Abstract
Background
To improve symptoms associated with testosterone deficiency, many testosterone therapies are available that aim to restore serum testosterone (T) levels to the normal physiologic range. The magnitude, frequency, and duration between peak and trough T concentrations vary with route of administration, and none reflect normal endogenous daily diurnal T variations.
Objective
To compare pharmacokinetic profiles of serum T from approved T formulations with endogenous diurnal T variations in young and older men, and to consider whether there may be value in mimicking the diurnal T rhythmicity with exogenous testosterone therapies as men age.
Materials and methods
A literature search of studies examining the diurnal variation of endogenous T in healthy men and men with testosterone deficiency was performed using PubMed in January 2020. Additional searches for serum T pharmacokinetic profiles of various testosterone therapy formulations were also conducted. Prescribing information for various T formulations was also reviewed.
Discussion and conclusion
Endogenous diurnal T variation is well described and appears to be blunted naturally as men age. Men with testosterone deficiency lack diurnal T variation and exhibit a flatter T profile compared with eugonadal men. Some T replacement options provide intraday T level variations similar to normal circadian secretion, and others provide a flatter exposure profile reflective of depot release. Others provide profiles that exceed the frequency and physiologic range of the natural diurnal variation of T. All exogenous T replacement dosing targets an increase in average T levels to within the normal physiologic range and improves symptoms associated with low T, but no single testosterone therapy can exactly mimic the normal diurnal T patterns seen in younger men and the blunted circadian T secretion of older men.
Keywords: diurnal variation, testosterone deficiency, testosterone therapy
1. INTRODUCTION
The United States Food and Drug Administration (USFDA) approves use of various formulations for testosterone therapy (TTh) for men with testosterone deficiency (TD) associated with certain medical conditions to achieve eugonadal levels of T seen in healthy men. 1 The Endocrine Society clinical practice guideline and American Urological Association recommend diagnosing TD in men with presence of clinical signs and symptoms of TD and testosterone (T) levels consistently <10.4 nmol/L (300 ng/dl). 2 , 3 Societies outside the United States largely are in agreement with the clinical practice guideline set by the Endocrine Society, with most societies recommending a cutoff of either 10.4 nmol/L (300 ng/dl) or 12–12.1 nmol/L (346–350 ng/dl) as the mean total T cutoff for TD diagnosis and during follow‐up. There are slight differences in diagnostic workup and follow‐up 4 ; for example, the Endocrine of Society of Australia has recommended separate mean total T (TT) cutoffs to diagnose TD for young men and men older than 70 years, possibly to reflect the lower mean total T levels seen in older men. 5 , 6 , 7 , 8 In addition, “adult‐onset hypogonadism” or “late‐onset hypogonadism” (LOH), as defined by the Sexual Medicine Society of North America (SMSNA), is a syndrome characterized by clinical and biochemical evidence of low T with advancing age. 7 Currently, only the European Academy of Andrology (EAA) and the SMSNA have provided recommendations for the short‐term treatment of LOH with TTh. Nevertheless, overall, these societies recommend that pre‐treatment serum T levels should be obtained and T levels should be regularly assessed following TTh. 9 , 10 , 11 , 12
In 2016, it was estimated that 2.4 million men in the United States 40 to 69 years old have symptomatic TD. 13 Globally, this prevalence ranges from 2% to 6%. 6 , 14 Results from the Hypogonadism in Males (HIM) study estimated that approximately 40% of men aged ≥45 years had T levels <10.4 nmol/L (300 ng/dl) and that a patient's risk for TD was estimated to increase by 17% for every 10‐year increase in age. Age‐related decline in T levels may be attributed to variances in T levels observed in men over 40. 15 TD may negatively impact men's health and quality of life, as symptoms of TD include impaired sexual function, depressed mood and fatigue, changes in bone mineral density, and body composition, and increased body fat. 2 , 16 The presence of certain comorbidities, such as obesity, type 2 diabetes mellitus, atherosclerotic cardiovascular disease, and metabolic syndrome, are also associated with low T levels, which may be ameliorated with long‐term TTh. 17 , 18 , 19 , 20 , 21 , 22 , 23 To improve symptoms associated with TD, many TTh are available to restore serum T levels to the middle tertile of the normal physiologic range (450–600 ng/dl). 3 , 24 Men across a wide age range seek treatment for low T, with an estimated 2.3% of men in their 40s and 3.8% of men in their 60s receiving some form of TTh in 2011. 25 When used appropriately, TTh can ameliorate signs and symptoms associated with hypogonadism. 26 , 27 There are many routes of administration for TTh approved for use by the FDA. Depending on the route of administration, the varying T formulation pharmacokinetics (PKs) contribute to the differences in time when improvements begin to take effect (ranging between 3 and 6 weeks), with maximum effects achieved within 6 months to 1 year after initiating therapy. 13 , 28 , 29 Furthermore, the amplitude between peak T (C max) and trough T (C min) concentrations following TTh may also vary widely from what is observed in daily diurnal endogenous T variations. In this review, we sought to compare the PK profiles of serum T from different exogenous T formulations with diurnal variations in endogenous serum T levels and consider whether, as men age, there may be clinical value in mimicking the diurnal T rhythmicity with exogenous TTh.
2. MATERIALS AND METHODS
A literature search using PubMed was performed in January 2020 with the following search terms: “diurnal variation” or “circadian rhythm,” and “testosterone,” “testosterone deficiency,” or “testosterone treatment.” Studies evaluating diurnal T variation in healthy young and older men, as well as men with low T, were reviewed. This article reviewed the prescribing information for various T preparations currently approved by the FDA, and additional searches were conducted using PubMed to identify primary PK studies for each TTh. Studies with clinical importance and T PK assessments were included. This review was funded by Antares Pharma, Inc., who provided review for medical accuracy.
3. PK PROFILES OF APPROVED EXOGENOUS T FORMULATIONS
3.1. Subcutaneous injections
In September 2018, the FDA approved subcutaneous (SC) testosterone enanthate (TE) as an option for TTh for men with TD. This product is supplied as a single‐dose auto‐injector that patients self‐administer in the abdominal region once a week. The recommended starting dose is 75 mg and may be adjusted in 25 mg increments to 50 or 100 mg based on trough‐concentration‐guided dosing. 30
In an open‐label, single‐arm, dose‐blinded, 52‐week phase 3 study, 150 patients were initiated on 75 mg SC TE administered weekly, with trough‐concentration‐guided dose adjustment. 31 At baseline, TT was 8.0 ± 3.3 nmol/L (230.4 ± 94.0 ng/dl). The primary endpoint was met, with 92.7% of overall patients achieving T levels within physiologic range of 10.4 to 38.1 nmol/L (300–1100 ng/dl). There were no patients with a measured TT C max ≥52 nmol/L (1500 ng/dl) at week 12. Mean C trough values >10.4 nmol/L (300 ng/dl) were maintained from week 6 (17.4 ± 6.0 nmol/L, or 501.9 ± 172.7 ng/dl) through week 52 (16.9 ± 5.3 nmol/L, or 487.2 ± 153.3 ng/dl) of the study. Following 12 weeks on SC TE treatment, the 7‐day mean total T concentration (C avg0‐168h) was 19.2 ± 4.4 nmol/L (553.3 ± 127.3 ng/dl). The C max (27.4 ± 7.5 nmol/L, or 789.8 ± 215.4 ng/dl; Table 1) and C min (15.1 ± 3.8 nmol/L, or 435.6 ± 109.2 ng/dl) measured during the 7‐day period at week 12 yielded a peak‐to‐trough ratio of 1.8. During the week 12 dosing interval, there was little fluctuation in dihydrotestosterone (DHT) and estradiol (E2). The mean DHT:TT ratio was 7.4 ± 2.7 at week 12. Consistent with a previous study of SC TE, 32 SC weekly dosing achieves stabilized physiologic T levels over a one‐week dosing interval after injection, and minimizes large peak and trough differences as seen with some other TTh. 31
TABLE 1.
Comparison of testosterone PK profile of different TTh preparations
| Route | Formulation | Dose | Mean C max | Time to C max | Mean C min | Mean C avg Between Treatment Doses | Recommended Starting Dose |
|---|---|---|---|---|---|---|---|
| SC | TE 31 , 32 (XYOSTED®) 30 | 50 mg/week | 29.5 ± 9.5 nmol/L (849.6 ± 274.8 ng/dl) | 12.0 h (median) | 15.9 ± 4.9 nmol/L (458.2 ± 139.9 ng/dl) | 20.7 ± 6.2 nmol/L (598.2 ± 177.7 ng/dl) |
|
| 75 mg/week | 26.3 ± 6.4 nmol/L (758.2 ± 185.9 ng/dl) | 11.9 h (median) | 14.9 ± 3.4 nmol/L (431.2 ± 97.5 ng/dl) | 18.6 ± 3.7 nmol/L (537.6 ± 108.1 ng/dl) | |||
| 100 mg/week | 30.0 ± 8.3 nmol/L (866.3 ± 238.6 ng/dl) | 24.1 h (median) | 14.8 ± 4.2 nmol/L (427.7 ± 120.9 ng/dl) | 19.8 ± 4.4 nmol/L (571.1 ± 127.2 ng/dl) | |||
| Overall | 27.4 ± 7.5 nmol/L (789.8 ± 215.4 ng/dl) | 11.9 h (median) | 15.1 ± 3.8 nmol/L (435.6 ± 109.2 ng/dl) | 19.2 ± 4.4 nmol/L (553.3 ± 127.3 ng/dl) | |||
| IM | TC 35 (Depo®‐Testosterone) 34 | 200 mg/2 weeks | 38.6 ± 10.3 nmol/L (1112 ± 297 ng/dl) | 4–5 days | n/a | n/a |
|
| TE 36 (DELATESTRYL®) 37 | 100 mg/week | >41.6 nmol/L or 1200 ng/dl | 24 h | n/a | 36.6 nmol/L (1055 ng/dl) |
|
|
| 200 mg/2 weeks | >41.6 nmol/L or 1200 ng/dl | 48 h | n/a | 32.7 nmol/L (943 ng/dl) | |||
| 300 mg/3 weeks | >41.6 nmol/L or 1200 ng/dl | 36–48 h | n/a | 30.6 nmol/L (883 ng/dl) | |||
| 400 mg/4 weeks | ∼55.5 nmol/L or 1600 ng/dl | 36–48 h | n/a | 25.3 nmol/L (729 ng/dl) | |||
| TU 38 , 39 (AVEED®) 40 | 750 mg | 30.9 ± 12.0 nmol/L (890.6 ± 345.1 ng/dl) | 10 days post‐injection |
Week 14: 11.8 ± 4.3 nmol/L (339.5 ± 122.7 ng/dl) Week 24:11.2 ± 3.4 nmol/L (323.5 ± 99.1 ng/dl) |
17.1 ± 4.9 nmol/L (494.5 ± 141.5 ng/dl) |
|
|
| TU (REANDRON®/ NEBIDO® 97 ) | 1000 mg | 45 nmol/L (1298 ng/dl) | 7 days | 17 nmol/L (490 ng/dl) | n/a |
|
|
| Subdermal implantation | T pellets 50 (TESTOPEL® at days 1–113 45 ) | 900 mg (12 pellets) | 35.4 ± 0.9 nmol/L (1021.6 ± 24.9 ng/dl) | 13.5 days (median) | n/a | 22.1 ± 4.3 nmol/L (638.3 ± 124.2 ng/dl) |
|
| T pellets—retrospective study 48 | 6–7 pellets (450–525 mg) | 33.5 nmol/L (966 ng/dl) | 1 month | 8.1 nmol/L (233 ng/dl) | 24.7 nmol/L (712 ng/dl) | ||
| 8–9 pellets (600–675 mg) | 37.4 nmol/L (1078 ng/dl) | 1 month | 7.7 nmol/L (221 ng/dl) | 24.9 nmol/L (719 ng/dl) | |||
| 10–12 pellets (750–900 mg) | 61.1 nmol/L (1762 ng/dl) | 1 month | 8.9 nmol/L (257 ng/dl) | 27.6 nmol/L (795 ng/dl) | |||
| Transdermal patch | TE patch 52 (ANDRODERM®) 51 | 5 mg/day (no longer available) | 26.5 ± 9.6 nmol/L (765 ± 277 ng/dl) | 8.2 ± 2.0 h | 9.7 ± 5.1 nmol/L (280 ± 146 ng/dl) | 17.9 ± 6.1 nmol/L (517 ± 176 ng/dl) |
|
| Topical gels and solutions | 1% T gel 64 (AndroGel® 1% at day 90) 62 | 50 mg/day | 29.3 ± 1.9 nmol/L (845 ± 55 ng/dl) | 4.0 h | 12.3 ± 0.6 nmol/L (355 ± 17 ng/dl) | 19.2 ± 1.1 nmol/L (554 ± 32 ng/dl) |
|
| 100 mg/day | 41.7 ± 2.3 nmol/L (1203 ± 66 ng/dl) | 7.9 h | 17.4 ± 0.8 nmol/L (502 ± 23 ng/dl) | 27.5 ± 1.1 nmol/L (793 ± 32 ng/dl) | |||
| Topical gels and solutions, continued | 1.62% T gel (AndroGel® 1.62% at day 112/TESTOGEL®) 63 | 20.25–81 mg | 29.3 ± 16.6 nmol/L (845 ± 480 ng/dl) | 8 h | n/a | 19.5 ± 9.0 nmol/L (561 ± 259 ng/dl) |
|
| 2% T gel (FORTESTA®) 67 | 40 mg/day | 29.6 ± 14.5 nmol/L (855 ± 417 ng/dl) | 2–4 h | n/a | 15.4 ± 6.1 nmol/L (444 ± 176 ng/dl) |
|
|
| 2% T gel (TESTAVAN®/TESTARZON®) 74 , 98 | 23 mg/day | 25 ± 8.8 nmol/L (721 ± 254 ng/dl) | 4 h | n/a | 12.8 ± 4.2 nmol/L (369 ± 121 ng/dl) |
|
|
| 1% T gel (TESTIM®/VOGELXO®) 71 , 72 | 50 mg/day | 18.7 ± 12.9 nmol/L (538 ± 371 ng/dl) | 8 h | 7.7 ± 4.4 nmol/L (223 ± 126 ng/dl) | 12.7 ± 6.5 nmol/L (365 ± 187 ng/dl) |
|
|
| 100 mg/day | 31.1 ± 19.6 nmol/L (897 ± 565 ng/dl) | 4–8 h | 13.7 ± 6.6 nmol/L (394 ± 189 ng/dl) | 21.2 ± 9.9 nmol/L (612 ± 286 ng/dl) | |||
| 2% T solution 76 (AXIRON® at day 120) 61 [DSC] | 30 mg/day | 27.0 ± 14.4 nmol/L (779 ± 416 ng/dl) | n/a | 10.1 ± 6.1 nmol/L (291 ± 175 ng/dl) | 17.1 ± 8.3 nmol/L (493 ± 239 ng/dl) |
|
|
| 60 mg/day | 29.1 ± 15.1 nmol/L (839 ± 436 ng/dl) | n/a | 10.0 ± 4.0 nmol/L (288 ± 115 ng/dl) | 17.5 ± 6.1 nmol/L (506 ± 175 ng/dl) | |||
| 90 mg/day | 23.0 ± 11.6 nmol/L (664 ± 336 ng/dl) | n/a | 8.0 ± 4.1 nmol/L (230 ± 119 ng/dl) | 14.4 ± 5.7 nmol/L (415 ± 165 ng/dl) | |||
| 120 mg/day | 22.8 ± 12.2 nmol/L (658 ± 353 ng/dl) | n/a | 9.1 ± 3.8 nmol/L (262 ± 111 ng/dl) | 13.5 ± 5.5 nmol/L (390 ± 160 ng/dl) | |||
| Overall | 27.5 ± 14.5 nmol/L (792 ± 417 ng/dl) | 2 h | n/a | 16.6 ± 6.1 nmol/L (480 ± 177 ng/dl) | |||
| Nasal T gel | 4.5% T nasal gel 79 (NATESTO®) 77 | 22 mg/day | 36.3 ± 16.2 nmol/L (1045.7 ± 467.1 ng/dl) | 1.4 h | 6.5 ± 3.2 nmol/L (186.3 ± 92.6 ng/dl) | 13.0 ± 4.5 nmol/L (375.3 ± 128.9 ng/dl) | |
| 33 mg/day | 32.4 ± 13.2 nmol/L (934.9 ± 381.2 ng/dl) | 1 h | 7.0 ± 2.5 nmol/L (200.9 ± 72.7 ng/dl) | 13.4 ± 3.9 nmol/L (386.9 ± 111.9 ng/dl) | 3 × 11 mg (3 daily dose; 2 pump actuations, 1 pump actuation in each nostril) 77 | ||
| Buccal T muco‐adhesives | T buccal system 83 (STRIANT®) 82 [DSC] | 30 mg/twice per day | 33.6 ± 15.3 nmol/L (969 ± 441 ng/dl) | 10.5 ± 9.3 h | 10.1 ± 4.5 nmol/L (291 ± 130 ng/dl) | 18.4 ± 7.1 nmol/L (531 ± 205 ng/dl) |
|
| Oral T | TU capsules (JATENZO®) 89 | 158–396 mg taken orally twice daily | 34.9 ± 20.1 nmol/L (1008 ± 581 ng/dl) | 2 h | n/a | 14.0 ± 4.4 nmol/L (403 ± 128 ng/dl) |
|
| TU capsules (Andriol® Testocaps®) 99 | 80–160 mg/day for 2–3 weeks | 40 nmol/L (1154 ng/dl) | 4–5 h | n/a | n/a |
|
DSC, discontinued.
3.2. Intramuscular injections
Intramuscular (IM) injection of T esters has been widely used as TTh. Unlike non‐esterified T, which has a short half‐life of 10 min when injected, esterification of T at the 17β‐carbon prolongs the duration of action. Esterification increases the solubility of T in oil, allowing for slower release of T with IM injection. 13 , 33 The three IM formulations that are approved by the FDA for use as TTh are testosterone cypionate (TC), TE, and testosterone undecanoate (TU). Depending on the formulation and dosage, following IM injection of TE or TC, supraphysiologic levels occur within a week after administration, decreasing to sub‐therapeutic levels in between dosing intervals, resulting in large TT peak‐to‐trough ratios. A longer interval to reach steady state is observed with IM TU. The Endocrine Society Clinical Practice Guideline for TTh recommends measuring serum T levels midway between injections for IM TE or TC and at the end of the dosing interval prior to the subsequent injection for IM TU, adjusting dose or frequency to target the low‐mid physiological range (12.1–20.8 nmol/L, or 350–600 ng/dl). 2
3.2.1. Testosterone cypionate
TC is available in two strengths, 100 and 200 mg/ml concentrations prepared in cottonseed oil. 34 The recommended dose is 50 to 400 mg administered every 2 to 4 weeks for IM TC. In a study of 11 men with hypogonadism, every‐other‐week administration of 200 mg IM TC caused a threefold rise in serum T, with peak values occurring between 2 to 3 days (38.4 ± 15.3 nmol/L; 1108 ± 440 ng/dl) and 4 to 5 days (38.6 ± 10.3 nmol/L, or 1112 ± 297 ng/dl) post‐injection. 35 Similarly, E2 levels also increased almost threefold. Within 2 weeks, mean T C avg approached 13.9 nmol/L (400 ng/dl). These large fluctuations in serum T over the 2‐week dosing period differ greatly from what is observed in normal, diurnal T variation of healthy young or older men.
3.2.2. Testosterone enanthate
A study evaluated the relative efficacy of four different dosage regimens in 23 men with primary hypogonadism. 36 The men received one of the four following regimens—100 mg weekly, 200 mg every 2 weeks, 300 mg every 3 weeks, or 400 mg every 4 weeks—and mean serum T concentrations were assessed once weekly during the initial 12‐week treatment period. For all dosing regimens, the mean C max was >41.6 nmol/L (1200 ng/dl) and occurred between 24 and 48 h. After the last dose of each regimen, mean C avg values were 36.6 nmol/L (1055 ng/dl), 32.7 nmol/L (943 ng/dl), 30.6 nmol/L (883 ng/dl), and 25.3 nmol/L (729 ng/dl) for the 100, 200, 300, and 400 mg doses, respectively. Treatment with either 100 mg/week or 200 mg every 2 weeks was able to lower the initially elevated luteinizing hormone (LH) and follicle‐stimulating hormone (FSH) concentrations. Based on these results, the authors recommend 200 mg IM TE injections every 2 weeks or 300 mg every 3 weeks, which seem to be effective to keep mean serum T levels within normal range (10.4–41.6 nmol/L, or 300–1200 ng/dl). The manufacturer of TE, supplied as 5 ml (200 mg/ml) in sesame oil and available in multiple‐dose vials, recommends that the starting dose of IM TE injections be 50 to 400 mg every 2 to 4 weeks. 37
3.2.3. Testosterone undecanoate
Esterification of T at the 17β‐position with undecanoic acid results in a longer‐acting IM TTh option that increases treatment intervals compared with that of other T esters. 33 , 38 The efficacy and safety of 750 mg IM TU were evaluated in an open‐label, 84‐week, phase 3 clinical trial of 130 men with TD. 39 Enrolled men received 750 mg TU in 3 ml of castor oil (250 mg/ml) by deep IM injections administered at baseline, week 4, and every 10 weeks thereafter through 9 injections. Serum T levels peaked approximately 7 days after each injection, with a mean C max of 30.9 ± 11.9 nmol/L (890.6 ng/dl) after the third IM TU injection. Serum T profiles were nearly identical after the third and fourth injection. Mean C min levels remained within the reference range (10.4–34.7 nmol/L, or 300–1000 ng/dl) at every time point measured throughout the study, and a peak‐to‐trough ratio was maintained around 2.6 to 2.8. During the 10 weeks after the third injection, 94% of patients treated with 750 mg IM TU had T C avg levels within 10.4 to 34.7 nmol/L, or 300 to 1000 ng/dl (mean C avg: 17.2 nmol/L or 494.9 ng/dl), which is consistent with a previous study where T levels were achieved within the normal range during a 10‐week dosing period. 38 , 39 The PK profile of IM TU does not demonstrate the supraphysiological peaks observed with IM TE and TC injections, and C min levels occur at later intervals after each injection. With fewer peaks and troughs, IM TU may be more acceptable for men with TD seeking TTh than IM TC or TE. Patients treated with IM TU also demonstrated average levels of DHT and E2, and DHT:T and E2:T ratios were within the reference range. The recommended dose of IM TU is an initial 750 mg injection, followed by 750 mg 4 weeks later, and 750 mg every 10 weeks thereafter, injected in the gluteus medius. 40
3.2.4. Testosterone ester mixtures
Globally, a variety of T ester mixtures are available for TTh outside of the United States (e.g., Testoviron Depot: T propionate and TE; Sustanon 250: 30 mg T propionate, 60 mg T phenylpropionate, 60 mg T isocaproate, and 100 T decanoate) 41 , 42 ; however, in silico simulations suggest that injections of T ester mixtures produce wider fluctuations of serum T concentrations than IM TE injections alone. These mixtures have not been recently studied, but in a 1974 study, 3 men with TD treated with 115.7 mg TE and 20 mg T propionate reported approximately 40 nmol/L (1154 ng/dl) increase of serum T over baseline 1 day post‐IM injection, suggesting combining these so‐called “short‐acting” T esters only worsens the PK profile and increases the initial undesired T peak. 33 , 43
3.3. Subdermal T pellets
Subdermal T pellet implantation was among the earliest methods developed for clinical applications of TTh since the late 1930s, and the original pellets were usually implanted under the skin of the lower abdominal wall. 44 Currently available T pellets require office visits and a minor procedure for T pellet implantation into the hip area. 33 These T pellets consist of crystalline T and are synthesized by high‐temperature molding which allows prolonged, steady, and low concentrations of T to be released over an extended period. 33 , 45 In an early crossover study, 43 men with primary or secondary hypogonadism were randomized to 1 of 3 initial dosing regimens (6 pellets x 100 mg; 6 pellets x 200 mg; 3 pellets x 200 mg). 46 Men were sequentially treated with the next regimen following an interval of ≥6 months until T levels returned to hypogonadal levels and completed all three dosing regimens. For all treatment regimens, peak T levels occurred at the first month after pellet insertion; serum T levels gradually declined to baseline by 6 months for the two 600 mg regimens, but remained significantly elevated after 6 months at the 1200 mg dose. The 75 mg implantable subdermal T pellet (TESTOPEL®) received FDA approval in 1972, and the recommended dosing regimen is 150 to 450 mg (2–6 pellets) implanted subcutaneously every 3 to 6 months; dosing is adjustable depending on the patient's age and diagnosis, and how the patient responds to treatment. 45 However, data from the medical literature suggest that insertion of at least 10 pellets (≥750 mg) may be common in clinical practice. 47 , 48 , 49 , 50
In a retrospective review of medical records collected from 6 different institutions from 380 men, the mean C max occurred 1 month post‐implantation, regardless of the number of pellets implanted (T C avg for 6–7 pellets, 24.7 nmol/L [712 ng/dl]; 8–9 pellets, 24.9 nmol/L [719 ng/dl]; ≥10 pellets, 27.6 nmol/L [795 ng/dl]). 48 The more T pellets (10–12; 750–900 mg) that were implanted, the higher and more sustained levels of T that could be achieved and maintained. A retrospective study of 273 men with TD treated with T pellets revealed that patients with 6 to 9 pellets implanted had significantly lower total T levels than men receiving 10 to 12 pellets (21.3 nmol/L [614 ng/dl] vs. 28.1 nmol/L [811 ng/dl]; p = 0.0006). 49 In an open‐label, three‐center study, implantation of 12 T pellets (900 mg) resulted in peak serum T levels within 2 weeks, with a mean C max of 35.4 nmol/L (1021.6 ng/dl) and a mean C avg of 22.1 nmol/L (638.3 ng/dl) by month 4. 50 Most patients (93%) did not have C max levels >52 nmol/L (1500 ng/dl), and 71% of patients had total T levels >10.4 nmol/L (300 ng/dl) for at least 3 months, consistent with results from an open‐label study in which 86% (24/28) of patients received 6 to 12 pellets (450–900 mg), maintaining T levels >10.9 nmol/L (315 ng/dl) at 4 months. 47 , 50
3.4. Transdermal T patch
The FDA originally approved ANDRODERM®, 51 a non‐scrotal transdermal T patch, in 1995, with the 2.5 mg/day and 5.0 mg/day systems. This method of delivery allows T to be continually absorbed without dose accumulations for 24 h. 52 , 53 , 54 , 55 , 56 In a 24‐week, multicenter, randomized 1:1, parallel‐group study comparing the PK, efficacy, and safety of a transdermal T system with IM TE injections in 66 men with TD, daily application of 2 transdermal T patches (5.0 mg/day total) resulted in morning T levels within the defined normal physiologic range (10.6–35.7 nmol/L, or 306–1031 ng/dl) in 96% of patients over weeks 2 to 24. 54 At week 16, T C avg was 17.9 ± 6.1 nmol/L (517 ± 176 ng/dl) compared with 1.9 ± 2.2 nmol/L (55.4 ± 62.8 ng/dl) at baseline. Peak T levels were reached approximately 8.2 h after application, with a C max of 26.5 ± 9.6 nmol/L (765 ± 277 ng/dl). In 34 men from a multicenter, phase 3 study of a transdermal T patch system for TD, nightly applications of 2 patches (5.0 mg/day) resulted in peak levels occurring in the morning after application and decreasing slowly until system removal, mimicking the circadian patterns reported in healthy, young men. 52
A reduced dosing regimen for either 2.0 mg/day or 4.0 mg/day systems applied nightly was evaluated in an interventional study enrolling 40 men with TD for 4 weeks (Clinicaltrials.gov identifier: NCT01104246). This reduced dosing regimen was approved in 2011, and manufacturer data show that following 28 days of transdermal T application, 97% (34/35) men with TD were able to achieve C avg within 10.4 to 35.7 nmol/L (300–1030 ng/dl). 51 Mean C max values with 2.0 mg/day and 4.0 mg/day treatment were 22.5 ± 5.0 nmol/L (648 ± 145 ng/dl) and 24.1 ± 5.5 nmol/L (696 ± 158 ng/dl), respectively. Similar to the 2.5 mg/day and 5.0 mg/day systems, peak T levels occurred 8 h post‐application, mimicking diurnal variation when the patch is applied at night.
3.5. Topical T gels and solutions
Because of their convenience and ease of application, transdermal T gels and solutions are among the most commonly used formulations and have been preferred by many patients. 57 , 58 Users of T gel have indicated a preference for a gel formulation that can dispense accurate T doses in a small gel volume in the inner thigh/abdomen and one with a shorter waiting time after application before showering and/or swimming. 59 Currently, there are four branded, FDA‐approved hydroalcoholic T gels available: AndroGel®, FORTESTA®, TESTIM®, and VOGELXO®. Despite its convenience, for most patients, serum T levels fall under the normal range within 24 h of applying transdermal 2% T gel, requiring daily application of product. 60 In addition to T gels, AXIRON® was offered as a T topical solution. 61
3.5.1. AndroGel®
AndroGel® is available in 1.0% and 1.62% concentrations. 62 , 63 Topical AndroGel® 1.0% is offered as a unit‐dose packet containing 2.5 g or 5.0 g of gel, equivalent to 25 or 50 mg of T, respectively. 62 A randomized, 180‐day study of 227 men with TD evaluated the PK profile and tolerability of AndroGel® 1.0% at two dosages (50 and 100 mg/day) compared with the T patch (5 mg/day). 64 The study was double‐blinded until day 90 for the T gel groups, after which patients could elect to continue with the long‐term follow‐up study and receive any dose adjustments as necessary. After the first application of either 5 g or 10 g T gel, mean C avg, C max, and C min T levels were within the normal physiological range (values ranged from 7.9 ± 0.5 to 25.9 ± 1.4 nmol/L; 228 ± 14 to 747 ± 40 ng/dl). 64 The C avg, C max, and C min following 90 days of 10 g T gel application were 27.5 nmol/L (793 ng/dl), 41.7 nmol/L (1203 ng/dl), and 17.4 nmol/L (502 ng/dl), respectively, compared with 19.2 nmol/L (554 ng/dl), 29.3 nmol/L (845 ng/dl), and 12.3 nmol/L (355 ng/dl) with 5 g T gel. 64 At day 90, peak T levels were reached after 4 and 8 h with 5 g and 10 g T gel application, respectively. Steady‐state serum T levels were achieved within a few days, and these levels were maintained with once‐daily applications. AndroGel® 1.62% is also available as unit‐dose packets (20.25 mg T in 1.25 g gel, or 40.5 mg T in 2.5 g gel), as well as a metered‐dose pump delivering 20.25 mg T in 1.25 g gel. 63 Compared with AndroGel® 1.0%, this formulation reduces the volume of gel and total mass of gel applied. 65 Efficacy and safety of 1.62% topical T gel at the initial dose of 2.5 g were evaluated in a two‐phase, 364‐day, randomized, double‐blind, placebo‐controlled study in 234 men with TD. 65 , 66 Serum T levels were assessed on days 14, 28, and 42, and adjustments to dose were made (increased or decreased) in 1.25 mg increments if TT levels were not within the pre‐specified range (12.1–26.0 nmol/L, or 350–750 ng/dl). The primary endpoint was met, with 81.6% (146/179) of patients achieving C avg within the pre‐specified range (10.4–34.7 nmol/L, or 300–1000 ng/dl) on day 112. Doses were distributed as follows after titration: 1.25 g (7.3%, 17/234), 2.5 g (25.6%, 60/234), 3.75 g (28.2%, 66/234), and 5.0 g (38.9%, 91/234). Combining all four doses on day 112, C avg and C max levels were 19.5 nmol/L (561 ng/dl) and 29.3 nmol/L (845 ng/dl), respectively. 63 Mean E2 mirrored changes observed in TT levels, but LH, FSH, and sex hormone binding globulin (SHBG) significantly decreased from baseline following T gel use. In a long‐term follow‐up to this study, DHT levels were generally within the normal reference range (0.4–3.3 nmol/L, or 11.2–95.5 ng/dl), and 77.9% of patients continuing with AndroGel® 1.62% treatment maintained C avg within the normal range on day 364 (mean total T concentration, 15.8 nmol/L, or 455 ng/dl), confirming the results observed in the double‐blind phase of the study. 66
3.5.2. FORTESTA®
FORTESTA® is available with a metered‐dose pump delivering 0.5 g gel (10 mg of T) per pump actuation, with the recommended starting dose of 40 mg of T (4 pump actuations) applied once daily to the thighs in the morning. 67 The PK profile and safety of FORTESTA®, a 2% T gel, were evaluated in a multicenter, 90‐day, open‐label non‐comparative trial of 149 men with TD. 68 Men started with a 40 mg dose, and adjustments were made on days 14, 35, and 60 in 10 mg increments to between 10 and 70 mg/day. The primary efficacy endpoint was met, with 77.5% (100/129) of patients achieving C avg within the normal range, defined as 10.4 to 39.5 nmol/L (300–1140 ng/dl), on day 90. Overall, C avg was 15.2 nmol/L (438.6 ng/dl) and C max was 28.7 mol/L (827.6 ng/dl) on day 90, with 94.6% (122/129) of patients with a C max ≤52.0 nmol/L (1500 ng/dl) and 2 patients with C max between 62.4 and 86.7 nmol/L (1800 and 2500 ng/dl). The 24‐h PK profile showed that peak T levels were reached approximately 2 to 4 h post‐application. 67 , 68
3.5.3. TESTIM® and VOGELXO®
TESTIM® 1% gel is available as a unit‐dose tube containing the recommended starting dose of 50 mg of T in 5 g of gel. 69 Serum T level measurements should be performed 14 days after starting treatment, and daily doses may be increased to 100 mg (2 tubes) if serum T levels are below the normal range (10.4–34.7 nmol/L, or 300–1000 ng/dl). VOGELXO® 1% gel is available as unit‐dose tubes, packets, or a multi‐dose metered pump containing the recommended starting dose of 50 mg of T, with the multi‐dose metered pump delivering 12.5 mg of T per actuation. 70 In 2015, VOGELXO® received an AB rating from the FDA, deeming it therapeutically equivalent to TESTIM® when substituted and used as indicated. The PK and clinical profile of TESTIM®/VOGELXO® 1% gel were evaluated in men with TD against other TTh in various studies. 71 , 72 , 73 There were 3 concentration maxima observed with both TESTIM®/VOGELXO® 1% and AndroGel® 1%; peak T levels were reached at 3 to 4 h, 8 to 10 h, and 18 to 24 h post‐application. However, with TESTIM®/VOGELXO® 1%, the C max was approximately 30% higher than with AndroGel® 1%. 71 In a randomized, blinded, parallel treatment group study of 406 men with TD, treatment with TESTIM®/VOGELXO® 1% gel normalized serum T levels with fewer skin irritations than the T patch. Within 30 days of treatment, the increase in mean C avg from baseline was similar in the 50 mg gel and T patch groups (50%). Treatment with the 100 mg gel resulted in a 173% increase in mean C avg from baseline compared with the T patch group, with 95% of patients in the 100 mg gel group achieving mean C avg above 10.4 nmol/L (300 ng/dl). At day 30, daily peak‐to‐trough ratios were 2.4 and 2.3 in patients treated with 50 mg and 100 mg gel, respectively, which was less than the fluctuation observed with the T patch (3.0). At day 90, more patients in both T gel groups had mean C avg values (50 mg: 75%; 100 mg: 80%) above 10.4 nmol/L (300 ng/dl) compared with the T patch‐treated patients (57%), and peak‐to‐trough ratios remained around 2.2 for both T gel groups but increased slightly, to 3.2, in the T patch group. Mean changes in DHT C avg from baseline to day 30 were four‐ to sevenfold greater for the 50 mg and 100 mg gel, respectively, than that observed for the T patch treatment group. While T gel treatment produced higher serum DHT levels at day 30 and day 90, DHT:T ratios remained stable and similar to that reported in normal men. In these studies, treatment with TESTIM® gel increased and maintained serum T levels to within the normal range, improved sexual function, and resulted in fewer application site reactions than patch formulations of TTh. 72
3.5.4. TESTAVAN®/TESTARZON®
TESTAVAN®/TESTARZON® 2% T gel (available outside the United States) is administered on the upper arm or shoulder with a cap applicator; the recommended starting dose is 23 mg (1 pump actuation) applied once daily, preferably in the morning. 74 The dose can be titrated based on serum T levels and presence of clinical signs and symptoms associated with TD. 74 This gel uses a hydroalcoholic and highly viscous topical formulation, and includes a cap applicator for hands‐free dispensing and application. In a phase 3 study evaluating the efficacy and safety of TESTAVAN® 2% gel over 90 days, 76.1% of men achieved average T concentration of 10.4–36.4 nmol/L (300–1050 ng/dl) on day 90. 75 Depending on dose, T levels peaked approximately 2–4 h post‐application and decreased to pre‐application levels within 12 h, mirroring the natural diurnal rhythm of male T.
3.5.5. AXIRON®
AXIRON® was supplied in a metered‐dose pump delivering 30 mg of T per pump applied once daily with an applicator to the underarms, with a recommended starting dose of 60 mg T (1 pump or twist actuation per axilla). 61 This delivery route with an applicator prevents users from touching the solution and minimizes the risk of transfer of T to others. The efficacy and safety of the 60 mg/day 2% topical T solution were evaluated in an open‐label trial enrolling 155 men with TD. Of the 155 enrolled men, 135 men completed the 120‐day study. 76 On day 15, 76.1% of patients had C avg within the pre‐defined normal range (10.4–36.4 nmol/L, or 300–1050 ng/dl), which increased to 84.1% by day 120. With the 60 mg/day dose, mean TT concentrations were 15.8 nmol/L (456 ng/dl) and 17.6 nmol/L (508 ng/dl) on days 15 and 120, respectively; peak T levels were reached at 2 h post‐application, and T peak‐to‐trough ratios were maintained around 3 from day 15 to 120. There were similar proportions of men with a C max <52.05 nmol/L (1500 ng/dl) on day 15 (95.6%) and day 120 (94.8%). At day 120, most men (n = 97) remained on the 60 mg/day dose; 3 had decreased to 30 mg/day, 25 patients increased to 90 mg/day, and 10 patients titrated up to receive 120 mg/day. By day 120, steady‐state levels of serum T, free T, and DHT were attained, and the DHT:T ratio was within the expected range of 0.05 to 0.33. This axillary application of T solution was well tolerated, and 84% of men achieved serum T within the normal range by day 120. 76 This product has since been discontinued.
3.6. Nasal administration
Approved by the FDA in May 2014, the nasal gel formulation of TTh (NATESTO®) is a product using a metered‐dose pump delivering 5.5 mg of T per pump actuation, with the recommended dosing of 2 pumps (11 mg; 1 actuation/nostril) administered intranasally 3 times daily (total 33 mg/day) 6 to 8 h apart. 77 Absorption occurs through the nasal mucosa, thereby avoiding hepatic first‐pass metabolism, with approximately 75% of administered T entering the blood. 78 Peak serum T levels were reached approximately 60 min post‐application and declined to near baseline before the next dose. 79 , 80 In a randomized, dose‐ranging, open‐label phase 3 study of 306 men with TD (ClinicalTrials.gov NCT01446042), 11 mg nasal T dosed two times or three times daily resulted in between 71% and 91% of patients achieving serum TT concentrations within the defined range (10.4–36.4 nmol/L; 300–1050 ng/dl). At day 90, mean TT C avg values were 13.0 and 14.6 nmol/L (375 and 421 ng/dl) for 11 mg dosed two or three times daily, respectively. 79 Compared with other routes of T delivery, nasal T gel administration results in rather low C avg and C max TT levels, as well as DHT and DHT:T ratios, even with administering 11 mg three times daily. While patients experience 3 C max peaks in 1 day because of the required 3 daily doses, only 3.3% of patients had a C max between 62.4 and 86.7 nmol/L (1800–2500 ng/dl). At day 90, the C max and C min were 32.4 nmol/L (934.9 ng/dl) and 7.0 nmol/L (200.9 ng/dl), respectively, with a peak‐to‐trough ratio of 4.7. Furthermore, an ongoing phase 4 clinical trial suggests that not only can T nasal gel increase serum T over time, but it can also maintain FSH, LH, and semen parameters. 81
3.7. Buccal mucoadhesives
A testosterone buccal system (TBS) was approved by the FDA in June 2003 (STRIANT®, Endo Pharmaceuticals). 82 Each TBS contains 30 mg T and is designed as a tablet‐like mucoadhesive system applied to the gum region twice daily. As the buccal system hydrates, it slowly forms a gel to allow sustained and controlled‐release of T over 12 h through the buccal mucosa. This transbuccal route bypasses liver metabolism and delivers T directly into the systemic circulation. 83 The efficacy and the PK profile of TBS were evaluated in a phase 3 study in 82 men with TD. Following 12 weeks of applying TBS twice daily, the mean T C avg increased to 20.1 to 24.9 nmol/L (580–718 ng/dl) at weeks 4, 8, and 12 from baseline levels of 5.2 ± 3.1 nmol/L (150 ± 89 ng/dl). At week 12, steady‐state T concentrations within 20.1 to 24.9 nmol/L (580–718 ng/dl) were achieved by 87% (71/82) of patients, a slightly lower percentage than in another study where 92% of men achieved a C avg within the normal range following TBS application. 84 The time‐averaged steady‐state C avg measured over the two consecutive 12‐h dosing intervals was 18.7 ± 5.9 nmol/L (540 ± 170 ng/dl), with a peak‐to‐trough ratio of 3.3 (C max of 34.3 ± 12.5 nmol/L, or 990 ± 360 ng/dl; C min of 10.4 ± 4.2 nmol/L, or 300 ± 120 ng/dl). Physiologic levels of T can be sustained with continued use, with peak T levels reached in the morning 10 to 12 h after evening application, mimicking circadian T patterns. Mean serum DHT and E2 levels were within the upper limit of the normal range. While men experience two C max peaks daily produced from two daily doses, there does not appear to be an accumulation of T over time. 85 This product has since been discontinued.
3.8. Oral testosterone undecanoate (TU)
Historically, oral TTh with non‐esterified T has been unsuccessful in delivering physiological T because of first‐pass hepatic metabolism; to overcome this, high doses were needed to achieve measurable serum T levels. 86 A new, oral TU formulation delivered via a self‐emulsifying drug delivery system was developed to promote solubilization and absorption of the lipophilic TU in the gastrointestinal tract, and in March 2019, became the first oral TTh approved by the FDA. In a phase 2 study, 200 mg oral TU administered twice a day resulted in 87% of men achieving average serum T levels within the physiological range (10.4–34.7 nmol/L, or 300–1000 ng/dl), and none of the men had serum T levels >52 nmol/L (1500 ng/dl). 87 Peak T levels were reached 4 to 5 h after administration, and levels steadily decreased to baseline at approximately 12 h unless a second dose was administered. As oral TU capsules are recommended to be taken with a meal, serum T levels appear to be modulated by dietary fat content. 88 C avg and mean C max serum T levels were approximately 2‐fold higher when 200 mg oral TU was administered with food compared with fasting. 87 Dietary fat was found to affect mean serum T levels achieved with oral TU; meals with higher fat content increased serum T concentrations. In a phase 3 study comparing the efficacy and safety of 237 mg oral TU given twice daily with once daily 60 mg topical T solution, 87% (145/166) of men with TD treated with oral TU were able to achieve a mean C avg within 8.7 to 31.4 nmol/L (252–907 ng/dl), meeting the primary objective. 89 At the final study visit on day 105, the mean C avg was 14.0 nmol/L (403 ng/dl) and C max was 34.9 nmol/L (1008 ng/dl). As oral TU is given twice daily, there were 2 serum T peaks between 20.8 and 24.3 nmol/L (600 and 700 ng/dl) approximately 4 h after administration, 2 sub‐therapeutic troughs (<6.9 nmol/L or <200 ng/dl) 12 h after administration, and a peak‐to‐trough ratio approaching 4.
4. DIURNAL VARIATION IN SERUM T LEVELS
Diurnal variation in endogenous serum T levels in healthy men is well documented, with highest T levels in the morning and lowest values in the afternoon and early evening, although the amplitudes of peak and trough levels vary by age. In 1983, a study by Bremner et al. showed that there was a clear difference between serum T levels in normal young men (mean age 25.2 years) and older men (mean age 71.0 years). 5 In young men, serum T levels were highest in the morning, falling to their lowest levels approximately 12 h later and gradually increasing again to peak levels the next morning. Furthermore, a study to determine how endogenous T levels vary over clinic hours revealed that in 30‐ to 40‐year‐old men, morning total T levels are 30% to 35% higher than levels measured in the mid to late afternoon. This amplitude in daily endogenous T variation decreases with age, with a morning‐to‐afternoon difference of only 10% in 70‐year‐old men. 90 The morning‐to‐afternoon total T ratios in young and older men were approximately 1.3 and 1.1, respectively, similar to what was observed in the Bremner study. These observations have led to recommendations in various clinical guidelines, including those of the American Urology Association in 2018, to obtain early morning blood tests for the diagnosis of TD, a threshold defined as <10.4 nmol/L (300 ng/dl). 3 While endogenous serum T exhibits a clear diurnal pattern in young men that appears to be blunted naturally as men age, there does not appear to be much diurnal variation for DHT, SHBG, LH, FSH, or E2, regardless of age. 90
Diurnal variation appears to be absent in men with TD. Using a population mixed‐effect analysis, Gupta et al. showed that no circadian rhythm was detected in men with TD, and the mean endogenous T levels in men with TD (serum T levels <10.4 nmol/L, or 300 ng/dl) were much lower than in healthy young (mean age, 28 years) or older (mean age, 71 years) men. 91 Consistent with the literature, their modeling of circadian T in healthy young and older men revealed peak‐to‐trough ratios of 1.3 and 1.2, respectively. Additionally, univariate analysis of cross‐sectional data from 3007 older men (≥40 years) showed that the proportion of men with serum T levels <10.4 nmol/L (300 ng/dl) did not significantly change during the day (p < 0.11), further supporting that endogenous T diurnal variation is absent in men with TD. 92 Results from a study by Shlykova et al. evaluating endogenous T levels over a 24‐h period in 21 healthy male volunteers showed that men with baseline serum T levels <10.4 nmol/L (300 ng/dl) did not demonstrate diurnal variation within the 24‐h sampling period. 93 To understand the circadian rhythmicity of T levels in men with TD, a population kinetic model built by Gonzales‐Sales et al., using baseline T profiles from 859 men with TD, predicted a base T value of 8.3 nmol/L (239 ng/dl), with the amplitude of oscillation estimated to be 1.1 nmol/L (32.4 ng/dl). 94 Their model also predicted that a stretched cosine function was more suitable to describe the circadian behavior of T levels of men with TD, as trough levels occurred approximately 5 h after peak T levels, and levels then increased until the next peak occurred approximately 19 h later. 94
A variety of exogenous TTh are available to increase serum T to physiologic levels in males with TD and may alleviate symptoms associated with TD. 2 Some T replacement options more closely mimic the circadian levels of T identified in older men, whereas other options seem to provide PK profiles closer to those of younger men (Figure 1 and Table 1). Some TTh options provide profiles that exceed the frequency of the natural T circadian rhythmicity. Once‐weekly SC TE injections bring mean T levels into the physiologic range within 24 h after the first dose, with a total T C max/C min ratio of 1.8. The PK profile appears to mimic the flatter profile of older males’ endogenous T. 95 IM T injections can cause both supratherapeutic T levels post‐injection and subtherapeutic levels during the dosing interval, and depending on the formulation and dosage, peak‐to‐trough ratios of IM TC and TE range between 2 and 5.3. Longer‐lasting TU injections do not demonstrate the supratherapeutic peaks of other IM formulations, with trough levels occurring at later time points after each injection and a peak‐to‐trough ratio of approximately 2.6 to 2.8. All IM TTh preparations result in PK profiles that are unlike those of the normal diurnal variation of healthy young or older men. Daily transdermal gels and solutions, and nasal and oral T products, provide a consistent serum T level within physiologic range in most patients. The daily dosing frequency of the topical gel products results in a PK profile with a resemblance to that of endogenous T in younger males. Men using nasal and oral T products are able to achieve mean serum T levels that are within the normal range, but they experience several T peaks and troughs throughout the day because of the multiple daily dosing regimens required (2 or 3 times/day). This results in a PK profile that significantly deviates from the endogenous PK profiles of both younger and older patients. No single formulation appears to provide an exposure profile that would resemble diurnal variation of both young and older men. The importance of diurnal variation of endogenous T is not fully understood, but there may be additional factors (eg, sleep quality and duration) that may influence endogenous T levels. 96 However, further clarification is needed for the association between the circadian timing of sleep loss and its effect on T levels.
FIGURE 1.
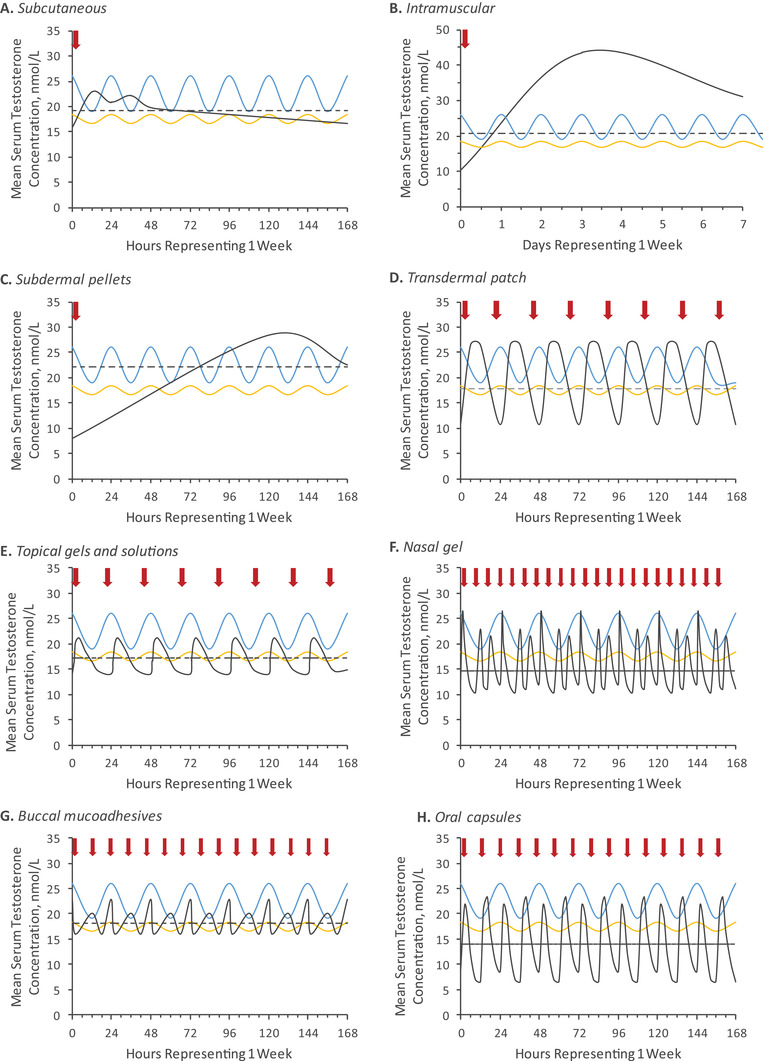
Extrapolateda mean steady‐state serum total testosterone concentrations. Estimated diurnal T in young men (blue, range: 23–28 years) and older men (age range 58–82 years). Red arrows indicate time of T administration. Dotted lines indicate C avg over a dosing interval of 168 h. Black lines in each panel represent mean steady‐state T profiles of: (A) weekly SC TE administration; (B) average mean T profiles for IM TC, TE, and TU over 2 weeks; (C) 900 mg subdermal T pellets; (D) 4.0 mg daily transdermal patch; (E) average mean T profiles of 100 mg TESTIM®/VOGELXO®, 40 mg FORTESTA®, 1.62% AndroGel®, and 2% AXIRON®; (F) nasal gel T at day 90 three times daily; (G) 30 mg buccal mucoadhesives applied twice daily; and (H) twice daily 200 mg oral TU capsules. aFor products with ≥1 daily dose, we extrapolated data over 1 week to compare all PK profiles consistently. For products with dosing regimens of longer than a week (eg, IM injections and T pellet implantation), only the PK profile in the first week following dosing is presented; these data do not reflect average concentrations throughout the entire dosing interval. IM, intramuscular; SC, subcutaneous; T, testosterone; TC, testosterone cypionate; TE, testosterone enanthate; TU, testosterone undecanoate
5. CONCLUSION
With testosterone therapy, serum T may be returned to levels within the normal physiologic range, and symptoms associated with low T may be improved. However, no testosterone therapy product precisely simulates the endogenous diurnal T variation observed in young and older men, and more clarification is needed on the impact of the variability in range between peak and trough exposure with each different testosterone therapy.
CONFLICT OF INTEREST
Alexander W. Pastuszak: consultant, speaker, research support, Endo Pharmaceuticals; founder and leadership role, Woven Health; leadership role, Vault Health; speaker, Bayer AG; advisor, Allotrope Medical, Inherent Biosciences. Marc Gittelman: consultant, speaker, research support, Clarus. James P. Tursi: former employee, Antares Pharma, Inc. Jonathan S. Jaffe: employee, Antares Pharma, Inc. David Schofield: employee, Antares Pharma, Inc. Martin M. Miner: leadership role, Vault Health; research support, Acerus Pharmaceuticals.
AUTHOR CONTRIBUTIONS
Alexander W. Pastuszak: conceptualization, methodology, investigation, writing—original draft, writing—review & editing; Marc Gittelman: conceptualization, methodology, investigation, writing—original draft, writing—review & editing; James P. Tursi: conceptualization, methodology, investigation, writing—original draft, writing—review & editing; Jonathan S. Jaffe: conceptualization, methodology, investigation, writing—original draft, writing—review & editing, funding acquisition, resources, supervision; David Schofield: conceptualization, methodology, investigation, writing—original draft, writing—review & editing, funding acquisition, resources, supervision; Martin M. Miner: conceptualization, methodology, investigation, writing—original draft, writing—review & editing.
ACKNOWLEDGMENTS
The authors thank Ying Hou of MedVal Scientific Information Services, LLC (Princeton, NJ), for medical writing and editorial assistance, which were funded by Antares Pharma, Inc. This manuscript was prepared according to the International Society for Medical Publication Professionals’ “Good Publication Practice for Communicating Company‐Sponsored Medical Research: GPP3.”
Pastuszak AW, Gittelman M, Tursi JP, Jaffe JS, Schofield D, Miner MM. Pharmacokinetics of testosterone therapies in relation to diurnal variation of serum testosterone levels as men age. Andrology. 2022;10:209–222. 10.1111/andr.13108
This work was previously presented in part at the 20th Annual Fall Scientific Meeting of the Sexual Medicine Society of North America (SMSNA).
DATA AVAILABILITY STATEMENT
All data analyzed during this review are included in this article or in the data listed in the References.
REFERENCES
- 1. US Food and Drug Administration . FDA Drug safety communication: FDA cautions about using testosterone products for low testosterone due to aging; requires labeling change to inform of possible increased risk of heart attack and stroke with use. 2018. Accessed February 9, 2020. https://www.fda.gov/drugs/drug‐safety‐and‐availability/fda‐drug‐safety‐communication‐fda‐cautions‐about‐using‐testosterone‐products‐low‐testosterone‐due. [DOI] [PubMed]
- 2. Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(5):1715‐1744. [DOI] [PubMed] [Google Scholar]
- 3. Mulhall JP, Trost LW, Brannigan RE, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol. 2018;200(2):423‐432. [DOI] [PubMed] [Google Scholar]
- 4. Giagulli VA, Castellana M, Lisco G, Triggiani V. Critical evaluation of different available guidelines for late‐onset hypogonadism. Andrology. 2020;8(6):1628‐1641. [DOI] [PubMed] [Google Scholar]
- 5. Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56(6):1278‐1281. [DOI] [PubMed] [Google Scholar]
- 6. Wu FC, Tajar A, Beynon JM, et al. Identification of late‐onset hypogonadism in middle‐aged and elderly men. N Engl J Med. 2010;363(2):123‐135. [DOI] [PubMed] [Google Scholar]
- 7. Khera M, Broderick GA, Carson CC, 3rd , et al. Adult‐onset hypogonadism. Mayo Clin Proc. 2016;91(7):908‐926. [DOI] [PubMed] [Google Scholar]
- 8. Corona G, Krausz C. Late‐onset hypogonadism a challenging task for the andrology field. Andrology. 2020;8(6):1504‐1505. [DOI] [PubMed] [Google Scholar]
- 9. Wang C, Nieschlag E, Swerdloff R, et al. Investigation, treatment and monitoring of late‐onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. Eur J Endocrinol. 2008;159(5):507‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morales A, Bebb RA, Manjoo P, et al. Diagnosis and management of testosterone deficiency syndrome in men: clinical practice guideline. CMAJ. 2015;187(18):1369‐1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hackett G, Kirby M, Edwards D, et al. British society for sexual medicine guidelines on adult testosterone deficiency, with statements for UK practice. J Sex Med. 2017;14(12):1504‐1523. [DOI] [PubMed] [Google Scholar]
- 12. Dohle GR, Arver S, Bettocchi C, Jones TH, Kliesch S, Punab M. EAU Guidelines on Male Hypogonadism. European Association of Urology; 2015. [Google Scholar]
- 13. Shoskes JJ, Wilson MK, Spinner ML. Pharmacology of testosterone replacement therapy preparations. Transl Androl Urol. 2016;5(6):834‐843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92(11):4241‐4247. [DOI] [PubMed] [Google Scholar]
- 15. Kelsey TW, Li LQ, Mitchell RT, Whelan A, Anderson RA, Wallace WHB. A validated age‐related normative model for male total testosterone shows increasing variance but no decline after age 40 years. PLoS One. 2014;9(10):e109346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536‐2559. [DOI] [PubMed] [Google Scholar]
- 17. Zarotsky V, Huang MY, Carman W, et al. Systematic literature review of the risk factors, comorbidities, and consequences of hypogonadism in men. Andrology. 2014;2(6):819‐834. [DOI] [PubMed] [Google Scholar]
- 18. Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60(7):762‐769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Traish AM, Haider A, Doros G, Saad F. Long‐term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syndrome: an observational, long‐term registry study. Int J Clin Pract. 2014;68(3):314‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhattacharya RK, Khera M, Blick G, Kushner H, Nguyen D, Miner MM. Effect of 12 months of testosterone replacement therapy on metabolic syndrome components in hypogonadal men: data from the Testim Registry in the US (TRiUS). BMC Endocr Disord. 2011;11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta‐analysis. J Clin Endocrinol Metab. 2011;96(10):3007‐3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kloner RA, Carson C, 3rd , Dobs A, Kopecky S, Mohler ER, 3rd. Testosterone and cardiovascular disease. J Am Coll Cardiol. 2016;67(5):545‐557. [DOI] [PubMed] [Google Scholar]
- 23. Elagizi A, Köhler TS, Lavie CJ. Testosterone and cardiovascular health. Mayo Clin Proc. 2018;93(1):83‐100. [DOI] [PubMed] [Google Scholar]
- 24. Corona G, Rastrelli G, Vignozzi L, Maggi M. Emerging medication for the treatment of male hypogonadism. Expert Opin Emerg Drugs. 2012;17(2):239‐259. [DOI] [PubMed] [Google Scholar]
- 25. Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173(15):1465‐1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Isidori AM, Buvat J, Corona G, et al. A critical analysis of the role of testosterone in erectile function: from pathophysiology to treatment‐a systematic review. Eur Urol. 2014;65(1):99‐112. [DOI] [PubMed] [Google Scholar]
- 27. Sansone A, Sansone M, Lenzi A, Romanelli F. Testosterone replacement therapy: the emperor's new clothes. Rejuvenation Res. 2017;20(1):9‐14. [DOI] [PubMed] [Google Scholar]
- 28. Saad F, Aversa A, Isidori AM, Zafalon L, Zitzmann M, Gooren L. Onset of effects of testosterone treatment and time span until maximum effects are achieved. Eur J Endocrinol. 2011;165(5):675‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Snyder PJ, Bhasin S, Cunningham GR, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016;374(7):611‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. XYOSTED (testosterone enanthate) injection, for subcutaneous use [prescribing information]. Antares Pharma, Inc.; September 2018. [Google Scholar]
- 31. Kaminetsky JC, McCullough A, Hwang K, Jaffe JS, Wang C, Swerdloff RS. A 52‐week study of dose adjusted subcutaneous testosterone enanthate in oil self‐administered via disposable auto‐injector. J Urol. 2019;201(3):587‐594. [DOI] [PubMed] [Google Scholar]
- 32. Kaminetsky J, Jaffe JS, Swerdloff RS. Pharmacokinetic profile of subcutaneous testosterone enanthate delivered via a novel, prefilled single‐use autoinjector: a phase II study. Sex Med. 2015;3(4):269‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Behre HM, Wang C, Handelsman DJ, Nieschlag E. Pharmacology of testosterone preparations. In: Nieschlag E, Behre H, eds. Testosterone: Action, Deficiency, Substitution. Cambridge University Press; 2004:405‐444. [Google Scholar]
- 34.Depo® – Testosterone testosterone cypionate injection [prescribing information]. Pfizer, Inc.; August 2018. [Google Scholar]
- 35. Nankin HR. Hormone kinetics after intramuscular testosterone cypionate. Fertil Steril. 1987;47(6):1004‐1009. [PubMed] [Google Scholar]
- 36. Snyder PJ, Lawrence DA. Treatment of male hypogonadism with testosterone enanthate. J Clin Endocrinol Metab. 1980;51(6):1335‐1339. [DOI] [PubMed] [Google Scholar]
- 37. Delatestryl® (testosterone enanthate injection, USP) [prescribing information]. Endo Pharmaceuticals Solutions Inc.; October 2016. [Google Scholar]
- 38. Morgentaler A, Dobs AS, Kaufman JM, et al. Long acting testosterone undecanoate therapy in men with hypogonadism: results of a pharmacokinetic clinical study. J Urol. 2008;180(6):2307‐2313. [DOI] [PubMed] [Google Scholar]
- 39. Wang C, Harnett M, Dobs AS, Swerdloff RS. Pharmacokinetics and safety of long‐acting testosterone undecanoate injections in hypogonadal men: an 84‐week phase III clinical trial. J Androl. 2010;31(5):457‐465. [DOI] [PubMed] [Google Scholar]
- 40. AVEED® (testosterone undecanoate) injection, for intramuscular use [prescribing information]. Endo Pharmaceuticals, Inc.; January 2018. [Google Scholar]
- 41. Testoviron® Depot [data sheet]. Bayer Israel Ltd; March 2020. [Google Scholar]
- 42. SUSTANON® ‘250’ [prescribing information]. Aspen Pharmacare; December 2020. [Google Scholar]
- 43. Fukutani K, Isurugi K, Takayasu H, Wakabayashi K, Tamaoki B. Effects of depot testosterone therapy on serum levels of luteinizing hormone and follicle‐stimulating hormone in patients with Klinefelter's syndrome and hypogonadotropic eunuchoidism. J Clin Endocrinol Metab. 1974;39(5):856‐864. [DOI] [PubMed] [Google Scholar]
- 44. Deanesly R, Parkes AS. Further experiments on the administration of hormones by the subcutaneous implantation of tablets. Lancet. 1938;232(6002):606‐609. [Google Scholar]
- 45. TESTOPEL® (testosterone pellets) [prescribing information]. Endo Pharmaceuticals, Inc.; August 2018. [Google Scholar]
- 46. Handelsman DJ, Conway AJ, Boylan LM. Pharmacokinetics and pharmacodynamics of testosterone pellets in man. J Clin Endocrinol Metab. 1990;71(1):216‐222. [DOI] [PubMed] [Google Scholar]
- 47. Kaminetsky JC, Moclair B, Hemani M, Sand M. A phase IV prospective evaluation of the safety and efficacy of extended release testosterone pellets for the treatment of male hypogonadism. J Sex Med. 2011;8(4):1186‐1196. [DOI] [PubMed] [Google Scholar]
- 48. McCullough AR, Khera M, Goldstein I, Hellstrom WJ, Morgentaler A, Levine LA. A multi‐institutional observational study of testosterone levels after testosterone pellet (Testopel®) insertion. J Sex Med. 2012;9(2):594‐601. [DOI] [PubMed] [Google Scholar]
- 49. Pastuszak AW, Mittakanti H, Liu JS, Gomez L, Lipshultz LI, Khera M. Pharmacokinetic evaluation and dosing of subcutaneous testosterone pellets. J Androl. 2012;33(5):927‐937. [DOI] [PubMed] [Google Scholar]
- 50. McMahon CG, Shusterman N, Cohen B. Pharmacokinetics, clinical efficacy, safety profile, and patient‐reported outcomes in patients receiving subcutaneous testosterone pellets 900 mg for treatment of symptoms associated with androgen deficiency. J Sex Med. 2017;14(7):883‐890. [DOI] [PubMed] [Google Scholar]
- 51. ANDRODERM® (testosterone transdermal system), for topical use CIII [prescribing information]. Allergan USA, Inc.; October 2016. [Google Scholar]
- 52. Meikle AW, Arver S, Dobs AS, Sanders SW, Rajaram L, Mazer NA. Pharmacokinetics and metabolism of a permeation‐enhanced testosterone transdermal system in hypogonadal men: Influence of application site—a clinical research center study. J Clin Endocrinol Metab. 1996;81(5):1832‐1840. [DOI] [PubMed] [Google Scholar]
- 53. Arver S, Dobs AS, Meikle AW, et al. Long‐term efficacy and safety of a permeation‐enhanced testosterone transdermal system in hypogonadal men. Clin Endocrinol (Oxf). 1997;47(6):727‐737. [DOI] [PubMed] [Google Scholar]
- 54. Dobs AS, Meikle AW, Arver S, Sanders SW, Caramelli KE, Mazer NA. Pharmacokinetics, efficacy, and safety of a permeation‐enhanced testosterone transdermal system in comparison with bi‐weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab. 1999;84(10):3469‐3478. [DOI] [PubMed] [Google Scholar]
- 55. Korbonits M, Slawik M, Cullen D, et al. A comparison of a novel testosterone bioadhesive buccal system, striant, with a testosterone adhesive patch in hypogonadal males. J Clin Endocrinol Metab. 2004;89(5):2039‐2043. [DOI] [PubMed] [Google Scholar]
- 56. Mazer N, Bell D, Wu J, Fischer J, Cosgrove M, Eilers B. Comparison of the steady‐state pharmacokinetics, metabolism, and variability of a transdermal testosterone patch versus a transdermal testosterone gel in hypogonadal men. J Sex Med. 2005;2(2):213‐226. [DOI] [PubMed] [Google Scholar]
- 57. Abadilla KA, Dobs AS. Topical testosterone supplementation for the treatment of male hypogonadism. Drugs. 2012;72(12):1591‐1603. [DOI] [PubMed] [Google Scholar]
- 58. Aydogdu A, Swerdloff RS. Emerging medication for the treatment of male hypogonadism. Expert Opin Emerg Drugs. 2016;21(3):255‐266. [DOI] [PubMed] [Google Scholar]
- 59. Retzler J, Smith AB, Oliveira Goncalves AS, Whitty JA. Preferences for the administration of testosterone gel: Evidence from a discrete choice experiment. Patient Prefer Adherence. 2019;13:657‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sansone A, Sansone M, Selleri R, et al. Monitoring testosterone replacement therapy with transdermal gel: When and how? J Endocrinol Invest. 2019;42(12):1491‐1496. [DOI] [PubMed] [Google Scholar]
- 61. AXIRON – Testosterone solution [prescribing information]. Eli Lilly and Company; July 2017. [Google Scholar]
- 62. AndroGel® (testosterone gel) 1% for topical use CIII [prescribing information]. AbbVie, Inc.; May 2019. [Google Scholar]
- 63. AndroGel® (testosterone gel) 1.62% for topical use CIII [prescribing information]. AbbVie, Inc.; May 2019. [Google Scholar]
- 64. Swerdloff RS, Wang C, Cunningham G, et al. Long‐term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab. 2000;85(12):4500‐4510. [DOI] [PubMed] [Google Scholar]
- 65. Kaufman JM, Miller MG, Garwin JL, Fitzpatrick S, McWhirter C, Brennan JJ. Efficacy and safety study of 1.62% testosterone gel for the treatment of hypogonadal men. J Sex Med. 2011;8(7):2079‐2089. [DOI] [PubMed] [Google Scholar]
- 66. Kaufman JM, Miller MG, Fitzpatrick S, McWhirter C, Brennan JJ. One‐year efficacy and safety study of a 1.62% testosterone gel in hypogonadal men: Results of a 182‐day open‐label extension of a 6‐month double‐blind study. J Sex Med. 2012;9(4):1149‐1161. [DOI] [PubMed] [Google Scholar]
- 67. FORTESTA™ (testosterone) gel for topical use [prescribing information]. Endo Pharmaceuticals, Inc.; September 2013. [Google Scholar]
- 68. Dobs AS, McGettigan J, Norwood P, Howell J, Waldie E, Chen Y. A novel testosterone 2% gel for the treatment of hypogonadal males. J Androl. 2012;33(4):601‐607. [DOI] [PubMed] [Google Scholar]
- 69. TESTIM® (testosterone gel) for topical use [prescribing information]. Endo Pharmaceuticals, Inc.; April 2018. [Google Scholar]
- 70. VOGELXO® (testosterone) gel, for topical use [prescribing information]. Upsher‐Smith Laboratories; August 2017. [Google Scholar]
- 71. Marbury T, Hamill E, Bachand R, Sebree T, Smith T. Evaluation of the pharmacokinetic profiles of the new testosterone topical gel formulation, testim, compared to AndroGel. Biopharm Drug Dispos. 2003;24(3):115‐120. [DOI] [PubMed] [Google Scholar]
- 72. Bouloux P. Testim® 1% testosterone gel for the treatment of male hypogonadism. Clin Ther. 2005;27(3):286‐298. [DOI] [PubMed] [Google Scholar]
- 73. Steidle C, Schwartz S, Jacoby K, et al. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab. 2003;88(6):2673‐2681. [DOI] [PubMed] [Google Scholar]
- 74. TESTAVAN® (testosterone) gel [product information]. Ferring Pharmaceuticals Pty Ltd; August 2020. [Google Scholar]
- 75. Cunningham G, Belkoff L, Brock G, et al. Efficacy and safety of a new topical testosterone replacement gel therapy for the treatment of male hypogonadism. Endocr Pract. 2017;23(5):557‐565. [DOI] [PubMed] [Google Scholar]
- 76. Wang C, Ilani N, Arver S, McLachlan RI, Soulis T, Watkinson A. Efficacy and safety of the 2% formulation of testosterone topical solution applied to the axillae in androgen‐deficient men. Clin Endocrinol (Oxf). 2011;75(6):836‐843. [DOI] [PubMed] [Google Scholar]
- 77. Natesto (testosterone) nasal gel [prescribing information]. Aytu BioScience, Inc.; December 2017. [Google Scholar]
- 78. Banks WA, Morley JE, Niehoff ML, Mattern C. Delivery of testosterone to the brain by intranasal administration: Comparison to intravenous testosterone. J Drug Target. 2009;17(2):91‐97. [DOI] [PubMed] [Google Scholar]
- 79. Rogol AD, Tkachenko N, Bryson N. Natesto, a novel testosterone nasal gel, normalizes androgen levels in hypogonadal men. Andrology. 2016;4(1):46‐54. [DOI] [PubMed] [Google Scholar]
- 80. Mattern C, Hoffmann C, Morley JE, Badiu C. Testosterone supplementation for hypogonadal men by the nasal route. Aging Male. 2008;11(4):171‐178. [DOI] [PubMed] [Google Scholar]
- 81. Masterson T, Molina M, Ibrahim E, Ramasamy R. Natesto effects on reproductive hormones and semen parameters: Results from an ongoing single‐center, investigator‐initiated phase IV clinical trial. Eur Urol Focus. 2018;4(3):333‐335. [DOI] [PubMed] [Google Scholar]
- 82. Striant (testosterone buccal system) mucoadhesive for buccal administration [prescribing information]. Endo Pharmaceuticals, Inc.; October 2016. [Google Scholar]
- 83. Wang C, Swerdloff R, Kipnes M, et al. New testosterone buccal system (Striant) delivers physiological testosterone levels: Pharmacokinetics study in hypogonadal men. J Clin Endocrinol Metab. 2004;89(8):3821‐3829. [DOI] [PubMed] [Google Scholar]
- 84. Dobs AS, Matsumoto AM, Wang C, Kipnes MS. Short‐term pharmacokinetic comparison of a novel testosterone buccal system and a testosterone gel in testosterone deficient men. Curr Med Res Opin. 2004;20(5):729‐738. [DOI] [PubMed] [Google Scholar]
- 85. Dinsmore WW, Wyllie MG. The long‐term efficacy and safety of a testosterone mucoadhesive buccal tablet in testosterone‐deficient men. BJU Int. 2012;110(2):162‐169. [DOI] [PubMed] [Google Scholar]
- 86. Daggett PR, Wheeler MJ, Nabarro JD. Oral testosterone, a reappraisal. Horm Res. 1978;9(3):121‐129. [DOI] [PubMed] [Google Scholar]
- 87. Yin AY, Htun M, Swerdloff RS, et al. Reexamination of pharmacokinetics of oral testosterone undecanoate in hypogonadal men with a new self‐emulsifying formulation. J Androl. 2012;33(2):190‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yin A, Alfadhli E, Htun M, et al. Dietary fat modulates the testosterone pharmacokinetics of a new self‐emulsifying formulation of oral testosterone undecanoate in hypogonadal men. J Androl. 2012;33(6):1282‐1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. JATENZO (testosterone undecanoate) capsules, for oral use [prescribing information]. Clarus Therapeutics, Inc.; May 2019. [Google Scholar]
- 90. Brambilla DJ, Matsumoto AM, Araujo AB, McKinlay JB. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94(3):907‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gupta SK, Lindemulder EA, Sathyan G. Modeling of circadian testosterone in healthy men and hypogonadal men. J Clin Pharmacol. 2000;40(7):731‐738. [DOI] [PubMed] [Google Scholar]
- 92. Crawford ED, Barqawi AB, O'Donnell C, Morgentaler A. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100(3):509‐513. [DOI] [PubMed] [Google Scholar]
- 93. Shlykova N, Davidson E, Krakowsky Y, Bolanos J, Traish A, Morgentaler A. Absent diurnal variation in serum testosterone in young men with testosterone deficiency. J Urol. 2020;203(4):817–823. [DOI] [PubMed] [Google Scholar]
- 94. Gonzalez‐Sales M, Barriere O, Tremblay PO, Nekka F, Desrochers J, Tanguay M. Modeling testosterone circadian rhythm in hypogonadal males: Effect of age and circannual variations. AAPS J. 2016;18(1):217‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kaminetsky J, Gittelman M, Swerdloff R, Longstreth J, Dudley R, Danoff T. Monitoring testosterone (T) levels in men receiving oral testosterone undecanoate (TU): Dealing with post‐collection conversion of TU to T [abstract]. J Urol. 2019;201(suppl 4):e859. [Google Scholar]
- 96. Liu PY. A clinical perspective of sleep and andrological health: Assessment, treatment considerations, and future research. J Clin Endocrinol Metab. 2019;104(10):4398‐4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. REANDRON® 1000 [product information]. Bayer Australia Ltd; July 2017. [Google Scholar]
- 98. Testarzon 20 mg/g Transdermal gel [prescribing information]. Ferring Ireland Ltd.; March 2020. [Google Scholar]
- 99. Andriol® Testocaps® [prescribing information]. Merck Sharp & Dohme (Australia) Pty. Limited; October 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analyzed during this review are included in this article or in the data listed in the References.


