Abstract
Recently published syntheses, reactions and characterizations of unusual unsaturated ring strained Group 4 metallocene metallacycles like metalla‐cyclocumulenes, ‐cycloallenes and ‐cycloalkynes with different ring size are updated for the last three years. There exist for some of these metallacycles, depending on the ring size, 7‐, 5‐ and 4‐membered compounds. The new results for these metallacycles are summarized here and considered in addition to the former published results. Additionally, several compounds of this type were now characterized by new reactions. For a better understanding of these compounds, some spectroscopical methods as well as theoretical calculations were published. Despite of these all‐C‐metallacycles, only in some cases the syntheses and reactions for the corresponding hetero‐metallacycles were published too. Examples for these metallaheterocyclic compounds will not be considered in this article. All these unusual ring strained compounds have a great potential for a lot of interesting synthetic applications in the future. Additionally, they are very interesting from the theoretical point of view.
Keywords: group 4 metals, organometallic compounds, organic syntheses, theoretical investigations, unusual metallacycles
Indeed interesting and important: Recently published contributions of unusual unsaturated ring strained Group 4 metallocene metallacycles show new aspects of these compounds. Several new investigations of 7‐ and 5‐membered metalla‐cyclocumulenes, 5‐ and 4‐membered metalla‐cycloallenes as well as metalla‐cyclopentynes, complete the understanding of these compounds. From the theoretical point of view and the reactivity they have a great potential for important synthetic applications.

1. Introduction
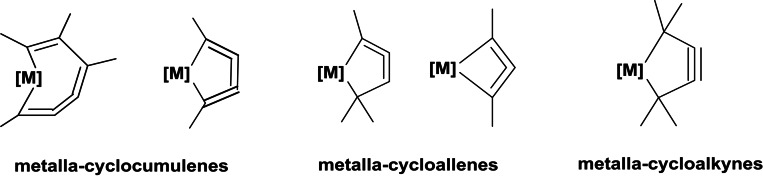
Several usual metallacycles of different ring size and unsaturation like metalla‐cyclopropanes, ‐cyclopropenes, ‐cyclopentanes, ‐cyclopentadienes and many others are often important to understand stochiometric and catalytic reactions for organometallic compounds. Regarding the syntheses and reactions of unusual unsaturated Group 4 metallocene metallacycles, the number is more restricted. Nevertheless, some examples were published in the past and summarized in several reviews. In the case of small ring strained metallacycles, which contain cumulenic and allenic double as well as triple bonds, the incorporation of unsaturation into smaller cyclic structures increases the ring strain. A number of these 7‐, 5‐ and 4‐membered metallacycles based on Group 4 metals were published with unusual structural motifs like metalla‐cyclocumulenes (1‐metalla‐cyclopenta‐2,3‐4‐trienes), metalla‐cycloallenes (1‐metallacyclopenta‐3,4‐dienes) and metalla‐cyclopentynes (1‐metallacyclopent‐3‐ynes). [1] The results of metalla‐cyclocumulenes, ‐cycloallenes and ‐cycloalkynes with different ring size were in the center of these older papers (Scheme 1).
Scheme 1.
Examples for metalla‐cyclocumulenes, ‐cycloallenes and ‐cycloalkynes with different ring size.
Only the data from metalla‐cycloalkynes and not from the group of similar metalla‐pentalynes, which exist not for Group 4 metals, are mentioned in this update. The Group 4 metallacycles are described by its syntheses, reactions as well as the bonding character. The characterizations of these unusual metallacycles by spectroscopical and theoretical methods are summarized in one chapter, because the different types of compounds were mostly analyzed together and in comparison to each other in a single paper. Additionally, not only all‐C‐metallacycles of these types are known, but also special cases of the corresponding hetero‐metallacycles exist. In this update, only the recent examples for all‐C‐metallacycles from the last three years for these compounds are collected and discussed. [1]
2. Reactions of Unusual Group 4 Metallacycles
2.1. Metalla‐cyclocumulenes
2.1.1. 7‐membered metalla‐cyclocumulenes
The first example of a 7‐membered metalla‐cyclocumulene was published by Buchwald and coworkers as a result from the reaction of the Negishi reagent Cp2Zr(η2‐butene), formed from Cp2Zr(n‐Bu)2, with 1,4‐bis(trimethylsilyl)buta‐1,3‐diyne Me3Si−C≡C−C≡C−SiMe3. It was obtained as a cyclic 1 : 2 adduct of Zr and two diyne molecules together with other products. [2] A lot of reactions with this and similar compounds were published later which were summarized in several reviews. [1]
Recently, Burlakov, Shur and coworkers described in addition to former obtained results several interesting new synthetic applications for compounds of this type. [3]
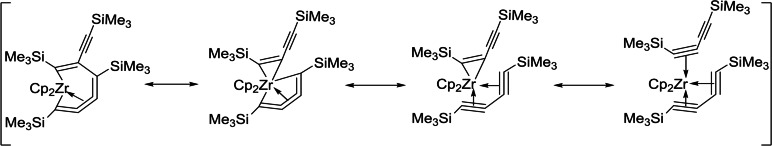
More recently, Burlakov, Shur and coworkers published other new applications for such compounds. [3] All these reactions proceed as the result of the different mesomeric descriptions and the equilibrium for 7‐membered metalla‐cyclocumulenes (Scheme 2). [1j]
Scheme 2.
Different mesomeric descriptions of 7‐membered metalla‐cyclocumulenes.
Additionally, an equilibrium exists for these complexes as well as the products of a butadiyne dissociation (Scheme 3). [1j]
Scheme 3.
Equilibrium for dissociation and association of 7‐membered metalla‐cyclocumulenes.
Following these descriptions of mesomerism and equilibrium, one can better understand the following different reactions in which the single considered species react in a different manner to obtain the later described products (Scheme 4).
Scheme 4.
Reactions of the 7‐membered metalla‐cyclocumulenes with benzophenone and acenaphthenequinone.
Despite of reactions with nitriles to heterocyclic products, the interaction with carbonyl compounds was investigated in detail. [3a] The reaction of the 7‐membered zircona‐cyclocumulene Cp2Zr[η4‐(Me3Si)C=C=C=C(SiMe3)−C(C≡CSiMe3)=C(SiMe3)−] with benzophenone gives after dissociation of the diyne Me3SiC≡C−C≡CSiMe3, the 9‐membered dioxazirconacycle Cp2Zr[η2‐O(CPh)2(SiMe3)C=C=C=C(SiMe3)(CPh)2O−]. It contains a cumulenic group in the metallacycle. Similar complexes were obtained with fluorenone and 4‐chlorobenzaldehyde. In the reaction of the products with HCl, the cis‐cumulenic diol HO(Ph2C)(Me3Si)C=C=C=C(SiMe3)(CPh2)OH and Cp2ZrCl2 were formed. The reaction of the starting zircona‐cyclocumulene with benzil PhC(=O)−C(=O)Ph gives the 9‐membered Cp2Zr[η2‐C(Me3Si)(C≡CSiMe3)−C(SiMe3)(C≡CSiMe3)OC(Ph)OC(Ph)O−] as a dioxazirconacycle. If the starting complex reacted with acenaphthenequinone, the obtained products depend on the temperature. At 20 °C a stable 11‐membered trioxazirconacycle with three double bonds in the cycle was formed, whereas at 80 °C a 10‐membered tetraoxadizirconacycle together with an octasubstituted cyclooctatetraene [−(Me3Si)=C(C≡CSiMe3)−]4 was isolated. Mechanistic suggestions for these reactions and the consequences for the formation of the obtained products were discussed. The basis for this were the different mesomeric descriptions (Scheme 2) and the equilibrium of dissociation and association (Scheme 3) of 7‐membered metalla‐cyclocumulenes as starting materials.
The same influence of mesomerism and equilibrium was observed in a further paper by Burlakov, Shur and coworkers for the protolysis of the 7‐membered zirconacyclocumulene Cp2Zr[η4‐(Me3Si)C=C=C=C(SiMe3)−C(C≡CSiMe3)=C(SiMe3)−] (Scheme 5). [3b]
Scheme 5.
Reactions of the 7‐membered metalla‐cyclocumulenes with HCl.
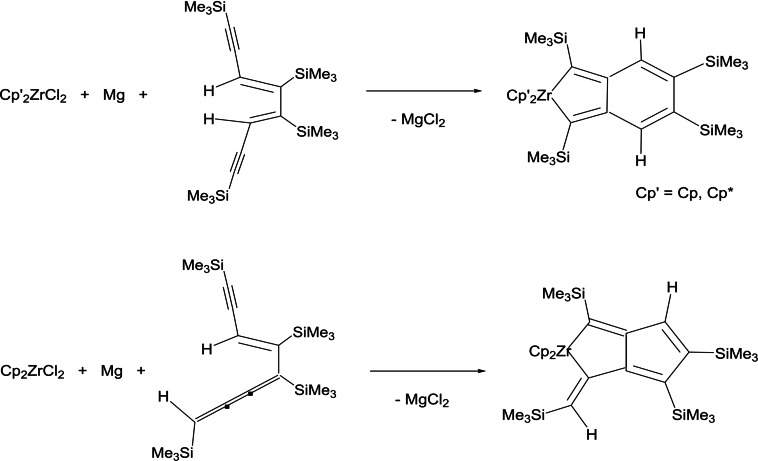
The reaction of the 7‐membered zirconacyclocumulene Cp2Zr[η4‐Me3SiC4(SiMe3)−C(C2SiMe3)=CSiMe3] as starting material with a stochiometric amount of HCl gives the complex Cp2Zr[(Cl)−C(C≡CSiMe3)=C(SiMe3)−C(C≡CSiMe3)=CH(SiMe3)] in which one C≡C group coordinates with zirconium. By the reaction of this compound with an excess of HCl, Cp2ZrCl2 and a mixture of cis,cis‐ and cis,trans‐unsaturated compounds of the type H(Me3Si)C=C(C≡CSiMe3)−C(SiMe3)=CH−(C≡CSiMe3) were formed. Protolysis with two equivalents of HCl leads to Cp2ZrCl2 together with a mixture of H(Me3SiC≡C)C=C(SiMe3)−C(SiMe3)=C(C≡CSiMe3)H and H(Me3SiC≡C)C=C(SiMe3)−C(SiMe3)=C=C=C(SiMe3)H. This mixture gives with Cp2ZrCl2 by the reduction with magnesium two zirconabicycles. From these is one a fused zircona‐cyclopentadiene with cyclohexadiene, and the other a fused zirconacyclopentene with cyclopentadiene units. The reaction of H(Me3SiC≡C)C=C(SiMe3)−C(SiMe3)=C(C≡CSiMe3)H with Cp*2ZrCl2 and magnesium affords the analogous zirconabicycles of fused zircona‐cyclopentadiene and cyclohexadiene rings (Scheme 6).
Scheme 6.
Reduction of Cp’2ZrCl2 (Cp’=Cp and Cp*) by magnesium in the presence of H(Me3SiC≡C)C=C(SiMe3)−C(SiMe3)=C(C≡CSiMe3)H or H(Me3SiC≡C)C=C(SiMe3)−C(SiMe3)=C=C=C(SiMe3)H to different zirconabicycles.
If another substituted 7‐membered zircona‐cyclocumulene Cp2Zr[η4‐(Ph)C=C=C=C(Ph)−(o‐C6H4)−] reacted with two equivalents of HCl as products Cp*2ZrCl2 and Ph2C=C(H)−C≡CPh were formed. The mechanism for the formation of the obtained product was discussed on the basis of the resonance form of the starting material. These results show, that in the protolysis of the zirconacyclopentadiene resonance form with an equimolar amount of HCl, a σ‐alkenyl complex was formed. The subsequent reaction of this compound with an excess of HCl or the direct reaction of the 7‐membered zircona‐cyclocumulene with two equivalents HCl, gave Cp2ZrCl2 and isomeric bis‐enynes. Both resonance forms of the zircona‐cyclocumulene react with HCl to a mixture of a bis‐enyne and a [3]cumulenic enyne. The other zircona‐cyclopentadiene resonance form of the zircona‐cyclocumulene reacts with HCl, but only to an enyne as a single product was produced.
In a recent review Tonks and coworkers summarized several examples for multicomponent syntheses of 5‐ and 6‐membered aromatic heterocycles by Group 4–8 transition metal catalysts. [3c] In this context they mentioned that a 7‐membered zirconacyclocumulene can serve with benzonitrile as a catalyst for the formation of the tetrasubstituted 4‐silylethynyl‐3‐silylpyrimidine. [3d] Again the dissociation of one diyne (Scheme 3) via a zirconacyclocumulene and a zirconacyclopropene with a coordinated benzonitrile was assumed. Insertion of two benzonitriles gives after reductive elimination the pyrimidine as product.
2.1.2. The 5‐membered metalla‐cyclocumulenes
The first 5‐membered metalla‐cyclocumulene was published in 1994. [4] Different methods to prepare such metallacycles are known like the complexation of 1,4‐disubstituded butadiene by Group 4 metallocenes or the coupling of two alkynyl ligands in the coordination sphere of Group 4 metallocenes. [1] A lot of reactions for such metallacycles were described in the past. [1] A special case for reaction is the dimerization by coupling of Group 4 metalla‐cyclocumulenes to organometallic [4]radialenes. [1] The symmetrical substituted tricyclic [4]radialene of titanocene with phenyl substituents was obtained in 1999 by the formal dimerization of the 5‐membered titana‐cyclocumulene (Scheme 7). As another product in this reaction, the unsymmetrical bicyclic complex was formed. A similar [4]radialene with methyl substituted Cp’=η5‐C5H4Me was obtained by the reaction of (η5‐C5H4Me)2Ti(η2‐Me3SiC2SiMe3) with PhC≡C−C≡CPh. A hafnium [4]radialene was formed by decomposition of di‐n‐butylhafnocene Cp2Hf(n‐Bu)2 in the presence of 1,4‐diphenylbutadiyne PhC≡C−C≡CPh at higher temperature. The analogous organozirconium [4]radialene was not known for a long time. In the above mentioned paper from 1993 by Buchwald and coworkers [2] gave not the expected tricyclic organozirconium [4]radialene. In the reaction of Cp2Zr(η2‐butene), formed from Cp2Zr(n‐Bu)2, with bis(trimethylsilyl)buta‐1,3‐diyne Me3Si−C≡C−C≡C‐SiMe3 indeed the 7‐membered zircona‐cyclocumulene was isolated.
Scheme 7.
Formation and reaction of a zirconocene [4]radialene of zirconocene.
In a very recently published paper by Burlakov, Aysin and coworkers, the first [4]radialene of zirconocene was realized (Scheme 7). [5]
It was obtained by the reaction of Cp2Zr(n‐Bu)2 via Cp2Zr(η2‐butene) with PhC≡C−C≡CPh. In the protolysis of this compound with HCl, the metal free [4]radialene was formed. [5] The molecular structure of the [4]radialene of zirconocene was investigated by X‐ray analysis. Additionally, the organometallic [4]radialenes of Group 4 metallocenes were also characterized by Raman spectroscopy and DFT calculations. The X‐ray data of the zirconium [4]radialene show a similar structure as found before for the known analogous Ti and Hf‐compounds. During Raman investigations, the zirconium [4]radialene and the hafnium [4]radialene both undergo under laser a partial photo‐dissociation, in which the corresponding 5‐membered metallacyclocumulenes were formed. The quantum‐chemical calculation for the dimerization of 5‐membered metallacyclocumulenes to metalla [4]radialenes show that the stability of the radialenes depend on the steric effects of the substituents. The metalla [4]radialenes of
Group 4 metallocenes are stable with H, Me and Ph but not with bulky substituents like t‐Bu and SiMe3. The GIMIC and EDDB criterions of aromaticity show a weak antiaromaticity in the cycle, and the antiaromaticity decreases for the metals Zr≥Hf>Ti. The results show a higher thermodynamic stability, and conjugation as well aromaticity of the organometallic radialenes with the metals Ti, Zr and Hf having several substituents.
2.2. Metalla‐cycloallenes
2.2.1. The 5‐membered metalla‐cycloallenes
It was proposed as early as in 2004 that 5‐membered metalla‐cycloallenes of Group 4 metals as 1‐metalla‐cyclopenta‐2,3‐dienes could formed by coupling of a σ‐alkenyl with a σ‐alkynyl ligands. [6] Later these metallacycles were realized and described as metalla‐cycloallenes or metalla‐cycloallenoides. As predicted, they were obtained either by coupling of a σ‐alkenyl with a σ‐alkynyl ligands or by coordination of 1,3‐enynes. This was published for such compounds by Erker [7a] and Suzuki [7b] together with their coworkers (Scheme 8). The obtained metallacycles were characterized by its molecular structures and published together with several reactions. [1]
Scheme 8.
Formation of 1‐metalla‐cyclopenta‐2,3‐dienes
Suzuki and coworkers reported later a lot of reactions with these compounds. [8] The chemistry of 5‐membered all‐C‐ and hetero‐metalla‐cycloallenes was summarized in several papers. [1]
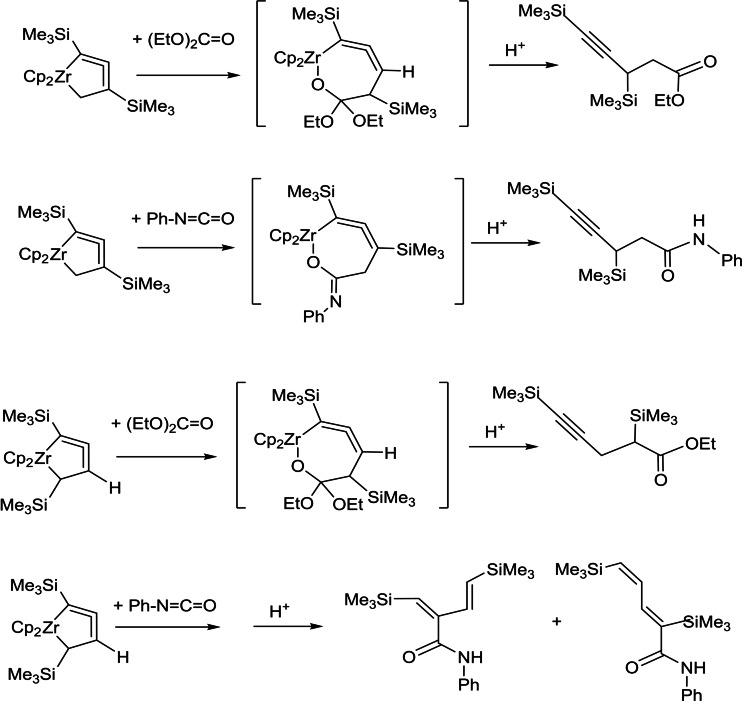
Recently, further interesting synthetic investigations with these metallacycles were described. [9] The 5‐membered metalla‐cycloallene compounds as 2,5‐disubstituted 1‐zircona‐cyclopenta‐2,3‐dienes were obtained by coordination of (E)‐1,4‐bis(trialkylsilyl)but‐1‐en‐3‐ynes. In previous studies for reactions of 2,4‐substituted 1‐zircona‐cyclopenta‐2,3‐dienes with ketones, after hydrolysis alkynyl alcohols. For the 2,5‐bis(trialkylsilyl) compounds with ketones, allenyl alcohols were formed. In the reactions with a nitrile via 7‐membered 1‐aza‐2‐zircona‐cyclohepta‐3,4,7‐trienes, pyrrole compounds were isolated (Scheme 9). If the (E)‐1,4‐bis(trialkylsilyl)but‐1‐en‐3‐ynes react with titanium isopropoxide / n‐butyllithium and after this with nitriles, alkynyl ketones were described (Schemes 9, 10, 11).
Scheme 9.
Reactions of 5‐membered metalla‐cycloallene compounds with ketones and nitriles.
Scheme 10.
Reactions of 5‐membered metalla‐cycloallene compounds with carboxylate esters and isocyanates.
Scheme 11.
Insertion of ketones and reaction of the formed intermediates via transmetallation by copper salts with a subsequent addition of allyl halides to allylated products.
The 5‐membered metalla‐cycloallenes reacted as 1‐zircona‐cyclopenta‐2,3‐dienes with esters and isocyanates (Scheme 10). From carboxylic esters like ethyl acetate and methyl benzoate, after hydrolysis, low yields of alkynyl ketones were formed. The diethyl carbonate gave moderate yields of alkynyl esters. By the insertion of phenylisocyanate into the Zr−C bond an alkynyl amide were isolated, if the starting material has substituents at the 2,4‐position. In contrast to these results, with silyl groups at the 2,5‐position, after hydrolysis dienyl amides were obtained. This was explained by the insertion of the carbonyl group into the Zr−Csp3 bond. Firstly, 7‐membered 1‐oxa‐2‐zircona‐cyclohepta‐3,4‐dienes as intermediates are assumed as the result of the insertion. For the reaction of 2,5‐disilyl‐1‐zircona‐cyclopenta‐2,3‐dienes with isocyanates were 5‐membered 1‐oxa‐2‐zircona‐cyclopent‐3‐ene intermediates assumed
Additionally, several C−C bond formation reactions of the described intermediates were realized.[ 8 , 9 ] A further example is the realization via transmetallation to copper salts and the subsequent addition of allyl halides to give allylated products (Scheme 11).
The new reactions of 1‐zircona‐cyclopenta‐2,3‐dienes with many different substrates were mostly investigated by Suzuki and coworkers, from which not all are reported here in this review. Only ketones, nitriles, carboxylate esters, diethyl carbonate and isocyanates were considered, which gave after hydrolysis ketones, esters and amides etc. Such compounds have a high potential for organic syntheses, and the obtained ketones and esters contain alkyne and the amides diene substituents.
2.2.2. The 4‐membered metalla‐cycloallenes
The comparison of 5‐membered metalla‐cyclocumulenes with three double bonds and 5‐membered metalla‐cycloallenes with only two double bonds in the ring system, led to the question, whether the smaller 4‐membered metalla‐cycloallenes (1‐metalla‐cyclobuta‐2,3‐dienes) could exist. The realization of these molecules for Group 4 metals was a problem, because the smaller highly unsaturated 4‐membered ring should have a much higher ring strain compared to the existing larger 5‐membered rings. The highly strained 4‐membered metalla‐cycloallenes of Group 4 metals were unknown for a longer time, connected with the question how to synthesize of such compounds is possible. The synthesis of the first 4‐membered metalla‐cycloallene of a Group 4 metal, the very unusual 1‐titana‐cyclobuta‐2,3‐diene [rac‐(ebthi)Ti[−C(SiMe3)=C=C(SiMe3)−] (rac‐ebthi=rac‐1,2‐ethylene‐1,1’‐bis(η5‐tetrahydroindenyl)) has a long story. [10] Such 4‐membered metalla‐cycloallenes were published before only for Group 6 metals. By coupling of a carbyne complex [M≡CR] with a terminal alkyne and deprotonation or in alkyne metathesis as decomposition products of the alkylidyne catalyst and a reaction with a terminal alkyne. Such 1‐metalla‐cyclobuta‐2,3‐dienes were published by Schrock and co‐workers and called as “deprotiometallacyclobutadienes”, like Cp(Cl)W[−C(t‐Bu)=C=C(t‐Bu)−] [11a] and [{(CF3)2CHO}2(py2)Mo(py2)[−C(t‐Bu)=C=C(t‐Bu)−]. [11b] The tungsten complex was a by‐product in the metathesis which explained why terminal alkynes are not metathesized. The molybdenum complex served as a model compound in reactions of alkylidyne complexes with terminal alkynes. During the decomposition of an alkylidene catalyst with a terminal alkyne, Fürstner and co‐workers observed the formation of a 1‐molybda‐cyclobuta‐2,3‐diene. [11c] Tamm and co‐workers published the complex [(F3C)3CO)]2Mo[−C(Mes)=C=C(Ph)−] as another compound of this type. [11d] These compounds were all described as more or less unusual by‐products connected with alkyne metathesis experiments and not as products of a purposeful synthesis.
It is of interest that the later in this paper mentioned theoretical prediction for the existence of 4‐menbered matalla‐cycloallenes of Group 4 metals was realized in 2019 by Reiss, Beweries and coworkers in a paper under the title “1‐Titanacyclobuta‐2,3‐diene ‐ an elusive fourmembered cyclic allene”. For the synthesis of a highly strained 1‐metalla‐cyclobuta‐2,3‐diene of a Group 4 metal, firstly the reaction of the allene precursor Li2(Me3Si‐C=C=C‐SiMe3) with Cp2ZrCl2 was investigated. [12] As products of this reaction were only the linear allene‐bridged dizirconocene complexes (Cp2ZrCl)2[‐μ‐(Me3Si)C3(SiMe3)‐] and (Cp2Zr)2[‐μ‐(Me3Si)C3(SiMe3)‐]2 obtained. These by two allenediyl units bridged two Zr centers are 1,5‐dizircona‐cyclooctatetra‐2,3,6,7‐enes with two cumulated double bonds. The dinuclear 1,3‐allenediyl bridged zirconocene complex (Cp2ZrCl)2[‐μ‐(Me3Si)C3(SiMe3)‐] was successfully used as precatalyst in the dehydropolymerisation of methylamine borane. The reaction with MeLi gave the complex (Cp2ZrMe)2[(‐μ‐(Me3Si)C3(SiMe3)‐]2 which showed high activity for this catalytic reaction.[ 13 , 14 ] Perhaps the 4‐membered metallacycloallene is formed as an intermediate during these transformations.
Reiß, Beweries and coworkers reported later for Group 4 metals the first 1‐titana‐cyclobuta‐2,3‐diene rac‐(ebthi)Ti[−C(SiMe3)=C=C(SiMe3)−] (rac‐ebthi=rac‐1,2‐ethylene‐1,1’‐bis(η5‐tetrahydroindenyl)) (Scheme 12). [15] The changing from the Cp ligand to the chelating rac‐(ebthi) ligand and from zirconium to titanium made this complex possible. By the reaction of rac‐(ebthi)TiCl2 with Li2(Me3Si−C=C=C−SiMe3), the successful synthesis of the first mononuclear 4‐membered all‐C‐metalla‐cyclooallene from a Group 4 metal was possible.
Scheme 12.
Reaction of rac‐(ebthi)TiCl2 with Li2(Me3Si−C=C=C−SiMe3) to rac‐(ebthi)Ti[−C(SiMe3)=C=C(SiMe3)−] (rac‐ebthi=rac‐1,2‐ethylene‐1,1’‐bis(η5‐tetrahydroindenyl)).
This smallest compound in the series of such small ring strained metallacycles reacts with ketones and aldehydes to give enynes by oxygen transfer to titanium.
It is a question of the future, by which methods and used Cp’ ligands as well as substituents the synthesis of similar mononuclear zirconium complexes would be possible, too.
2.3. Metallacyclopentynes
In the above mentioned recent review by Hu and Li it was again summarized, that the incorporation of a metal center is an elegant method to reduce the ring strain and to stabilize highly strained acetylenic carbon‐containing compounds. [1l] This article
gives an overview on two types of complexes for 5‐membered metallacycles with one triple bond like metallacycloalkynes and metallapentalynes. These metallacycles show some similarities and are interesting due to their abnormal structures and unusual reactivities as well as intermediates in many transition‐metal‐catalyzed organic reactions. The metallapentalynes are not considered in this review.
The first substituted 5‐membered metallacycloalkyne of Group 4 metals was published by Suzuki and coworkers. [16] Later, the synthesis of such a non‐substituted 1‐titanacyclopentyne together with a reaction was described. Since these days, many of reactions of these metallacycles were described. [1]
In an very interesting recently published paper, the generation of masked Ti(II) intermediates from Ti(IV) amides by a β‐H abstraction and deprotonation under formation of a titanacyclopentyne was discussed by Tonks and coworkers (Scheme 13). [17]
Scheme 13.
Twofold C−H activation of the propargylic positions of 3‐hexyne and the formation of a titanacyclopentyne.
They proposed a mechanism for nitrene‐coupled transfer hydrogenation. Starting from TiCl2(NMe2)2, the coordination of 3‐hexyne and by subsequent deprotonation of both propargylic C−H positions, the formation of a titanacyclopentyne was assumed. By the substitution of the formed 2,3,4‐hexatriene MeCH=C=C=CHMe and coordination of azobenzene a hydrazido complex could be formed, but the 2,3,4‐hexatriene was not found in the reaction mixtures. An alternative mechanistic suggestion including other dehydrogenation products of 3‐hexyne could not ruled out. Alternately, the TiCl2(NMe2)2 could undergo β‐H abstraction under formation of an η2‐titanaziridine, which could gives by a substitution with azobenzene the N‐methylformimine and the titanium η2‐hydrazido complex. Nevertheless, the proposed reaction of titanium amides like TiCl2(NMe2)2 and the deprotonation of two propargylic C−H groups of 3‐hexyne with the formation of a titanacyclopentyne could be a new method to prepare such unusual metallacycles. The future will show, if this could be a general method to make metallacyclopentynes.
The assumed formation of a titanacyclopentyne [Cp2Ti(η2‐MeCH−C2−CHMe)] by Tonks and coworkers during their mechanistic discussion for the reaction of L n Ti(NRR’)2 with hexyne Me−CH2C≡CCH2−Me by a twofold C−H activation of the propargylic abstraction in the β‐H positions of the alkyne is very interesting. This suggestion has some similarities to the formation of the first non‐substituted titanacyclopentyne Cp2Ti(η2‐H2C−C2−CH2−). This was formed by the twofold β‐chloro abstraction to Cp2TiCl2 in the reaction of Cp2Ti(η2‐Me3SiC2SiMe3) with Cl−CH2C≡CCH2−Cl. [1]
3. New Bonding Description for Unusual Metallacycles
The series of ring strained unusual Group 4 metallacycles were investigated to understand better the bonding of these complexes. Because all these complexes of the 7‐, 5‐ and 4‐membered ring systems have several aspects of similarity, in some publications this was described not only for compounds of one type, but together for reasons of comparison from several examples with overlapping in only one paper. This is the reason why the description and the discussion for the bonding character was considered here together in one chapter and not under the single before described chapters. In nearly all cases, a longer time ago some relevant data were published as the basic for recently described extensions of the investigations. These results are mentioned here only for a better understanding and to show the progress of the last three years.
3.1. The 5‐membered metallacyclocumulenes, ‐cycloallenes and ‐cycloalkynes
5‐membered Group 4 metallacyclocumulenes and ‐cycloalkynes were calculated firstly by Jemmis and coworkers [18a] as well as by Lam and Lin. [18b] These investigations were later extended and compared to the metallacycloallenes. [8] The theoretical investigations on these ring‐strained all‐carbon metallacycles gave several similarities regarding the electronic structures of metallacycloallenes, metallacyclopentynes and phenylallenyl complexes. [19] These were explained by similar kinds of in‐plane and out‐of‐plane molecular orbitals of the acetylene and allene moieties. The strong interaction of the two central carbon atoms along with the terminal carbon atoms was described. This type of interaction is the main driving force for the experimental realization of metallacyclocumulenes. The similarity of bonding in the five‐membered metallacycloallene to that of the metallacyclocumulene and metallacyclopentyne is the result of the bent metallocene moiety Cp2M to stabilize the strained organic π‐systems. Several other theoretical investigations of these very unusual and interesting group of compounds were obtained later.
3.2. The 7‐membered zirconacyclocumulenes
Aysin and Bukalov published very recently the electronic structure of 7‐membered zirconacyclocumulenes together with its characterization by vibrational spectra and QTAIM (Quantum Theory of Atoms In Molecules). [20] The IR and Raman spectra for 7‐membered zirconacyclocumulenes were studied by normal coordinate analysis. It was shown by this spectroscopic investigations and the QTAIM analysis, that the 7‐membered cyclocumulenes consist of two independent fragments. These are a double bond C=C and a coordinated cumulene bond C=C=C=C. Three Raman lines are typical vibrations for a cumulene group which were also observed in IR spectra. By means of Raman spectroscopy in a laser beam, an irreversible isomerization of a 7‐membered cyclocumulene to a zirconacyclopentadiene with alkyne substituents was detected. For another 7‐membered cyclocumulene it was shown on the basis of IR spectra that the cumulene structure is stable in its isomerization. These results were very useful for the better understanding of several reaction of this class of organometallics, as described before. [3]
In addition to this description, the aromaticity of 5‐ and 7‐membered metallacyclocumulenes of Group 4 metals was very recently published by Aysin and coworkers.[21] Modern criteria were used to study the conjugation and aromaticity in 5‐membered metallacyclocumulenes of Group 4 metals for the two types Cp2M[(η4‐(R)C=C=C=C=C(R)−] and Cp2M[(η4‐(R)C=C=C=C=C(R)−](RC≡CR). The data of the ISE, NICS‐scan, EDDB, and GIMIC methods for both types were obtained. After all these results, the π‐delocalization in the metallacycle was excluded. In plane aromaticity in the whole 5‐membered metallacyclocumulenes was shown by the EDDB and GIMIC investigations. The authors wrote, that this rare aromaticity type has some peculiarities for the IC distribution. The aromatic diatropic ICs are located inside and outside of the metallacycle. the ring. The electron density of the delocalized bonds (EDDB) is unusual with a high density inside the metallacyclecycle. The aromaticity decreases from Ti to Zr and Hf as shown by the EDDB and GIMIC data.
3.3. The 4‐membered metallacycloallenes
Several years ago, Jemmis, Schulz and coworkers calculated the theoretical evidence for the existence of an unusual 4‐membered metallacycloallene by a Group 4 transition‐metal fragment.[ 19 , 22 ] As a result of these comprehensive theoretical studies it was shown, that a Group 4 metal can stabilize the exotic 4‐membered metallacycloallene. The interaction of the metal with the central carbon atom of the MC3 ring along with two strong terminal MC bonds stabilizes the existence of such an extremely ring strained metallacycle. It was predicted, that acyclic and cyclic alkylated amines like NMe2, piperidino, and NHCs as substituents would be capable of donating two electrons to the complex and stabilizes this type of compounds significantly to make it synthetically viable. The existence of the MC2 bond in the MC3 ring was supported by MO and NBO analyses as well as by the enhanced stability of the complexes.
After the very recently reported successful synthesis of the first 4‐membered metallacycloallene of a Group 4 metal by Reiß, Beweries and coworkers, a more realistic theoretical description in comparison to their experimentally obtained data became possible. The X‐ray molecular structure of the 1‐titanacyclobuta‐2,3‐diene [rac‐(ebthi)Ti[−C(SiMe3)=C=C(SiMe3)−] with rac‐ebthi=rac‐1,2‐ethylene‐1,1’‐bis(h5‐tetrahydroindenyl)) was reported and analyzed. After X‐ray molecular structure investigations is this metallacycle not planar, showing electronic and structural differences compared to the above mentioned Group 6 complexes. For the structures of 1‐metallacyclobuta‐2,3‐dienes are different descriptions depending on the metal center possible. Schrock and Churchill favored for Group 6 complexes an interaction of MoX2 2+ with a dianion [R−C=C=C−R]2−. By a reorganization of the π‐electrons in the xy plane of the MoC3 ring is the allenic character of the C3 unit destroyed. This is reason why in the structural formula the delocalization was introduced. Changing from Group 6 (Mo, W) to Group 4 (Ti) metals leads to a different bonding situation in these metallacycloallenes. The former mentioned theoretical calculations by Jemmis, Schulz and coworkers support this description for such planar substituted metallacycles. An additional stabilization in 4‐membered Group 4 metallacycloallenes is possible by interaction of the central carbon atoms with the metal of the in‐plane aromatic MC3 ring. The mesomeric description for these interactions in the 4‐membered is shown in Scheme 14.
Scheme 14.
Mesomeric description for 4‐membered titanacycloallenes unsaturated and ring strained four membered MC3.
Further investigations and calculations were presented in the new paper of Reiß, Beweries and coworkers for the 4‐membered titanacycloallene, to understand better these unusual metallacycles (Scheme 15).
Scheme 15.
Bonding situation of rac‐(ebthi)Ti[η2‐C(SiMe3)=C=C(SiMe3)−] as a singlet biradical of Ti(III) coupled with a monoanionic allene radical.
There is a short distance between titanium and the β‐carbon atom, but no significant bonding interaction between both atoms. The ring critical point was found near the centre of the TiC3 ring. Scheme 15 shows the main resonance structures which best describe the bonding situation in this unusual complex. This is a singlet biradical of the Ti(III) center which is antiferromagnetically coupled with a monoanionic allene radical.
4. Actinide Metallocene Bis(trimethylsilyl)acetylene Complexes
Typical reactions of Group 4 metallocene metallacyclocumulenes were extended in the past to such actinide metallacycles, too. For this were several papers from Zi, Walter et al. [23] as well as Kiplinger and co‐workers [24] published.
Additionally, exist some examples of new investigations concerning this chemistry, which show the extension of Group 4 metallacycles to actinide metallocene complexes. [25] Zi, Walter and coworkers described the synthesis and reactivity of the metallacyclocumulene Cp’2U(η4‐PhC4Ph) by the reaction of Cp’2U(η2‐PhC2Ph) with Cp’=η5‐1,2,4‐(Me3C)3C5H2 and the diyne PhC≡C−C≡CPh. [25a]
Zhang and coworkers, very recently reported other substitution and coupling reactions to metallacycles of lutetium and dysprosium. [25b] Some of these metallacycles were the first examples in rare‐earth organometallic chemistry. [25] These papers show that this chemistry is not restricted to Group 4 metals. Several similarities exist for such ring strained metallacycles of Group 4 metallocene complexes with actinide metallacycles.
5. Conclusions
There exist some recently published examples for new syntheses, reactions and characterizations of unusual unsaturated ring strained Group 4 metallocene metallacycles. For the 7‐ and 5‐membered metalla‐cyclocumulenes, the 5‐ and 4‐membered metalla‐cycloallenes as well as 5‐membered metalla‐cycloalkynes were several new results of its preparation and reactions published. Additionally, some spectroscopic and theoretical investigations were mentioned which are connected mostly with the aromatic character of these compounds. These considerations like mesomerism and equilibrium have a great impact to understand the new described reactions. For these ring strained compounds, the influence of the Group 4 metals, the ring‐size and the substituents were studied. The described results show, that these compounds are not only exotic. They are interesting from the theoretical point of view and have a great potential for some synthetic applications.
Conflict of interest
The authors declare no conflict of interest.
Biographical Information
Uwe Rosenthal studied chemistry (1968‐72), received his Ph.D. under supervision of E. Kurras (1976), and completed his habilitation (1991) at the University of Rostock. After postdoctoral time at the A. N. Nesmeyanov Institute of Organoelement Compounds (INEOS) at the Russian Academy of Sciences in Moscow with M. E. Vol′pin and V. B. Shur (1988) he was a visiting research scientist at the Max Planck Institute of Kohlenforschung in Mülheim/Ruhr with G. Wilke and K. Pörschke (1990‐91), He headed (1992‐96) the Max Planck Research group “Complex Catalysis”, became Professor of Inorganic Chemistry at the University of Rostock (1993), was Deputy Director of the Leibniz Institute of Catalysis at the University of Rostock (2003‐16) and his scientific interests are the basics of Organometallic Chemistry (unusual metallacycles) for applications in Homogeneous Catalysis (selective oligomerization of ethylene).

Acknowledgements
I would like to particularly thank all my former Ph.D. students, postdocs, assistants, cooperation partners and other people whose names are mentioned in the list of references, for all of her excellent scientific results, giving the basis of this review. Open Access funding enabled and organized by Projekt DEAL.
U. Rosenthal, Chem. Eur. J. 2021, 27, 17751.
Dedicated to my many years friend Dr. sc. Vladimir V. Burlakov
References
- 1. Selected Reviews:
- 1a. Ohff A., Pulst S., Lefeber C., Peulecke N., Arndt P., Burlakov V. V., Rosenthal U., Synlett 1996, 111–118; [Google Scholar]
- 1b. Rosenthal U., Burlakov V. V., Arndt P., Baumann W., Spannenberg A., Organometallics 2005, 24, 456–471; [Google Scholar]
- 1c. Rosenthal U., Burlakov V. V., Bach M. A., Beweries T., Chem. Soc. Rev. 2007, 36, 689–820; [DOI] [PubMed] [Google Scholar]
- 1d. Rosenthal U., Angew. Chem. 2008, 120, 5196–5199; [Google Scholar]; Angew. Chem. Int. Ed. 2008, 47, 5118–5121; [DOI] [PubMed] [Google Scholar]
- 1e. Beweries T., Rosenthal U., Sci. Synth. 2011/4; [Google Scholar]
- 1f. Beweries T., Hähnel M., Rosenthal U. Catal. Sci. Technol. 2013, 3, 18–28; [Google Scholar]
- 1g. Roy S., Rosenthal U., Jemmis E. D., Acc. Chem. Res. 2014, 47, 2917–2930; [DOI] [PubMed] [Google Scholar]
- 1h. Rosenthal U., Eur. J. Inorg. Chem. 2019, 895–919; [Google Scholar]
- 1i. Rosenthal U., ChemOpen 2019, 8, 1036–1047; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1j. Rosenthal U., Chem. Soc. Rev. 2020, 49, 2119–2139; [DOI] [PubMed] [Google Scholar]
- 1k. Komarov I. V., Russ. Chem. Rev. 2001, 70, 991–1016; [Google Scholar]
- 1l. Hu B., Li C., Catalysts 2020, 10, 1268; [Google Scholar]
- 1m. Rosenthal U., Organometallics 2020, 39, 4403–4411. [Google Scholar]
- 2. Hsu D. P., Davis W. M., Buchwald S. L., J. Am. Chem. Soc. 1993, 115, 10394–10395. [Google Scholar]
- 3.
- 3a. Burlakov V. V., Andreev M. V., Bogdanov V. S., Smol'yakov A. F., Minacheva M. Kh., Shur V. B., Organometallics 2019, 38, 2636–2646; [Google Scholar]
- 3b. Burlakov V. V., Andreev M. A., Bogdanov V. S., Arndt P., Baumann W., Spannenberg A., Rosenthal U., Shur V. B., Organometallics 2020, 39, 2365–2374; [Google Scholar]
- 3c. Huh D. N., Cheng Y., Frye C. W., Egger D. T., Tonks I. A., Chem. Sci. 2021, in print; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3d. Burlakov V. V., Becker L., Bogdanov V. S., Andreev M. V., Arndt P., Spannenberg A., Baumann W., Rosenthal U., Eur. J. Inorg. Chem. 2014, 5304–5310. [Google Scholar]
- 4.
- 4a. Rosenthal U., Ohff A., Baumann W., Kempe R., Tillack A., Burlakov V. V., Angew. Chem. Int. Ed. 1994, 33, 1605–1608; [Google Scholar]; Angew. Chem. 1994, 106, 1678–1680; [Google Scholar]
- 4b. Pellny P. M., Kirchbauer F. G., Burlakov V. V., Baumann W., Spannenberg A., Rosenthal U., J. Am. Chem. Soc. 1999, 121, 8313–8323. [Google Scholar]
- 5.
- 5a. Aysin R. R., Andreev M. V., Bogdanov V. S., Bukalov S. S., Korlyukov A. A., Dorovatovskii P. V., Burlakov V. V., Organometallics 2021, 40, 1344–1350. [Google Scholar]
- 6. Rosenthal U., Angew. Chem. 2004, 116, 3972–3977; [Google Scholar]; Angew. Chem. Int. Ed. 2004, 43, 3882–3887. [DOI] [PubMed] [Google Scholar]
- 7.
- 7a. Ugolotti J., Dierker G., Kehr G., Fröhlich R., Grimme S., Erker G., Angew. Chem. Int. Ed. 2008, 47, 2622–2625; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2008, 120, 2662–2665; [Google Scholar]
- 7b. Suzuki N., Hashizume D., Koshino H., Chihara T., Angew. Chem. 2008, 120, 5276–5280; [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2008, 47, 5198–5202. [DOI] [PubMed] [Google Scholar]
- 8. Suzuki N., Kurita R., Kawamura A., Akiko O., Tomoyuki M., Masuyama Y., J. Organomet. Chem. 2018, 874, 40–48. [Google Scholar]
- 9. Suzuki N., Hosoya M., Makoto O., Tomoyuki M., Mochizuki A., Masuyama Y., J. Organomet. Chem. 2020, 923, 121410. [Google Scholar]
- 10. Rosenthal U., Eur. J. Inorg. Chem. 2019, 3456–3461. [Google Scholar]
- 11.
- 11a. McCullough L. G., Listemann M. L., Schrock R. R., Churchill M. R., Ziller J. W., J. Am. Chem. Soc. 1983, 105, 6729–6730; [Google Scholar]
- 11b. Churchill M. R., Ziller J. W., J. Organomet. Chem. 1985, 281, 237–248; [Google Scholar]
- 11c. McCullough L. G., Schrock R. R., Dewan J. C., Murdzek J. C., J. Am. Chem. Soc. 1985, 107, 5987–5998; [Google Scholar]
- 11d. Heppekausen J., Stade R., Kondoh A., Seidel G., Goddard R., Fürstner A., Chem. Eur. J. 2012, 18, 10281–10299; [DOI] [PubMed] [Google Scholar]
- 11e. Ehrhorn H., Bockfeld D., Freytag M., Banneberg T., Kefalidis C. E., Maron L., Tamm M., Organometallics 2019, 38, 1627–1639. [Google Scholar]
- 12. Reiß F., Reiß M., Spannenberg A., Jiao H., Baumann W., Arndt P., Rosenthal U., Beweries T., Chem. Eur. J. 2018, 24, 5667–5674. [DOI] [PubMed] [Google Scholar]
- 13. Trose M., Reiß M., Reiß F., Spannenberg A., Boye S., Lederer A., Arndt P., Beweries T., Dalton Trans. 2018, 47, 12858–12862. [DOI] [PubMed] [Google Scholar]
- 14. Han D., Anke F., Trose M., Beweries T., Coord. Chem. Rev. 2019, 380, 260–286. [Google Scholar]
- 15. Reiß F., Reiß M., Bresien J., Spannenberg A., Jiao H., Baumann W., Arndt P., Beweries T., Chem. Sci. 2019, 10, 5319–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.
- 16a. Suzuki N., Nishiura M., Wakatsuki Y., Science 2002, 295, 660–663; [DOI] [PubMed] [Google Scholar]
- 16b. Hashizumi D., Suzuki N., Chihara T., Chem. Commun. 2006, 1233–1235; [DOI] [PubMed] [Google Scholar]
- 16c. Suzuki N., Hashizume D., Coord. Chem. Rev. 2010, 254, 1307–1326. [Google Scholar]
- 17. Pearce A. J., Cheng Y., Dunscomb R. J., Tonks I. A., Organometallics 2020, 39, 3771–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.
- 18a. Jemmis E. D., Phukan A. K., Giju K. T., Organometallics 2002, 21, 2254; [Google Scholar]
- 18b. Lam K. C., Lin Z., Organometallics 2003, 22, 3466–3470; [Google Scholar]
- 18c. Roy S., Jemmis E. D., Ruhmann M., Schulz Axel, Kaleta K., Beweries T., Rosenthal U., Organometallics 2011, 30, 2670–2679. [Google Scholar]
- 19. Roy S., Rosenthal U., Jemmis E. D., Acc. Chem. Res. 2014, 47, 2917–2930. [DOI] [PubMed] [Google Scholar]
- 20. Aysin R. R., Bukalov S. S., J. Mol. Struct. 2021, 1231, 130002. [Google Scholar]
- 21. Aysin R. R., Bukalov S. S., Organometallics 2021, 40, 938–947. [Google Scholar]
- 22. Roy S., Jemmis E. D., Schulz A., Beweries T., Rosenthal U., Angew. Chem. Int. Ed. 2012, 51, 5347–5350; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 5442–5446. [Google Scholar]
- 23. Selected Examples:
- 23a. Zhang L., Fang B., Hou G., Ai L., Ding W., Walter M. D., Zi G., Dalton Trans. 2016, 45, 16441–16452; [DOI] [PubMed] [Google Scholar]
- 23b. Fang B., Zhang L., Hou G., Zi G., Fang D. C., Walter M. D., Organometallics 2015, 34, 5669–5681; [Google Scholar]
- 23c. Zi G., Chem. Commun. 2018, 54, 7412–7430; [DOI] [PubMed] [Google Scholar]
- 23d. Lv Z.-J., Huang Z., Shen J., Zhang W.-X., Xi Z., J. Am. Chem. Soc. 2019,141, 20547–20555; [DOI] [PubMed] [Google Scholar]
- 23e. Zhang L., Hou G., Zi G., Ding W., Walter M. D., Dalton Trans. 2017, 46, 3716–3728. [DOI] [PubMed] [Google Scholar]
- 24. Pagano J. K., Erickson K. E., Scott B. L., Morris D. E., Waterman R., Kiplinger J. L., J. Organomet. Chem. 2017, 829, 79–84. [Google Scholar]
- 25. Examples :
- 25a. Wang D., Ding W., Hou G., Zi G., Walter M. D., Chem. Eur. J. 2021, 27, 6767–6782; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25b. Lv Z.-J., Chai Z., Zhu M., Wei J., Zhang W.-X., J. Am. Chem. Soc. 2021, 143, 9151–9161. [DOI] [PubMed] [Google Scholar]