the medians and interquartile ranges (IQR). Kruskal–Wallis tests were used to assess differences among groups. **p < .01, ***p < .001; ns, not significant; Stage I, Stage I periodontitis; Stage II, Stage II periodontitis; Stage III, Stage III periodontitis; Stage IV, Stage IV periodontitis
Subjects with generalized gingival inflammation (BOP% > 30%) reported more frequently GBoB compared with those with localized gingival inflammation (10% ≤ BOP% ≤ 30%) and minimal inflammation (BOP% < 10%). Notably, the distribution of Hb in TPS among subjects with different gingival inflammatory statuses was in agreement with self‐reported GBoB. The concentration and total amount of Hb increased with the extent of gingival inflammation (minimal inflammation < localized inflammation < generalized inflammation, p < .001).
A logistic regression analysis was conducted to investigate the association between the GBoB test and periodontal status (Table 2). Subjects with self‐reported GBoB exhibited increased DORs for having all periodontal diagnoses (gingivitis, periodontitis, periodontal disease, and varying degrees of gingival inflammation). Subjects with elevated concentrations and total amounts of Hb also had increased DORs for having all periodontal diagnoses.
TABLE 2.
Univariate logistic regression analysis for the association of GBoB estimates with different periodontal status
| Variables | Periodontal case definitions | ||
|---|---|---|---|
| Gingivitis vs. periodontal health | Periodontitis vs. periodontal health | Periodontal disease vs. periodontal health | |
| Crude OR (95% CI) | Crude OR (95% CI) | Crude OR (95% CI) | |
| Self‐reported GBoB | 8.87 (3.81–20.64)*** | 3.31 (1.62–6.77)*** | 3.98 (1.96–8.06)*** |
| Hb concentration | 1.005 (1.002–1.009)** | 1.004 (1.001–1.007)* | 1.004 (1.001–1.007)** |
| Total amount of Hb | 1.033 (1.019–1.047)*** | 1.018 (1.010–1.026)*** | 1.019 (1.011–1.027)*** |
| Variables | Gingival inflammatory status | ||
|---|---|---|---|
| Gingival inflammation vs. minimal inflammation | Localized gingival inflammation vs. minimal inflammation | Generalized gingival inflammation vs. minimal inflammation | |
| Crude OR (95% CI) | Crude OR (95% CI) | Crude OR (95% CI) | |
| Self‐reported GBoB | 4.16 (2.26–7.67)*** | 3.16 (1.68–5.95)*** | 7.55 (3.76–15.15)*** |
| Hb concentration | 1.005 (1.002–1.008)** | 1.004 (1.001–1.007)** | 1.009 (1.005–1.013)*** |
| Total amount of Hb | 1.024 (1.016–1.032)*** | 1.023 (1.015–1.031)*** | 1.035 (1.023–1.046)*** |
Note: Please see text for details of the periodontal case definitions and the gingival inflammatory status. ***p < .001; **p < .01; *p < .05.
Abbreviations: 95% CI, confidence interval of 95%; OR, odds ratio.
3.4. Diagnostic utility of self‐reported GBoB, concentrations, and total amounts of Hb in the detection of different periodontal conditions
The diagnostic accuracy of self‐reported GBoB, concentration, and total amount of Hb are reported in Table 3. The total amount of Hb in TPS performed better than self‐reported GBoB or the Hb concentration. The total amount of Hb (amount of blood loss) in GBoB test showed moderate to high accuracy in detecting gingival inflammation, especially generalized gingival inflammation, with a moderate sensitivity of 75.5%, a high specificity of 95.6%, and a high AUROC of 0.901. Additionally, this test showed acceptable performances for discriminating gingivitis (AUROC = 0.847), periodontitis (AUROC = 0.739), and periodontal disease (AUROC = 0.754) from periodontal health.
TABLE 3.
Diagnostic accuracy of self‐reported GBoB, concentration, and the total amount of Hb in saliva/toothpaste slurry to discriminate different periodontal status
| GBoB estimates | Periodontal health (from the population) | Gingivitis (from periodontal health) | Periodontitis (from periodontal health) | Periodontal disease (from periodontal health) | Gingival inflammation (from minimal inflammation) | Generalized gingival inflammation (from minimal inflammation) |
|---|---|---|---|---|---|---|
| (1) Self‐reported GBoB | ||||||
| AUROC (95% CI) | 0.632 (0.565–0.699) | 0.731 (0.641–0.820) | 0.610 (0.540–0.680) | 0.632 (0.565–0.699) | 0.639 (0.579–0.700) | 0.713 (0.639–0.788) |
| Cut‐off value | Absence | Presence | Presence | Presence | Presence | Presence |
| Sensitivity | 84.8% | 61.3% | 37.1% | 41.5% | 43.4% | 58.2% |
| Specificity | 41.5% | 84.4% | 84.8% | 84.8% | 84.4% | 84.4% |
| PPV | 21.9% | 79.2% | 91.2% | 93.4% | 90.8% | 80.3% |
| NPV | 93.4% | 70.0% | 24.1% | 21.9% | 29.7% | 65.0% |
| (2) Hb concentration | ||||||
| AUROC (95% CI) | 0.659 (0.587–0.730) | 0.692 (0.602–0.783) | 0.651 (0.578–0.725) | 0.659 (0.587–0.730) | 0.680 (0.619–0.741) | 0.804 (0.744–0.864) |
| Cut‐off value | <52.35 μg/ml | ≥52.88 μg/ml | ≥52.35 μg/ml | ≥52.35 μg/ml | ≥52.35 μg/ml | ≥124.99 μg/ml |
| Sensitivity | 84.8% | 83.9% | 78.1% | 41.5% | 81.1% | 60.2% |
| Specificity | 41.5% | 48.5% | 48.5% | 84.8% | 47.8% | 83.3% |
| PPV | 21.9% | 60.5% | 86.5% | 93.4% | 84.5% | 79.7% |
| NPV | 93.4% | 76.2% | 34.4% | 21.9% | 41.7% | 65.8% |
| (3) Total amount of Hb | ||||||
| AUROC (95% CI) | 0.754 (0.705–0.803) | 0.847 (0.776–0.917) | 0.739 (0.686–0.792) | 0.754 (0.705–0.803) | 0.799 (0.755–0.842) | 0.901 (0.854–0.948) |
| Cut‐off value | <92.32 μg | ≥72.69 μg | ≥92.33 μg | ≥92.32 μg | ≥71.61 μg | ≥92.98 μg |
| Sensitivity | 93.5% | 77.4% | 50.2% | 52.5% | 65.3% | 75.5% |
| Specificity | 52.5% | 84.8% | 95.5% | 93.5% | 86.7% | 95.6% |
| PPV | 28.0% | 82.8% | 97.9% | 98.4% | 94.5% | 94.9% |
| NPV | 98.4% | 80.0% | 31.2% | 28.0% | 41.5% | 78.2% |
Note: Diagnostic accuracy is calculated based on univariate logistic regression models of GBoB estimates. The thresholds for generalized gingival inflammation were above the cut‐off values (Hb concentration: 90.08; total amount of Hb: 77.05 μg) for visual detection of GBoB, while the threshold for gingival inflammation was below the cut‐off values (Hb concentration: 90.08; total amount of Hb: 77.05 μg), suggesting that Hb analysis contributed to the additional identification of subjects with gingival inflammation.
Abbreviations: 95% CI, confidence interval of 95%; AUROC, area under receiver operator characteristic curve; NPV, negative predictive value; PPV, positive predictive value.
4. DISCUSSION
Visual self‐detection of GBoB or quantitative Hb measurements in TPS could be a sentinel sign of periodontal health and disease. Indeed, the presence of self‐reported GBoB had significantly increased DORs of 3–8 for varying degrees of gingival inflammation and periodontal disease (gingivitis and periodontitis), while the absence of self‐reported GBoB had >90% predictive value for periodontal health. Similar results were observed for quantitative Hb analyses in TPS. Interestingly, GBoB was self‐detectable even after minor blood loss (Hb concentration: 90.58 μg/ml corresponding to a blood volume of 0.51 μl).
This is the first validation study on self‐reported GBoB in a supervised environment using a population seeking dental care in the context of a high‐quality diagnostic trial. Of importance is the observation that quantitative analyses of Hb in TPS performed better than the self‐reported qualitative test in the discrimination of various periodontal case diagnoses. Furthermore, the specific diagnostic profile of GBoB indicates that it can be incorporated into a multi‐test approach to improve screening. More investigations are in progress in these areas. A companion paper from this trial will report additional validation of the test as performed with manual or pressure‐sensitive powered toothbrushes and according to the brushing behaviour of the subjects (data not shown). These findings are in agreement with earlier reports (Kallio et al., 1994; Taani & Alhaija, 2003; Chatzopoulos et al., 2018), but sensitivity and specificity values were slightly better (Kallio et al., 1990; Kallio, 1996; Gilbert & Nuttall, 1999; Buhlin et al., 2002; Dietrich et al., 2005; Weintraub et al., 2013; Romano et al., 2020; Goulão et al., 2021). This may be partly explained by the recall bias in self‐administrated questionnaires or interviews used in previous studies and support the principle that training and practicing are necessary for effective deployment of GBoB at the population level. It should be stressed, however, that different criteria used to define gingival inflammation may hamper comparability among studies.
Although elevated Hb concentrations have been detected in unstimulated and paraffin‐stimulated saliva in periodontitis subjects (Nomura et al., 2006, 2012, 2016; Kugahara et al., 2008; Pham et al., 2011; Nam et al., 2015), the diagnostic utility of Hb levels after stimulation by a toothbrushing session—one of the most prevalent oral home care behaviours—had not been previously assessed. In the present study, the total amount of Hb in TPS performed slightly better than salivary Hb concentrations in previous studies. The different diagnostic performance is also related to variations in periodontal case definitions and study design. Furthermore, in previous studies elevation of Hb concentration in saliva is primarily a result of resting bleeding from ulcerated periodontal pockets without mechanical stimulation. It is expected that, in the current study, Hb measurements are the results of bleeding from ulcerated periodontal pockets combined with the marginal bleeding elicited by toothbrushing. Moreover, it is noteworthy that the total amount of Hb performed better for detecting various periodontal statuses than its concentration in TPS. This may be partly due to the fact that the extravasation of erythrocytes originating from the blood vessels of inflamed gingival tissues into the oral cavity may be diluted by the volume of stimulated saliva while brushing. These important observations, after adequate validation, may suggest the potential for developing biosensors or rapid tests to detect blood loss in oral fluids. Additional work is needed in this area.
Another important aspect of this study is the interpretation of the role of GBoB in periodontal health and disease. GBoB may be a reliable indicator of gingival inflammation measured by BOP, and thus it does not specifically discriminate between periodontitis and gingivitis, as both are characterized by varying degrees of gingival inflammation. As such, GBoB is conceivably associated with the extent of gingival inflammation defined by BOP% with different thresholds, but it does not necessarily have an association with the stages of periodontitis defined by severity of the periodontal attachment loss and the complexity of case management. Of note, the discrimination of periodontal health and disease by GBoB could improve the self‐awareness of periodontal health and early detection of the disease status in home settings. Identification of the disease status, especially gingivitis and incipient periodontitis, may contribute to more cost‐effective health gains. In this respect, the recent release from the Economist Intelligence Unit of data on the high returns on investment of treating and preventing gingivitis to prevent periodontitis emphasizes the importance of early self‐detection of gingival inflammation for the implementation of such strategy (Bishop, 2021).
Pending validation in independent populations from various health systems, introduction of self‐reported GBoB as a sentinel sign for periodontal health and disease may result in a profound reduction of the burden of periodontal disease despite its imperfect diagnostic accuracy (GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018). In other areas of medicine, the self‐detection of sentinel signs with comparable accuracy has had major health impacts in the early diagnosis of a variety of diseases. Perhaps one of the early success stories that has radically changed the management of breast cancer in the late 1970s and 1980s has been breast self‐examination (BSE). Its impact—besides allowing detection of smaller tumours and shortening the diagnostic process (Greenwald et al., 1978), which regrettably did not translate into increased survival (Thomas et al., 2002)—has been manifold: increased disease awareness, bringing more patients for clinical exams, and, lastly, paving the way for routine mammography and more targeted and effective treatment. In the future, a quantitative analysis of GBoB may bring benefits similar to the detection of occult blood in stools for screening of colorectal cancer (Lin et al., 2016).
Despite the promising role of GBoB, several limitations should be addressed in the present study. First, the study population consisted of a cohort of hospital dental attendees and therefore is not representative of the general population. Second, although the study was designed to test the validity of GBoB elicited by both manual and powered toothbrushes, the selection of specific toothbrushes and toothpaste might compromise its generalizability to some degree. Additionally, the subjects' brushing performance and GBoB detection in a supervised situation may deviate from their daily practices and evaluation at home despite our maximum effort in simulation of the real‐life situation. Furthermore, this study excludeed subjects with recent periodontal treatment for ease of analysis. As GBoB is believed to work as a warning signal for the general population, the inclusion of these subjects would have maximized the generalizability of the results. Finally, it remains unclear whether cigarette smoking, steroid hormone fluctuations, or the use of anticoagulants will affect the test results of GBoB, as evidence shows that smoking has a suppressive effect on BOP and that steroid hormone levels may modify gingival inflammatory response (Dietrich et al., 2004; Murakami et al., 2018). Taken together, further studies (including longitudinal studies) are highly needed to test its application in different settings and verify the external validity in different populations using more representative samples.
Despite its low sensitivity for the discrimination of periodontitis, self‐reported GBoB may be a promising sentinel sign for periodontal health and disease, and gingival inflammation in particular. GBoB is visually detectable even after minor blood loss. After pending validation in independent populations, the recognition of GBoB may improve the self‐awareness of the individual periodontal health status, which could translate into better prevention, earlier detection, and effective treatment of periodontal disease, thereby eventually reducing the high global burden of disease.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Ke Deng contributed to design, data acquisition and interpretation, statistical analyses, drafted and critically revised the manuscript; George Pelekos and Lijian Jin contributed to data interpretation and critically revised the manuscript; Maurizio S. Tonetti contributed to concept, design, data interpretation, drafted and critically revised the manuscript. All authors gave their final approval and agree to be accountable for all aspects of the work.
ETHICS STATEMENT
The study was conducted according to the Declaration of Helsinki and approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (IRB Approval Number: UW19‐188). Informed consent was obtained from all participants. The study protocol was registered both on ClinicalTrials.gov (NCT03928080) and the HKU Clinical Trials Registry (HKUCTR‐2631). This study followed the Standards for Reporting Diagnostic Accuracy (STARD) guidelines (Cohen et al., 2016).
Supporting information
Appendix Figure S1 Relation between self‐reported gingival bleeding on brushing (GBoB) and the total amount of Hb in the TPS samples. (A) Distribution of the total amount of Hb in subjects with or without self‐reported GBoB. Each point denotes the value for a participant. The horizontal bars display the medians and interquartile ranges (IQR). The horizontal green line denotes the threshold value for GBoB being self‐detected (Hb ≥77.05 μg). (B) ROC analysis of Hb, comparing total amount in subjects with self‐reported GBoB to those without GBoB.
Deng, K. , Pelekos, G. , Jin, L. , & Tonetti, M. S. (2021). Gingival bleeding on brushing as a sentinel sign of gingival inflammation: A diagnostic accuracy trial for the discrimination of periodontal health and disease. Journal of Clinical Periodontology, 48(12), 1537–1548. 10.1111/jcpe.13545
Funding information The Hong Kong Health & Medical Research Fund (HMRF) grant No. 07182796 to Maurizio S. Tonetti supported this study. Ke Deng is the recipient of a Hong Kong Higher Education Fellowship.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abrams, K. , Caton, J. , & Polson, A. (1984). Histologic comparisons of interproximal gingival tissues related to the presence or absence of bleeding. Journal of Periodontology, 55(11), 629–632. [DOI] [PubMed] [Google Scholar]
- Arias‐Bujanda, N. , Regueira‐Iglesias, A. , Balsa‐Castro, C. , Nibali, L. , Donos, N. , & Tomás, I. (2020). Accuracy of single molecular biomarkers in saliva for the diagnosis of periodontitis: A systematic review and meta‐analysis. Journal of Clinical Periodontology, 47(1), 2–18. [DOI] [PubMed] [Google Scholar]
- Bain, B. J. (2006). Blood cells [electronic resource]: A practical guide. Blackwell. [Google Scholar]
- Bishop, C. (2021). Time to take gum disease seriously: The societal and economic impact of periodontitis. The Economist Intelligence Unit (pp. 1–46). Retrieved from https://eiuperspectives.economist.com/sites/default/files/eiu-efp-oralb-gum-disease.pdf
- Buhlin, K. , Gustafsson, A. , Andersson, K. , Håkansson, J. , & Klinge, B. (2002). Validity and limitations of self‐reported periodontal health. Community Dentistry and Oral Epidemiology, 30(6), 431–437. [DOI] [PubMed] [Google Scholar]
- Caton, J. G. , Armitage, G. , Berglundh, T. , Chapple, I. L. C. , Jepsen, S. , Kornman, K. S. , Mealey, B. L. , Papapanou, P. N. , Sanz, M. , & Tonetti, M. S. (2018). A new classification scheme for periodontal and peri‐implant diseases and conditions—Introduction and key changes from the 1999 classification. Journal of Clinical Periodontology, 45(Suppl. 20), S1–S8. [DOI] [PubMed] [Google Scholar]
- Chapple, I. L. C. , Mealey, B. L. , VanDyke, T. E. , Bartold, P. M. , Dommisch, H. , Eickholz, P. , Geisinger, M. L. , Genco, R. J. , Glogauer, M. , Goldstein, M. , Griffin, T. J. , Holmstrup, P. , Johnson, J. K. , Kapila, Y. , Lang, N. P. , Meyl, J. , Murakami, S. , Plemons, J. , Romito, G. A. , … Yoshie, H . (2018). Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 world workshop on the classification of periodontal and peri‐implant diseases and conditions. Journal of Clinical Periodontology, 45(Suppl. 20), S68–s77. [DOI] [PubMed] [Google Scholar]
- Chatzopoulos, G. S. , Cisneros, A. , Sanchez, M. , Lunos, S. , & Wolff, L. F. (2018). Validity of self‐reported periodontal measures, demographic characteristics, and systemic medical conditions. Journal of Periodontology, 89(8), 924–932. [DOI] [PubMed] [Google Scholar]
- Cohen, J. F. , Korevaar, D. A. , Altman, D. G. , Bruns, D. E. , Gatsonis, C. A. , Hooft, L. , Irwig, L. , Levine, D. , Reitsma, J. B. , de Vet, H. C. , & Bossuyt, P. M. (2016). Stard 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. BMJ Open, 6(11), e012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport, R. H., Jr. , Simpson, D. M. , & Hassell, T. M. (1982). Histometric comparison of active and inactive lesions of advanced periodontitis. Journal of Periodontology, 53(5), 285–295. [DOI] [PubMed] [Google Scholar]
- Deng, K. , Pelekos, G. , Jin, L. , & Tonetti, M. S. (2021). Diagnostic accuracy of self‐reported measures of periodontal disease: A clinical validation study using the 2017 care definitions. Journal of Clinical Periodontology, 48, 1037–1050. 10.1111/jcpe.13484 [DOI] [PubMed] [Google Scholar]
- Dietrich, T. , Bernimoulin, J. P. , & Glynn, R. J. (2004). The effect of cigarette smoking on gingival bleeding. Journal of Periodontology, 75(1), 16–22. [DOI] [PubMed] [Google Scholar]
- Dietrich, T. , Stosch, U. , Dietrich, D. , Schamberger, D. , Bernimoulin, J. P. , & Joshipura, K. (2005). The accuracy of individual self‐reported items to determine periodontal disease history. European Journal of Oral Sciences, 113(2), 135–140. [DOI] [PubMed] [Google Scholar]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England), 392(10159), 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert, A. D. , & Nuttall, N. M. (1999). Self‐reporting of periodontal health status. British Dental Journal, 186(5), 241–244. [DOI] [PubMed] [Google Scholar]
- Goulão, B. , MacLennan, G. S. , & Ramsay, C. R. (2021). Have you had bleeding from your gums? Self‐report to identify gingival inflammation (the sing diagnostic accuracy and diagnostic model development study). Journal of Clinical Periodontology, 48, 919–928. 10.1111/jcpe.13455 [DOI] [PubMed] [Google Scholar]
- Greenstein, G. , Caton, J. , & Polson, A. M. (1981). Histologic characteristics associated with bleeding after probing and visual signs of inflammation. Journal of Periodontology, 52(8), 420–425. [DOI] [PubMed] [Google Scholar]
- Greenwald, P. , Nasca, P. C. , Lawrence, C. E. , Horton, J. , McGarrah, R. P. , Gabriele, T. , & Carlton, K. (1978). Estimated effect of breast self‐examination and routine physician examinations on breast‐cancer mortality. The New England Journal of Medicine, 299(6), 271–273. [DOI] [PubMed] [Google Scholar]
- Ito, H. , Numabe, Y. , Hashimoto, S. , Sekino, S. , Murakashi, E. , Ishiguro, H. , Sasaki, D. , Yaegashi, T. , Takai, H. , Mezawa, M. , Ogata, Y. , Watanabe, H. , Hagiwara, S. , Izumi, Y. , Hiroshima, Y. , Kido, J. I. , Nagata, T. , & Kunimatsu, K. (2016). Correlation between gingival crevicular fluid hemoglobin content and periodontal clinical parameters. Journal of Periodontology, 87(11), 1314–1319. [DOI] [PubMed] [Google Scholar]
- Kallio, P. (1996). Self‐assessed bleeding in monitoring gingival health among adolescents. Community Dentistry and Oral Epidemiology, 24(2), 128–132. [DOI] [PubMed] [Google Scholar]
- Kallio, P. , Ainamo, J. , & Dusadeepan, A. (1990). Self‐assessment of gingival bleeding. International Dental Journal, 40(4), 231–236. [PubMed] [Google Scholar]
- Kallio, P. , Nordblad, A. , Croucher, R. , & Ainamo, J. (1994). Self‐reported gingivitis and bleeding gums among adolescents in Helsinki. Community Dentistry and Oral Epidemiology, 22(5 Pt. 1), 277–282. [DOI] [PubMed] [Google Scholar]
- Kilian, M. , Chapple, I. L. , Hannig, M. , Marsh, P. D. , Meuric, V. , Pedersen, A. M. , Tonetti, M. S. , Wade, W. G. , & Zaura, E. (2016). The oral microbiome—An update for oral healthcare professionals. British Dental Journal, 221(10), 657–666. [DOI] [PubMed] [Google Scholar]
- Kugahara, T. , Shosenji, Y. , & Ohashi, K. (2008). Screening for periodontitis in pregnant women with salivary enzymes. The Journal of Obstetrics and Gynaecology Research, 34(1), 40–46. [DOI] [PubMed] [Google Scholar]
- Lin, J. S. , Piper, M. A. , Perdue, L. A. , Rutter, C. M. , Webber, E. M. , O'Connor, E. , Smith, N. , & Whitlock, E. P. (2016). Screening for colorectal cancer: Updated evidence report and systematic review for the us preventive services task force. JAMA, 315(23), 2576–2594. [DOI] [PubMed] [Google Scholar]
- Mariath, A. A. , Haas, A. N. , Fischer, C. M. , de Araujo, F. B. , & Rösing, C. K. (2009). Professional toothbrushing as a method for diagnosing gingivitis in 3‐ to 6‐year‐old preschool children. Oral Health & Preventive Dentistry, 7(4), 315–321. [PubMed] [Google Scholar]
- Murakami, S. , Mealey, B. L. , Mariotti, A. , & Chapple, I. (2018). Dental plaque‐induced gingival conditions. Journal of Periodontology, 89(Suppl. 1), S17–S27. [DOI] [PubMed] [Google Scholar]
- Nam, S. H. , Jung, H. I. , Kang, S. M. , Inaba, D. , Kwon, H. K. , & Kim, B. I. (2015). Validity of screening methods for periodontitis using salivary hemoglobin level and self‐report questionnaires in people with disabilities. Journal of Periodontology, 86(4), 536–545. [DOI] [PubMed] [Google Scholar]
- Nomura, Y. , Okada, A. , Kakuta, E. , Gunji, T. , Kajiura, S. , & Hanada, N. (2016). A new screening method for periodontitis: An alternative to the community periodontal index. BMC Oral Health, 16(1), 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura, Y. , Tamaki, Y. , Eto, A. , Kakuta, E. , Ogino, D. , Nakamura, Y. , Takahashi, N. , Hino, F. , Koresawa, K. , Hanada, N. , & Shimizu, K. (2012). Screening for periodontal diseases using salivary lactate dehydrogenase, hemoglobin level, and statistical modeling. Journal of Dental Sciences, 7(4), 379–383. [Google Scholar]
- Nomura, Y. , Tamaki, Y. , Tanaka, T. , Arakawa, H. , Tsurumoto, A. , Kirimura, K. , Sato, T. , Hanada, N. , & Kamoi, K. (2006). Screening of periodontitis with salivary enzyme tests. Journal of Oral Science, 48(4), 177–183. [DOI] [PubMed] [Google Scholar]
- Papapanou, P. N. , Sanz, M. , Buduneli, N. , Dietrich, T. , Feres, M. , Fine, D. H. , Flemmig, T. F. , Garcia, R. , Giannobile, W. V. , Graziani, F. , Greenwell, H. , Herrera, D. , Kao, R. T. , Kebschull, M. , Kinane, D. F. , Kirkwood, K. L. , Kocher, T. , Kornman, K. S. , Kumar, P. S. , … Tonetti, M. S. (2018). Periodontitis: Consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri‐implant diseases and conditions. Journal of Periodontology, 89(Suppl. 1), S173–s182. [DOI] [PubMed] [Google Scholar]
- Pham, T. A. , Ueno, M. , Shinada, K. , Yanagisawa, T. , Wright, F. A. , & Kawaguchi, Y. (2011). Periodontal disease and related factors among vietnamese dental patients. Oral Health & Preventive Dentistry, 9(2), 185–194. [PubMed] [Google Scholar]
- Romano, F. , Perotto, S. , Bianco, L. , Parducci, F. , Mariani, G. M. , & Aimetti, M. (2020). Self‐perception of periodontal health and associated factors: A cross‐sectional population‐based study. International Journal of Environmental Research and Public Health, 17(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taani, D. Q. , & Alhaija, E. S. (2003). Self‐assessed bleeding as an indicator of gingival health among 12–14‐year‐old children. Journal of Oral Rehabilitation, 30(1), 78–81. [DOI] [PubMed] [Google Scholar]
- Thomas, D. B. , Gao, D. L. , Ray, R. M. , Wang, W. W. , Allison, C. J. , Chen, F. L. , Porter, P. , Hu, Y. W. , Zhao, G. L. , Pan, L. D. , Li, W. , Wu, C. , Coriaty, Z. , Evans, I. , Lin, M. G. , Stalsberg, H. , & Self, S. G. (2002). Randomized trial of breast self‐examination in shanghai: Final results. Journal of the National Cancer Institute, 94(19), 1445–1457. [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S. , Chapple, I. L. , Jepsen, S. , & Sanz, M. (2015). Primary and secondary prevention of periodontal and peri‐implant diseases: Introduction to, and objectives of the 11th european workshop on periodontology consensus conference. Journal of Clinical Periodontology, 42(Suppl. 16), S1–S4. [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S. , Deng, K. , Christiansen, A. , Bogetti, K. , Nicora, C. , Thurnay, S. , & Cortellini, P. (2020). Self‐reported bleeding on brushing as a predictor of bleeding on probing: Early observations from the deployment of an internet of things network of intelligent power‐driven toothbrushes in a supportive periodontal care population. Journal of Clinical Periodontology, 47(10), 1219–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonetti, M. S. , Greenwell, H. , & Kornman, K. S. (2018). Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. Journal of Clinical Periodontology, 45(Suppl. 20), S149–S161. [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S. , Jepsen, S. , Jin, L. , & Otomo‐Corgel, J. (2017). Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. Journal of Clinical Periodontology, 44(5), 456–462. [DOI] [PubMed] [Google Scholar]
- Trombelli, L. , Farina, R. , Silva, C. O. , & Tatakis, D. N. (2018). Plaque‐induced gingivitis: Case definition and diagnostic considerations. Journal of Clinical Periodontology, 45(Suppl. 20), S44–S67. [DOI] [PubMed] [Google Scholar]
- van Kampen, E. J. , & Zijlstra, W. G. (1983). Spectrophotometry of hemoglobin and hemoglobin derivatives. Advances in Clinical Chemistry, 23, 199–257. [DOI] [PubMed] [Google Scholar]
- Weintraub, J. A. , Finlayson, T. L. , Gansky, S. A. , Santo, W. , & Ramos‐Gomez, F. (2013). Clinically determined and self‐reported dental status during and after pregnancy among low‐income hispanic women. Journal of Public Health Dentistry, 73(4), 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Figure S1 Relation between self‐reported gingival bleeding on brushing (GBoB) and the total amount of Hb in the TPS samples. (A) Distribution of the total amount of Hb in subjects with or without self‐reported GBoB. Each point denotes the value for a participant. The horizontal bars display the medians and interquartile ranges (IQR). The horizontal green line denotes the threshold value for GBoB being self‐detected (Hb ≥77.05 μg). (B) ROC analysis of Hb, comparing total amount in subjects with self‐reported GBoB to those without GBoB.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


