Abstract
Purpose
Both metabolic dysfunction-associated fatty liver disease (MAFLD) and hepatitis B virus (HBV) are risk factors for hepatocellular carcinoma (HCC). Although concurrent MAFLD is common in patients with HBV-related HCC, whether MAFLD increases the risk of poor prognosis in patients with HBV-related HCC remains unclear. This study aimed to investigate the impact of MAFLD on prognosis in patients with HBV-related HCC.
Patients and Methods
In this retrospective cohort study, 549 patients with HBV-related HCC were enrolled from January 2010 to April 2020 in Guangdong Provincial Hospital of Chinese Medicine, including 169 patients with MAFLD (MAFLD group) and 380 patients without MAFLD (Non-MAFLD group). Propensity score matching (PSM) analysis was performed to balance the baseline characteristics. Kaplan–Meier survival curves were performed to compare the prognosis between the two matched groups. A multivariate Cox proportional hazards model was used to determine the risk factors for poor prognosis.
Results
The median follow-up time for all patients was 20 (interquartile range 8–40) months. We found concurrent MAFLD was associated with a significantly decreased PFS rate before and after PSM analysis. The 1-year, 2-year, and 3-year PFS rates for the MAFLD and Non-MAFLD groups after PSM were 61.3% and 70.8%, 43.9% and 54.5%, 31.1% and 41.8%, respectively. Cox multivariable analysis showed that concurrent MAFLD was an independent risk factor for poor prognosis (death or progression) (HR = 1.49, P = 0.001). More interestingly, the risk of poor prognosis was significantly higher in the MAFLD subtype with metabolic components ≥2 compared to those with metabolic components <2 (HR = 1.97, P < 0.001).
Conclusion
Concurrent MAFLD was associated with a higher risk of poor prognosis in patients with HBV-related HCC, especially MAFLD with metabolic components ≥2.
Keywords: hepatocellular carcinoma, hepatitis B virus, metabolic dysfunction-associated fatty liver disease, poor prognosis
Introduction
To date, liver cancer remains a significant public health problem worldwide. Following the GLOBOCAN estimates,1 liver cancer is the third leading cause of cancer death globally, with approximately830000deaths in 2020. Hepatocellular carcinoma (HCC), the most common form of liver cancer, has become the second leading cause of cancer death in China,2 with nearly 400,000 new cases every year. In recent years, the prevalence of metabolic dysfunction-associated fatty liver disease (MAFLD, a new definition of fatty liver disease) has increased rapidly in China, where Hepatitis B virus (HBV) infection is quite common. Therefore, the population of having these two concomitant diseases is also increasing year by year, which might result in a higher risk of HCC since both MAFLD and HBV are among the most common etiologies for HCC.3,4
Most evidence supports that chronic hepatitis B (CHB) and MAFLD can affect each other. Their interaction may involve multiple pathways or factors in metabolism, immunity, and genetics. However, their interplay was very complicated and whether the coexistence of MAFLD and CHB has a synergistic effect on the risk of HCC and poor prognosis is still elusive. Some studies5,6 suggest fatty liver disease increases the risk of HCC and mortality in CHB patients. A cohort study7 of 1076 CHB patients found that MAFLD was a more significant risk factor for HCC, liver-related events, and death. In a retrospective study8 with a mean follow-up of 6 years, liver steatosis was a strong predictor of mortality and cancer in CHB regardless of viral load. Nevertheless, other researchers hold the opposite opinion. Yoon et al9 revealed that concurrent non-alcoholic fatty liver disease (NAFLD) was not associated with overall survival in patients with CHB-related HCC after adjusting for baseline characteristics. In another study for patients with HBV-related early-stage HCC,10 no significant difference was found in terms of HCC recurrence and death or liver transplantation between patients with and without MAFLD. Therefore, whether MAFLD increases the risk of poor prognosis in HBV-related HCC remains unclear. Moreover, considering MAFLD, the new definition of the fatty liver just proposed in 2020, data on the association of MAFLD and the prognosis in patients with HBV-related HCC is limited. Due to this issue, this current study was designed to determine the impact of MAFLD on the prognosis of patients with HBV-related HCC based on propensity score matching (PSM) analysis.
Materials and Methods
Study Design and Patient Population
In this retrospective cohort study, HBV-related HCC patients with MAFLD or without MAFLD were enrolled from January 2010 to April 2020 in Guangdong Provincial Hospital of Chinese Medicine. This study was conducted by the Declaration of Helsinki and approved by the Ethics Committee of the Guangdong Provincial Hospital of Chinese Medicine (YE2021-200-01). Written informed consent was obtained from all patients through follow-up in the outpatient department or by telephone and letter follow-up before this study.
The inclusion criteria were as follows: 18 years or older; diagnosis of HCC (according to histological examination or radiological criteria11); evidence of serum HBsAg positive for at least six months; patients in the MAFLD group should have a diagnosis of MAFLD for at least six months prior this study. Patients who met the following criteria were excluded: history of other malignant tumors; co-infection with other hepatitis virus or human immunodeficiency virus; patients with severe systemic dysfunction; lost to follow-up within three months after the index date.
According to the international expert consensus statement,12 diagnosis of MAFLD was established radiologically by evidence of hepatic steatosis combined with BMI ≥ 23 kg/m2, or with diabetes, or with at least two of the following metabolic risk factors: (1) blood pressure ≥ 130/85 mmHg or receiving drug treatment for hypertension; (2) plasma triglycerides ≥ 1.7 mmol/L or receiving lipid-lowering drugs treatment; (3) plasma high-density lipoprotein cholesterol (HDL-C)< 1.0 mmol/L in men or < 1.3 mmol/L in women or lipid-lowering drug treatment; (4) prediabetes defined as fasting blood glucose 5.6–6.9 mmol/L; (5) waist circumference ≥ 90 cm in men or ≥ 80 cm in women; (6) homeostatic model assessment of insulin resistance ≥2.5; or (7) plasma C-reactive protein level > 2 mg/L. Data for the last three criteria were unavailable.
Diabetes was defined in patients with a fasting blood glucose ≥7.0 mmol/L or a history of diabetes diagnosis. Hypertriglyceridemia was defined as a triglycerides ≥1.7 mmol/L. Metabolic components in the MAFLD subgroup were four: BMI ≥28kg/m2, hypertension, diabetes, and hypertriglyceridemia. According to the above diagnostic criteria of MAFLD and the high-risk population with poor prognosis of MAFLD reported in the literature, the following MAFLD subtypes were chosen for subtype analysis, including MAFLD with or without BMI ≥23kg/m2, diabetes, BMI ≥23kg/m2 plus diabetes, metabolic risk factors ≥3, and metabolic components≥2.
Data Collection
Clinical and laboratory data were obtained through a retrospective review of outpatient case notes and inpatient case notes. Baseline clinical data were collected at the index date, including age, sex, alcohol intake habit, body mass index (BMI), history of antiviral treatment, presence of cirrhosis, past medical history (diabetes mellitus and hypertension), Hepatitis B e antigen (HBeAg), HBV DNA levels, alpha-fetoprotein (AFP), Barcelona Clinic Liver Cancer (BCLC) stage, and blood chemistry parameters. The following blood chemistry parameters were recorded: alanine aminotransferase (ALT), albumin, total bilirubin (TBIL), fasting glucose, triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C).
Follow-Up
The primary endpoint of the retrospective study was progression-free survival (PFS),13 which was defined as the time from the index date to progression, or death, or end of follow-up (February 1, 2022). We defined the index date as the date of diagnosis for HBV-related HCC. Tumor progression was defined according to the modified Response Evaluation Criteria In Solid Tumors (mRECIST).14
Statistical Analysis
Propensity score matching (PSM) analysis with a nearest-neighbor 1:1 matching scheme and a caliper size of 0.1 was used to reduce the impact of potential confounding factors and selection bias between the MAFLD group and Non-MAFLD group. The Shapiro–Wilk normality test was used to evaluate the normality. Continuous variables were shown as median with interquartile range (p25-p75) or mean ± standard deviation (SD), assessed by student-test or non-parametric test (Mann–Whitney) as appropriate. Categorical variables were presented as frequencies (n) with percentages (%), analyzed by the chi-squared test. Kaplan–Meier survival curves and the cumulative hazard curves were performed to compare the prognosis between the two matched groups. The univariable and multivariable proportional models were performed to determine risk factors for poor prognosis (progression or death).
Statistical analyses were conducted using R software (V.4.1.1, and match it package V.4.3.0) and STATA (V.16.0, Copyright 1985–2019 Stata Corp MP-Parallel Edition). A value of P <0.05 (two-sided) was considered statistically significant.
Results
Baseline Characteristics of the Patients
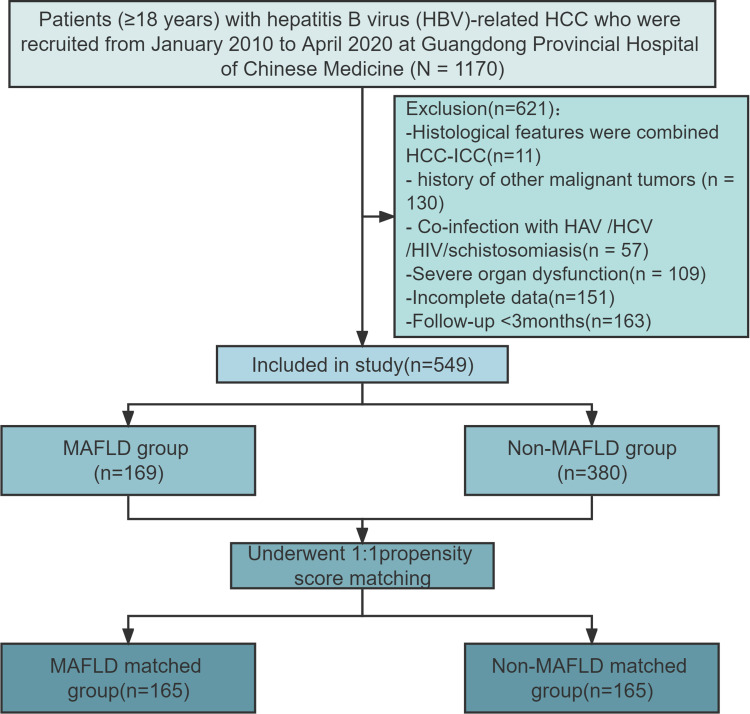
From January 2010 to April 2020, a total of 549 patients with hepatitis B virus-related HCC were enrolled in this study, including 169 (30.8%) patients with MAFLD (MAFLD group) and 380 (69.2%) patients without MAFLD (Non-MAFLD group). More detailed information about the study flow chart is shown in Figure 1.
Figure 1.
Flowchart of this study.
Of the 549 patients studied, most were male (85.2%), and the mean age was 55.32±11.48. Compared to the Non-MAFLD group, the patients in the MAFLD group had a significantly larger BMI, a higher proportion of diabetes and hypertension, higher levels of TC, TG, LDL-C, and lower levels of serum HBV-DNA. More detailed baseline characteristics of the enrolled patients are shown in Table 1.
Table 1.
Baseline Characteristics of Patients Before and After PSM
| Variables | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| MAFLD (N=169) | Non-MAFLD (N=380) | P | MAFLD (N=165) | Non-MAFLD (N=165) | P | |
| Male, n (%) | 140(82.8) | 327(86.1) | 0.330 | 139(84.2) | 140(84.8) | 0.879 |
Age (years), 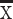 ±SD ±SD |
55.7±11.7 | 55.2±11.8 | 0.606 | 55.5±10.7 | 56.2±11.5 | 0.588 |
BMI (Kg/m2), 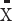 ±SD ±SD |
25.50±3.91 | 22.47±3.45 | <0.001 | |||
| Excessive alcohol, n (%) | 20(11.8) | 38(10.0) | 0.519 | |||
| Diabetes, n (%) | 43(25.4) | 59(15.5) | 0.006 | |||
| Hypertension, n (%) | 56(33.1) | 79(20.8) | 0.002 | |||
| Cirrhosis, n (%) | 111(65.7) | 223(58.7) | 0.121 | 107 (64.8) | 105(63.6) | 0.818 |
| Antiviral treatment | ||||||
| No | 104(61.5) | 250 (65.8) | 0.255 | 104 (63.0) | 110 (66.7) | 0.785 |
| <1 year | 28(16.6) | 69 (18.2) | 27 (16.4) | 24 (14.5) | ||
| ≥1 year | 37 (21.9) | 61 (16.1) | 34 (20.6) | 31 (18.8) | ||
ALT (U/L), 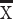 ±SD ±SD |
33 (22.0,52.0) | 35 (22.25, 53.0) | 0.545 | 33 (22.5,53.0) | 34 (21,53.5) | 0.950 |
ALB (g/L), 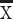 ± SD ± SD |
40.7±5.9 | 38.0±6.5 | <0.001 | 40.6±5.9 | 39.5±5.7 | 0.067 |
| TBIL (μmol/L) | 13.6 (9.4,18.5) | 14.4 (10.2, 23.0) | 0.054 | 13.6 (9.4,18.3) | 13.4 (8.9,21.0) | 0.846 |
Glucose (mmol/L), 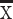 ±SD ±SD |
5.9±2.1 | 5.6±2.0 | 0.092 | |||
TC (mmol/L), 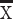 ±SD ±SD |
4.8±1.2 | 4.3±1.1 | <0.001 | |||
TG (mmol/L), 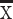 ±SD ±SD |
1.3±0.7 | 1.0±0.5 | <0.001 | |||
| HDL-C (mmol/L) | 1.2±0.5 | 1.1±0.4 | 0.081 | |||
| LDL-C (mmol/L) | 3.2±1.1 | 2.7±0.99 | <0.001 | |||
| HBeAg-positive, n(%) | 27 (16.0) | 67 (17.6) | 0.635 | 27(16.4) | 23(13.9) | 0.539 |
| HBVDNA, log10, IU/mL | 5.5 (3.9,11. 8) | 8.8 (5.5,12.3) | 0.007 | 8.0±4.2 | 8.0±3.8 | 0.623 |
| AFP>20 ng/mL | 91(53.8) | 233(61.3) | 0.100 | 91(55.2) | 87(52.7) | 0.659 |
| BCLC stage | ||||||
| 0 | 10 (5.9) | 34 (8.9) | 0.672 | 10(6.1) | 11(6.7) | 0.889 |
| A | 83 (49.1) | 166 (43.7) | 81(49.1) | 82(49.7) | ||
| B | 31 (18.3) | 70 (18.4) | 31(18.8) | 29(17.6) | ||
| C | 44 (26.0) | 108 (28.4) | 42(25.5) | 43(26.1) | ||
| D | 1 (0.6) | 2 (0.5) | 1(0.6) | 0 | ||
| With hepatectomy | ||||||
| Yes | 117 (69.2) | 184 (48.4) | <0.001 | 113(68.5) | 112(67.9) | 0.906 |
| No | 52 (30.8) | 196 (51.6) | 52(31.5) | 53(32.1) | ||
Abbreviations: MAFLD, metabolic dysfunction-associated fatty liver disease; BMI, body mass index; ALT, alanine aminotransferase; ALB, albumin; TBIL, total bilirubin; TC, cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; AFP, alpha-fetoprotein; BCLC, Barcelona clinic liver cancer; SD, standard deviation.
In the MAFLD group, the proportions of patients with BMI ≥23 Kg/m2, with BMI ≤23Kg/m2, and metabolic dysfunctions, with BMI ≤23 Kg/m2 and diabetes were 76.2%, 18.3%, and 5.9%, respectively.
Reducing the differences in baseline characteristics between the MAFLD group and the Non-MAFLD group, which might affect the prognosis, PSM analysis was performed with 165 pairs matched. As shown in Table 1, the baseline characteristics, including gender, age, presence of cirrhosis, antiviral treatment, ALT, albumin, TBIL, HBeAg status, HBV DNA, AFP, Barcelona stage, and treatment with hepatectomy, were well balanced between the two groups. However, some MAFLD-related factors, including BMI, diabetes, hypertension, etc., were not balanced in PSM analysis.
Comparison of PFS Rate and Cumulative Hazard Ratio Between HBV-Related HCC Patients with and without MAFLD After PSM Analysis
After a median follow-up of 20 months (interquartile range 8–40 months), a total of 348 (63.4%) patients showed death or progression, including 141 deaths and 207 patients with progression. The proportion of deaths or progression in the MAFLD group was significantly higher than that in the Non-MAFLD group (71.6% vs 59.7%, P=0.08).
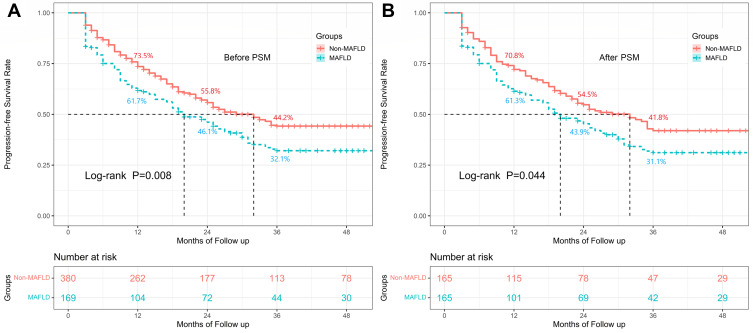
Before PSM, MAFLD was associated with a significantly decreased PFS rate (Figure 2A, P=0.008). The 1-year, 2-year, and 3-year PFS rates for the MAFLD and Non-MAFLD groups were 61.7% and 73.5%, 46.1% and 55.8%, 32.1% and 44.2%, respectively. A similar result was found after PSM. The PFS rate in the MAFLD group was also significantly lower than that in the Non-MAFLD group after PSM (Figure 2B, P=0.044). The 1-year, 2-year, and 3-year PFS rates for the MAFLD and Non-MAFLD groups were 61.3% and 70.8%, 43.9% and 54.5%, 31.1% and 41.8%, respectively. MAFLD group showed a higher cumulative hazard ratio (HR) than that of the Non-MAFLD group, with a higher risk for poor prognosis at the 3-year (HR=1.37, P=0.029).
Figure 2.
Survival analysis among HBV-related HCC patients with and without MAFLD before and after PSM analysis. The Kaplan–Meier curves indicated that the progression-free survival rates for the MAFLD group were lower than those in the Non-MAFLD group both before PSM analysis ((A), P=0.008) and after PSM analysis ((B), P=0.044).
Factors Associated with Poor Prognosis in Patients with Hepatitis B Virus-Related HCC
As shown in Table 2, Cox multivariable analysis showed that concurrent MAFLD was an independent risk factor for poor prognosis (death or progression) (HR=1.49, P=0.001) in patients with Hepatitis B Virus-Related HCC. Other independent significant risk factors for poor prognosis were the presence of cirrhosis (HR=1.40, P=0.005), without antiviral treatment before (HR=1.67, P<0.001), BCLC stage C or D (HR=1.83, P<0.001) and the year of diagnosis ≥2018 (HR=1.33, P=0.022). Meanwhile, the albumin level at baseline was a protective factor for poor prognosis (HR=0.98, P=0.018).
Table 2.
Univariate and Multivariate Analyses of Factors Associated with Poor Prognosis in Patients with Hepatitis B Virus-Related HCC
| Variables | Univariate Analyses | Multivariate Analyses | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| MAFLD (with/without) | 1.35 (1.09, 1.69) | 0.007 | 1.49(1.19,1.87) | 0.001 |
| Sex (male/female) | 1.34 (0.97, 1.85) | 0.078 | ||
| Age (year) | 1.01 (0.99, 1.02) | 0.085 | ||
| Body mass index (Kg/m2) | 1.00 (0.97, 1.02) | 0.770 | ||
| Excessive alcohol (yes/no) | 1.21 (0.85, 1.71) | 0.288 | ||
| Diabetes l (yes/no) | 1.35 (1.04, 1.76) | 0.023 | ||
| Hypertension, (yes/no) | 1.10 (0.86, 1.40) | 0.439 | ||
| Cirrhosis (yes/no) | 1.43 (1.15, 1.78) | 0.002 | 1.40(1.11,1.76) | 0.005 |
| Antiviral treatment (no/yes) | 1.81 (1.44, 2.28) | <0.001 | 1.67(1.31,2.12) | <0.001 |
| ALT (>50/≤50U/L) | 1.56 (1.24, 1.96) | <0.001 | ||
| Albumin (g/L) | 0.98 (0.96,0.99) | 0.004 | 0.98(0.96,0.99) | 0.018 |
| TBIL (μmol/L) | 1.001(0.998, 1.004) | 0.455 | ||
| Blood glucose (mmol/L) | 1.05(0.99,1.10) | 0.064 | ||
| Cholesterol (mmol/L) | 1.12(1.01,1.23) | 0.025 | ||
| Triglycerides (mmol/L) | 1.24(1.06, 1.45) | 0.007 | ||
| HDL-C (mmol/L) | 0.90(0.70,1.16) | 0.424 | ||
| LDL-C (mmol/L) | 1.10(0.98,1.22) | 0.098 | ||
| HBeAg (positive/ negative) | 1.04(0.78,1.37) | 0.809 | ||
| HBVDNA (log10IU/mL) | 1.03(0.99,1.05) | 0.056 | ||
| AFP (>20/≤20ng/mL) | 1.33(1.07,1.65) | 0.010 | ||
| BCLC stage (C or D /A or B) | 2.08(1.65, 2.62) | <0.001 | 1.83(1.44,2.32) | <0.001 |
| Hepatectomy (yes/no) | 0.77(0.62,0.95) | 0.016 | ||
| Year of diagnosis (≥2018/<2018) | 1.60 (1.27, 2.01) | <0.001 | 1.33(1.04,1.69) | 0.022 |
Abbreviations: MAFLD, metabolic dysfunction-associated fatty liver disease; BMI, body mass index; ALT, alanine aminotransferase; TBIL, total bilirubin; TC, cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; AFP, alpha-fetoprotein; BCLC, Barcelona clinic liver cancer; HR, hazard ratio; CI, confidence interval.
MAFLD Subtype with Metabolic Components ≥2 is a Risk Factor Associated with Poor Prognosis in Patients with Hepatitis B Virus-Related HCC
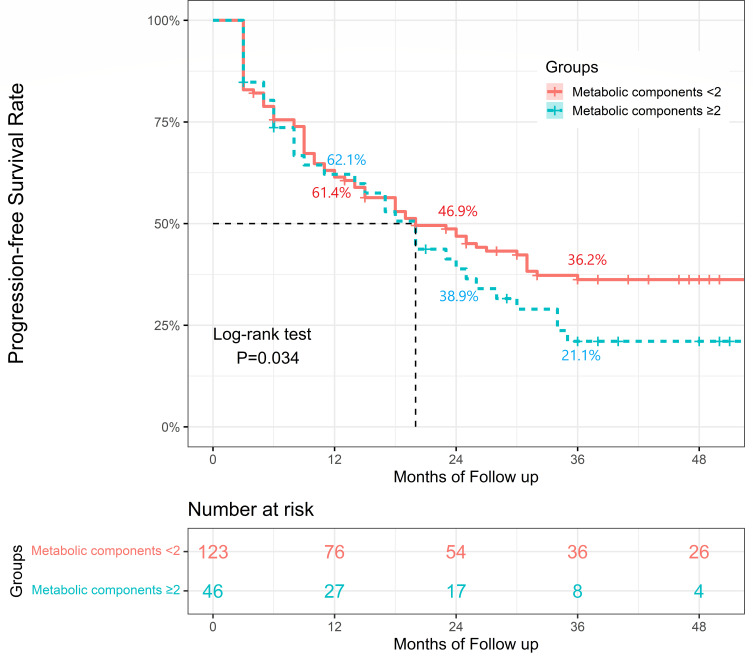
In terms of MAFLD subtype multivariate analysis, MAFLD with metabolic components ≥2 was determined to be an independent risk factor for poor prognosis. The risk of poor prognosis was significantly higher in the MAFLD subtype with metabolic components ≥2 compared to those without (HR=1.49, P=0.045) after adjusting for the above independent factors associated with poor prognosis, including the presence of cirrhosis, antiviral treatment, BCLC stage, the year of diagnosis and albumin level. Compared to the Non-MAFLD group, the MAFLD subtype with metabolic components <2 indicated a higher risk of poor prognosis (HR=1.32, P=0.035), whereas MAFLD with metabolic components ≥2 indicated a much more increased risk of poor prognosis (HR=1.97, P<0.001). As shown in Figure 3, the PFS rate in the MAFLD subtype with metabolic components ≥2 was significantly lower than that in the MAFLD subtype with metabolic components <2 (P=0.034). However, the other MAFLD subtypes were not found to be significantly associated with poor prognosis. No significant difference was found between the MAFLD subtype with BMI ≥23 and BMI <23, between the MAFLD subtype with and without diabetes, between the MAFLD subtype with and without BMI ≥23 plus diabetes, as well as between the MAFLD subtype with metabolic risk factors ≥3 and <3 (Table 3).
Figure 3.
Survival analysis among HBV-related HCC patients in MAFLD subgroup. The Kaplan–Meier curves indicated that the progression-free survival rates for the MAFLD subtype with metabolic components ≥2 were lower than those with metabolic components<2.
Table 3.
Association of Different MAFLD Subtypes with Poor Prognosis in Patients with Hepatitis B Virus-Related HCC
| Variables | Unadjusted HR (95% CI) | P | Adjusted# HR (95% CI) | P | |
|---|---|---|---|---|---|
| MAFLD with BMI <23 | Reference | ||||
| MAFLD with BMI ≥23 | 0.89 (0.59,1.36) | 0.897 | |||
| MAFLD without diabetes | Reference | ||||
| MAFLD with diabetes | 1.25(0.84,1.86) | 0.280 | |||
| MAFLD without BMI ≥23 and diabetes | Reference | ||||
| MAFLD with BMI≥23 and diabetes | 1.29 (0.83,2.02) | 0.254 | |||
| MAFLD with metabolic risk factors <3 | Reference | ||||
| MAFLD with metabolic risk factors ≥3 | 1.52(0.71,3.27) | 0.282 | |||
| MAFLD with metabolic components<2 | Reference | ||||
| MAFLD with metabolic components ≥2 | 1.54(1.05, 2.26) | 0.026 | 1.49(1.01, 2.19) | 0.045 | |
| Non-MAFLD group | Reference | ||||
| MAFLD with metabolic components<2 | 1.19(0.92, 1.54) | 0.178 | 1.32(1.02,1.72) | 0.035 | |
| MAFLD with metabolic components ≥2 | 1.88(1.34, 2.64) | <0.001 | 1.97(1.39, 2.78) | <0.001 | |
Notes: By Cox multivariable analysis; #Adjusted for baseline characteristics, including presence of cirrhosis, antiviral treatment, BCLC stage, the year of diagnosis and ALB level.
Abbreviations: MAFLD, metabolic dysfunction-associated fatty liver disease; BMI, body mass index; HR, hazard ratio; CI, confidence interval.
Discussion
Alongside the growing number of patients with metabolic syndrome and related disorders worldwide, MAFLD has become more common in patients with HBV-related HCC. Previous evidence supported that there is a complex interplay between CHB and MAFLD. However, the impact of MAFLD on prognosis in HBV-related HCC is still a controversial topic. This retrospective cohort study enrolled 549 patients with HBV-related HCC for a median follow-up of 20 months. We found that concurrent MAFLD was associated with a higher risk of poor prognosis in patients with HBV-related HCC, especially the MAFLD subtype with metabolic components ≥2.
Some significant findings should be concerned. Firstly, before and after PSM analysis, we found concurrent MAFLD was associated with a significantly decreased PFS rate. After PSM, the 1-year, 2-year, and 3-year PFS rates for the MAFLD group were lower than those in the Non-MAFLD group (61.3% and 70.8%, 43.9% and 54.5%, 31.1% and 41.8%). More importantly, results from Cox multivariable analysis revealed that concurrent MAFLD was an independent risk factor for poor prognosis (death or progression) (HR=1.49, P=0.001). The above findings confirmed that concurrent MAFLD increases the risk of poor prognosis in patients with HBV-related HCC. It indicated that management of MAFLD should be paid more attention to and strengthened in these patients.
These results seemed similar to some previous studies on the association between fatty liver disease and the prognosis of HBV-related HCC. Wong et al15 and Peleg et al8 demonstrated that the simultaneous presence of HBV and hepatic steatosis was related to an increased risk of disease progression to hepatic and extra-hepatic malignancies. In a multicenter study,7 clinical outcomes were worse in patients with combined steatohepatitis and CHB than in patients with CHB only. In van et al’s study, MAFLD increased the risk of HCC (HR 1.96) and was associated with decreased event-free survival (HR 2.00) in patients with CHB. Interestingly, this study showed that individuals without metabolic dysfunction were not at increased risk of adverse outcomes. This was similar to our findings.
However, other researchers present inconsistent findings. Yoon et al9 reported that concurrent NAFLD was not associated with overall survival in patients with CHB-related HCC. Heterogeneous patient populations may partially explain the discrepancy. Yoon et al concentrated on NAFLD patients who underwent curative surgical resection, while our study focused on MAFLD patients. NHANES III showed that patients with MAFLD had a greater increased risk of all-cause mortality than those with NAFLD.16 More importantly, some NAFLD-related factors, including BMI, diabetes, and hypertension, were well balanced in PSM analysis in Yoon et al’s study so that the impact of NAFLD on prognosis on HCC might not be found. Lin et al’s study10 also did not find that concurrent MAFLD increased the risk of poor prognosis on HBV-Related Stage 0/A HCC after curative resection. It might be due to the non-balanced baseline characteristics between the MAFLD group and the Non-MAFLD group in their study. The MAFLD group had a significantly higher proportion of patients with microvascular invasion than the non-MAFLD group in Lin et al’s study. Nevertheless, the association between MAFLD and the prognosis of HBV-related HCC should be confirmed in future multicenter prospective studies.
Secondly, we found without antiviral treatment before (HR=1.67, P<0.001) were other independent significant risk factors for poor prognosis of HBV-related HCC. But in this current study, only 35% of patients have been treated with antiviral drugs before. Previous studies17,18 have demonstrated that standardized antiviral treatment effectively improved the prognosis of liver cancer. It indicated that antiviral treatment should be started earlier in CHB patients. Meanwhile, BCLC stage C or D (HR=1.83, P<0.001) and cirrhosis (HR=1.40, P=0.005) were also independent risk factors for HCC, similar to previous studies’ results.19,20 It indicated that CHB patients with MAFLD or without MAFLD should be treated with antiviral treatment earlier to prevent progression to cirrhosis and HCC. Furthermore, the year of diagnosis ≥2018 (HR=1.33, P=0.022) was another independent risk factor for poor prognosis. It may be due to the markedly increased number of severe patients admitted and the improved technical level of diagnosis in our hospital after 2018.
Thirdly, some interesting results were found in the MAFLD subtype analysis. After adjusting for baseline characteristics, MAFLD with metabolic components ≥2 had a significantly higher risk of poor prognosis than those without MAFLD (HR=1.97, P<0.001). This finding was similar to Yu et al’s study,21 which revealed that metabolic risk factors had the most significant effect on HCC risk in CHB patients. It suggested that reducing the metabolic risk factors was quite crucial in managing MAFLD in patients with HBV-related HCC.
There are several limitations of this study. Firstly, there may be potential selection bias in a retrospective cohort study. However, PSM and multivariable-adjusted analyses were used to reduce the effect of selection bias and adjust for underlying confounding factors in the current study. Secondly, due to the limited samples of lean-MAFLD, this subtype was not evaluated in this study. However, the results from our study provided an essential basis for future studies. In future studies, the association between lean-MAFLD and the poor prognosis of HBV-related HCC should be determined.
Conclusion
In conclusion, concurrent MAFLD was associated with a higher risk of poor prognosis in patients with HBV-related HCC, especially MAFLD with metabolic components ≥2. It indicated that management of MAFLD should not be ignored to improve the prognosis of HBV-related HCC. However, multicenter, large-scale studies should be designed in the future to confirm our findings and focus more on the impact of the lean-MAFLD subtype on the prognosis of HBV-related HCC.
Funding Statement
The current study was funded by the Thirteen Five-Year Plan for Major and Special Program of the National Science and Technology of China (2018ZX10725506-003 and 2018ZX10725505-004); the biological resources project collaborated by Guangdong Provincial Hospital of Chinese Medicine and Shanghai chip National Engineering Center (YN2016XP03); the Clinical research projects of Guangdong Provincial Hospital of Chinese Medicine (YN10101903) and the Science and Technology research project of Traditional Chinese Medicine of Guangdong Provincial Hospital of Chinese Medicine (YN2022DB04).
Abbreviations
HCC, hepatocellular carcinoma; MAFLD, metabolic dysfunction-associated fatty liver disease; HBV, hepatitis B virus, HBV-related HCC, HBV-related hepatocellular carcinoma; CHB, chronic hepatitis B; NASH, non-alcoholic steatohepatitis; NAFLD, non-alcoholic fatty liver disease; PSM, propensity score matching; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; HBeAg, hepatitis B e antigen; HBV DNA, hepatitis B virus deoxyribonucleic acid; AFP, alpha-fetoprotein; ALT, alanine aminotransferase; TBIL, total bilirubin; TG, triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; PFS, progression-free survival; SD, standard deviation; CI, confidence interval; HR, hazard ratio; BCLC, Barcelona clinic liver cancer; MR, magnetic resonance; CT, computed tomography.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Ethical Approval
All procedures performed in this retrospective study were following the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Approval for investigations was obtained from the Ethics Committee of the Guangdong Provincial Hospital of Chinese Medicine (YE2021-200-01). Written informed consents were obtained from all patients through following up in outpatient department or by telephone and letter follow-up before this study.
Disclosure
The authors declare that they have no conflicts of interest.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Zhou J, Sun H, Wang Z, et al. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer. 2020;9(6):682–720. doi: 10.1159/000509424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen CL, Yang HI, Yang WS, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135(1):111–121. doi: 10.1053/j.gastro.2008.03.073 [DOI] [PubMed] [Google Scholar]
- 4.Golabi P, Rhea L, Henry L, Younossi ZM. Hepatocellular carcinoma and non-alcoholic fatty liver disease. Hepatol Int. 2019;13(6):688–694. doi: 10.1007/s12072-019-09995-8 [DOI] [PubMed] [Google Scholar]
- 5.Kim MN, Han K, Yoo J, Hwang SG, Ahn SH. Increased risk of hepatocellular carcinoma and mortality in chronic viral hepatitis with concurrent fatty liver. Aliment Pharmacol Ther. 2022;55(1):97–107. doi: 10.1111/apt.16706 [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Wang KF, Zhao Z, You QH, Wu YY, Sun J. Study on the relationship between non-alcoholic fatty liver disease and hepatocellular carcinoma in patients with prior hepatitis B virus infection. Zhonghua Gan Zang Bing Za Zhi. 2022;30(1):52–56. doi: 10.3760/cma.j.cn501113-20191227-00482 [DOI] [PubMed] [Google Scholar]
- 7.van Kleef LA, Choi HSJ, Brouwer WP, et al. Metabolic dysfunction-associated fatty liver disease increases risk of adverse outcomes in patients with chronic hepatitis B. JHEP Rep. 2021;3(5):100350. doi: 10.1016/j.jhepr.2021.100350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peleg N, Issachar A, Sneh Arbib O, et al. Liver steatosis is a strong predictor of mortality and cancer in chronic hepatitis B regardless of viral load. JHEP Rep. 2019;1(1):9–16. doi: 10.1016/j.jhepr.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon JS, Lee HY, Chung SW, et al. Prognostic impact of concurrent non-alcoholic fatty liver disease in patients with chronic hepatitis B-related hepatocellular carcinoma. J Gastroenterol Hepatol. 2020;35(11):1960–1968. doi: 10.1111/jgh.15026 [DOI] [PubMed] [Google Scholar]
- 10.Lin YP, Lin SH, Wang CC, et al. Impact of MAFLD on HBV-Related Stage 0/A Hepatocellular Carcinoma after Curative Resection. J Pers Med. 2021;11(8):548. doi: 10.3390/jpm11080684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9(4):452–463. doi: 10.21037/hbsn-20-480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi: 10.1016/j.jhep.2020.03.039 [DOI] [PubMed] [Google Scholar]
- 13.Llovet JM, Montal R, Villanueva A. Randomized trials and endpoints in advanced HCC: role of PFS as a surrogate of survival. J Hepatol. 2019;70(6):1262–1277. doi: 10.1016/j.jhep.2019.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi: 10.1055/s-0030-1247132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong GL, Chan HL, Yu Z, et al. Coincidental metabolic syndrome increases the risk of liver fibrosis progression in patients with chronic hepatitis B–a prospective cohort study with paired transient elastography examinations. Aliment Pharmacol Ther. 2014;39(8):883–893. doi: 10.1111/apt.12658 [DOI] [PubMed] [Google Scholar]
- 16.Huang Q, Zou X, Wen X, Zhou X, Ji L. NAFLD or MAFLD: which Has Closer Association With All-Cause and Cause-Specific Mortality?-Results From NHANES III. Front Med. 2021;8:693507. doi: 10.3389/fmed.2021.693507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi J, Jo C, Lim YS. Tenofovir Versus Entecavir on Recurrence of Hepatitis B Virus-Related Hepatocellular Carcinoma After Surgical Resection. Hepatology. 2021;73(2):661–673. doi: 10.1002/hep.31289 [DOI] [PubMed] [Google Scholar]
- 18.Jang JW, Yoo SH, Nam HC, et al. Association of Prophylactic Anti-Hepatitis B Virus Therapy With Improved Long-term Survival in Patients With Hepatocellular Carcinoma Undergoing Transarterial Therapy. Clin Infect Dis. 2020;71(3):546–555. doi: 10.1093/cid/ciz860 [DOI] [PubMed] [Google Scholar]
- 19.Aliyari Ghasabeh M, Shaghaghi M, Pandey A, et al. Integrating baseline MR imaging biomarkers into BCLC and CLIP improves overall survival prediction of patients with hepatocellular carcinoma (HCC). Eur Radiol. 2021;31(3):1630–1641. doi: 10.1007/s00330-020-07251-4 [DOI] [PubMed] [Google Scholar]
- 20.Xu XF, Xing H, Han J, et al. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma: a Multicenter Study From China. JAMA Surg. 2019;154(3):209–217. doi: 10.1001/jamasurg.2018.4334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu MW, Lin CL, Liu CJ, Yang SH, Tseng YL, Wu CF. Influence of Metabolic Risk Factors on Risk of Hepatocellular Carcinoma and Liver-Related Death in Men With Chronic Hepatitis B: a Large Cohort Study. Gastroenterology. 2017;153(4):1006–1017 e1005. doi: 10.1053/j.gastro.2017.07.001 [DOI] [PubMed] [Google Scholar]