Abstract
Objectives
The aim was to evaluate the role of resorbable membranes applied over customized titanium meshes related to soft tissue healing and bone regeneration after vertical/horizontal bone augmentation.
Materials and Methods
Thirty patients with partial edentulism of the maxilla/mandible, with vertical/horizontal reabsorption of the alveolar bone, and needing implant‐supported restorations, were randomly divided into two groups: Group A was treated using only custom‐made meshes (Mesh‐) and Group B using custom‐made meshes with cross‐linked collagen membranes (Mesh+). Data collection included surgical/technical and healing complications, “pseudo‐periosteum” thickness, bone density, planned bone volume (PBV), regenerated bone volume (RBV), regeneration rate (RR), vertical bone gain (VBG), and implant survival in regenerated areas. Statistical analysis was performed between the two study groups using a significance level of α = .05.
Results
Regarding the healing complications, the noninferiority analysis proved to be inconclusive, despite the better results of group Mesh+ (13%) compared to group Mesh‐ (33%): estimated value −1.13 CI‐95% from −0.44 to 0.17. Superiority approach confirmed the absence of significant differences (p = .39). RBV was 803.27 mm3 and 843.13 mm3, respectively, and higher RR was observed in group Mesh+ (82.3%) compared to Mesh‐ (74.3%), although this value did not reach a statistical significance (p = .44). All 30 patients completed the study, receiving 71 implants; 68 out of them were clinically stable and in function.
Conclusion
The results showed that customized meshes alone do not appear to be inferior to customized meshes covered by cross‐linked collagen membranes in terms of healing complication rates and regeneration rates, although superior results were observed in group Mesh+compared to group Mesh‐ for all variables.
Keywords: alveolar ridge augmentation, collagen membrane, healing complication, osseointegrated implants, titanium mesh
1. INTRODUCTION
The use of osseointegrated implants has allowed an extension of treatment solutions to prosthetically rehabilitate patients suffering from partial or total edentulism, with the simultaneous presence of maxillary atrophy. Despite the development of various techniques and augmentation materials, the re‐establishment of an adequate amount of bone, especially in vertical defects, remains challenging (Lim G. et al. 2018).
Many surgical procedures have been proposed: block bone grafts (inlays and onlays), osteodistraction, and, in particular, guided bone regeneration (GBR) (Urban IA. et al. 2019; Chiapasco & Zaniboni, 2009; Milinkovic & Cordaro, 2014; Aghaloo 2016).
Although there is ample scientific evidence of its effectiveness, GBR with nonresorbable membranes has always been at the center of debate linked to the poor reproducibility of the results obtained, because this technique is quite operator dependent. This statement can be also applied to titanium meshes and other technique for vertical ridge augmentation (Urban et al. 2019). The main cause of failure in the GBR is related to the early or late exposure of the membrane and/or biomaterials as well as the consequent contamination and infection of the materials themselves, which irreversibly compromise bone regeneration (Rocchietta I. et al. 2008; Fontana F. et al. 2011). Later, the introduction of resorbable membranes has significantly reduced biological complications in GBR, making it a highly predictable and effective technique in the treatment of alveolar atrophy of the upper and lower jaw; however, resorbable membranes have been shown to be less effective in vertical ridge augmentation rather than horizontal augmentation (Merli M. et al., 2013 and 2014, Urban IA. Et al. 2013). Otherwise, in the late 1980s, titanium meshes were first used for osseous restoration of deficient edentulous maxillary alveolar ridges, in order to have a better support of complete dentures. (Boyne et al., 1985; Gongloff 1986).
In the late 1990s, Von Arx et al. (1996) introduced the use of titanium mesh, positioned before or during implant placement in order to maintain adequate volume in which the bone graft can reorganize and create new bone. More recently, some authors have proposed the execution of custom‐made meshes, using CAD‐CAM technology, in order to have the device planned and produced before the surgery, with rounded corner and margins, accurate fitting, and adaption in situ, consequently, having intrinsic stability (Sumida T. et al. 2015; Sagheb et al., 2017; Seiler et al., 2018; Ciocca L. et al., 2015; Hartmann A. et al. 2019; Cucchi A. et al. 2020; Chiapasco et al., 2021).
Despite the advantages of customized meshes, many authors have still reported high complication rates, i.e., early or late exposures, and lacking bone volume due to pseudo‐periosteum formation (Ciocca L. et al. 2018; Hartmann A. et al. 2019). In order to improve clinical outcomes of titanium meshes, some authors evaluated the use of membrane barriers over titanium micro‐mesh in a small group of patients. For all patients, the postoperative healing was uneventful, with no dehiscence. At re‐entry in all cases, the space under the titanium mesh was completely filled by newly formed bone and no residual bone defects were observed (Assenza B. et al. 2001; Degidi M. et al. 2003).
However, according to authors’ current knowledge, there are no studies that have investigated the role of the resorbable membrane over the titanium mesh which compare outcomes with and without membranes.
For the aforementioned reasons, the present study was primarily aimed at evaluating the role of resorbable membranes over customized CAD/CAM titanium mesh in regard to (i) the surgical/technical and healing complications rates, (ii) the bone density, (iii) the pseudo‐periosteum between the mesh and the newly formed bone, and (iv) the volumetric bone gain and regeneration rates, in patients with horizontal and/or vertical bone defects.
2. MATERIALS AND METHODS
2.1. Study design and patient selection
The study was designed as an independent, monocentric, parallel‐group, randomized, controlled, and noninferiority clinical trial, in which the variables were prospectively analyzed. The study was conducted in accordance with the Declaration of Helsinki guidelines and approved by the International Ethical Committee of Bologna‐Imola (CE 17139 06/12/2017).
The experimental design followed the Consolidated Standard of Reporting Trials (CONSORT) statement (Moher et al., 2012) and the study protocol was registered on Clinical Trial.gov with the registration number NCT04286334.
As for the sample size, there was not any other clinical study comparing the two surgical techniques; therefore, two similar studies of the research group were considered: one of which reported 15% of healing complications using conventional titanium meshes covered by resorbable membranes (Cucchi A. et al. 2017), and the other reported 66% of complications using a customized mesh without membranes (Ciocca L. et al. 2018). The primary outcome of the study was to evaluate the percentage of healing complications in the two different groups.
The research hypothesis in the noninferiority study is that technique A (Mesh‐) (custom‐made mesh alone) is not inferior to technique B (Mesh+) (custom‐made mesh with membrane), considering a statistical power of 80% at one level of significance of 5% and a noninferiority margin of 10%. If the complications of A are equal to or less than the complications of B + 10%, the research hypothesis will be confirmed. A statistician calculated that the sample size necessary to support the research hypothesis was 15 subjects per group.
The inclusion criteria were as follows: (1) partial edentulism of upper maxilla or mandible with vertical and horizontal bone resorption of the alveolar ridge requiring three‐dimensional bone regeneration for prosthetically guided implant placement and (2) capacity to understand and accept the written conditions of the study.
The exclusion criteria were as follows: (1) insufficient oral hygiene; (2) smoking habit of >10 cigarettes/day; (3) abuse of alcohol or drugs; (4) pregnancy, acute local or systemic infections; (5) uncontrolled diabetes or other metabolic disease; (6) severe hepatic or renal dysfunction; (7) autoimmune disorders; (8) patients who underwent radiotherapy in the last 5 years; and (9) patients undergoing immunosuppressive therapy or immunocompromised patients.
Depending on a previous computer‐generated randomization sequence, the first statistician created the randomized patient codes and 30 subjects were enrolled, planned, and treated from 2017 to 2019: 15 patients were assigned to group A (Mesh‐), treated by means of custom‐made titanium mesh alone, and 15 patients were assigned to group B (Mesh+) treated by means of custom‐made mesh and resorbable membranes. Each patient received written information and provided written consent, before any study‐related procedure.
2.2. Digital planning and device production
The digital planning of augmented volume was performed using software for virtual modeling and analysis; stl files and dicom files were superimposed to verify the accuracy of the augmentation; and finally, the customized devices were realized using Sintered Laser‐Melting (SLM) technology, as described previously (Cucchi A. et al. 2019; Cucchi A. et al. 2020) (Figure 1a–c). The roughness parameters of mesh surface were 1.63 ± 0.19 μm and 2.08 ± 0.20 μm for Ra and Rq, respectively (Cruz et al. 2020).
FIGURE 1.
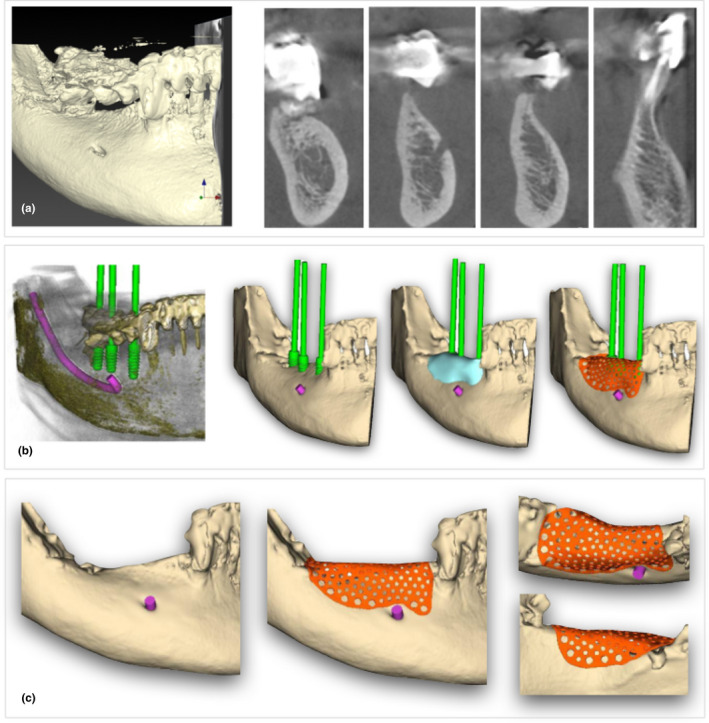
(a) A dedicated software for.dicom files allowed to have the 3D rendering and the ortho‐radial slices for digital planning; (b) digital work‐flow included the following steps: creation of.stl model, implant‐prosthetic planning, bone augmentation planning, and mesh customization; and (c) approval of the customized mesh after buccal, crestal, and lingual evaluation [Colour figure can be viewed at wileyonlinelibrary.com]
2.3. Clinical procedures
The study involved three main surgical phases: reconstructive surgery (T0), mesh removal and implant placement (T1), and implant re‐opening and soft tissue management (T2).
On the day of the first surgery (T0), local anesthesia was administered: articaine 4% with epinephrine 1:100.000. A medium‐crestal horizontal incision was performed from the oblique external line to the gingival sulcus of the two adjacent teeth; subsequently, two vertical mesial and distal vertical releasing incisions are performed on the vestibular side and a mesial oblique releasing incision on the lingual/palatal side; and finally, the detachment and the skeletonization of the region to be treated are performed, carefully isolating the mental nerve, the infra‐orbital nerve, the nasopalatine nerve, the major palatal artery, the nasal cavities, or the maxillary sinus (depending on which region is treated), and not to tear or perforate the surgical flaps (Figure 2a–d).
FIGURE 2.
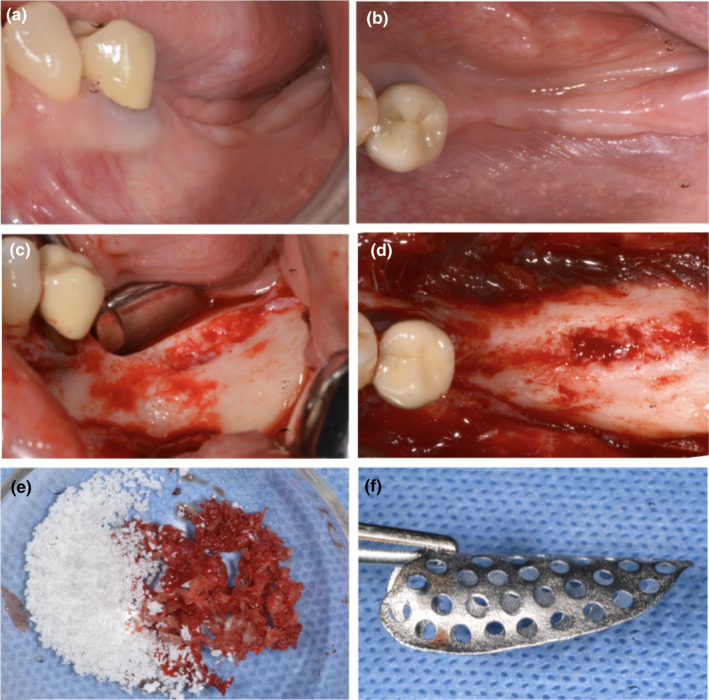
(a and b) Preoperative evaluation of soft tissue defect—lateral and occlusal views; (c and d) intraoperative evaluation of bone defect—lateral and occlusal views; (e) mixture 50:50 of autogenous bone and high porosity xenograft; (f) presentation of customized mesh [Colour figure can be viewed at wileyonlinelibrary.com]
Subsequently, the mobilization of the buccal flap in the maxilla was performed, by means of a periosteal releasing incision at the vestibular level which was made approximately 5 mm or more from the gingival margin, and a superficial dissection with partial thickness of the mucous component with respect to the muscle‐periosteal component (Urban IA. et al. 2017); in the mandible, the lingual flap was mobilized through the detachment of the flap up to the mylohyoid line and the accessory mylohyoid muscle was released (Ronda M. et al. 2014); however, the buccal flap was managed using a brushing technique (Ronda M. et al. 2015); and finally, in the posterior maxilla, if necessary, a buccal fat pad (BFP) flap was used to reduce the risk of wound dehiscence.
Perforations of the cortical bone were performed in order to promote the migration of osteogenic and osteoprogenitor cells and favor the revascularization of the bone graft; moreover, approximately 0.5–1.0 g of autologous bone was harvested with a bone scraper (Safescraper®, Meta) from the posterior mandible.
The titanium mesh (3D‐Mesh®, BTK, Biotec Srl, Dueville) was taken from their sterile envelopment and was filled using a mix 50:50 of autogenous bone and bone xenograft (Zcore®, Osteogenics Biomedical), previously hydrated with peripheral venous blood, and finally were fixed using osteosynthesis screws (Profix® System, Biomedical) (Figures 2e,f) and (Figure 3a–c). In order to avoid any gap between the mesh and the initial residual bone, the mesh was over‐filled with grafting material and it was placed in situ and compressed with force; finally, the space under the mesh was carefully checked for any voids after the placement of the mini‐screws.
FIGURE 3.
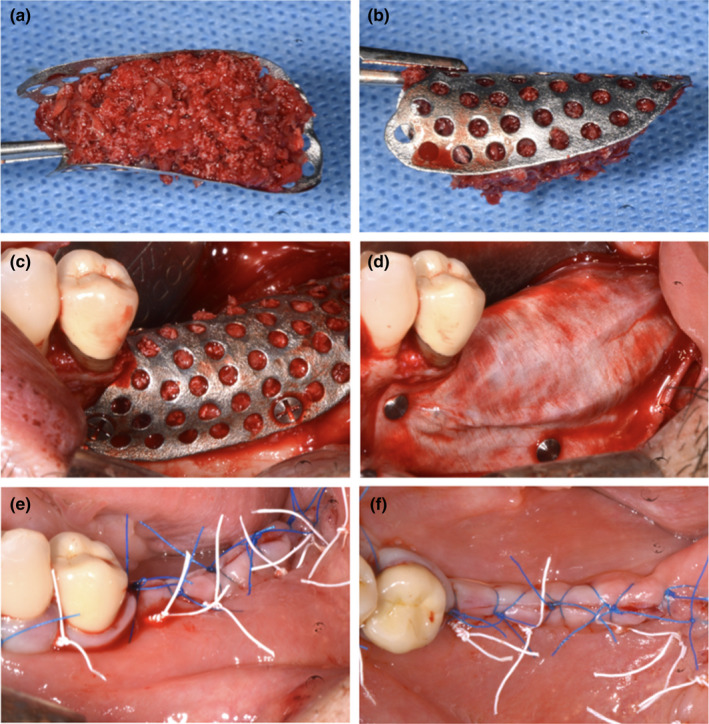
(a and b) Customized mesh filled using bone graft—lateral and occlusal views; (c) fixation of the customized mesh using self‐tapping 5‐mm titanium mini‐screws; (d) application of a cross‐linked collagen membrane over the mesh using titanium tacks; (e and f) primary closure of surgical flaps using horizontal maîtresse sutures (white) and single interrupted sutures (blue)—lateral and occlusal views [Colour figure can be viewed at wileyonlinelibrary.com]
At the end of this first phase, the envelope with the patient code was opened and each patient was assigned to group Mesh‐ or group Mesh+, thus determining application or nonapplication of a cross‐linked collagen membranes (Cytoplast® RTM, Osteogenics Biomedical) over the titanium mesh. Finally, the intervention ended with the first intention closure of the surgical flaps by means of horizontal mattress 5/0 suture and interrupted 5/0 sutures (Figure 3d,f) (Cucchi et al. 2019).
All patients were prescribed 2 g of amoxicillin +Clavulanic acid and 500 mg metronidazole as a premedication, starting 1 hour before the operation and for consecutive 6 days after; a nonsteroidal anti‐inflammatory drug was also prescribed for 6 consecutive days; and a proton pump inhibitor, as a gastroprotector, once per day for 10 days. Postoperative instructions included a 2 weeks soft diet combined with adequate oral hygiene with 4 rinses a day with a 0.2% chlorhexidine‐based mouthwash. During the entire healing period, no removable partial dentures were used, which could have interfered with the healing of the soft tissues involved in the surgery. The sutures were removed 15 days after the operation.
After 6 months (T1), a CBCT was obtained and the augmented sites were reopened: osteosynthesis screws and titanium meshes were removed and a sample of bone tissue was taken with a core drill for histological and micro‐CT analysis. The thickness of the pseudoperiosteum, a layer of dense connective tissue with low cellularity and no mineralization, that is usually found under titanium mesh or PTFE membranes, was measured using a UNC‐15 periodontal probe at the implant sites; the density of newly formed bone was also measured.
Finally, after clinical measurements, the planned titanium implants (BT Safe®, BTK, Biotec Srl, Dueville) were placed according to the manufacturer's instructions and the surgical sites were closed using a 5/0 suture (Figure 4a–f).
FIGURE 4.
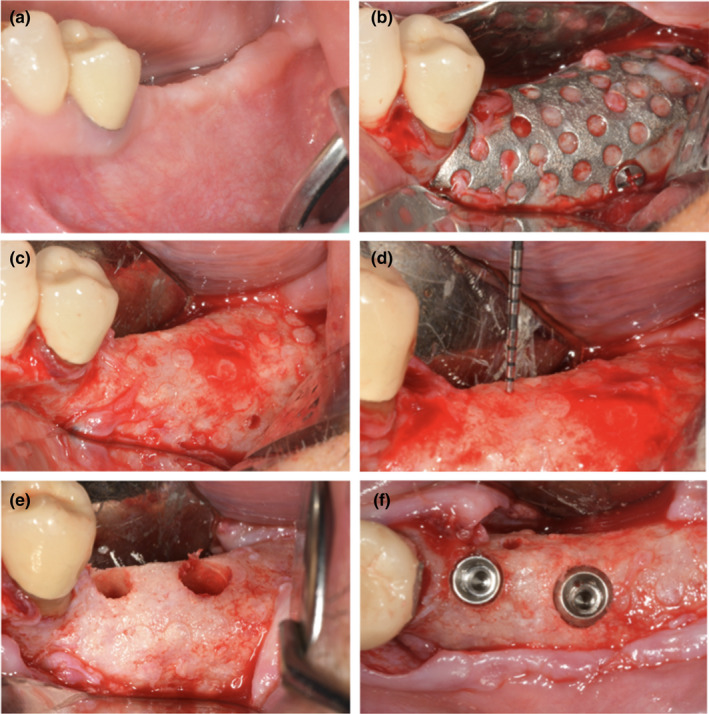
(a) Preoperative evaluation of soft tissue defect 6 months after surgery; (b) re‐entry surgery after flap elevation and mesh identification; (c and d) intraoperative evaluation of bone augmentation, bone density, and pseudoperiosteum; (e) preparation of implant sites using drilling burs; (f) placement of two implants in the molar region [Colour figure can be viewed at wileyonlinelibrary.com]
After 3 months (T2), the sites were re‐opened and the implants were checked for osteointegration applying a counter torque of 25 Nw/cm; finally, soft tissues were managed to improve the quality or the quantity of peri‐implant mucosa, if needed.
2.4. Data collection
Before and during surgery as well as in postoperative visits, all clinical and healing data were recorded on a specific data collection form (CRF).
Surgical complications were evaluated during T0 and included (a) failure to close the surgical flaps by primary intention, (b) flap lacerations or perforations, (c) severe bleeding or hemorrhage, (d) neurological injuries (transient or permanent paresthesia), and (e) other intraoperative problems. All complications were classified according to Fontana et al. (2011). With regards to technical complication, it was considered partial or total mesh fracture and partial or total mesh misfitting.
Healing complications were evaluated from T0 to T1 which included early exposure of the mesh (within the first month), late exposure of the mesh (beyond the first month), abscess without exposure, or other infections. All complications were classified according to the criteria proposed by Fontana et al. (2011).
Bone density and Pseudo‐periosteum type: The bone density was assessed at T1 using a calibrated probing force of 30 gm, based on the resistance of the newly formed bone to probe penetration at the top of the crest in the vertical direction and at the buccal side in the horizontal direction in the planned implant site. Bone density was classified into 3 classes: high density, medium density, and low density. The pseudo‐periosteum was clinically evaluated and assessed with a UNC‐15 periodontal probe at T1 and it was classified into 3 types according to Cucchi et al. (2019). A precalibrated operator that had previous experience with these evaluations took all these measurements (Cucchi et al. 2020).
Bone Volumes and Regeneration Rate (RR): The bone volumes at the level of the treated region were measured as linear and cubic measures on cone beam computed tomography (CBCT) obtained before and after bone regeneration (T0 and T1) (Figure 5a,b), thus determining the planned bone volume (PBV), the effective regenerated bone volume (RBV), the difference between the PBV and the bone volume obtained which is the lacking bone volume (LBV), and finally the regeneration rates (RR) that is the percentage of regenerated bone volume with respect to the bone volume planned (RBV/PBV*100). For each of the values obtained, the mean, median, standard deviation, and range were calculated. Vertical bone gain (VBG) was also measured on CBCT in a linear dimension. The steps for the volumetric analyses are described and reported in a previous article (Cucchi A. et al. 2020).
FIGURE 5.
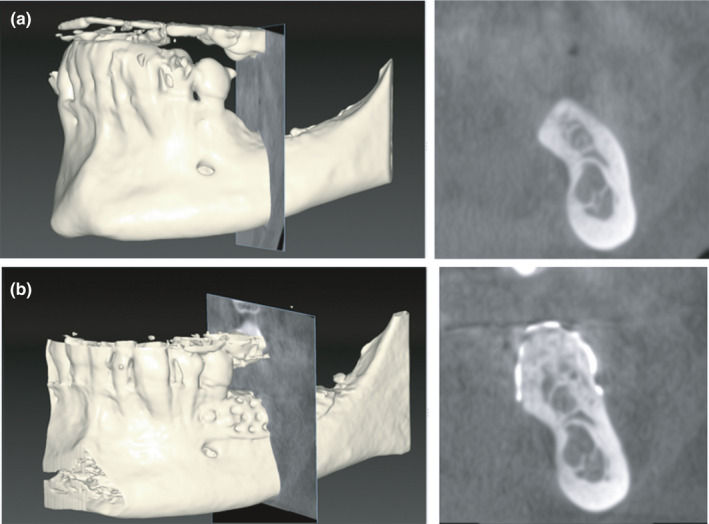
(a and b) Cone beam computed tomography (CBCT) before and after bone augmentation in correspondence with maximum vertical defect and calculation of vertical bone gain (VBG)
Implant Stability and Osteointegration Rate: Implant stability was measured with a surgical handpiece at T1 and implants were divided into low torque (<15 N/cm), medium torque (15‐<x<35 N/cm), and high torque (>35 N/cm). Finally, implant osteointegration was evaluated at T2 applying a counter torque of 25 N/cm.
2.5. Data management and statistical analysis
An Excel data collection form and data management system were used (Microsoft Excel 2011; Windows, ver. 14.0.0; Microsoft Corp.,). All data were entered by a single blinded operator. Prior to entry, all data were evaluated in terms of accuracy and completeness. For each continuous variable, the mean, median, standard deviation (SD), interquartile range (IQR), and the 95% confidence interval (95%CI) were reported.
This study was based on the hypothesis that Group A (Mesh‐) would not be inferior to Group B (Mesh+) in relation to the healing complication rate (primary outcome). The threshold for noninferiority of Group A with respect to Group B was decided at less than 10% difference in the mean change. The noninferiority test was only performed for the healing, surgical/technical complications, and RR outcomes (one‐sided 95% confidence interval approach), specifying for each outcome the largest difference that was clinically acceptable (delta =Δ). The null hypothesis (H0) of superiority analysis was that there is no true difference in terms of healing complication rates between groups Mesh‐ and Mesh+, and the alternative hypothesis (H1) states that there is a difference between the intervention groups. Test of normality was carried out with the Skewness/Kurtosis tests (normal distribution if p‐value >.05). For continuous numerical variables, the mean and the median were reported, highlighting the data that best represent their distribution. In bivariate analysis, proportions were compared using Qui‐squared (X2) tests or Fisher's exact test where appropriate. The comparison of means to evaluate statistically significant differences was performed by t‐test, Wilcoxon rank sum test, and Wilcoxon matched‐pairs signed‐ranks test where necessary.
The aforementioned procedures were carried out considering each patient as well as the statistical unit in order to have 30 observations (n = 30). All tables and graphs were realized after a patient‐level analysis: a mean value for each patient was used in the statistical analysis for all implant‐related variables, considering multiple observations within patients (30 patients and 71 implants). The threshold value decided for determining the statistical significance corresponds to a p‐value of .05 (5%). The statistician was blinded and external to the working group. Data analysis was performed with STATA/IC software (StataCorp LLC).
3. RESULTS
A total of 30 partially edentulous patients with vertical and/or horizontal bone defects were enrolled and treated between 2017 and 2019. The defects were divided as follows: 7 (23.3%) were in the anterior region (1 mandible, 6 maxilla), whereas 23 (76.7%) were in the posterior region (15 mandible, 8 maxilla). The majority of the sites (n = 25, 83.3%) showed a vertical defect; five sites (16.7%) had only a horizontal defect. Characteristics of treated sites are reported in Table 1.
TABLE 1.
Distribution of demographic and intervention characteristics in the study population
| Group M‐ | Group M+ | |
|---|---|---|
| Intervention | Mesh Alone | Mesh+Membr. |
| Patients n° | 15 (50%) | 15 (50%) |
| Male | 7 (46.7%) | 8 (53.3%) |
| Female | 8 (53.3%) | 7 (46.7%) |
| Maxilla | 5 (33.3%) | 9 (60%) |
| Mandible | 10 (66.7%) | 6 (40%) |
| Anterior | 2 (13.33%) | 4 (26.67%) |
| Posterior | 13 (86.67%) | 11 (73.33%) |
| wImplants n° | 34 (47.9%) | 37 (52.1%) |
The table shows the absolute and (percentage) frequency.
Concerning the surgical complications, no failures to move the surgical flaps over the mesh and closure by primary intention were observed; moreover, no vascular or flap lesions occurred during surgery; however, four neurological lesions were reported, classified as paresthesia: three involved the mental nerve and were resolved within 1 month and one, involving the infra‐orbital nerve, was still present after 1 year. All these patients were treated, at time of sutures removal, by means of alfa‐lipoic acid, vitamin B‐C‐E complex, and N‐ acetil‐carnitine. Three technical complication were observed, referring to two partial mesh fractures and one partial mesh misfitting.
Surgical/technical complication rates were 13.3% and 26.7% for groups Mesh‐ and Mesh+, respectively, and no statistically significant differences were observed between the two groups (p‐value = .65).
Regarding healing complications, three early and two late exposure of the meshes occurred for a total of 5 (two class 2; three class 3), and two infections without exposure (two class 4) were observed during healing time. In these seven cases, complications were managed as follows: early exposed meshes were removed from one to three months after surgery; in cases of late exposure or infections, the meshes were removed within seven days after the complications were observed. In all cases, implants were placed as planned but in two cases an adjunctive GBR procedure was required.
The noninferiority analysis proved inconclusive, despite the superior performance of group Mesh+ (13.3%) compared to Mesh‐ (33.3%). Healing complications did not show statistical differences between the two study groups (Fisher's exact = 0.39). The difference in exposure between the two groups was not statistically significant (Fisher's exact = 0.33), with less exposure rate when a resorbable membrane was used (6.7%) compared to mesh alone (26.7%). Statistical analysis is reported in Table 2 and Figure 6.
TABLE 2.
Observed frequencies of complications (surgical/technical and healing), pseudoperiosteum, bone density, and implant stability in the two groups (n = 30)
| Group M‐ | Group M+ | p‐value |
|---|---|---|
| Surgical/technical Complications | ||
| No. of subjects (relative percentage) | ||
| Yes: 2 (13.3%) | 4 (26.7%) | .65 |
| No: 13 (86.7%) | 11 (73.3%) | |
| Healing complications | ||
| No. of subjects (relative percentage) | ||
| Yes: 5 (33.3%) | 2 (13.3%) | .39 |
| No: 10 (68.7%) | 13 (86.7%) | |
| Pseudo‐Periosteum | ||
| No. of subjects (relative percentage) | ||
| Type 1: 7 (46.7%) | 10 (66.7%) | .46 |
| Type 2: 4 (26.7%) | 4 (26.7%) | |
| Type 3: 4 (26.7%) | 1 (6.7%) | |
| Bone Density | ||
| No. of subjects (relative percentage) | ||
| Hard: 4 (26.7%) | 3 (20%) | .75 |
| Med.: 10 (66.7%) | 9 (60%) | |
| Soft: 1 (6.7%) | 3 (20%) | |
| Implant Stability | ||
| No. of subjects (relative percentage) | ||
| ≥35 N: 10 (66.7%) | 9 (60%) | 1.00 |
| <35 N: 5 (33.3%) | 6 (40%) | |
The table summarizes absolute and percentage frequency found in the two study groups. Fisher's exact test was conducted due to an inadequate sample size for the chi‐square test of homogeneity. No significant statistical differences were observed between the arms.
FIGURE 6.
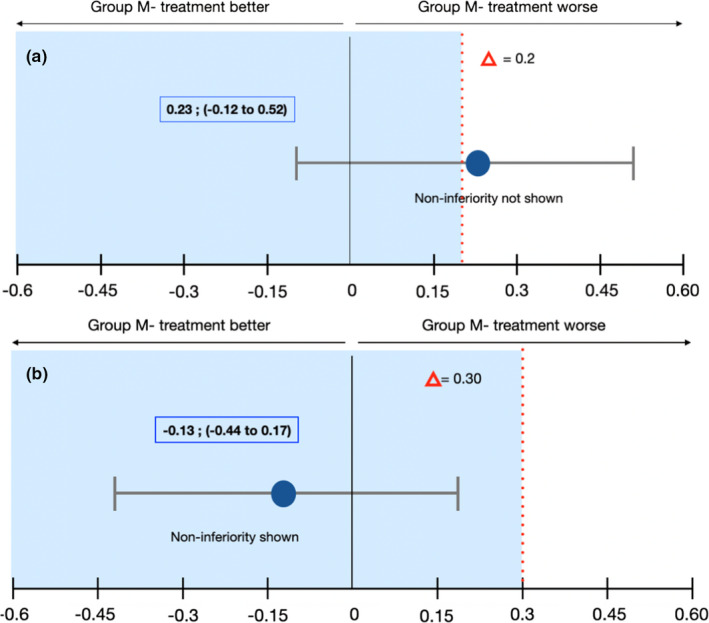
Healing and Surgical/technical Complications: Noninferiority Analysis. Error bars indicated one‐sided 95% confidence intervals of the difference in the healing complication mean values between the M‐ and M+ groups (Mesh‐ minus Mesh+). The red broken line delineating the difference in the score shows the noninferiority margin (delta); tinted area indicates the zone of noninferiority. (a) Healing Complications: The CI includes Δ and zero and the data do not prove noninferiority of Mesh‐ group compared with Mesh+group. Although there is no statistically significant difference between the two treatments, Mesh‐ group tends to be worse than Mesh+group in terms of healing complications. (b) Surgical/technical Complications: The CI does not include Δ and the data prove noninferiority of Mesh‐ group compared with Mesh+group. Although there is no statistically significant difference between the two treatments, Mesh‐ group tends to be superior to Mesh+group in terms of surgical/technical complications [Colour figure can be viewed at wileyonlinelibrary.com]
With regard to pseudo‐periosteum, 17 sites were classified as type 1 (7 in Group Mesh‐ and 10 in Group Mesh+), 8 as type 2 (4 in Group Mesh‐ and 4 in Group Mesh+), and 5 as type 3 (4 in Group Mesh‐ and 1 in Group Mesh+). The differences between the two groups were not statistically significant (Fisher's exact = 0.47). Similar results were observed in regard to bone densities, without any statistical differences (Fisher's exact = 0.75). Types of pseudo‐periosteum and bone density are summarized in Table 2.
Regarding bone volumes, group Mesh‐ showed values of 1019.33 mm3, 216.27 mm3, and 803.07 mm3 for PBV, LBV, and RBV, respectively; in the group Mesh+, values of 1022.0 mm3, 178.87 mm3, and 843.13 mm3 were measured for PBV, LBV, and RBV, respectively. No statistical differences were observed between the two groups (p > .05 for all variables), confirming that volumes of bone augmentation at the baseline and at 6 months were similar in the two groups.
Regarding RR, although group Mesh+showed higher RR (82.3%) than the group Mesh‐ (74.3%), this difference was not statistically significant (Mann–Whitney = 0.44). The noninferiority analysis regarding RR proved to be inconclusive, despite the superior performance of group Mesh+compared to Mesh‐, as shown in Figure 7. Moreover, the mean RR of patients with uneventful healing was 82.6 ± 17.9%, whereas in patient who had complications, the mean RR was 64.2 ± 22.5%. Finally, the VBG value was superior in group Mesh+ (6.4 mm) compared to that in the group Mesh‐ (4.7 mm), even if it did not achieve statistical significance (Mann‐Whitney = 0.08). Statistical data about bone volumes, RR, and VBG are reported in Table 3.
FIGURE 7.
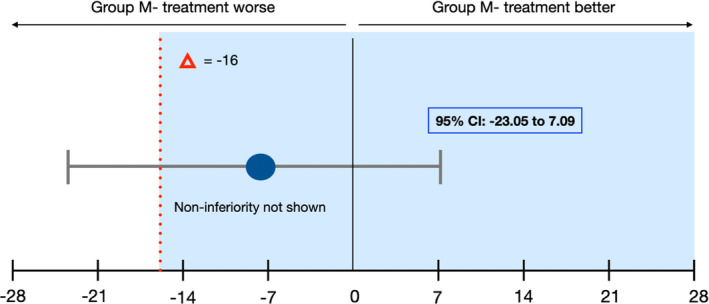
Regeneration Rates (RR): Noninferiority Analysis. Error bars indicated one‐sided 95% confidence intervals of the difference in the healing complication mean values between the M‐ and M+ groups (M‐ minus M+). The red broken line delineating the difference in the score (Δ = −16) shows the noninferiority margin (delta); tinted area indicates the zone of noninferiority. The CI includes Δ and zero and the data do not prove noninferiority of group M‐ compared with group M+ [Colour figure can be viewed at wileyonlinelibrary.com]
TABLE 3.
Complications (as continuous numeric variables) and volumetric bone measurements in the two study groups (n = 30)
| Group M‐ | Group M+ | Estimated | p‐value | ||||
|---|---|---|---|---|---|---|---|
| Mean/Median | (SD) 95% CI | Mean/Median | (SD) 95% CI | Mean | 95% CI | ||
| Surgical/technical Compl | 0.13/ 0 | (0.35) | 0.27/ 0 | (0.46) | 0.23 | −1.12; 0.52 | 0.37¥ |
| −0.06; 0.33 | 0.01; 0.52 | ||||||
| Healing Compl | 0.33/ 0 | (0.49) | 0.13/ 0 | (0.43) | −1.13 | −0.44; 0.17 | 0.20¥ |
| 0.06; 0.60 | −0.06; 0.33 | ||||||
| Vol_Pre (mm3) | 3350.07/ 2343 | (2695.33) | 1749.67/ 1768 | (1243.95) | 1600.4 | 30.35; 3170.5 | 0.24¥ |
| 1857.44; 4842.7 | 1060.8; 2438.5 | ||||||
| Vol_Post (mm3) | 4153.13/ 3284 | (2924.74) | 2592.80/ 2309 | (1447.25) | 1560.33 | −165.6; 3286.2 | 0.22¥ |
| 2533.46; 5772.8 | 1791.3; 3394.3 | ||||||
| PBV (mm3) | 1019.33/ 910 | (488.44) | 1022/ 970 | (362.34) | −2.67 | −324.32; 318.99 | 0.48¥ |
| 748.84; 1289.83 | |||||||
| (821.3; 1222.6) | |||||||
| LBV (mm3) | 216.27/ 163 | (204.36) | 178.87/ 133 | (205.95) | 37.4 | −116.05; 190.85 | 0.46¥ |
| 64.82; 292.92 | |||||||
| 103.10; 329.44 | |||||||
| RBV (mm3) | 803.07/ 759 | (553.78) | 843.13/ 766 | (389.52) | −40.07 | −398.16; 318.02 | 0.53¥ |
| 627.42; 1058.8 | |||||||
| 496.40; 1109.74 | |||||||
| RR (%) | 74.32/ 74.54 | (22.10) | 82.30/ 88.05 | (17.98) | −7.98 | −23.05; 7.09 | 0.44¥ |
| 62.08; 86.55 | 72.34; 92.26 | ||||||
| VBG (mm) | 4.74 /4.02 | (2.56) | 6.36 /6.28 | (2.31) | −1.62 | −3.63; 0.40 | 0.11¢ |
| 3.02; 6.46 | 5.03; 7.69 | ||||||
The table shows the values of mean, median, standard deviation (sd), and 95%CIs in groups M‐ and M+. Estimated mean difference and relative 95% confidence intervals between treatment groups were reported. Vol_Pre and Vol_Post: preoperative and postoperative bone volume; PBV: Planned Bone Volume; LBV: Lacking Bone Volume; RBV: Regenerated Bone Volume; RR: Regeneration Rates; VBG: Vertical Bone Gain. Wilcoxon matched‐pairs signed‐ranks and Student's T Test were used where necessary.
¥: Wilcoxon matched‐pairs signed‐ranks; ¢: Student's T Test.
Finally, 71 implants were placed in 30 patients (34 in group Mesh‐ and 37 in group Mesh+). Most of implants (n = 43) showed a stability superior to 35 N/cm; 27 implants had a medium stability (15‐<x<35 N/cm); and only one implant had a torque value less than 15 N/cm (2).
Three implants failed to achieve osteointegration, of which two implants in one patient of the group Mesh‐ and one implant in a patient of the group Mesh+. Based on implant analysis, survival rates in regenerated areas were 94.0% and 97.0% for the two groups, respectively, with no statistical differences.
4. DISCUSSION
GBR was introduced in 1988 by Dahlin et al. 1988 by means of nonresorbable PTFE membranes for the treatment of alveolar bone defects; in the following decades, many authors have confirmed the efficacy and the predictability of GBR for vertical ridge augmentation (Rezepi M. and Donos N. 2010; Lutz R. et al. 2015; Elnayef B. et al. 2017; Urban IA. et al. 2019). As an alternative to nonresorbable membranes, some authors introduced the use of titanium meshes for reconstruction of localized and extensive defects (Boyne PJ. et al. 1985; Von Arx T. et al., 1996 and 1998).
Despite favorable clinical and radiographic outcomes, both surgical techniques presented high complication rates due to early or late exposure, and consequent infections are considered operator‐dependent procedures (Simion M. et al. 1994; Garcia J. et al. 2018; Lim G. et al. 2018).
In the recent years, the introduction of customized titanium meshes has simplified and reduced the duration of surgery (Sumida T. et al. 2015; Cucchi A. et al. 2020), even though the complication rates during the healing phase still remain high (Ciocca L. et al. 2018; Hartmann et al., 2019).
Regarding the aforementioned, the significant question which must be considered and answered is the usefulness of application of resorbable membranes over titanium meshes. According to GBR principles (Elgali I. et al. 2017), a barrier membrane plays the key role in reducing soft tissue formation and improving bone reconstruction; therefore, some clinicians recommend covering titanium meshes with resorbable membranes to improve clinical outcomes (Cucchi A. et al. 2019).
Nonetheless, in the scientific literature, there are no clinical randomized or nonrandomized studies on human patients that compare the results obtained by means of titanium meshes with or without collagen membranes. Similarly, there are no animal studies related to this topic, except for an interesting study which observed that when resorbable membranes were applied over titanium meshes, the newly formed bone rates (%) at 8 and 16 weeks were twice or more if the membranes were not used (Shin SI. et al. 2013).
Consequently, the main aim of this study was to investigate the influence of resorbable membranes over titanium meshes for both vertical and/or horizontal alveolar ridge augmentations.
The authors chose to use customized titanium meshes and not conventional ones, because their intra‐operative benefits are evident; the custom‐made meshes represent a digital evolution of conventional ones, realized using SLM technology after planning and virtual project (Hartmann A. and Seiler M. 2020; Sagheb K. et al. 2017; Revilla‐León M. et al. 2020).
In the present study, cross‐linked collagen membranes in a single layer were used to cover titanium meshes filled with a mix 50:50 of autogenous bone and bone xenograft with high porosity. The authors demonstrated that in a previous randomized clinical trial, the effectiveness of covering a titanium mesh with cross‐linked collagen membranes achieves similar outcomes in comparison to titanium‐reinforced PTFE membranes (Cucchi A. et al. 2017,2019,2020).
Since the significant problem of customized meshes remains the healing complications, the healing complication rates were considered the primary outcome and regeneration rates the secondary outcome. The noninferiority design was chosen because of the absence of previous comparative studies, demonstrating the positive effect of resorbable membranes over titanium meshes. In this study, healing complication rates presented a value of about 33% in the group without membrane, whereas in the group with a combined application of mesh and membrane, the value was approximately 13% which suggests that the application of the membrane is favorable, although these values showed no statistically significant differences. These results compared favorably with other studies reporting exposure rates in the order of 25% (Harmann A. et al. (2019) and 33% in the study of Sagheb K. et al. (2017); in both studies, a native collagen membrane was used over customized titanium meshes. On the other hand, Ciocca L. et al. (2018) did not apply any membranes on customized meshes and their healing complication rate was about 66%.
Healing complications of titanium meshes represent the most severe adverse events that often lead to partial or complete failure of the bone augmentation; other surgical techniques, such as GBR or bone block grafts, have been reported to have similar post‐operative complications. In this regard, Urban et al. (2019), in the most recent systematic review and metanalysis, reported that the type of procedure influenced the rate of complications with a 47.3% rate for distraction osteogenesis, 12.1% for GBR, and 23.9% for the use of blocks. Within GBR, nonresorbable membranes showed a complication rate of 6.9% and resorbable membranes supported by titanium meshes or plates of 23.3%, which is similar to those reported in the present study.
In this clinical study, a trend of a reduction in healing complications, when a resorbable membrane was used to cover the mesh, was observed and its possible reasons may be due to the following: protection of the clot in the early healing phase, protection of inner surfaces of the flaps from trauma due to some parts of the mesh, protection of the graft from bacteria penetration in the early healing phase, and thickening of soft tissues (Zeng N. et al. 2016; Janner SFM. et al. 2017). It is to consider that a buccal fat pad flap was used in two patients of group M‐ and in three patients of group M+ to favor the primary closure in the most challenging maxillary cases.
The digital planning of bone augmentation and customized mesh allowed the evaluation of the so‐called planned bone volume that represents 100% of potential regeneration. The customized mesh was used as a reference tool to measure the regenerated bone volume and lacking bone volume, as well as obtaining the regeneration rates in the two groups in order to evaluate the effect of the membrane. Other authors have used volumetric analysis to investigate the effectiveness of bone augmentation but they did not calculate the ratio between PLV and RBV (Lizio et al., 2014; Merli M. et al. 2017; Alayan J. and Ivanovski S. 2018).
In the present study, CBCT was obtained before surgery and 6 months after surgery in order to calculate the bone augmentation; a further CBCT is planned after 1 year to evaluate the bone resorption of augmented sites. No CBCT was taken immediately after the surgery to avoid excessive radiation exposure to the patients (Bornstein et al., 2014). Without a CBCT immediately after surgery, it is not possible to know the “real” bone volume obtained immediately after surgery and the bone volume at the end of surgery might be less or more than the planned one. However, the volumetric comparisons between baseline and 6‐month volumes offer information about the superiority or noninferiority of two surgical approaches.
It is important to emphasize that the two different approaches were tested both in the mandible and the maxilla as well as in the anterior and in the posterior region. The majority of the defects were predominantly vertical and only a few were horizontal; moreover, as a result of the randomization in the two different groups, the baseline volume of the bone defects was similar.
A trend of an increase in bone formation with the use of a resorbable membrane was observed; in fact, the application of a membrane resulted in a higher regeneration rate (88% compared to 75% of the Group without membrane), i.e., more regenerated bone volume in relation to a planned bone volume, even if the difference was not statistically significant. Obviously, the results obtained are influenced by the treated sites, the surgical technique, and the potential healing of the patient in this study was not possible to determine (Monje et al. 2017; Plonka et al. 2018).
No influences were found on pseudo‐periosteum and bone density formation with the use of the resorbable membrane although a tendency versus a better pseudo‐periosteum type was observed with its use. The pseudo‐periosteum formed under a titanium mesh represents a lack of bone regeneration with respect to the planned bone augmentation. Since it remains unclear whether the soft tissue under the nonresorbable devices undergoing mineralization over time and this tissue could protect the bone and prevent graft infection and resorption, the tissue was left in place after barrier removal but at implants sites, irrespective of the tissue type (Cucchi et al. 2019).
Further studies regarding the formation of pseudo‐periosteum in augmented sites could be interesting to compare the outcomes with different resorbable or nonresorbable membranes and/or titanium meshes (Soldatos et al., 2017; Khojasteh A et al. 2019).
An interesting issue observed in this study was the difficulty to remove the mesh, because the peripheral margins of the mesh were often embedded in newly formed bone and although all the titanium meshes were completely removed, a frequent finding was that the meshes positioned in the posterior mandible were more challenging to remove on the lingual side due to mylohyoid muscle and fibrous adherences.
Considering the research hypothesis of this noninferiority study, the statistical results confirmed that custom‐made meshes alone are not inferior to custom‐made meshes with membranes. Also, the following analysis based on a superiority approach did not observe significant differences. However, the authors cannot conceal that all variables showed better results in Mesh+group compared to Mesh‐, suggesting that membranes over titanium meshes have a positive role. A possible reason could be due to a small sample size that is too small and further studies with a higher number of patients should be carried out to draw strong conclusions.
The most significant strengths related to the present study were the novelty of the investigation, the study design (double blinded randomized clinical trial), the variety of treated sites (both mandible and maxilla; both anterior and posterior), the extension of treated defects (about 1 cm3), and finally with respect to previously established surgical and prosthetic protocols; the main drawbacks of the study were the small number of patients due to the absence of previous studies for sample size calculation and the experience of a surgeon using novel surgical devices, i.e., customized titanium meshes. It is important to underline that the absence of follow‐up after the implant loading represents a relevant limitation of this study as well as the short follow‐up regarding to the bone volume changes over time.
Within the limitations of this randomized clinical trial, the results showed that custom‐made titanium meshes are effective for horizontal and vertical ridge augmentation both with and without resorbable membranes. The application of membranes did not improve the outcomes of bone augmentation significantly; however, a trend of more favorable results was observed when a membrane was applied. Consequently, further clinical investigations with higher number of patients are needed to assess the role of resorbable membranes applied over titanium meshes.
As conclusion, the customized titanium meshes can be considered as a reliable solution to have horizontal and vertical bone augmentation before implant placement using both mesh alone and mesh covered by a long‐lasting membrane. The use of mesh alone was demonstrated noninferior to the mesh plus membrane. Although statistical analysis did not reveal significant differences, the application of membrane seems to favor a better trend in the healing complication rates and in regeneration rates. Short‐ and long‐term follow‐up of regenerated bone volumes and peri‐implant bone levels after functional loading will be mandatory to confirm the reliability of this augmentation procedure and the effective role of the membrane.
AUTHOR CONTRIBUTION
Alessandro Cucchi: Conceptualization (lead); Investigation (lead); Resources (equal); Validation (supporting). Elisabetta Vignudelli: Data curation (equal); Methodology (lead); Project administration (lead); Resources (supporting). Debora Franceschi: Methodology (equal); Writing‐original draft (lead); Writing‐review & editing (equal). Emanuele Randellini: Investigation (equal); Project administration (equal); Resources (supporting); Software (supporting). Antonino Fiorino: Data curation (supporting); Formal analysis (lead); Methodology (supporting); Software (lead); Visualization (supporting). Giuseppe Corinaldesi: Funding acquisition (lead); Project administration (lead); Resources (equal); Supervision (lead).
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to Dr. Lisa Rinaldi for their effort during the data collection and parameter measurements for this study. The English of this manuscript was checked by a professional native‐speaker Teacher, Nathalie Ann De Vito. Finally, the authors want to thank Prof. Maria Rosaria Gatto for her help and support at the beginning of the study.
Cucchi, A. , Vignudelli, E. , Franceschi, D. , Randellini, E. , Lizio, G. , Fiorino, A. , & Corinaldesi, G. (2021). Vertical and horizontal ridge augmentation using customized CAD/CAM titanium mesh with versus without resorbable membranes. A randomized clinical trial. Clinical Oral Implants Research, 32, 1411–1424. 10.1111/clr.13841
Registered in www.clinicaltrials.gov ‐ Identifier: NCT04286334
REFERENCES
- Aghaloo, T. L. , Misch, C. , Lin, G. H. , Iacono, V. J. , & Wang, H. L. Bone augmentation of the edentulous maxilla for implant placement: A systematic review. The International Journal of Oral & Maxillofacial Implants, 31, s19–s30. 10.11607/jomi.16suppl.g1 [DOI] [PubMed] [Google Scholar]
- Alayan, J. , & Ivanovski, S. (2018). A prospective controlled trial comparing xenograft/autogenous bone and collagen‐stabilized xenograft for maxillary sinus augmentation‐complications, patient‐reported outcomes and volumetric analysis. Clinical Oral Implants Research, 29(2), 248–262. 10.1111/clr.13107 [DOI] [PubMed] [Google Scholar]
- Assenza, B. , Piattelli, M. , Scarano, A. , Lezzi, G. , Petrone, G. , & Piattelli, A. (2001). Localized ridge augmentation using titanium micromesh. Journal of Oral Implantology, 27(6), 287–292. [DOI] [PubMed] [Google Scholar]
- Bornstein, M. M. , Al‐Nawas, B. , Kuchler, U. , & Tahmaseb, A. (2014). Consensus statements and recommended clinical procedures regarding contemporary surgical and radiographic techniques in implant dentistry. The International Journal of Oral & Maxillofacial Implants, 29S, 78–82. 10.11607/jomi.2013.g1 [DOI] [PubMed] [Google Scholar]
- Boyne, P. J. , Cole, M. D. , Stringer, D. , & Shafqat, J. P. (1985). A technique for osseous restoration of deficient edentulous maxillary ridges. Journal of Oral and Maxillofacial Surgery, 43, 87–91. 10.1016/0278-2391(85)90054-0 [DOI] [PubMed] [Google Scholar]
- Chiapasco, M. , Casentini, P. , Tommasato, G. , Dellavia, C. , & Del Fabbro, M. (2021). Customized CAD/CAM titanium meshes for the guided bone regeneration of severe alveolar ridge defects: Preliminary results of a retrospective clinical study in humans. Clinical Oral Implants Research, 32(4), 498–510. 10.1111/clr.13720 [DOI] [PubMed] [Google Scholar]
- Chiapasco, M. , & Zaniboni, M. (2009). Clinical outcomes of GBR procedures to correct peri‐implant dehiscences and fenestrations: a systematic review. Clinical oral implants research, 20(Suppl 4), 113–123. 10.1111/j.1600-0501.2009.01781.x [DOI] [PubMed] [Google Scholar]
- Ciocca, L. , Lizio, G. , Baldissara, P. , Sambuco, A. , Scotti, R. , & Corinaldesi, G. (2018). Prosthetically CAD‐CAM‐guided bone augmentation of atrophic jaws using customized titanium mesh: Preliminary results of an open prospective study. Journal of Oral Implantology, 44(2), 131–137. 10.1563/aaid-joi-D-17-00125 [DOI] [PubMed] [Google Scholar]
- Ciocca, L. , Ragazzini, S. , Fantini, M. , Corinaldesi, G. , & Scotti, R. (2015). Work flow for the prosthetic rehabilitation of atrophic patients with a minimal‐intervention CAD/CAM approach. The Journal of Prosthetic Dentistry, 114(1), 22–26. 10.1016/j.prosdent.2014.11.014 [DOI] [PubMed] [Google Scholar]
- Cruz, N. , Martins, M. I. , Domingos Santos, J. , Gil Mur, J. , & Tondela, J. P. (2020). Surface comparison of three different commercial custom‐made titanium meshes produced by SLM for dental applications. Materials, 13(9), 2177. 10.3390/ma13092177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucchi, A. , Bianchi, A. , Calamai, P. , Rinaldi, L. , Mangano, F. , Vignudelli, E. , & Corinaldesi, G. (2020). Clinical and volumetric outcomes after vertical ridge augmentation using computer‐aided‐design/computer‐aided manufacturing (CAD/CAM) customized titanium meshes: A pilot study. BMC Oral Health, 20, 2020a. 10.1186/s12903-020-01205-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucchi, A. , Chierico, A. , Fontana, F. , Mazzocco, F. , Cinquegrana, C. , Belleggia, F. , Rossetti, P. , Soardi, C. M. , Todisco, M. , Luongo, R. , Signorini, L. , Ronda, M. , & Pistilli, R. (2019). Statements and recommendations for guided bone regeneration. Implant Dentistry, 28(4), 388–399. 10.1097/ID.0000000000000909 [DOI] [PubMed] [Google Scholar]
- Cucchi, A. , Giavatto, M. A. , Giannatiempo, J. , Lizio, G. , & Corinaldesi, G. (2019). Custom‐made titanium mesh for maxillary bone augmentation with immediate implants and delayed loading. Journal of Oral Implantology, 45(1), 59–64. 10.1563/aaid-joi-D-18-00141 [DOI] [PubMed] [Google Scholar]
- Cucchi, A. , Sartori, M. , Aldini, N. N. , Vignudelli, E. , & Corinaldesi, G. (2019). A proposal of pseudo‐periosteum classification after GBR by means of titanium‐reinforced d‐PTFE membranes or titanium meshes plus cross‐linked collagen membranes. The International Journal of Periodontics & Restorative Dentistry, 39(4), e157–e165. 10.11607/prd.3598 [DOI] [PubMed] [Google Scholar]
- Cucchi, A. , Sartori, M. , Parrilli, A. , Aldini, N. N. , Vignudelli, E. , & Corinaldesi, G. (2019). Histological and histomorphometric analysis of bone tissue after guided bone regeneration with non‐resorbable membranes vs resorbable membranes and titanium mesh. Clinical Implant Dentistry and Related Research, 21(4), 693–701. 10.1111/cid.12814 [DOI] [PubMed] [Google Scholar]
- Cucchi, A. , Vignudelli, E. , Fiorino, A. , Pellegrino, G. , & Corinaldesi, G. (2020). Vertical ridge augmentation (VRA) with Ti‐reinforced d‐PTFE membranes or Ti meshes and collagen membranes: 1‐year results of a randomized clinical trial. Clinical Oral Implants Research, 32(1), 1–14. 10.1111/clr.13673 [DOI] [PubMed] [Google Scholar]
- Cucchi, A. , Vignudelli, E. , Napolitano, A. , Marchetti, C. , & Corinaldesi, G. (2017). Evaluation of complication rates and vertical bone gain after guided bone regeneration with non‐resorbable membranes versus titanium meshes and resorbable membranes. A randomized clinical trial. Clinical Implant Dentistry and Related Research, 19(5), 821–832. 10.1111/cid.12520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin, C. , Linde, A. , Gottlow, J. , & Nyman, S. (1988). Healing of bone defects by guided tissue regeneration. Plastic and Reconstructive Surgery, 81(5), 672–676. 10.1097/00006534-198805000-00004 [DOI] [PubMed] [Google Scholar]
- Degidi, M. , Scarano, A. , & Piattelli, A. (2003). Regeneration of the alveolar crest using titanium micromesh with autologous bone and resorbable membrane. Journal of oral implantology, 29, 86–90. [DOI] [PubMed] [Google Scholar]
- Elgali, I. , Omar, O. , Dahlin, C. , & Thomsen, P. (2017). Guided bone regeneration: Materials and biological mechanisms revisited. European Journal of Oral Sciences, 125(5), 315–337. 10.1111/eos.12364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elnayef, B. , Monje, A. , Gargallo‐Albiol, J. , Galindo‐Moreno, P. , Wang, H. L. , & Hernández‐Alfaro, F. (2017). Vertical ridge augmentation in the atrophic mandible: A systematic review and meta‐analysis. International Journal of Oral and Maxillofacial Implants, 32, 291–312. [DOI] [PubMed] [Google Scholar]
- Fontana, F. , Maschera, E. , Rocchietta, I. , & Simion, M. (2011). Clinical classification of complications in guided bone regeneration procedures by means of a non‐resorbable membrane. International Journal of Periodontics and Restorative Dentistry, 31(3), 265–273. [PubMed] [Google Scholar]
- Garcia, J. , Dodge, A. , Luepke, P. , Wang, H. L. , Kapila, Y. , & Lin, G. H. (2018). Effect of membrane exposure on guided bone regeneration: A systematic review and meta‐analysis. Clinical oral implants research, 29, 328–338. [DOI] [PubMed] [Google Scholar]
- Gongloff, R. K. , Cole, M. , Whitlow, W. , & Boyne, P. J. (1986). Titanium mesh and particulate cancellous bone and marrow grafts to augment the maxillary alveolar ridge. International Journal of Oral and Maxillofacial Surgery, 15(3), 263–268. 10.1016/S0300-9785(86)80083-7 [DOI] [PubMed] [Google Scholar]
- Hartmann, A. , Hildebrandt, H. , Schmohl, J. U. , & Kämmerer, P. W. (2019). Evaluation of risk parameters in bone regeneration using a customized titanium mesh: results of a clinical study. Implant Dentistry, 28(6), 543–550. 10.1097/ID.0000000000000933 [DOI] [PubMed] [Google Scholar]
- Hartmann, A. , & Seiler, M. (2020). Minimizing risk of customized titanium mesh exposures ‐ a retrospective analysis. BMC Oral Health, 20(1), 36. 10.1186/s12903-020-1023-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janner, S. F. M. , Bosshardt, D. D. , Cochran, D. L. , Chappuis, V. , Huynh‐Ba, G. , Jones, A. A. , & Buser, D. (2017). The influence of collagen membrane and autogenous bone chips on bone augmentation in the anterior maxilla: A preclinical study. Clinical Oral Implants Research, 28(11), 1368–1380. 10.1111/clr.12996 [DOI] [PubMed] [Google Scholar]
- Khojasteh, A. , Hosseinpour, S. , Rezai Rad, M. , Alikhasi, M. , & Zadeh, H. H. (2019). Buccal fat pad‐derived stem cells with anorganic bovine bone mineral scaffold for augmentation of atrophic posterior mandible: An exploratory prospective clinical study. Clinical Implant Dentistry and Related Research, 21(2), 292–300. 10.1111/cid.12729 [DOI] [PubMed] [Google Scholar]
- Lim, G. , Lin, G. H. , Monje, A. , Chan, H. L. , & Wang, H. L. (2018). Wound healing complications following guided bone regeneration for ridge augmentation: A systematic review and meta‐analysis. The International Journal of Oral & Maxillofacial Implants, 33, 41–50. [DOI] [PubMed] [Google Scholar]
- Lizio, G. , Corinaldesi, G. , & Marchetti, C. (2014). Alveolar ridge reconstruction with titanium mesh: A three‐dimensional evaluation of factors affecting bone augmentation. The International Journal of Oral & Maxillofacial Implants, 29(6), 1354–1363. 10.11607/jomi.3417 [DOI] [PubMed] [Google Scholar]
- Lutz, R. , Neukam, F. W. , Simion, M. , & Schmitt, C. M. (2015). Long‐term outcomes of bone augmentation on soft and hard‐tissue stability: A systematic review. Clinical oral implants research, 26(Suppl. 11), 103–122. [DOI] [PubMed] [Google Scholar]
- Merli, M. , Moscatelli, M. , Mariotti, G. , Motroni, A. , Mazzoni, A. , Mazzoni, S. , Breschi, L. , & Nieri, M. (2017). A novel approach to bone reconstruction: The wafer technique. The International Journal of Periodontics & Restorative Dentistry, 37(3), 317–325. 10.11607/prd.3055 [DOI] [PubMed] [Google Scholar]
- Merli, M. , Moscatelli, M. , Mariotti, G. , Rotundo, R. , Bernardelli, F. , & Nieri, M. (2014). Bone level variation after vertical ridge augmentation: resorbable barriers versus titanium‐reinforced barriers. A 6‐year double‐blind randomized clinical trial. International Journal of Oral and Maxillofacial Implants, 29(4), 905–913. 10.11607/jomi.3203 [DOI] [PubMed] [Google Scholar]
- Merli, M. , Moscatelli, M. , Mazzoni, A. , Mazzoni, S. , Pagliaro, U. , Breschi, L. , Motroni, A. , & Nieri, M. (2013). Fence technique: Guided bone regeneration for extensive three‐dimensional augmentation. The International Journal of Periodontics and Restorative Dentistry, 33, 129–136. 10.11607/prd.1175 [DOI] [PubMed] [Google Scholar]
- Milinkovic, I. , & Cordaro, L. (2014). Are there specific indications for the different alveolare bone augmentation procedures for implant placement? A systematic review. International Journal of Oral and Maxillofacial Surgery, 43(5), 606–625. [DOI] [PubMed] [Google Scholar]
- Moher, D. , Hopewell, S. , Schulz, K. F. , Montori, V. , Gøtzsche, P. C. , Devereaux, P. J. , Elbourne, D. , Egger, M. , & Altman, D. G. (2012). CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. International journal of surgery (London, England), 10(1), 28–55. 10.1016/j.ijsu.2011.10.001 [DOI] [PubMed] [Google Scholar]
- Monje, A. , Urban, I. A. , Miron, R. J. , Caballe‐Serrano, J. , Buser, D. , & Wang, H. L. (2017). Morphologic patterns of the atrophic posterior maxilla and clinical implications for bone regenerative therapy. The International Journal of Periodontics & Restorative Dentistry, 37(5), e279–e289. 10.11607/prd.3228 [DOI] [PubMed] [Google Scholar]
- Plonka, A. B. , Urban, I. A. , & Wang, H. L. (2018). Decision tree for vertical ridge augmentation. The International Journal of Periodontics & Restorative Dentistry, 38(2), 269–275. 10.11607/prd.3280 [DOI] [PubMed] [Google Scholar]
- Retzepi, M. , & Donos, N. (2010). Guided bone regeneration: Biological principle and therapeutic applications. Clinical Oral Implants Research, 21(6), 567–576. 10.1111/j.1600-0501.2010.01922.x. [DOI] [PubMed] [Google Scholar]
- Revilla‐León, M. , Sadeghpour, M. , & Özcan, M. (2020). A review of the applications of additive manufacturing technologies used to fabricate metals in implant dentistry. Journal of Prosthodontics, 29(7), 579–593. 10.1111/jopr.13212 [DOI] [PubMed] [Google Scholar]
- Rocchietta, I. , Fontana, F. , & Simion, M. (2008). Clinical outcomes of vertical bone augmentation to enable dental implant placement: A systematic review. Journal of Clinical Periodontology, 35(Suppl. 8), 203–215. 10.1111/j.1600-051X.2008.01271.x [DOI] [PubMed] [Google Scholar]
- Ronda, M. , Rebaudi, A. , Torelli, L. , & Stacchi, C. (2014). Expanded vs. dense polytetrafluoroethylene membranes in vertical ridge augmentation around dental implants: A prospective randomized controlled clinical trial. Clinical Oral Implants Research, 25(7), 859–866. 10.1111/clr.12157 [DOI] [PubMed] [Google Scholar]
- Ronda, M. , & Stacchi, C. (2015). A novel approach for the coronal advancement of the buccal flap. The International Journal of Periodontics & Restorative Dentistry, 35, 795–801. 10.11607/prd.2232 [DOI] [PubMed] [Google Scholar]
- Sagheb, K. , Schiegnitz, E. , Moergel, M. , Walter, C. , Al‐Nawas, B. , & Wagner, W. (2017). Clinical outcome of alveolar ridge augmentation with individualized CAD‐CAM‐produced titanium mesh. International Journal of Implant Dentistry, 3(1), 36. 10.1186/s40729-017-0097-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler, M. , Kämmerer, P. W. , Peetz, M. , & Hartmann, A. (2018). Customized lattice structure in reconstruction of three‐dimensional alveolar defects. International journal of computerized dentistry, 21(3), 261–267. [PubMed] [Google Scholar]
- Shin, S. I. , Herr, Y. , Kwon, Y. H. , & Chung, J. H. (2013). Effect of a collagen membrane combined with a porous titanium membrane on exophytic new bone formation in a rabbit calvarial model. Journal of Periodontology, 84(1), 110–116. [DOI] [PubMed] [Google Scholar]
- Simion, M. , Baldoni, M. , Rossi, P. , & Zaffe, D. (1994). A comparative study of the effectiveness of a e‐PTFE memnranes with and without early exposure during the healing period. International Journal of Periodontics and Restorative Dentistry, 14, 166–180. [PubMed] [Google Scholar]
- Soldatos, N. K. , Stylianou, P. , Koidou, V. P. , Angelov, N. , Yukna, R. , & Romanos, G. E. (2017). Limitations and options using resorbable versus nonresorbable membranes for successful guided bone regeneration. Quintessence International, 48(2), 131–147. [DOI] [PubMed] [Google Scholar]
- Sumida, T. , Otawa, N. , Kamata, Y. U. , Kamakura, S. , Mtsushita, T. , Kitagaki, H. , Mori, S. , Sasaki, K. , Fujibayashi, S. , Takemoto, M. , Yamaguchi, A. , Sohmura, T. , Nakamura, T. , & Mori, Y. (2015). Custom‐made titanium devices as membranes for bone augmentation in implant treatment: Clinical application and the comparison with conventional titanium mesh. Journal of Cranio‐Maxillo‐Facial Surgery, 43(10), 2183–2218. 10.1016/j.jcms.2015.10.020 [DOI] [PubMed] [Google Scholar]
- Urban, I. , Monje, A. , Lozada, J. , & Wang, H. L. (2017). Principles for vertical ridge augmentation in the atrophic posterior mandible: A technical review. The International Journal of Periodontics & Restorative Dentistry, 37, 639–645. 10.11607/prd.3200 [DOI] [PubMed] [Google Scholar]
- Urban, I. A. , Montero, E. , Monje, A. , & Sanz‐Sánchez, I. (2019). Effectiveness of vertical ridge augmentation interventions: A systematic review and meta‐analysis. Journal of Clinical Periodontology, 46(Suppl. 21), 319–339. 10.1111/jcpe.13061 [DOI] [PubMed] [Google Scholar]
- Urban, I. A. , Nagursky, H. , Lozada, J. L. , & Nagy, K. (2013). Horizontal ridge augmentation with a collagen membrane and a combination of particulated autogenous bone and anorganic bovine bone‐derived mineral: a prospective case series in 25 patients. The International Journal of Periodontics and Restorative Dentistry, 33(3), 299–307. 10.11607/prd.1407 [DOI] [PubMed] [Google Scholar]
- Von Arx, T. , Hardt, N. , & Wallkamm, B. (1996). The TIME technique: A new method for localized alveolar ridge augmentation prior to placement of dental implants. International Journal of Oral and Maxillofacial Implants, 11(3):387–394. [PubMed] [Google Scholar]
- Zeng, N. , van Leeuwen, A. , Yuan, H. , Bos, R. R. , Grijpma, D. W. , & Kuijer, R. (2016). Evaluation of novel resorbable membranes for bone augmentation in a rat model. Clinical Oral Implants Research, 27, e8–e14. 10.1111/clr.12519 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


