Abstract
Background and purpose
Real‐world data on alemtuzumab are limited and do not provide evidence of its effectiveness after various disease‐modifying therapies (DMTs). Our aim was to provide real‐world data on the impact of clinical variables and previous DMTs on clinical response to alemtuzumab.
Methods
Sixteen Italian multiple sclerosis centers retrospectively included patients who started alemtuzumab from January 2015 to December 2018, and recorded demographics, previous therapies, washout duration, relapses, Expanded Disability Status Scale (EDSS) score, and magnetic resonance imaging data. Negative binomial regression models were used to assess the effect of factors on annualized relapse (ARR) after alemtuzumab initiation.
Results
We studied 322 patients (mean age 36.8 years, median EDSS score 3, median follow‐up 1.94 years). Previous treatments were: fingolimod (106), natalizumab (80), first‐line oral agents (56), first‐line injectables (interferon/glatiramer acetate; 30), and other drugs (15). Thirty‐five patients were treatment‐naïve. The pre‐alemtuzumab ARR was 0.99 and decreased to 0.13 during alemtuzumab treatment (p < 0.001). The number of previous‐year relapses was associated with alemtuzumab ARR (adjusted risk ratio [RR] 1.38, p = 0.009). Progression‐free survival was 94.5% after 1 year, and 89.2% after 2 years of alemtuzumab treatment. EDSS score improvement occurred in 13.5% after 1 year, and 20.6% after 2 years. Re‐baselining patients after 6 months of alemtuzumab treatment, led to no evidence of disease activity status in 71.6% after 1 year and 58.9% after 2 years.
Conclusions
Alemtuzumab decreases ARR independent of previous therapy, including patients with disease activity during natalizumab treatment. Overall, 90% of patients showed no disease progression, and 20% an improvement after 2 years of alemtuzumab.
Keywords: alemtuzumab, cohort, efficacy, real‐world evidence, safety
Alemtuzumab decreases annualized relapse independent of previous therapy, including in patients with disease activity during natalizumab treatment. Overall, 90% of patients show no disease progression, and 20% an improvement after 2 years of alemtuzumab. Re‐baselining patients after 6 months, led to no evidence of disease activity status in 71.6% after 1 year and 58.9% after 2 years.

INTRODUCTION
Alemtuzumab is a humanized monoclonal antibody that selectively targets the cell surface antigen CD52 [1, 2]. This causes a massive depletion of both circulating T and B cells, which is followed by a repopulation pattern different from all other treatments [3]. Although the exact mechanism of action remains unknown, this is thought to cause a more favorable, less inflammatory and autoreactive pool of lymphocytes [4].
When compared to interferon β1a, alemtuzumab proved to be effective in two phase 3 clinical trials in relapsing‐remitting multiple sclerosis (RRMS) patients with regard to both clinical and radiological measures [5, 6]. This performance was confirmed in the open‐label phase of the study and in a long‐term follow‐up [7, 8]. Alemtuzumab is approved for two treatment courses, 1 year apart [9]. The first course requires 5 consecutive days of treatment with one intravenous injection of 12 mg each, while the second requires 3 consecutive days of infusions [5, 6]. In case of disease activity occurring at least 12 months after the last course, a third, or further, 3‐day course was later approved. Additional courses were able to improve outcomes in patients showing disease activity after the second course, without reducing overall safety [10, 11].
Real‐world evidence (RWE) studies are very few for alemtuzumab, and data on the impact of washout and on‐therapy relapses on the future course of the disease are lacking. The objective of the present study was to provide RWE on the use of alemtuzumab in Italian multiple sclerosis centers. This included patient profile (previous DMT use and reason for switch), real‐life retention, efficacy of alemtuzumab, the impact of clinical variables and previous DMTs on clinical response, impact of washout and initial relapses on future response to alemtuzumab, and no evidence of disease activity (NEDA) measures.
PATIENTS AND METHODS
Study design
We designed a multicenter, retrospective study including 16 Italian multiple sclerosis centers. The ethics committee of the coordinating center (Genova) approved the study. Raw data collection was approved by the local ethics committees at all centers. We included all patients treated with alemtuzumab from January 2015 to December 2018, and for whom clinical data were available. There were no specific exclusion criteria. We obtained written informed consent from all patients for a bigger project on real‐world data collection that started earlier.
We collected data using a centralized electronic case report form stored at the University Federico II. All electronic case report form entries were reviewed and queries generated for inconsistencies or missing data.
We collected demographics (age, gender, level of education) and clinical data at the time of treatment start (baseline): time from disease onset and diagnosis; previous DMT use; reason for previous DMT discontinuation; relapses during previous DMT; relapses during washout period from previous DMT to alemtuzumab initiation; relapses during alemtuzumab treatment; washout duration; number of alemtuzumab courses; Expanded Disability Status Scale (EDSS) score before and during alemtuzumab treatment; and magnetic resonance imaging (MRI) data before alemtuzumab initiation, at alemtuzumab initiation and during follow‐up (presence of gadolinium‐enhancing lesions, increase in T2 lesion load). The Lorscheider criteria for secondary progressive multiple sclerosis (SPMS) diagnosis were verified at treatment start.
Alemtuzumab was used at all centers according to the summary of product characteristics approved for alemtuzumab [9].
Follow‐up visits were not scheduled at fixed time points for all patients and there may be inter‐visit heterogeneity. If patients discontinued alemtuzumab treatment, we collected data and reason for treatment end. Drop‐out from alemtuzumab treatment was defined as decision by the treating physician to terminate alemtuzumab follow‐up and switch the patient to another DMT. We also captured all reported adverse events during alemtuzumab follow‐up, as well as infusion‐associated reactions (IARs). No evidence of disease activity (NEDA) was defined as the absence of new relapses, new gadolinium‐enhancing lesions, and EDSS score worsening after alemtuzumab initiation.
Anti‐John Cunningham virus (JCV) antibody test results had been previously collected at each center using standard clinically available services (i.e. the Unilabs Stratify JCV website).
Statistical methods
We report descriptive analyses as mean with standard deviation (SD), or median with interquartile range (IQR), or range for quantitative characteristics, and count with relative frequency for categorical ones.
We used a chi‐squared test for trend to test the possible increase in the proportion of treatment‐naïve and pretreated with first‐line therapies, as compared with a decrease in the proportion of patients switched from natalizumab or fingolimod.
We compared the annualized relapse (ARRs) pre‐alemtuzumab and during alemtuzumab using the non‐parametric Wilcoxon matched‐pairs signed‐rank test.
We used a negative binomial regression model, with log of time while on alemtuzumab as offset, to assess variables associated with relapse activity in alemtuzumab (age, gender, disease duration, EDSS, previous treatment, length of last treatment, washout period pre‐alemtuzumab and last MRI activity pre‐alemtuzumab). The interaction between washout period and previous DMT was also considered since washout was heterogeneous. We used a multivariable model including variables with a p value <0.10 in the univariable analysis. Analysis was also corrected for center of treatment.
Progression‐free survival, cumulative probability of improvement and disease‐activity free probability (NEDA) were calculated using the Kaplan–Meier approach.
We used stata (v.16; StataCorp.) for the computation.
RESULTS
We included 322 patients (71.1% women) with a mean age of 37.8 years, a median EDSS score of 3, a mean disease duration of 7.4 years, and a median number of previous therapies of 3 (Table 1). A total of 195 patients (60.6%) were from three centers and equally distributed. The remaining 13 centers contributed a total of 5% of patients or fewer. Heterogeneity among the centers was observed with regard to baseline EDSS score (p < 0.001) and ARR in the year prior to alemtuzumab initiation (p < 0.001). Patients in seven centers had a mean EDSS score below 3, in six centers it was in the range of 3–4 and in the remaining 3 centers it was greater than 4. Nine centers had a mean ARR below 1 and ranging between 0.31 and 0.9. The small sample included from some centers and differences in DMTs before alemtuzumab treatment among the centers would have contributed to the observed heterogeneity for baseline EDSS and ARR.
TABLE 1.
Demographic and clinical characteristics of the whole cohort (n = 322)
| Age, years | 37.8 (9.5) |
| Women, n (%) | 229 (71.1) |
| Median (IQR) EDSS | 3 (2–5) |
| Median (IQR; range) years since onset | 9.0 (4.4–14.7; 0.2–38.9) |
| Median (IQR; range) no. of previous therapies | 3 (1–4; 0–10) |
| ARR 1‐year pre‐treatment | 0.99 (1.02) |
| SPMS diagnosis (Lorscheider criteria), n (%) | 16 (5) |
| Median (range) year of alemtuzumab treatment | 2016 (2015–2018) |
| Active MRI 1‐year pre‐treatment start, n (%) | 156/249 (62.7) |
| DMT pre‐treatment | |
| Naive | 35 (10.9) |
| Fingolimod | 106 (32.9) |
| Natalizumab | 80 (24.8) |
| DMF | 46 (14.2) |
| IFN | 15 (4.7) |
| GA | 15 (4.7) |
| Teriflunomide | 10 (3.1) |
| Other | 15 (4.7) |
| Reason for previous DMT discontinuation | |
| Inefficacy | 196/288 (68.1) |
| Relapse | 117 (40.6) |
| MRI activity | 65 (22.6) |
| EDSS progression | 14 (4.9) |
| Safety | 69 (24.0) |
| JCV+ or natalizumab antibodies | 57 (19.8) |
| Adverse events | 12 (4.2) |
| Other (pregnancy, end of treatment cycle, no adherence) | 23 (7.9) |
| Median (IQR) length of previous DMT, years | 1.7 (0.9–2.9) |
| Median (range) washout, months | 2.7 (0–29.0) |
Abbreviations: DMF, dimethylfumarate; DMT, disease‐modifying therapy; EDSS, Expanded Disability Status Scale; GA, glatiramer acetate; INF, interferon; IQR, interquartile range; JCV, John Cunningham virus; MRI, magnetic resonance imaging; n, total number; SD, standard deviation; SPMS, secondary progressive multiple sclerosis.
All patients were judged as having RRMS by clinicians, while 16 patients (5%) satisfied the Lorscheider criteria for SPMS diagnosis at treatment start.
The most frequent DMT was fingolimod (n = 106; Figure 1), followed by first‐line therapies (n = 86; dimethylfumarate [n = 46], interferon/glatiramer acetate [n = 30], teriflunomide [n = 10]), natalizumab (n = 80) and other DMTs (n = 15 [of these, six were pretreated with daclizumab, six with mitoxantrone, three with cyclophosphamide]). Thirty‐five patients were treatment‐naïve. During the most recent years there was a trend towards an increase in the number of treatment‐naïve patients and in patients pretreated with first‐line DMTs. In parallel, we witnessed a percentual decrease in the number of patients pretreated with fingolimod and natalizumab (chi‐squared test for trend: p = 0.0065).
FIGURE 1.

Frequency distribution of pre‐alemtuzumab disease‐modifying therapies (DMTs)
The reasons for switch from pre‐alemtuzumab DMTs were relapse (40.6%), MRI activity (22.6%), JCV+ or anti‐natalizumab antibodies (19.8%), EDSS score progression (4.9%), adverse events (4.2%) and other (7.9%). Figure S1 shows the reasons for switch for all four pretreatment categories. Disease activity (relapses, MRI activity, EDSS score worsening) was the predominant cause of switch from first‐line DMTs and from fingolimod. Disease activity and anti‐natalizumab antibodies were the switch trigger in almost 30% of patients pretreated with natalizumab, albeit less frequent than anti‐JCV antibodies. Pregnancy was also a drop‐out reason for nine patients treated with different DMTs (four switched from natalizumab [the previous DMT was fingolimod or mitoxantrone, while two patients were naïve before natalizumab], two from dimethylfumarate [preceded by fingolimod], two from fingolimod [preceded by natalizumab] and one from nitoxantrone [as first therapy]).
Follow‐up data showed an excellent retention rate, with 24 patients dropping out (Figure S2) for the following reasons: adverse events (n = 7); relapse (n = 6); MRI activity (n = 5); compliance (n = 3); other (n = 3). Of these, 10 patients switched to ocrelizumab, nine did not receive other DMTs until the last available follow‐up, two switched to fingolimod, one to natalizumab, one received hematopoietic stem‐cell transplantation, and one a third course of alemtuzumab 2 years after the second dose because of high disease activity.
The pre‐alemtuzumab ARR was 0.99 and decreased to 0.13 during alemtuzumab treatment (p < 0.001; Figure 2a). This was also true for patients pretreated with natalizumab (ARR 0.65 last year on natalizumab vs. 0.16 during alemtuzumab; p < 0.001 [Figure 2b]). In a multivariable model, patients pretreated with fingolimod (ARR 0.18; p = 0.038) or natalizumab (ARR 0.16; p = 0.029) had a higher ARR during alemtuzumab treatment as compared to naïve patients (ARR 0.02). Number of previous year relapses was also associated with a higher ARR during alemtuzumab treatment (risk ratio [RR ]1.43, 95% confidence interval [CI] 1.11–1.84; p = 0.005). Between the first two alemtuzumab courses, 13.9% experienced a relapse, and this was linked to higher ARR during the remaining follow‐up (RR 5.48, 95% CI 2.63–11.41; p < 0.001 [Figure 2c]).
FIGURE 2.
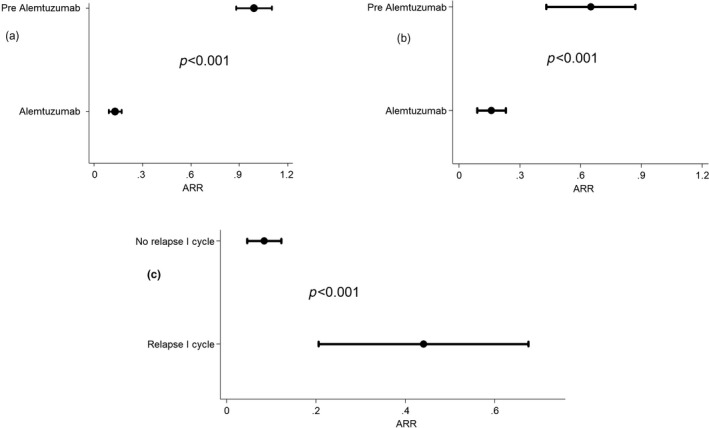
Annualized relapse (ARR) in the overall population (a), in patients switching form natalizumab (b) and from the second year for patients showing relapses between the first and second course of alemtuzumab (c)
Washout from previous DMT (median 80 days) did not impact the ARR during alemtuzumab treatment (p = 0.46). This was also confirmed when taking into account the previous treatment (p for interaction washout/previous DMT = 0.69).
Relapses were equally distributed during the first 90 days of washout without evidence of preferential timing for disease reactivation (Figure 3).
FIGURE 3.
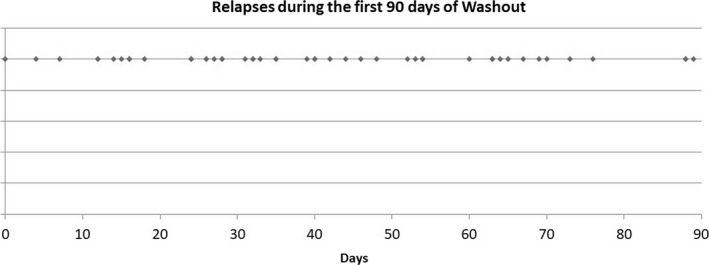
Temporal distribution of relapses during pre‐alemtuzumab washout
The median follow‐up was 1.94 years after the first alemtuzumab course. The median (IQR; range) time between subsequent visits was 5.6 (4.2–8.9;1.4–22.3) months, and the median (IQR) annual visits over 2 years was 1.5 (1–2.5) with, respectively, 69 (21.4%) and 145 (45%) patients with 2 and ≥3 visits in the first 2 years.
Progression‐free survival was 95.4% (95% CI 92.1–97.3) after 1 year, 89.2% (95% CI 84.4–92.6) after 2 years, and 86.1% (95% CI 80.3–90.3) after 3 years of alemtuzumab treatment (Figure 4a). We did not detect any impact of DMTs on progression (p = 0.99). The cumulative probability of improvement was 13.5% (95% CI 9.9–18.2) at 1 year and 20.6% (95% CI 15.9–26.3) at 2 years from the first alemtuzumab course (Figure 4b).
FIGURE 4.

Impact of alemtuzumab on disability progression and improvement
Magnetic resonance imaging 3 months after alemtuzumab start was available for 287 patients (89.1%). Four to twelve months after the first Alemtuzumab course (i.e., excluding MRI performed in first 3 months) 36/286 scans (12.6%) had gadolinium‐enhancing lesions and 67/287 (23.3%) had new T2 lesions. Between month 12 to month 24, 42/286 scans (14.7%) had gadolinium‐enhancing lesions and 77/287 (26.8%) had new T2 lesions. Globally 73/287 (25.4%) patients had an activity observed on MRI during the first year, and 83/287 (28.9%) during the second year after alemtuzumab start.
The number of patients with NEDA during the first year of alemtuzumab treatment was 183/290, and was 164/290 over 2 years (Figure 5). Nineteen patients with NEDA (6.6%) during the first year had active disease during the second year. The disease activity‐free probability calculated using the Kaplan–Meier method on time to NEDA was 63.3% (95% CI 57.4–68.7) at 1 year and 54.8% (95% CI 48.6–60.6) after 2 years. We then considered re‐baselining patients after 6 months into alemtuzumab therapy and found NEDA status in 71.6% (65.9–76.6%) after 1 year and 58.9% (52.5–64.6) after 2 years.
FIGURE 5.
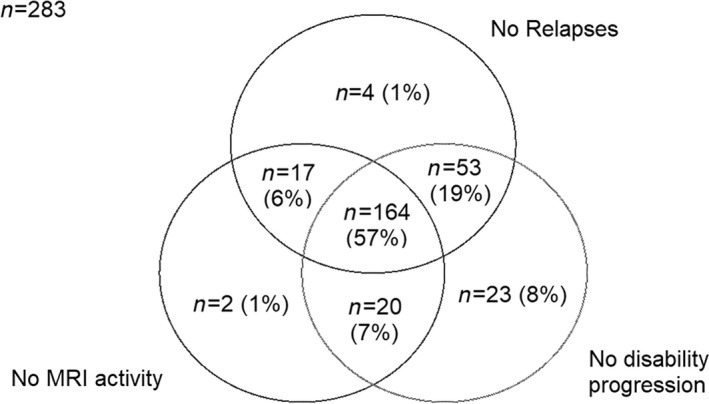
Impact of alemtuzumab on disease activity. MRI, magnetic resonance imaging
A total of 173 patients (57.8%) had adverse events during the study, 130 (43.3%) were IARs, 79 (26.3%) were registered during follow‐up, and 36 patients (12%) had both IARs and adverse events during follow‐up. The most commonly reported IAR was urticaria (n = 63; 21%), followed by headache (n = 45; 15%), fever (n = 37; 12.3%), cutaneous rash (n = 32; 10.7%), and nausea (n = 9; 3%). The most frequently reported adverse events during follow‐up were autoimmune thyroid disorders (n = 34; 11.3%), cutaneous rash (n = 7; 2.3%), respiratory infections (n = 6; 2%), urinary infections (n = 4; 1.3%) and cytomegalovirus (CMV) infections (n = 3; 1%). Adverse events leading to alemtuzumab drop‐out were allergic reaction, severe pancytopenia, gastrostomy infection, anaphylactic shock, and autoimmune hemolytic anemia.
We recorded one death secondary to invasive aspergillosis occurring 3 weeks after alemtuzumab infusion [12].
DISCUSSION
We report the results of a large RWE study investigating the clinical use, effectiveness, and safety of alemtuzumab treatment in Italian multiple sclerosis centers. Our study reveals how alemtuzumab was preferentially considered after fingolimod treatment failure, primarily due to disease relapse or MRI activity, or after natalizumab treatment when JCV positivity caused safety concerns. Due to the prevalent use of fingolimod and natalizumab as second‐line DMTs, we could conclude that alemtuzumab has been considered, shortly after its launch, as a third‐line therapy. Before 2018, alemtuzumab was the only approved alternative for patients failing second‐line DMTs (i.e., fingolimod and natalizumab), as ocrelizumab and cladribine were not available in Italy, and rituximab was still off‐label.
During the last years of observation, switches from first‐line therapies increased, as well as the number of treatment‐naïve patients initiating alemtuzumab. We could speculate that the increased availability of alternative treatments, namely, ocrelizumab, may have reduced the number of switches from second‐line DMTs to alemtuzumab. Also, increased confidence in the use of alemtuzumab may have led Italian neurologists to use it earlier in the disease course.
Fingolimod pretreatment has been associated with severe disease reactivation soon after the initiation of alemtuzumab [13, 14, 15, 16, 17]. However, results are contrasting and scarce [14, 18]. In a German multicenter observational study [18], 50 RRMS patients beneficially switched from fingolimod to alemtuzumab, and the authors concluded that alemtuzumab could be a rescue strategy in patients with high disease activity during fingolimod treatment [18]. In an Italian multicenter observational study [19], alemtuzumab reduced relapses, new T2/gadolinium‐enhancing lesions, and EDSS score, compared to fingolimod, and results were related to washout length or lymphocyte count.
In addition to disease activity and safety, compliance should also be considered when switching to alemtuzumab. Hospital and yearly administrations make alemtuzumab an obvious choice to ensure good treatment adherence, although frequent monitoring of laboratory variables still requires a certain degree of compliance.
As for safety and tolerability, 40% of our patients reported IARs, which is lower than the previously reported incidence of 90–97% [5, 6]. Peri‐infusion acute events include cardiotoxicity, pulmonary alveolar hemorrhage, myocardial infarction, and cervicocephalic arterial dissection [20]. We did not observe any of these rare events, but one patient in this cohort died as a result of fulminating aspergillosis 3 weeks after infusion. This was caused by an unexpected early neutropenia that was not captured and thus remained unmanaged [12].
As for autoimmune disorders, we report 11.3% of patients developing autoimmune thyroid disorders. Previous studies and reports found up to 40% of patients developing autoimmune thyroid disease after alemtuzumab treatment [5, 6, 21, 22, 23, 24, 25].
During follow‐up, infective adverse events were reported in 4.3% of our patients and consisted of respiratory, urinary and CMV infections. This shows an acceptable safety profile, although infective adverse events are frequently reported in alemtuzumab‐treated patients, and more frequently than with anti‐CD20 DMTs, and should be carefully evaluated. Taken together, this should lead to an accurate and cautious selection of candidates able to comply with monitoring procedures.
In our cohort, treatment retention was excellent, with fewer than 10% of patients dropping out, mainly for safety or lack of efficacy. This figure is lower than previously reported in open‐label extension studies [11] and for Italian cohorts [26].
Alemtuzumab was extremely effective, leading to disease relapse suppression independently from previous treatment. This is not surprising for first‐line DMTs [5, 6] or for fingolimod [19], despite the fact that, in a recent retrospective study [31] a suboptimal therapeutic response to alemtuzumab in fingolimod pretreated patients was observed. Regarding the post‐natalizumab phase, where treatment choices are very difficult, our results are novel. The efficacy of alemtuzumab was evident for all patients pretreated with natalizumab, including patients switching for JCV positivity, and patients non‐responsive to natalizumab. Only treatment‐naïve patients had a lower ARR than those on natalizumab and fingolimod. This adds value to the efficacy of alemtuzumab as it can be successfully used in this setting as a rescue strategy.
The ability to halt disability progression, and stimulate disability improvement, are crucial for every DMT and excellent results have been previously reported in RWE studies for natalizumab and rituximab [27, 28]. In a comparative, propensity score‐matched study, alemtuzumab showed similar reduction of disease progression with an inferior ability to favor disease improvement, when compared to natalizumab (hazard ratio 0.35) [29]. A possible explanation for this inferior ability may lie in the population enrolled in that study as it collected patients treated soon after alemtuzumab became available, and a limited number of patients were treated for more than 1 year. This should be carefully considered as the phenotype of patients treated with alemtuzumab changed considerably during recent years to a more favorable one (i.e., first‐line switches or treatment‐naïve). In a more recent retrospective study [31] a higher probability of disability progression at 48 months was found for patients previously treated with fingolimod as compared with naïve patients. This result was not confirmed from our cohort where no heterogeneity among DMTs pre‐alemtuzumab on disability progression over 24 months was detected.
No evidence of disease activity was low when compared to other second‐line DMTs, being 63.3% at 1 year and 54.8% after 2 years of therapy. This is partially due to disease activity during the first 6 months, as re‐baselining after that point increased NEDA status to 71.6% after 1 year and 58.9% after 2 years. This also diverges from the excellent finding of progression‐free survival of 95.4% after 1 year, and 89.2% after 2 years, and also of cumulative improvement of 13.5% at 1 year and 20.6% at 2 years from initiation of alemtuzumab treatment. This is comparable to the well‐known effect of natalizumab on disease improvement [28], but it should be noted that alemtuzumab was used in more adverse conditions, namely, as third‐line or rescue therapy.
We should also consider that disease activity during the year preceding alemtuzumab treatment negatively impacted on the ARR during alemtuzumab treatment (RR 1.38), and that relapses between alemtuzumab courses increased the risk of future relapses. This is confirmed by the fact that, globally, 25% of treated patients had activity on MRI during the first year, and 29% during the second year after alemtuzumab start. Altogether, this shows that alemtuzumab is less effective on disease activity than it is on blocking disability worsening and in stimulating disability improvement.
The recent change in the alemtuzumab summary of product characteristics did not impact on the present population, as all of our patients had been treated before April 2019. The impact of this change will be evaluated by future studies. It is possible that excluding patients with autoimmune and cardiovascular diseases, and previous episodes of arterial dissection will increase the safety of alemtuzumab. Excluding patients with cardiovascular disease may also lower the mean age of patients treated with alemtuzumab, thus increasing the overall safety and efficacy of the DMT. Unfortunately, the exclusion of patients with autoimmune diseases may limit its use as an induction DMT in younger patients.
Some limitations of the present study should be noted. First, our 2‐year follow‐up was too short to observe serious adverse events such as secondary autoimmunity that could require additional courses of treatment. Second, the absence of fixed time points for visits could impact on the ability to collect all adverse events and clinical relapses. Third, disability improvement in a chronic and progressive disease can be a transient condition and should be confirmed at subsequent visits in order to observe the duration [30] of the improvement. Further, some MRI scans were missing due to the retrospective nature of the study and this may have influenced the interpretation of disease activity.
In conclusion, we have provided RWE that alemtuzumab is effective regardless of pretreatment status based on clinical variables and previously used DMTs. Washout, on‐treatment relapses, or MRI activity did not translate into disability progression or reduce the possibility of disability improvement, and should not trigger withdrawal from alemtuzumab.
CONFLICT OF INTEREST
Francesco Saccà has received honoraria for public speaking and/or for advisory boards from Alexion, Almirall, Argenx, Avexis, Biogen, Forward Pharma, Lexeo, Merck, Mylan, Novartis, Pomona, Sanofi, Roche, Takeda and Teva. Cinzia Valeria Russo has nothing to disclose. Roberta Grasso has received honoraria for public speaking and/or for advisory boards from Merck, Biogen, Sanofi and Novartis. Jessica Frau serves on scientific advisory boards for Biogen and Genzyme, and has received honoraria as a speaker from Merck Serono, Genzyme, Biogen and Teva. Pietro Annovazzi has received personal compensation for speaking at meetings or participating in advisory boards from Almirall, Biogen, Merck, Mylan, Novartis, Roche, Sanofi and TEVA. Elisabetta Signoriello has received personal compensation from Almirall, Biogen, Genzyme, Novartis and Teva for traveling and advisory boards. Simona Bonavita has received honoraria for public speaking and/or for advisory boards from Roche, Novartis, Teva, Genzyme, Merck Serono and Biogen. Roberta Grasso has received compensation for serving on advisory boards for Merck, Biogen, Sanofi and Novartis. Marinella Clerico has received personal compensation as an invited speaker to conferences or for participating on advisory committee or boards from Merck, Biogen, Novartis, Sanofi‐ Genzyme and Pomona, and has received sponsorship to attend congresses from Merck, Biogen, Novartis, Sanofi‐Genzyme and Almirall. Cinzia Cordioli has received personal compensation for speaking and travel grants from Biogen, Novartis, TEVA, Merck Serono and Almirall. Alice Laroni has received financial support for travel and attending meetings from Merck, Sanofi Genzyme, Teva, Biogen and Novartis. Marco Capobianco has received personal compensation for speaking at meetings or participating on advisory boards from Almirall, Biogen, Merck, Novartis, Roche, Sanofi and TEVA. Valentina Torri Clerici acted as an Advisory Board member of Teva, Merck, Roche, Biogen, Novartis and Almirall, has received funding for traveling by Genzyme, Merck and Roche, and has received honoraria for speaking or writing from Genzyme, Novartis and Almirall. She received support for research projects from Almirall. Arianna Sartori has received funding for travel and/or speaker honoraria from Novartis, Teva, Merk, Genzyme, Roche and Biogen. Paola Cavalla received support for participation in scientific meetings or personal compensation for speaking at meetings or on advisory boards from Almirall, Biogen, Merck, Novartis, Roche, Sanofi and TEVA. Giorgia Teresa Maniscalco received personal compensation from Novartis, Genzyme, Biogen, Merck Serono, and TEVA for public speaking and advisory boards. Sara La Gioia has nothing to disclose. Francesca Caleri has received honoraria for advisory boards and/or for public speaking, and/or travel grants from Biogen, Merck, Teva, Sanofi‐Genzyme and Roche. Alessia Giugno has received sponsorship for travel and attending meeting from Sanofi‐Genzyme. Rosa Iodice has received honoraria for advisory boards and/or travel grants and/or public speaking fees from Biogen, Merck, Mylan, Sanofi and Roche. Antonio Carotenuto has received research grants from ALMIRALL and ECTRIMS‐MAGNIMS society, and honoraria form Novartis, Merck and Biogen. Eleonora Cocco has received research grants and honoraria as a speaker and member of advisory boards from Almirall, Bayer, Biogen Idec, Merck Serono, Novartis, Sanofi Genzyme, Teva and Roche. Giuseppe Fenu has received honoraria for consultancy from Novartis and Biogen and for speaking from Merck and Teva. Mauro Zaffaroni has received financial support for attending scientific meetings from Biogen, Genzyme, Merck Serono, Novartis, Sanofi‐Aventis and Teva, and received funds for his department from Novartis. Damiano Baroncini has received honoraria from Almirall for the creation of editorial publications, and travel grants for participation to international congresses from Genzyme and TEVA. Giacomo Lus has received personal compensation for activities with Biogen Idec, Merck Serono, Novartis and Sanofi‐Aventis Pharmaceuticals, and with Teva Neuroscience as a consultant and speaker, and has received research support from Biogen Idec, Merck Serono and Novartis. Antonio Gallo A.G. received honoraria for speaking and travel grants from Biogen, Sanofi‐Aventis, Merck, Genzyme, Teva and Novartis. Stefania De Mercanti has received sponsorship to attend congresses from Merck, Biogen, Novartis, Sanofi‐Genzyme and Almirall. Caterina Lapucci has nothing to disclose. Valeria Di Francescantonio has nothing to disclose. Maria Pia Sormani has received consulting fees from Biogen, Merck, Teva, Genzyme, Roche, Novartis, GeNeuro and Medday. Laura Brambilla has received honoraria for speaking from Novartis and for traveling from Sanofi‐Genzyme and Roche, and has acted as an Advisory Board member of Sanofi‐Genzyme and is involved as principal investigator in clinical trials for Roche and Merck‐Serono. Alessio Signori has received teaching honoraria from Novartis outside this work.
AUTHOR CONTRIBUTIONS
Cinzia Valeria Russo: Data curation (equal); Writing ‐‐ original draft (equal); Writing ‐‐ review and editing (equal). Francesco Saccà: Conceptualization (lead); Data curation (lead); Software (lead); Writing ‐‐ original draft (lead); Writing ‐‐ review and editing (equal). Jessica Frau: Data curation (equal); Writing ‐‐ original draft (equal). Pietro Annovazzi: Data curation (equal); Writing ‐‐ original draft (equal). Elisabetta Signoriello: Data curation (equal); Writing ‐‐ original draft (equal). Simona Bonavita: Data curation (equal); Writing ‐‐ original draft (equal). Roberta Grasso: Data curation (equal); Writing ‐‐ original draft (equal). Marinella Clerico: Data curation (equal); Writing ‐‐ original draft (equal). Cinzia Cordioli: Data curation (equal); Writing ‐‐ original draft (equal). Alice Laroni: Data curation (equal); Writing ‐‐ original draft (equal). Marco Capobianco: Data curation (equal); Writing ‐‐ original draft (equal). Valentina Torri Clerici: Data curation (equal); Writing ‐‐ original draft (equal). Arianna Sartori: Data curation (equal); Writing ‐‐ original draft (equal). Paola Cavalla: Data curation (equal); Writing ‐‐ original draft (equal). Giorgia Teresa Maniscalco: Data curation (equal); Writing ‐‐ original draft (equal). Sara La Gioia: Data curation (equal); Writing ‐‐ original draft (equal). Francesca Caleri: Data curation (equal); Writing ‐‐ original draft (equal). Alessia Giugno: Data curation (equal); Writing ‐‐ original draft (equal). Rosa Iodice: Data curation (equal); Writing ‐‐ original draft (equal). Antonio Carotenuto: Data curation (equal); Writing ‐‐ original draft (equal). Eleonora Cocco: Data curation (equal); Writing ‐‐ original draft (equal). Giuseppe Fenu: Data curation (equal); Writing ‐‐ original draft (equal). Mauro Zaffaroni: Data curation (equal); Writing ‐‐ original draft (equal). Damiano Baroncini: Data curation (equal); Writing ‐‐ original draft (equal). Giacomo Lus: Data curation (equal); Writing ‐‐ original draft (equal). Antonio Gallo: Data curation (equal); Writing ‐‐ original draft (equal). Stefania De Mercanti: Data curation (equal); Writing ‐‐ original draft (equal). Caterina Lapucci: Data curation (equal); Writing ‐‐ original draft (equal). Valeria Di Francescantonio: Data curation (equal); Writing ‐‐ original draft (equal). Laura Brambilla: Data curation (equal); Writing ‐‐ original draft (equal). Maria Pia Sormani: Resources (supporting); Writing ‐‐ original draft (equal). Alessio Signori: Data curation (lead); Formal analysis (lead); Methodology (lead); Software (lead); Writing ‐‐ original draft (lead); Writing ‐‐ review and editing (lead).
Supporting information
ACKNOWLEDGEMENTS
This study did not receive any financial support. Open Access funding provided by Universita degli Studi di Napoli Federico II within the CRUI‐CARE Agreement. [Correction added on 21 May 2022, after first online publication: CRUI funding statement has been added.]
Russo CV, Saccà F, Frau J, et al. A real‐world study of alemtuzumab in a cohort of Italian patients. Eur J Neurol. 2022;29:257–266. 10.1111/ene.15121
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Cox AL, Thompson SA, Jones JL, et al. Lymphocyte homeostasis following therapeutic lymphocyte depletion in multiple sclerosis. Eur J Immunol. 2005;35(11):3332‐3342. [DOI] [PubMed] [Google Scholar]
- 2. Hu Y, Turner MJ, Shields J, et al. Investigation of the mechanism of action of alemtuzumab in a human CD52 transgenic mouse model. Immunology. 2009;128(2):260‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gilmore W, Lund BT, Li P, et al. Repopulation of T, B, and NK cells following alemtuzumab treatment in relapsing‐remitting multiple sclerosis. J Neuroinflammation. 2020;17(1):189. 10.1186/s12974-020-01847-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rolla S, Maglione A, De Mercanti SF, Clerico M. The meaning of immune reconstitution after alemtuzumab therapy in multiple sclerosis. Cells. 2020;9:1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first‐line treatment for patients with relapsing‐remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1819‐1828. [DOI] [PubMed] [Google Scholar]
- 6. Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease‐modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1829‐1839. [DOI] [PubMed] [Google Scholar]
- 7. Coles AJ, Boyko AN, De Seze J, et al. Alemtuzumab durably improves clinical outcomes in patients with active RRMS in the absence of continuous treatment: 7‐year follow‐up of CARE‐MS I patients (TOPAZ study). Mult Scler. 2017;23(3 Suppl.):P1188. [Google Scholar]
- 8. Havrdova E, Arnold DL, Cohen JA, et al. Alemtuzumab CARE‐MS I 5‐year follow‐up: durable efficacy in the absence of continuous MS therapy. Neurology. 2017;89(11):1107‐1116. 10.1212/WNL.0000000000004313. Epub 2017 Aug 23. Erratum in: Neurology. 2018 Apr 17;90(16):755. PMID: 28835401; PMCID: PMC5595278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. European Public Assessment Report (EPAR), European Medicines Agency (EMA). https://www.ema.europa.eu/en/medicines/human/EPAR/lemtrada. Accessed September 24, 2020.
- 10. Ziemssen T, Bass AD, Berkovich R, et al. Efficacy and safety of alemtuzumab through 9 years of follow‐up in patients with highly active disease: post hoc analysis of CARE‐MS I and II patients in the TOPAZ extension study. CNS Drugs. 2020;34(9):973‐988. 10.1007/s40263-020-00749-x. PMID: 32710396; PMCID: PMC7447657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Comi G, Alroughani R, Boster AL, et al. Efficacy of alemtuzumab in relapsing‐ remitting MS patients who received additional courses after the initial two courses: pooled analysis of the CARE‐MS, extension, and TOPAZ studies. Mult Scler. 2019;26(14):1866‐1876. Nov 25:1352458519888610. 10.1177/1352458519888610. PMID: 31762387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Russo CV, Saccà F, Paternoster M, et al. Post‐mortem diagnosis of invasive pulmonary aspergillosis after alemtuzumab treatment for multiple sclerosis. Mult Scler. 2020;26(1):123‐126. 10.1177/1352458518813110. Epub 2019 Mar 18 PMID:30882274. [DOI] [PubMed] [Google Scholar]
- 13. Notas K, Papadaki E, Orologas A, et al. Switching from fingolimod to alemtuzumab in patients with highly active relapsing‐remitting multiple sclerosis: α case series. Mult Scler Relat Disord. 2020;38:101517. [DOI] [PubMed] [Google Scholar]
- 14. Haghikia A, Dendrou CA, Schneider R, et al. Severe B‐cell‐mediated CNS disease secondary to alemtuzumab therapy. Lancet Neurol. 2017;16(2):104‐106. [DOI] [PubMed] [Google Scholar]
- 15. Willis M, Pearson O, Illes Z, et al. An observational study of alemtuzumab following fingolimod for multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2017;4(2):e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bernard‐Valnet R, Pignolet B, Biotti D, et al. Unexpected high multiple sclerosis activity after switching from fingolimod to alemtuzumab. Mult Scler Relat Disord. 2018;25:216‐218. [DOI] [PubMed] [Google Scholar]
- 17. Wehrum T, Beume LA, Stich O, et al. Activation of disease during therapy with alemtuzumab in 3 patients with multiple sclerosis. Neurology. 2018;90(7):e601‐e605. [DOI] [PubMed] [Google Scholar]
- 18. Huhn K, Bayas A, Doerck S, et al. Alemtuzumab as rescue therapy in a cohort of 50 relapsing–remitting MS patients with breakthrough disease on fingolimod: a multi‐center observational study. J Neurol. 2018;265(7):1521‐1527. [DOI] [PubMed] [Google Scholar]
- 19. Frau J, Saccà F, Signori A, et al. Outcomes after fingolimod to alemtuzumab treatment shift in relapsing–remitting MS patients: a multicentre cohort study. J Neurol. 2019;266:2440‐2446. 10.1007/s00415-019-09424-8 [DOI] [PubMed] [Google Scholar]
- 20. Hartung HP, Mares J, Barnett MH. Alemtuzumab: rare serious adverse events of a high‐efficacy drug. Mult Scler. 2020;26:737‐740. [DOI] [PubMed] [Google Scholar]
- 21. Willis MD, Harding KE, Pickersgill TP, et al. Alemtuzumab for multiple sclerosis: long term follow‐up in a multi‐centre cohort. Mult Scler. 2016;22(9):1215‐1223. [DOI] [PubMed] [Google Scholar]
- 22. Tuohy O, Costelloe L, Hill‐Cawthorne G, et al. Alemtuzumab treatment of multiple sclerosis: long‐term safety and efficacy. J Neurol Neurosurg Psychiatry. 2015;86(2):208‐215. [DOI] [PubMed] [Google Scholar]
- 23. Coles AJ, Fox E, Vladic A, et al. Alemtuzumab more effective than interferon β‐1a at 5‐year follow‐up of CAMMS223 clinical trial. Neurology. 2012;78(14):1069‐1078. [DOI] [PubMed] [Google Scholar]
- 24. Scappaticcio L, Castellana M, Virili C, et al. Alemtuzumab‐ induced thyroid events in multiple sclerosis: a systematic review and meta‐analysis. J Endocrinol Invest. 2020;43(2):219‐229. [DOI] [PubMed] [Google Scholar]
- 25. Pariani N, Willis M, Muller I, et al. Alemtuzumab‐induced thy‐ roid dysfunction exhibits distinctive clinical and immunological features. J Clin Endocrinol Metab. 2018;103(8):3010‐3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saccà F, Lanzillo R, Signori A, et al. Determinants of therapy switch in multiple sclerosis treatment‐naïve patients: a real‐life study. Mult Scler. 2019;25(9):1263‐1272. 10.1177/1352458518790390 [DOI] [PubMed] [Google Scholar]
- 27. Zecca C, Bovis F, Novi G, et al. Treatment of multiple sclerosis with rituximab: a multicentric Italian‐Swiss experience. Mult Scler. 2019;1:1352458519872889. 10.1177/1352458519872889 [DOI] [PubMed] [Google Scholar]
- 28. Baroncini D, Ghezzi A, Annovazzi PO, et al. Natalizumab versus fingolimod in patients with relapsing‐remitting multiple sclerosis non‐responding to first‐line injectable therapies. Mult Scler. 2016;22(10):1315‐1326. 10.1177/1352458516650736 [DOI] [PubMed] [Google Scholar]
- 29. Kalincik T, Brown JWL, Robertson N, et al. Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing‐remitting multiple sclerosis: a cohort study. Lancet Neurol. 2017;16(4):271‐281. 10.1016/S1474-4422(17)30007-8 [DOI] [PubMed] [Google Scholar]
- 30. Signori A, Boffa G, Bovis F, et al. Prevalence of disability improvement as a potential outcome for multiple sclerosis trials. Mult Scler. 2020;27(5):706‐711 10.1177/1352458520936236 [DOI] [PubMed] [Google Scholar]
- 31. Pfeuffer S, Ruck T, Pul R, et al. Impact of previous disease‐modifying treatment on effectiveness and safety outcomes, among patients with multiple sclerosis treated with alemtuzumab. J Neurol Neurosurg Psychiatry. 2021;92(9):1007‐1013. 10.1136/jnnp-2020-325304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


