Abstract
Aims and Objectives
To identify and compare frailty instruments used with hospitalised orthopaedic patients aged over 65.
Background
Frailty predicts clinical events in orthopaedic patients aged over 65. However, the strengths and limitations of different approaches to measuring frailty in this population are rarely discussed. As such, a comprehensive review to address the gap is needed.
Design
Scoping review using Arksey and O’Malley framework.
Methods
PubMed, CINAHL, PsycINFO, Scopus and EMBASE databases were searched to identify studies published from 2006 to 2020 regarding frailty instruments in older orthopaedic patients. The Preferred Reporting Items for Systematic Reviews and Meta‐analyses were followed.
Results
The initial search resulted in 1,471 articles. After review against inclusion and exclusion criteria, a final set of 31 articles containing 15 unique frailty instruments were evaluated. Most of the articles were from Western countries. Fried's phenotype and Frailty Index were commonly used. The frailty index was mostly modified to measure frailty. In hip fracture, physical function items were frequently modified in the measurement of frailty. Trained physicians and nurses administered most frailty instruments. Frailty screening was commonly conducted at hospital admission and used to prognosticate both postoperative complications and hospital outcomes. Most instruments could be completed within 10 min. Reported psychometrics had acceptable reliability and validity.
Conclusion
Many reliable frailty measures have been used in the inpatient orthopaedic settings; however, evidence is still lacking for a gold standard frailty instrument. More research is needed to identify the best‐performing measure. Frailty evaluation in patients with physical limitations is challenging with existing instruments. Clinical context, resources required and instrument quality are essential factors in selecting a frailty instrument.
Relevance to clinical practice
Musculoskeletal symptoms in older patients may bias frailty assessment. Proactive frailty screening with valid and practical instruments is vital to strengthen preoperative risk stratification and improve post‐surgical outcomes.
Keywords: aged, frailty, hospital, measurement, older adults, orthopaedic, outcomes, patients, scoping review, surgical
What does this paper contribute to the broader global clinical community?
Frailty is a common syndrome associated with poorer health outcomes in older orthopaedic patients. The clinical manifestations of musculoskeletal (MSK) conditions in this population may bias frailty classification; thus, tailored frailty assessment to be specific frailty instruments might be efficient in improving quality of care.
The scoping review identified 15 valid frailty instruments used in orthopaedic settings to guide the clinicians to stratify risk before operation. The Reported Edmonton Frail Scale, FRAIL Scale, PRISMA‐7 and Groningen Frailty Index may be practical with time efficient and less requirements for administration; however, further validated instruments for specific orthopaedic conditions or settings are crucial.
Current evidence is insufficient to prioritise one frailty instrument over another for screening older orthopaedic patients; therefore, clinical context, resources and pragmatic considerations should guide the decision for frailty instrument selection. Integrating additional resources—such as family member input or biomarkers—might be beneficial for monitoring frailty trajectories.
1. INTRODUCTION
As life expectancy has increased, promotion of healthy ageing, maintenance of functional ability and support of well‐being in older adults has become a global priority (World Health Organization, 2017). In older adults, musculoskeletal (MSK) conditions are expected, have a negative impact on functional ability and lead to an increased burden of disease (Briggs et al., 2016; Briggs & Dreinhofer, 2017). To slow down or reverse the functional decline, surgical treatment may be required. Orthopaedic surgeries for conditions like hip, knee and spine degeneration have increased, with a parallel rise in postoperative complications and mortality seen in older populations (Gleason et al., 2017; McIsaac et al., 2018; Rothrock et al., 2018; Segal et al., 2018; Wilson et al., 2018). Frailty is a syndrome that has emerged and been identified as an important concept that captures older adults' vulnerability to adverse health events (Fried et al., 2001, 2004). Although the prevalence of frailty is low in MSK conditions [approximately 4.9–10.7%; (Choi et al., 2015; Nguyen et al., 2015)], it is associated with degenerative MSK conditions such as rheumatoid arthritis, osteoporosis and osteoarthritis (Milte & Crotty, 2014; Zlobina et al., 2015). Frailty is also linked to an increase in adverse surgical outcomes and postoperative complications (Ondeck et al., 2018; Theou et al., 2018). Frail older adults with MSK conditions are at higher risk of mortality, fall‐related injury, disability and hospital readmission (Bellamy et al., 2017; Charest‐Morin et al., 2018; Ondeck et al., 2018; Shin et al., 2016; Walters et al., 2016). There is no proven pharmacological treatment to reverse frailty. Prevention and early screening of frailty can improve clinical care if used for risk stratification (Dent et al., 2016; Theou et al., 2018), treatment decision‐making and surgical planning. Hence, identifying and using efficient frailty screening instruments could delay dependency, promote health and support the well‐being of older orthopaedic populations.
Despite the opportunities of screening for frailty in orthopaedic populations, challenges remain. The first challenge is to identify which instruments provide accurate frailty identification in orthopaedic patients. Evidence underlined that the musculoskeletal ageing phenotype, comprising osteoporosis, osteoarthritis (OA) and sarcopenia may affect the accuracy of a frailty evaluation (Dasgupta et al., 2009; Kistler et al., 2015; Krishnan et al., 2014; Kua et al., 2016). The predominant clinical characteristics of orthopaedic patients, particularly physical limitations, weakness or immobility due to pain and neuromuscular impairment may cause a misinterpretation of someone being frail (Beaudart et al., 2015; Chen et al., 2014; Collino et al., 2013). As such, orthopaedic patients who are frail may present either as highly sensitive or insensitive to frailty measures. A recent study underlined that selecting a frailty instrument that fits with a specific orthopaedic population may be the best for clinical risk stratification (Mahmooth et al., 2020). Awareness of frailty emerged for more than two decades; however, no previous evidence regarding frailty instruments has been closely investigated in the orthopaedic population. The second challenge in assessing frailty is how to apply assessment tools in diverse inpatient settings. Although various measures have been developed, frailty instruments are unavailable in many geographical areas and languages (Buta et al., 2016). Overcoming language barriers and cultural issues to assess frailty is vital for better care.
Commonly used frailty measures may not be suitable for all hospital settings because of resource limitations, clinical context, instrument quality and cultural sensitivity considerations (Buta et al., 2016; Theou et al., 2018). Clinical contexts might be specific to equipment availability, time to complete the assessment, the measure's quality and cultural sensitivity. Recently, a scoping review (Church et al., 2020) was published focusing on the Clinical Frailty Scale, but reviews assessing other frailty instruments have not been assessed. To gain better knowledge about frailty instruments used in the orthopaedic population, conducting a new scoping review has advantages over other forms of review in providing a broad perspective (Grant & Booth, 2009) and clarifying a basis for currently implementing frailty instruments in this population. Research into frailty assessment has a long history; therefore, analysing different frailty instruments in the inpatient orthopaedic population is critical to inform care by applying clinical judgment‐based frailty instruments correctly. This review covers the gaps mentioned above and provides information about frailty instruments used by clinical specialists and healthcare providers caring for patients with orthopaedic conditions.
2. METHODS
2.1. Aim
To identify and compare frailty instruments used with hospitalised patients aged over 65.
2.2. Design
The Arksey and O'Malley framework (2005) was used as a guide for this review. The framework has five steps: 1) identifying the research question; 2) identifying relevant studies; 3) study selection; 4) charting the data; and 5) assembling, summarising and reporting the results.
2.3. Methods and search strategy
Stage 1: identifying the research question
The first stage included a preliminary exploration of the literature to identify knowledge gaps on frailty in hospitalised orthopaedic patients. Research questions for this review were as follows: 1) What frailty instruments are currently in use in inpatient orthopaedic settings?; 2) Which instruments are reliable and practical to measure frailty in an inpatient orthopaedic setting?
Stage 2: identifying relevant studies/search strategy
Two co‐authors (IR & OZ) performed the literature search in consultation with a health science librarian. The search was conducted in the main health databases—PubMed, CINAHL, PsycINFO, Scopus and EMBASE. The combination of Medical Subject Headings (MeSH) and keywords were modified for each database to optimise search strategies. The search included publications from 1 July 2006 to 31 December 2020. Keywords used were ‘frailty’, ‘orthopaedic’, ‘instrument’ or ‘scale’ or ‘indicator’, and ‘older adults’. The search strategies are presented in the supplement (Appendix S2). Last, the co‐authors (IR, OZ and SA) independently verified search terms and discussed initial results to confirm the strategies performed.
Stage 3: study selection
Peer‐reviewed original research articles and hand‐searched articles retrieved from the databases were considered eligible for review. The articles that have not been formally published or archived in a peer‐review format, such as conference proceedings, preprints, policy or hospital reports, and the grey literature was not included.
Eligibility criteria
The inclusion criteria were as follows: 1) article included frailty instrument(s); 2) the instrument was completed by hospitalised older adults or also by healthcare providers; 3)the average age of study participants was 65 years or older; 4) setting was hospital orthopaedic settings (units, wards or surgical department including orthopaedics); 5) article was written in English; and 6) published between July 2006 and December 2020. The exclusion criteria were articles: 1) unrelated to frailty; 2) focused on frailty in community settings; and 3) mentioned frail patients without measuring frailty.
Selection of studies for inclusion
Following the initial search, two co‐authors (IR and SA) independently reviewed the titles and abstracts to determine eligibility. Next, they independently reviewed full‐text articles and identified potential articles for inclusion. A third person arbitrated any disagreement among the initial reviewers. The third person (OZ) reviewed the articles in which there was disagreement about inclusion between initial reviewers, discussed and decided to include or exclude. The workflow was summarised using the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA 2020) guideline for scoping reviews (Figure 1) (Page et al., 2021; Tricco et al., 2018). Checklist of Preferred Reporting Items for Systematic reviews and Meta‐Analyses extension for Scoping Reviews (PRISMA‐ScR) are presented in File S1.
FIGURE 1.
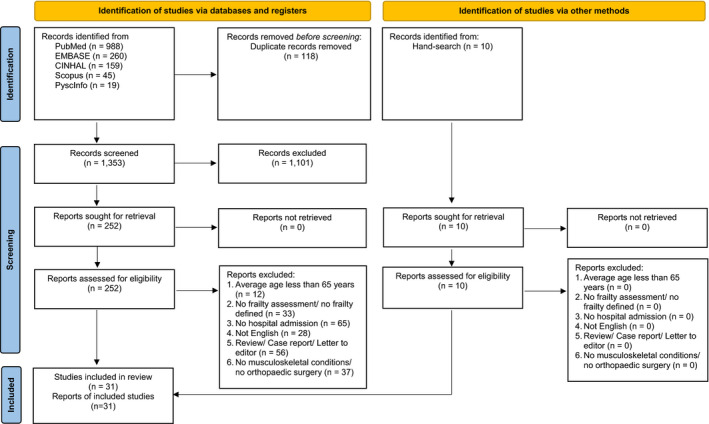
PRISMA 2020 flow diagram [Color figure can be viewed at wileyonlinelibrary.com]
Stage 4: charting the data
The combination of Microsoft Excel 2016, EndNote X7 and Rayyan application was used to remove duplicates and build a summary table focusing on: the country the study was conducted in, study design, name of frailty instrument, number and type of items, administration instructions and time to complete the instrument, scoring, population, health outcomes, instrument requirement and quality of the instrument. A subset of five studies was selected to pilot data extraction and valid agreement evaluation before beginning the complete review processes.
Stage 5: assembling, summarising and reporting the results
The identified studies were summarised, and relevant data were abstracted (Table 1, Appendix S2). Following Arksey and O’Malley (2005), no quality appraisal of the included studies was conducted. The reliability, responsiveness and validity of each frailty instrument were evaluated based on the standard measurement properties of health questionnaires (Terwee et al., 2007).
TABLE 1.
Summary of characteristics of frailty instruments commonly used in orthopaedic patients
|
Frailty Instrument |
Domains | Population/References | Outcome of interest |
|---|---|---|---|
| EFS | Nine domains (9 items): Cognitive impairment, dependence on daily activity living, recent burden illness, self‐perceived health, depression, weight loss, medication issues, incontinence, inadequate social support and mobility difficulty. | A single non‐cardiac surgery (mean age 77)/(Dasgupta et al., 2009) |
|
| EFS | Vertebral fracture (mean age 80)/(Walters et al., 2016) |
|
|
| mFI | Eleven domains (11 items) from the Canadian Study of Health and Aging Frailty Index (CSHA‐FI) matched to items from the American College of Surgeons National Surgical Improvement Program (NSQIP): change in everyday activity, problems with getting dressed, history of diabetes mellitus, lung problems, respiratory problems, congestive heart failure, myocardial infarction, cardiac problem, cerebrovascular problem, history of stroke and decrease peripheral pulses. | Primary TKA & THA (mean age 66)/(Shin, 2016) |
|
| mFI | THA (mean age 82)/(Ondeck et al., 2018) |
|
|
| mFI | Orthopaedic surgery (mean age 79.5)/(Vu et al., 2017) |
|
|
| mFI | THA (mean age 71.2)/(Bellamy, 2017) |
|
|
| mFI | Primary TKA (mean age 70.75)/(Runner, 2017) |
|
|
| mFI | Non‐complex lumbar spine surgery (median age 72)/(Charest‐Morin et al., 2018) |
|
|
| mFI | Intertrochanteric femur fractures (mean age 73)/(Boissonneault et al., 2019). |
|
|
| FP criteria | Five domains (5 items) and adapted some domains by using self‐report due to patient conditions: Shrinking (self‐reported), exhaustion (self‐reported), slowness (self‐reported), weakness (grip strength) and physical activity (Minnesota leisure time questionnaire) | Hip fracture (mean age 86)/(Kistler et al., 2015) |
|
| FP criteria | Five domains (5 items) as CHS and Women's Health and Aging Study (WHAS): Slow gait (3m‐walk), weakness (grip strength), low activity (energy expenditure), involuntary weight loss and exhaustion | Orthopaedic surgery (mean age 77)/(Cooper et al., 2016) |
|
| FP criteria | Lumbar spine stenosis (mean age 71)/(Kim et al., 2018) |
|
|
| FP criteria | General elective non‐cardiac surgical patients (mean age 74)/(Birkelbach O. et al., 2019). |
|
|
| FI | Multiple domains (51 deficit domains) based on deficits identified at the time of assessment | Hip fracture (mean age 81)/(Krishnan et al., 2014) |
|
| FI | Multiples domains (42 deficits domains) | Orthopaedic surgery (mean age 77)/(Cooper, 2016) |
|
| FI | Multiple domains (70 deficits domains) | Patients with elective surgeries for spinal disorders (mean age 65)/(Yagi et al., 2018) |
|
| FI | Multiple domains (29 deficits domains) | Fragility fracture in DM type 2 patients (mean age 65)/(Li et al., 2019) |
|
| FI | Multiple domains (32 deficits domains) | Patients who were undergoing unilateral primary or revision THA (median age 68)/(Johnson et al., 2019b) |
|
| FI | Multiple domains (32 deficits domains) | Patients undergoing unilateral primary or revision TKA (median age 69)/(Johnson et al., 2019a). |
|
| MFC | Modified five domains from FP criteria (5items): Exhaustion, weight loss, weakness, modified slowness and physical activity level | Hip fracture (mean age 79.1)/(Kua, 2016) |
|
| Modified Fried Index | Modified five domains from CHS (5 items): Weight loss (>101 lbs unintentionally in the prior year), grip strength (lowest 20% by gender and body mass index), exhaustion (self‐report), slowness (asking about 15 feet walking ability speed by gender and height) and low activity (kilocalories per week male<383, female<270). | Surgical patients included orthopaedic (mean age 73)/(McIsaac et al., 2018) |
|
| REFS | Nine domains (13 items): General health status, nutrition, self‐reported performance, functional independence, cognition, social support, medication use, mood and continence | Hip fracture (mean age 79.1)/(Kua, 2016) |
|
| REFS‐Thai | Older adults who scheduled for elective orthopaedic surgery (mean age 72)/(Roopsawang et al., 2020) |
|
|
| Hip‐MFS | Eight domains (8items): Serum albumin level, mid‐arm circumference, Charlson comorbidity index, walking dependency, cognitive function, risk of fallings, nutrition status and sex | Hip fracture (mean age 80.4)/(Choi, 2017) |
|
| MFST‐HP | Three domains (15 items): physical (9 items), psychological (4 items) and social (2 items). | Hospitalised older adults including orthopaedic (mean age 76.7)/(Warnier, 2016) |
|
| 5 items mFI | Five Domains (5 items): History of Diabetes Mellitus, Congestive Heart Failure (new diagnosis or exacerbation of chronic congestive heart failure within 30 days of surgery), hypertension requiring medication, history of chronic pulmonary disease or pneumonia, and non‐independent functional status (wholly or partially dependent in activities of daily living within the last 30 days prior to surgery). | Patients undergoing distal radius fracture procedure (mean age 65)/(Wilson et al., 2018) |
|
| 5 items mFI | Patients undergoing Kyphoplasty vertebral augmentation (mean age 73.98)/(Segal et al., 2018) |
|
|
| 5 items mFI | Patients undergoing total joint arthroplasty (mean age 66) (Traven, Reeves, Sekar, Slone, & Walton, 2019) |
|
|
| 5 items mFI | Patients undergoing total shoulder arthroplasty (mean age 70.4) (Holzgrefe et al., 2019) |
|
|
| CFS | Four domains (N/A items): Mobility, energy, physical activity and function | Surgical patients included orthopaedic (mean age 73)/(McIsaac et al., 2018) |
|
| CSHA‐CFS | Hip fracture (mean age 78) (Chen et al., 2019). |
|
|
| FRAIL scale | Five Domains (5 items): Fatigue, resistance, aerobic capacity, illness and weight loss. | Spine surgery (median age 71)/(Rothrock et al., 2018) |
|
| FRAIL scale | Orthopaedic trauma surgery(mean age 82.3)/(Gleason et al., 2017) |
|
|
| PRISMA−7 | Seven Domains (7 items): Age >85 years, male gender, health problems that limit activities, needs for support by others, health problems that require staying home, or someone taking care of, using a walker or wheelchair. | Vertebral fracture (mean age 80)/(Walters et al., 2016) |
|
| GFI | Four Domains (15 items): Physical, cognition, social and psychological. | Vertebral fracture (mean age 80)/(Walters et al., 2016) |
|
| GFI | Patients who underwent hip fracture surgery (mean age 83)/(Winters, Hartog, Roijen, Brohet, & Kamper, 2018). |
|
Abbreviations: 5 items mFI, 5 items modified Frailty Index; CFS, Clinical Frailty Scale; CSHA‐CFS, Chinese‐Canadian Study of Health and Aging Clinical Frailty Scale; EFS, Edmonton Frail Scale; FI, Frailty Index; FP criteria: Fried's Frailty Phenotype criteria; FRAIL scale, Fatigue, Resistance Ambulation, Illness and Loss of Weight scale; GFI, Groningen Frailty Indicator, THA, Total hip arthroplasty; Hip‐MFS, Hip‐Multidimensional Frailty Score; MFC, Modified Fried's Criteria; mFI, Modified/Simplified Frailty Index; MFST‐HP, Maastrich Frailty Screening Tool for Hospitalized Patients; REFS, Reported Edmonton Frail Scale; TKA, Total knee arthroplasty.
3. RESULTS
3.1. Characteristics of publications
The search initially identified a total of 1,471 articles (Figure 1). After duplicate articles were removed and abstracts screened, 262 articles were included for final review, with 95 percent agreement between three reviewers. After a full‐text review, 31 articles were retained and evaluated for this review. Studies in the final sample included hospitalised older adults in orthopaedic settings (Tables 1 and 2). Data for the articles were collected in the United States (n = 9); United Kingdom (n = 4); Canada (n = 3); Korea (n = 2); Singapore (n = 2); Taiwan (n = 1); and Thailand (n = 1). Sixteen percent of the studies used retrospective designs (n = 5). Of all included studies, there were 681,684 orthopaedic patients; the average age of participants was 81 (range 65–92) years old; nearly 60% of these participants were female. Study participants had multiple comorbidities and underwent orthopaedic surgical procedures, mostly were related to the knee (51.59%), hip (39.53%) and non‐specific orthopaedic conditions (5.91%) (Figure 2). We identified 15 unique frailty instruments. Translation and cross‐cultural validation were mentioned for 8 instruments (53.33%; Table 2).
TABLE 2.
Comparison and evaluation of frailty instruments commonly used in orthopaedic settings
| Comparison | Measurement | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EFS | mFI | PF Criteria | FI | Modified Fried Index | MFC | REFS | Hip‐MFS | MFST‐HP | 5 items mFI | CFS | CSHA‐CFS | FRAIL‐Scale | PRISMA−7 | GFI | |
| Country of study | US/UK | CA/US | US/KR | US/UK | CA | SG |
SG TH |
KR | US | US | US | TW | US | US | UK |
| Retrospective study design | √ | √ | √ | √ | √ | ||||||||||
| Administration: | |||||||||||||||
| Observation | |||||||||||||||
| Self‐reported only | √ | √ | √ | √ | |||||||||||
| Self‐reported +performance | √ | √ | √ | √ | |||||||||||
| Self‐reported +other standard assessments | √ | √ | √ | √ | √ | √ | √ | ||||||||
| Frailty Domains | 10 | 11 | 5 | 10+ | 5 | 5 | 9 | 8 | 3 | 5 | 4 | 2 | 5 | 7 | 7 |
| Number of items | 10 | 11 | 5 | 51, 42 | 5 | 5 | 13 | 8 | 15 | 5 | N/A | N/A | 5 | 7 | 15 |
| Cut‐off point of frailty | ≥ 7 | ≥ 0.25 | ≥ 3 | Varies | ≥ 3 | ≥ 3 | ≥ 8 | ≥ 8 | High score, more frail | ≥ 2 | ≥ 4 | ≥ 5 | ≥ 3 | ≥ 3 | ≥ 4 |
| Modified from the original measurement | √ | √ | √ | √ | √ | √ | |||||||||
| Requirement of measurement: | |||||||||||||||
| Other assessment information | X | √ | X | X | X | X | √ | √ | √ | √ | X | X | X | X | X |
| Specific equipment | X | X | √ | X | √ | √ | X | √ | X | X | X | X | X | X | X |
| Specific training | √ | √ | √ | √ | √ | √ | X | √ | X | √ | √ | √ | X | X | X |
| Time preference for measuring frailty | |||||||||||||||
| Preoperative | √ | √ | √ | √ | √ | √ | √ | √ | 48 h. post‐admission | √ | √ | √ | 1st day of admission | √ | √ |
| Human resources involved | |||||||||||||||
| Researcher/Training | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| Nurses | √ | ||||||||||||||
| Content validity | √ | X | √ | X | X | X | √ | X | X | X | X | X | X | X | X |
| Internal consistency | X | X | X | √ | X | X | √ | X | X | X | X | X | X | X | √ |
| Criterion validity | √ | √ | √ | √ | √ | √ | √ | √ | X | √ | X | √ | √ | √ | √ |
| Construct validity | X | X | X | X | X | X | √ | X | √ | X | X | X | X | X | X |
| Reliability | X | X | X | √ | X | X | √ | X | √ | X | X | X | X | X | X |
| Responsiveness | X | √ | X | √ | √ | X | √ | √ | X | X | √ | X | X | X | X |
| Time to complete (minutes) | N/A | N/A | N/A | <10 | <6 | 3–5 | ≤5 | N/A | <3 | N/A | <1 | <3 | N/A | N/A | N/A |
| Cross‐culture validation study | √ | N/A | N/A | √ | N/A | N/A | √ | N/A | N/A | N/A | √ | √ | √ | √ | √ |
Abbreviations: CA, Canada; CFS, Clinical Frailty Scale; CSHA‐CFS, Chinese‐Canadian Study of Health and Aging Clinical Frailty Scale; EFS, Edmonton Frail Scale; Fatigue, Resistance Ambulation, Illness and Loss of Weight scale; FI, Frailty Index; FP criteria: Fried's Phenotype Criteria; GFI, Groningen Frailty; Hip‐MFS, Hip‐Multidimensional Frailty Score; KR, South Korea; MFC, Modified Fried's Criteria; mFI, Modified/Simplified Frailty Index; MFST‐HP, Maastrich Frailty Screening Tool for Hospitalized Patients; REFS, Reported Edmonton Frail Scale; SG, Singapore; TH, Thailand; TW, Taiwan; UK, United Kingdom; US, The United States of America; √, report; X, no report; N/A, No information.
FIGURE 2.
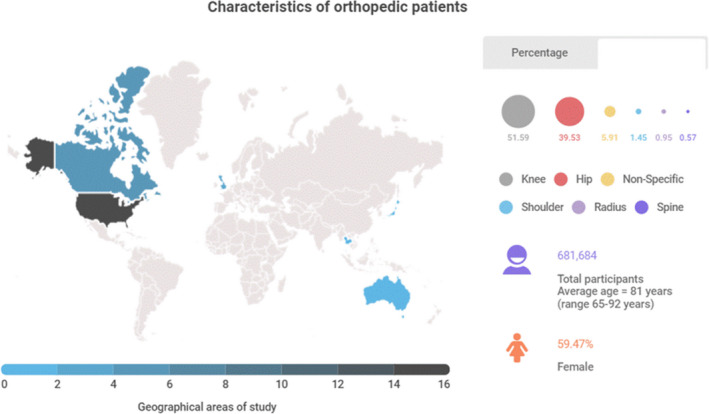
Characteristics of orthopaedic patients and geographical locations of studies [Color figure can be viewed at wileyonlinelibrary.com]
3.2. Frailty instruments used in an orthopaedic setting
Different frailty concepts led to differences in operational definition, structural domains, scales and scorings across the studies. Fifteen frailty instruments were identified (Table 1): the Edmonton Frailty Scale (EFS); modified Frailty Index (mFI)/Simplified Frailty Index; Fried's Phenotype criteria (FP criteria); Frailty Index (FI); Modified Fried Index; Modified Fried's Criteria (MFC); Reported Edmonton Frailty Scale (REFS); Hip‐Multidimensional Frailty Score (Hip‐MFS); Maastricht Frailty Screening Tool for Hospitalized Patients (MFST‐HP); 5‐item mFI; Clinical Frailty Scale (CFS); Chinese‐Canadian Study of Health and Aging Clinical Frailty Scale (CSHA‐CFS); FRAIL Scale (Fatigue, Resistance, Ambulation, Illnesses and Loss of Weight); PRISMA‐7; and Groningen Frailty Index (GFI). Across all instruments, the number of frailty domains varied from two to more than ten domains. The number of items ranged from 5 (e.g. FP criteria) to 51 items (e.g. Frailty Index). Self‐report combined with other assessment methods (66.67%, n = 10) were used. One tool—the CFS stated using clinical descriptors, pictographs of activity and functional assessment for frailty screening. Six frailty instruments (40%) were modified from the originally published instruments (Table 2).
Deficit accumulation (n = 6) and phenotype‐informed (n = 4) were the main approaches to determine frailty; notably, the deficit accumulation of frailty was widely modified for measuring frailty (n = 11). Physical function, fatigue, weight loss, cognitive function, limitation of activities and comorbidity were common criteria across instruments (n = 14). Two instruments, the FP criteria (Kistler et al., 2015) and MFC (Kua et al., 2016), were used to measure frailty in hip fracture patients and modified physical function/walking performance measures in order to allow for self‐report (Table 1). Most instruments used a binary cut‐off point (frail/non‐frail), but the FI had more than one cut‐off to quantify frailty severity. Frailty was commonly measured pre‐operatively (100%). Frailty assessments were used to predict short and long‐term outcomes such as postoperative complications (19 studies), length of stay (LOS [15 studies]), mortality rate (13 studies), discharge disposition (12 studies), physical or cognitive function (10 studies), readmission (9 studies), any adverse events (7 studies) and reoperation (5 studies).
3.3. Data sources, equipment and training
Assessing frailty required different resources (Table 2). Most frailty instruments required training for use (n = 10, 66.66%), while many used other standard assessment information (n = 5, 33.33%) or additional equipment (n = 4, 26.66%). The mFI, FI, MFST‐HP and 5 items mFI required information from medical records. The Hip‐MFS relied on many sources, including standard assessment information, physical performance, laboratory testing, mid‐arm circumference and specific training to evaluate frailty. The PF criteria required training, physical performance testing and specific equipment (a hand‐grip strength dynamometer). The Modified Fried Index and MFC also needed specific training and a dynamometer. In contrast, only four frailty instruments (REFS, Frail Scale, PRISMA‐7 and GFI) obviated the need for specific equipment and training to measure clinical frailty.
3.4. Measurement occasion and time
All frailty instruments were utilised for preoperative assessment on hospital admission. Of these, in two instruments, the authors selected a time point to measure frailty: the MFST‐HP was used to assess frailty at 48 hrs post‐hospital admission, while the FRAIL Scale was used to evaluate frail status on the first day of hospital admission.
The time spent to complete the frailty measures was reported in less than half the studies (n = 6; 47%), with time to complete ranging between 1 and 10 mins (n = 6). The MFC, MFST‐HP and Modified Fried Index were completed within <6 mins. Employing the REFS, however, the patients needed approximately 5 mins to complete it. The CFS and CSHA‐CFS were reportedly completed within 3 mins (Table 2).
3.5. Human resources
Evaluating frailty, the MFST‐HP was used by registered nurses (RNs) without additional training (Warnier et al., 2016). The other frailty instruments used trained research staff, such as physicians, to administer the frailty instruments (n = 14). All frailty instruments assessed the frailty status of the patients, yet none of these studies mentioned other people such as proxy, caregivers or family members who might be involved in the evaluation. Staff requested assistance with using frailty instruments that included data from other sources and/or trained personnel.
3.6. Quality of instrument properties
One article referred to content validity testing of the REFS‐Thai (Roopsawang et al., 2020a, 2020b). Two articles that included the REFS‐Thai (Roopsawang et al., 2020a, 2020b) and MFST‐HP mentioned construct validity testing (Warnier et al., 2016). Eighty‐three per cent of the articles (n = 25) reported criterion validity; the American Society of Anesthesiologists (ASA), Charlson Comorbidity Index (CCI) and other standard instruments were frequently selected to confirm validity testing (Bellamy et al., 2017; Choi et al., 2017; Cooper et al., 2016; Dasgupta et al., 2009; Holzgrefe et al., 2019; Kua et al., 2016; Roopsawang et al., 2020a; Runner et al., 2017; Shin et al., 2016; Vu et al., 2017). Three instruments (EFS, PRISMA‐7 and GFI) tested criterion validity with other frailty instruments (Walters et al., 2016). The FP criteria, FI, MFST‐HP and CSHA‐CFS were verified for reliability. The MFST‐HP demonstrated excellent reliability, both intra‐rater and inter‐rater (ICC (intra‐rater) =0.93, ICC (inter‐rater) =0.95) (Warnier et al., 2016) (Appendix S2). Poor reliability (weighted Kappa <0.6) was reported in FP criteria and FI (K = 0.53 (95% CI 0.44–0.61), 0.42 (95% CI 0.36–0.49), respectively) (Cooper et al., 2016). The MFC mentioned reliability from original studies, but not in the orthopaedic population (Kua et al., 2016). Forty per cent of frailty instruments (n = 6) were evaluated for responsiveness: EFS (Dasgupta et al., 2009), mFI (Boissonneault et al., 2019; Ondeck et al., 2018), Hip‐MFS (Choi et al., 2017), Modified Fried's Index and CFS (McIsaac et al., 2018) demonstrated intermediate quality; however, the REFS‐Thai (Roopsawang et al., 2020a) indicated excellent quality. The FI (Krishnan et al., 2014) and REFS‐Thai (Roopsawang et al., 2020a) showed good quality in predicting most of the adverse clinical outcomes (Figure 3 and Appendix S2). The findings of this scoping review demonstrated that most of the frailty instruments were valid, but more investigation is needed regarding reproducibility [agreement or reliability (36.6%)], responsiveness (34.14%) and cross‐cultural validation (23.17%) (Figure 3, Table 2 and Appendix S2). These results suggest that the FI, REFS‐Thai and MFST‐HP demonstrated an acceptable to good quality and predictability; while FRAIL Scale, REFS‐Thai, PRISMA‐7 and GFI may be practical tools for evaluating frailty in orthopaedic patients as they require no equipment nor training for administration (Figure 3, Table 2 and Appendix S2). Notably, current evidence is insufficient to prioritise one frailty instrument over another for screening older orthopaedic patients.
FIGURE 3.
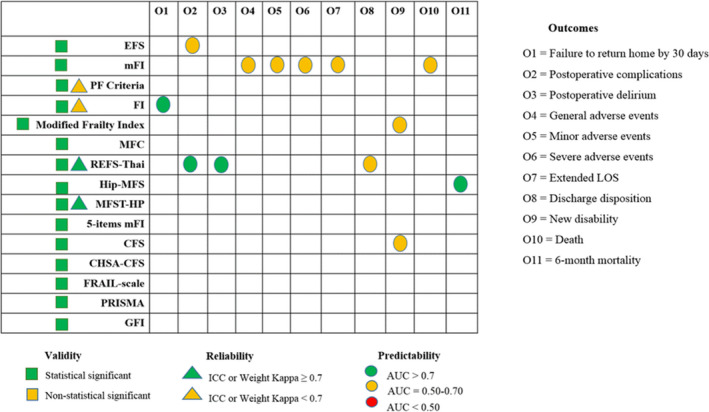
Quality of frailty instruments used in orthopaedic patients [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
This scoping review identified and evaluated 15 unique, reliable frailty instruments used with hospitalised older adults in orthopaedic settings. The Frailty Index and Fried phenotype were the most commonly used. Modification of frailty instrument, particularly physical function assessment, was frequently identified in hip fracture patients. This review adds to the literature by critically examining frailty instruments when used in older inpatient orthopaedic populations.
Across all studies, regardless of instrument, where the outcome was measured, frailty resulted in increased postoperative complications, adverse events, reoperation, readmission, mortality rate and prolonged LOS, and differences in discharge disposition. In orthopaedic settings, however, more evidence is needed to identify the best‐performing frailty instrument.
Based on our review, there are three potential concerns in selecting an instrument for identifying frailty in orthopaedic patients. First, understanding the clinical context, such as the clinical orthopaedic characteristics, is essential in measuring frailty. Although our findings revealed few commonalities across frailty instruments, adjustment of these instruments may be necessary in orthopaedic clinical populations. The review revealed that some authors modified existing frailty instruments, including tailoring scores, changing cut‐off points and adapting components from the original version. The FP criteria were altered. Its name was changed to measure frailty: MFC and Modified Fried Index. These findings are similar to a meta‐analysis analysing current frailty instruments that indicated that there were 262 different versions of the FP criteria used in clinical settings (Theou et al., 2015). Clinical characteristics of orthopaedic patients such as poor physical function, muscle loss/weakness and posture imbalances may impede interpretation of a frailty assessment. A few frailty instruments have been created for specific MSK conditions like the Hip‐MFS for hip fractures (Choi et al., 2017). The FP criteria (Kistler et al., 2015) and MFC (Kua et al., 2016) modified physical function components with the aim to precisely measure frailty in older adults with physical limitations. Our findings emphasise that identifying frailty using existing instruments in an orthopaedic population could be complicated due to the overlap between physical limitations and frailty, which impacts the interpretation of these measures (Dasgupta et al., 2009; Fried et al., 2004; Kistler et al., 2015; Krishnan et al., 2014; Kua et al., 2016). Tailored frailty assessment that suits specific orthopaedic populations may provide more in‐depth clinical information (Mahmooth et al., 2020).
Second, it is crucial to identify the means needed to use a frailty instrument, such as equipment requirements and human resources. It is also essential to factor in the amount of time to complete the measure. Equipment costs for measuring frailty should be considered when selecting an instrument for use in limited‐resource hospitals and clinics. The FP criteria, Modified Fried Index and MFC required objective measurement via a dynamometer to evaluate one component of frailty. The Hip‐MFS used surrogate markers and specific laboratory values in evaluating frailty. Using only self‐report for assessment of frailty has advantages; however, it may increase biases affecting frailty classification. Using a more sophisticated instrument that requires additional human resources requirements and equipment makes frailty evaluation less practical—in hospitals with staffing limitations, using instruments that do not require additional training, such as the REFS, FRAIL Scale, PRISMA‐7 and GFI, may be most appealing. None of the studies mentioned other people—proxy, caregivers or family members who might be involved in frailty evaluation in this review. Integrating family members and caregivers to evaluate frailty may provide additional contextual and clinical information.
Our findings revealed a range of completion time for frailty screening (1–10 mins). Increasing the time of frailty assessment may depend on factors including the number of items, clinical experience, the specific MSK limitations and the complexity of the assessment. The majority of frailty instruments required the clinical experience of the users; thus, novice clinicians spent more time than experts in administering an instrument. Notably, functional limitation due to MSK conditions may increase the time to complete a frailty evaluation, especially one that involves physical performance. Hospitals should be concerned about fostering early detection and screening for frailty as one means to promote health outcomes and control costs (Grimes et al., 2018; World Health Organization, 2017).
Third, focusing on the quality of instruments is significant in accurately detecting frailty. In our review, poor inter‐rater reliability was discovered in several common frailty instruments: FP criteria and FI (Cooper et al., 2016). The poor inter‐rater reliability indicated a difference in the judgment of frailty in orthopaedic patients. These findings echo previous literature findings that musculoskeletal ageing phenotype or clinical symptoms interfere with the accuracy of frailty evaluation (Dasgupta et al., 2009; Kistler et al., 2015; Krishnan et al., 2014; Kua et al., 2016) and confirm overlap of frailty and disability (Fried et al., 2001, 2004). In an ageing society, MSK conditions are a significant health problem, so frailty identification is needed to promote health and equity. Our findings are consistent with a recent systematic review of frailty instruments on the most instruments used in acute care (Theou et al., 2018). Despite the research into frailty having a long history, possibly two decades, there remains a paucity of evidence on frailty instruments in diverse geographical areas. As most instruments integrate self‐report in measuring frailty, clear communication is of concern (Fick & Lundebjerg, 2017). Promoting effective frailty screening through translation and cultural adaptation across different settings will promote health equity.
5. REVIEW LIMITATIONS
This review had several limitations. First, it only considered studies in English. Second, based on the search terms and selection criteria used, some relevant studies might have been missed, such as emergency orthopaedic surgery and surgery for bone tumour/sarcoma and adults less than 65 years of age. Another source of limitation is due to some health databases were not included for identifying research articles. However, we are confident that the findings provide helpful evidence on frailty instruments used for hospitalised older adult orthopaedic patients.
6. CONCLUSIONS
Early screening for frailty in the preoperative period is essential to prognosticate negative outcomes and provide better care in hospitalised older adults undergoing orthopaedic surgery. Current frailty instruments may be useful in inpatient orthopaedic settings, although evidence is lacking for the best frailty measure to use. Considerations when selecting a frailty instrument include clinical context, resource requirement, instrument quality and cultural sensitivity. Applying frailty screening in regular preoperative care or training family members to monitor frailty trajectories may enhance health outcomes. Future research that explores the feasibility and acceptability of incorporating family members into frailty assessment and using frailty instruments in hospital settings is crucial for providing equity and quality of care for all older adults.
7. RELEVANCE TO CLINICAL PRACTICE
The clinical spectrum of musculoskeletal manifestations in orthogeriatric patients may bias frailty classification. Proactive care and early identification of frailty in this population are more challenging yet are essential in promoting optimal health outcomes in hospital settings. Routine frailty screening with a practical and valid instrument is crucial to strengthen preoperative risk stratification for improving surgical care in older adults. Ultimately, specific or modified instruments may be needed for accurately identifying frail older adults who have physical limitations, which is concordant with the core clinical presentation of frailty.
CONFLICT OF INTEREST
All authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTION
Study design: IR, OZ, HT and BB; Implementation of the search strategy: IR, OZ and SA; Analysis of the results: IR, OZ and SA; Writing of the manuscript: IR, OZ, HT, SA, BB and RK.
Supporting information
File S1
App S2
ACKNOWLEDGEMENTS
Inthira Roopsawang received funding support from Ramathibodi School of Nursing, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Thailand; and the de Tornyay Center for Healthy Aging, University of Washington, Seattle.
Roopsawang, I. , Zaslavsky, O. , Thompson, H. , Aree‐Ue, S. , Kwan, R. Y. C. , & Belza, B. (2022). Frailty measurements in hospitalised orthopaedic populations age 65 and older: A scoping review. Journal of Clinical Nursing, 31, 1149–1163. 10.1111/jocn.16035
REFERENCES
- Arksey, H. , & O’Malley, L. (2005). Scoping studies: Towards a methodological framework. International Journal of Social Research Methodology, 8(1), 19–32. 10.1080/1364557032000119616 [DOI] [Google Scholar]
- Beaudart, C. , Reginster, J. Y. , Petermans, J. , Gillain, S. , Quabron, A. , Locquet, M. , Slomian, J. , Buckinx, F. , & Bruyère, O. (2015). Clinical components linked to sarcopenia: The sarcophage study. Osteoporosis International, 26(1), S144. [DOI] [PubMed] [Google Scholar]
- Bellamy, J. L. , Runner, R. P. , Vu, C. C. L. , Schenker, M. L. , Bradbury, T. L. , & Roberson, J. R. (2017). Modified frailty index is an effective risk assessment tool in primary total hip arthroplasty. Journal of Arthroplasty, 32(10), 2963–2968. 10.1016/j.arth.2017.04.056 [DOI] [PubMed] [Google Scholar]
- Birkelbach, O. , Mörgeli, R. , Spies, C. , Olbert, M. , Weiss, B. , Brauner, M. , Neuner, B. , Francis, R. , Treskatsch, S. , & Balzer, F. (2019). Routine frailty assessment predicts postoperative complications in elderly patients across surgical disciplines ‐ a retrospective observational study. BMC anesthesiology, 19(1), 204. 10.1186/s12871-019-0880-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissonneault, A. , Mener, A. , Schwartz, A. , Wilson, J. , Staley, C. , & Schenker, M. (2019). Impact of frailty on 30‐day morbidity and mortality of patients with intertrochanteric femur fractures. Orthopedics, 42(6), 344–348. 10.3928/01477447-20191001-05 [DOI] [PubMed] [Google Scholar]
- Briggs, A. M. , Cross, M. J. , Hoy, D. G. , Sanchez‐Riera, L. , Blyth, F. M. , Woolf, A. D. , & March, L. (2016). Musculoskeletal health conditions represent a global threat to healthy aging: A report for the 2015 World Health Organization world report on ageing and health. Gerontologist, 56(Suppl 2), S243–S255. 10.1093/geront/gnw002 [DOI] [PubMed] [Google Scholar]
- Briggs, A. M. , & Dreinhofer, K. E. (2017). Rehabilitation 2030: A Ccall to action relevant to improving musculoskeletal health care globally. Journal of Orthopaedic & Sports Physical Therapy, 47(5), 297–300. 10.2519/jospt.2017.0105 [DOI] [PubMed] [Google Scholar]
- Buta, B. J. , Walston, J. D. , Godino, J. G. , Park, M. , Kalyani, R. R. , Xue, Q. L. , Bandeen‐Roche, K. , & Varadhan, R. (2016). Frailty assessment instruments: Systematic characterization of the uses and contexts of highly‐cited instruments. Ageing Research Reviews, 26, 53–61. 10.1016/j.arr.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest‐Morin, R. , Street, J. , Zhang, H. , Roughead, T. , Ailon, T. , Boyd, M. , Dvorak, M. , Kwon, B. , Paquette, S. , Dea, N. , Fisher, C. G. , & Flexman, A. M. (2018). Frailty and sarcopenia do not predict adverse events in an elderly population undergoing non‐complex primary elective surgery for degenerative conditions of the lumbar spine. Spine Journal, 18(2), 245–254. 10.1016/j.spinee.2017.07.003 [DOI] [PubMed] [Google Scholar]
- Chen, C. L. , Chen, C. M. , Wang, C. Y. , Ko, P. W. , Chen, C. H. , Hsieh, C. P. , & Chiu, H. C. (2019). Frailty is Associated with an Increased Risk of Major Adverse Outcomes in Elderly Patients Following Surgical Treatment of Hip Fracture. Scientific reports, 9(1), 19135. 10.1038/s41598-019-55459-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. , Mao, G. , & Leng, S. X. (2014). Frailty syndrome: An overview. Clinical Interventions in Aging, 9, 433–441. 10.2147/cia.s45300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, J. , Ahn, A. , Kim, S. , & Wong, C. W. (2015). Global prevalence of physical frailty by Fried’s criteria in community‐dwelling elderly with national population‐based surveys. Journal of the American Medical Directors Association, 16(7), 548–550. 10.1016/j.jamda.2015.02.004 [DOI] [PubMed] [Google Scholar]
- Choi, J. Y. , Cho, K. J. , Kim, S. W. , Yoon, S. J. , Kang, M. G. , Kim, K. I. , Lee, Y. K. , Koo, K. H. , & Kim, C. H. (2017). Prediction of mortality and postoperative complications using the hip‐multidimensional frailty score in elderly patients with hip fracture. Scientific Reports, 7, 42966. 10.1038/srep42966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church, S. , Rogers, E. , Rockwood, K. , & Theou, O. (2020). A scoping review of the clinical frailty scale. BMC Geriatrics, 20(1), 393. 10.1186/s12877-020-01801-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collino, S. , Martin, F. P. , Karagounis, L. G. , Horcajada, M. N. , Moco, S. , Franceschi, C. , Kussmann, M. , & Offord, E. (2013). Musculoskeletal system in the old age and the demand for healthy ageing biomarkers. Mechanisms of Ageing and Development, 134(11–12), 541–547. 10.1016/j.mad.2013.11.003 [DOI] [PubMed] [Google Scholar]
- Cooper, Z. , Rogers, S. O. Jr , Ngo, L. , Guess, J. , Schmitt, E. , Jones, R. N. , Ayres, D. K. , Walston, J. D. , Gill, T. M. , Gleason, L. J. , Inouye, S. K. , & Marcantonio, E. R. (2016). Comparison of frailty measures as predictors of outcomes after orthopedic surgery. Journal of the American Geriatrics Society, 64(12), 2464–2471. 10.1111/jgs.14387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta, M. , Rolfson, D. B. , Stolee, P. , Borrie, M. J. , & Speechley, M. (2009). Frailty is associated with postoperative complications in older adults with medical problems. Archives of Gerontology and Geriatrics, 48(1), 78–83. 10.1016/j.archger.2007.10.007 [DOI] [PubMed] [Google Scholar]
- Dent, E. , Kowal, P. , & Hoogendijk, E. O. (2016). Frailty measurement in research and clinical practice: A review. European journal of internal medicine, 31, 3–10. 10.1016/j.ejim.2016.03.007 [DOI] [PubMed] [Google Scholar]
- Fick, D. M. , & Lundebjerg, N. E. (2017). When it comes to older adults, language matters. Journal of Gerontological Nursing, 43(9), 2–4. 10.3928/00989134-20170811-01 [DOI] [PubMed] [Google Scholar]
- Fried, L. P. , Ferrucci, L. , Darer, J. , Williamson, J. D. , & Anderson, G. (2004). Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 59(3), 255–263. 10.1093/gerona/59.3.M255 [DOI] [PubMed] [Google Scholar]
- Fried, L. P. , Tangen, C. M. , Walston, J. , Newman, A. B. , Hirsch, C. , Gottdiener, J. , Seeman, T. , Tracy, R. , Kop, W. J. , Burke, G. , & McBurnie, M. A. (2001). Frailty in older adults: Evidence for a phenotype. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 56(3), M146–M156. 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- Gleason, L. J. , Benton, E. A. , Alvarez‐Nebreda, M. L. , Weaver, M. J. , Harris, M. B. , & Javedan, H. (2017). FRAIL questionnaire screening tool and short‐term outcomes in geriatric fracture patients. Journal of the American Medical Directors Association, 18(12), 1082–1086. 10.1016/j.jamda.2017.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, M. J. , & Booth, A. (2009). A typology of reviews: An analysis of 14 review types and associated methodologies. Health Information and Libraries Journal, 26(2), 91–108. 10.1111/j.1471-1842.2009.00848.x [DOI] [PubMed] [Google Scholar]
- Grimes, K. , Kitts, J. , Tholl, B. , Samuelson‐Kiraly, C. , & Mitchell, J. I. (2018). Policy and economic considerations for frailty screening in the Canadian healthcare system. The Journal of Frailty & Aging, 7(4), 233–239. 10.14283/jfa.2018.32 [DOI] [PubMed] [Google Scholar]
- Holzgrefe, R. E. , Wilson, J. M. , Staley, C. A. , Anderson, T. L. , Wagner, E. R. , & Gottschalk, M. B. (2019). Modified frailty index is an effective risk‐stratification tool for patients undergoing total shoulder arthroplasty. Journal of Shoulder and Elbow Surgery, 28(7), 1232–1240. 10.1016/j.jse.2018.12.004 [DOI] [PubMed] [Google Scholar]
- Johnson, R. L. , Abdel, M. P. , Frank, R. D. , Chamberlain, A. M. , Habermann, E. B. , & Mantilla, C. B. (2019a). Frailty Index Is Associated With Periprosthetic Fracture and Mortality After Total Knee Arthroplasty. Orthopedics, 42(6), 335–343. 10.3928/01477447-20190812-05 [DOI] [PubMed] [Google Scholar]
- Johnson, R. L. , Abdel, M. P. , Frank, R. D. , Chamberlain, A. M. , Habermann, E. B. , & Mantilla, C. B. (2019b). Impact of Frailty on Outcomes After Primary and Revision Total Hip Arthroplasty. The Journal of arthroplasty, 34(1), 56–64.e5. 10.1016/j.arth.2018.09.078 [DOI] [PubMed] [Google Scholar]
- Kim, H. J. , Park, S. , Park, S. H. , Lee, J. H. , Chang, B. S. , Lee, C. K. , & Yeom, J. S. (2019). The prevalence and impact of frailty in patients with symptomatic lumbar spinal stenosis. European spine journal, 28(1), 46–54. https://doi.org/10.1007/s00586‐018‐5710‐1 [DOI] [PubMed] [Google Scholar]
- Kistler, E. A. , Nicholas, J. A. , Kates, S. L. , & Friedman, S. M. (2015). Frailty and short‐term outcomes in patients with hip fracture. Geriatric Orthopaedic Surgery & Rehabilitation, 6(3), 209–214. 10.1177/2151458515591170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan, M. , Beck, S. , Havelock, W. , Eeles, E. , Hubbard, R. E. , & Johansen, A. (2014). Predicting outcome after hip fracture: Using a frailty index to integrate comprehensive geriatric assessment results. Age and Ageing, 43(1), 122–126. 10.1093/ageing/aft084 [DOI] [PubMed] [Google Scholar]
- Kua, J. , Ramason, R. , Rajamoney, G. , & Chong, M. S. (2016). Which frailty measure is a good predictor of early postoperative complications in elderly hip fracture patients? Archives of Orthopaedic and Trauma Surgery, 136(5), 639–647. 10.1007/s00402-016-2435-7 [DOI] [PubMed] [Google Scholar]
- Li, G. , Prior, J. C. , Leslie, W. D. , Thabane, L. , Papaioannou, A. , Josse, R. G. , Kaiser, S. M. , Kovacs, C. S. , Anastassiades, T. , Towheed, T. , Davison, K. S. , Levine, M. , Goltzman, D. , Adachi, J. D. , & CaMos Research Group (2019). Frailty and Risk of Fractures in Patients With Type 2 Diabetes. Diabetes care, 42(4), 507–513. 10.2337/dc18-1965 [DOI] [PubMed] [Google Scholar]
- Mahmooth, Z. , Carpenter, E. , Lin, E. , Foster, M. , Haack, C. , Sharma, J. , Patel, D. , Sarmiento, J. , Sweeney, J. , Greene, W. , & Elwood, D. (2020). Frailty assessment in the acute care surgery population‐the agreement and predictive value on length of stay and readmission of 3 different instruments in a prospective cohort. The American Journal of Surgery, 220(4), 1058–1063. 10.1016/j.amjsurg.2020.03.026 [DOI] [PubMed] [Google Scholar]
- McIsaac, D. I. , Taljaard, M. , Bryson, G. L. , Beaule, P. E. , Gagne, S. , Hamilton, G. , Hladkowicz, E. , Huang, A. , Joanisse, J. A. , Lavallee, L. T. , MacDonald, D. , Moloo, H. , Thavorn, K. , van Walraven, C. , Yang, H. , & Forster, A. J. (2018). Frailty as a predictor of death or new disability after surgery: A prospective cohort study. Annals of Surgery, 271(2), 283–289. 10.1097/sla.0000000000002967 [DOI] [PubMed] [Google Scholar]
- Milte, R. , & Crotty, M. (2014). Musculoskeletal health, frailty and functional decline. Bailliere’s Best Practice & Research in Clinical Rheumatology, 28(3), 395–410. 10.1016/j.berh.2014.07.005 [DOI] [PubMed] [Google Scholar]
- Nguyen, T. N. , Cumming, R. G. , & Hilmer, S. N. (2015). A review of frailty in developing countries. Journal of Nutrition, Health & Aging, 19(9), 941–946. 10.1007/s12603-015-0503-2 [DOI] [PubMed] [Google Scholar]
- Ondeck, N. T. , Bovonratwet, P. , Ibe, I. K. , Bohl, D. D. , McLynn, R. P. , Cui, J. J. , Baumgaertner, M. R. , & Grauer, J. N. (2018). Discriminative ability for adverse outcomes after surgical management of hip fractures: A comparison of the charlson comorbidity index, elixhauser comorbidity measure, and modified frailty index. Journal of Orthopaedic Trauma, 32(5), 231–237. 10.1097/bot.0000000000001140 [DOI] [PubMed] [Google Scholar]
- Page, M. J. , McKenzie, J. E. , Bossuyt, P. M. , Boutron, I. , Hoffmann, T. C. , Mulrow, C. D. , Shamseer, L. , Tetzlaff, J. M. , Akl, E. A. , Brennan, S. E. , Chou, R. , Glanville, J. , Grimshaw, J. M. , Hróbjartsson, A. , Lalu, M. M. , Li, T. , Loder, E. W. , Mayo‐Wilson, E. , McDonald, S. , … Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ, 372. n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopsawang, I. , Thompson, H. , Zaslavsky, O. , & Belza, B. (2020a). Predicting hospital outcomes with the reported Edmonton Frail Scale‐Thai version in orthopaedic older patients. Journal of Clinical Nursing, 29(23–24), 4708–4719. 10.1111/jocn.15512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopsawang, I. , Thompson, H. , Zaslavsky, O. , & Belza, B. (2020b). The reported Edmonton Frail Scale‐Thai version: Development and validation of a culturally‐sensitive instrument. Nursing & Health Sciences, 22(3), 685–693. 10.1111/nhs.12713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothrock, R. J. , Steinberger, J. M. , Badgery, H. , Hecht, A. C. , Cho, S. K. , Caridi, J. M. , & Deiner, S. (2018). Frailty status as a predictor of three month cognitive and functional recovery following spinal surgery: A prospective pilot study. Spine Journal, 19(1):104–112. 10.1016/j.spinee.2018.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runner, R. P. , Bellamy, J. L. , Vu, C. C. L. , Erens, G. A. , Schenker, M. L. , & Guild, G. N. 3rd (2017). Modified frailty index is an effective risk assessment tool in primary total knee arthroplasty. Journal of Arthroplasty, 32(9), S177–S182. 10.1016/j.arth.2017.03.046 [DOI] [PubMed] [Google Scholar]
- Segal, D. N. , Wilson, J. M. , Staley, C. , & Michael, K. W. (2018). The 5‐item modified frailty index is predictive of 30‐day postoperative complications in patients undergoing kyphoplasty vertebral augmentation. World Neurosurgery, 116, e225–e231. 10.1016/j.wneu.2018.04.172 [DOI] [PubMed] [Google Scholar]
- Shin, J. I. , Keswani, A. , Lovy, A. J. , & Moucha, C. S. (2016). Simplified frailty index as a predictor of adverse outcomes in total hip and knee arthroplasty. Journal of Arthroplasty, 31(11), 2389–2394. 10.1016/j.arth.2016.04.020 [DOI] [PubMed] [Google Scholar]
- Terwee, C. B. , Bot, S. D. , de Boer, M. R. , van der Windt, D. A. , Knol, D. L. , Dekker, J. , Bouter, L. M. , & de Vet, H. C. (2007). Quality criteria were proposed for measurement properties of health status questionnaires. Journal of Clinical Epidemiology, 60(1), 34–42. 10.1016/j.jclinepi.2006.03.012 [DOI] [PubMed] [Google Scholar]
- Theou, O. , Cann, L. , Blodgett, J. , Wallace, L. M. , Brothers, T. D. , & Rockwood, K. (2015). Modifications to the frailty phenotype criteria: Systematic review of the current literature and investigation of 262 frailty phenotypes in the survey of health, ageing, and retirement in Europe. Ageing Research Reviews, 21, 78–94. 10.1016/j.arr.2015.04.001 [DOI] [PubMed] [Google Scholar]
- Theou, O. , Squires, E. , Mallery, K. , Lee, J. S. , Fay, S. , Goldstein, J. , Armstrong, J. J. , & Rockwood, K. (2018). What do we know about frailty in the acute care setting? A scoping review. BMC Geriatrics, 18(1), 139. 10.1186/s12877-018-0823-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traven, S. A. , Reeves, R. A. , Sekar, M. G. , Slone, H. S. , & Walton, Z. J. (2019). New 5‐Factor Modified Frailty Index Predicts Morbidity and Mortality in Primary Hip and Knee Arthroplasty. The Journal of arthroplasty, 34(1), 140–144. 10.1016/j.arth.2018.09.040 [DOI] [PubMed] [Google Scholar]
- Tricco, A. C. , Lillie, E. , Zarin, W. , O'Brien, K. K. , Colquhoun, H. , Levac, D. , Moher, D. , Peters, M. D. J. , Horsley, T. , Weeks, L. , Hempel, S. , Akl, E. A. , Chang, C. , McGowan, J. , Stewart, L. , Hartling, L. , Aldcroft, A. , Wilson, M. G. , Garritty, C. , … Straus, S. E. (2018). PRISMA extension for scoping reviews (PRISMA‐ScR): Checklist and explanation. Annals of Internal Medicine, 169(7), 467–473. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- Vu, H. T. T. , Nguyen, T. X. , Nguyen, T. N. , Nguyen, A. T. , Cumming, R. , Hilmer, S. , & Pham, T. (2017). Prevalence of frailty and its associated factors in older hospitalised patients in Vietnam. BMC Geriatrics, 17(1), 216. 10.1186/s12877-017-0609-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters, S. , Chan, S. , Goh, L. , Ong, T. , & Sahota, O. (2016). The prevalence of frailty in patients admitted to hospital with vertebral fragility fractures. Current Rheumatology Reviews, 12(3), 244–247. 10.2174/1573397112666160619190744 [DOI] [PubMed] [Google Scholar]
- Warnier, R. M. , van Rossum, E. , van Leendert, J. A. , Pijls, N. A. , Mulder, W. J. , Schols, J. M. , & Kempen, G. I. (2016). Screening for frailty in hospitalized older adults: Reliability and feasibility of the Maastricht frailty screening tool for hospitalized patients (MFST‐HP). Research in Gerontological Nursing, 9(5), 243–251. 10.3928/19404921-20160906-01 [DOI] [PubMed] [Google Scholar]
- Wilson, J. M. , Holzgrefe, R. E. , Staley, C. A. , Schenker, M. L. , & Meals, C. G. (2018). Use of a 5‐item modified frailty index for risk stratification in patients undergoing surgical management of distal radius fractures. The Journal of Hand Surgery, 43(8), 701–709. 10.1016/j.jhsa.2018.05.029 [DOI] [PubMed] [Google Scholar]
- Winters, A. M. , Hartog, L. C. , Roijen, H. , Brohet, R. M. , & Kamper, A. M. (2018). Relationship between clinical outcomes and Dutch frailty score among elderly patients who underwent surgery for hip fracture. Clinical interventions in aging, 13, 2481–2486. 10.2147/CIA.S181497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2017). The Global strategy and action plan on ageing and health. Retrieved from http://who.int/ageing/global‐strategy/en/ [Google Scholar]
- Yagi, M. , Fujita, N. , Okada, E. , Tsuji, O. , Nagoshi, N. , Tsuji, T. , Asazuma, T. , Nakamura, M. , Matsumoto, M. , & Watanabe, K. (2018). Impact of Frailty and Comorbidities on Surgical Outcomes and Complications in Adult Spinal Disorders. Spine, 43(18), 1259–1267. 10.1097/BRS.0000000000002596 [DOI] [PubMed] [Google Scholar]
- Zlobina, I. A. , Krivtsunov, A. N. , Bogat, S. V. , & Prashchayeu, K. I. (2015). Musculoskeletal system as a target organ of a frailty processes. Advances in Gerontology, 28(4), 725–728. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1
App S2


